Uveoscleral Outflow Routes after MicroPulse Laser Therapy for Refractory Glaucoma: An Optical Coherence Tomography Study of the Sclera
Abstract
1. Introduction
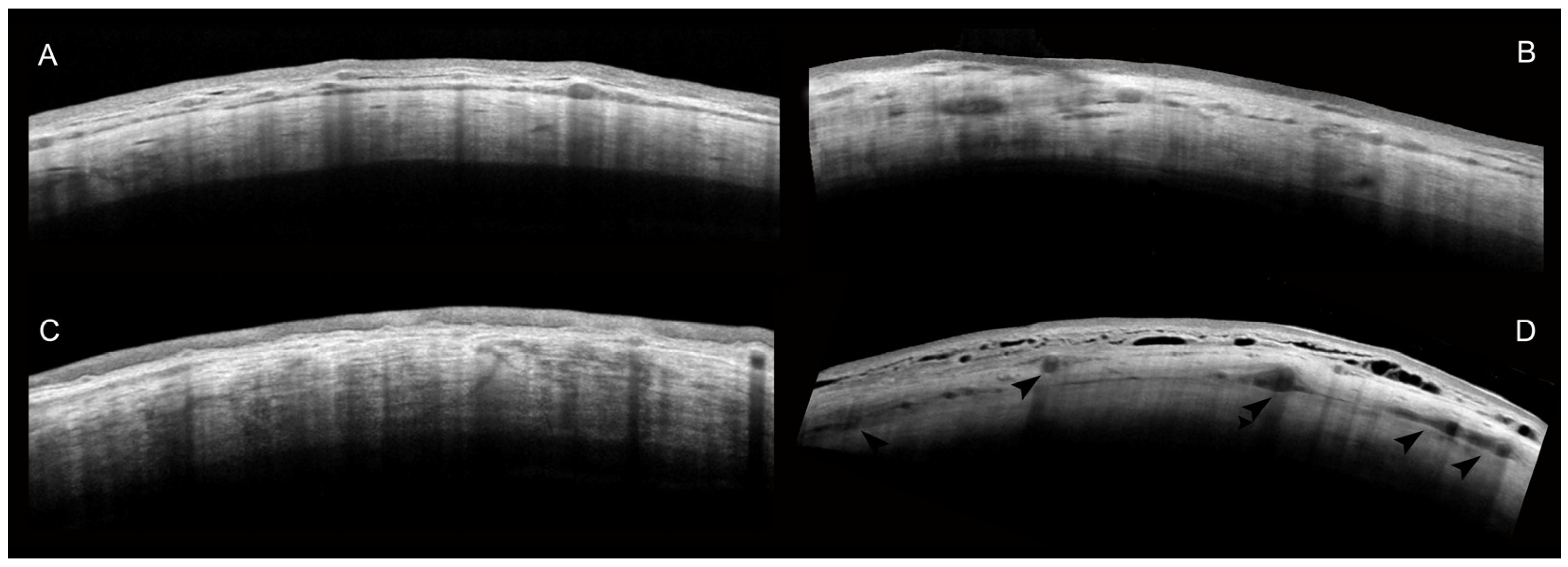
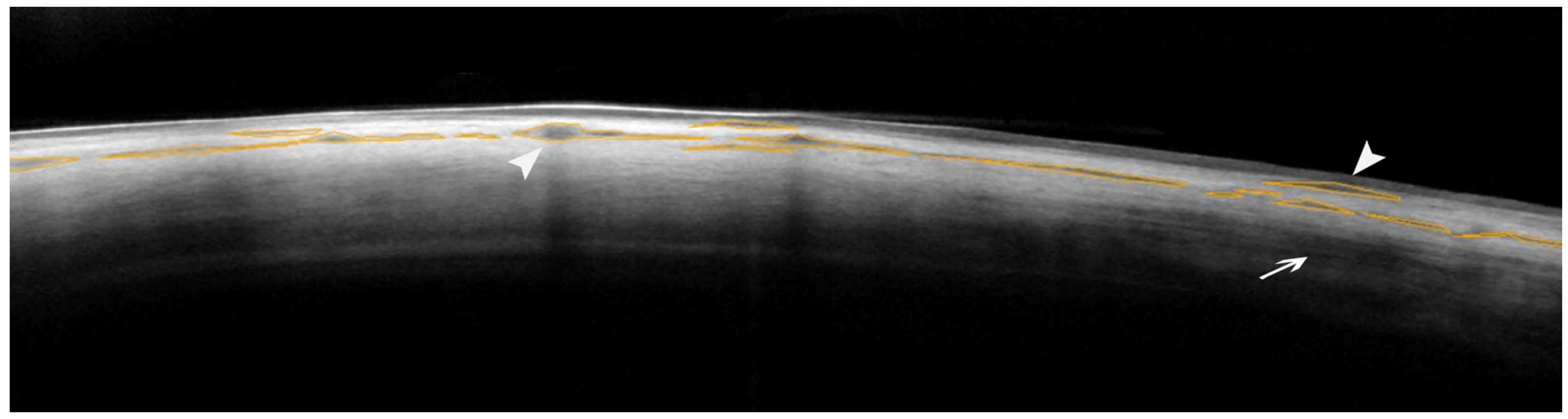
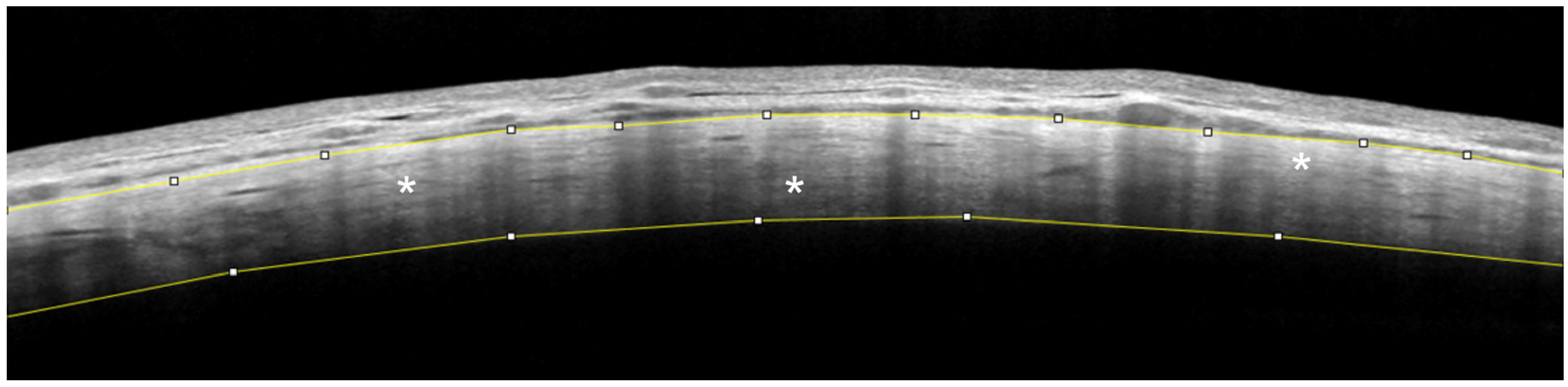
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients’ Enrolment
4.2. Surgical Procedure
4.3. Examinations
AS-OCT of the Sclera
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bengtsson, B.; Lindén, C.; Heijl, A.; Andersson-Geimer, S.; Aspberg, J.; Jóhannesson, G. The Glaucoma Intensive Treatment Study: Interim Results from an Ongoing Longitudinal Randomized Clinical Trial. Acta Ophthalmol. 2021, 100, E455–E462. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A. Reduction of Intraocular Pressure and Glaucoma Progression. Arch. Ophthalmol. 2002, 120, 1268. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, A.; Haukka, J.; Loukovaara, S.; Harju, M. Incidence of Glaucoma Filtration Surgery from Disease Onset of Open-Angle Glaucoma. Acta Ophthalmol. 2023, 102, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Gedde, S.J.; Heuer, D.K.; Parrish, R.K. Review of Results from the Tube versus Trabeculectomy Study. Curr. Opin. Ophthalmol. 2010, 21, 123–128. [Google Scholar] [CrossRef]
- Landers, J.; Martin, K.; Sarkies, N.; Bourne, R.; Watson, P. A Twenty-Year Follow-up Study of Trabeculectomy: Risk Factors and Outcomes. Ophthalmology 2012, 119, 694–702. [Google Scholar] [CrossRef]
- Dastiridou, A.I.; Katsanos, A.; Denis, P.; Francis, B.A.; Mikropoulos, D.G.; Teus, M.A.; Konstas, A.-G. Cyclodestructive Procedures in Glaucoma: A Review of Current and Emerging Options. Adv. Ther. 2018, 35, 2103–2127. [Google Scholar] [CrossRef] [PubMed]
- Anand, N.; Klug, E.; Nirappel, A.; Solá-Del Valle, D. A Review of Cyclodestructive Procedures for the Treatment of Glaucoma. Semin. Ophthalmol. 2020, 35, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Michelessi, M.; Bicket, A.K.; Lindsley, K. Cyclodestructive Procedures for Non-Refractory Glaucoma. Cochrane Database Syst. Rev. 2018, 2018, CD009313. [Google Scholar] [CrossRef]
