The Application of Rho Kinase Inhibitors in the Management of Glaucoma
Abstract
1. Introduction
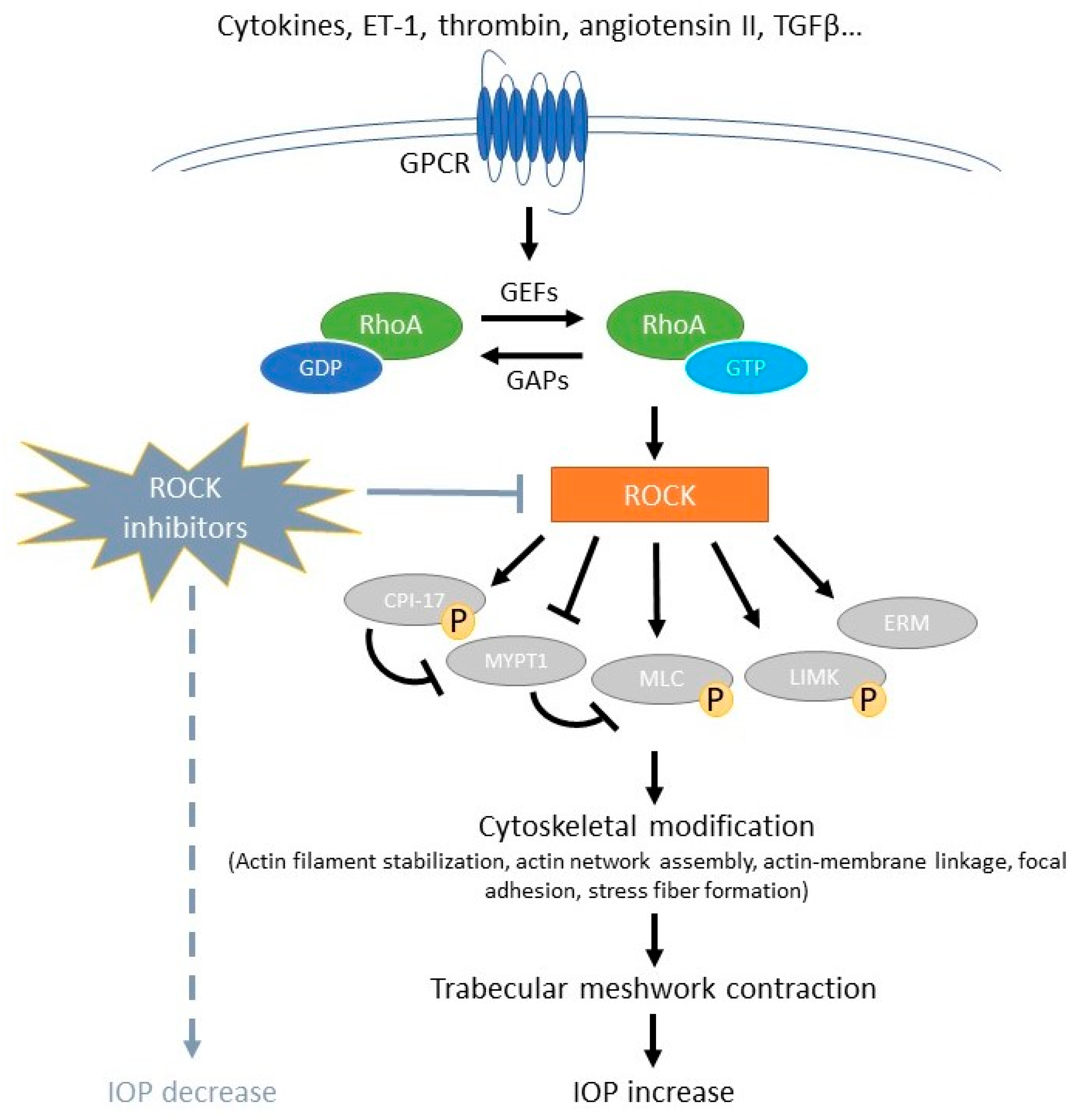
2. Rho/Rho Kinase Pathway in the Role of Glaucoma
2.1. Role of Rho/Rho Kinase in Aqueous Outflow Pathway
2.2. Role of Rho/Rho Kinase in Optic Nerve and Retina
2.3. Role of Rho/Rho Kinase in Tenon Fibroblast
3. Rho Kinase Inhibitors
4. Recent Trials of Rho Kinase Inhibitors in Glaucoma Treatment
4.1. Ripasudil (K-115)
4.2. Netarsudil (AR-13324)
4.3. SNJ-1656 (Y-39983)
4.4. AR-12286
4.5. Sovesudil (PHP-201, AMA0076)
4.6. Fasudil (HA-1077)
4.7. Other ROCK Inhibitors in Preclinical Studies or under Clinical Trials
5. ROCK Inhibitors in Other Ocular Diseases
6. Drug Delivery System of Rho Kinase Inhibitors
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Allison, K.; Patel, D.; Alabi, O. Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future. Cureus 2020, 12, e11686. [Google Scholar] [CrossRef]
- Winkler, N.S.; Fautsch, M.P. Effects of prostaglandin analogues on aqueous humor outflow pathways. J. Ocul. Pharmacol. Ther. 2014, 30, 102–109. [Google Scholar] [CrossRef]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef]
- Tamm, E.R.; Braunger, B.M.; Fuchshofer, R. Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways. Prog. Mol. Biol. Transl. Sci. 2015, 134, 301–314. [Google Scholar]
- Buffault, J.; Labbé, A.; Hamard, P.; Brignole-Baudouin, F.; Baudouin, C. The trabecular meshwork: Structure, function and clinical implications. A review of the literature. J. Fr. Ophtalmol. 2020, 43, e217–e230. [Google Scholar] [CrossRef]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Keller, K.E. Tunneling nanotubes and actin cytoskeleton dynamics in glaucoma. Neural Regen. Res. 2020, 15, 2031–2032. [Google Scholar] [CrossRef]
- Liu, B.; McNally, S.; Kilpatrick, J.I.; Jarvis, S.P.; O’Brien, C.J. Aging and ocular tissue stiffness in glaucoma. Surv. Ophthalmol. 2018, 63, 56–74. [Google Scholar] [CrossRef]
- Moshirfar, M.; Parker, L.; Birdsong, O.C.; Ronquillo, Y.C.; Hofstedt, D.; Shah, T.J.; Gomez, A.T.; Hoopes, P.C.S. Use of Rho kinase Inhibitors in Ophthalmology: A Review of the Literature. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 101–111. [Google Scholar]
- Sturdivant, J.M.; Royalty, S.M.; Lin, C.W.; Moore, L.A.; Yingling, J.D.; Laethem, C.L.; Sherman, B.; Heintzelman, G.R.; Kopczynski, C.C.; deLong, M.A. Discovery of the ROCK inhibitor netarsudil for the treatment of open-angle glaucoma. Bioorg. Med. Chem. Lett. 2016, 26, 2475–2480. [Google Scholar] [CrossRef]
- Inoue, T.; Tanihara, H. Rho-associated kinase inhibitors: A novel glaucoma therapy. Prog. Retin. Eye Res. 2013, 37, 1–12. [Google Scholar] [CrossRef]
- Saha, B.C.; Kumari, R.; Kushumesh, R.; Ambasta, A.; Sinha, B.P. Status of Rho kinase inhibitors in glaucoma therapeutics—An overview. Int. Ophthalmol. 2022, 42, 281–294. [Google Scholar] [CrossRef]
- Tanna, A.P.; Johnson, M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology 2018, 125, 1741–1756. [Google Scholar] [CrossRef]
