A Testis-Specific DMRT1 (Double Sex and Mab-3-Related Transcription Factor 1) Plays a Role in Spermatogenesis and Gonadal Development in the Hermaphrodite Boring Giant Clam Tridacna crocea
Abstract
1. Introduction
2. Results
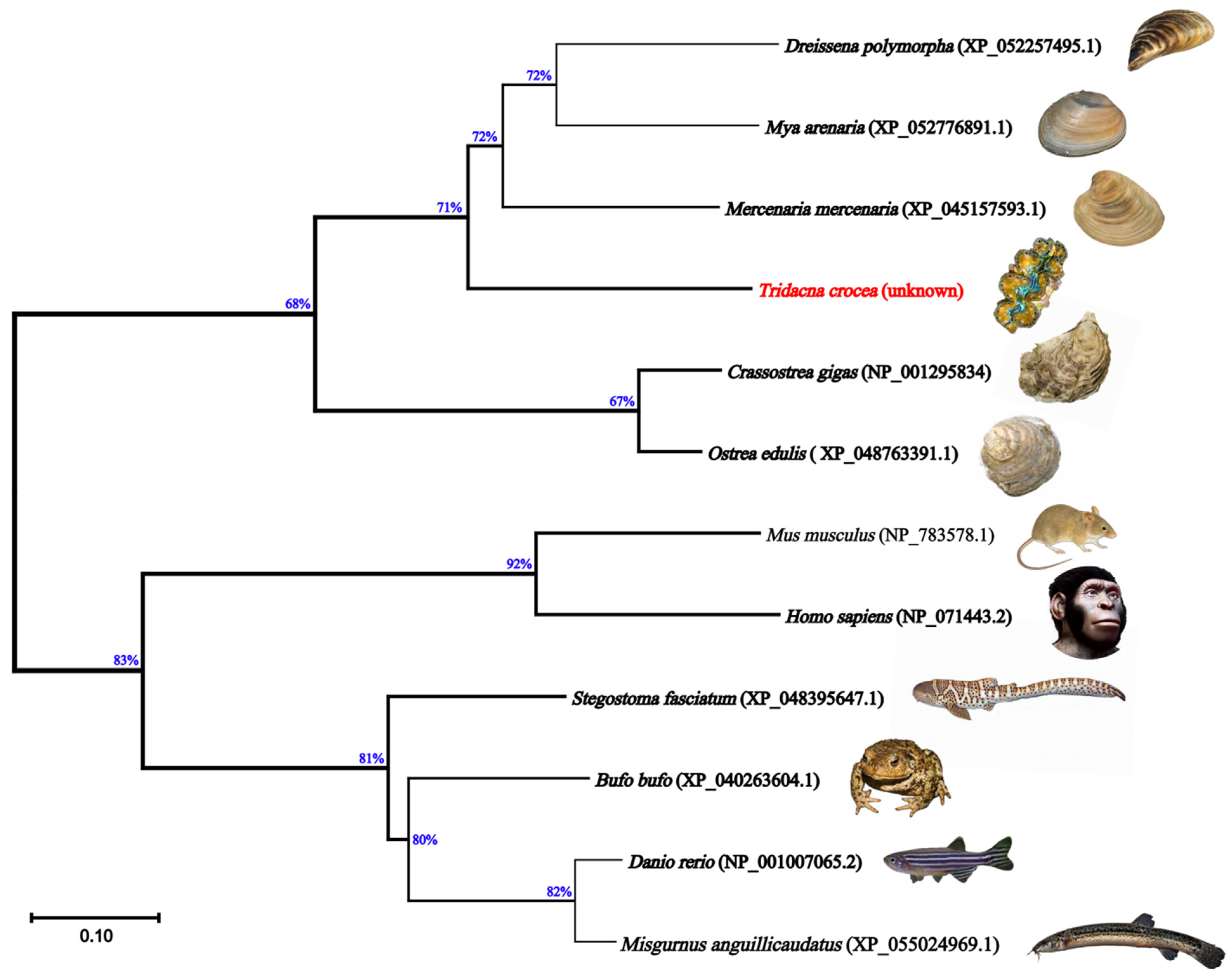
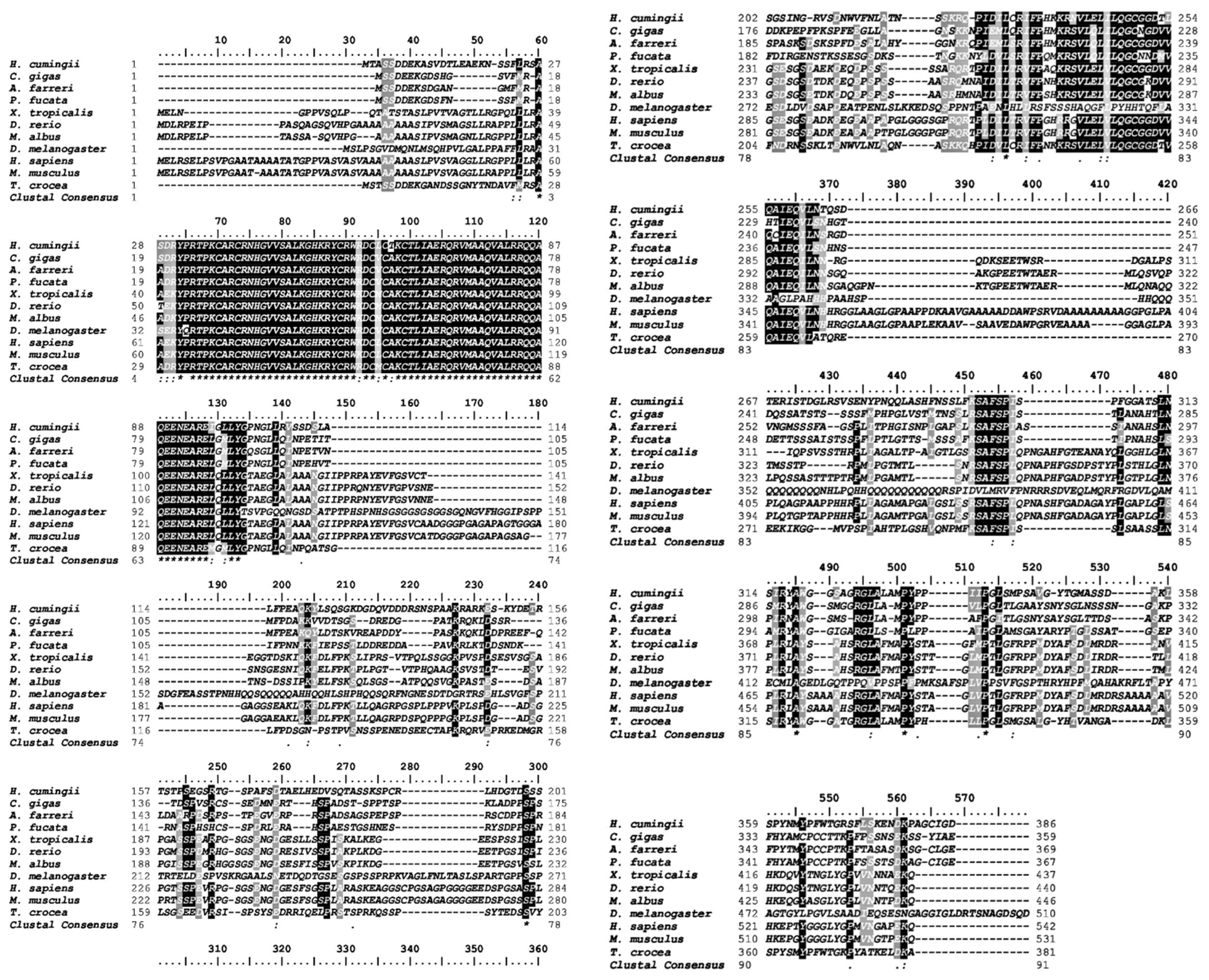
2.1. Nucleotides, Translated Amino Acid Profile, and Phylogeny of Tc-DMRT1 Gene
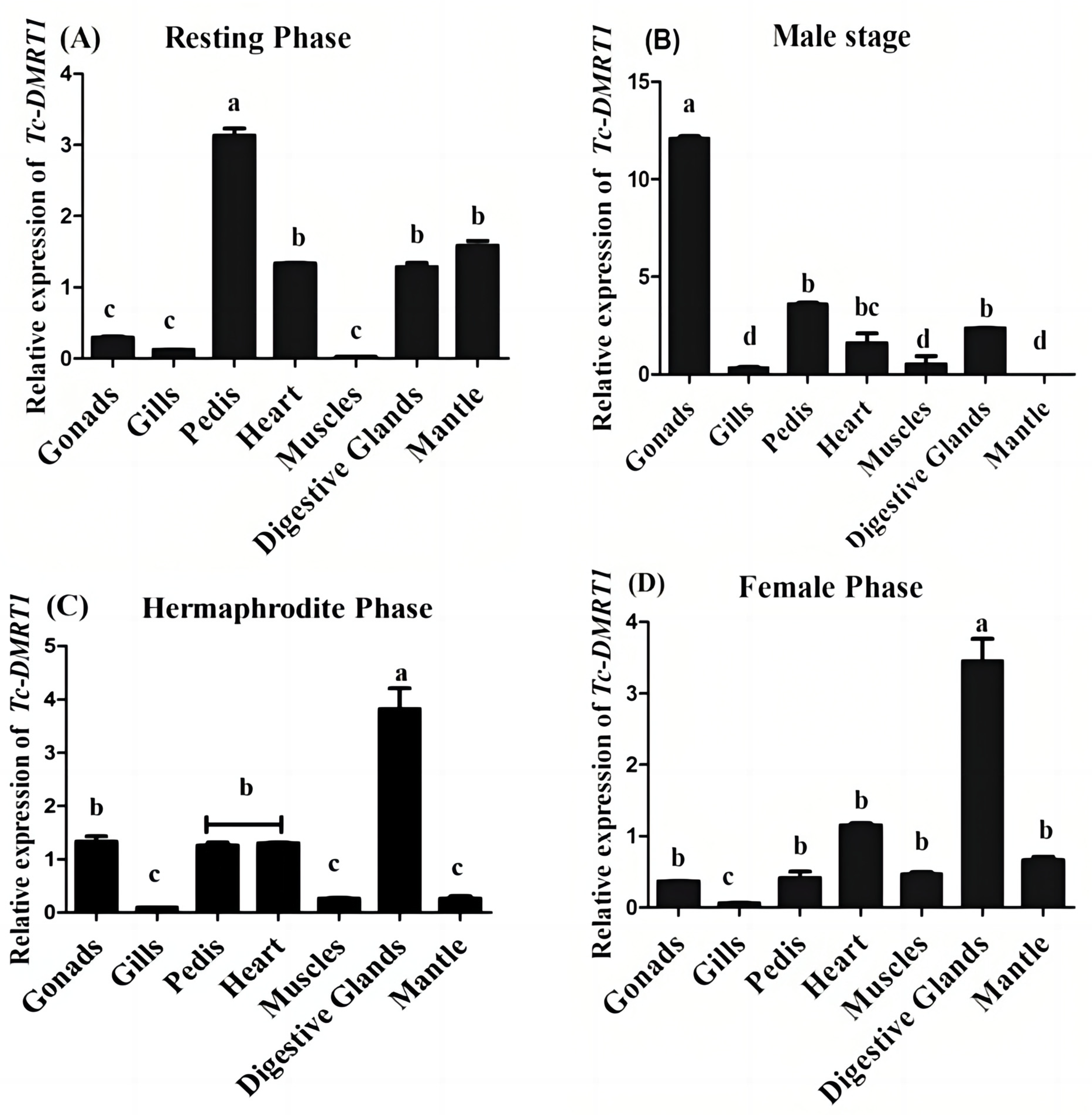
2.2. Expression Pattern of Tc-DMRTI during Various Reproductive Stages
2.3. In-Situ Hybridization for the Localization of Tc-DMRT1 mRNA during Various Reproductive Stages
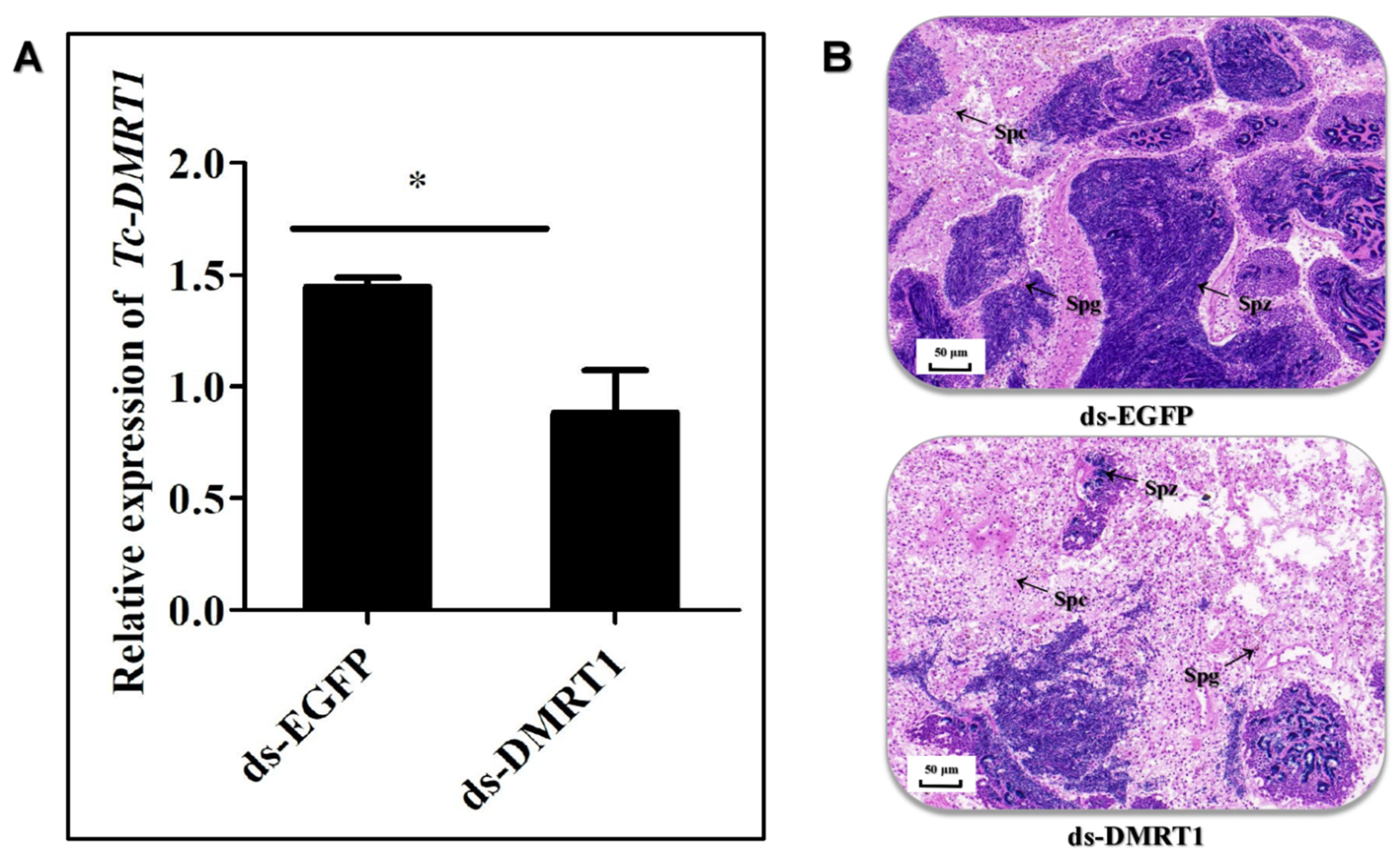
2.4. ds-DMRT1 Injection
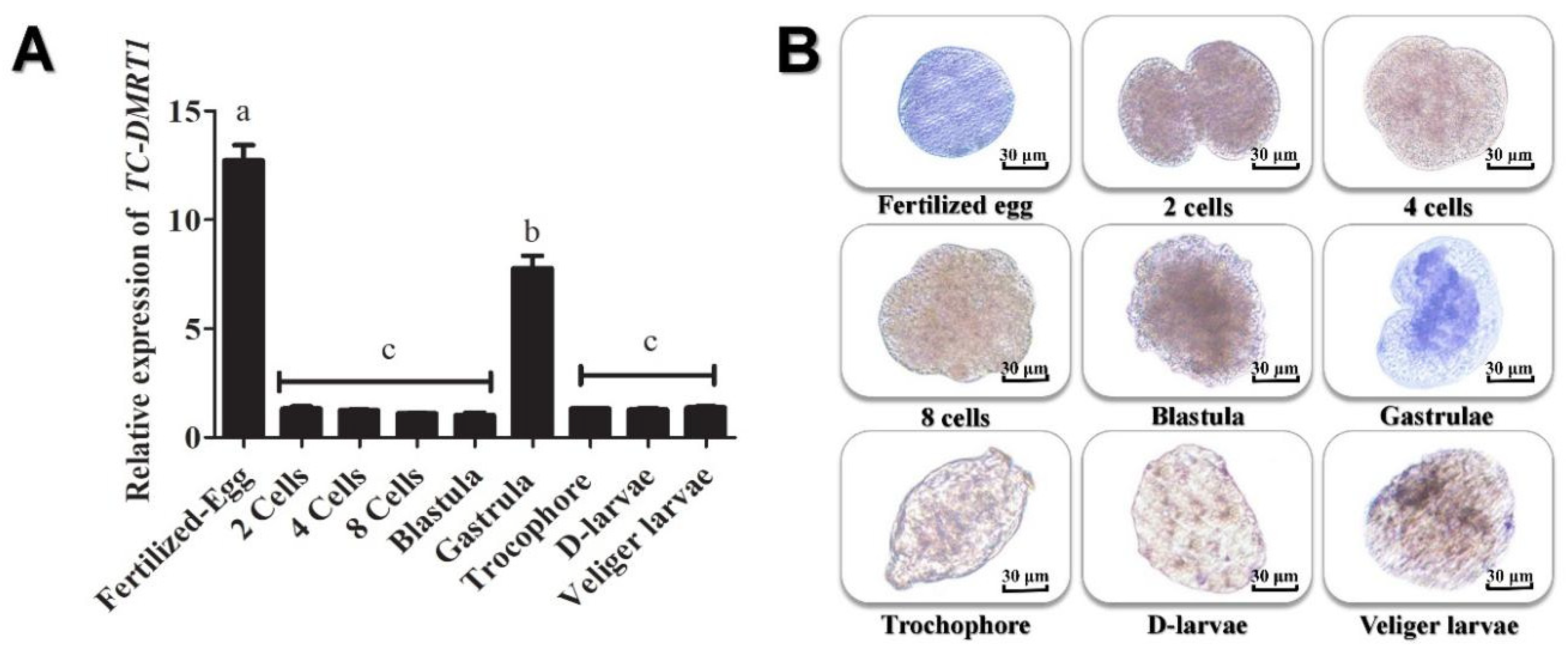
2.5. Spatial and Temporal Expression Pattern of Tc-DMRT1 during Embryogenesis
3. Discussion
4. Materials and Methods
4.1. Giant Clam Procurements and Maintenance Conditions
4.2. Tissue Collection
4.3. Total RNA Extraction, cDNA Synthesis, PCR, Cloning, and RACE-PCR
4.4. Quantitative Polymerase Chain Reaction
4.5. Synthesis of dsRNA
4.6. In-Situ Hybridization (ISH) and Whole-Mount In-Situ Hybridization (WISH)
4.7. Histology Sections
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramah, S.; Taleb-Hossenkhan, N.; Todd, P.A.; Neo, M.L.; Bhagooli, R.J.M.B. Drastic decline in giant clams (Bivalvia: Tridasscninae) around Mauritius Island, Western Indian Ocean: Implications for conservation and management. Mar. Biodivers. 2019, 49, 815–823. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Ma, H.; Qin, Y.; Zhou, Y.; Wei, J.; Deng, Y.; Li, X.; Xiao, S.; Xiang, Z.; et al. Artificial interspecific hybridization of two giant clams, Tridacna squamosa and Tridacna crocea, in the south China sea. Aquaculture 2019, 515, 734581. [Google Scholar] [CrossRef]