- Ma, A.; Yu, S.W.Y.; Wong, J.K.W. Micropulse Laser for the Treatment of Glaucoma: A Literature Review. Surv. Ophthalmol. 2019, 64, 486–497. [Google Scholar] [CrossRef]
- Souissi, S.; Le Mer, Y.; Metge, F.; Portmann, A.; Baudouin, C.; Labbé, A.; Hamard, P. An Update on Continuous-Wave Cyclophotocoagulation (CW-CPC) and Micropulse Transscleral Laser Treatment (MP-TLT) for Adult and Paediatric Refractory Glaucoma. Acta Ophthalmol. 2021, 99, E621–E653. [Google Scholar] [CrossRef]
- Johnstone, M.A.; Padilla, S.; Wen, K. Transcleral Laser, Ciliary Muscle Shortening & Outflow Pathway Reorganization. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3468. [Google Scholar]
- Johnstone, M.A.; SONG, S.; Padilla, S.; Wen, K.; Xin, C.; Wen, J.C.; Martin, E.; Wang, R.K. Microscope Real-Time Video (MRTV), High- Resolution OCT (HR-OCT) & Histopathology (HP) to Assess How Transcleral Micropulse Laser (TML) Affects the Sclera, Ciliary Body (CB), Muscle (CM), Secretory Epithelium (CBSE), Suprachoroidal Space (SCS) & Aqueous Outflow System. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2825. [Google Scholar]
- Moussa, K.; Feinstein, M.; Pekmezci, M.; Lee, J.H.; Bloomer, M.; Oldenburg, C.; Sun, Z.; Lee, R.K.; Ying, G.S.; Han, Y. Histologic Changes Following Continuous Wave and Micropulse Transscleral Cyclophotocoagulation: A Randomized Comparative Study. Transl. Vis. Sci. Technol. 2020, 9, 22. [Google Scholar] [CrossRef]
- Tsujisawa, T.; Ishikawa, H.; Uga, S.; Asakawa, K.; Kono, Y.; Mashimo, K.; Shoji, N. Morphological Changes and Potential Mechanisms of Intraocular Pressure Reduction after Micropulse Transscleral Cyclophotocoagulation in Rabbits. Ophthalmic Res. 2022, 65, 595–602. [Google Scholar] [CrossRef]
- Barac, R.; Vuzitas, M.; Balta, F. Choroidal Thickness Increase after Micropulse Transscleral Cyclophotocoagulation. Rom. J. Ophthalmol. 2018, 62, 144–148. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Agnifili, L.; Fasanella, V.; Toto, L.; Brescia, L.; Di Staso, S.; Doronzo, E.; Marchini, G. Uveo-Scleral Outflow Pathways after Ultrasonic Cyclocoagulation in Refractory Glaucoma: An Anterior Segment Optical Coherence Tomography and in Vivo Confocal Study. Br. J. Ophthalmol. 