Plant-Based HSP90 Inhibitors in Breast Cancer Models: A Systematic Review
Abstract
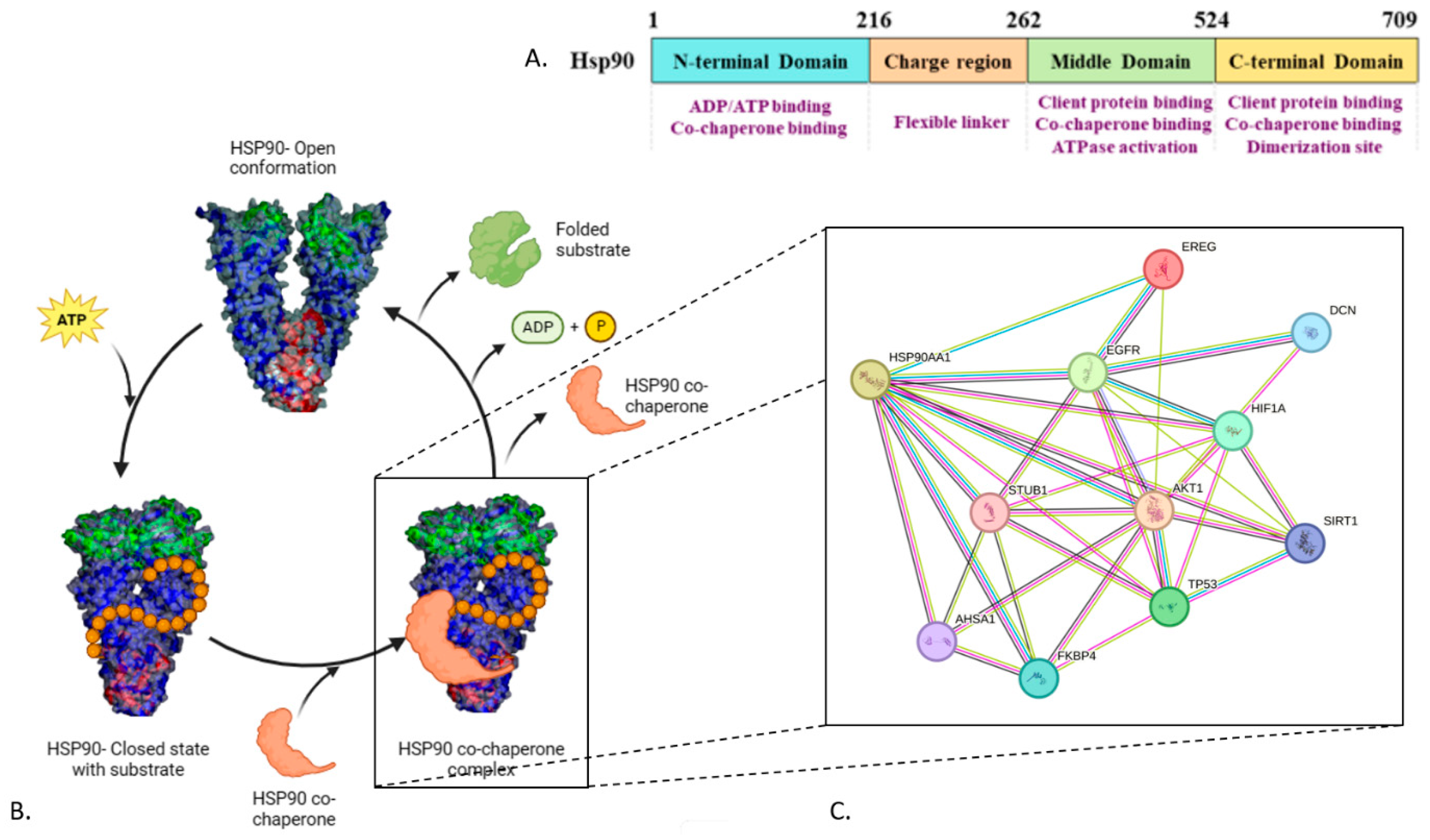
1. Introduction
2. Material and Methods
2.1. Search Strategy
2.2. Research Questions
2.3. Articles Selection
2.4. Data Extraction
2.5. Risk-of-Bias Evaluation
3. Results
3.1. Search Results
3.2. Risk-of-Bias Evaluation
3.3. Plant Extracts
3.4. Compounds
3.5. Techniques Used to Explore the HSP90 Inhibitory Effect of Plant Extracts
3.6. Breast Cancer Models
3.7. HSP90 Inhibition Pathways Explored In Vitro
3.8. HSP90 Inhibition Pathways Explored In Vivo
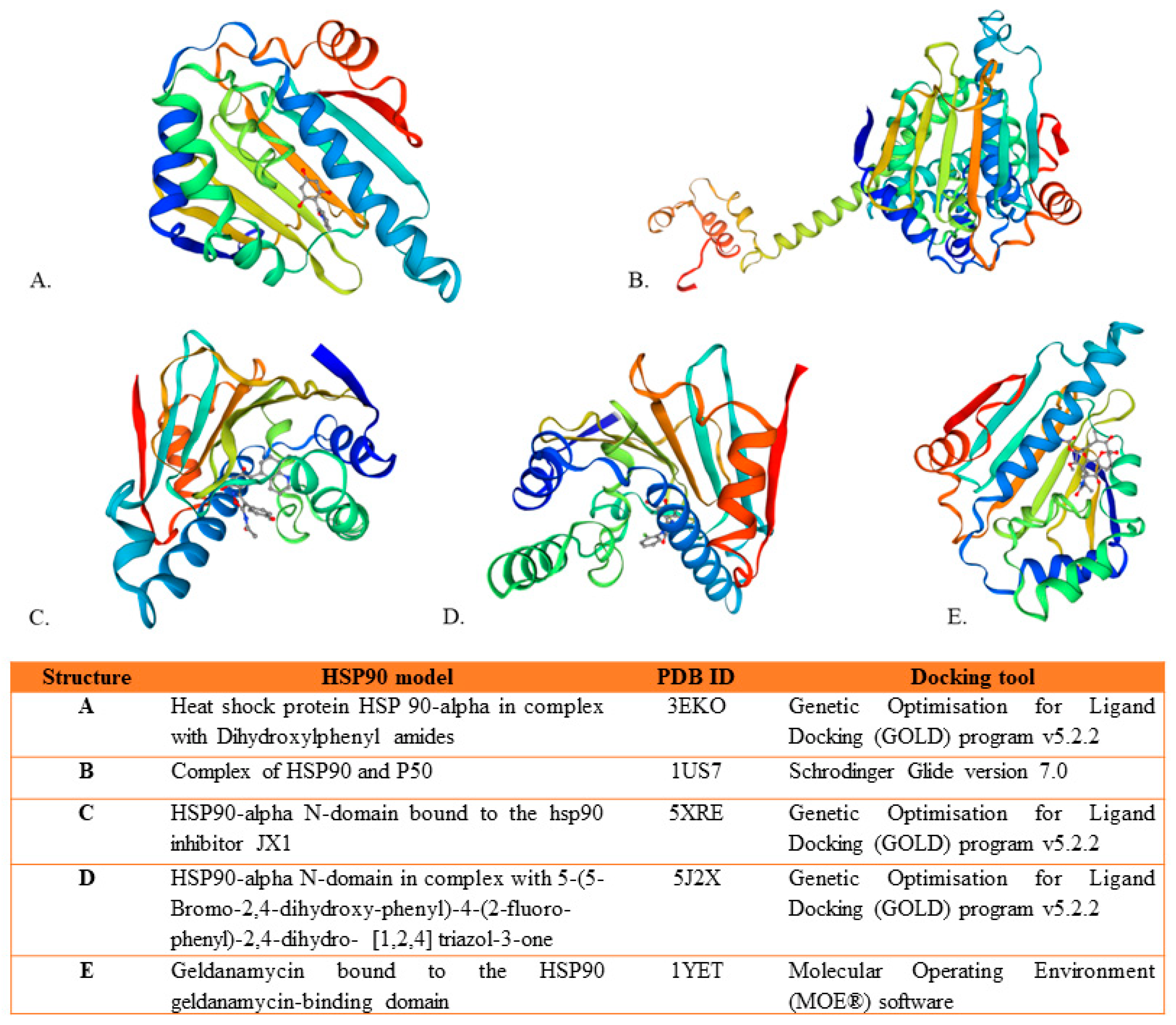
3.9. HSP90 Binding Explored In Silico
4. Discussion
5. Conclusions
Limitations and Future Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSP90 | heat-shock protein |
| IC50 | half-maximal inhibitory concentration |
| NTD | N-terminal domain |
| MD | middle domain |
| CTD | C-terminal domain |
| GRP94 | glucose-regulated protein 94 |
| TRAP1 | tumor necrosis factor receptor-associated protein 1 |
| ER | estrogen receptor |
| Akt | antiapoptotic kinase |
| GA | Geldanamycin |
| HSR | heat-shock response |
Appendix A
| Equation | Database | N° of Articles |
| “HSP90 Heat-Shock Proteins” [Mesh] AND “Plant Extracts” [Mesh] AND “Breast Neoplasms” [Mesh] | PubMed | 4 |
| “HSP90 Heat-Shock Proteins” AND “Plant Extracts” AND “Breast Neoplasms” | Scopus | 3 |
| “HSP90 Heat-Shock Proteins” AND “Plant Extracts” AND “Breast Neoplasms” | Dimensions | 3 |
| ((ALL = (HSP90 Heat Shock Proteins)) AND ALL = (Breast cancer)) AND ALL = (Plant extracts) | WOS | 4 |
| ((“Breast Neoplasms” OR “Breast Neoplasm” OR “Neoplasm, Breast” OR “Breast Tumors” OR “Breast Tumor” OR “Tumor, Breast” OR “Tumors, Breast” OR “Neoplasms, Breast” OR “Breast Cancer” OR “Cancer, Breast” OR “Mammary Cancer” OR “Cancer, Mammary” OR “Cancers, Mammary” OR “Mammary Cancers” OR “Malignant Neoplasm of Breast” OR “Breast Malignant Neoplasm” OR “Breast Malignant Neoplasms” OR “Malignant Tumor of Breast” OR “Breast Malignant Tumor” OR “Breast Malignant Tumors” OR “Cancer of Breast” OR “Cancer of the Breast” OR “Mammary Carcinoma, Human” OR “Carcinoma, Human Mammary” OR “Carcinomas, Human Mammary” OR “Human Mammary Carcinomas” OR “Mammary Carcinomas, Human” OR “Human Mammary Carcinoma” OR “Mammary Neoplasms, Human” OR “Human Mammary Neoplasm” OR “Human Mammary Neoplasms” OR “Neoplasm, Human Mammary” OR “Neoplasms, Human Mammary” OR “Mammary Neoplasm, Human” OR “Breast Carcinoma” OR “Breast Carcinomas” OR “Carcinoma, Breast” OR “Carcinomas, Breast”) AND (“Plant Extracts” OR “Extracts, Plant” OR “Plant Extract” OR “Extract, Plant” OR “Herbal Medicines” OR “Medicines, Herbal” OR “Phytochemicals” OR “Dietary Phytochemical” OR “Phytochemical, Dietary” OR “Plant Bioactive Compound” OR “Bioactive Compound, Plant” OR “Compound, Plant Bioactive” OR “Plant Biologically Active Compound” OR “Dietary Phytochemicals” OR “Phytochemicals, Dietary” OR “Plant Bioactive Compounds” OR “Bioactive Compounds, Plant” OR “Compounds, Plant Bioactive” OR “Plant Biologically Active Compounds” OR “Plant-Derived Chemical” OR “Chemical, Plant-Derived” OR “Plant Derived Chemical” OR “Bioactive Compounds, Plant” OR “Compounds, Plant Bioactive” OR “Plant Bioactive Compounds” OR “Biologically Active Compounds, Plant” OR “Phytonutrient” OR “Plant-Derived Chemicals” OR “Chemicals, Plant-Derived” OR “Plant Derived Chemicals” OR “Phytonutrients” OR “Plant-Derived Compounds” OR “Compounds, Plant-Derived” OR “Plant Derived Compounds” OR “Plant-Derived Compound” OR “Compound, Plant-Derived” OR “Plant Derived Compound”) AND (“HSP90 Heat-Shock Proteins” OR “ HSP90 Heat Shock Proteins”)) | PubMed | 8 |
| Dimensions | 6 | |
| (“HSP90 Heat-Shock Proteins” OR “HSP90 Heat Shock Proteins”) AND (“Breast Neoplasm” OR “Breast Tumor” OR “Breast Cancer” OR “Mammary Cancer”) AND (“Plant Extract” OR “Phytochemical” OR “Plant-Derived Chemical” OR “Bioactive Compound, Plant”) | Scopus | 6 |
| Dimensions | 4 | |
| PubMed | 6 | |
| (ALL = ((HSP90 Heat-Shock Proteins) OR (HSP90 Heat Shock Proteins))) AND (ALL = ((Breast Neoplasm) OR (Breast Tumor) OR (Breast Cancer) OR (Mammary Cancer))) AND (ALL = ((Plant Extract) OR (Phytochemical) OR (Plant-Derived Chemical) OR (Bioactive Compound, Plant))) | WOS | 7 |