- Soo, P.; Todd, P.A.J.M.b. The behaviour of giant clams (Bivalvia: Cardiidae: Tridacninae). Mar. Biol. 2014, 161, 2699–2717. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; et al. Global Trajectories of the Long-Term Decline of Coral Reef Ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Z.; Qin, Y.; Li, X.; Ma, H.; Wei, J.; Zhou, Y.; Xiao, S.; Xiang, Z.; Noor, Z.; et al. Phenotypic traits of two boring giant clam (Tridacna crocea) populations and their reciprocal hybrids in the South China Sea. Aquaculture 2019, 519, 734890. [Google Scholar] [CrossRef]
- Billeter, J.-C.; Rideout, E.J.; Dornan, A.J.; Goodwin, S.F. Control of Male Sexual Behavior in Drosophila by the Sex Determination Pathway. Curr. Biol. 2006, 16, R766–R776. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Pilgrim, D. Sex and the single worm: Sex determination in the nematode C. elegans. Mech. Dev. 1999, 83, 3–15. [Google Scholar] [CrossRef]
- Fu, C.; Li, F.; Wang, L.; Wu, F.; Wang, J.; Fan, X.; Liu, T. Molecular characteristics and abundance of insulin-like androgenic gland hormone and effects of RNA interference in Eriocheir sinensis. Anim. Reprod. Sci. 2020, 215, 106332. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, K.; Qiu, G. Identification of an insulin-like androgenic gland hormone transcript highly expressed in male precociousness of the Chinese mitten crab Eriocheir sinensis. Aquac. Res. 2018, 50, 304–311. [Google Scholar] [CrossRef]
- Ma, K.-Y.; Lin, J.-Y.; Guo, S.-Z.; Chen, Y.; Li, J.-L.; Qiu, G.-F. Molecular characterization and expression analysis of an insulin-like gene from the androgenic gland of the oriental river prawn, Macrobrachium nipponense. Gen. Comp. Endocrinol. 2013, 185, 90–96. [Google Scholar] [CrossRef]