2016, 100, 1668–1675. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Fasanella, V.; Mastropasqua, A.; Ciancaglini, M.; Agnifili, L. High-Intensity Focused Ultrasound Circular Cyclocoagulation in Glaucoma: A Step Forward for Cyclodestruction? J. Ophthalmol. 2017, 2017, 7136275. [Google Scholar] [CrossRef]
- Tan, N.; Ang, M.; Chan, A.; Barathi, V.A.; Tham, C.C.; Barton, K.; Chelvin, C.A. Sng Transscleral Cyclophotocoagulation and Its Histological Effects on the Conjunctiva. Sci. Rep. 2019, 9, 18703. [Google Scholar] [CrossRef]
- Nemoto, H.; Honjo, M.; Okamoto, M.; Sugimoto, K.; Aihara, M. Potential Mechanisms of Intraocular Pressure Reduction by Micropulse Transscleral Cyclophotocoagulation in Rabbit Eyes. Investig. Opthalmology Vis. Sci. 2022, 63, 3. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S. Gender Difference in the Pathophysiology and Treatment of Glaucoma. Curr. Eye Res. 2014, 40, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.S.; Russell, R. A Novel Human Sex Difference: Male Sclera Are Redder and Yellower than Female Sclera. Arch. Sex. Behav. 2022, 51, 2733–2740. [Google Scholar] [CrossRef]
- Issiaka, M.; Zrikem, K.; Mchachi, A.; Benhmidoune, L.; Rachid, R.; Mohamed, E.L.; Belhadji Salam, A.; Amza, A. Micropulse Diode Laser Therapy in Refractory Glaucoma. Adv. Ophthalmol. Pract. Res. 2023, 3, 23–28. [Google Scholar] [CrossRef]
- Zemba, M.; Dumitrescu, O.-M.; Vaida, F.; Dimirache, E.-A.; Pistolea, I.; Stamate, A.; Burcea, M.; Branisteanu, D.; Balta, F.; Barac, I. Micropulse vs. Continuous Wave Transscleral Cyclophotocoagulation in Neovascular Glaucoma. Exp. Ther. Med. 2022, 23, 278. [Google Scholar] [CrossRef]
- Garcia, G.A.; Nguyen, C.V.; Yelenskiy, A.; Akiyama, G.; McKnight, B.; Chopra, V.; Lu, K.; Huang, A.; Tan, J.C.H.; Francis, B.A. Micropulse Transscleral Diode Laser Cyclophotocoagulation in Refractory Glaucoma. Ophthalmol. Glaucoma 2019, 2, 402–412. [Google Scholar] [CrossRef]