References
- Piaz, F.D.; Terracciano, S.; De Tommasi, N.; Braca, A. Hsp90 Activity Modulation by Plant Secondary Metabolites. Planta Medica 2015, 81, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H. Antitumor agents based on natural product models. J. Pharm. Sci. 1982, 71, 478–479. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.E.; Raghavendra, N.M.; Penido, C. Natural heat shock protein 90 inhibitors in cancer and inflammation. Eur. J. Med. Chem. 2020, 189, 112063. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Jodha, B.; Prabina, K.E.; Aggarwal, N.; Patel, S. Recent advances in phytochemical based nano-drug delivery systems to combat breast cancer: A review. J. Drug Deliv. Sci. Technol. 2022, 77, 103832. [Google Scholar] [CrossRef]
- Bray, F.; McCarron, P.; Parkin, D.M. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004, 6, 229–239. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, J.C.C. Carcinogenesis and Natural History of Breast Cancer. In Breast Diseases: An Evidence-Based Pocket Guide; Novita, G., Frasson, A.L., Millen, E.C., Zerwes, F., Cavalcante, F.P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 227–235. [Google Scholar] [CrossRef]
- Shrihastini, V.; Muthuramalingam, P.; Adarshan, S.; Sujitha, M.; Chen, J.-T.; Shin, H.; Ramesh, M. Plant Derived Bioactive Compounds, Their Anti-Cancer Effects and In Silico Approaches as an Alternative Target Treatment Strategy for Breast Cancer: An Updated Overview. Cancers 2021, 13, 6222. [Google Scholar] [CrossRef] [PubMed]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Khan, M.I.; Bouyahya, A.; Hachlafi, N.E.L.; El Menyiy, N.; Akram, M.; Sultana, S.; Zengin, G.; Ponomareva, L.; Shariati, M.A.; Ojo, O.A.; et al. Anticancer properties of medicinal plants and their bioactive compounds against breast cancer: A review on recent investigations. Environ. Sci. Pollut. Res. 2022, 29, 24411–24444. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Chen, J.; Yang, F.; Zhou, Z.; Liu, Y.; Guo, Y.; Hu, H.; Gao, H.; Li, H.; Zhou, W.; et al. BJ-B11, an Hsp90 Inhibitor, Constrains the Proliferation and Invasion of Breast Cancer Cells. Front. Oncol. 2019, 9, 1447. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2019.01447 (accessed on 22 July 2023). [CrossRef] [PubMed]
- Jafarinezhad, S.; Darban, R.A.; Javid, H.; Hashemy, S.I. The SP/NK1R system promotes the proliferation of breast cancer cells through NF-κB-mediated inflammatory responses. Cell Biochem. Biophys. 2023, 81, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bi, X. Heat Shock Proteins and Breast Cancer. Int. J. Mol. Sci. 2024, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Rampogu, S.P.S. Natural compounds as potential Hsp90 inhibitors for breast cancer-Pharmacophore guided molecular modelling studies. Comput. Biol. Chem. 2019, 83, 107113. [Google Scholar] [CrossRef] [PubMed]
- Yufu, Y.; Nishimura, J.; Nawata, H. High constitutive expression of heat shock protein 90α in human acute leukemia cells. Leuk. Res. 1992, 16, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef]
- Birbo, B.; Madu, E.E.; Madu, C.O.; Jain, A.; Lu, Y. Role of HSP90 in Cancer. Int. J. Mol. Sci. 2021, 22, 10317. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Schnaider, T.; Soti, C.; Prohászka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef]
- Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Jackson, S.E. Hsp90: Structure and Function. In Molecular Chaperones; Jackson, S., Ed.; In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2013; pp. 155–240. [Google Scholar] [CrossRef]
- Seo, Y.H. Organelle-specific Hsp90 inhibitors. Arch. Pharmacal Res. 2015, 38, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Tummalapalli, S.R.; Rotella, D.P. Progress in the discovery and development of heat shock protein 90 (Hsp90) inhibitors. J. Med. Chem. 2014, 57, 8718–8728. [Google Scholar] [CrossRef]
