Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model
Abstract
1. Introduction
2. Results
2.1. Hydroxytyrosol Improves Intestinal Barrier Function
2.2. Hydroxytyrosol Attenuates Intestinal Inflammation and Enhances Antioxidant Capacity
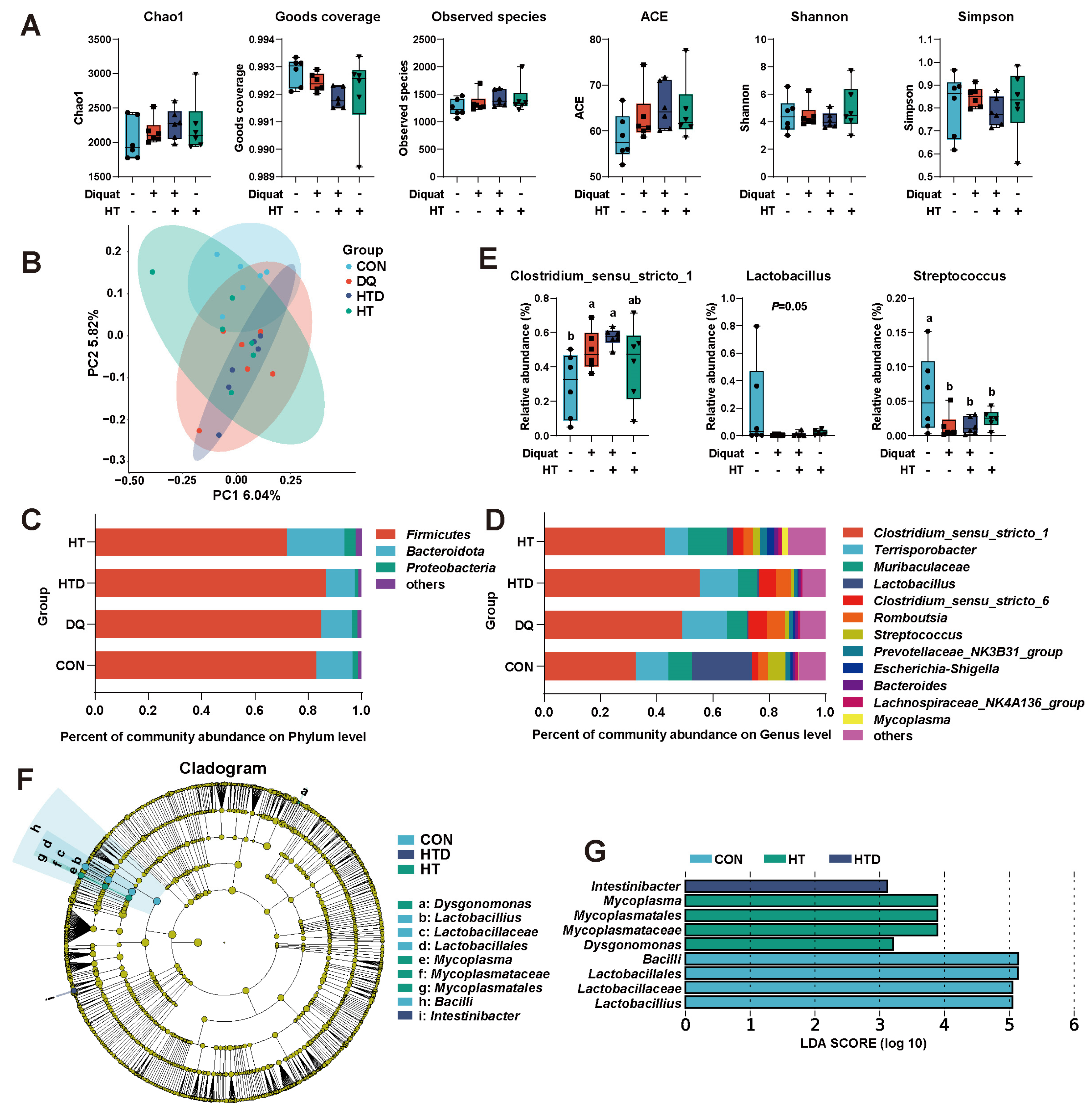
2.3. Hydroxytyrosol Regulates Microbial Composition
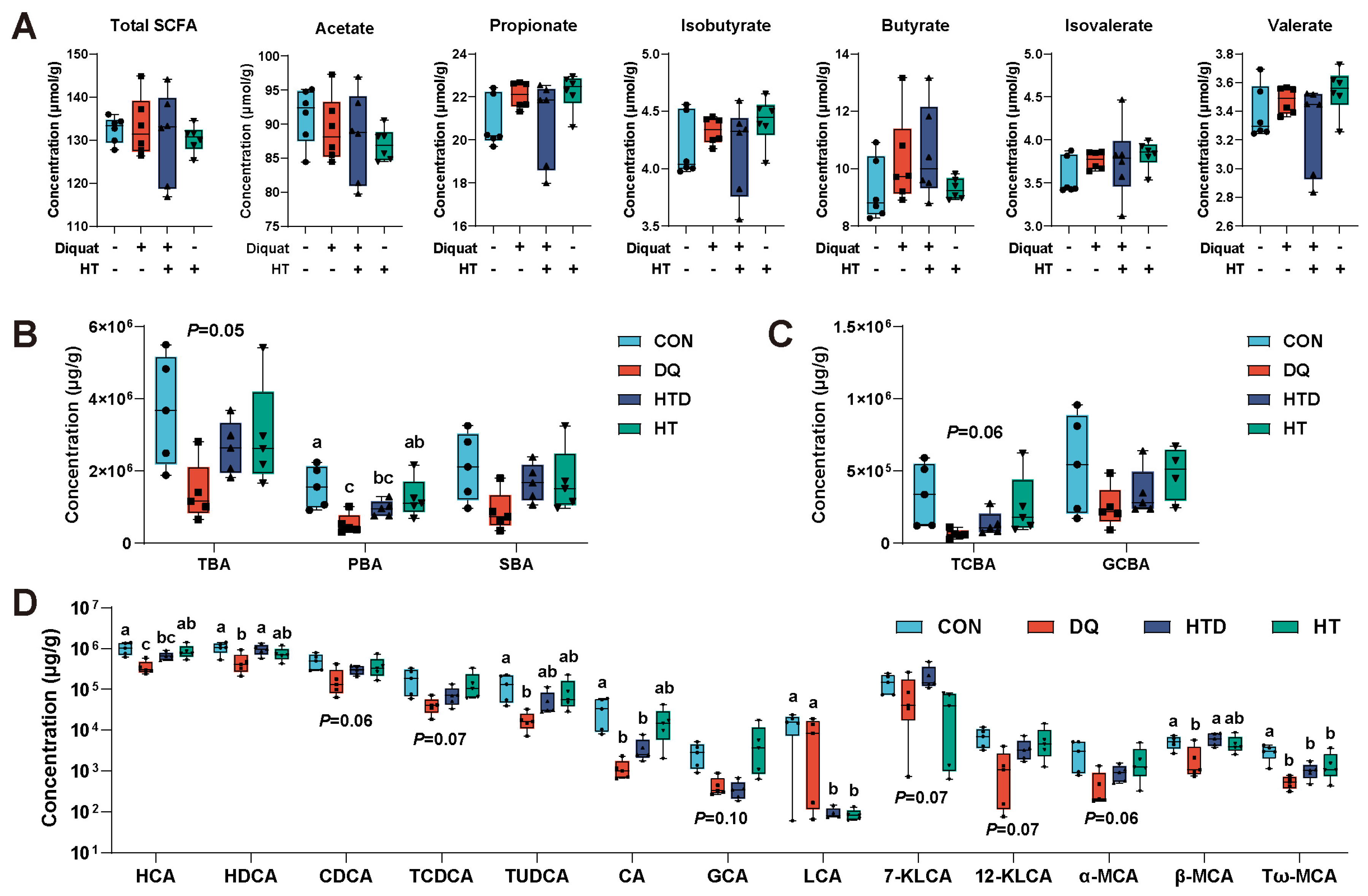
2.4. Hydroxytyrosol Changes BAs Metabolism
2.5. Correlation Analysis between Differential Ileal Microbiota, BAs and Gene Expression
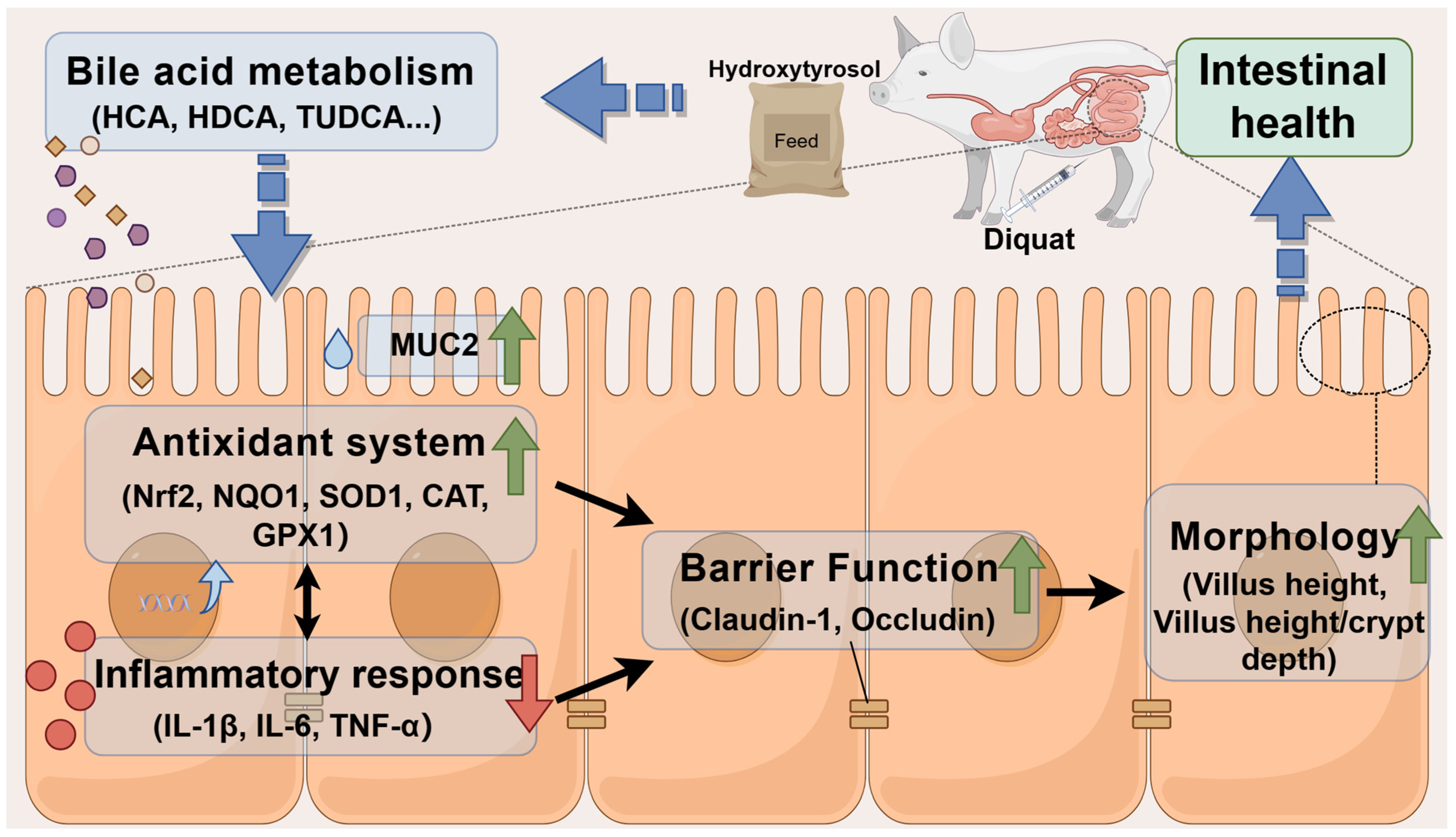
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Sample Collection
4.3. Histological Analysis
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Microbial 16S rRNA Analysis
4.6. Quantification of SCFAs and BAs
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Upadhyay, M.; Milliner, C.; Bell, B.A.; Bonilha, V.L. Oxidative stress in the retina and retinal pigment epithelium (RPE): Role of aging, and DJ-1. Redox Biol. 2020, 37, 101623. [Google Scholar] [CrossRef]
- Attia, M.; Essa, E.A.; Zaki, R.M.; Elkordy, A.A. An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (in Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants 2020, 9, 359. [Google Scholar] [CrossRef]
- Wen, Z.S.; Du, M.; Tang, Z.; Zhou, T.Y.; Zhang, Z.S.; Song, H.H.; Xiang, X.W.; Han, X.Y. Low Molecular Seleno-Aminopolysaccharides Protect the Intestinal Mucosal Barrier of Rats under Weaning Stress. Int. J. Mol. Sci. 2019, 20, 5727. [Google Scholar] [CrossRef]
- Huang, C.; Fan, Z.; Han, D.; Johnston, L.J.; Ma, X.; Wang, F. Pyrroloquinoline quinone regulates the redox status in vitro and in vivo of weaned pigs via the Nrf2/HO-1 pathway. J. Anim. Sci. Biotechnol. 2021, 12, 77. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Chen, Y.; Yu, C.; Azevedo, P.; Gong, J.; Yang, C. Bacillus licheniformis PF9 improves barrier function and alleviates inflammatory responses against enterotoxigenic Escherichia coli F4 infection in the porcine intestinal epithelial cells. J. Anim. Sci. Biotechnol. 2022, 13, 86. [Google Scholar] [CrossRef]
