The Basic Requirement of Tight Junction Proteins in Blood-Brain Barrier Function and Their Role in Pathologies
Abstract
:1. Introduction
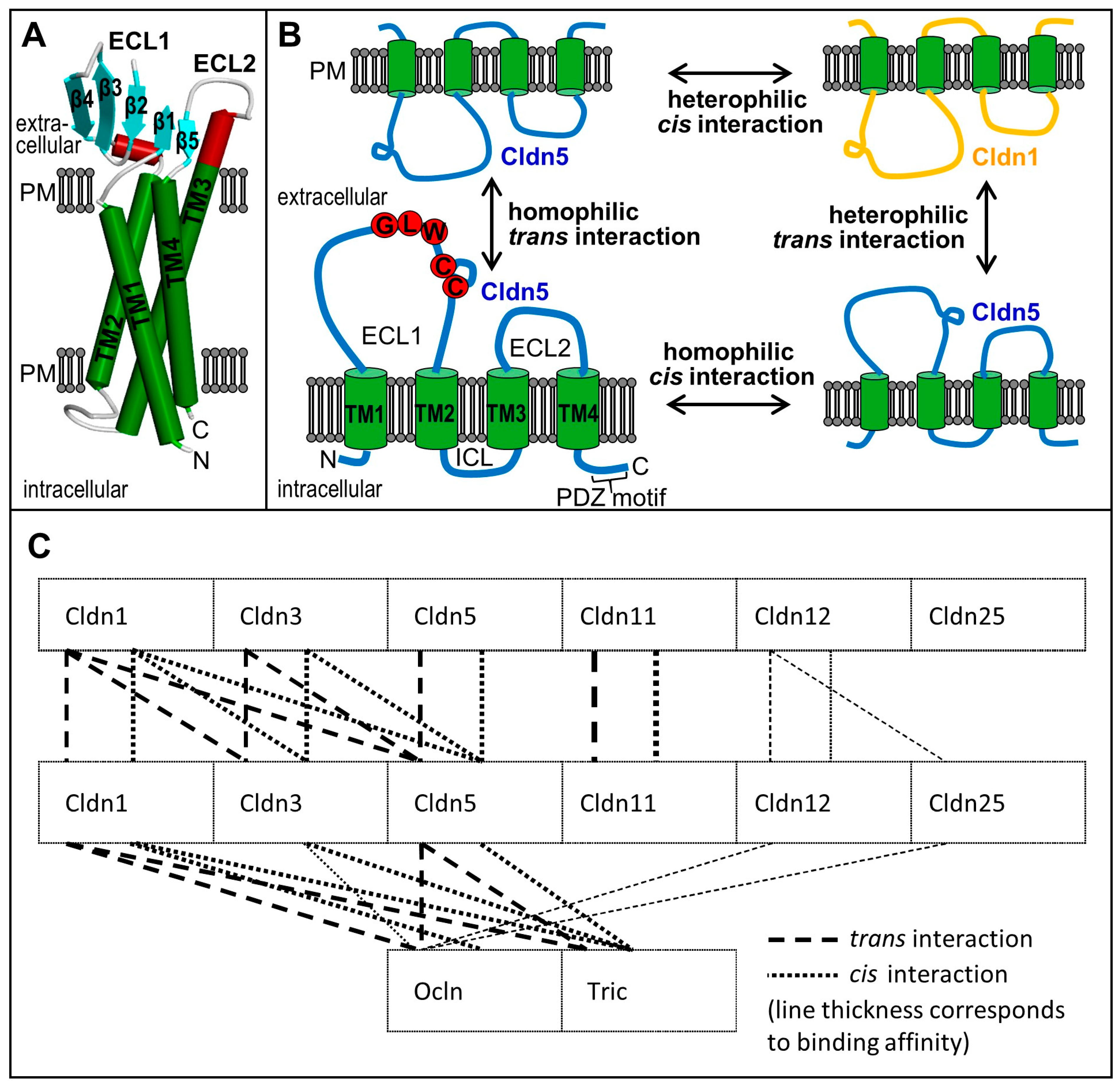
2. Tight Junctions: Proteins, Functions, and Structures
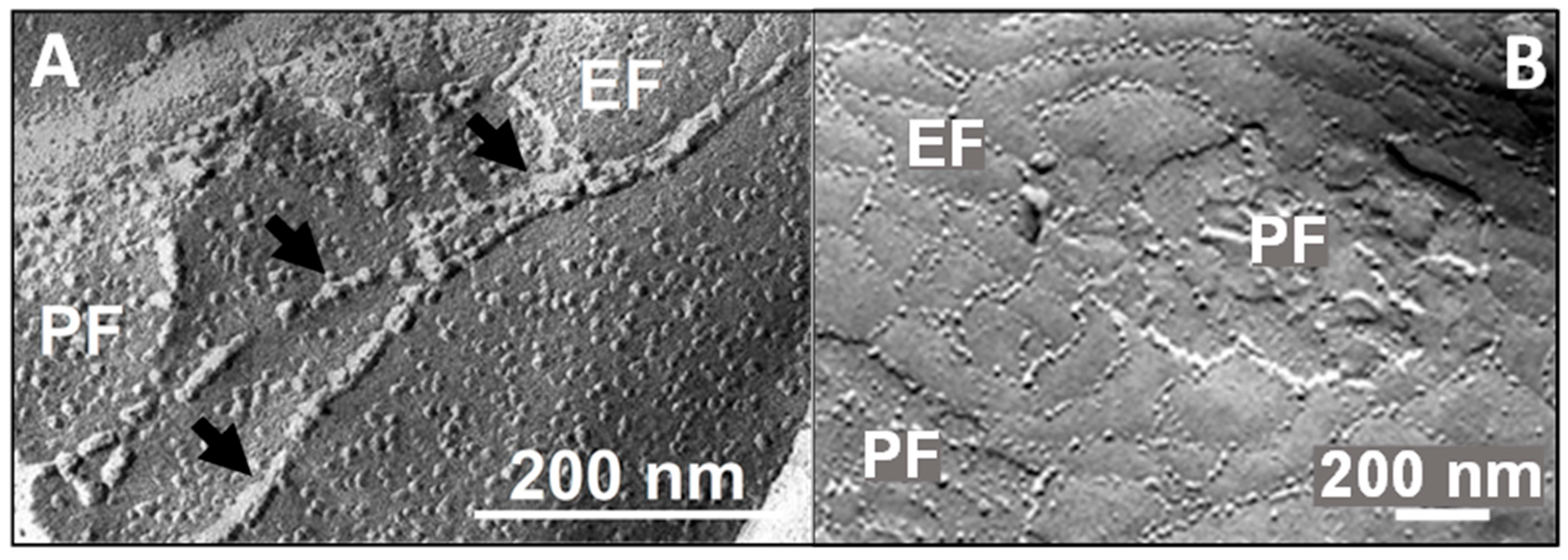
| Class | Paracellular Sealing | Function Paracellular Sealing/Channel-Forming | Other | Not Clear |
|---|---|---|---|---|
| classic * | Cldn1 [56] | Cldn2 [57]/(Na+, K+) [58] | ||
| Cldn3 [59] | ||||
| Cldn4 [60] | Cldn4 [61]/Na+ [62] | |||
| Cldn5 [63] | Cldn7 [64]/Na+ [65] | |||
| Cldn6 [66] | Cldn10 sealing/-10a an-, -10b cat+ [67] | Cldn6 [68] | ||
| Cldn8 [69] | Cldn15 sealing/Na+, K+ [62] | |||
| Cldn9 [66] | Cldn17 sealing/an- [70] | Cldn9 [68] | ||
| Cldn14 [71] | ||||
| Cldn19 [72] | ||||
| non-classic | Cldn11 [73] | Cldn16 sealing/cat++ [72] | Cldn13 [68] | Cldn12, -13, -20 [50] |
| Cldn18 [74] | Cldn21 sealing/Na+, K+, solutes ≤ 0.56 nm [75] | Cldn22, -23, -24 [50] | ||
| Cldn25 indirectly [76] via structure of TJ [50] | Cldn25, -26, -27 [50] |
| Expression | Function | Structure/Interactions | Regulation/Signalling |
|---|---|---|---|
| Claudin-1 (Senescence-associated epithelial membrane protein) | |||
| - gene CLDN1, chromosome 3 (human), -16 (mouse) - protein: human [77,78], mouse [78,79] - cell membranal at TJs [80] and cytoso- lic [50] localisation [53] - KO mouse: postnatal dehydration, lethal [81] | - causes tightness (TER) [56,82], sealing [56,82,83] - receptor for hepatitis C- virus [84] | - 211 aa; M.W., 22.7 kDa; pI, 8.41; N-/C-terminal tail, 7/27 aa; ECL1/ECL2, 53/27 aa (human) - homophilic cis/trans interactions [85,86], dissociation constant ECL1 to Cldn1 47 ± 0.6 nM [86] - heterophilic cis Cldn3, -5 [87], Ocln, Tric, MD3 [55], PDZ1 of ZO-1 [88]; trans Cldn3,-5 [87], Ocln, Tric, [55] - continuous P-face TJ-strands [55] - low membrane mobility [55] | - GPR30 via ERK and/or Akt-domain [89] - dehydroepiandrosteron/Gnα11 [90] - hypoxia inducible factor-complex [91] - cAMP/PKA, down-regulation and cytosolic localisation [92] - down-regulated by hypoxia, focal cerebral ischemia [50], glioblastoma [93] - down-regulated by TGFβ [93], Cu [94], miR212/132 [95] - differentiated regulation upon virus infection [96,97,98,99,100] |
| Claudin-3 (Clostridium perfringens enterotoxin receptor 2) | |||
| - gene CLDN3, chromosome 7 (human), 5 (mouse) - protein: human [77,78], mouse [78,79], rat [101] - KO mouse: amount of Cldn5 and Ocln, paracellular permeability reduced [53]; no changes found by other authors [102] | - enhances BBB integrity in vivo [78], increases complex-ity of TJ-strand network [53] - controls paracellular tightness [59,103,104] (particularly small molecules/ions) - limits endocytosis; pro-motes infarction/oedema [53] - supports embryogenesis/ postnatal development, stabilises BBB/TJ [105] | - 220 aa; M.W., 23.3 kDa; pI, 8.37; N/C-terminal tail, 8/40 aa; ECL1/ECL2, 51/23 aa (human) - homophilic interaction cis/trans [87] - heterophilic cis Cldn1, -5 [87], Tric, MD3 [55], associates ZO-1-PDZ1 [40]; trans Cldn1, -5 [87], Tric, MD3 [55] - continuous P-face strands [87,106] - high membrane mobility >Cldn5 [87] - strengthens TJ strand network/-branching [53] | - Wnt/β-catenin controlled barrier development [105] - expression modulated by Na/K-ATPase [107] - down-regulated by hypoxia/middle cerebral artery occlusion [50] - down (haemorrhage) (PI3K, sphingosine 1-phosphate receptor 1) [104] - loss in EAE, glioblastoma [78] - down-regulated at low Cu [94] |
| Claudin-5 (Transmembrane protein deleted in velocardiofacial syndrome) | |||
| - gene CLDN5, chromosome 22 (human), 16 (mouse) - very high expression [50,78,108], embryonically starting with cerebral angiogenesis [109] - KO mouse: abnormal TJs, brain capillaries permeable for molecules < 800 Da, lethal 10 h postnatally [63] - KD: BBB breakdown in tissue culture, human BEC [110] - t1/2 70 min [111] - protein amount: Cldn5 > −25, Ocln, Cldn1 > −11, −12 (isolated brain capillaries, TX-100 extract) [50] | - causes paracellular tightness for molecules < 800 Da [63] - induces/maintains TJ tightness [21,112] mediated via ECL1 [113] and ECL2 [54] | - 218 aa; M.W., 23.1 Da; pI, 8.25; N-/C-terminal tail, 7/38 aa; ECL1/ECL2, 53/16 aa (human) - homophilic cis/trans interaction [54,87] - heterophilic cis Cldn1, -3 [87], Tric, MD3; trans Cldn1, -3, Ocln, Tric [55] - discontinuous E-face-associated TJ-strands (in TJ-free cells) [21,54] - low membrane mobility [87] - mixed E-/P-face strands by Cldn3 [87] - associates ZO-1-PDZ1 [40] - no effect on ZO-1 clustering [114] - C54S, C64S (mouse ECL1, aa exchange) weaken barrier [113] - ECL1-G60R, human channelopathy: Cl-/small molecule flux [115] - transferred from BEC to leukocytes in EAE, possibly supporting transmigration into CNS [116] | - Thr(207)-phosphorylation opens porcine BBB, protein kinase A [117] - TGF-β/activin signalling increases Cldn5 [118] - VE-cadherin via Akt-activation: phosphorylation of FoxO1 induces Cldn5 [119] - adrenomedullin: enhanced expression and TER, decreases permeability [112] - increase (mRNA, protein, promoter): gluco-corticoids TER up [120,121], estrogen [122] - ROCK via EphA2: down-regulation [123] - ROCK up in dementia: Cldn5 down [124] - C/EBP-α (stimulated by JAM-A) up-regulation, reduced permeability [125] - serum Cldn5 up: autistic children [126], severe stroke [127] -down-regulated by EphA4/Tie2/Akap12 signalling mediating microvascular dysfunction and trauma [128] - down-regulated at low Cu [94] - oxidative stress inhibitor improves Cldn5, ZO-1, TER via Nrf2/HO-1 [129] |
| Claudin-11 (Oligodendrocyte-specific protein) | |||
| - gene CLDN11, chromosome 3 (human), 3 (mouse) - mRNA/protein: very high expression in BEC (human, mouse, rat) in vivo equal to Cldn5, in vitro strongly down-regulated [50,130] - less expressed in human brain oligodendrocytes [50] - KO mouse: mild neurological deficits [131], deafness (low endocochlear potential) [47] - KD: enhanced dextran permeability through BEC layers [130] | - contributes to tightness of BEC layers [50,130] and BBB [132] | - 207 aa; M.W., 22.0 Da; pI, 8.22; N-/C-terminal tail, 1/29 aa; ECL1/ECL2, 50/14 aa (human) - very strong homophilic cis/trans interaction (Cldn11 >> other Cldns, Ocln, Tric [55,133]) - no heterophilic binding [55]; Cldn5 colocalisation in junctions [50,130] - continuous P-face oriented TJ-strands, modulated by Ocln [50] - very low membrane mobility <other Cldns [50], Ocln, Tric, MD3 [55] | - reduced in multiple sclerosis [130] - decreased in EAE by activated annexin A2 signalling (brain capillaries) [134] - decrease in BEC by podocalyxin KD [135] - increased in blood of human autism spectrum disorder [126] - ischemia reduces Cldn11; KO of leucine-rich alpha-2 glycoprotein 1 improves Cldn11 and BBB in ischemia [132] |
| Claudin-12 | |||
| - gene CLDN12, chromosome 7 (human), 5 (mouse) - in BEC [63,94,136]; mRNA in vivo > in vitro [50] - expressed at TJs [50,63] - lack of Cldn12: intact BBB; neurological/behavioral changes [137] - knock-in mouse: mRNA in BEC, pericytes, oligodendrocytes, smooth muscle cells, astrocytes [137] | - not crucial in establishing or maintaining BBB TJ integrity [137] | - 244 aa; M.W., 27,1 kDa; pI, 8.80; N-/C-terminal tail, 10/49 aa; ECL1/ECL2, 56/18 aa (human) - homophilic: no cis- [87], but weak trans interaction [55] - heterophilic: weak trans interactions with Cldn22, -24, -25, Ocln [50] - no C-terminal PDZ-binding motif [87] - no strand formation [87], very high paracellu-lar flux in TJ-free cells [138] | - ouabain-activated Na/K-ATPase reduces expression [107] - high-energy diet decreases mRNA-, increases hippocampal permeability [139] - hyperammonia reduces mRNA in vitro [140] - down-regulated in hypoxia/ischemia [50,53] and in diet-induced diabetes (in latter case attenuated by carbonic anhydrase inhibitor [141]) - regulated by Cu exposure [94] |
| Claudin-25 (Claudin domain-containing protein 1) | |||
| - gene CLDND1, chromosom 3 (human), 16 (mouse), - very high mRNA expression in vivo in BEC [50] - in human BEC localised at TJs [76] - KD: reduces mRNA/protein without cytotoxicity, paracellular permeability raises for small molecules [76]; P-face strands less structured, reduced mesh number, i.e., less particles, larger meshes] [50]. | - contribution to cell adhe-sion and tightness for small molecules [76] | - 229 aa; M.W., 25.4 Da; pI, 5.37; N-/C-terminal tail, 10/44 aa; ECL1/ECL2, 50/19 aa (human) - no homophilic trans interaction in BEC [50] - weak heterophilic trans interaction (Cldn12, -22, -24, Ocln) [50] - no TJ strand formation, but strands supported indirectly (via Ocln) [50] | - xenobiotics-activated arylhydrocarbon-receptor [142], retinoic acid receptor-related orphan receptor α [143], and myeloid zinc finger 1 [144] increase mRNA expression - transcription inhibition by miR-124 [145] - cerebellar haemorrhage decreases expression in mouse BEC by [76] |
3. Tight Junctions and Their Proteins at the Blood-Brain Barrier
3.1. Claudins
3.2. Tight Junction-Associated MARVEL Proteins
| Expression | Function | Structure/Interactions | Regulation/Signalling |
|---|---|---|---|
| Occludin | |||
| - gene OCLN, chromosome 5 (human), -13 (mouse) - expression < Cldn5 [50] - increased expression in co-culture of BEC with astro-/pericytes [230], neurons [231] - half-life 6.2 h [189] - KO mouse: TJ-morphology unchanged, calcification of brain [212] - Ocln/Tric-double KO: lowers TJ-strand branches/ barrier integrity [209] | - TJ-regulation postulated [192] - redox sensor in TJs [204] - facilitates TJ branching/barrier tightness [55,209] - C-terminal CC-domain required for maintenance and regulation of macromolecule flux through TJs [215] - regulates centrosomes in cortex genesis [232] - regulates apoptosis via caspase-3 transcription [233] - controls HIV-transcription [234], glucose uptake/ATP-synthesis [235] of BBB pericytes - Ocln/caveolin-1/Alix-complex regulates HIV-permeation through BBB [192] - required for cytokine-mediated signal transduction [236] | - human: 522 aa; M.W. 59.1 kDa (polyphosphorylated ≤ 65 kDa [219]); pI 5.77 - cytosolic N-/C-termini 66/257 aa; ECL1 46 aa (11 Tyr, 19 Gly—potentially hydrophobic interactions, flexibility); ICL 11 aa; ECL2 48 aa (2 Cys, disulfide bridge, hypoxia-/redox-sensitive) [204] - interactions: homophilic trans, cis (CC do- main dimerises [214]); heterophilic cis Cldn1, MD3/trans Cldn1, -5 [55], -25 [50] - 3D-structures: cytosolic C-terminal region [237], complex ZO-1 (PDZ3-SH3-U5-GuK)/ Ocln (CC-domain) [222] - CC-domain binds ZO-1 (SH3-hinge-GuK) [216], possibly interacts with ZO-1 [215] - peptide sequence of CC-domain associates PKC ζ, Tyr-kinase c-Yes, PI3K, connexin 26 [218] - MARVEL-domain: in membrane appositions, cholesterol-rich microdomains [206]; mediates interaction of TJs with membrane lipids, cis-oligomerisation via Cys and membrane insertion [238] | - Tyr398, Ser408: high-conserved phosphorylation sites for PKCs, CK2, Tyr-kinase Src [217] - thrombin: Tyr-phosphorylation, Ocln-ZO-1-/TJ disruption, BBB leakage; angiopoietin-1 inhibits this Tyr-phosphorylation, stabilises TJs [239] - VEGF-activated atypical PKC opens BBB [240] - VEGF/hypoxia activate PLCγ, PI3K/Akt, PKG: rearrange Ocln, ZO-1, -2; open BBB [241] - EGFR-activation: p38 MAPK/NFκB signal pathway reduce Ocln expression [242] - ubiquitinated by E3A Nedd4-2 [243]/Itch [244] (prevented by γ-secretase blockade [245] - KD of E3A MARCH3 tightens BEC barrier, induces Ocln-/Cldn5 by FoxO1 deactivation [246] - reduction: TGF-β via MMPs [93], IL-17 [247] - degradation: MMP [248,249], calpain (Zn2+-dependent) [250], proteasome [244,251] - microwave radiation: reduced Ocln/Ocln-ZO-1 binding, TJ broadening/fracture, BBB opening (VEGF/Flk-1-ERK Tyr-phosphorylation mediated) [252] - ischemia/reperfusion: Ser490 phosphorylation/ ubiquitination via VEGFR2 [253] - Netrin-1 protects BBB, activates Kruppel-like factor 2/Ocln path (ischemia/reperfusion) [221] - hypoxia: MMP9 caused Ocln rearrangement in TJs, BBB leakage [254] - diet-induced diabetes: Ocln/ZO-1 down, BBB leak; lessened by carboanhydrase inhibitor [141] - autistic children: serum Ocln increase [126] |
| Tricellulin (MARVEL domain-containing protein 2) | |||
| - gene MARVELD2, chromosome 5 (human), 13 (mouse) - particular isoform Tric a [50] - expression <Ocln, <<Cldn5 [50] - brain [223], retina [203,255] - membranal in tricellular [256], bicellular cell contacts [257], likewise nuclear, perinuclear localised [223] - KO mouse: hearing loss, degenerated cochlea hair cells [258] - Tric/Occl-double KO lowers TJ-strand branches/ barrier integrity [209] | - sealing of macromolecules but not ions in tricellular TJs [211] - regulates H2O-permeability [259] - role in regulating blood-cerebrospinal fluid barrier [255] - facilitates TJ branching/barrier integrity [55,209] - redox-regulation in TJs [260] | - mouse: 558 aa, M.W. 64.2 kDa; pI 7.21 - cytosolic N-/C-terminal 194/196 aa; very short ECL1/ECL2 8/16 aa; ECL2: disulfide bridge, hypoxia-/redox-sensitive [260] - homophilic: cis interaction in 2- and 3-cell TJs; trans in 3-cellular TJs [55,260] - heterophilic interaction: cis Cldn1, -3, -5, MD3; trans Cldn1, -5 [55] - continous P-face strand network in 3-cell TJs [260]; intensifies Cldn1 TJs [55] - C-terminal CC-domain: crystal structure (2.2 Å), dimer with polar interface [261] - angulins bind/recruit Tric in TJs [225] - N-terminus associates dynamin-binding protein (=scaffold protein Tuba) [262], human plasminogen [263] | - ubiquitination by Itch [264] - MAPK-, PKC-activation causes nuclear localisation in weakly differentiated tissue [265] - toxin ESX-1 secretion-associated protein EspG1 reduces expression [266] - induction by mirRNA-203 (microRNA binding motif on Tric) inhibitor, weakening Pb-induced blood-cerebrospinal fluid barrier leak [255] - down-regulated: interleukin-13 (via IL-13-receptor α2) [267], choleratoxin [268] - degradation: by MMP2/3 [269] - apoptosis: degraded at Asp487, Asp 441 (C-terminal CC-domain, caspase cleavage) [270] - OGD: Tric down in BEC [200] - increase in cortex: autism spectrum disorders (Cldn3, -5, -12) [271] |