- Ventura, T.; Manor, R.; Aflalo, E.D.; Weil, S.; Raviv, S.; Glazer, L.; Sagi, A. Temporal Silencing of an Androgenic Gland-Specific Insulin-Like Gene Affecting Phenotypical Gender Differences and Spermatogenesis. Endocrinology 2008, 150, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Naimi, A.; Martinez, A.-S.; Specq, M.-L.; Mrac, A.; Diss, B.; Mathieu, M.; Sourdaine, P. Identification and expression of a factor of the DM family in the oyster Crassostrea gigas. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.J.T.i.G. Dmrt genes in the development and evolution of sexual dimorphism. Trends Genet. 2012, 28, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Matson, C.K.; Zarkower, D. Sex and the singular DM domain: Insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 2012, 13, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.A.-L.; Cosseau, C.; Mouahid, G.; Duval, D.; Grunau, C.; Toulza, E.; Allienne, J.-F.; Boissier, J. The roles of Dmrt (Double sex/Male-abnormal-3 Related Transcription factor) genes in sex determination and differentiation mechanisms: Ubiquity and diversity across the animal kingdom. Comptes Rendus Biol. 2015, 338, 451–462. [Google Scholar] [CrossRef]
- Baker, B.S.; Ridge, K.A.J.G. Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 1980, 94, 383–423. [Google Scholar] [CrossRef]
- Burtis, K.; Coschigano, K.; Baker, B.; Wensink, P. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 1991, 10, 2577–2582. [Google Scholar] [CrossRef]
- Raymond, C.S.; Shamu, C.E.; Shen, M.M.; Seifert, K.J.; Hirsch, B.; Hodgkin, J.; Zarkower, D. Evidence for evolutionary conservation of sex-determining genes. Nature 1998, 391, 691–695. [Google Scholar] [CrossRef]
- Shen, M.M.; Hodgkin, J. mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell 1988, 54, 1019–1031. [Google Scholar] [CrossRef]
- Masuyama, H.; Yamada, M.; Kamei, Y.; Fujiwara-Ishikawa, T.; Todo, T.; Nagahama, Y.; Matsuda, M. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosom. Res. 2011, 20, 163–176. [Google Scholar] [CrossRef]