- Carpineto, P.; Agnifili, L.; Senatore, A.; Agbeanda, A.G.; Lappa, A.; Borrelli, E.; Di Martino, G.; Oddone, F.; Mastropasqua, R. Scleral and Conjunctival Features in Patients with Rhegmatogenous Retinal Detachment Undergoing Scleral Buckling: An Anterior Segment Optical Coherence Tomography and in Vivo Confocal Microscopy Study. Acta Ophthalmol. 2019, 97, E1069–E1076. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Maslin, J.; Noecker, R.J. Early Results of Micropulse Transscleral Cyclophotocoagulation for the Treatment of Glaucoma. Eur. J. Ophthalmol. 2019, 30, 700–705. [Google Scholar] [CrossRef]
- Oya Tekeli; Helin Ceren Köse Comparative Efficacy and Safety of Micropulse Transscleral Laser Cyclophotocoagulation Using Different Duration Protocols in Eyes with Good Visual Acuity. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 3359–3369. [CrossRef]
- Hodapp, E.; Parrish, R.K.; Anderson, D.R. Clinical Decisions in Glaucoma; Mosby: St. Louis, MO, USA, 1993. [Google Scholar]
- Aquino, M.C.; Lim, D.; Chew, P.T. Micropulse P3TM (MP3) Laser for Glaucoma: An Innovative Therapy. J. Curr. Glaucoma Pract. DVD 2018, 12, 51–52. [Google Scholar] [CrossRef]


| Complications | Fs (n = 19) | Ss (n = 23) |
|---|---|---|
| Transient IOP spike * | 2 | 1 |
| Transient VA decrease § | 1 | 2 |
| Conjunctival hyperemia | 5 | 7 |
| Conjunctival chemosis | 2 | 4 |
| Sub-conjunctival hemorrhage | 1 | 0 |
| Corneal abrasion | 1 | 0 |
| Anterior chamber inflammation | 4 | 5 |
| Eyelid hematoma | 0 | 2 |
| Macular edema | 1 | 0 |
| Variables | Fs | Ss | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 Months | p-Value | Baseline | 6 Months | p-Value | |
| IOP | 25.0 [22.0;30.5] | 20.0 [18.0;24.5] | 0.013 | 29.0 [26.0;34.5] | 19.0 [14.0;21.5] | <0.001 |
| I-MISHA | 0.05 [0.04;0.07] | 0.05 [0.04;0.06] | 0.895 | 0.05 [0.03;0.08] | 0.05 [0.04;0.06] | 0.334 |
| S-MISHA | 0.03 [0.02;0.05] | 0.06 [0.04;0.08] | 0.037 | 0.03 [0.02;0.04] | 0.06 [0.05;0.08] | <0.001 |
| T-MISHA | 0.05 [0.03;0.06] | 0.05 [0.04;0.07] | 0.358 | 0.04 [0.03;0.06] | 0.06 [0.05;0.06] | 0.102 |
| I-SR | 99.3 [89.8;106] | 101 [89.8;103] | 0.609 | 102 [95.2;113] | 93.5 [86.5;98.4] | 0.002 |
| S-SR | 97.7 [92.3;105] | 96.4 [92.9;102] | 0.511 | 106 [99.3;114] * | 98.3 [90.4;107] | 0.052 |
| T-SR | 97.6 [92.5;104] | 98.0 [92.5;101] | 0.422 | 104 [99.8;109] * | 94.1 [90.9;101] | 0.001 |