| MarvelD3 (MARVEL domain-containing protein 3) | |||
| - gene MARVELD3, chromosome 16 (human) - KD retards TJ formation [22] | - may partially replace Ocln, Tric [22] | - human 401 aa, M.W. 44.9 Da; pI 8.84; ECL1/2 24/39, N-/C-terminal 226/39 aa - cis binding: MD3, Ocln, Tric, Cldn1, -5 [55] | - down-regulated by OGD in bovine BEC [55] |
3.3. Junctional Adhesion Molecules
3.4. Cytosolic Tight Junction-Associated Proteins
4. Tight Junctions and Pathologies of the Brain
5. Conclusions and Perspectives
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Rodriguez-Boulan, E.; Macara, I.G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 2014, 15, 225–242. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Junctional Complexes in Various Epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef] [PubMed]
- Irie, K.; Shimizu, K.; Sakisaka, T.; Ikeda, W.; Takai, Y. Roles and modes of action of nectins in cell-cell adhesion. Semin. Cell Dev. Biol. 2004, 15, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta Biomembr. 2008, 1778, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.; Georgiou, M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 2011, 192, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Firth, J.A. Immunohistochemical localization of adherens junction components in blood-brain-barrier microvessels of the rat. J. Cell Sci. 1993, 104, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, F.; Sluysmans, S.; Flinois, A.; Shah, J.; Vasileva, E.; Citi, S. Scaffolding proteins of vertebrate apical junctions: Structure, functions and biophysics. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183399. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, K.; Chiba, H.; Fujita, H.; Kojima, T.; Saito, T.; Endo, T.; Sawada, N. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J. Cell. Physiol. 2006, 208, 123–132. [Google Scholar] [CrossRef]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta Biomembr. 2009, 1788, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, L.A. Further observations on fine-structure of freeze-cleaved tight junctions. J. Cell Sci. 1973, 13, 763–786. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Tumer, J.R. Tight, Junction Pore and Leak Pathways: A Dynamic Duo. In Annual Review of Physiology; Julius, D., Clapham, D.E., Eds.; Annual Reviews: San Mateo, CA, USA, 2011; Volume 73, pp. 283–309. [Google Scholar]
- Kirschner, N.; Rosenthal, R.; Furuse, M.; Moll, I.; Fromm, M.; Brandner, J.M. Contribution of Tight Junction Proteins to Ion, Macromolecule, and Water Barrier in Keratinocytes. J. Investig. Dermatol. 2013, 133, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta Biomembr. 2008, 1778, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Van Itallie, C.M. Physiology and Function of the Tight Junction. Cold Spring Harbor Perspect. Biol. 2009, 1, a002584. [Google Scholar] [CrossRef] [PubMed]
- Kooij, G.; Kopplin, K.; Blasig, R.; Stuiver, M.; Koning, N.; Goverse, G.; van der Pol, S.M.A.; Hof, B.V.; Gollasch, M.; Drexhage, J.A.R.; et al. Disturbed function of the blood-cerebrospinal fluid barrier aggravates neuro-inflammation. Acta Neuropathol. 2014, 128, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Namorado, M.C.; Martin, D.; Luna, J.; Alarcon, L.; Islas, S.; Valencia, L.; Muriel, P.; Ponce, L.; Reyes, J.L. Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int. 2000, 57, 2386–2402. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.W.; Surma, M.A.; Simons, K. Polarized sorting and trafficking in epithelial cells. Cell Res. 2012, 22, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Valdes, J.; Shoshani, L.; Contreras, R.G. Role of tight junctions in establishing and maintaining cell polarity. Annu. Rev. Physiol. 1998, 60, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Matter, K.; Aijaz, S.; Tsapara, A.; Balda, M.S. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr. Opin. Cell Biol. 2005, 17, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Furuse, M.; Fujimoto, K.; Tsukita, S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA 1999, 96, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Raleigh, D.R.; Marchiando, A.M.; Zhang, Y.; Shen, L.; Sasaki, H.; Wang, Y.M.; Long, M.Y.; Turner, J.R. Tight Junction-associated MARVEL Proteins MarvelD3, Tricellulin, and Occludin Have Distinct but Overlapping Functions. Mol. Biol. Cell 2010, 21, 1200–1213. [Google Scholar] [CrossRef] [PubMed]
- Martin-Padura, I.; Lostaglio, S.; Schneemann, M.; Williams, L.; Romano, M.; Fruscella, P.; Panzeri, C.; Stoppacciaro, A.; Ruco, L.; Villa, A.; et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998, 142, 117–127. [Google Scholar] [CrossRef]
- Guillemot, L.; Paschoud, S.; Pulimeno, P.; Foglia, A.; Citi, S. The cytoplasmic plaque of tight junctions: A scaffolding and signalling center. Biochim. Biophys. Acta Biomembr. 2008, 1778, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I.; Nusrat, A.; Parkos, C.A. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol. Biol. Cell 2004, 15, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Zwanziger, D.; Staat, C.; Andjelkovic, A.V.; Blasig, I.E. Claudin-derived peptides are internalized via specific endocytosis pathways. In Barriers and Channels Formed by Tight Junction Proteins I; Fromm, M., Schulzke, J.D., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2012; Volume 1257, pp. 29–37. [Google Scholar]
- Stamatovic, S.M.; Keep, R.F.; Wang, M.M.; Jankovic, I.; Andjelkovic, A.V. Caveolae-mediated Internalization of Occludin and Claudin-5 during CCL2-induced Tight Junction Remodeling in Brain Endothelial Cells. J. Biol. Chem. 2009, 284, 19053–19066. [Google Scholar] [CrossRef] [PubMed]
- Bruewer, M.; Utech, M.; Ivanov, A.I.; Hopkins, A.M.; Parkos, C.A.; Nusrat, A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005, 19, 923–933. [Google Scholar] [CrossRef]
- Takahashi, S.; Iwamoto, N.; Sasaki, H.; Ohashi, M.; Oda, Y.; Tsukita, S.; Furuse, M. The E3 ubiquitin ligase LNX1p80 promotes the removal of claudins from tight junctions in MDCK cells. J. Cell Sci. 2009, 122, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Gehne, N.; Lamik, A.; Lehmann, M.; Haseloff, R.F.; Andjelkovic, A.V.; Blasig, I.E. Cross-over endocytosis of claudins is mediated by interactions via their extracellular loops. PLoS ONE 2017, 12, e0182106. [Google Scholar] [CrossRef] [PubMed]
- Mineta, K.; Yamamoto, Y.; Yamazaki, Y.; Tanaka, H.; Tada, Y.; Saito, K.; Tamura, A.; Igarashi, M.; Endo, T.; Takeuchi, K.; et al. Predicted expansion of the claudin multigene family. FEBS Lett. 2011, 585, 606–612. [Google Scholar] [CrossRef]
- Furuse, M.; Tsukita, S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006, 16, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Nishizawa, T.; Tani, K.; Yamazaki, Y.; Tamura, A.; Ishitani, R.; Dohmae, N.; Tsukita, S.; Nureki, O.; Fujiyoshi, Y. Crystal Structure of a Claudin Provides Insight into the Architecture of Tight Junctions. Science 2014, 344, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, Y.; Suzuki, H.; Tani, K.; Nishikawa, K.; Irie, K.; Ogura, Y.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 2015, 347, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, T.; Shinya, N.; Ito, K.; Ohsawa, N.; Terada, T.; Hirata, K.; Kawano, Y.; Yamamoto, M.; Kimura-Someya, T.; Yokoyama, S.; et al. Structural basis for disruption of claudin assembly in tight junctions by an enterotoxin. Sci. Rep. 2016, 6, 33632. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, A.J.; Stroud, R.M. Claudin-9 structures reveal mechanism for toxin-induced gut barrier breakdown. Proc. Natl. Acad. Sci. USA 2019, 116, 17817–17824. [Google Scholar] [CrossRef] [PubMed]
- Gunzel, D.; Yu, A.S.L. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Tanaka, H.; Tamura, A. The Claudins: From Tight Junctions to Biological Systems. Trends Biochem. Sci. 2019, 44, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Yeh, S.; Appleton, B.A.; Held, H.A.; Kausalya, P.J.; Phua, D.C.Y.; Wong, W.L.; Lasky, L.A.; Wiesmann, C.; Hunziker, W.; et al. Convergent and divergent ligand specificity among PDZ domains of the LAP and zonula occludens (ZO) families. J. Biol. Chem. 2006, 281, 22299–22311. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2 and ZO-3, with the COOH termini of claudins. J. Cell Biol. 1999, 147, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Ruffer, C.; Gerke, V. The C-terminal cytoplasmic tail of claudins 1 and 5 but not its PDZ-binding motif is required for apical localization at epithelial and endothelial tight junctions. Eur. J. Cell Biol. 2004, 83, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Weaver, J.; Jin, X.C.; Zhang, Y.; Xu, J.; Liu, K.J.; Li, W.P.; Liu, W.L. Nitric Oxide Interacts with Caveolin-1 to Facilitate Autophagy-Lysosome-Mediated Claudin-5 Degradation in Oxygen-Glucose Deprivation-Treated Endothelial Cells. Mol. Neurobiol. 2016, 53, 5935–5947. [Google Scholar] [CrossRef]
- Tanaka, M.; Kamata, R.; Sakai, R. EphA2 phosphorylates the cytoplasmic tail of claudin-4 and mediates paracellular permeability. J. Biol. Chem. 2005, 280, 42375–42382. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Gambling, T.M.; Carson, J.L.; Anderson, J.M. Palmitoylation of claudins is required for efficient tight-junction localization. J. Cell Sci. 2005, 118, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Lohrberg, D.; Krause, E.; Schumann, M.; Piontek, J.; Winkler, L.; Blasig, I.E.; Haseloff, R.F. A strategy for enrichment of claudins based on their affinity to Clostridium perfringens enterotoxin. BMC Mol. Biol. 2009, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Gow, A.; Southwood, C.M.; Li, J.S.; Pariali, M.; Riordan, G.P.; Brodie, S.E.; Danias, J.; Bronstein, J.M.; Kachar, B.; Lazzarini, R.A. CNS myelin and Sertoli cell tight junction strands are absent in Osp/Claudin-11 null mice. Cell 1999, 99, 649–659. [Google Scholar] [CrossRef]
- Gow, A.; Davies, C.; Southwood, C.M.; Frolenkov, G.; Chrustowski, M.; Ng, L.; Yamauchi, D.; Marcus, D.C.; Kachar, B. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J. Neurosci. 2004, 24, 7051–7062. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.R.; Ryan, A.K. Claudins: Unlocking the code to tight junction function during embryogenesis and in disease. Clin. Genet. 2010, 77, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood-brain barrier: Structural and functional aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.; Dufresne, J.; Hermo, L.; Cyr, D.G. Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology 2001, 142, 854–863. [Google Scholar] [CrossRef]
- Inai, T.; Sengoku, A.; Hirose, E.; Iida, H.; Shibata, Y. Claudin-7 expressed on lateral membrane of rat epididymal epithelium does not form aberrant tight junction strands. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 1431–1438. [Google Scholar] [CrossRef]
- Winkler, L.; Blasig, R.; Breitkreuz-Korff, O.; Berndt, P.; Dithmer, S.; Helms, H.C.; Puchkov, D.; Devraj, K.; Kaya, M.; Qin, Z.; et al. Tight junctions in the blood-brain barrier promote edema formation and infarct size in stroke—Ambivalent effects of sealing proteins. J. Cereb. Blood Flow Metab. 2020, 41, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Piontek, J.; Winkler, L.; Wolburg, H.; Müller, S.L.; Zuleger, N.; Piehl, C.; Wiesner, B.; Krause, G.; Blasig, I.E. Formation of tight junction: Determinants of homophilic interaction between classical claudins. Faseb J. 2008, 22, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Cording, J.; Berg, J.; Kading, N.; Bellmann, C.; Tscheik, C.; Westphal, J.K.; Milatz, S.; Gunzel, D.; Wolburg, H.; Piontek, J.; et al. In tight junctions, claudins regulate the interactions between occludin, tricellulin and marvelD3, which, inversely, modulate claudin oligomerization. J. Cell Sci. 2013, 126, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Inai, T.; Kobayashi, J.; Shibata, Y. Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol. 1999, 78, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S. Conversion of Zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell Biol. 2001, 153, 263–272. [Google Scholar] [CrossRef]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schoneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef]