- Song, X.; Pi, S.; Gao, Y.; Zhou, F.; Yan, S.; Chen, Y.; Qiao, L.; Dou, X.; Shao, D.; Xu, C. The Role of Vasoactive Intestinal Peptide and Mast Cells in the Regulatory Effect of Lactobacillus casei ATCC 393 on Intestinal Mucosal Immune Barrier. Front. Immunol. 2021, 12, 723173. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Gao, R.; Wang, J.; Zhu, W. Early-life galacto-oligosaccharides supplementation alleviates the small intestinal oxidative stress and dysfunction of lipopolysaccharide-challenged suckling piglets. J. Anim. Sci. Biotechnol. 2022, 13, 70. [Google Scholar] [CrossRef]
- Lauridsen, C.; Schönherz, A.A.; Højsgaard, S. Effect of Maternal Dietary Redox Levels on Antioxidative Status and Immunity of the Suckling Off-Spring. Antioxidants 2021, 10, 478. [Google Scholar] [CrossRef]
- Wang, Y.; An, M.; Zhang, Z.; Zhang, W.; Kulyar, M.F.; Iqbal, M.; He, Y.; Li, F.; An, T.; Li, H.; et al. Effects of Milk Replacer-Based Lactobacillus on Growth and Gut Development of Yaks’ Calves: A Gut Microbiome and Metabolic Study. Microbiol. Spectr. 2022, 10, e0115522. [Google Scholar] [CrossRef]
- Sambiagio, N.; Sauvain, J.J.; Berthet, A.; Auer, R.; Schoeni, A.; Hopf, N.B. Rapid Liquid Chromatography-Tandem Mass Spectrometry Analysis of Two Urinary Oxidative Stress Biomarkers: 8-oxodG and 8-isoprostane. Antioxidants 2020, 10, 38. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, D.D.; Wu, J.; Liu, J.; Zhou, Y.C.; Tan, Y.Z.; Feng, W.W.; Peng, C. Interactions between gut microbiota and polyphenols: A mechanistic and metabolomic review. Phytomedicine 2023, 119, 154979. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef]
- Yang, J.; Pei, G.; Sun, X.; Xiao, Y.; Miao, C.; Zhou, L.; Wang, B.; Yang, L.; Yu, M.; Zhang, Z.S.; et al. RhoB affects colitis through modulating cell signaling and intestinal microbiome. Microbiome 2022, 10, 149. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, M.; Cani, P.D.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Mansell, T.J. Production and Sensing of Butyrate in a Probiotic Escherichia coli Strain. Int. J. Mol. Sci. 2020, 21, 3615. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Jia, C.; Lin, X.; Hao, H.; Li, S.; Li, F.; Cui, Q.; Chen, Y.; Wu, F.; Xiao, X. The inhibition of enterocyte proliferation by lithocholic acid exacerbates necrotizing enterocolitis through downregulating the Wnt/β-catenin signalling pathway. Cell Prolif. 