NPC Intracellular Cholesterol Transporter 1 Regulates Ovarian Maturation and Molting in Female Macrobrachium nipponense
Abstract
1. Introduction
2. Results
2.1. Sequence Analysis of Mn-NPC1
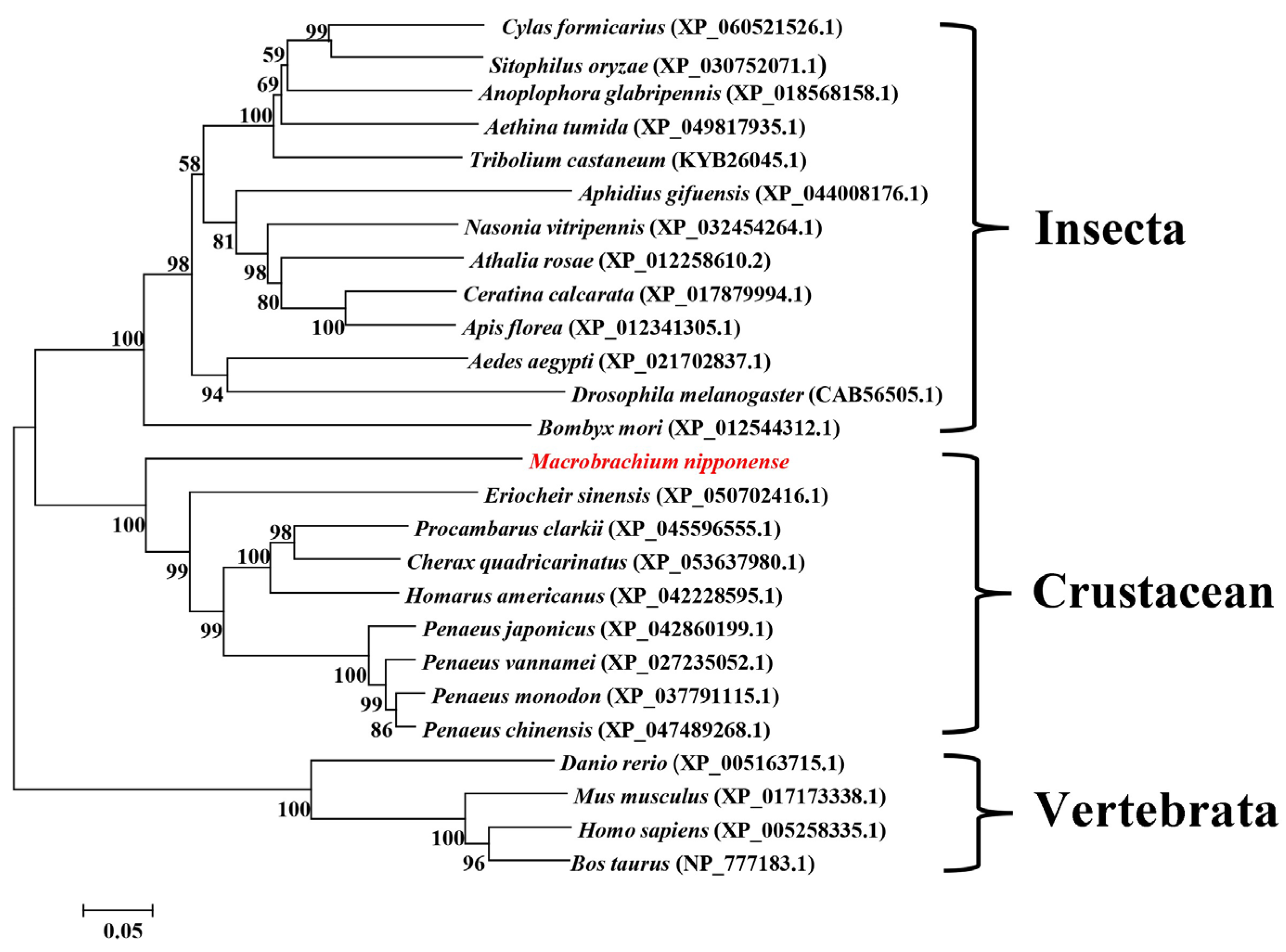
2.2. Sequence Alignment and Phylogenetic Analysis
2.3. Expression Analysis of Mn-NPC1
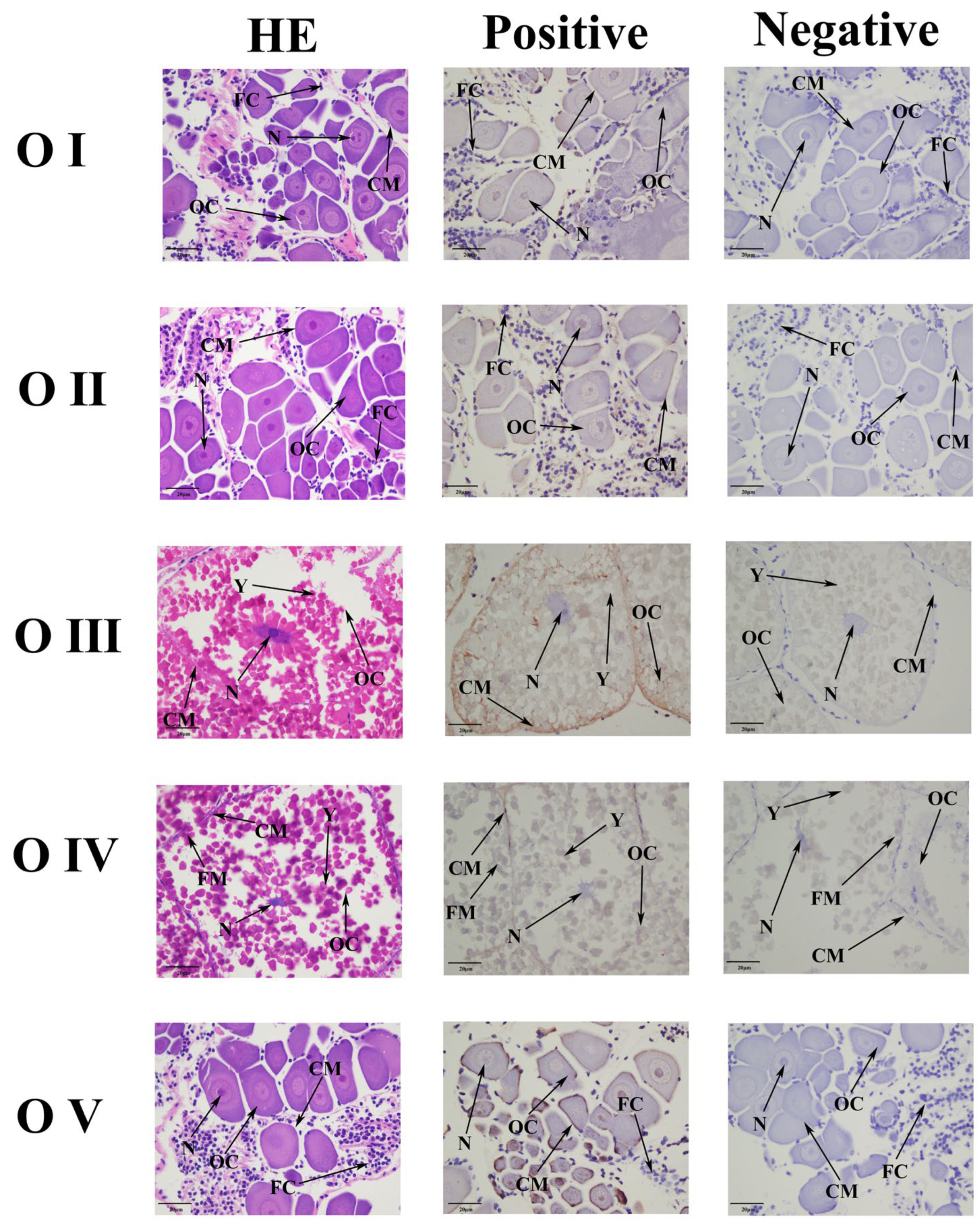
2.4. Subcellular Localization of Mn-NPC1
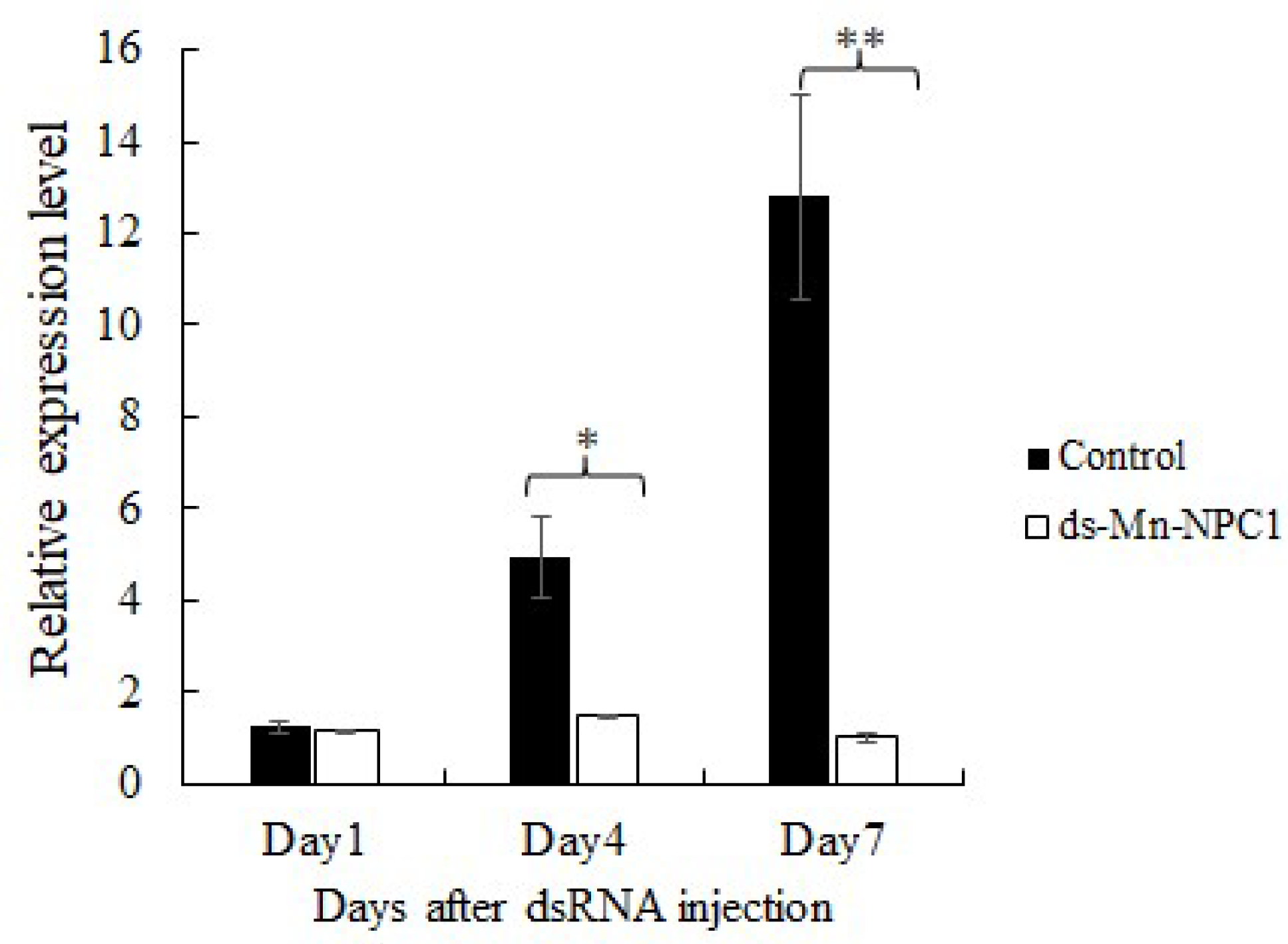
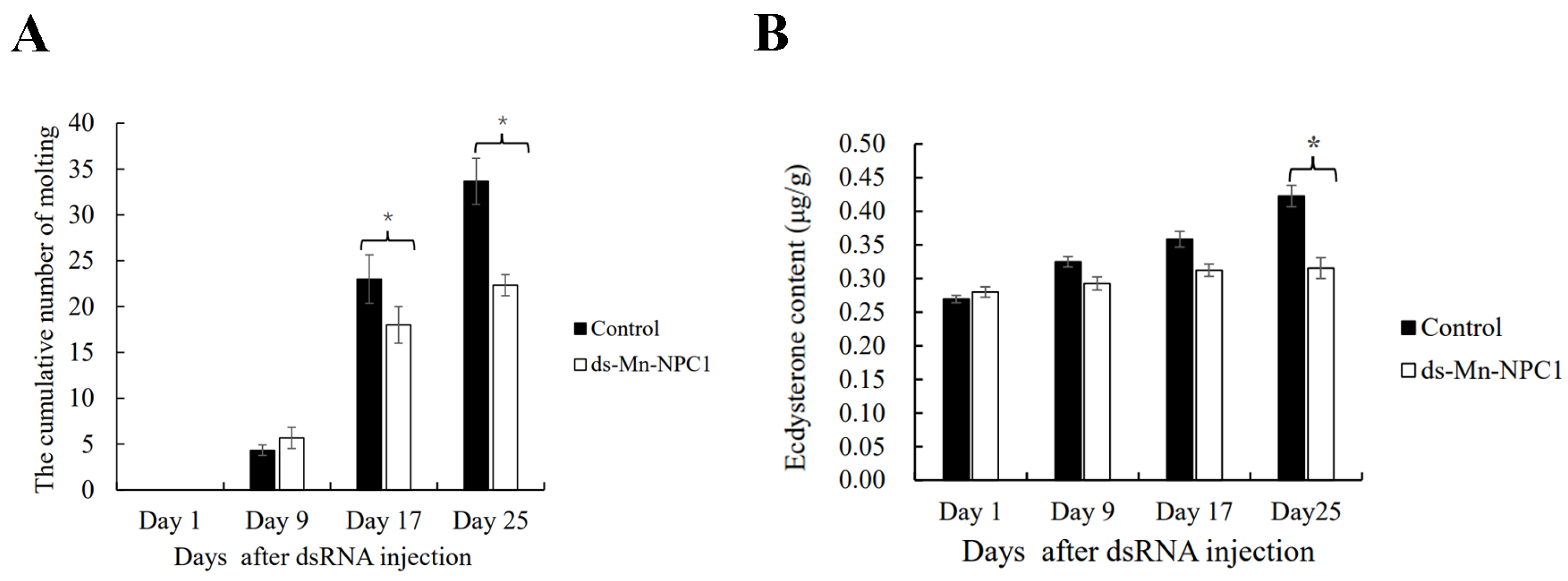
2.5. Functional Analysis of Mn-NPC1 in Ovarian Maturation Regulation
3. Discussion
4. Materials and Methods
4.1. Experimental Prawns and Sample Collection
4.2. RNA Extraction and Amplification of Mn-NPC1
4.3. Bioinformatics Sequence Analysis
4.4. In Situ Hybridization
4.5. RNA Interference
4.6. qPCR and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, P.; Qi, D.; Chen, L.Q.; Zhang, H.; Zhang, X.W.; Qin, J.G.; Hu, S.N. Gene discovery from an ovary cDNA library of oriental river prawn Macrobrachium nipponense by ESTs annotation. Comp. Biochem. Physiol. D 2009, 4, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.Q.; Ding, Z.L.; Zhang, Y.X.; Zhou, P.X.; Wu, C.B.; Zhu, M.H.; Ye, J.Y. Types of carbohydrate in feed affect the growth performance, antioxidant capacity, immunity, and activity of digestive and carbohydrate metabolism enzymes in juvenile Macrobrachium nipponense. Aquaculture 2019, 512, 734282. [Google Scholar] [CrossRef]
- Jiang, H.X.; Li, X.L.; Sun, Y.H.; Hou, F.J.; Zhang, Y.F.; Li, F.; Gu, Z.M.; Liu, X.L. Insights into sexual precocity of female Oriental River prawn Macrobrachium nipponense through transcriptome analysis. PLoS ONE 2016, 11, e0157173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.P.; Fu, H.T.; Qiao, H.; Jin, S.B.; Zhang, W.Y.; Jiang, S.F.; Gong, Y.S.; Xiong, Y.W. Expression and functional analysis of cathepsin L1 in ovarian development of the oriental river prawn, Macrobrachium nipponense. Aquac. Rep. 2021, 20, 100724. [Google Scholar] [CrossRef]