- Milatz, S.; Krug, S.M.; Rosenthal, R.; Gunzel, D.; Muller, D.; Schulzke, J.D.; Amasheh, S.; Fromm, M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta Biomembr. 2010, 1798, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.; Rahner, C.; Anderson, J.M. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J. Clin. Investig. 2001, 107, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Renigunta, A.; Yang, J.; Waldegger, S. Claudin-4 forms paracellular chloride channel in the kidney and requires claudin-8 for tight junction localization. Proc. Natl. Acad. Sci. USA 2010, 107, 18010–18015. [Google Scholar] [CrossRef]
- Colegio, O.R.; Van Itallie, C.M.; McCrea, H.J.; Rahner, C.; Anderson, J.M. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am. J. Physiol. Cell Physiol. 2002, 283, C142–C147. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Gomes, A.S.; Paul, D.L.; Goodenough, D.A. Study of claudin function by RNA interference. J. Biol. Chem. 2006, 281, 36117–36123. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, M.D.; Lu, Q.; Chen, Y.H. Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 2005, 118, 2683–2693. [Google Scholar] [CrossRef] [PubMed]
- Sas, D.; Hu, M.C.; Moe, O.W.; Baum, M. Effect of claudins 6 and 9 on paracellular permeability in MDCK II cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1713–R1719. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Rogan, S.; Yu, A.; Vidal, L.S.; Holmes, J.; Anderson, J.M. Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am. J. Physiol. Renal Physiol. 2006, 291, F1288–F1299. [Google Scholar] [CrossRef] [PubMed]
- Abuazza, G.; Becker, A.; Williams, S.S.; Chakravarty, S.; Truong, H.T.; Lin, F.M.; Baum, M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am. J. Physiol. Renal Physiol. 2006, 291, F1132–F1141. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.L.; Enck, A.H.; Lencer, W.I.; Schneeberger, E.E. Claudin-8 expression in Madin-Darby canine kidney cells augments the paracellular barrier to cation permeation. J. Biol. Chem. 2003, 278, 17350–17359. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Gunzel, D.; Conrad, M.P.; Rosenthal, R.; Fromm, A.; Amasheh, S.; Schulzke, J.D.; Fromm, M. Claudin-17 forms tight junction channels with distinct anion selectivity. Cell. Mol. Life Sci. 2012, 69, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yosef, T.; Belyantseva, I.A.; Saunders, T.L.; Hughes, E.D.; Kawamoto, K.; Van Itallie, C.M.; Beyer, L.A.; Halsey, K.; Gardner, D.J.; Wilcox, E.R.; et al. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 2003, 12, 2049–2061. [Google Scholar] [CrossRef]
- Hou, J.H.; Renigunta, A.; Konrad, M.; Gornes, A.S.; Schneeberger, E.E.; Paul, D.L.; Waldegger, S.; Goodenough, D.A. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J. Clin. Investig. 2008, 118, 619–628. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.J.; Foo, C.F.H.; Dinger, M.E.; Smooker, P.M.; Stanton, P.G. Claudin-11 and occludin are major contributors to Sertoli cell tight junction function, in vitro. Asian J. Androl. 2016, 18, 620–626. [Google Scholar] [PubMed]
- Jovov, B.; Van Itallie, C.M.; Shaheen, N.J.; Carson, J.L.; Gambling, T.M.; Anderson, J.M.; Orlando, R.C. Claudin-18: A dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am. J. Physiol. Gastroint. Liver Physiol. 2007, 293, G1106–G1113. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yamamoto, Y.; Kashihara, H.; Yamazaki, Y.; Tani, K.; Fujiyoshi, Y.; Mineta, K.; Takeuchi, K.; Tamura, A.; Tsukita, S. Claudin-21 Has a Paracellular Channel Role at Tight Junctions. Mol. Cell. Biol. 2016, 36, 954–964. [Google Scholar] [CrossRef]
- Ohnishi, M.; Ochiai, H.; Matsuoka, K.; Akagi, M.; Nakayama, Y.; Shima, A.; Uda, A.; Matsuoka, H.; Kamishikiryo, J.; Michihara, A.; et al. Claudin Domain Containing 1 Contributing to Endothelial Cell Adhesion Decreases in Presence of Cerebellar Hemorrhage. J. Neurosci. Res. 2017, 95, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.D.; Aalinkeel, R.; Sykes, D.E.; Reynolds, J.L.; Bindukumar, B.; Adal, A.; Qi, M.; Toh, J.; Xu, G.; Prasad, P.N.; et al. Methamphetamine alters blood brain barrier permeability via the modulation of tight junction expression: Implication for HIV-1 neuropathogenesis in the context of drug abuse. Brain Res. 2008, 1203, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Wolburg-Buchholz, K.; Kraus, J.; Rascher-Eggstein, G.; Liebner, S.; Hamm, S.; Duffner, F.; Grote, E.H.; Risau, W.; Engelhardt, B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003, 105, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Hanske, S.; Dyrna, F.; Bechmann, I.; Krueger, M. Different segments of the cerebral vasculature reveal specific endothelial specifications, while tight junction proteins appear equally distributed. Brain Struct. Funct. 2017, 222, 1179–1192. [Google Scholar] [CrossRef]
- Liebner, S.; Fischmann, A.; Rascher, G.; Duffner, F.; Grote, E.H.; Kalbacher, H.; Wolburg, H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000, 100, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.M.; Francis, S.A.; McCormack, J.M.; Lai, J.; Rogers, R.A.; Skare, I.B.; Lynch, R.D.; Schneeberger, E.E. Inducible expression of claudin-1-myc but not occludin-VSV-G results in aberrant tight junction strand formation in MDCK cells. J. Cell Sci. 2000, 113, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, F.; Schafer, J.; Lyck, R.; Makrides, V.; Brunner, S.; Schaeren-Wiemers, N.; Deutsch, U.; Engelhardt, B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011, 122, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Mailly, L.; Baumert, T.F. Hepatitis C virus infection and tight junction proteins: The ties that bind. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183296. [Google Scholar] [CrossRef] [PubMed]
- Milatz, S.; Piontek, J.; Schulzke, J.D.; Blasig, I.E.; Fromm, M.; Gunzel, D. Probing the cis-arrangement of prototype tight junction proteins claudin-1 and claudin-3. Biochem. J. 2015, 468, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, S.; Staat, C.; Zwanziger, D.; Sauer, R.S.; Bellmann, C.; Guenther, R.; Krause, E.; Haseloff, R.F.; Rittner, H.; Blasig, I.E. Redox-Sensitive Structure and Function of the First Extracellular Loop of the Cell-Cell Contact Protein Claudin-1: Lessons from Molecular Structure to Animals. Antioxid. Redox Signal. 2015, 22, 1–14. [Google Scholar] [CrossRef]
- Piontek, J.; Fritzsche, S.; Cording, J.; Richter, S.; Hartwig, J.; Walter, M.; Yu, D.; Turner, J.R.; Gehring, C.; Rahn, H.P.; et al. Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell. Mol. Life Sci. 2011, 68, 3903–3918. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, H.E.; Gahlaut, N.; Mohandessi, S.; Yu, D.; Turner, J.R.; Miller, L.W. Time-resolved luminescence resonance energy transfer imaging of protein-protein interactions in living cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13582–13587. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Takasawa, A.; Takasawa, K.; Aoyama, T.; Murata, M.; Osanai, M.; Saito, T.; Sawada, N. Estrogen/GPR30 Signaling Contributes to the Malignant Potentials of ER-Negative Cervical Adenocarcinoma via Regulation of Claudin-1 Expression. Neoplasia 2018, 20, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Upmanyu, N.; Bulldan, A.; Papadopoulos, D.; Dietze, R.; Malviya, V.N.; Scheiner-Bobis, G. Impairment of the Gn alpha 11-controlled expression of claudin-1 and MMP-9 and collective migration of human breast cancer MCF-7 cells by DHEAS. J. Steroid Biochem. Mol. Biol. 2018, 182, 50–61. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Kao, D.J.; Kitzenberg, D.A.; Dobrinskikh, E.; Schwisow, K.D.; Masterson, J.C.; Kendrick, A.A.; Kelly, C.J.; Bayless, A.J.; Kominsky, D.J.; et al. HIF-dependent regulation of claudin-1 is central to intestinal epithelial tight junction integrity. Mol. Biol. Cell 2015, 26, 2252–2262. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, H.; Kojima, T.; Fujibe, M.; Soma, T.; Miyajima, H.; Nagasawa, K.; Wada, I.; Sawada, N. Cyclic AMP induces phosphorylation of claudin-5 immunoprecipitates and expression of claudin-5 gene in blood-brain-barrier endothelial cells via protein kinase A-dependent and -independent pathways. Exp. Cell Res. 2003, 290, 275–288. [Google Scholar] [CrossRef]
- Ishihara, H.; Kubota, H.; Lindberg, R.L.P.; Leppert, D.; Gloor, S.M.; Errede, M.; Virgintino, D.; Fontana, A.; Yonekawa, Y.; Frei, K. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta(2) involves matrix metalloproteinases and tight junction proteins. J. Neuropathol. Exp. Neurol. 2008, 67, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.Q.; Tang, Z.X.; Li, Y.; Hu, L.M.; Pan, J.Q. The Effects of Copper on Brain Microvascular Endothelial Cells and Claudin Via Apoptosis and Oxidative Stress. Biol. Trace Elem. Res. 2016, 174, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Burek, M.; Konig, A.; Lang, M.; Fiedler, J.; Oerter, S.; Roewer, N.; Bohnert, M.; Thal, S.C.; Blecharz-Lang, K.G.; Woitzik, J.; et al. Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity Through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells. Transl. Stroke Res. 2019, 10, 672–683. [Google Scholar] [CrossRef]
- Velandia-Romero, M.L.; Calderon-Pelaez, M.A.; Castellanos, J.E. In Vitro Infection with Dengue Virus Induces Changes in the Structure and Function of the Mouse Brain Endothelium. PLoS ONE 2016, 11, e0157786. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, A.M.; Como, C.N.; Bubak, A.N.; Mescher, T.; Jones, D.; Nagel, M.A. Varicella Zoster Virus Alters Expression of Cell Adhesion Proteins in Human Perineurial Cells via Interleukin 6. J. Infect. Dis. 2019, 220, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Yumine, N.; Matsumoto, Y.; Ohta, K.; Fukasawa, M.; Nishio, M. Claudin-1 inhibits human parainfluenza virus type 2 dissemination. Virology 2019, 531, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zi, X.; Peng, Y.; Wang, Z.; Hong, H.; Yan, Y.; Guan, W.; Tan, K.S.; Liu, J.; Ong, H.H.; et al. H3N2 influenza virus infection enhances oncostatin M expression in human nasal epithelium. Exp. Cell Res. 2018, 371, 322–329. [Google Scholar] [CrossRef]
- Kast, J.I.; McFarlane, A.J.; Globinska, A.; Sokolowska, M.; Wawrzyniak, P.; Sanak, M.; Schwarze, J.; Akdis, C.A.; Wanke, K. Respiratory syncytial virus infection influences tight junction integrity. Clin. Exp. Immunol. 2017, 190, 351–359. [Google Scholar] [CrossRef]
- Wachter, B.; Schurger, S.; Schmid, A.; Groger, A.; Sadler, R.; Speidel, A.; Rolinger, J.; Pichler, B.J.; Berg, D.; Wagner, H.J.; et al. 6-Hydroxydopamine leads to T2 hyperintensity, decreased claudin-3 immunoreactivity and altered aquaporin 4 expression in the striatum. Behav. Brain Res. 2012, 232, 148–158. [Google Scholar] [CrossRef]
- Dias, M.C.; Coisne, C.; Lazarevic, I.; Baden, P.; Hata, M.; Iwamoto, N.; Francisco, D.M.F.; Vanlandewijck, M.; He, L.; Baier, F.A.; et al. Publisher Correction: Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci. Rep. 2019, 9, 10702. [Google Scholar] [CrossRef]
- Hashimoto, K.; Oshima, T.; Tomita, T.; Kim, Y.; Matsumoto, T.; Joh, T.; Miwa, H. Oxidative stress induces gastric epithelial permeability through claudin-3. Biochem. Biophys. Res. Commun. 2008, 376, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.L.; Ge, H.F.; Li, Q.; Zhang, X.; Hu, R.; Hu, S.L.; Liu, X.; Zhang, J.H.; Chen, Y.J.; Feng, H. Artesunate Protected Blood-Brain Barrier via Sphingosine 1 Phosphate Receptor 1/Phosphatidylinositol 3 Kinase Pathway After Subarachnoid Hemorrhage in Rats. Mol. Neurobiol. 2017, 54, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Corada, M.; Bangsow, T.; Babbage, J.; Taddei, A.; Czupalla, C.J.; Reis, M.; Felici, A.; Wolburg, H.; Fruttiger, M.; et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008, 183, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Sasaki, H.; Tsukita, S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J. Cell Biol. 1999, 147, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Fedorova, A.A.; Kravtsova, V.V.; Bikmurzina, A.E.; Okorokova, L.S.; Matchkov, V.V.; Cornelius, V.; Amasheh, S.; Krivoi, I.I. Circulating Ouabain Modulates Expression of Claudins in Rat Intestine and Cerebral Blood Vessels. Int. J. Mol. Sci. 2020, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Ikeda, C.; Uchida, Y.; Sakamoto, Y.; Miller, F.; Glacial, F.; Decleves, X.; Scherrmann, J.M.; Couraud, P.O.; Kubo, Y.; et al. Quantitative Targeted Absolute Proteomic Analysis of Transporters, Receptors and Junction Proteins for Validation of Human Cerebral Microvascular Endothelial Cell Line hCMEC/D3 as a Human Blood-Brain Barrier Model. Mol. Pharm. 2013, 10, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ek, C.J.; Dziegielewska, K.M.; Stolp, H.; Saunders, N.R. Functional effectiveness of the blood brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica). J. Comp. Neurol. 2006, 496, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.C.; Federici, C.; Guillonneau, F.; Chretien, F.; Camoin, L.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.O. Guanine nucleotide-binding protein G alpha i2: A new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells. J. Cereb. Blood Flow Metab. 2012, 32, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Mandel, I.; Paperna, T.; Volkowich, A.; Merhav, M.; Glass-Marmor, L.; Miller, A. The Ubiquitin-Proteasome Pathway Regulates Claudin 5 Degradation. J. Cell. Biochem. 2012, 113, 2415–2423. [Google Scholar] [CrossRef]