- Önay-Uçar, E. Heat Shock Proteins and Cancer: Plant Based Therapy. In Heat Shock Protein-Based Therapies; Asea, A.A.A., Almasoud, N.N., Krishnan, S., Kaur, P., Eds.; In Heat Shock Proteins; Springer International Publishing: Cham, Switzerland, 2015; pp. 27–48. [Google Scholar] [CrossRef]
- Kamal, A.; Thao, L.; Sensintaffar, J.; Zhang, L.; Boehm, M.F.; Fritz, L.C.; Burrows, F.J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003, 425, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 2002, 8, S55–S61. [Google Scholar] [CrossRef]
- Yuno, A.; Lee, M.J.; Lee, S.; Tomita, Y.; Rekhtman, D.; Moore, B.; Trepel, J.B. Clinical Evaluation and Biomarker Profiling of Hsp90 Inhibitors. Methods Mol. Biol. 2018, 1709, 423–441. [Google Scholar] [CrossRef]
- Sanchez, J.; Carter, T.R.; Cohen, M.S.; Blagg, B.S.J. Old and New Approaches to Target the Hsp90 Chaperone. Curr. Cancer Drug Targets 2020, 20, 253–270. [Google Scholar] [CrossRef]
- Mishra, S.J.; Khandelwal, A.; Bannerjee, M.; Balch, M.; Peng, S.; Davis, R.E.; Merfeld, T.; Munthali, V.; Deng, J.; Matts, R.L.; et al. Selective Inhibition of the Hsp90α Isoform. Angew. Chem. Int. Ed. 2021, 60, 10547–10551. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, L.; Svartsjö, S.; Bäck, V.; de Thonel, A.; Mezger, V.; Sabéran-Djoneidi, D.; Roos-Mattjus, P. Gambogic acid and gambogenic acid induce a thiol-dependent heat shock response and disrupt the interaction between HSP90 and HSF1 or HSF2. Cell Stress Chaperon 2021, 26, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Santagata, S.; Lin, N.U. Inhibiting HSP90 to treat cancer: A strategy in evolution. Curr. Mol. Med. 2012, 12, 1108–1124. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Hatial, I.; Keegan, B.M.; Blagg, B.S.J. Assay design and development strategies for finding Hsp90 inhibitors and their role in human diseases. Pharmacol. Ther. 2020, 221, 107747. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C. HSP90 inhibition without heat shock response. Blood 2018, 132, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Mimnaugh, E.G.; De Costa, B.; Myers, C.E.; Neckers, L.M. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: Essential role for stress proteins in oncogenic transformation. Proc. Natl. Acad. Sci. USA 1994, 91, 8324–8328. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, C.; Meulman, P.A.; Wnuk, R.J.; Peterson, D.H. Geldanamycin, a new antibiotic. J. Antibiot. 1970, 23, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, J.M.; Veeraraghavan, V.P.; Kullappan, M.; Velmurugan, D.; Vennila, R.; Rupert, S.; Dorairaj, S.; Surapaneni, K.M. Molecular modeling studies of the effects of withaferin A and its derivatives against oncoproteins associated with breast cancer stem cell activity. Process. Biochem. 2021, 111, 186–199. [Google Scholar] [CrossRef]
- Nagaprashantha, L.D.; Singhal, J.; Chikara, S.; Gugiu, G.; Horne, D.; Awasthi, S.; Salgia, R.; Singhal, S.S. 2 ’-Hydroxyflavanone induced changes in the proteomic profile of breast cancer cells. J. Proteom. 2019, 192, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bishayee, A. Trianthema portulacastrum Linn. Displays Anti-Inflammatory Responses during Chemically Induced Rat Mammary Tumorigenesis through Simultaneous and Differential Regulation of NF-kappa B and Nrf2 Signaling Pathways. Int. J. Mol. Sci. 2015, 16, 2426–2445. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Han, S.; Wang, N.; Mo, F.; Loo, T.Y.; Shen, J.; Huang, H.; Chen, J. Bioactivity-Guided Identification and Cell Signaling Technology to Delineate the Lactate Dehydrogenase A Inhibition Effects of Spatholobus suberectus on Breast Cancer. PLoS ONE 2013, 8, e56631. [Google Scholar] [CrossRef]
- Wang, H.-C.; Tsai, Y.-L.; Wu, Y.-C.; Chang, F.-R.; Liu, M.-H.; Chen, W.-Y.; Wu, C.-C. Withanolides-induced breast cancer cell death is correlated with their ability to inhibit heat protein 90. PLoS ONE 2012, 7, e37764. [Google Scholar] [CrossRef] [PubMed]
