Oleuropein, a Component of Extra Virgin Olive Oil, Improves Liver Steatosis and Lobular Inflammation by Lipopolysaccharides–TLR4 Axis Downregulation
Abstract
1. Introduction
2. Results
2.1. Oleuropein Reduces HFD-Induced Increased Platelet Activation and Higher Concentrations of Circulating LPS
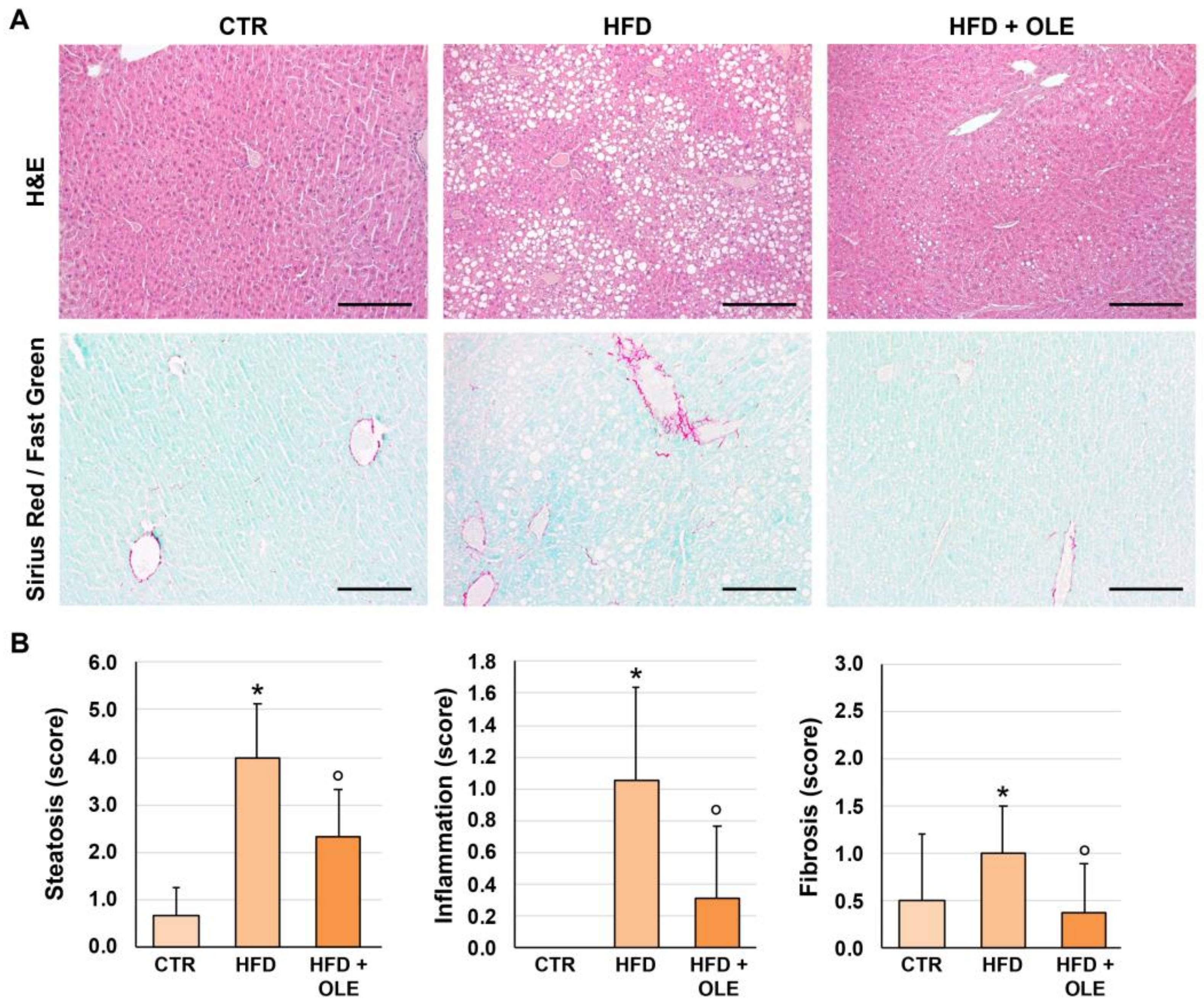
2.2. Oleuropein Reduces HFD-Induced Hepatic Steatosis, Lobular Inflammation, and Fibrosis
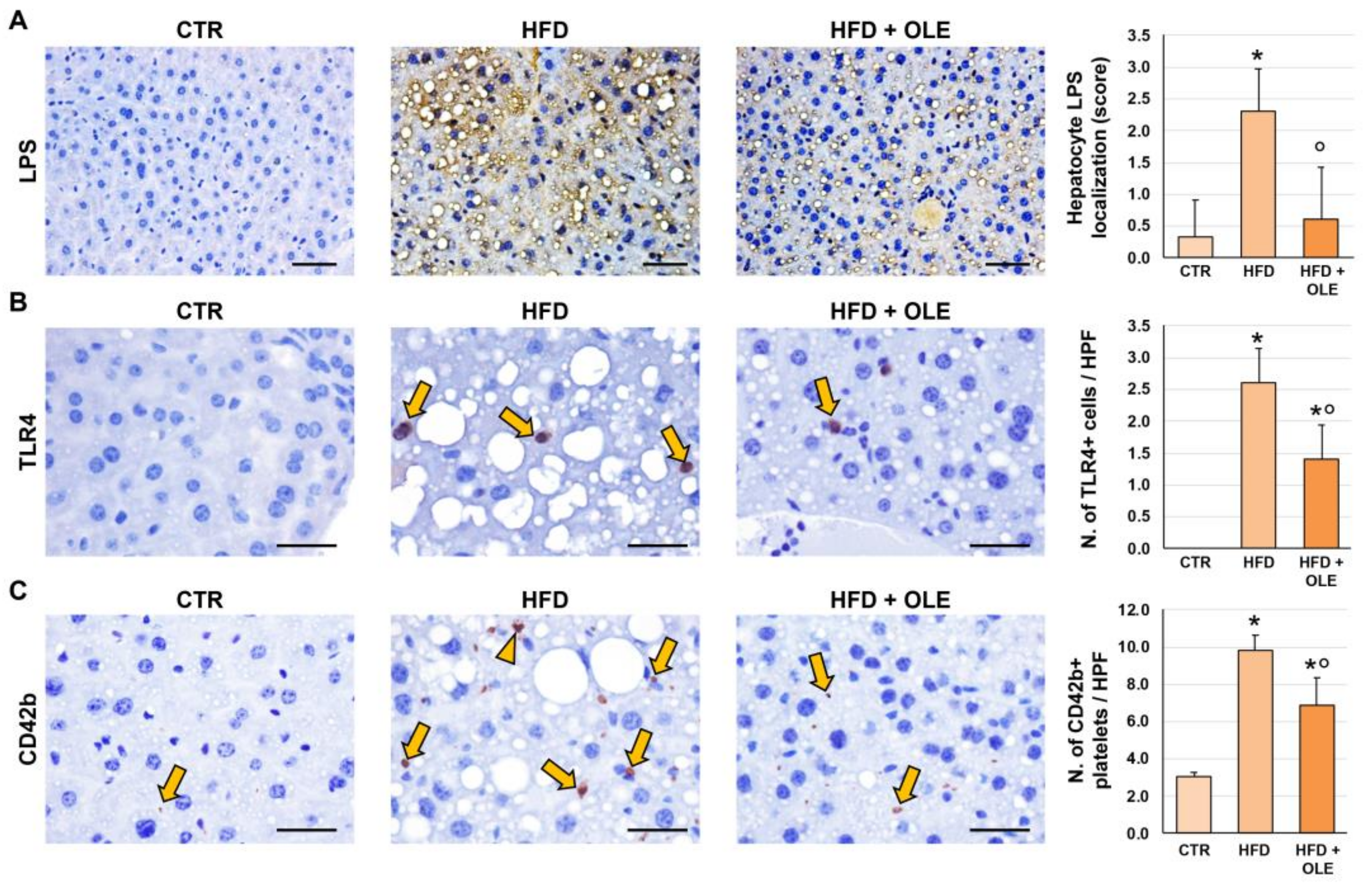
2.3. Oleuropein Reduces HFD-Induced Hepatic LPS Localization and TLR4+ Macrophage Increase
2.4. Oleuropein Reduces HFD-Induced Hepatic CD42b+ Platelet Increase
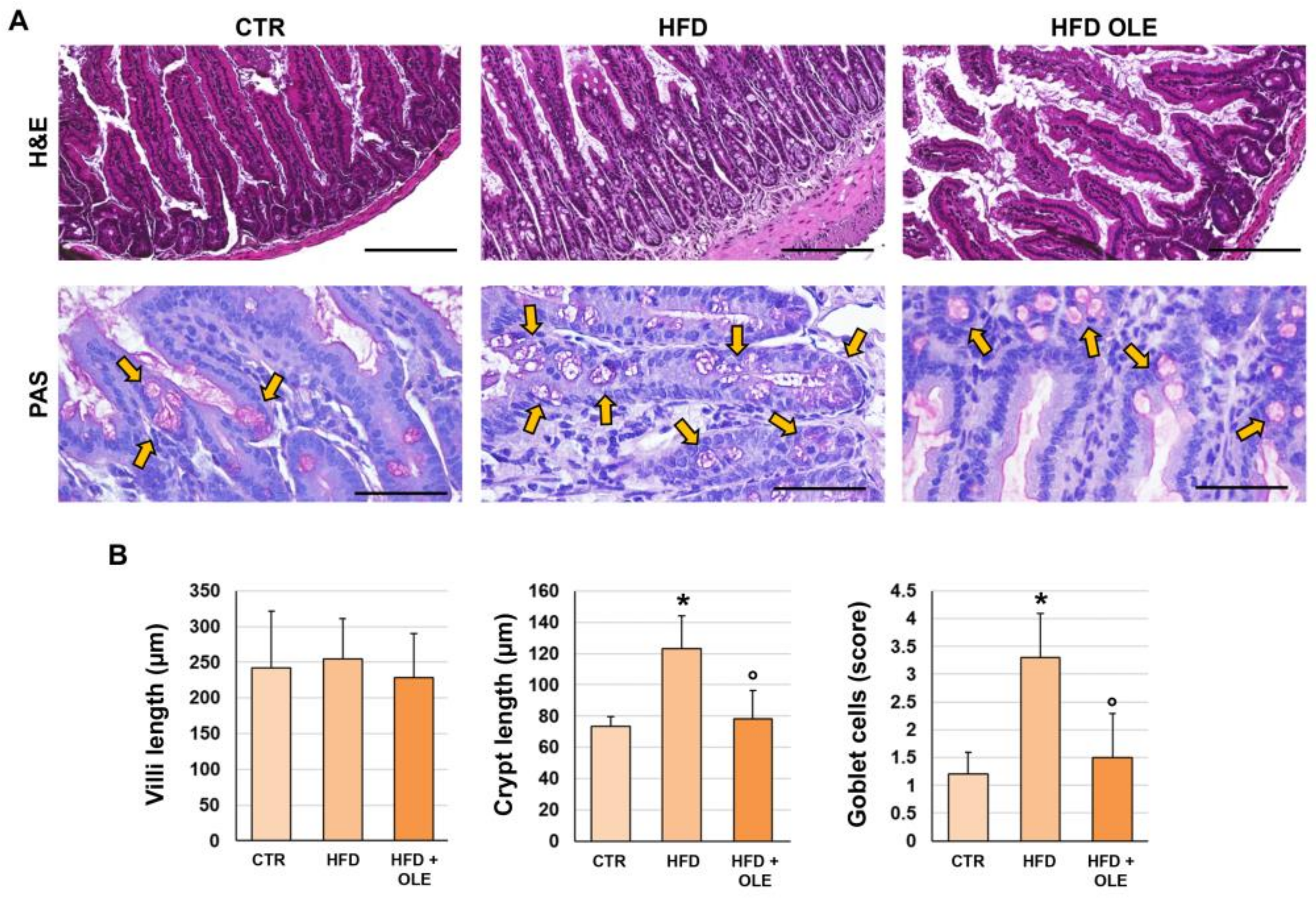
2.5. Oleuropein Reduces HFD-Induced Intestinal Morphological Derangements
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. HDL3 Assay
4.3. Tissue Sampling and Histo-Morphological and Immunohistochemical Stains
4.4. Histopathology and Immunophenotype of Liver and Intestine
4.5. Serum LPS
4.6. Soluble Platelet Selectin (sP-Selectin) Assay
4.7. Zonulin Assay
4.8. Interferon γ (IFN-γ) Assay
4.9. Tumor Necrosis Factor-α (TNF-α) Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, J.M.; Diehl, A.M. Nonalcoholic fatty liver disease: An underrecognized cause of cryptogenic cirrhosis. JAMA J. Am. Med. Assoc. 2003, 289, 3000–3004. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Niess, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Carpino, G.; Del Ben, M.; Pastori, D.; Carnevale, R.; Baratta, F.; Overi, D.; Francis, H.; Cardinale, V.; Onori, P.; Safarikia, S.; et al. Increased Liver Localization of Lipopolysaccharides in Human and Experimental NAFLD. Hepatology 2020, 72, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Baker, S.S.; Gill, C.; Liu, W.; Alkhouri, R.; Baker, R.D.; Gill, S.R. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology 2013, 57, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shanab, A.; Quigley, E.M. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; De Maria, S.; Carteni, M.; Nardone, G. Gut—liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar] [CrossRef]