- Honda, M.; Nakagawa, S.; Hayashi, K.; Kitagawa, N.; Tsutsumi, K.; Nagata, I.; Niwa, M. Adrenomedullin improves the blood-brain barrier function through the expression of claudin-5. Cell. Mol. Neurobiol. 2006, 26, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.J.; Watry, D.D.; Marcondes, M.C.G.; Fox, H.S. Selective decrease in paracellular conductance of tight junctions: Role of the first extracellular domain of claudin-5. Mol. Cell. Biol. 2004, 24, 8408–8417. [Google Scholar] [CrossRef] [PubMed]
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R.; et al. Nano-scale architecture of blood-brain barrier tight-junctions. eLife 2021, 10, e63253. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Poirier, K.; Boddaert, N.; Hubert, L.; Aubart, M.; Kaminska, A.; Alison, M.; Desguerre, I.; Munnich, A.; Campbell, M. Recurrent de novo mutations in CLDN5 induce an anion-selective blood-brain barrier and alternating hemiplegia. Brain 2022, 145, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, D.; Paul, D.; Ge, S.; Jellison, E.; Pachter, J.S. Appearance of claudin-5(+) leukocyte subtypes in the blood and CNS during progression of EAE. J. Neuroinflamm. 2021, 18, 296. [Google Scholar] [CrossRef] [PubMed]
- Soma, T.; Chiba, H.; Kato-Mori, Y.; Wada, T.; Yamashita, T.; Kojima, T.; Sawada, N. Thr(207) of claudin-5 is involved in size-selective loosening of the endothelial barrier by cyclic AMP. Exp. Cell Res. 2004, 300, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Nishihara, A.; Mishima, K.; Yamashita, J.; Shimizu, K.; Miyazawa, K.; Nishikawa, S.; Miyazono, K. TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J. Cell Biol. 2003, 163, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Taddei, A.; Giampietro, C.; Conti, A.; Orsenigo, F.; Breviario, F.; Pirazzoli, V.; Potente, M.; Daly, C.; Dimmeler, S.; Dejana, E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat. Cell Biol. 2008, 10, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, G.B.; Malaeb, S.N.; Stonestreet, B.S. Maternal glucocorticoid exposure alters tight junction protein expression in the brain of fetal sheep. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H179–H188. [Google Scholar] [CrossRef] [PubMed]
- Felinski, E.A.; Cox, A.E.; Phillips, B.E.; Antonetti, D.A. Glucocorticoids induce transactivation of tight junction genes occludin and claudin-5 in retinal endothelial cells via a novel cis-element. Exp. Eye Res. 2008, 86, 867–878. [Google Scholar] [CrossRef]
- Burek, M.; Arias-Loza, P.A.; Roewer, N.; Forster, C.Y. Claudin-5 as a Novel Estrogen Target in Vascular Endothelium. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Li, W.M.; Song, J.N.; Zhang, M.; Huang, T.Q.; Wei, X. High expression of EphA2 led to secondary injury by destruction of BBB integrity though the ROCK pathway after diffuse axonal injury. Neurosci. Lett. 2020, 736, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Hong, D.Y.; Lee, D.H.; Park, S.W.; Lee, J.Y.; Jeong, J.H.; Kim, E.Y.; Chung, H.M.; Hong, K.S.; Park, S.P.; et al. Inflammation and Rho-Associated Protein Kinase-Induced Brain Changes in Vascular Dementia. Biomedicines 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Kakogiannos, N.; Ferrari, L.; Giampietro, C.; Scalise, A.A.; Maderna, C.; Rava, M.; Taddei, A.; Lampugnani, M.G.; Pisati, F.; Malinverno, M.; et al. JAM-A Acts via C/EBP-alpha to Promote Claudin-5 Expression and Enhance Endothelial Barrier Function. Circ. Res. 2020, 127, 1056–1073. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, A.; Ferahkaya, H.; Karagoz, H.; Kilinc, I.; Energin, V.M. Serum claudin-5, claudin-11, occludin, vinculin, paxillin, and beta-catenin levels in preschool children with autism spectrum disorder. Nord. J. Psychiatry 2023, 77, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Kazmierski, R.; Michalak, S.; Wencel-Warot, A.; Nowinski, W.L. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology 2012, 79, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; de Jager, C.; Brickler, T.; Soliman, E.; Ladner, L.; Kaloss, A.M.; Zhu, Y.; Pridham, K.J.; Mills, J.; Ju, J.; et al. Endothelial deletion of EPH receptor A4 alters single-cell profile and Tie2/Akap12 signaling to preserve blood-brain barrier integrity. Proc. Natl. Acad. Sci. USA 2023, 120, e2204700120. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; An, Z.; Fu, Z.; Chen, X.; Tong, Q.; Zhang, Y.; Ren, H. Kinsenoside alleviates oxidative stress-induced blood-brain barrier dysfunction via promoting Nrf2/HO-1 pathway in ischemic stroke. Eur. J. Pharmacol. 2023, 949, 175717. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Sumiya, T.; Tachikawa, M.; Yamakawa, T.; Murata, S.; Yagi, Y.; Sato, K.; Stephan, A.; Ito, K.; Ohtsuki, S.; et al. Involvement of Claudin-11 in Disruption of Blood-Brain, -Spinal Cord, and -Arachnoid Barriers in Multiple Sclerosis. Mol. Neurobiol. 2019, 56, 2039–2056. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.; Mottahedeh, J.; Prins, M.; Ridder, W.; Nusinowitz, S.; Bronstein, J.M. Disrupted compaction of CNS myelin in an OSP/claudin-11 and PLP/DM20 double knockout mouse. Mol. Cell. Neurosci. 2005, 29, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Cao, G.; Qian, Y.; Fu, L.; Hu, J.; Xu, T.; Wu, Y.; Lv, Y. Single-cell RNA sequencing unveils Lrg1’s role in cerebral ischemia—reperfusion injury by modulating various cells. J. Neuroinflamm. 2023, 20, 285. [Google Scholar] [CrossRef] [PubMed]
- Cording, J.; Gunther, R.; Vigolo, E.; Tscheik, C.; Winkler, L.; Schlattner, I.; Lorenz, D.; Haseloff, R.F.; Schmidt-Ott, K.M.; Wolburg, H.; et al. Redox Regulation of Cell Contacts by Tricellulin and Occludin: Redox-Sensitive Cysteine Sites in Tricellulin Regulate Both Tri- and Bicellular Junctions in Tissue Barriers as Shown in Hypoxia and Ischemia. Antioxid. Redox Signal. 2015, 23, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, K.; Suzuki, M.; Sato, R.; Kawarada, S.; Terasaki, T.; Uchida, Y. Activation of Annexin A2 signaling at the blood-brain barrier in a mouse model of multiple sclerosis. J. Neurochem. 2022, 160, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Nagano, H.; Ogata, S.; Ito, S.; Masuda, T.; Ohtsuki, S. Knockdown of Podocalyxin Post-Transcriptionally Induces the Expression and Activity of ABCB1/MDR1 in Human Brain Microvascular Endothelial Cells. J. Pharm. Sci. 2022, 111, 1812–1819. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, S.; Yamaguchi, H.; Katsukura, Y.; Asashima, T.; Terasaki, T. mRNA expression levels of tight junction protein genes in mouse brain capillary endothelial cells highly purified by magnetic cell sorting. J. Neurochem. 2008, 104, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Castro Dias, M.; Coisne, C.; Baden, P.; Enzmann, G.; Garrett, L.; Becker, L.; Holter, S.M.; German Mouse Clinic, C.; Hrabe de Angelis, M.; Deutsch, U.; et al. Claudin-12 is not required for blood-brain barrier tight junction function. Fluids Barriers CNS 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Nakatsu, D.; Hempstock, W.; Sugioka, S.; Ishizuka, N.; Furuse, K.; Sugawara, T.; Fukazawa, Y.; Hayashi, H. Reconstitution of functional tight junctions with individual claudin subtypes in epithelial cells. Cell Struct. Funct. 2023, 48, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Zhang, Y.S.; Zheng, W.; Davidson, T.L. The Effects of a High-Energy Diet on Hippocampal Function and Blood-Brain Barrier Integrity in the Rat. J. Alzheimers Dis. 2010, 21, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.; Asashima, T.; Ohtsuki, S.; Yamaguchi, H.; Ito, S.; Terasaki, T. Hyperammonemia induces transport of taurine and creatine and suppresses claudin-12 gene expression in brain capillary endothelial cells in vitro. Neurochem. Int. 2007, 50, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Salameh, T.S.; Mortell, W.G.; Logsdon, A.F.; Butterfield, D.A.; Banks, W.A. Disruption of the hippocampal and hypothalamic blood-brain barrier in a diet-induced obese model of type II diabetes: Prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS 2019, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Lee, K.L.; Furness, S.G.B.; Bosdotter, C.; Poellinger, L.; Whitelaw, M.L. Xenobiotics and Loss of Cell Adhesion Drive Distinct Transcriptional Outcomes by Aryl Hydrocarbon Receptor Signaling. Mol. Pharmacol. 2012, 82, 1082–1093. [Google Scholar] [CrossRef]
- Matsuoka, H.; Shima, A.; Uda, A.; Ezaki, H.; Michihara, A. The retinoic acid receptor-related orphan receptor alpha positively regulates tight junction protein claudin domain-containing 1 mRNA expression in human brain endothelial cells. J. Biochem. 2017, 161, 441–450. [Google Scholar] [PubMed]
- Shima, A.; Matsuoka, H.; Yamaoka, A.; Michihara, A. Transcription of CLDND1 in human brain endothelial cells is regulated by the myeloid zinc finger 1. Clin. Exp. Pharmacol. Physiol. 2021, 10, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Tamura, A.; Kinehara, M.; Shima, A.; Uda, A.; Tahara, H.; Michihara, A. Levels of tight junction protein CLDND1 are regulated by microRNA-124 in the cerebellum of stroke-prone spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2018, 498, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The Perivascular Astroglial Sheath Provides a Complete Covering of the Brain Microvessels: An Electron Microscopic 3D Reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Readnower, R.D.; Chavko, M.; Adeeb, S.; Conroy, M.D.; Pauly, J.R.; McCarron, R.M.; Sullivan, P.G. Increase in Blood-Brain Barrier Permeability, Oxidative Stress, and Activated Microglia in a Rat Model of Blast-Induced Traumatic Brain Injury. J. Neurosci. Res. 2010, 88, 3530–3539. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Wang, F.; Fan, Y.X.; Ping, G.F.; Yang, J.Y.; Wu, C.F. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav. Brain Res. 2013, 236, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. Understanding and circumventing the blood-brain barrier. Acta Paediatr. 2003, 92, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Risau, W.; Wolburg, H. Development of the blood-brain-barrier. Trends Neurosci. 1990, 13, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Kimelberg, H.K. Water homeostasis in the brain: Basic concepts. Neuroscience 2004, 129, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.A.; Dallas, A.D.; Davies, S. Measurement of filtration coefficient in single cerebral microvessels of the frog. J. Physiol. 1990, 423, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain-barrier in anesthetized rats—A developmental-study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef]
- Lauschke, K.; Frederiksen, L.; Hall, V.J. Paving the Way toward Complex Blood-Brain Barrier Models Using Pluripotent Stem Cells. Stem Cells Dev. 2017, 26, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Villegas, J.C.; Broadwell, R.D. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. II. Adsorptive transcytosis of WGA-HRP and the blood-brain and brain blood barriers. J. Neurocytol. 1993, 22, 67–80. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.Z.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 17. [Google Scholar] [CrossRef]
- Mark, K.S.; Davis, T.P. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1485–H1494. [Google Scholar] [CrossRef] [PubMed]
- van der Goes, A.; Wouters, D.; van der Pol, S.M.A.; Huizinga, R.; Ronken, E.; Adamson, P.; Greenwood, J.; Dijkstra, C.D.; de Vries, H.E. Reactive oxygen species enhance the migration of monocytes across the blood-brain barrier in vitro. FASEB J. 2001, 15, 1852–1854. [Google Scholar] [CrossRef] [PubMed]
- El-Bacha, R.S.; Minn, A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell. Mol. Biol. 1999, 45, 15–23. [Google Scholar] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx 2005, 2, 54–62. [Google Scholar] [CrossRef]
- Nalecz, K.A. Solute Carriers in the Blood-Brain Barier: Safety in Abundance. Neurochem. Res. 2017, 42, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC Transporters: Expression, Localization, and Species Differences at the Blood-Brain and the Blood-Cerebrospinal Fluid Barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Stieger, B.; Noe, B.; Fritschy, J.M.; Meier, P.J. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J. Histochem. Cytochem. 1999, 47, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J.; Sanchez-Covarrubias, L.; Slosky, L.M.; Zhang, Y.F.; Laracuente, M.L.; Ronaldson, P.T. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: Relevance to CNS drug delivery. J. Cereb. Blood Flow Metab. 2014, 34, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B.; Meier, P.J. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta Biomembr. 2003, 1609, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sajja, R.K.; Naik, P.; Cucullo, L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J. Pharmacovigil. 2014, 2, 125. [Google Scholar] [PubMed]