2022, 55, e13228. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, L.; Zhang, Y.; Zheng, L.; Xu, M.; Rong, R.; Zhu, T. Baicalin ameliorates H2O2 induced cytotoxicity in HK-2 cells through the inhibition of ER stress and the activation of Nrf2 signaling. Int. J. Mol. Sci. 2014, 15, 12507–12522. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yang, W.L.; Xing, M.X.; Zhang, H.; Ai, L.Z. Natural polyphenols-gut microbiota interactions and effects on glycolipid metabolism via polyphenols-gut-brain axis: A state-of-the-art review. Trends Food Sci. Technol. 2023, 140, 104171. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef]
- Martin, M.A.; Ramos, S.; Granado-Serrano, A.B.; Rodriguez-Ramiro, I.; Trujillo, M.; Bravo, L.; Goya, L. Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells. Mol. Nutr. Food Res. 2010, 54, 956–966. [Google Scholar] [CrossRef]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of GSH synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Z.; Feng, Z.; Hao, J.; Shen, W.; Li, X.; Sun, L.; Sharman, E.; Wang, Y.; Wertz, K.; et al. Hydroxytyrosol protects against oxidative damage by simultaneous activation of mitochondrial biogenesis and phase II detoxifying enzyme systems in retinal pigment epithelial cells. J. Nutr. Biochem. 2010, 21, 1089–1098. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Li, L.; Espe, M.; Lu, K.L.; Rahimnejad, S. Hydroxytyrosol attenuates hepatic fat accumulation via activating mitochondrial biogenesis and autophagy through the AMPK pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhong, R.Q.; Zhang, S.F.; Wang, M.Y.; Wen, X.B.; Yi, B.; Zhao, Y.; Chen, L.; Zhang, H.F. Hydroxytyrosol attenuates diquat-induced oxidative stress by activating Nrf2 pathway and modulating colonic microbiota in mice. J. Nutr. Biochem. 2023, 113, 109256. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhang, S.F.; Zhong, R.Q.; Wan, F.; Chen, L.; Liu, L.; Yi, B.; Zhang, H.F. Olive fruit extracts supplement improve antioxidant capacity via altering colonic microbiota composition in mice. Front. Nutr. 2021, 8, 645099. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; King, J.A.; Meyer, S.A.; Abdelwahed, K.; Busnena, B.; El Sayed, K. Safety Evaluations of Single Dose of the Olive Secoiridoid S-(-)-Oleocanthal in Swiss Albino Mice. Nutrients 2020, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Biolato, M.; Manca, F.; Marrone, G.; Cefalo, C.; Racco, S.; Miggiano, G.A.; Valenza, V.; Gasbarrini, A.; Miele, L.; Grieco, A. Intestinal permeability after Mediterranean diet and low-fat diet in non-alcoholic fatty liver disease. World J. Gastroenterol. 2019, 25, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Heinritz, S.N.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Xu, S.; Chen, L.; Deng, Y.; Feng, J. Periplaneta americana Extract Pretreatment Alleviates Oxidative Stress and Inflammation and Increases the Abundance of Gut Akkermansia muciniphila in Diquat-Induced Mice. Antioxidants 2022, 11, 1806. [Google Scholar] [CrossRef]
- Wen, Z.S.; Tang, Z.; Ma, L.; Zhu, T.L.; Wang, Y.M.; Xiang, X.W.; Zheng, B. Protective Effect of Low Molecular Weight Seleno-Aminopolysaccharide on the Intestinal Mucosal Oxidative Damage. Mar. Drugs 2019, 17, 64. [Google Scholar] [CrossRef]
- Cao, S.; Wang, C.; Yan, J.; Li, X.; Wen, J.; Hu, C. Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting Parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 2020, 147, 8–22. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, Y.; Chen, D.; Yu, B.; He, J. Chlorogenic Acid Attenuates Oxidative Stress-Induced Intestinal Epithelium Injury by Co-Regulating the PI3K/Akt and IκBα/NF-κB Signaling. Antioxidants 2021, 10, 1915. [Google Scholar] [CrossRef]
- Wu, T.; Li, K.; Yi, D.; Wang, L.; Zhao, D.; Lv, Y.; Zhang, L.; Chen, H.; Ding, B.; Hou, Y.; et al. Dietary Supplementation with Trihexanoin Enhances Intestinal Function of Weaned Piglets. Int. J. Mol. Sci. 2018, 19, 3277. [Google Scholar] [CrossRef] [PubMed]
- Olivier, S.; Pochard, C.; Diounou, H.; Castillo, V.; Divoux, J.; Alcantara, J.; Leclerc, J.; Guilmeau, S.; Huet, C.; Charifi, W.; et al. Deletion of intestinal epithelial AMP-activated protein kinase alters distal colon permeability but not glucose homeostasis. Mol. Metab. 2021, 47, 101183. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, C.J.; Abdullah; Tian, W.N.; Qiu, Z.Y.; Song, M.Y.; Cao, Y.; Xiao, J. Hydroxytyrosol alleviates dextran sulfate sodium-induced colitis by modulating inflammatory responses, intestinal barrier, and microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.C.; Lee, Y.J.; Wang, Y.T.; Cheng, S.Y.; Cheng, H.L. Cytotoxic and Anti-Inflammatory Triterpenoids in the Vines and Leaves of Momordica charantia. Int. J. Mol. Sci. 2022, 23, 1071. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, X.; Zheng, D.; Li, C.; Chen, Y.; Kong, K.; Xu, W.; Shi, B.; Chen, X.; Dai, F.; et al. Ultrasmall PtAu(2) nanoclusters activate endogenous anti-inflammatory and anti-oxidative systems to prevent inflammatory osteolysis. Theranostics 2023, 13, 1010–1027. [Google Scholar] [CrossRef] [PubMed]
- Arundina, I.; Diyatri, I.; Surboyo, M.D.C.; Monica, E.; Afanda, N.M. Growth factor stimulation for the healing of traumatic ulcers with liquid rice hull smoke. J. Taibah Univ. Med. Sci. 2021, 16, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Pompili, S.; Tempia Valenta, S.; Salvi, V.; Volpe, U. C-Reactive Protein as a Biomarker for Major Depressive Disorder? Int. J. Mol. Sci. 2022, 23, 1616. [Google Scholar] [CrossRef] [PubMed]
- Kamai, T.; Murakami, S.; Arai, K.; Ishida, K.; Kijima, T. Association of Nrf2 expression and mutation with Weiss and Helsinki scores in adrenocortical carcinoma. Cancer Sci. 2022, 113, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, H.; Zhao, Y.; Wang, R.; Li, G.; Zhang, G.; Zhang, Y. Effects of Dietary Lysophospholipid Inclusion on the Growth Performance, Nutrient Digestibility, Nitrogen Utilization, and Blood Metabolites of Finishing Beef Cattle. Antioxidants 2022, 11, 1486. [Google Scholar] [CrossRef]
- de S. Ribeiro, B.C.; de C. Faria, R.V.; de S. Nogueira, J.; Santos Valença, S.; Chen, L.; Romana-Souza, B. Olive oil promotes the survival and migration of dermal fibroblasts through Nrf2 pathway activation. Lipids 2023, 58, 59–68. [Google Scholar] [CrossRef]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cuzzocrea, S.; Di Paola, R.; et al. Hidrox(®) Roles in Neuroprotection: Biochemical Links between Traumatic Brain Injury and Alzheimer’s Disease. Antioxidants 2021, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.; Kim, D.H.; Chon, J.W.; Song, K.Y.; Seo, K.H. Synergistic effects of the early administration of Lactobacillus kefiranofaciens DN1 and Kluyveromyces marxianus KU140723-05 on the inhibition of Salmonella Enteritidis colonization in young chickens. Poult. Sci. 2020, 99, 5999–6006. [Google Scholar] [CrossRef]