- Kapil, S.; Duseja, A.; Sharma, B.K.; Singla, B.; Chakraborti, A.; Das, A.; Ray, P.; Dhiman, R.K.; Chawla, Y. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016, 31, 213–221. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Karwoski, C.B. Endotoxin-mediated hepatic lipid accumulation during parenteral nutrition in rats. J. Am. Coll. Nutr. 2002, 21, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.J.; Engstler, A.J.; Ziegenhardt, D.; Bischoff, S.C.; Trautwein, C.; Bergheim, I. Loss of lipopolysaccharide-binding protein attenuates the development of diet-induced non-alcoholic fatty liver disease in mice. J. Gastroenterol. Hepatol. 2017, 32, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhuang, Z.J.; Bian, D.X.; Ma, X.J.; Xun, Y.H.; Yang, W.J.; Luo, Y.; Liu, Y.L.; Jia, L.; Wang, Y.; et al. Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clin. Exp. Pharmacol. Physiol. 2014, 41, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.; Okamoto, S.; Kimura, A.; Uema, T.; Higa, M.; Yonamine, M.; Namba, T.; Ogata, E.; Yamazaki, S.; Shimabukuro, M.; et al. Metabolically and immunologically beneficial impact of extra virgin olive and flaxseed oils on composition of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 2411–2425. [Google Scholar] [CrossRef]
- Bartimoccia, S.; Cammisotto, V.; Nocella, C.; Del Ben, M.; D‘Amico, A.; Castellani, V.; Baratta, F.; Pignatelli, P.; Loffredo, L.; Violi, F.; et al. Extra Virgin Olive Oil Reduces Gut Permeability and Metabolic Endotoxemia in Diabetic Patients. Nutrients 2022, 14, 2153. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Pastori, D.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Novo, M.; Del Ben, M.; Farcomeni, A.; Angelico, F.; Violi, F. Gut-derived lipopolysaccharides increase post-prandial oxidative stress via Nox2 activation in patients with impaired fasting glucose tolerance: Effect of extra-virgin olive oil. Eur. J. Nutr. 2019, 58, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. BioMed Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, Y.; Um, S.J.; Yoon, S.K.; Park, T. Oleuropein attenuates hepatic steatosis induced by high-fat diet in mice. J. Hepatol. 2011, 54, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, Y.; Park, T. Hepatoprotective effect of oleuropein in mice: Mechanisms uncovered by gene expression profiling. Biotechnol. J. 2010, 5, 950–960. [Google Scholar] [CrossRef]