- Tian, B.; Geiger, B.; Epstein, D.L.; Kaufman, P.L. Cytoskeletal involvement in the regulation of aqueous humor outflow. Investig. Ophthalmol. Vis. Sci. 2000, 41, 619–623. [Google Scholar]
- Peterson, J.A.; Tian, B.; Geiger, B.; Kaufman, P.L. Effect of latrunculin-B on outflow facility in monkeys. Exp. Eye Res. 2000, 70, 307–313. [Google Scholar] [CrossRef]
- Tian, B.; Kaufman, P.L.; Volberg, T.; Gabelt, B.T.; Geiger, B. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch. Ophthalmol. 1998, 116, 633–643. [Google Scholar] [CrossRef]
- Julian, L.; Olson, M.F. Rho-associated coiled-coil containing kinases (ROCK): Structure, regulation, and functions. Small GTPases 2014, 5, e29846. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Dang, Y. Rho-Kinase Inhibitors as Emerging Targets for Glaucoma Therapy. Ophthalmol. Ther. 2023, 12, 2943–2957. [Google Scholar] [CrossRef]
- Tian, B.; Gabelt, B.T.; Geiger, B.; Kaufman, P.L. The role of the actomyosin system in regulating trabecular fluid outflow. Exp. Eye Res. 2009, 88, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, E.; Nakajima, T.; Minagawa, Y.; Shearer, T.R.; Azuma, M. Contribution of ROCK in contraction of trabecular meshwork: Proposed mechanism for regulating aqueous outflow in monkey and human eyes. J. Pharm. Sci. 2005, 94, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Thieme, H.; Nuskovski, M.; Nass, J.U.; Pleyer, U.; Strauss, O.; Wiederholt, M. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4240–4246. [Google Scholar]
- Schehlein, E.M.; Robin, A.L. Rho-Associated Kinase Inhibitors: Evolving Strategies in Glaucoma Treatment. Drugs 2019, 79, 1031–1036. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-associated kinase pathway in glaucoma (Review). Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [CrossRef]
- Zhang, M.; Maddala, R.; Rao, P.V. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am. J. Physiol. Cell Physiol. 2008, 295, C1057–C1070. [Google Scholar] [CrossRef]
- Pattabiraman, P.P.; Rao, P.V. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 2010, 298, C749–C763. [Google Scholar] [CrossRef]
- Goldhagen, B.; Proia, A.D.; Epstein, D.L.; Rao, P.V. Elevated levels of RhoA in the optic nerve head of human eyes with glaucoma. J. Glaucoma 2012, 21, 530–538. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Miao, H.; Lukas, T. Astrocytes in glaucomatous optic neuropathy. Prog. Brain Res. 2008, 173, 353–373. [Google Scholar]
- Tang, Y.; Chen, Y.; Chen, D. The heterogeneity of astrocytes in glaucoma. Front. Neuroanat. 2022, 16, 995369. [Google Scholar] [CrossRef]
- Lukas, T.J.; Miao, H.; Chen, L.; Riordan, S.M.; Li, W.; Crabb, A.M.; Wise, A.; Du, P.; Lin, S.M.; Hernandez, M.R. Susceptibility to glaucoma: Differential comparison of the astrocyte transcriptome from glaucomatous African American and Caucasian American donors. Genome Biol. 2008, 9, R111. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, Y.; Kitaoka, Y.; Kumai, T.; Lam, T.T.; Kuribayashi, K.; Isenoumi, K.; Munemasa, Y.; Motoki, M.; Kobayashi, S.; Ueno, S. Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina. Brain Res. 2004, 1018, 111–118. [Google Scholar] [CrossRef]