- Jiang, H.X.; Liu, X.W.; Li, Y.Z.; Zhang, R.; Liu, H.F.; Ma, X.; Wu, L.M.; Qiao, Z.G.; Li, X.J. Identification of ribosomal protein L24 (RPL24) from the oriental river prawn, Macrobrachium nipponense, and its roles in ovarian development. Comp. Biochem. Phys. A 2022, 266, 111154. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Li, Y.Z.; Zhang, S.S.; Li, H.X.; Liu, X.W.; Zhang, R.; Zhang, M.; Wang, L.; Yu, M.; Qiao, Z.G.; et al. Identification of the cyclooxygenase (COX) gene and its role in ovarian development and ovulation of the oriental river prawn Macrobrachium nipponense. Aquac. Rep. 2023, 29, 101488. [Google Scholar] [CrossRef]
- Jiang, H.X.; Li, X.L.; Sun, Y.H.; Hou, F.J.; Zhang, Y.F.; Li, F.; Guo, J.L.; Wang, Y.C.; Gu, Z.M.; Liu, X.L. Molecular and functional characterization of nucleoside diphosphate kinase (nm23) gene in oriental river prawn Macrobrachium nipponense during ovarian development. Aquac. Res. 2018, 49, 1219–1231. [Google Scholar] [CrossRef]
- Montes-Dominguez, A.L.; Avena-Soto, J.A.; Lizarraga-Rodriguez, J.L.; Perez-Gala1, R.J.; Jimenez-Gutierrez1, S.; SoteloFalomir, J.A.; Pinzon-Miranda, F.M.; Martinez-Perez, F.; Muñoz-Rubi, H.A.; Chavez-Herrera, D.; et al. Comparison between cultured and wild Pacific white shrimp (Penaeus vannamei) vitellogenesis: Next-generation sequencing and relative expression of genes directly and indirectly related to reproduction. PeerJ 2021, 9, e10694. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Chen, L.Q.; Zhou, Z.L.; Li, E.C.; Zhao, X.Q.; Guo, H. The site of vitellogenin synthesis in Chinese mitten-handed crab Eriocheir sinensis. Comp. Biochem. Phys. B 2006, 143, 453–458. [Google Scholar] [CrossRef]