- Adair, K.L.; Bost, A.; Bueno, E.; Kaunisto, S.; Kortet, R.; Peters-Schulze, G.; Martinson, V.G.; Douglas, A.E. Host determinants of among-species variation in microbiome composition in drosophilid flies. ISME J. 2020, 14, 217–229. [Google Scholar] [CrossRef]
- Chen, C.; Niu, M.; Pan, J.; Du, N.; Liu, S.; Li, H.; He, Q.; Mao, J.; Duan, Y.; Du, Y. Bacteroides, butyric acid and t10,c12-CLA changes in colorectal adenomatous polyp patients. Gut Pathog. 2021, 13, 1. [Google Scholar] [CrossRef]
- Wen, X.; Wan, F.; Wu, Y.; Liu, L.; Liu, Y.; Zhong, R.; Chen, L.; Zhang, H. Caffeic acid supplementation ameliorates intestinal injury by modulating intestinal microbiota in LPS-challenged piglets. Food Funct. 2023, 14, 7705–7717. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, W.; Yu, J.; Liu, Y.; Ma, H.; Ji, C.; Zhang, C.; Xue, J.; Li, R.; Cui, H. Astaxanthin From Haematococcus pluvialis Prevents High-Fat Diet-Induced Hepatic Steatosis and Oxidative Stress in Mice by Gut-Liver Axis Modulating Properties. Front. Nutr. 2022, 9, 840648. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Li, Y.; Jiang, Y.; Hu, Y.; Liu, T.; Tian, X.; Zhao, X.; Zhu, Y.; Wang, S.; et al. Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat. Commun. 2020, 11, 3218. [Google Scholar] [CrossRef]
- Zhong, J.; He, X.; Gao, X.; Liu, Q.; Zhao, Y.; Hong, Y.; Zhu, W.; Yan, J.; Li, Y.; Li, Y.; et al. Hyodeoxycholic acid ameliorates nonalcoholic fatty liver disease by inhibiting RAN-mediated PPARα nucleus-cytoplasm shuttling. Nat. Commun. 2023, 14, 5451. [Google Scholar] [CrossRef]
- Li, C.X.; Wang, X.Q.; Cheng, F.F.; Yan, X.; Luo, J.; Wang, Q.G. Hyodeoxycholic acid protects the neurovascular unit against oxygen-glucose deprivation and reoxygenation-induced injury in vitro. Neural Regen. Res. 2019, 14, 1941–1949. [Google Scholar]