- Domitrović, R.; Jakovac, H.; Marchesi, V.V.; Šain, I.; Romić, Ž.; Rahelić, D. Preventive and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacol. Res. 2012, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; Mahmoudi, A.; Feryeni, A.; Fki, I.; Bouallagui, Z.; Choura, S.; Chamkha, M.; Sayadi, S. Hepatoprotective Effect of Oleuropein-Rich Extract from Olive Leaves against Cadmium-Induced Toxicity in Mice. BioMed Res. Int. 2020, 2020, 4398924. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pignatelli, P.; Castellani, V.; Carnevale, R.; Cammisotto, V. Gut dysbiosis, endotoxemia and clotting activation: A dangerous trio for portal vein thrombosis in cirrhosis. Blood Rev. 2023, 57, 100998. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Kuchay, M.S. Toll-like receptors and metabolic (dysfunction)-associated fatty liver disease. Pharmacol. Res. 2022, 185, 106507. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gao, H.; Han, S.; Yuan, R.; He, J.; Zhuo, Y.; Feng, Y.L.; Tang, M.; Feng, J.; Yang, S. Oleuropein Attenuates Lipopolysaccharide-Induced Acute Kidney Injury In Vitro and In Vivo by Regulating Toll-Like Receptor 4 Dimerization. Front. Pharmacol. 2021, 12, 617314. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, L.; Sun, K.; Li, J.; Han, S.; Gao, X.; Wang, Q.Q.; Yang, S.; Sun, W.; Gao, H. Oleuropein alleviates myocardial ischemia-reperfusion injury by suppressing oxidative stress and excessive autophagy via TLR4/MAPK signaling pathway. Chin. Med. 2024, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Hur, W.; Li, T.Z.; Lee, Y.K.; Choi, J.E.; Hong, S.W.; Lyoo, K.S.; You, C.R.; Jung, E.S.; Jung, C.K.; et al. Oleuropein prevents the progression of steatohepatitis to hepatic fibrosis induced by a high-fat diet in mice. Exp. Mol. Med. 2014, 46, e92. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Kubo, A.; Furuse, M.; Tsukita, S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 2004, 117 Pt 7, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Wood Heickman, L.K.; DeBoer, M.D.; Fasano, A. Zonulin as a potential putative biomarker of risk for shared type 1 diabetes and celiac disease autoimmunity. Diabetes Metab. Res. Rev. 2020, 36, e3309. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Aasbrenn, M.; Lydersen, S.; Farup, P.G. Changes in serum zonulin in individuals with morbid obesity after weight-loss interventions: A prospective cohort study. BMC Endocr. Disord. 2020, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.; Bibin, Y.S.; Prabhu, D.; Shanthirani, C.S.; Gokulakrishnan, K.; Lakshmi, B.S.; Mohan, V.; Balasubramanyam, M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol. Cell Biochem. 2014, 388, 203–210. [Google Scholar] [CrossRef]
- Li, X.; Atkinson, M.A. The role for gut permeability in the pathogenesis of type 1 diabetes--a solid or leaky concept? Pediatr. Diabetes 2015, 16, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Sciarretta, S.; Valenti, V.; di Nonno, F.; Calvieri, C.; Nocella, C.; Frati, G.; Forte, M.; d‘Amati, G.; Pignataro, M.G.; et al. Low-grade endotoxaemia enhances artery thrombus growth via Toll-like receptor 4: Implication for myocardial infarction. Eur. Heart J. 2020, 41, 3156–3165. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, P.; Klag, T.; Malek, N.P.; Heikenwalder, M. Platelets in chronic liver disease, from bench to bedside. JHEP Rep. 2019, 1, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Cammisotto, V.; Bartimoccia, S.; Pignatelli, P.; Carnevale, R.; Nocella, C. Gut-derived low-grade endotoxaemia, atherothrombosis and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, I.; Sutti, S.; Jindal, A.; Vacchiano, M.; Bozzola, C.; Reutelingsperger, C.; Kusters, D.; Bena, S.; Parola, M.; Paternostro, C.; et al. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology 2014, 60, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]