- Mendis, A.S.; Thabrew, I.; Samarakoon, S.R.; Tennekoon, K.H. Modulation of expression of heat shock proteins and apoptosis by Flueggea leucopyrus (Willd) decoction in three breast cancer phenotypes. BMC Complement. Altern. Med. 2015, 15, 404. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; El-Hefnawy, H.M.; Osman, S.M.; El-Raey, M.A.; Mokhtar, F.A.; Ibrahim, H.A. Antioxidant, anti-inflammatory and cytotoxic activities of Jasminum Multiflorum (Burm. F.) Andrews leaves towards MCF-7 breast cancer and HCT 116 colorectal cell lines and identification of bioactive metabolites. Anti-Cancer Agents Med. Chem. 2021, 21, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Yu, J.-H.; Wu, J.-N.; Tashiro, S.-I.; Onodera, S.; Minami, M.; Ikejima, T. P53-mediated cell cycle arrest and apoptosis through a caspase-3- independent, but caspase-9-dependent pathway in oridonin-treated MCF-7 human breast cancer cells. Acta Pharmacol. Sin. 2007, 28, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Roudbari, L.S.; Eslami, M.; Movahedi, M.; Golab, F. Evaluation of the Anti-Metastatic Effect of Foeniculum Vulgare on the Protein Expression of HSP 70 & 90 in Balb/c Mice with 4t1 Model of Breast Cancer. Asian Pac. J. Cancer Prev. 2023, 24, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Chang, F.-R.; Huang, Z.-Y.; Chen, J.-H.; Wu, Y.-C.; Wu, C.-C. Tubocapsenolide A, a novel withanolide, inhibits proliferation and induces apoptosis in MDA-MB-231 cells by thiol oxidation of heat shock proteins. J. Biol. Chem. 2008, 283, 17184–17193. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, J.M.; van der Ende, J.; Limpens, J.; de Vries, H.J.C.; Schallig, H.D.F.H. Safety and efficacy of allylamines in the treatment of cutaneous and mucocutaneous leishmaniasis: A systematic review. PLoS ONE 2021, 16, e0249628. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; Patnode, C.D.; Berkman, N.D.; Bass, E.B.; Chang, S.; Hartling, L.; Murad, M.H.; Treadwell, J.R.; Kane, R.L. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J. Clin. Epidemiol. 2018, 97, 26–34. [Google Scholar] [CrossRef]
- Hsieh, P.-W.; Huang, Z.-Y.; Chen, J.-H.; Chang, F.-R.; Wu, C.-C.; Yang, Y.-L.; Chiang, M.Y.; Yen, M.-H.; Chen, S.-L.; Yen, H.-F.; et al. Cytotoxic Withanolides from Tubocapsicum anomalum. J. Nat. Prod. 2007, 70, 747–753. [Google Scholar] [CrossRef]
- Xiang, K.; Li, C.; Li, M.-X.; Song, Z.-R.; Ma, X.-X.; Sun, D.-J.; Li, H.; Chen, L.-X. Withanolides isolated from Tubocapsicum anomalum and their antiproliferative activity. Bioorganic Chem. 2021, 110, 104809. [Google Scholar] [CrossRef] [PubMed]
- Liew, H.Y.; Tan, X.Y.; Chan, H.H.; Khaw, K.Y.; Ong, Y.S. Natural HSP90 inhibitors as a potential therapeutic intervention in treating cancers: A comprehensive review. Pharmacol. Res. 2022, 181, 106260. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Kim, Y.-J.; Park, S.; Park, M.; Farrand, L.; Nguyen, C.-T.; Ann, J.; Nam, G.; Park, H.-J.; Lee, J.; et al. A novel HSP90 inhibitor targeting the C-terminal domain attenuates trastuzumab resistance in HER2-positive breast cancer. Mol. Cancer 2020, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Huang, W.; Liu, R.; Kong, Y.; Liu, Y.; Chen, X.; Xu, J. Synergistic Activity of the HSP90 Inhibitor Ganetespib with Lapatinib Reverses Acquired Lapatinib Resistance in HER2-Positive Breast Cancer Cells. Front. Pharmacol. 2021, 12, 651516. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2021.651516 (accessed on 11 July 2023). [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y. Recent Advances in the Discovery of Novel HSP90 Inhibitors: An Update from 2014. Curr. Drug Targets 2020, 21, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.J.; Liu, W.; Beebe, K.; Banerjee, M.; Kent, C.N.; Munthali, V.; Koren, J.; Taylor, J.A.; Neckers, L.M.; Holzbeierlein, J.; et al. The Development of Hsp90β-Selective Inhibitors to Overcome Detriments Associated with pan-Hsp90 Inhibition. J. Med. Chem. 2021, 64, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Tomašič, T.; Durcik, M.; Keegan, B.M.; Skledar, D.G.; Zajec, Ž.; Blagg, B.S.J.; Bryant, S.D. Discovery of Novel Hsp90 C-Terminal Inhibitors Using 3D-Pharmacophores Derived from Molecular Dynamics Simulations. Int. J. Mol. Sci. 2020, 21, 6898. [Google Scholar] [CrossRef] [PubMed]
- Arba, M.; Ruslin, R.; Kartasasmita, R.E.; Ibrahiim, S.; Tjahjono, D.H. Insight into the Interaction of Cationic Porphyrin-Anthraquinone Hybrids with Hsp90: In Silico Analysis. J. Math. Fundam. Sci. 2018, 50, 303–314. [Google Scholar] [CrossRef]