- Raymond, C.S.; Murphy, M.W.; O’Sullivan, M.G.; Bardwell, V.J.; Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000, 14, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Y.; Zhou, Z.; Lin, C.; Wei, J.; Qin, Y.; Xiang, Z.; Ma, H.; Zhang, Y.; Zhang, Y.; et al. Comparative transcriptome analysis of three gonadal development stages reveals potential genes involved in gametogenesis of the fluted giant clam (Tridacna squamosa). BMC Genom. 2020, 21, 872. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Okada, E.; Umemoto, H.; Tamura, K.; Uno, Y.; Nishida-Umehara, C.; Matsuda, Y.; Takamatsu, N.; Shiba, T.; Ito, M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA 2008, 105, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kettlewell, J.R.; Anderson, R.C.; Bardwell, V.J.; Zarkower, D. Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad. Gene Expr. Patterns 2003, 3, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.D.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev. Biol. 2011, 356, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lei, N.; Hornbaker, K.I.; Rice, D.A.; Karpova, T.; Agbor, V.A.; Heckert, L.L. Sex-Specific Differences in Mouse DMRT1 Expression Are Both Cell Type- and Stage-Dependent During Gonad Development1. Biol. Reprod. 2007, 77, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The Mammalian Doublesex Homolog DMRT1 Is a Transcriptional Gatekeeper that Controls the Mitosis versus Meiosis Decision in Male Germ Cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011, 476, 101–104. [Google Scholar] [CrossRef]
- Zarkower, D. DMRT genes in vertebrate gametogenesis. Curr. Top. Dev. Biol. 2013, 102, 327–356. [Google Scholar]