| Antibody | Host Species | Manufacturer | Code | Dilution | Application |
|---|---|---|---|---|---|
| E. coli LPS | Mouse | abcam | ab35654 | 1:50 | IHC |
| CD42b | Rabbit | abcam | ab183345 | 1:100 | IHC |
| TLR4 | Rabbit | Atlas Antibodies | HPA049174 | 1:200 | IHC |
| Occludin | Rabbit | abcam | ab216327 | 1:100 | IF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirone, L.; Overi, D.; Carpino, G.; Carnevale, R.; De Falco, E.; Nocella, C.; D’Amico, A.; Bartimoccia, S.; Cammisotto, V.; Castellani, V.; et al. Oleuropein, a Component of Extra Virgin Olive Oil, Improves Liver Steatosis and Lobular Inflammation by Lipopolysaccharides–TLR4 Axis Downregulation. Int. J. Mol. Sci. 2024, 25, 5580. https://doi.org/10.3390/ijms25115580
Schirone L, Overi D, Carpino G, Carnevale R, De Falco E, Nocella C, D’Amico A, Bartimoccia S, Cammisotto V, Castellani V, et al. Oleuropein, a Component of Extra Virgin Olive Oil, Improves Liver Steatosis and Lobular Inflammation by Lipopolysaccharides–TLR4 Axis Downregulation. International Journal of Molecular Sciences. 2024; 25(11):5580. https://doi.org/10.3390/ijms25115580
Chicago/Turabian StyleSchirone, Leonardo, Diletta Overi, Guido Carpino, Roberto Carnevale, Elena De Falco, Cristina Nocella, Alessandra D’Amico, Simona Bartimoccia, Vittoria Cammisotto, Valentina Castellani, and et al. 2024. "Oleuropein, a Component of Extra Virgin Olive Oil, Improves Liver Steatosis and Lobular Inflammation by Lipopolysaccharides–TLR4 Axis Downregulation" International Journal of Molecular Sciences 25, no. 11: 5580. https://doi.org/10.3390/ijms25115580
APA StyleSchirone, L., Overi, D., Carpino, G., Carnevale, R., De Falco, E., Nocella, C., D’Amico, A., Bartimoccia, S., Cammisotto, V., Castellani, V., Frati, G., Sciarretta, S., Gaudio, E., Pignatelli, P., Alvaro, D., & Violi, F. (2024). Oleuropein, a Component of Extra Virgin Olive Oil, Improves Liver Steatosis and Lobular Inflammation by Lipopolysaccharides–TLR4 Axis Downregulation. International Journal of Molecular Sciences, 25(11), 5580. https://doi.org/10.3390/ijms25115580