- Zhao, Y.H.; Li, D.D.; Zhao, J.J.; Song, J.N.; Zhao, Y.L. The role of the low-density lipoprotein receptor-related protein 1 (LRP-1) in regulating blood-brain barrier integrity. Rev. Neurosci. 2016, 27, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pardridge, W.M. Rapid transferrin efflux from brain to blood across the blood-brain barrier. J. Neurochem. 2001, 76, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Bjorbaek, C.; Elmquist, J.K.; Michl, P.; Ahima, R.S.; van Bueren, A.; McCall, A.L.; Flier, J.S. Expression of leptin receptor isoforms in rat brain microvessels. Endocrinology 1998, 139, 3485–3491. [Google Scholar] [CrossRef]
- Moura, R.P.; Martins, C.; Pinto, S.; Sousa, F.; Sarmento, B. Blood-brain barrier receptors and transporters: An insight on their function and how to exploit them through nanotechnology. Expert Opin. Drug Deliv. 2019, 16, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.; Miller, D.W.; Elmquist, W.F. Effect of the P-Glycoprotein Inhibitor, Cyclosporine-A, on the Distribution of Rhodamine-123 to the Brain—An in-vivo Microdialysis Study in Freely Moving Rats. Biochem. Biophys. Res. Commun. 1995, 211, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Sparreboom, A.; vanAsperen, J.; Mayer, U.; Schinkel, A.H.; Smit, J.W.; Meijer, D.K.F.; Borst, P.; Nooijen, W.J.; Beijnen, J.H.; vanTellingen, O. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA 1997, 94, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Tamai, I.; Sakanaka, K.; Sakata, A.; Yamashima, T.; Yamashita, J.; Tsuji, A. In vivo and in vitro evidence for ATP dependency of P-glycoprotein-mediated efflux of doxorubicin at the blood-brain barrier. Biochem. Pharmacol. 1995, 49, 1541–1544. [Google Scholar] [CrossRef]
- Loscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.A.; Hawkins, B.T.; Huber, J.D.; Egleton, R.D.; Davis, T.P. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H738–H743. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Sasaki, H.; Furuse, M.; Tsukita, S. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999, 147, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, S.; Schmidt, T.; Mahn, M.; Florian, P.; Mankertz, J.; Tavalali, S.; Gitter, A.; Schulzke, J.D.; Fromm, M. Contribution of claudin-5 to barrier properties in tight junctions of epithelial cells. Cell Tissue Res. 2005, 321, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Kiang, A.S.; Kenna, P.F.; Kerskens, C.; Blau, C.; O’Dwyer, L.; Tivnan, A.; Kelly, J.A.; Brankin, B.; Farrar, G.J.; et al. RNAi-mediated reversible opening of the blood-brain barrier. J. Gene. Med. 2008, 10, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Berselli, A.; Alberini, G.; Benfenati, F.; Maragliano, L. The impact of pathogenic and artificial mutations on Claudin-5 selectivity from molecular dynamics simulations. Comput. Struct. Biotechnol. J. 2023, 21, 2640–2653. [Google Scholar] [CrossRef] [PubMed]
- Rossa, J.; Ploeger, C.; Vorreiter, F.; Saleh, T.; Protze, J.; Gunzel, D.; Wolburg, H.; Krause, G.; Piontek, J. Claudin-3 and Claudin-5 Protein Folding and Assembly into the Tight Junction Are Controlled by Non-conserved Residues in the Transmembrane 3 (TM3) and Extracellular Loop 2 (ECL2) Segments. J. Biol. Chem. 2014, 289, 7641–7653. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Noell, S.; Mack, A.; Wolburg-Buchholz, K.; Fallier-Becker, P. Brain endothelial cells and the glio-vascular complex. Cell Tissue Res. 2009, 335, 75–96. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Fan, S.S.; Dykstra, H.; Rom, S.; Mercer, A.; Reichenbach, N.L.; Gofman, L.; Persidsky, Y. Inhibition of Glycogen Synthase Kinase 3 beta Promotes Tight Junction Stability in Brain Endothelial Cells by Half-Life Extension of Occludin and Claudin-5. PLoS ONE 2013, 8, e55972. [Google Scholar] [CrossRef]
- Bocsik, A.; Walter, F.R.; Gyebrovszki, A.; Fulop, L.; Blasig, I.; Dabrowski, S.; Otvos, F.; Toth, A.; Rakhely, G.; Veszelka, S.; et al. Reversible Opening of Intercellular Junctions of Intestinal Epithelial and Brain Endothelial Cells With Tight Junction Modulator Peptides. J. Pharm. Sci. 2016, 105, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Yang, Y.S.; Zhang, J.H.; Ji, P.; Du, W.J.; Jiang, P.; Xie, D.H.; Huang, H.D.; Wu, M.; Zhang, G.Z.; et al. Domain-swapped dimerization of the second PDZ domain of ZO2 may provide a structural basis for the polymerization of claudins. J. Biol. Chem. 2007, 282, 35988–35999. [Google Scholar] [CrossRef]
- Torices, S.; Roberts, S.A.; Park, M.; Malhotra, A.; Toborek, M. Occludin, caveolin-1, and Alix form a multi-protein complex and regulate HIV-1 infection of brain pericytes. Faseb J. 2020, 34, 16319–16332. [Google Scholar] [CrossRef]
- Koval, M. Claudin Heterogeneity and Control of Lung Tight Junctions. Ann. Rev. Physiol. 2013, 75, 551–567. [Google Scholar]
- Wollscheid, B.; Bausch-Fluck, D.; Henderson, C.; O’Brien, R.; Bibel, M.; Schiess, R.; Aebersold, R.; Watts, J.D. Mass-spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat. Biotechnol. 2009, 27, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Matsumoto, H.; Zhao, X.M.; Das, S.K.; Paria, B.C. Embryonic signals direct the formation of tight junctional permeability barrier in the decidualizing stroma during embryo implantation. J. Cell Sci. 2004, 117, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, M.; Stephenne, X.; Gilis, A.; Jacquemin, E.; Henrion-Caude, A.; Girard, M.; Gonzales, E.; Revencu, N.; Reding, R.; Wanty, C.; et al. Neonatal Ichthyosis and Sclerosing Cholangitis Syndrome: Extremely Variable Liver Disease Severity from Claudin-1 Deficiency. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Staat, C.; Coisne, C.; Dabrowski, S.; Stamatovic, S.M.; Andjelkovic, A.V.; Wolburg, H.; Engelhardt, B.; Blasig, I.E. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials 2015, 54, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Dithmer, S.; Staat, C.; Muller, C.; Ku, M.C.; Pohlmann, A.; Niendorf, T.; Gehne, N.; Fallier-Becker, P.; Kittel, A.; Walter, F.R.; et al. Claudin peptidomimetics modulate tissue barriers for enhanced drug delivery. Ann. N. Y. Acad. Sci. 2017, 1397, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Tornabene, E.; Helms, H.C.C.; Pedersen, S.F.; Brodin, B. Effects of oxygen-glucose deprivation (OGD) on barrier properties and mRNA transcript levels of selected marker proteins in brain endothelial cells/astrocyte co-cultures. PLoS ONE 2019, 14, e0221103. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin—A novel integral membrane-protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Wong, V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am. J. Physiol. 1997, 273, C1859–C1867. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Higashi, T.; Furuse, M. Localization of Angulin-1/LSR and Tricellulin at Tricellular Contacts of Brain and Retinal Endothelial Cells in vivo. Cell Struct. Funct. 2014, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, C.; Schreivogel, S.; Gunther, R.; Dabrowski, S.; Schumann, M.; Wolburg, H.; Blasig, I.E. Highly Conserved Cysteines Are Involved in the Oligomerization of Occludin-Redox Dependency of the Second Extracellular Loop. Antioxid. Redox Signal. 2014, 20, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Blasig, I.E.; Bellmann, C.; Cording, J.; del Vecchio, G.; Zwanziger, D.; Huber, O.; Haseloff, R.F. Occludin Protein Family: Oxidative Stress and Reducing Conditions. Antioxid. Redox Signal. 2011, 15, 1195–1219. [Google Scholar] [CrossRef] [PubMed]
- Bosse, F.; Hasse, B.; Pippirs, U.; Greiner-Petter, R.; Muller, H.W. Proteolipid plasmolipin: Localization in polarized cells, regulated expression and lipid raft association in CNS and PNS myelin. J. Neurochem. 2003, 86, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Whitney, J.A.; Flores, C.; Gonzalez, S.; Cereijido, M.; Matter, K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996, 134, 1031–1049. [Google Scholar] [CrossRef] [PubMed]
- Hirase, T.; Staddon, J.M.; Saitou, M.; AndoAkatsuka, Y.; Itoh, M.; Furuse, M.; Fujimoto, K.; Tsukita, S.; Rubin, L.L. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell Sci. 1997, 110, 1603–1613. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.C.; Higashi, T.; Fukazawa, Y.; Otani, T.; Tauchi, M.; Higashi, A.Y.; Furuse, M.; Chiba, H. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell 2021, 32, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Ninomiya, T.; Konno, T.; Kohno, T.; Taniguchi, M.; Sawada, N. Expression of tricellulin in epithelial cells and non-epithelial cells. Histol. Histopath. 2013, 28, 1383–1392. [Google Scholar]
- Krug, S.M.; Amasheh, S.; Richter, J.F.; Milatz, S.; Gunzel, D.; Westphal, J.K.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin Forms a Barrier to Macromolecules in Tricellular Tight Junctions without Affecting Ion Permeability. Mol. Biol. Cell 2009, 20, 3713–3724. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef] [PubMed]
- Schulzke, J.D.; Gitter, A.H.; Mankertz, J.; Spiegel, S.; Seidler, U.; Amasheh, S.; Saitou, M.; Tsukita, S.; Fromm, M. Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta Biomembr. 2005, 1669, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.K.; Rueckert, C.; Voss, M.; Mueller, S.L.; Piontek, J.; Gast, K.; Blasig, I.E. The oligomerization of the coiled coil-domain of occludin is redox sensitive. Ann. N. Y. Acad. Sci 2009, 1165, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.M.; Shen, L.; Rajapakse, H.; Raleigh, D.R.; Wang, Y.T.; Wang, Y.M.; Lingaraju, A.; Zha, J.M.; Abbott, E.; McAuley, E.M.; et al. Occludin OCEL-domain interactions are required for maintenance and regulation of the tight junction barrier to macromolecular flux. Mol. Biol. Cell 2013, 24, 3056–3068. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.L.; Portwich, M.; Schmidt, A.; Utepbergenov, D.I.; Huber, O.; Blasig, I.E.; Krause, G. The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J. Biol. Chem. 2005, 280, 3747–3756. [Google Scholar] [CrossRef] [PubMed]
- Dorfel, M.J.; Huber, O. A phosphorylation hotspot within the occludin C-terminal domain. In Barriers and Channels Formed by Tight Junction Proteins I; Fromm, M., Schulzke, J.D., Eds.; Blackwell Science Publishing: Oxford, UK, 2012; Volume 1257, pp. 38–44. [Google Scholar]
- Nusrat, A.; Chen, J.A.; Foley, C.S.; Liang, T.W.; Tom, J.; Cromwell, M.; Quan, C.; Mrsny, R.J. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J. Biol. Chem. 2000, 275, 29816–29822. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.Y.; Krause, E.; Muller, E.C.; Blasig, I.E.; Utepbergenov, D.I. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J. Biol. Chem. 2001, 276, 38480–38486. [Google Scholar] [CrossRef] [PubMed]
- Reiche, J.; Huber, O. Post-translational modifications of tight junction transmembrane proteins and their direct effect on barrier function. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183330. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Chen, Z.; Lin, H.; Li, X. Netrin-1 protects blood-brain barrier (BBB) integrity after cerebral ischemia-reperfusion by activating the Kruppel-like factor 2 (KLF2)/occludin pathway. J. Biochem. Mol. Toxicol. 2024, 38, e23623. [Google Scholar] [CrossRef] [PubMed]
- Tash, B.R.; Bewley, M.C.; Russo, M.; Keil, J.M.; Griffin, K.A.; Sundstrom, J.M.; Antonetti, D.A.; Tian, F.; Flanagan, J.M. The occludin and ZO-1 complex, defined by small angle X-ray scattering and NMR, has implications for modulating tight junction permeability. Proc. Natl. Acad. Sci. USA 2012, 109, 10855–10860. [Google Scholar] [CrossRef] [PubMed]
- Mariano, C.; Palmela, I.; Pereira, P.; Fernandes, A.; Falcao, A.S.; Cardoso, F.L.; Vaz, A.R.; Campos, A.R.; Goncalves-Ferreira, A.; Kim, K.S.; et al. Tricellulin expression in brain endothelial and neural cells. Cell Tissue Res. 2013, 351, 397–407. [Google Scholar] [CrossRef]
- Tachibana, K.; Kondoh, M. A Method to Prepare a Bioprobe for Regulatory Science of the Drug Delivery System to the Brain: An Angulin Binder to Modulate Tricellular Tight Junction-Seal. Methods Mol. Biol. 2021, 2367, 291–304. [Google Scholar] [PubMed]
- Higashi, T.; Tokuda, S.; Kitajiri, S.; Masuda, S.; Nakamura, H.; Oda, Y.; Furuse, M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2-tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J. Cell Sci. 2013, 126, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Izumi, Y.; Oda, Y.; Higashi, T.; Iwamoto, N. Molecular organization of tricellular tight junctions. Tissue Barriers 2014, 2, e28960. [Google Scholar] [CrossRef] [PubMed]
- Mesli, S.; Javorschi, S.; Berard, A.M.; Landry, M.; Priddle, H.; Kivlichan, D.; Smith, A.J.; Yen, F.T.; Bihain, B.E.; Darmon, M. Distribution of the lipolysis stimulated receptor in adult and embryonic murine tissues and lethality of LSR−/− embryos at 12.5 to 14.5 days of gestation. Eur. J. Biochem. 2004, 271, 3103–3114. [Google Scholar] [CrossRef]
- Sohet, F.; Lin, C.; Munji, R.N.; Lee, S.Y.; Ruderisch, N.; Soung, A.; Arnold, T.D.; Derugin, N.; Vexler, Z.S.; Yen, F.T.; et al. LSR/angulin-1 is a tricellular tight junction protein involved in blood-brain barrier formation. J. Cell Biol. 2015, 208, 703–711. [Google Scholar] [CrossRef]