| Variables | Fs (n = 19) | Ss (n = 23) | p-Value |
|---|---|---|---|
| DIOP | −5.00 [−7.00;−4.00] | −12.00 [−13.00;−10.00] | <0.001 |
| DI-MISHA | 0.00 [−0.02;0.01] | −0.01 [−0.02;0.01] | 0.299 |
| DS-MISHA | 0.02 [−0.01;0.03] | 0.03 [0.02;0.05] | 0.061 |
| DT-MISHA | 0.00 [−0.01;0.02] | 0.01 [0.00;0.02] | 0.719 |
| DI-SR | −0.28 [−14.53;9.37] | −10.43 [−18.06;0.15] | 0.096 |
| DS-SR | −0.83 [−9.41;6.37] | −2.76 [−9.14;−0.05] | 0.598 |
| DT-SR | −3.08 [−10.17;8.13] | −10.75 [−12.89;0.40] | 0.188 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnifili, L.; Palamini, A.; Brescia, L.; Porreca, A.; Oddone, F.; Tanga, L.; Ruggeri, M.L.; Quarta, A.; Mastropasqua, R.; Di Nicola, M.; et al. Uveoscleral Outflow Routes after MicroPulse Laser Therapy for Refractory Glaucoma: An Optical Coherence Tomography Study of the Sclera. Int. J. Mol. Sci. 2024, 25, 5913. https://doi.org/10.3390/ijms25115913
Agnifili L, Palamini A, Brescia L, Porreca A, Oddone F, Tanga L, Ruggeri ML, Quarta A, Mastropasqua R, Di Nicola M, et al. Uveoscleral Outflow Routes after MicroPulse Laser Therapy for Refractory Glaucoma: An Optical Coherence Tomography Study of the Sclera. International Journal of Molecular Sciences. 2024; 25(11):5913. https://doi.org/10.3390/ijms25115913
Chicago/Turabian StyleAgnifili, Luca, Andrea Palamini, Lorenza Brescia, Annamaria Porreca, Francesco Oddone, Lucia Tanga, Maria Ludovica Ruggeri, Alberto Quarta, Rodolfo Mastropasqua, Marta Di Nicola, and et al. 2024. "Uveoscleral Outflow Routes after MicroPulse Laser Therapy for Refractory Glaucoma: An Optical Coherence Tomography Study of the Sclera" International Journal of Molecular Sciences 25, no. 11: 5913. https://doi.org/10.3390/ijms25115913
APA StyleAgnifili, L., Palamini, A., Brescia, L., Porreca, A., Oddone, F., Tanga, L., Ruggeri, M. L., Quarta, A., Mastropasqua, R., Di Nicola, M., & Mastropasqua, L. (2024). Uveoscleral Outflow Routes after MicroPulse Laser Therapy for Refractory Glaucoma: An Optical Coherence Tomography Study of the Sclera. International Journal of Molecular Sciences, 25(11), 5913. https://doi.org/10.3390/ijms25115913