- Li, J.; Chen, Y.; Li, R.; Zhang, X.; Chen, T.; Mei, F.; Liu, R.; Chen, M.; Ge, Y.; Hu, H.; et al. Gut microbial metabolite hyodeoxycholic acid targets the TLR4/MD2 complex to attenuate inflammation and protect against sepsis. Mol. Ther. 2023, 31, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Paumgartner, G.; Beuers, U. Ursodeoxycholic acid in cholestatic liver disease: Mechanisms of action and therapeutic use revisited. Hepatology 2002, 36, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M. Tauroursodeoxycholate-Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef] [PubMed]
- Zangerolamo, L.; Vettorazzi, J.F.; Rosa, L.R.O.; Carneiro, E.M.; Barbosa, H.C.L. The bile acid TUDCA and neurodegenerative disorders: An overview. Life Sci. 2021, 272, 119252. [Google Scholar] [CrossRef] [PubMed]
- Ferrebee, C.B.; Li, J.; Haywood, J.; Pachura, K.; Robinson, B.S.; Hinrichs, B.H.; Jones, R.M.; Rao, A.; Dawson, P.A. Organic Solute Transporter α-β Protects Ileal Enterocytes From Bile Acid-Induced Injury. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Santos-López, J.A.; Garcimartín, A.; Bastida, S.; Bautista-Ávila, M.; González-Muñoz, M.J.; Benedí, J.; Sánchez-Muniz, F.J. Lipoprotein Profile in Aged Rats Fed Chia Oil- or Hydroxytyrosol-Enriched Pork in High Cholesterol/High Saturated Fat Diets. Nutrients 2018, 10, 1830. [Google Scholar] [CrossRef]
- Hu, R.; He, Z.; Liu, M.; Tan, J.; Zhang, H.; Hou, D.X.; He, J.; Wu, S. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J. Anim. Sci. Biotechnol. 2020, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, B.; Chen, D.W. Duration and indicators of oxidation stress induced by diquat in growing pigs. Sci. Agric. Sin. 2008, 41, 4359–4364. (In Chinese) [Google Scholar]
- Tang, S.; Zhang, S.; Zhong, R.; Su, D.; Xia, B.; Liu, L.; Chen, L.; Zhang, H. Time-course alterations of gut microbiota and short-chain fatty acids after short-term lincomycin exposure in young swine. Appl. Microbiol. Biotechnol. 2021, 105, 8441–8456. [Google Scholar] [CrossRef]
- Tang, S.; Chen, Y.; Deng, F.; Yan, X.; Zhong, R.; Meng, Q.; Liu, L.; Zhao, Y.; Zhang, S.; Chen, L.; et al. Xylooligosaccharide-mediated gut microbiota enhances gut barrier and modulates gut immunity associated with alterations of biological processes in a pig model. Carbohydr. Polym. 2022, 294, 119776. [Google Scholar] [CrossRef]


| Genes | Accession No. | Primer Sequences |
|---|---|---|
| ZO-1 | XM_003480423.4 | F: GGGGTAGGGGTCCTTCCTAT R: CATTTGCTTGGCAGTCAGGTT |