- Sugawara, T.; Furuse, K.; Otani, T.; Wakayama, T.; Furuse, M. Angulin-1 seals tricellular contacts independently of tricellulin and claudins. J. Cell Biol. 2021, 220, e202005062. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Ohtsuki, S.; Hosoya, K.; Nakashima, E.; Terasaki, T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J. Neurochem. 2004, 89, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Savettieri, G.; Di Liegro, I.; Catania, C.; Licata, L.; Pitarresi, G.L.; D’Agostino, S.; Schiera, G.; De Caro, V.; Giandalia, G.; Giannola, L.I.; et al. Neurons and ECM regulate occludin localization in brain endothelial cells. Neuroreport 2000, 11, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Bendriem, R.M.; Singh, S.; Aleem, A.A.; Antonetti, D.A.; Ross, M.E. Tight junction protein occludin regulates progenitor Self-Renewal and survival in developing cortex. eLife 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Shen, L.; Zuo, L.; Shashikanth, N.; Ong, M.; Wu, L.C.; Zha, J.M.; Edelblum, K.L.; Wang, Y.T.; Wang, Y.M.; et al. Inflammation-induced Occludin Downregulation Limits Epithelial Apoptosis by Suppressing Caspase-3 Expression. Gastroenterology 2019, 157, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Bertrand, L.; Luethen, M.; Dabrowski, S.; Lombardi, J.; Morgan, L.; Sharova, N.; Stevenson, M.; Blasig, I.E.; Toborek, M. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J. 2016, 30, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.; Skowronska, M.; Lombardi, J.; He, J.; Seth, N.; Velichkovska, M.; Toborek, M. Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J. Cereb. Blood Flow Metab. 2018, 38, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Fanning, A.S.; Holmes, J.; Anderson, J.M. Occludin is required for cytokine-induced regulation of tight junction barriers. J. Cell Sci. 2010, 123, 2844–2852. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Fanning, A.S.; Anderson, J.M.; Lavie, A. Structure of the conserved cytoplasmic C-terminal domain of occludin: Identification of the ZO-1 binding surface. J. Mol. Biol. 2005, 352, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, Y.; Shepshelovitch, J.; Nevo-Yassaf, I.; Yeheskel, A.; Shmerling, H.; Kwiatek, J.M.; Gaus, K.; Pasmanik-Chor, M.; Hirschberg, K. The MARVEL transmembrane motif of occludin mediates oligomerization and targeting to the basolateral surface in epithelia. J. Cell Sci. 2012, 125, 3545–3556. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.; Mayanil, C.S.; Kim, K.S.; Tomita, T. Angiopoietin-1 Regulates Brain Endothelial Permeability through PTPN-2 Mediated Tyrosine Dephosphorylation of Occludin. PLoS ONE 2015, 10, e0130857. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Lin, C.M.; Keil, J.M.; Sundstrom, J.M.; Smith, C.D.; Antonetti, D.A. Novel atypical PKC inhibitors prevent vascular endothelial growth factor-induced blood-retinal barrier dysfunction. Biochem. J. 2012, 446, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Wiesnet, M.; Marti, H.H.; Renz, D.; Schaper, W. Simultaneous activation of several second messengers in hypoxia-induced hyperpermeability of brain derived endothelial cells. J. Cell. Physiol. 2004, 198, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hori, T.; Ohashi, N.; Baine, A.M.; Eckman, C.B.; Nguyen, J.H. Occludin Is Regulated by Epidermal Growth Factor Receptor Activation in Brain Endothelial Cells and Brains of Mice with Acute Liver Failure. Hepatology 2011, 53, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Raikwar, N.S.; Vandewalle, A.; Thomas, C.P. Nedd4-2 interacts with occludin to inhibit tight junction formation and enhance paracellular conductance in collecting duct epithelia. Am. J. Physiol. Renal Physiol. 2010, 299, F436–F444. [Google Scholar] [CrossRef] [PubMed]
- Traweger, A.; Fang, D.; Liu, Y.C.; Stelzhammer, W.; Krizbai, I.A.; Fresser, F.; Bauer, H.C.; Bauer, H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J. Biol. Chem. 2002, 277, 10201–10208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Tian, Y.; Huang, J.Y.; Tao, R.R.; Liao, M.H.; Lu, Y.M.; Ye, W.F.; Wang, R.; Fukunaga, K.; Lou, Y.J.; et al. The g-Secretase Blocker DAPT Reduces the Permeability of the Blood-Brain Barrier by Decreasing the Ubiquitination and Degradation of Occludin During Permanent Brain Ischemia. CNS Neurosci. Ther. 2013, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Leclair, H.M.; Andre-Gregoire, G.; Treps, L.; Azzi, S.; Bidere, N.; Gavard, J. The E3 ubiquitin ligase MARCH3 controls the endothelial barrier. FEBS Lett. 2016, 590, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human T(H)17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, C.; Krischke, M.; Wegener, J.; Galla, H.J. Tyrosine phosphatase inhibition induces loss of blood-brain barrier integrity by matrix metalloproteinase-dependent and -independent pathways. Brain Res. 2004, 995, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jin, X.C.; Liu, K.J.; Liu, W.L. Matrix Metalloproteinase-2-Mediated Occludin Degradation and Caveolin-1-Mediated Claudin-5 Redistribution Contribute to Blood-Brain Barrier Damage in Early Ischemic Stroke Stage. J. Neurosci. 2012, 32, 3044–3057. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Tanabe, S.; Suzuki, T. Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and occludin expression. Am. J. Physiol. Gastroint. Liver Physiol. 2016, 311, G105–G116. [Google Scholar] [CrossRef] [PubMed]
- Li, R.R.; Qi, Y.N.; Jiang, M.; Zhang, T.H.; Wang, H.W.; Wang, L.G.; Han, M.Y. Primary tumor-secreted VEGF induces vascular hyperpermeability in premetastatic lung via the occludin phosphorylation/ubiquitination pathway. Mol. Carcinog. 2019, 58, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Li, X.; Gao, Y.B.; Wang, S.M.; Zhao, L.; Dong, J.; Yao, B.W.; Xu, X.P.; Chang, G.M.; Zhou, H.M.; et al. Activation of VEGF/Flk-1-ERK Pathway Induced Blood-Brain Barrier Injury after Microwave Exposure. Mol. Neurobiol. 2015, 52, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, A.; Lin, C.M.; Shanmugam, S.; Lindner, H.M.; Abcouwer, S.F.; Antonetti, D.A. Ischemia-reperfusion injury induces occludin phosphorylation/ubiquitination and retinal vascular permeability in a VEGFR-2-dependent manner. J. Cereb. Blood Flow Metab. 2014, 34, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.T.; Burgers, H.F.; Rabie, T.; Marti, H.H. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J. Cereb. Blood Flow Metab. 2010, 30, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Zhao, F.; Cao, Z.P.; Zhang, J.B.; Aschner, M.; Luo, W.J. Mir-203-mediated tricellulin mediates lead-induced in vitro loss of blood-cerebrospinal fluid barrier (BCB) function. Toxicol. Vitr. 2015, 29, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Sasaki, H.; Tsukita, S.; Furuse, M.; Tsukita, S. Loss of Occludin Affects Tricellular Localization of Tricellulin. Mol. Biol. Cell 2008, 19, 4687–4693. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, T.; Sakaguchi, H.; Tamura, A.; Miyashita, T.; Yamazaki, Y.; Tokumasu, R.; Inamoto, R.; Matsubara, A.; Mori, N.; Hisa, Y.; et al. Deletion of Tricellulin Causes Progressive Hearing Loss Associated with Degeneration of Cochlear Hair Cells. Sci. Rep. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Torres, C.; Krug, S.M.; Schulzke, J.D.; Rosenthal, R.; Fromm, M. Tricellulin Effect on Paracellular Water Transport. Int. J. Mol. Sci. 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Cording, J.; Arslan, B.; Staat, C.; Dithmer, S.; Krug, S.M.; Kruger, A.; Berndt, P.; Gunther, R.; Winkler, L.; Blasig, I.E.; et al. Trictide, a tricellulin-derived peptide to overcome cellular barriers. Ann. N. Y. Acad. Sci. 2017, 1405, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, A.; Radusheva, V.; Krug, S.M.; Heinemann, U. Crystal structure of the tricellulin C-terminal coiled-coil domain reveals a unique mode of dimerization. Ann. N. Y. Acad. Sci. 2017, 1405, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Otani, T.; Ikenouchi, J.; Furuse, M. Tricellulin regulates junctional tension of epithelial cells at tricellular contacts through Cdc42. J. Cell Sci. 2014, 127, 4201–4212. [Google Scholar] [PubMed]
- Sumitomo, T.; Nakata, M.; Higashino, M.; Yamaguchi, M.; Kawabata, S. Group A Streptococcus exploits human plasminogen for bacterial translocation across epithelial barrier via tricellular tight junctions. Sci. Rep. 2016, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Jennek, S.; Mittag, S.; Reiche, J.; Westphal, J.K.; Seelk, S.; Dorfel, M.J.; Pfirrmann, T.; Friedrich, K.; Schutz, A.; Heinemann, U.; et al. Tricellulin is a target of the ubiquitin ligase Itch. Ann. N. Y. Acad. Sci. 2017, 1397, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Takasawa, A.; Murata, M.; Takasawa, K.; Ono, Y.; Osanai, M.; Tanaka, S.; Nojima, M.; Kono, T.; Hirata, K.; Kojima, T.; et al. Nuclear localization of tricellulin promotes the oncogenic property of pancreatic cancer. Sci. Rep. 2016, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Morampudi, V.; Graef, F.A.; Stahl, M.; Dalwadi, U.; Conlin, V.S.; Huang, T.; Vallance, B.A.; Yu, H.B.; Jacobson, K. Tricellular Tight Junction Protein Tricellulin Is Targeted by the Enteropathogenic Escherichia coli Effector EspG1, Leading to Epithelial Barrier Disruption. Infect. Immun. 2017, 85, 20. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Bojarski, C.; Fromm, A.; Lee, I.M.; Dames, P.; Richter, J.F.; Turner, J.R.; Fromm, M.; Schulzke, J.D. Tricellulin is regulated via interleukin-13-receptor alpha 2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018, 11, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Vishnevskaya, O.N.; Okorokova, L.S.; Fedorova, A.A.; Kruglova, N.M.; Rybalchenko, O.V.; Aschenbach, J.R.; Amasheh, S. Cholera toxin perturbs the paracellular barrier in the small intestinal epithelium of rats by affecting claudin-2 and tricellulin. Pflugers Arch. 2019, 471, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Eum, S.Y.; Jaraki, D.; Bertrand, L.; Andras, I.E.; Toborek, M. Disruption of epithelial barrier by quorum-sensing N-3-(oxododecanoyl)-homoserine lactone is mediated by matrix metalloproteinases. Am. J. Physiol. Gastroint. Liver Physiol. 2014, 306, G992–G1001. [Google Scholar] [CrossRef] [PubMed]
- Janke, S.; Mittag, S.; Reiche, J.; Huber, O. Apoptotic Fragmentation of Tricellulin. Int. J. Mol. Sci. 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, M.; Sapone, A.; Senger, S.; Camhi, S.S.; Kadzielski, S.M.; Buie, T.M.; Kelly, D.L.; Cascella, N.; Fasano, A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol. Autism 2016, 7, 49. [Google Scholar] [CrossRef]
- Severson, E.A.; Jiang, L.; Ivanov, A.I.; Mandell, K.J.; Nusrat, A.; Parkos, C.A. Cis-dimerization mediates function of junctional adhesion molecule A. Mol. Biol. Cell 2008, 19, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Sasaki, H.; Furuse, M.; Ozaki, H.; Kita, T.; Tsukita, S. Junctional adhesion molecule (JAM) binds to PAR-3: A possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell Biol. 2001, 154, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bazzoni, G.; Martinez-Estrada, O.M.; Mueller, F.; Nelboeck, P.; Schmid, G.; Bartfai, T.; Dejana, E.; Brockhaus, M. Homophilic interaction of junctional adhesion molecule. J. Biol. Chem. 2000, 275, 30970–30976. [Google Scholar] [CrossRef]
- Lamagna, C.; Meda, P.; Mandicourt, G.; Brown, J.; Gilbert, R.J.C.; Jones, E.Y.; Kiefer, F.; Ruga, P.; Imhof, B.A.; Aurrand-Lions, M. Dual interaction of JAM-C with JAM-B and alpha(M)beta(2) integrin: Function in junctional complexes and leukocyte adhesion. Mol. Biol. Cell 2005, 16, 4992–5003. [Google Scholar] [CrossRef] [PubMed]
- Yeung, D.; Manias, J.L.; Stewart, D.J.; Nag, S. Decreased junctional adhesion molecule-A expression during blood-brain barrier breakdown. Acta Neuropathol. 2008, 115, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Aurrand-Lions, M.; Johnson-Leger, C.; Wong, C.; Du Pasquier, L.; Imhof, B.A. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood 2001, 98, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Martin, T.A.; Zhang, G.B.; Jiang, W.G. Junctional Adhesion Molecules in Cerebral Endothelial Tight Junction and Brain Metastasis. Anticancer Res. 2013, 33, 2353–2359. [Google Scholar] [PubMed]
- Kummer, D.; Ebnet, K. Junctional Adhesion Molecules (JAMs): The JAM-Integrin Connection. Cells 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.U.; Naik, U.P. Junctional adhesion molecule-A-induced endothelial cell migration on vitronectin is integrin alpha(v)beta(3) specific. J. Cell Sci. 2006, 119, 490–499. [Google Scholar] [CrossRef]
- Ostermann, G.; Weber, K.S.C.; Zernecke, A.; Schroder, A.; Weber, C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat. Immunol. 2002, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Santoso, S.; Sachs, U.J.H.; Kroll, H.; Linder, M.; Ruf, A.; Preissner, K.T.; Chavakis, T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J. Exp. Med. 2002, 196, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Tornavaca, O.; Chia, M.; Dufton, N.; Almagro, L.O.; Conway, D.E.; Randi, A.M.; Schwartz, M.A.; Matter, K.; Balda, M.S. ZO-1 controls endothelial adherens junctions, cell-cell tension, angiogenesis, and barrier formation. J. Cell Biol. 2015, 208, 821–838. [Google Scholar] [CrossRef]