- Bertrand, J.; Winton, M.J.; Rodriguez-Hernandez, N.; Campenot, R.B.; McKerracher, L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J. Neurosci. 2005, 25, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Takaseki, S.; Yoshitomi, T. Effects of ripasudil hydrochloride hydrate (K-115), a Rho-kinase inhibitor, on ocular blood flow and ciliary artery smooth muscle contraction in rabbits. Jpn. J. Ophthalmol. 2017, 61, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S. Trabeculectomy and antimetabolites. Br. J. Ophthalmol. 2004, 88, 855–856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibrahim, D.G.; Ko, J.A.; Iwata, W.; Okumichi, H.; Kiuchi, Y. An in vitro study of scarring formation mediated by human Tenon fibroblasts: Effect of Y-27632, a Rho kinase inhibitor. Cell Biochem. Funct. 2019, 37, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Futakuchi, A.; Inoue, T.; Fujimoto, T.; Inoue-Mochita, M.; Kawai, M.; Tanihara, H. The effects of ripasudil (K-115), a Rho kinase inhibitor, on activation of human conjunctival fibroblasts. Exp. Eye Res. 2016, 149, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Honjo, M.; Tanihara, H.; Kameda, T.; Kawaji, T.; Yoshimura, N.; Araie, M. Potential Role of Rho-Associated Protein Kinase Inhibitor Y-27632 in Glaucoma Filtration Surgery. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Van de Velde, S.; Van Bergen, T.; Vandewalle, E.; Kindt, N.; Castermans, K.; Moons, L.; Stalmans, I. Chapter 14—Rho kinase inhibitor AMA0526 improves surgical outcome in a rabbit model of glaucoma filtration surgery. In Progress in Brain Research; Bagetta, G., Nucci, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 220, pp. 283–297. [Google Scholar]
- Mizuno, Y.; Komatsu, K.; Tokumo, K.; Okada, N.; Onoe, H.; Okumichi, H.; Hirooka, K.; Aoki, G.; Miura, Y.; Kiuchi, Y. Safety and Efficacy of the Rho-Kinase Inhibitor (Ripasudil) in Bleb Needling after Trabeculectomy: A Prospective Multicenter Study. J. Clin. Med. 2023, 13, 75. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, D.; Guo, J.; Ren, Z.; Zhou, L.; Wang, S.; Xu, B.; Wang, R. Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol. Med. Chir. 2006, 46, 421–428. [Google Scholar] [CrossRef]
- Satoh, K.; Fukumoto, Y.; Shimokawa, H. Rho-kinase: Important new therapeutic target in cardiovascular diseases. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H287–H296. [Google Scholar] [CrossRef]
- Zhang, Y.; Saradna, A.; Ratan, R.; Ke, X.; Tu, W.; Do, D.C.; Hu, C.; Gao, P. RhoA/Rho-kinases in asthma: From pathogenesis to therapeutic targets. Clin. Transl. Immunol. 2020, 9, e01134. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, S.; Jiang, Q. Role of Rho kinase signal pathway in inflammatory bowel disease. Int. J. Clin. Exp. Med. 2015, 8, 3089–3097. [Google Scholar] [PubMed]