- Ding, X.; Nagaraju, G.P.C.; Novotney, D.; Lovett, D.L.; Borst, D.W. Yolk protein expression in the green crab, Carcinus maenas. Aquaculture 2010, 298, 325–331. [Google Scholar] [CrossRef]
- Hiransuchalert, R.; Thamniemdee, N.; Khamnamtong, B.; Yamano, K.; Klinbunga, S. Expression profiles and localization of vitellogenin mRNA and protein during ovarian development of the giant tiger shrimp Penaeus monodon. Aquaculture 2013, 412, 193–201. [Google Scholar] [CrossRef]
- Jia, X.W.; Chen, Y.D.; Zou, Z.H.; Lin, P.; Wang, Y.L.; Zhang, Z.P. Characterization and expression profile of Vitellogenin gene from Scylla paramamosain. Gene 2013, 520, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.F.; Qiao, H.; Fu, H.T.; Gu, Z.M. Hepatopancreas Proteomic Analysis Reveals Key Proteins and Pathways in Regulatory of Ovary Maturation of Macrobrachium nipponense. Animals 2023, 13, 977. [Google Scholar] [CrossRef] [PubMed]
- Winkler, M.B.L.; Nel, L.; Frain, K.M.; Dedic, E.; Olesen, E.; Pedersen, B.P. Sterol uptake by the NPC system in eukaryotes: A Saccharomyces cerevisiae perspective. FEBS Lett. 2022, 596, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Cheruku, S.R.; Xu, Z.; Dutia, R.; Lobel, P.; Storch, J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J. Biol. Chem. 2006, 281, 31594–31604. [Google Scholar] [CrossRef]
- Mukherjee, S.; Maxfield, F.R. Lipid and cholesterol trafficking in NPC. BBA-Mol. Cell Biol. Lipids 2004, 1685, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Friedland, N.; Liou, H.L.; Lobel, P.; Stock, A.M. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc. Natl. Acad. Sci. USA 2003, 100, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, M.B.; Ban, L.P.; Song, L.M.; Liu, Y.; Pelosi, P.; Wang, G.R. Niemann-Pick C2 proteins: A new function for an old family. Front. Physiol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Deffieu, M.S.; Pfeffer, S.R. Niemann-Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc. Natl. Acad. Sci. USA 2011, 108, 18932–18936. [Google Scholar] [CrossRef]
- Huang, X.; Suyama, K.; Buchanan, J.; Zhu, A.J.; Scott, M.P. A Drosophila model of the Niemann-Pick type C lysosome storage disease: Dnpc1a is required for molting and sterol homeostasis. Development 2005, 132, 5115–5124. [Google Scholar] [CrossRef]
- Voght, S.P.; Fluegel, M.L.; Andrews, L.A.; Pallanck, L.J. Drosophila NPC1b promotes an early step in sterol absorption from the midgut epithelium. Cell Metab. 2007, 5, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, Z.; Scott, M.P.; Huang, X. The cholesterol trafficking protein NPC1 is required for Drosophila spermatogenesis. Dev. Biol. 2011, 351, 146–155. [Google Scholar] [CrossRef]
- Ke, X.X.; Chao, H.J.; Abbas, M.N.; Kausar, S.; Gul, I.; Ji, H.Y.; Yang, L.Q.; Cui, H.J. Niemann-Pick type C1 regulates cholesterol transport and metamorphosis in silkworm, Bombyx mori (Dazao). Int. J. Biol. Macromol. 2020, 152, 525–534. [Google Scholar] [CrossRef]
- Yu, H.; Wang, L.H.; Liu, S.X.; Chen, X.H.; Wei, X.Y.; Niu, C.X.; Volodymyr, V.; Song, Q.S.; Zhang, H.W. Functional analysis of a NPC1 gene from the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Arch. Insect Biochem. Physiol. 2023, 114, e22048. [Google Scholar] [CrossRef] [PubMed]
- Behmer, S.T.; Nes, W.D. Insect sterol nutrition and physiology: A global overview. Adv. Insect Physiol. 2003, 31, 1–72. [Google Scholar]
- Xu, S.J.; Benoff, B.; Liou, H.L.; Lobel, P.; Stock, A.M. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann- Pick type C2 disease. J. Biol. Chem. 2007, 282, 23525–23531. [Google Scholar] [CrossRef]