- Murphy, M.W.; Sarver, A.L.; Rice, D.; Hatzi, K.; Ye, K.; Melnick, A.; Heckert, L.L.; Zarkower, D.; Bardwell, V.J. Genome-wide analysis of DNA binding and transcriptional regulation by the mammalian Doublesex homolog DMRT1 in the juvenile testis. Proc. Natl. Acad. Sci. USA 2010, 107, 13360–13365. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.-R.; Wu, G.-C.; Yueh, W.-S.; Kuo, S.-F.; Dufour, S.; Chang, C.-F. Dmrt1 (doublesex and mab-3-related transcription factor 1) expression during gonadal development and spermatogenesis in the Japanese eel. Gen. Comp. Endocrinol. 2019, 279, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Teaniniuraitemoana, V.; Huvet, A.; Levy, P.; Klopp, C.; Lhuillier, E.; Gaertner-Mazouni, N.; Gueguen, Y.; Le Moullac, G. Gonad transcriptome analysis of pearl oyster Pinctada margaritifera: Identification of potential sex differentiation and sex determining genes. BMC Genom. 2014, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Marchand, O.; Govoroun, M.; D’cotta, H.; McMeel, O.; Lareyre, J.-J.; Bernot, A.; Laudet, V.; Guiguen, Y. DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 2000, 1493, 180–187. [Google Scholar] [CrossRef]
- Fernandino, J.I.; Guilgur, L.G.; Somoza, G.M. Dmrt1 expression analysis during spermatogenesis in pejerrey, Odontesthes bonariensis. Fish Physiol. Biochem. 2006, 32, 231–240. [Google Scholar] [CrossRef]
- Liu, B.Z.; Cong, J.J.; Su, W.Y.; Hao, Z.L.; Sun, Z.H.; Chang, Y.Q. Identification and functional analysis of the Dmrt1 gene and the SoxE gene in the sexual development of sea cucumber, Apostichopus japonicus. Front. Genet. 2023, 14, 1097825. [Google Scholar] [CrossRef] [PubMed]