- Wei, L.; Surma, M.; Shi, S.; Lambert-Cheatham, N.; Shi, J. Novel Insights into the Roles of Rho Kinase in Cancer. Arch. Immunol. Ther. Exp. 2016, 64, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Loirand, G. Rho Kinases in Health and Disease: From Basic Science to Translational Research. Pharmacol. Rev. 2015, 67, 1074–1095. [Google Scholar] [CrossRef]
- Hsu, C.R.; Chen, Y.H.; Liu, C.P.; Chen, C.H.; Huang, K.K.; Huang, J.W.; Lin, M.N.; Lin, C.L.; Chen, W.R.; Hsu, Y.L.; et al. A Highly Selective Rho-Kinase Inhibitor (ITRI-E-212) Potentially Treats Glaucoma Upon Topical Administration with Low Incidence of Ocular Hyperemia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.; Kim, Y.K.; Jeoung, J.W.; Satyal, S.; Kim, J.; Kim, S.; Park, K.H. Sovesudil (locally acting rho kinase inhibitor) for the treatment of normal-tension glaucoma: The randomized phase II study. Acta Ophthalmol. 2022, 100, e470–e477. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 1 clinical trials of a selective Rho kinase inhibitor, K-115. JAMA Ophthalmol. 2013, 131, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am. J. Ophthalmol. 2013, 156, 731–736. [Google Scholar] [CrossRef]
- Tanihara, H.; Kakuda, T.; Sano, T.; Kanno, T.; Gunji, R. Safety and efficacy of ripasudil in Japanese patients with glaucoma or ocular hypertension: 12-month interim analysis of ROCK-J, a post-marketing surveillance study. BMC Ophthalmol. 2020, 20, 275. [Google Scholar]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Fukushima, A.; Suganami, H.; Araie, M. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016, 94, e26–e34. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined with Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2015, 133, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Okayama, R.; Shiokawa, M.; Ishida, K.; Tomita, G. Efficacy and safety of adding ripasudil to existing treatment regimens for reducing intraocular pressure. Int. Ophthalmol. 2018, 38, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Yamamoto, T.; Aihara, M.; Koizumi, N.; Minami, H.; Kojima, S.; Isobe, T.; Kanazawa, M.; Suganami, H. Crossover Randomized Study of Pharmacologic Effects of Ripasudil-Brimonidine Fixed-Dose Combination Versus Ripasudil or Brimonidine. Adv. Ther. 2023, 40, 3559–3573. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Yamamoto, T.; Aihara, M.; Kawakita, K.; Kojima, S.; Kanazawa, M.; Nojima, T.; Suganami, H. Ripasudil-Brimonidine Fixed-Dose Combination vs Ripasudil or Brimonidine: Two Phase 3 Randomized Clinical Trials. Am. J. Ophthalmol. 