- Behmer, S.T. Overturning dogma: Tolerance of insects to mixed-sterol diets is not universal. Curr. Opin. Insect Sci. 2017, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.N.; Liang, H.H.; Kausar, S.; Dong, Z.; Cui, H.J. Zinc finger protein RP-8, the Bombyx mori ortholog of programmed cell death 2, regulates cell proliferation. Dev. Comp. Immunol. 2020, 104, 103542. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.N.; Zhu, B.J.; Kausar, S.; Dai, L.S.; Sun, Y.X.; Tian, J.W.; Liu, C.L. Suppressor of cytokine signaling 2-12 regulates antimicrobial peptides and ecdysteroid signaling pathways in Bombyx mori (Dazao). J. Insect Physiol. 2017, 103, 47–56. [Google Scholar] [CrossRef]
- Kausar, S.; Abbas, M.N.; Zhao, Y.; Cui, H. Immune strategies of silkworm, Bombyx mori against microbial infections. Invertebr. Surviv. J. 2019, 16, 130–140. [Google Scholar]
- Zheng, J.C.; Sun, S.L.; Yue, X.R.; Liu, T.X.; Jing, X.F. Phylogeny and evolution of the cholesterol transporter NPC1 in insects. J. Insect Physiol. 2018, 107, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.R.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.J.; Yao, X.R.; Lyer, S.P.N.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wan, B.; Jin, M.R.; Wang, J.; Xin, T.R.; Zou, Z.W.; Xia, B. The loss of Halloween gene function seriously affects the development and reproduction of Diaphorina citri (Hemiptera: Liviidae) and increases its susceptibility to pesticides. Pestic. Biochem. Physiol. 2023, 191, 105361. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.W.; Qiao, H.; Fu, Y.; Fu, H.T.; Zhang, W.Y.; Jin, S.B.; Gong, Y.S.; Jiang, S.F.; Xiong, Y.W.; Wu, Y.N.; et al. RNA interference shows that Spook, the precursor gene of 20-hydroxyecdysone (20E), regulates the molting of Macrobrachium nipponense. J. Steroid Biochem. Mol. Biol. 2021, 213, 105976. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.Y.; Fu, Y.; Zhang, W.Y.; Jiang, S.F.; Xiong, Y.W.; Yan, Y.; Gong, Y.S.; Qiao, H.; Fu, H.T. Characterization, expression and functional analysis of CYP306a1 in the oriental river prawn, Macrobrachium nipponense. Aquac. Rep. 2022, 22, 101009. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Zhang, W.Y.; Xiong, Y.W.; Wang, J.S.; Jin, S.B.; Qiao, H.; Jiang, S.F.; Fu, H.T. Dual roles of CYP302A1 in regulating ovarian maturation and molting in Macrobrachium nipponense. J. Steroid Biochem. Mol. Biol. 2023, 232, 106336. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Xiong, Y.W.; Zhang, W.Y.; Fu, H.T.; Jiang, S.F.; Bai, H.K.; Sun, S.M.; Jin, S.B.; Gong, Y.S. Characterization, expression, and function analysis of gonad-inhibiting hormone in Oriental River prawn, Macrobrachium nipponense and its induced expression by temperature. Comp. Biochem. Phys. A 2015, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.B.; Jiang, P.; Wang, Z.Q.; Long, S.R.; Liu, R.D.; Zhang, X.; Yang, W.; Ren, H.J.; Cui, J. DsRNA-mediated Silencing of Nudix hydrolase in Trichinella Spiralis inhibits the larval invasion and survival in mice. Exp. Parasitol. 2016, 162, 35–42. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Primer | Primer Sequence (5′-3′) |
|---|---|
| Mn-NPC1 F1(ORF) | CTACTCTGTTTCTTTTGGCGAC |
| Mn-NPC1 R1(ORF) | TAGGTGACAGAGGAGCATAGCA |
| Mn-NPC1 F2(ORF) | GATATCGTCCACCTCGGAGACA |
| Mn-NPC1 R2(ORF) | ATGACGCTGGCTGGGCGATGTA |
| Mn-NPC1 F3 (ORF) | CTTCTATGATACCGAACGTGAC |
| Mn-NPC1 R3(ORF) | TTACCAGAAACATTGTCATCAG |
| Mn-NPC1 R (5′) | AGCAGGCTTGGGTGGGCCGTTG |
| Mn-NPC1 F (3′) | CCTGAAAGTCTTCAAGAATCAA |
| Mn-NPC1 F(qPCR) | GATCCTACACTAAGCATCCCCTG |