| Occludin | NM_001163647.2 | F: CAGGTGCACCCTCCAGATTG R: TATGTCGTTGCTGGGTGCAT |
| Claudin-1 | NM_001243483.1 | F: TTTCCTCAATACAGGAGGGAAGC R: CCCTCTCCCCACATTCGAG |
| MUC2 | XM_021082584.1 | F: CGCATGGATGGCTGTTTCTG R: ATTGCTCGCAGTTGTTGGTG |
| IL-1β | NM_214055.1 | F: CCAGCCAGTCTTCATTGTT R: CATCTCTTTGGGGCCAT |
| IL-6 | NM_001252429.1 | F: TCCAATCTGGGTTCAATCA R: TCTTTCCCTTTTGCCTCA |
| IL-10 | NM_214041.1 | F: CCGACTCAACGAAGAAGG R: CTGGTTGGGAAGTGGATG |
| TNF-α | NM_214022.1 | F: CCGACAGATGGGCTGTA R: TCTTGATGGCAGAGAGGAG |
| Nrf2 | XM_013984303.2 | F: TAAGGGTGCTCCTTTGCGAG R: ATCAAATCCATGTCCTTGGCG |
| Keap1 | XM_021076667.1 | F: CGCCTCATCGAGTTCGCTTACAC R: GCACGGACCACACTGTCAATCTG |
| HO-1 | NM_001004027.1 | F: GTTTGAGGAGGTGCAGGAG R: GGTTGTCACGGGAGTGG |
| NQO1 | NM_001159613.1 | F: CAGCATTGGGCACACTC R: GGCGCAAAGTACAGTGG |
| SOD1 | NM_001190422.1 | F: GCCAAAGGATCAAGAGAGG R: GAGAGGGCGATCACAGAA |
| CAT | NM_214301.2 | F: GCTGAGTCCGAAGTCGTCTA R: GTCAGGATATCAGGTTTCTGCG |
| GPX1 | NM_214201.1 | F: AACGGTGCGGGACTACA R: TCGGACGTACTTGAGGCA |
| GPX2 | NM_001115136.1 | F: CAACACATTCCGAGGCA R: GAAGCCAAGAACCACCAG |
| β-actin | XM_001928093.1 | F: GCGTAGCATTTGCTGCATGA R: GCGTGTGTGTAACTAGGGGT |
| GAPDH | NM_001206359.1 | F: CGTGTCGGTTGTGGATCTGA R: TGACGAAGTGGTCGTTGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Wan, F.; Zhong, R.; Chen, L.; Zhang, H. Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model. Int. J. Mol. Sci. 2024, 25, 5590. https://doi.org/10.3390/ijms25115590
Wen X, Wan F, Zhong R, Chen L, Zhang H. Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model. International Journal of Molecular Sciences. 2024; 25(11):5590. https://doi.org/10.3390/ijms25115590
Chicago/Turabian StyleWen, Xiaobin, Fan Wan, Ruqing Zhong, Liang Chen, and Hongfu Zhang. 2024. "Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model" International Journal of Molecular Sciences 25, no. 11: 5590. https://doi.org/10.3390/ijms25115590
APA StyleWen, X., Wan, F., Zhong, R., Chen, L., & Zhang, H. (2024). Hydroxytyrosol Alleviates Intestinal Oxidative Stress by Regulating Bile Acid Metabolism in a Piglet Model. International Journal of Molecular Sciences, 25(11), 5590. https://doi.org/10.3390/ijms25115590