- Zajec, Ž.; Dernovšek, J.; Gobec, M.; Tomašič, T. In Silico Discovery and Optimisation of a Novel Structural Class of Hsp90 C-Terminal Domain Inhibitors. Biomolecules 2022, 12, 884. [Google Scholar] [CrossRef]

| PICOS Elements | Criteria |
|---|---|
| Population | Plant-based Hsp90 compounds |
| Intervention | Anticancer effect on breast cancer cells and HSP90 inhibition |
| Comparators | Other HSP90 inhibitors (including nonplant-based HSP90 inhibitors) or no treatment |
| Outcome | Effectiveness of plant-based HSP90 inhibitors in blocking breast cancer cell growth |
| Study design | in vitro, in vivo, and in silico studies |
| Criteria | Questions to Consider |
|---|---|
| Selection bias |
|
| Performance bias |
|
| Detection bias |
|
| Reporting bias |
|
| Articles | Selection Bias | Performance Bias | Detection Bias | Reporting Bias | Overall Score | Quality |
|---|---|---|---|---|---|---|
| Study 1 [42] | 🟡 2 | 🟡 2.75 | 🟢 3 | 🟢 3 | 2.68 | Low risk of bias |
| Study 2 [41] | 🟡 2.5 | 🟡 2 | 🟡 2.75 | 🟢 3 | 2.56 | Low risk of bias |
| Study 3 [43] | 🟡 2 | ⚪0.75 | 🟢 3 | 🟢 3 | 2.18 | Moderate risk of bias |
| Study 4 [44] | 🟡 2.5 | ⚪0.75 | 🟢 3 | 🟢 3 | 2.31 | Moderate risk of bias |
| Study 5 [40] | 🟡 2.75 | 🟢 3 | 🟡 2.5 | 🟢 3 | 2.81 | Low risk of bias |
| Study 6 [6] | 🟡 2.25 | 🟢 3 | 🔴 1.75 | 🟡 2.33 | 2.33 | Moderate risk of bias |
| Study 7 [45] | 🔴 1.75 | ⚪ 0.75 | 🟢 3 | 🟢 3 | 2.12 | Moderate risk of bias |
| Study 8 [46] | 🟡 2.25 | ⚪ 0.75 | 🟢 3 | 🟢 3 | 2.25 | Moderate risk of bias |
| Study 9 [47] | 🟢 3 | 🔴 1.5 | 🟢 3 | 🟢 3 | 2.62 | Low risk of bias |
| Study 10 [48] | 🟡 2.25 | 🔴 1.5 | 🟢 3 | 🟢 3 | 2.43 | Moderate risk of bias |
| Study 11 [49] | 🟡 2.5 | 🔴 1.5 | 🟢 3 | 🟢 3 | 2.5 | Low risk of bias |
| Plant Name | Geographical Localization | Part Used | Type of Extract | Study Design | Breast Cancer Model | Treatment Dose | Duration of Treatment | IC50 Value | Outcome | Study |
|---|---|---|---|---|---|---|---|---|---|---|
| Foeniculum vulgare | Iran | Seeds | Aqueous | In vivo | Female BALB/c mice challenged with 4T1 cells | 50, 100, and 200 mg/kg; IP injection | Daily, for two weeks | ND | Decreased HSP90 expression | [48] |
| Spatholobus suberectus Dunn | China | All plant | Aqueous ethanol | In vitro | MCF-7, MDA-MB-231 | 0 μg/mL to 100 μg/mL | Up to 48 h | ND | Inhibits Hsp90/HIF-1a interactions | [43] |
| In vivo | Xenograft mouse model | 1 g/kg/d, oral intake | Every 3 days for 25 days | |||||||
| Flueggea leucopyrus (Willd.) | Sri Lanka | Aerial parts | Aqueous | In vitro | MCF-7 | 1, 2, 5, 10, and 20 μM | 12 h for Western blot; 48 h for IC50 | 27.89 μg/mL | Inhibits HSP90 | [44] |
| MDA-MB-231 | 99.43 μg/mL | |||||||||
| SKBR-3 | 121.43 μg/mL | |||||||||
| Tubocapsicum anomalum (Solanaceae) | China | Leaves, Stems | methanol | In vitro | MDA-MB-231 | 0.1, 1, and 10 μM | 24 h and 48 h | 2.7 μM (1.26 μg/mL) | Induced thiol oxidation and aggregation of Hsp90-Hsp70 | [46] |
| Trianthema portulacastrum Linn | Southeast Asia, tropical America, and Africa | Leaves | Ethanol | In vitro, in vivo | DMBA-induced mammary carcinogenesis in Sprague-Dawley rats | 50, 100, and 200 mg/kg in the diet | 16 weeks | ND | Decreased HSP90 expression | [45] |
| Jasminum multiflorum (Burm. f.) Andrews | Egypt | Leaves | Methanol | In vitro, in silico | MCF-7 | 1000–7.81 µg/mL | 1 h | 24.81 µg/mL | Compounds showed high affinity scores toward HSP90 | [41] |
| Class | Compound | Source | Breast Cancer Model | Mechanism of Inhibition | Method of Investigation | Comparator | Study |
|---|---|---|---|---|---|---|---|
| Diarylheptanoid | Epicalyxins C | ND | MCF-7, MDA-MB-231 | Binds to the NTD of HSP90 | Molecular docking | Geldanamycin, Radicicol | [6] |
| Calyxins A | ND | MCF-7, MDA-MB-231 | Binds to the NTD of HSP90 | Molecular docking | Geldanamycin, Radicicol | [6] | |
| 6-hydroxycalyxin F | ND | NA | Binds to the NTD of HSP90 | Molecular docking | Geldanamycin, Radicicol | [6] | |
| Diterpenoid | Oridonin | Rabdosian rubescens | MCF-7 cell line | ND | Western blot | ND | [49] |