- Bazzoni, G. The JAM family of junctional adhesion molecules. Curr. Opin. Cell Biol. 2003, 15, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Ebnet, K. Junctional adhesion molecules (JAMs): Cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol. Rev. 2017, 97, 1529–1554. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kundu, R.K.; Yang, E.; Hirata, K.; Ho, Y.D.; Quertermous, T. Targeted disruption of endothelial cell-selective adhesion molecule inhibits angiogenic processes in vitro and in vivo. J. Biol. Chem. 2003, 278, 34598–34604. [Google Scholar] [CrossRef] [PubMed]
- Wegmann, F.; Petri, B.; Khandoga, A.G.; Moser, C.; Khandoga, A.; Volkery, S.; Li, H.; Nasdala, I.; Brandau, O.; Fassler, R.; et al. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J. Exp. Med. 2006, 203, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.N.; Nottebaum, A.F.; Butz, S.; Volkery, S.; Zeuschner, D.; Stehling, M.; Vestweber, D. Interference with ESAM (Endothelial Cell-Selective Adhesion Molecule) Plus Vascular Endothelial-Cadherin Causes Immediate Lethality and Lung-Specific Blood Coagulation. Arter. Thromb. Vasc. Biol. 2020, 40, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1—A high-molecular-weight polypeptide associated with the tight junction (Zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Stevenson, B.R.; Jesaitis, L.A.; Goodenough, D.A.; Mooseker, M.S. Characterization of ZO-1, a Protein-Component of the Tight Junction from Mouse Liver and Madin-Darby Canine Kidney Cells. J. Cell Biol. 1988, 106, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Jesaitis, L.A.; Goodenough, D.A. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the drosophila disks-large tumor-suppressor protein. J. Cell Biol. 1994, 124, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Haskins, J.; Gu, L.J.; Wittchen, E.S.; Hibbard, J.; Stevenson, B.R. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 1998, 141, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Umeda, K.; Tsukita, S.; Furuse, M.; Tsukita, S. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J. Cell Biol. 2007, 176, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Giepmans, B.N.G.; Moolenaar, W.H. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 1998, 8, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Anderson, J.M. Zonula Occludens-1 and-2 Are Cytosolic Scaffolds That Regulate the Assembly of Cellular Junctions. In Molecular Structure and Function of the Tight Junction: From Basic Mechanisms to Clinical Manifestations; Fromm, M., Schulzke, J.D., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2009; Volume 1165, pp. 113–120. [Google Scholar]
- Fanning, A.S.; Lye, M.F.; Anderson, J.M.; Lavie, A. Domain swapping within PDZ2 is responsible for dimerization of ZO proteins. J. Biol. Chem. 2007, 282, 37710–37716. [Google Scholar] [CrossRef] [PubMed]
- Balda, M.S.; Matter, K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000, 19, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Bal, M.S.; Castro, V.; Piontek, J.; Rueckert, C.; Walter, J.K.; Shymanets, A.; Kurig, B.; Haase, H.; Nurnberg, B.; Blasig, I.E. The hinge region of the scaffolding protein of cell contacts, zonula occludens protein 1, regulates interacting with various signaling proteins. J. Cell. Biochem. 2012, 113, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, Y.; Cahill, H.; Yu, M.; Badea, T.C.; Smallwood, P.M.; Peachey, N.S.; Nathans, J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 2009, 139, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Ma, T.Y.; Anderson, J.M. Isolation and functional characterization of the actin-binding region in the tight junction protein ZO-1. Faseb J. 2002, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, C.J.; Arpin, M.; Fanning, A.S.; Louvard, D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc. Natl. Acad. Sci. USA 1996, 93, 10779–10784. [Google Scholar] [CrossRef]
- Traweger, A.; Fuchs, R.; Krizbai, I.A.; Weiger, T.M.; Bauer, H.C.; Bauer, H. The tight junction protein ZO-2 localizes to the nucleus and interacts with the heterogeneous nuclear ribonucleoprotein scaffold attachment factor-B. J. Biol. Chem. 2003, 278, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Ikenouchi, J.; Katahira-Tayama, S.; Furuse, K.; Sasaki, H.; Nakayama, M.; Matsui, T.; Tsukita, S.; Furuse, M.; Tsukita, S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 2006, 126, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Van Itallie, C.M.; Anderson, J.M. Zonula occludens-1 and-2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol. Biol. Cell 2012, 23, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Huxham, J.; Tabaries, S.; Siegel, P.M. Afadin (AF6) in cancer progression: A multidomain scaffold protein with complex and contradictory roles. Bioessays 2020, 17, e2000221. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, E.; Sluysmans, S.; Bochaton-Piallat, M.L.; Citi, S. Cell-specific diversity in the expression and organization of cytoplasmic plaque proteins of apical junctions. Ann. N. Y. Acad. Sci. 2017, 1405, 160–176. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, Y.L.; Liang, P.; Li, L.S.; Zhou, Y.D.; Zhang, W.D.; Wang, D.F.; Wei, G.H. PI3K/AKT/Afadin signaling pathway contributes to pathological vascularization in glioblastomas. Oncol. Lett. 2018, 15, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Coureuil, M.; Mikaty, G.; Miller, F.; Lecuyer, H.; Bernard, C.; Bourdoulous, S.; Dumenil, G.; Mege, R.M.; Weksler, B.B.; Romero, I.A.; et al. Meningococcal Type IV Pili Recruit the Polarity Complex to Cross the Brain Endothelium. Science 2009, 325, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Schwaninger, M. Apicobasal polarity of brain endothelial cells. J. Cereb. Blood Flow Metab. 2016, 36, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Wang, Y.; Smallwood, P.M.; Williams, J.; Nathans, J. Dlg1 activates beta-catenin signaling to regulate retinal angiogenesis and the blood-retina and blood-brain barriers. eLife 2019, 8, e45542. [Google Scholar] [CrossRef] [PubMed]
- Sewduth, R.N.; Kovacic, H.; Jaspard-Vinassa, B.; Jecko, V.; Wavasseur, T.; Fritsch, N.; Pernot, M.; Jeaningros, S.; Roux, E.; Dufourcq, P.; et al. PDZRN3 destabilizes endothelial cell-cell junctions through a PKC zeta-containing polarity complex to increase vascular permeability. Sci. Signal. 2017, 10, eaag3209. [Google Scholar] [CrossRef] [PubMed]
- Chrifi, I.; Hermkens, D.; Brandt, M.M.; van Dijk, C.G.M.; Burgisser, P.E.; Haasdijk, R.; Pei, J.Y.; van de Kamp, E.H.M.; Zhu, C.B.; Blonden, L.; et al. Cgnl1, an endothelial junction complex protein, regulates GTPase mediated angiogenesis. Cardiovasc. Res. 2017, 113, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.D.; Witt, K.A.; Hom, S.; Egleton, R.D.; Mark, K.S.; Davis, T.P. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1241–H1248. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Kealy, J.; Humphries, M.M.; Gong, Y.; Hou, J.; Hudson, N.; Cassidy, L.M.; Martiniano, R.; Shashi, V.; Hooper, S.R.; et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol. Psychiatr. 2018, 23, 2156–2166. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.J. Human-Induced Pluripotent Stem Cell-Based Model of the Blood-Brain at 10 Years: A Retrospective on Past and Current Disease Models. Handb. Exp. Pharmacol. 2023, 281, 141–156. [Google Scholar] [PubMed]
- Biron, K.E.; Dickstein, D.L.; Gopaul, R.; Jefferies, W.A. Amyloid Triggers Extensive Cerebral Angiogenesis Causing Blood Brain Barrier Permeability and Hypervascularity in Alzheimer’s Disease. PLoS ONE 2011, 6, e23789. [Google Scholar] [CrossRef] [PubMed]
- Hartz, A.M.S.; Bauer, B.; Soldner, E.L.B.; Wolf, A.; Boy, S.; Backhaus, R.; Mihaljevic, I.; Bogdahn, U.; Klunemann, H.H.; Schuierer, G.; et al. Amyloid-beta Contributes to Blood-Brain Barrier Leakage in Transgenic Human Amyloid Precursor Protein Mice and in Humans With Cerebral Amyloid Angiopathy. Stroke 2012, 43, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Oikari, L.E.; Pandit, R.; Stewart, R.; Cuni-Lopez, C.; Quek, H.; Sutharsan, R.; Rantanen, L.M.; Oksanen, M.; Lehtonen, S.; de Boer, C.M.; et al. Altered Brain Endothelial Cell Phenotype from a Familial Alzheimer Mutation and Its Potential Implications for Amyloid Clearance and Drug Delivery. Stem Cell Rep. 2020, 14, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.I.; Cayrol, R.; Prat, A. Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2011, 1812, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.S.; Beers, D.R.; Wen, S.; Bowser, R.; Appel, S.H. Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology 2009, 72, 1614–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Deane, R.; Ali, Z.; Parisi, M.; Shapovalov, Y.; O’Banion, M.K.; Stojanovic, K.; Sagare, A.; Boillee, S.; Cleveland, D.W.; et al. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat. Neurosci. 2008, 11, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.T.; Woulfe, J.M. Striatal blood-brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow Metab. 2015, 35, 747–750. [Google Scholar] [CrossRef]
- Wu, X.L.; Wang, P.; Liu, Y.H.; Xue, Y.X. Effects of poly (ADP-ribose) polymerase inhibitor 3-aminobenzamide on blood-brain barrier and dopaminergic neurons of rats with lipopolysaccharide-induced Parkinson’s disease. J. Mol. Neurosci. 2014, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Ouellet, J.; Sawiak, S.J.; Cisbani, G.; Lagace, M.; Kuan, W.L.; Saint-Pierre, M.; Dury, R.J.; Alata, W.; St-Amour, I.; Mason, S.L.; et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann. Neurol. 2015, 78, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Linville, R.M.; Nerenberg, R.F.; Grifno, G.; Arevalo, D.; Guo, Z.; Searson, P.C. Brain microvascular endothelial cell dysfunction in an isogenic juvenile iPSC model of Huntington’s disease. Fluids Barriers CNS 2022, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Reschke, C.R.; Reddy, A.; Mae, M.A.; Connolly, R.; Behan, C.; O’Keeffe, E.; Bolger, I.; Hudson, N.; et al. Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat. Commun. 2022, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Kealy, J.; Greene, C.; Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. 2020, 726, 14. [Google Scholar] [CrossRef]
- On, N.H.; Mitchell, R.; Savant, S.D.; Bachmeier, C.J.; Hatch, G.M.; Miller, D.W. Examination of blood-brain barrier (BBB) integrity in a mouse brain tumor model. J. Neuro Oncol. 2013, 111, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.J.; Tan, Y.A.; Dai, S.H.; Zhu, Y.; Meng, T.T.; Yang, X.Q.; Liu, Y.P.; Liu, X.; Yuan, H.; Hu, F.Q. VEGF-mediated tight junctions pathological fenestration enhances doxorubicin-loaded glycolipid-like nanoparticles traversing BBB for glioblastoma-targeting therapy. Drug Deliv. 2017, 24, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Higashida, T.; Kreipke, C.W.; Rafols, J.A.; Peng, C.Y.; Schafer, S.; Schafer, P.; Ding, J.Y.; Dornbos, D.; Li, X.H.; Guthikonda, M.; et al. The role of hypoxia-inducible factor-la, aquaporin-4, and matrix metalloproteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injury Laboratory investigation. J. Neurosurg. 2011, 114, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Jungner, M.; Siemund, R.; Venturoli, D.; Reinstrup, P.; Schalen, W.; Bentzer, P. Blood-brain barrier permeability following traumatic brain injury. Minerva Anestesiol. 2016, 82, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Hanrahan, F.; Gobbo, O.L.; Kelly, M.E.; Kiang, A.S.; Humphries, M.M.; Nguyen, A.T.H.; Ozaki, E.; Keaney, J.; Blau, C.W.; et al. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat. Commun. 2012, 3, 849. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cao, Y.; Chang, S. An inhibitor of claudin-5 interactions, M01, alleviates neuroinflammation and vasogenic edema after blood-spinal cord barrier dysfunction. NeuroReport 2023, 34, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Huang, K.; Mikulis, D.; Silver, F.; Kassner, A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS ONE 2017, 12, e0171558. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.X.; Wang, Z.H.; Liu, Y.H.; Wang, P.; Xue, Y.X. Specific Role of Tight Junction Proteins Claudin-5, Occludin, and ZO-1 of the Blood-Brain Barrier in a Focal Cerebral Ischemic Insult. J. Mol. Neurosci. 2011, 44, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.M.; Taher, M.; Chin, K.Y.; Grace, M.; McIntyre, P.; Miller, A.A. Characterisation of a mouse cerebral microvascular endothelial cell line (bEnd.3) after oxygen glucose deprivation and reoxygenation. Clin. Exp. Pharmacol. Physiol. 2016, 43, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Venkat, P.; Chopp, M.; Chen, J. Blood-Brain Barrier Disruption, Vascular Impairment, and Ischemia/Reperfusion Damage in Diabetic Stroke. J. Am. Heart Assoc. 2017, 6, e005819. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, C.; Meng, Z.; Gan, L.; Guo, R.; Liu, J.; Bond Lau, W.; Xie, D.; Zhao, J.; Lopez, B.L.; et al. C1q/TNF-Related Protein 3 Prevents Diabetic Retinopathy via AMPK-Dependent Stabilization of Blood-Retinal Barrier Tight Junctions. Cells 2022, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Arba, F.; Leigh, R.; Inzitari, D.; Warach, S.J.; Luby, M.; Lees, K.R.; Collaboration, S.V.I. Blood-brain barrier leakage increases with small vessel disease in acute ischemic stroke. Neurology 2017, 89, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Al-Thani, M.; Goodwin-Trotman, M.; Bell, S.; Patel, K.; Fleming, L.K.; Vilain, C.; Abramowicz, M.; Allan, S.M.; Wang, T.; Cader, M.Z.; et al. A novel human iPSC model of COL4A1/A2 small vessel disease unveils a key pathogenic role of matrix metalloproteinases. Stem Cell Rep. 2023, 18, 2386–2399. [Google Scholar] [CrossRef] [PubMed]