- Klinbunga, S.; Amparyup, P.; Khamnamtong, B.; Hirono, I.; Aoki, T.; Jarayabhand, P. Isolation and Characterization of Testis-Specific DMRT1 in the Tropical Abalone (Haliotis asinina). Biochem. Genet. 2008, 47, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Katz, M.; Sinclair, A.H. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol. Reprod. 2003, 68, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhou, L.; Yao, B.; Li, C.-J.; Gui, J.-F. Differential and spermatogenic cell-specific expression of DMRT1 during sex reversal in protogynous hermaphroditic groupers. Mol. Cell. Endocrinol. 2007, 263, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-B.; Park, J.-G.; Park, Y.-J.; Takemura, A.; Hur, S.-P.; Lee, Y.-D.; Kim, S.-J. Isolation and characterization of DMRT1 and its putative regulatory region in the protogynous wrasse, Halichoeres tenuispinis. Gene 2009, 438, 8–16. [Google Scholar] [CrossRef]
- Omotehara, T.; Smith, C.A.; Mantani, Y.; Kobayashi, Y.; Tatsumi, A.; Nagahara, D.; Hashimoto, R.; Hirano, T.; Umemura, Y.; Yokoyama, T.; et al. Spatiotemporal expression patterns of doublesex and mab-3 related transcription factor 1 in the chicken developing gonads and Müllerian ducts. Poult. Sci. 2014, 93, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Mawaribuchi, S.; Musashijima, M.; Wada, M.; Izutsu, Y.; Kurakata, E.; Park, M.K.; Takamatsu, N.; Ito, M. Molecular Evolution of Two Distinct dmrt1 Promoters for Germ and Somatic Cells in Vertebrate Gonads. Mol. Biol. Evol. 2016, 34, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, H.; Seppola, M.; Torgersen, J.S.; Delghandi, M.; Andersen, Ø. Sexually dimorphic expression of dmrt1 in immature and mature Atlantic cod (Gadus morhua L.). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 156, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cheng, H.; Huang, X.; Gao, S.; Yu, H.; Zhou, R. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochem. Biophys. Res. Commun. 2005, 330, 950–957. [Google Scholar] [CrossRef]