| Flavonoid | Epicatechin | Spatholobus suberectus Dunn | MCF-7, MDA-MB-231 cell lines; xenograft mouse model | HSP90/HIF-1a cochaperone interaction | Western blot, RT-PCR Immunohistochemistry and TUNEL | ND | [43] |
| 2′-hydroxyflavanone | ND | MCF-7, MDA-MB-231, and SKBR3 cell lines | ND | Western blot, LC-MS/MS | ND | [42] | |
| Gallocatechin | Spatholobus suberectus Dunn | MCF-7, MDA-MB-231 cell lines; xenograft mouse model | HSP90/HIF-1a cochaperone interaction | Western blot, RT-PCR, immunohistochemistry, and TUNEL | ND | [43] | |
| Kaempferol neohesperidoside | Jasminum multiflorum (Burm. f.) Andrews | MCF-7 | Binds to the NTD of HSP90 | Molecular docking | Geldanamycin | [41] | |
| Catechin | Spatholobus suberectus Dunn | MCF-7, MDA-MB-231 cell lines; xenograft mouse model | HSP90/HIF-1a cochaperone interaction | Western blot, RT-PCR, immunohistochemistry, and TUNEL | ND | [43] | |
| Epigallocatechin | Spatholobus suberectus Dunn | MCF-7, MDA-MB-231 cell lines; xenograft mouse model | HSP90/HIF-1a cochaperone interaction | Western blot, RT-PCR, immunohistochemistry, and TUNEL | ND | [43] | |
| Phenylethanoid | Tyrosol glucoside | Jasminum multiflorum (Burm. f.) Andrews | MCF-7 | Binds to the NTD of HSP90 | Molecular docking | Geldanamycin | [41] |
| 4-hydroxytyrosol | Jasminum multiflorum (Burm. f.) Andrews | MCF-7 | Binds to the NTD of HSP90 | Molecular docking | Geldanamycin | [41] | |
| Secoiridoid | Oleuropein aglycone | Jasminum multiflorum (Burm. f.) Andrews | MCF-7 | Binds to the NTD of HSP90 | Molecular docking | Geldanamycin | [41] |
| Withanolide | Withanolide E | Physalis peruviana | MCF-7, MDA-MB-231 | ND | Western blot, luciferase-based assays, shRNA knockdown | Geldanamycin | [47] |
| Tubocapsenolide A | Tubocapsicum anomalum | MCF-7, MDA-MB-231 | Hsp90/Hsp70 cochaperone interaction | Western blot, luciferase-based assays, shRNA knockdown, and detection of intracellular reactive oxygen species accumulation | Geldanamycin | [46,47] | |
| Tubocapsenolide B | Tubocapsicum anomalum | MCF-7, MDA-MB-231 | ND | Western blot, luciferase-based assays, shRNA knockdown | Geldanamycin | [47] | |
| Tubocapsanolide C | Tubocapsicum anomalum | MCF-7, MDA-MB-231 | ND | Western blot, luciferase-based assays, shRNA knockdown | Geldanamycin | [47] | |
| Tubocapsanolide E | Tubocapsicum anomalum | MCF-7, MDA-MB-231 | ND | Western blot, luciferase-based assays, shRNA knockdown | Geldanamycin | [47] | |
| Anomanolide A | Tubocapsicum anomalum | MCF-7, MDA-MB-231 | ND | Western blot, luciferase-based assays, shRNA knockdown | Geldanamycin | [47] | |
| 4b-hydroxywithanolide | Physalis peruviana | MCF-7, MDA-MB-231 | ND | Western blot, luciferase-based assays, shRNA knockdown | Geldanamycin | [47] | |
| Peruvianolide H | Physalis peruviana | MCF-7, MDA-MB-231 | ND | Western blot, luciferase-based assays, shRNA knockdown | Geldanamycin | [47] | |
| Withaferin A | ND | MCF-7, MDA-MB-231, BrCSCs | Binds to the NTD of HSP90 | Western blot, luciferase-based assays, shRNA knockdown, molecular docking | Doxorubicin | [40,47] | |
| Withaferin A diacetate | ND | MCF-7, BrCSCs | Binds to the NTD of HSP90 | Molecular docking | Doxorubicin | [40] | |
| 2,3-dihydrowithaferin A | ND | MCF-7, BrCSCs | Binds to the NTD of HSP90 | Molecular docking | Doxorubicin | [40] |
| Study | Type of Analysis | Technique | Study Type | Outcome |
|---|---|---|---|---|
| [46] | Anticancer effect analysis | MTT antiproliferative assay | Quantitative | Dose-dependent decrease in cell viability upon TA treatment. |
| Caspase-3, -8, and -9 activity assays | Quantitative | Increase in caspase-3, -8, and -9 activities upon TA treatment. | ||
| PARP cleavage assay | Quantitative | Increase in PARP cleavage upon TA treatment. | ||
| Flow cytometry | Quantitative | Cell cycle arrest at G1 phase upon TA treatment. | ||
| Western blotting | Qualitative | Proteasome-dependent degradation of Cdk4, cyclin D1, Raf-1, Akt, and mutant p53, Hsp90 client proteins upon TA treatment. | ||
| HSP90 inhibition effect analysis | Nonreducing SDS-PAGE | Qualitative | Rapid and selective induction of thiol oxidation and aggregation of Hsp90 and Hsp70 upon TA treatment. | |