2023, 248, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Yamamoto, T.; Aihara, M.; Koizumi, N.; Fukushima, A.; Kawakita, K.; Kojima, S.; Nakamura, T.; Suganami, H. Long-term intraocular pressure-lowering efficacy and safety of ripasudil-brimonidine fixed-dose combination for glaucoma and ocular hypertension: A multicentre, open-label, phase 3 study. Graefes Arch. Clin. Exp. Ophthalmol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Namekata, K.; Noro, T.; Nishijima, E.; Sotozono, A.; Guo, X.; Harada, C.; Shinozaki, Y.; Mitamura, Y.; Nakano, T.; Harada, T. Drug combination of topical ripasudil and brimonidine enhances neuroprotection in a mouse model of optic nerve injury. J. Pharmacol. Sci. 2024, 154, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Futakuchi, A.; Morimoto, T.; Ikeda, Y.; Tanihara, H.; Inoue, T. Intraocular pressure-lowering effects of ripasudil in uveitic glaucoma, exfoliation glaucoma, and steroid-induced glaucoma patients: ROCK-S, a multicentre historical cohort study. Sci. Rep. 2020, 10, 10308. [Google Scholar] [CrossRef] [PubMed]
- Harvinder Nagpal, M.K. Topical Ripasudil as First Line Treatment for Ocular Hypertension in Uveitis Cases: A Prospective Study. J. Clin. Diagn. Res. 2021, 15, NC01–NC03. [Google Scholar]
- Mimura, T.; Noma, H.; Inoue, Y.; Kawashima, M.; Kitsu, K.; Mizota, A. Early Postoperative Effect of Ripasudil Hydrochloride After Trabeculectomy on Secondary Glaucoma: A Randomized Controlled Trial. Open Ophthalmol. J. 2022, 16, e2206201. [Google Scholar] [CrossRef]
- Saito, H.; Kagami, S.; Mishima, K.; Mataki, N.; Fukushima, A.; Araie, M. Long-term Side Effects Including Blepharitis Leading to Discontinuation of Ripasudil. J. Glaucoma 2019, 28, 289–293. [Google Scholar] [CrossRef]
- Tanihara, H.; Kakuda, T.; Sano, T.; Kanno, T.; Kurihara, Y. Long-Term Intraocular Pressure-Lowering Effects and Adverse Events of Ripasudil in Patients with Glaucoma or Ocular Hypertension over 24 Months. Adv. Ther. 2022, 39, 1659–1677. [Google Scholar] [CrossRef]
- Singh, I.P.; Fechtner, R.D.; Myers, J.S.; Kim, T.; Usner, D.W.; McKee, H.; Sheng, H.; Lewis, R.A.; Heah, T.; Kopczynski, C.C. Pooled Efficacy and Safety Profile of Netarsudil Ophthalmic Solution 0.02% in Patients with Open-angle Glaucoma or Ocular Hypertension. J. Glaucoma 2020, 29, 878–884. [Google Scholar] [CrossRef]
- Kiel, J.W.; Kopczynski, C.C. Effect of AR-13324 on episcleral venous pressure in Dutch belted rabbits. J. Ocul. Pharmacol. Ther. 2015, 31, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-F.; Williamson, J.E.; Kopczynski, C.; Serle, J.B. Effect of 0.04% AR-13324, a ROCK, and norepinephrine transporter inhibitor, on aqueous humor dynamics in normotensive monkey eyes. J. Glaucoma 2015, 24, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Ramirez, N.; Novack, G.D.; Kopczynski, C. Ocular hypotensive safety and systemic absorption of AR-13324 ophthalmic solution in normal volunteers. Am. J. Ophthalmol. 2015, 159, 980–985.e1. [Google Scholar] [CrossRef]
- Bacharach, J.; Dubiner, H.B.; Levy, B.; Kopczynski, C.C.; Novack, G.D. Double-masked, randomized, dose-response study of AR-13324 versus latanoprost in patients with elevated intraocular pressure. Ophthalmology 2015, 122, 302–307. [Google Scholar] [CrossRef]