- Yi, W.; Zarkower, D. Similarity of DNA binding and transcriptional regulation by Caenorhabditis elegans MAB-3 and Drosophila melanogaster DSX suggests conservation of sex determining mechanisms. Development 1999, 126, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Liao, Q.; Shi, G.; Qin, Y.; Zhang, Y.; Ma, H.; Li, J.; Yu, Z. Developmental Expression Pattern of the Piwi1 Gene, Timing of Sex Differentiation and Maturation in Artificially Produced Juvenile Boring Giant Clam, Tridacna crocea. Front. Mar. Sci. 2022, 9, 883661. [Google Scholar] [CrossRef]
- Poo, J.S.T.; Choo, C.Y.L.; Hiong, K.C.; Boo, M.V.; Wong, W.P.; Chew, S.F.; Ip, Y.K. Phototrophic potential and form II ribulose-1,5-bisphosphate carboxylase/oxygenase expression in five organs of the fluted giant clam, Tridacna squamosa. Coral Reefs 2020, 39, 361–374. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2 2 DD C T Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Noor, Z.; Guo, S.; Zhao, Z.; Qin, Y.; Shi, G.; Ma, H.; Zhang, Y.; Li, J.; Yu, Z. Identification and involvement of DAX1 gene in spermatogenesis of boring giant clam Tridacna crocea. Gene 2024, 911, 148338. [Google Scholar] [CrossRef]
- Fabioux, C.; Pouvreau, S.; Le Roux, F.; Huvet, A. The oyster vasa-like gene: A specific marker of the germline in Crassostrea gigas. Biochem. Biophys. Res. Commun. 2004, 315, 897–904. [Google Scholar] [CrossRef]


| Primer | Sequence (5′-3′) | Purpose |
|---|---|---|
| Dmrt1_5′ RACE1 | CCATCTCTCTTGCCTCGTTTTCCT | 5′ RACE of Tc-DMRT1 |
| DMRT1_5′ RACE2 | GTCAGTGGCTCCTTTTTCGTCATC | |
| DMRT1_3′ RACE1 | ATCTTCGCTCAGTGCTGCCAGTTCTC | 3′ RACE of Tc-DMRT1 |
| DMRT1_3′ RACE2 | CTGGAAAACCGTATGCTACCAAGGA | |
| qDMRT1 5′ | CTTGGCATTTATGACAATCAAGC | Quantification of DMRT1 |
| qDMRT1 3′ | ACGGCGTCGTTCGTGTAGTT | |
| qRPL5_5′ | CATACTCACACGAACTGCCTC | Reference ribosomal protein L5 gene for reproductive stages |