| Luciferase refolding assay | Qualitative | Inhibition of the chaperone activity of Hsp90-Hsp70 complex in the luciferase refolding assay. | ||
| [42] | Anticancer effect analysis | MTT antiproliferative assay | Quantitative | Decrease in cell viability in all three subtypes of breast cancer cells treated with 2HF. |
| Proteomic analysis | Quantitative | Significant changes in the proteins responsible for breast cancer incidence, metastases, and therapeutic sensitivity in breast cancer cells. | ||
| HSP90 inhibition effect analysis | Western blotting | Qualitative | Decrease in HSP90 protein expression in all three subtypes of breast cancer cells treated with 2HF. | |
| [48] | Anticancer effect analysis | Serum GR and GPx measurement by ELISA | Quantitative | Fennel extract increased the level of serum GR in mice.Fennel extract did not increase GPx in all treated groups. |
| Immunofluorescence (IFS) | Qualitative | Decreased expression of HSP 70 and 90 proteins in mice. | ||
| Her2 gene expression by QRT-PCR | Quantitative | Fennel extract inhibited the expression of the Her2 gene in breast cancer. | ||
| HSP90 inhibition effect analysis | Immunohistochemistry (IHC) | Qualitative | Decreased the expression of HSP70 and HSP90 in mice treated with fennel extract. | |
| [41] | Anticancer effect analysis | Neutral red uptake assay | Quantitative | Determined the IC50 = 24.81 µg/mL value of the plant extract. |
| HSP90 inhibition effect analysis | Molecular docking | Quantitative | Kaempferol neohesperidoside and oleuropein aglycon showed superior affinity toward HSP90 compared to Geldanamycin. | |
| [40] | Anticancer effect analysis | MTT antiproliferative assay | Quantitative | WFA showed lower IC50 value than that of Doxorubicin. |
| HSP90 inhibition effect analysis | Molecular docking | Quantitative | WFA and withaferin A diacetate exhibited strong receptor–ligand interactions against HSP90. | |
| [6] | Anticancer effect analysis | Virtual screening | Quantitative | 135 phytochemicals retrieved with satisfying pharmacophore features. |
| ADME/T properties analysis | Quantitative | 95 natural compounds identified as candidates to inhibit Hsp90. | ||
| HSP90 inhibition effect analysis | Molecular docking | Quantitative | Three compounds identified as better inhibitors than Geldanamycin and Radicicol. | |
| Pharmacophore modeling | Qualitative | A structure-based pharmacophore model was generated with features complementary to residues required for Hsp90 inhibition. | ||
| Molecular dynamics simulations | Quantitative | The hit compounds retained their intermolecular interactions and position in the binding pocket. | ||
| [44] | Anticancer effect analysis | Sulphorhodamine (SRB) assay | Quantitative | Decoction mediates significant cytotoxic effects in all three breast cancer cells phenotypes. |
| Fluorescent microscopic examination of apoptosis-related morphological changes | Qualitative | Apoptotic morphological changes observed in all three breast cancer cell lines. | ||
| DNA fragmentation | Qualitative | DNA fragmentation observed in all three breast cancer cell lines. | ||
| Caspase-3/7 assay | Quantitative | Caspase-3/7 were significantly activated in MDA-MB-231 and SKBR-3 cells, indicating caspase-dependent apoptosis in these cells and caspase-independent apoptosis in MCF-7 cells. | ||
| HSP90 inhibition effect analysis | Real-time reverse transcription PCR (RT-PCR) | Quantitative | Inhibition of HSP90 expression mediated by the decoction in MCF-7 and MDA-MB-231, with little effect in the SKBR-3 cells. | |
| Immunofluorescence analysis of HSP protein expression | Qualitative | No significant effects compared to the controls. | ||
| [45] | Anticancer effect analysis/HSP90 inhibition effect analysis | Immunohistochemistry | Qualitative | TPE downregulated COX-2 and HSP90, blocked IκBα degradation, hampered NF-κB translocation, and upregulated Nrf2 expression and nuclear translocation during DMBA mammary carcinogenesis.TPE treatment reduced HSP90 expression and increased Nrf2-positive cells in DMBA-induced mammary tumors in rats. |