- Lewis, R.A.; Levy, B.; Ramirez, N.; Kopczynski, C.C.; Usner, D.W.; Novack, G.D. Fixed-dose combination of AR-13324 and latanoprost: A double-masked, 28-day, randomised, controlled study in patients with open-angle glaucoma or ocular hypertension. Br. J. Ophthalmol. 2016, 100, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Clement Freiberg, J.; von Spreckelsen, A.; Kolko, M.; Azuara-Blanco, A.; Virgili, G. Rho kinase inhibitor for primary open-angle glaucoma and ocular hypertension. Cochrane Database Syst. Rev. 2022, 6, Cd013817. [Google Scholar]
- Asrani, S.; Robin, A.L.; Serle, J.B.; Lewis, R.A.; Usner, D.W.; Kopczynski, C.C.; Heah, T. Netarsudil/Latanoprost Fixed-Dose Combination for Elevated Intraocular Pressure: Three-Month Data from a Randomized Phase 3 Trial. Am. J. Ophthalmol. 2019, 207, 248–257. [Google Scholar] [CrossRef]
- Walters, T.R.; Ahmed, I.I.K.; Lewis, R.A.; Usner, D.W.; Lopez, J.; Kopczynski, C.C.; Heah, T. Once-Daily Netarsudil/Latanoprost Fixed-Dose Combination for Elevated Intraocular Pressure in the Randomized Phase 3 MERCURY-2 Study. Ophthalmol. Glaucoma 2019, 2, 280–289. [Google Scholar] [CrossRef]
- Asrani, S.; Bacharach, J.; Holland, E.; McKee, H.; Sheng, H.; Lewis, R.A.; Kopczynski, C.C.; Heah, T. Fixed-Dose Combination of Netarsudil and Latanoprost in Ocular Hypertension and Open-Angle Glaucoma: Pooled Efficacy/Safety Analysis of Phase 3 MERCURY-1 and -2. Adv. Ther. 2020, 37, 1620–1631. [Google Scholar] [CrossRef]
- Stalmans, I.; Lim, K.S.; Oddone, F.; Fichtl, M.; Belda, J.I.; Hommer, A.; Laganovska, G.; Schweitzer, C.; Voykov, B.; Zarnowski, T.; et al. MERCURY-3: A randomized comparison of netarsudil/latanoprost and bimatoprost/timolol in open-angle glaucoma and ocular hypertension. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 179–190. [Google Scholar] [CrossRef]
- Tanihara, H.; Inatani, M.; Honjo, M.; Tokushige, H.; Azuma, J.; Araie, M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol. 2008, 126, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Tanihara, H.; Tokushige, H.; Araie, M. Efficacy and safety of SNJ-1656 in primary open-angle glaucoma or ocular hypertension. Acta Ophthalmol. 2015, 93, e393–e395. [Google Scholar] [CrossRef]
- Kopczynski, C.; Novack, G.D.; Swearingen, D.; van Haarlem, T. Ocular hypotensive efficacy, safety and systemic absorption of AR-12286 ophthalmic solution in normal volunteers. Br. J. Ophthalmol. 2013, 97, 567–572. [Google Scholar] [CrossRef]
- Williams, R.D.; Novack, G.D.; van Haarlem, T.; Kopczynski, C. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am. J. Ophthalmol. 2011, 152, 834–841.e1. [Google Scholar] [CrossRef]
- Ren, R.; Humphrey, A.A.; Kopczynski, C.; Gong, H. Rho Kinase Inhibitor AR-12286 Reverses Steroid-Induced Changes in Intraocular Pressure, Effective Filtration Areas, and Morphology in Mouse Eyes. Investig. Ophthalmol. Vis. Sci. 2023, 64, 7. [Google Scholar] [CrossRef]