| qRPL5_3′ | CTCCGTCTACTTCTGTCTGTCC | |
| DMRT1_RNAi_5′ | TCAGCAGCAGACCGTTATCCGGATCCTAATACGACTCACTATAGG | RNA interference |
| DMRT1_RNAi_3′ | GGATCCTAATACGACTCACTATAGGTCGGCACCATTCCACCTTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, Z.; Zhao, Z.; Guo, S.; Wei, Z.; Cai, B.; Qin, Y.; Ma, H.; Yu, Z.; Li, J.; Zhang, Y. A Testis-Specific DMRT1 (Double Sex and Mab-3-Related Transcription Factor 1) Plays a Role in Spermatogenesis and Gonadal Development in the Hermaphrodite Boring Giant Clam Tridacna crocea. Int. J. Mol. Sci. 2024, 25, 5574. https://doi.org/10.3390/ijms25115574
Noor Z, Zhao Z, Guo S, Wei Z, Cai B, Qin Y, Ma H, Yu Z, Li J, Zhang Y. A Testis-Specific DMRT1 (Double Sex and Mab-3-Related Transcription Factor 1) Plays a Role in Spermatogenesis and Gonadal Development in the Hermaphrodite Boring Giant Clam Tridacna crocea. International Journal of Molecular Sciences. 2024; 25(11):5574. https://doi.org/10.3390/ijms25115574
Chicago/Turabian StyleNoor, Zohaib, Zhen Zhao, Shuming Guo, Zonglu Wei, Borui Cai, Yanping Qin, Haitao Ma, Ziniu Yu, Jun Li, and Yuehuan Zhang. 2024. "A Testis-Specific DMRT1 (Double Sex and Mab-3-Related Transcription Factor 1) Plays a Role in Spermatogenesis and Gonadal Development in the Hermaphrodite Boring Giant Clam Tridacna crocea" International Journal of Molecular Sciences 25, no. 11: 5574. https://doi.org/10.3390/ijms25115574
APA StyleNoor, Z., Zhao, Z., Guo, S., Wei, Z., Cai, B., Qin, Y., Ma, H., Yu, Z., Li, J., & Zhang, Y. (2024). A Testis-Specific DMRT1 (Double Sex and Mab-3-Related Transcription Factor 1) Plays a Role in Spermatogenesis and Gonadal Development in the Hermaphrodite Boring Giant Clam Tridacna crocea. International Journal of Molecular Sciences, 25(11), 5574. https://doi.org/10.3390/ijms25115574