- El-Khouly, F.E.; Haumann, R.; Breur, M.; Veldhuijzen van Zanten, S.E.M.; Kaspers, G.J.L.; Hendrikse, N.H.; Hulleman, E.; van Vuurden, D.G.; Bugiani, M. The neurovascular unit in diffuse intrinsic pontine gliomas. Free Neuropathol. 2021, 2, 2–17. [Google Scholar] [PubMed]
- Li, C.; Jiang, Z.; Lu, W.; Arrick, D.; McCarter, K.; Sun, H. Effect of obesity on early blood-brain barrier disruption following transient focal cerebral ischemia. Obes. Sci. Pract. 2016, 2, 58–68. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.Y.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, A.E.; Buist, R.; Del Bigio, M.R.; Toft-Hansen, H.; Khorooshi, R.; Owens, T.; Peeling, J. Blood-brain barrier disruption in CCL2 transgenic mice during pertussis toxin-induced brain inflammation. Fluids Barriers CNS 2012, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xue, Y.X.; Liu, L.B.; Liu, Y.H. Endothelial-monocyte-activating polypeptide II increases blood-tumor barrier permeability by down-regulating the expression levels of tight junction associated proteins. Brain Res. 2010, 1319, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, L.; Yang, X.; Li, M.; Zhao, Q.; Wu, H. Irisin improves BBB dysfunction in SAP rats by inhibiting MMP-9 via the ERK/NF-kappaB signaling pathway. Cell. Signal. 2022, 93, 110300. [Google Scholar] [CrossRef]
- Leda, A.R.; Bertrand, L.; Andras, I.E.; El-Hage, N.; Nair, M.; Toborek, M. Selective Disruption of the Blood-Brain Barrier by Zika Virus. Front. Microbiol. 2019, 10, 2158. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, U.; Singh, S.K. Zika Virus NS1 Suppresses VE-Cadherin and Claudin-5 via hsa-miR-101-3p in Human Brain Microvascular Endothelial Cells. Mol. Neurobiol. 2021, 58, 6290–6303. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Paul, D.; Pachter, J.S. Alterations in tight junction protein and IgG permeability accompany leukocyte extravasation across the choroid plexus during neuroinflammation. J. Neuropathol. Exp. Neurol. 2014, 73, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Huggins, M.A.; Johnson, H.L.; Jin, F.; N’Songo, A.; Hanson, L.M.; LaFrance, S.J.; Butler, N.S.; Harty, J.T.; Johnson, A.J. Perforin Expression by CD8 T Cells Is Sufficient To Cause Fatal Brain Edema during Experimental Cerebral Malaria. Infect. Immun. 2017, 85, e00985-16. [Google Scholar] [CrossRef] [PubMed]
- Endres, L.M.; Jungblut, M.; Divyapicigil, M.; Sauer, M.; Stigloher, C.; Christodoulides, M.; Kim, B.J.; Schubert-Unkmeir, A. Development of a multicellular in vitro model of the meningeal blood-CSF barrier to study Neisseria meningitidis infection. Fluids Barriers CNS 2022, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Connolly, R.; Brennan, D.; Laffan, A.; O’Keeffe, E.; Zaporojan, L.; O’Callaghan, J.; Thomson, B.; Connolly, E.; Argue, R.; et al. Blood-brain barrier disruption and sustained systemic inflammation in individuals with long COVID-associated cognitive impairment. Nat. Neurosci. 2024, 27, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Amruta, N.; Ismael, S.; Leist, S.R.; Gressett, T.E.; Srivastava, A.; Dinnon, K.H., III; Engler-Chiurazzi, E.B.; Maness, N.J.; Qin, X.; Kolls, J.K.; et al. Mouse Adapted SARS-CoV-2 (MA10) Viral Infection Induces Neuroinflammation in Standard Laboratory Mice. Viruses 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.T.; Dehghani, G.A. Acute hypertension induces brain injury and blood-brain barrier disruption through reduction of claudins mRNA expression in rat. Pathol. Res. Pract. 2014, 210, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Araiz, A.; Porcu, F.; Perez-Hernandez, M.; Garcia-Gutierrez, M.S.; Aracil-Fernandez, M.A.; Gutierrez-Lopez, M.D.; Guerri, C.; Manzanares, J.; O’Shea, E.; Colado, M.I. Disruption of blood-brain barrier integrity in postmortem alcoholic brain: Preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict. Biol. 2017, 22, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Costea, L.; Meszaros, A.; Bauer, H.; Bauer, H.C.; Traweger, A.; Wilhelm, I.; Farkas, A.E.; Krizbai, I.A. The Blood-Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int. J. Mol. Sci. 2019, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Hirayama, R.; Sato, N.; Hattori, K.; Kato, T.; Takeda, H.; Kondoh, M. Association of Plasma Claudin-5 with Age and Alzheimer Disease. Int. J. Mol. Sci. 2024, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Wang, C.; Zhang, J.; Qu, L.; Liu, X.; Lu, Y.; Yang, W.; Deng, J.; Lorenz, D.; Gao, P.; et al. Interferon-gamma safeguards blood-brain barrier during experimental autoimmune encephalomyelitis. Am. J. Pathol. 2014, 184, 3308–3320. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of Brain Metastases. Curr. Oncol. Rep. 2012, 14, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Koyama, Y. Pathogenesis of Brain Edema and Investigation into Anti-Edema Drugs. Int. J. Mol. Sci. 2015, 16, 9949–9975. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, K.E.; Witt, K.A. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 2008, 32, 200–219. [Google Scholar] [CrossRef]
- Tian, Y.; Milic, J.; Monasor, L.S.; Chakraborty, R.; Wang, S.; Yuan, Y.; Asare, Y.; Behrends, C.; Tahirovic, S.; Bernhagen, J. The COP9 signalosome reduces neuroinflammation and attenuates ischemic neuronal stress in organotypic brain slice culture model. Cell. Mol. Life Sci. 2023, 80, 262. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, L.; Qu, M.; Liang, H.; Li, W.; Li, Y.; Deng, L.; Zhang, Z.; Yang, G.Y. Mesenchymal stem cells attenuate blood-brain barrier leakage after cerebral ischemia in mice. J. Neuroinflamm. 2018, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Duan, X.; Liu, Y.; Li, F.; Ma, S.; Shang, X.; Wang, X.; Liu, Y.; Xue, R.; Qin, Z. Tie2-expressing monocytes/macrophages promote cerebral revascularization in peri-infarct lesions upon ischemic insult. Signal Transduct. Target. Ther. 2021, 6, 295. [Google Scholar] [CrossRef] [PubMed]
- Easton, A.S.; Fraser, P.A. Variable restriction of albumin diffusion across inflamed cerebral microvessels of the anaesthetized rat. J. Physiol. 1994, 475, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Breitkreuz-Korff, O.; Tscheik, C.; Del Vecchio, G.; Dithmer, S.; Walther, W.; Orthmann, A.; Wolburg, H.; Haseloff, R.F.; Schroder, L.; Blasig, I.E.; et al. M01 as a novel drug enhancer for specifically targeting the blood-brain barrier. J. Control. Release 2021, 338, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Humphries, M.M.; Kiang, A.S.; Nguyen, A.T.; Gobbo, O.L.; Tam, L.C.; Suzuki, M.; Hanrahan, F.; Ozaki, E.; Farrar, G.J.; et al. Systemic low-molecular weight drug delivery to pre-selected neuronal regions. EMBO Mol. Med. 2011, 3, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Shirakura, K.; Okada, Y.; Takeda, H.; Endo, K.; Tamura, M.; Watari, A.; Sadamura, Y.; Sawasaki, T.; Doi, T.; et al. Claudin-5-Binders Enhance Permeation of Solutes across the Blood-Brain Barrier in a Mammalian Model. J. Pharmacol. Exp. Ther. 2017, 363, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.H.; Quay, S.C. Advances in nasal drug delivery through tight junction technology. Expert Opin. Drug Deliv. 2005, 2, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.E.; Makienko, E.G.; Prieve, M.G.; Fuller, M.; Houston, M.E.; Johnson, P.H. Phage display screening of epithelial cell monolayers treated with EGTA: Identification of peptide FDFWITP that modulates tight junction activity. J. Biomol. Screen 2007, 12, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Eiting, K.; Cui, K.Y.; Leonard, A.K.; Morris, D.; Li, C.Y.; Farber, K.; Sileno, A.P.; Houston, M.E.; Johnson, P.H.; et al. Therapeutic utility of a novel tight junction modulating peptide for enhancing intranasal drug delivery. J. Pharm. Sci. 2006, 95, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Zeniya, S.; Kuwahara, H.; Daizo, K.; Watari, A.; Kondoh, M.; Yoshida-Tanaka, K.; Kaburagi, H.; Asada, K.; Nagata, T.; Nagahama, M.; et al. Angubindin-1 opens the blood-brain barrier in vivo for delivery of antisense oligonucleotide to the central nervous system. J. Control. Release 2018, 283, 126–134. [Google Scholar] [CrossRef] [PubMed]
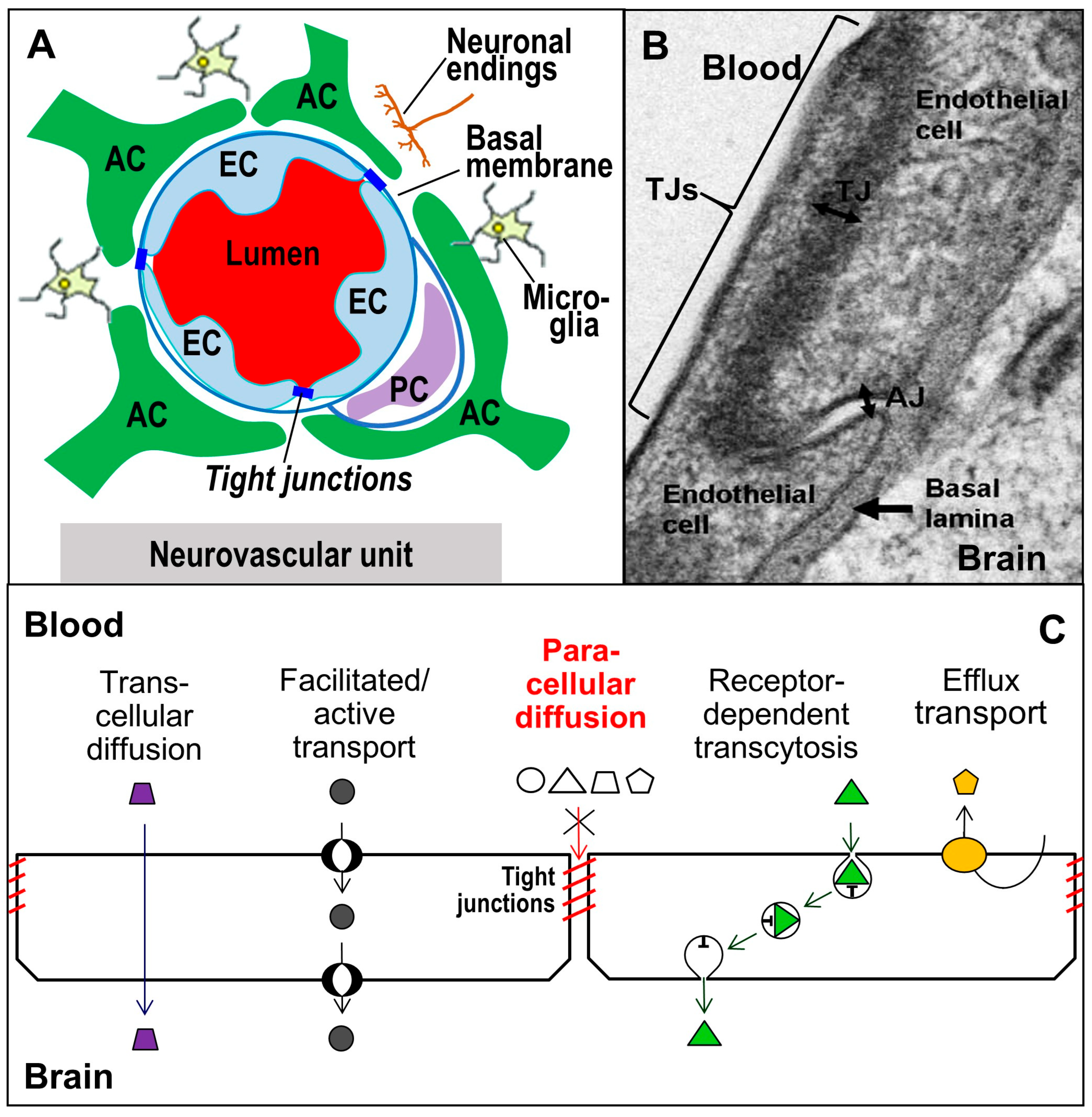

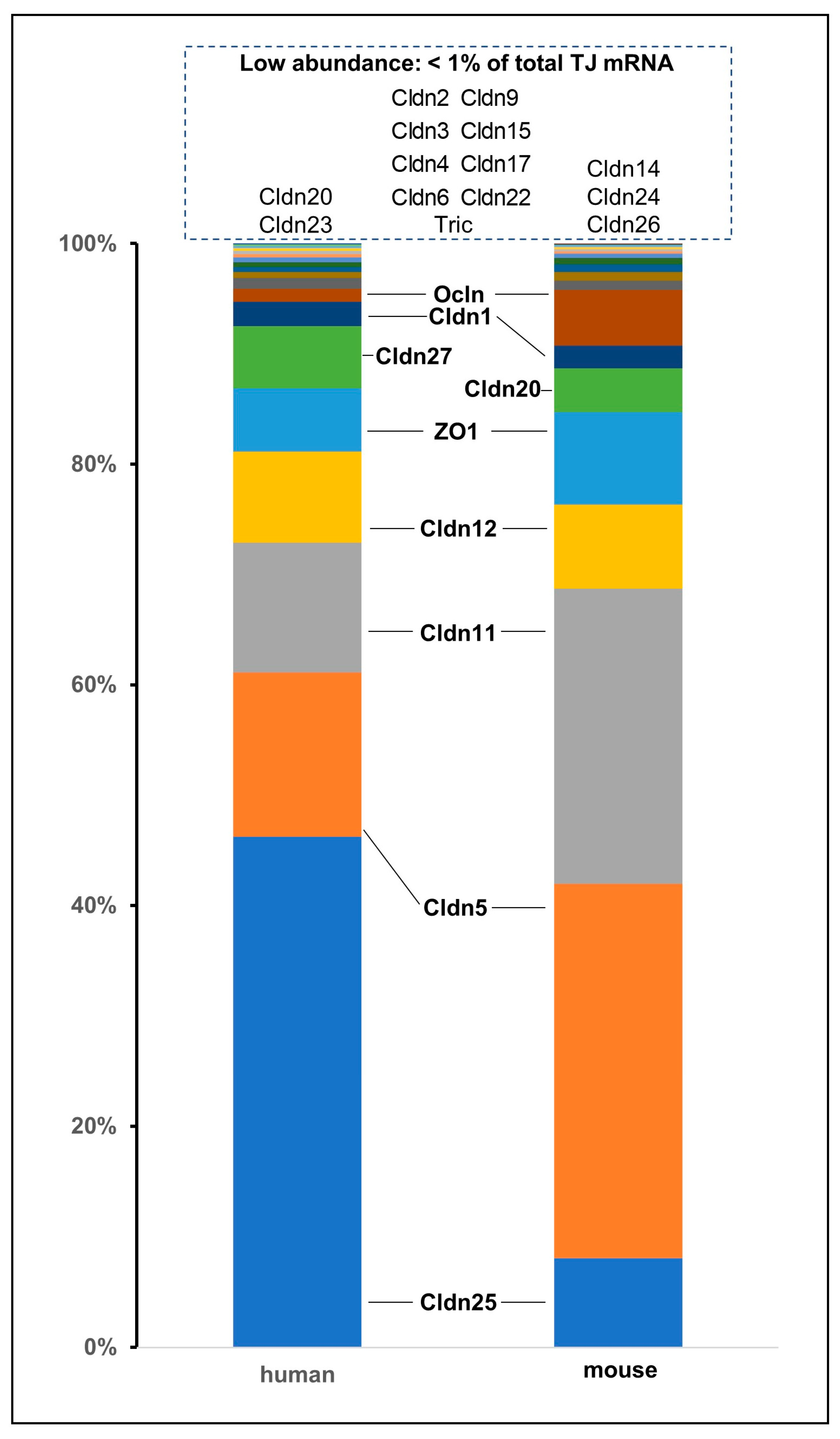
| Type of Disorder | Leakage of Blood-Brain Barrier | Tight Junction Alteration |
|---|---|---|
| neurodegeneration | - m. Alzheimer mouse model [319,320]; iBEC layer, mutant transfected [321] | - ß-amyloid triggered angiogenesis → Cldn1, -5 down-regulated [319,320] → Cldn3, -5 up-regulated [321] |
| - multiple sclerosis [322] - EAE [228] | → down-regulated: Cldn3 (EAE) [78], Cldn11 (patient, EAE/mouse) [130] → angulin-1 down, 3-cell contacts [228] | |
| - amyotrophic lateral sclerosis [323] | - mouse model → BCSFB: loss of Cldn5, Ocln, ZO-1 [324] | |
| - m. Parkinson, extravasation in striatum [325] | - rat model → Cldn5, Ocln, ZO-1 up-regulated (substantia nigra) [326] | |
| - chorea Huntington, human and mouse model [327,328] | → Occl, ZO-1 reduced in iBEC [328] | |
| epilepsy | - cainic acid-induced seizures, temporal lope epilepsy [329] | - resected brain → Cldn5, Ocln, ZO-1 reduced [329] |
| psychiatric disorders | - schizophrenia, autism spectrum disorder (ADS), affective disorders [330] - ADS, cortex | → Cldn12, Ocln, ZO-1 down-regulated [330] → Cldn3, -5, -12, Tric up [271] |
| - schizophrenia associated with Cldn5 gene variant [314] | → lessens Cldn5 in BEC; antipsychotic drug enhances Cldn5 [314] | |
| brain tumours | - advanced tumour grades [78,93,331] | → reduction of Cldn1, -3, -5, Ocln (glioblastoma) [78,80,332] |
| traumatic brain injury | - rat model [333] - patients [334] - Cldn5-si/shRNA enhanced leakage, reduced swelling [335] | →Ocln, ZO-1 reduced [333] → Cldn5 reversibly down-regulated [335] → ZO-1 degradation [336] |
| ischemia/stroke | - acute ischemic stroke, human [337] - ischemia/reperfusion, mouse [129] | - at clinical worsening → Cldn5, Cldn5:ZO-1 ratio increased in blood [127] |
| - middle cerebral artery occlusion [228,338] | → 3 h: Cldn1, -3, -12, Ocln dropped, but Cldn5 rose [50,53]; 5 d: Cldn5, Ocln, ZO-1 reduced [338] → angulin-1 down, 3-cell contact [228] | |
| - hypoxia/glucose lack, BEC [200] | → Cldn5, Ocln, ZO-1 down [339] | |
| - haemorrhage [76,104] | → Cldn25 down-regulated (BEC) [76] → Cldn3, -5 down; improvement/reduced leak by anti-malaria drug [104] | |
| - reinforced by | - d. mellitus [340] - BRB leakage in murine diabetic retinopathy [341] - microangiopathy: small vessel disease, stroke imaging [342]; iBEC layer, mutant transfected [343] | → Cldn5, Ocln depressed [341] → Cldn5 and ZO-1 expression reduced (autopsy samples) [344] → Cldn5-, Ocln-junctions affected [343] |
| high-fat diet | - diet-induced obese diabetic mice [141]; obesity [345] | → Cldn12, Ocln, ZO-1 reduced [141] |
| inflammation | - thrombin-caused, BEC layer [239] - astrocyte-derived VEGF [346] | → Ocln-ZO-1 binding lost [239] → Cldn5 decrease in BEC [332] |
| - peripheral (CFA) [182,347,348] - pancreatitis [349] | → Ocln down/Cldn3, -5 up (CFA) [182] → degradation of ZO-1, Cldn5 [349] | |
| infection | - Zika virus in mice [350] | → Cldn5 down [350], via miR-101-3p [351] |
| - bacterial pertussis toxin [347] | → ZO-1 down (BCSFB) [352] | |
| - Plasmodium falciparum [353] - Neisseria meningitidis, iBEC [354] - long COVID patients [355] | → Cldn5, Ocln down (mouse) [353] → Cldn5, Ocln down (cell layer) [354] → Cldn5 down (mouse) [356] | |
| hypertonia | - acute in rat [357] | → Cldn3, -5, -12 depression [357] |
| alcohol abuse | - hippocampal IgG extravasation [358] | → Cldn5 down [358] |
| microcephaly | - small molecule flux higher [115] | → human Cldn5-G60R (ECL1) [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dithmer, S.; Blasig, I.E.; Fraser, P.A.; Qin, Z.; Haseloff, R.F. The Basic Requirement of Tight Junction Proteins in Blood-Brain Barrier Function and Their Role in Pathologies. Int. J. Mol. Sci. 2024, 25, 5601. https://doi.org/10.3390/ijms25115601
Dithmer S, Blasig IE, Fraser PA, Qin Z, Haseloff RF. The Basic Requirement of Tight Junction Proteins in Blood-Brain Barrier Function and Their Role in Pathologies. International Journal of Molecular Sciences. 2024; 25(11):5601. https://doi.org/10.3390/ijms25115601
Chicago/Turabian StyleDithmer, Sophie, Ingolf E. Blasig, Paul A. Fraser, Zhihai Qin, and Reiner F. Haseloff. 2024. "The Basic Requirement of Tight Junction Proteins in Blood-Brain Barrier Function and Their Role in Pathologies" International Journal of Molecular Sciences 25, no. 11: 5601. https://doi.org/10.3390/ijms25115601
APA StyleDithmer, S., Blasig, I. E., Fraser, P. A., Qin, Z., & Haseloff, R. F. (2024). The Basic Requirement of Tight Junction Proteins in Blood-Brain Barrier Function and Their Role in Pathologies. International Journal of Molecular Sciences, 25(11), 5601. https://doi.org/10.3390/ijms25115601