- Van de Velde, S.; Van Bergen, T.; Sijnave, D.; Hollanders, K.; Castermans, K.; Defert, O.; Leysen, D.; Vandewalle, E.; Moons, L.; Stalmans, I. AMA0076, a novel, locally acting Rho kinase inhibitor, potently lowers intraocular pressure in New Zealand white rabbits with minimal hyperemia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1006–1016. [Google Scholar] [CrossRef]
- Pakravan, M.; Beni, A.N.; Ghahari, E.; Varshochian, R.; Yazdani, S.; Esfandiari, H.; Ahmadieh, H. The Ocular Hypotensive Efficacy of Topical Fasudil, a Rho-Associated Protein Kinase Inhibitor, in Patients with End-Stage Glaucoma. Am. J. Ther. 2017, 24, e676–e680. [Google Scholar] [CrossRef]
- Mietzner, R.; Kade, C.; Froemel, F.; Pauly, D.; Stamer, W.D.; Ohlmann, A.; Wegener, J.; Fuchshofer, R.; Breunig, M. Fasudil Loaded PLGA Microspheres as Potential Intravitreal Depot Formulation for Glaucoma Therapy. Pharmaceutics 2020, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Osi, B.; Al-Kinani, A.A.; Al-Qaysi, Z.K.; Khoder, M.; Alany, R.G. Exploring the Ocular Absorption Pathway of Fasudil Hydrochloride towards Developing a Nanoparticulate Formulation with Improved Performance. Pharmaceutics 2024, 16, 112. [Google Scholar] [CrossRef]
- Khallaf, A.M.; El-Moslemany, R.M.; Ahmed, M.F.; Morsi, M.H.; Khalafallah, N.M. Exploring a Novel Fasudil-Phospholipid Complex Formulated as Liposomal Thermosensitive in situ Gel for Glaucoma. Int. J. Nanomed. 2022, 17, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Hartman, P.J.; Cooke, D.L.; Hsu, H.H.; Stewart, J.; Sumi, K.; Yoshida, Y.; Hidaka, H.; Novack, G.D. Phase I/II, Double-Masked, Randomized, Vehicle-Controlled Study of H-1337 Ophthalmic Solution for Glaucoma and Ocular Hypertension. Ophthalmol. Glaucoma 2023, 6, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Wirta, D.; Li, X.Y.; Shen, W.; Lu, C.; Novack, G.D. Double-Masked, Vehicle-Controlled, Randomized, Phase II Study of the Ocular Hypotensive Activity and Safety of VVN539 Ophthalmic Solution. Ophthalmol. Sci. 2024, 4, 100426. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Hirata, K.; Torii, R.; Hamuro, J.; Kinoshita, S. Enhancement of corneal endothelium wound healing by Rho-associated kinase (ROCK) inhibitor eye drops. Br. J. Ophthalmol. 2011, 95, 1006–1009. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Kay, E.P.; Ueno, M.; Sakamoto, Y.; Nakamura, S.; Hamuro, J.; Kinoshita, S. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2493–2502. [Google Scholar] [CrossRef]
- Kinoshita, S.; Colby, K.A.; Kruse, F.E. A Close Look at the Clinical Efficacy of Rho-Associated Protein Kinase Inhibitor Eye Drops for Fuchs Endothelial Corneal Dystrophy. Cornea 2021, 40, 1225–1228. [Google Scholar] [CrossRef]
- Mateos-Olivares, M.; García-Onrubia, L.; Valentín-Bravo, F.J.; González-Sarmiento, R.; Lopez-Galvez, M.; Pastor, J.C.; Usategui-Martín, R.; Pastor-Idoate, S. Rho-Kinase Inhibitors for the Treatment of Refractory Diabetic Macular Oedema. Cells 2021, 10, 1683. [Google Scholar] [CrossRef]