| [43] | Anticancer effect analysis | Apoptosis assay | Quantitative | SS manifested apoptosis-inducing activity in both MCF-7 and MDA-MB-231 cells. |
| JC-1 staining | Quantitative | SS activated the mitochondrial pathway apoptosis in breast cancer cells. | ||
| Cell cycle analysis | Quantitative | SS arrested the G2/M checkpoint in breast cancer cells. | ||
| Tumor growth assay | Quantitative | Oral herbal extracts (1 g/kg/d) administration attenuated tumor growth in breast cancer xenografts. | ||
| LDH-A activity assay | Quantitative | SS possessed significant anticancer effects via LDH-A inhibition both in vitro and in vivo. | ||
| HSP90 inhibition effect analysis | Co-immunoprecipitation assay | Quantitative | Epigallocatechin disassociated Hsp90 from HIF-1a. | |
| Western blot analysis | Quantitative | Epigallocatechin accelerated HIF-1a proteasome degradation. | ||
| Immunohistochemistry assay | Quantitative | Epigallocatechin downregulated HIF-1a expression in breast cancer xenografts. | ||
| LDH-A expression assay | Quantitative | Epigallocatechin downregulated LDH-A expression in breast cancer xenografts. | ||
| Apoptosis assay | Quantitative | Epigallocatechin elevated apoptosis ratio in breast cancer xenografts. | ||
| [47] | Anticancer effect analysis | MTT antiproliferative assay | Quantitative | Withanolides reduced cell viability in MDA-MB-231 and MCF-7 cells. |
| Apoptosis assay | Qualitative | Withanolides induced cell cycle arrest and apoptosis in MDA-MB-231 and MCF-7 cells. | ||
| Anti-caspase activity analysis | Qualitative | Withanolides induced caspase-3 and PARP cleavage, indicating activation of caspase-dependent apoptosis. | ||
| HSP90 inhibition effect analysis | Western blotting | Qualitative | Withanolides selectively depleted HSP90 client proteins and induced HSP70. | |
| Luciferase-based assays | Qualitative | Withanolides inhibited HSP90 chaperone activity. | ||
| shRNA knockdown | Qualitative | Knockdown of HSP70 by shRNA enhanced the cytotoxicity of withanolides in MBA-MB-231 cells. | ||
| [49] | Anticancer effect analysis | Apoptosis assay | Quantitative | Oridonin induced apoptosis in MCF-7 cells. |
| Cell viability assay | Quantitative | Oridonin inhibited cell growth and proliferation in MCF-7 cells. | ||
| Caspase activity assay | Quantitative | Oridonin activated the caspase cascade, leading to apoptosis in MCF-7 cells. | ||
| Observation of morphological changes in cells using phase contrast microscopy | Qualitative | Oridonin induced morphological changes in MCF-7 cells. | ||
| Membrane leakage assay | Qualitative | Oridonin induced membrane leakage in MCF-7 cells. | ||
| Mitochondrial transmembrane potential alternation | Qualitative | Oridonin induced mitochondrial alternations, amplifying the activation of the caspase cascade in MCF-7 cells. | ||
| Calpain-facilitated cell death | Qualitative | Oridonin induced cell death through a caspase-3-independent but caspase-9-dependent pathway in MCF-7 cells. | ||
| HSP90 inhibition effect analysis | Western blot analysis for HSP90 expression | Quantitative | Oridonin downregulated HSP90 expression in MCF-7 cells. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarguan, I.; Ghoul, S.; Belayachi, L.; Benjouad, A. Plant-Based HSP90 Inhibitors in Breast Cancer Models: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 5468. https://doi.org/10.3390/ijms25105468
Zarguan I, Ghoul S, Belayachi L, Benjouad A. Plant-Based HSP90 Inhibitors in Breast Cancer Models: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(10):5468. https://doi.org/10.3390/ijms25105468
Chicago/Turabian StyleZarguan, Ilham, Sonia Ghoul, Lamiae Belayachi, and Abdelaziz Benjouad. 2024. "Plant-Based HSP90 Inhibitors in Breast Cancer Models: A Systematic Review" International Journal of Molecular Sciences 25, no. 10: 5468. https://doi.org/10.3390/ijms25105468
APA StyleZarguan, I., Ghoul, S., Belayachi, L., & Benjouad, A. (2024). Plant-Based HSP90 Inhibitors in Breast Cancer Models: A Systematic Review. International Journal of Molecular Sciences, 25(10), 5468. https://doi.org/10.3390/ijms25105468