| Mn-NPC1 R(qPCR) | GATCCTACACTAAGCATCCCCTG |
| EIF-F (qPCR) | CATGGATGTACCTGTGGTGAAAC |
| EIF-R (qPCR) | CTGTCAGCAGAAGGTCCTCATTA |
| Mn-NPC1 probe | CGGTTTCTCGCCTTTTTCATTTGATTTTGTCTATG |
| Mn-NPC1 anti-probe | CATAGACAAAATCAAATGAAAAAGGCGAGAAACCG |
| Mn-NPC1 iF (RNAi) | TAATACGACTCACTATAGGGGTCGTGGCTCTCTCTTACGG |
| Mn-NPC1 iR (RNAi) | TAATACGACTCACTATAGGGGACACTTTGTGTTGCGCAGT |
| GFP iF (RNAi) | GATCACTAATACGACTCACTATAGGGTCCTGGTCGAGCTGGACGG |
| GFP iR (RNAi) | GATCACTAATACGACTCACTATAGGGCGCTTCTCGTTGGGGTCTTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Zhang, W.; Xiong, Y.; Zhang, M.; Yuan, H.; Niu, Y.; Qiao, H.; Fu, H. NPC Intracellular Cholesterol Transporter 1 Regulates Ovarian Maturation and Molting in Female Macrobrachium nipponense. Int. J. Mol. Sci. 2024, 25, 6049. https://doi.org/10.3390/ijms25116049
Jiang S, Zhang W, Xiong Y, Zhang M, Yuan H, Niu Y, Qiao H, Fu H. NPC Intracellular Cholesterol Transporter 1 Regulates Ovarian Maturation and Molting in Female Macrobrachium nipponense. International Journal of Molecular Sciences. 2024; 25(11):6049. https://doi.org/10.3390/ijms25116049
Chicago/Turabian StyleJiang, Sufei, Wenyi Zhang, Yiwei Xiong, Mengying Zhang, Huwei Yuan, Yunpeng Niu, Hui Qiao, and Hongtuo Fu. 2024. "NPC Intracellular Cholesterol Transporter 1 Regulates Ovarian Maturation and Molting in Female Macrobrachium nipponense" International Journal of Molecular Sciences 25, no. 11: 6049. https://doi.org/10.3390/ijms25116049
APA StyleJiang, S., Zhang, W., Xiong, Y., Zhang, M., Yuan, H., Niu, Y., Qiao, H., & Fu, H. (2024). NPC Intracellular Cholesterol Transporter 1 Regulates Ovarian Maturation and Molting in Female Macrobrachium nipponense. International Journal of Molecular Sciences, 25(11), 6049. https://doi.org/10.3390/ijms25116049