- Ahmadieh, H.; Nourinia, R.; Hafezi-Moghadam, A.; Sabbaghi, H.; Nakao, S.; Zandi, S.; Yaseri, M.; Tofighi, Z.; Akbarian, S. Intravitreal injection of a Rho-kinase inhibitor (fasudil) combined with bevacizumab versus bevacizumab monotherapy for diabetic macular oedema: A pilot randomised clinical trial. Br. J. Ophthalmol. 2019, 103, 922–927. [Google Scholar] [CrossRef]
- Ding, J.; Crews, K.; Carbajal, K.; Weksler, M.; Moore, L.; Carlson, E.C.; Lin, C.-W. Ocular Tissue Distribution and Duration of Release of AR-13503 Following Administration of AR-13503 Sustained Release Intravitreal Implant in Rabbits and Miniature Swine. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5387. [Google Scholar]
- Chan, W.; Akhbanbetova, A.; Quantock, A.J.; Heard, C.M. Topical delivery of a Rho-kinase inhibitor to the cornea via mucoadhesive film. Eur. J. Pharm. Sci. 2016, 91, 256–264. [Google Scholar] [CrossRef][Green Version]
- Lin, L.; Lin, Q.; Li, J.; Han, Y.; Chang, P.; Lu, F.; Zhao, Y.E. ROCK inhibitor modified intraocular lens as an approach for inhibiting the proliferation and migration of lens epithelial cells and posterior capsule opacification. Biomater. Sci. 2019, 7, 4208–4217. [Google Scholar] [CrossRef]
- Mehta, A.A.; Kanu, L.N.; Sood-Mendiratta, S.; Quinones, R.; Hawkins, A.; Lehrer, R.A.; Malhotra, K.; Papas, R.; Hillman, D.; Wilensky, J.T.; et al. Experience with netarsudil 0.02% and latanoprostene bunod 0.024% as adjunctive therapy for glaucoma. Eur. J. Ophthalmol. 2022, 32, 322–326. [Google Scholar] [CrossRef]
- Bahr, T.; Woolf, S.; Favre, H.; Waldman, C. Comparison of netarsudil and latanoprostene bunod as adjuncts to maximum medical therapy in primary open-angle glaucoma. Can. J. Ophthalmol. 2023, 58, 356–360. [Google Scholar] [CrossRef]
| Intervention Compound | Phase/Trial ID | Conditions | Year |
|---|---|---|---|
| INS115644 | Phase 1/NCT00443924 | POAG and OH | 2007 |
| INS117548 | Phase 1/NCT00767793 | Bilateral OH or Early POAG | 2008 |
| LX7101 | Phase 1 and 2/NCT01528111 | POAG and OH | 2012 |
| ATS907 | Phase 1/2a/NCT01520116 | POAG and OH | 2012 |
| Phase 2/NCT01668524 | POAG and OH | 2012 | |
| H-1337 | Phase 2/NCT05913232 | POAG and OH | 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-C.; Chen, Y.-H.; Lu, D.-W. The Application of Rho Kinase Inhibitors in the Management of Glaucoma. Int. J. Mol. Sci. 2024, 25, 5576. https://doi.org/10.3390/ijms25115576
Liu L-C, Chen Y-H, Lu D-W. The Application of Rho Kinase Inhibitors in the Management of Glaucoma. International Journal of Molecular Sciences. 2024; 25(11):5576. https://doi.org/10.3390/ijms25115576
Chicago/Turabian StyleLiu, Li-Ching, Yi-Hao Chen, and Da-Wen Lu. 2024. "The Application of Rho Kinase Inhibitors in the Management of Glaucoma" International Journal of Molecular Sciences 25, no. 11: 5576. https://doi.org/10.3390/ijms25115576
APA StyleLiu, L.-C., Chen, Y.-H., & Lu, D.-W. (2024). The Application of Rho Kinase Inhibitors in the Management of Glaucoma. International Journal of Molecular Sciences, 25(11), 5576. https://doi.org/10.3390/ijms25115576





