Nanostructure-Mediated Transport of Therapeutics through Epithelial Barriers
Abstract
1. Introduction
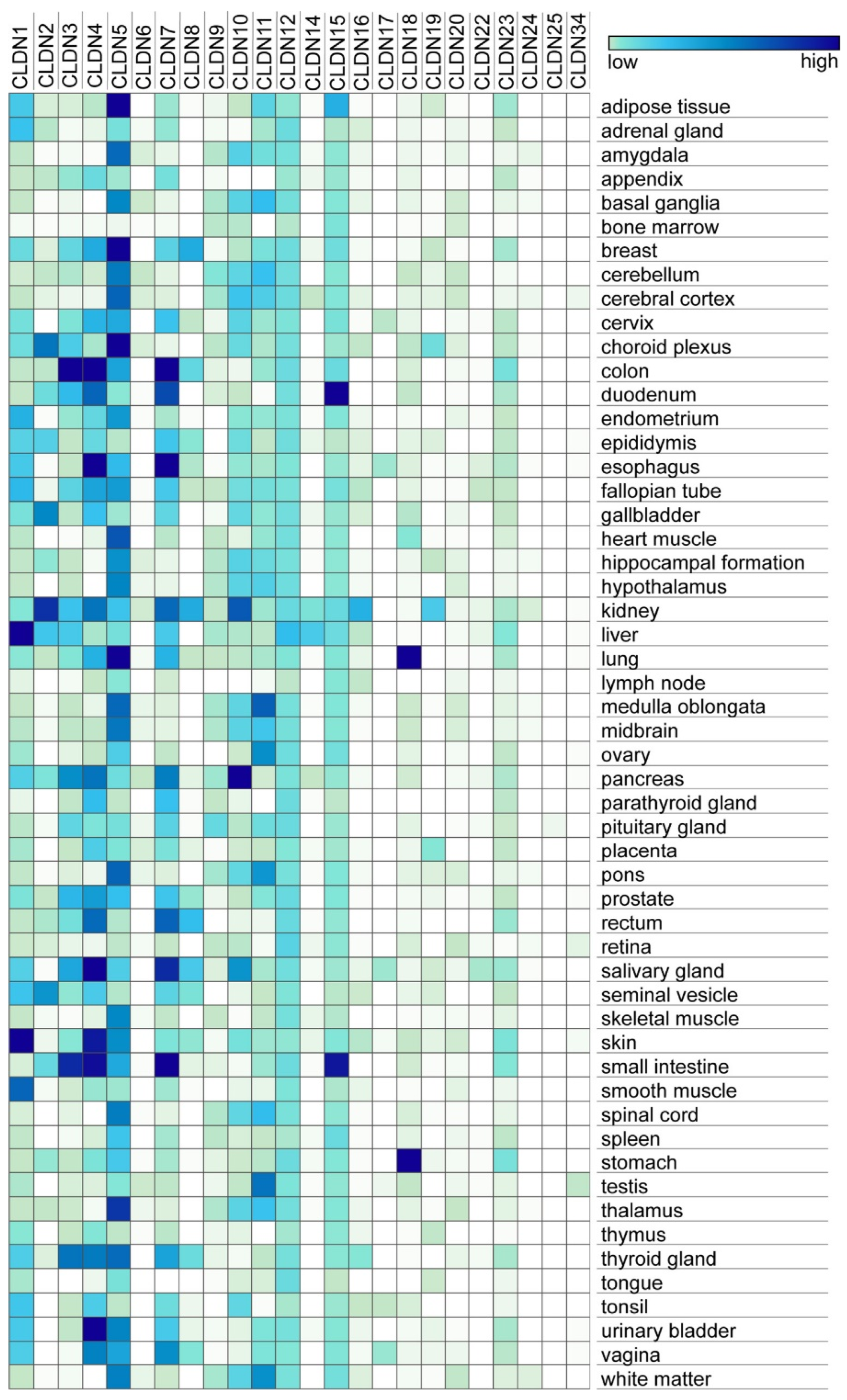
2. Tight Junctions and Paracellular Diffusion
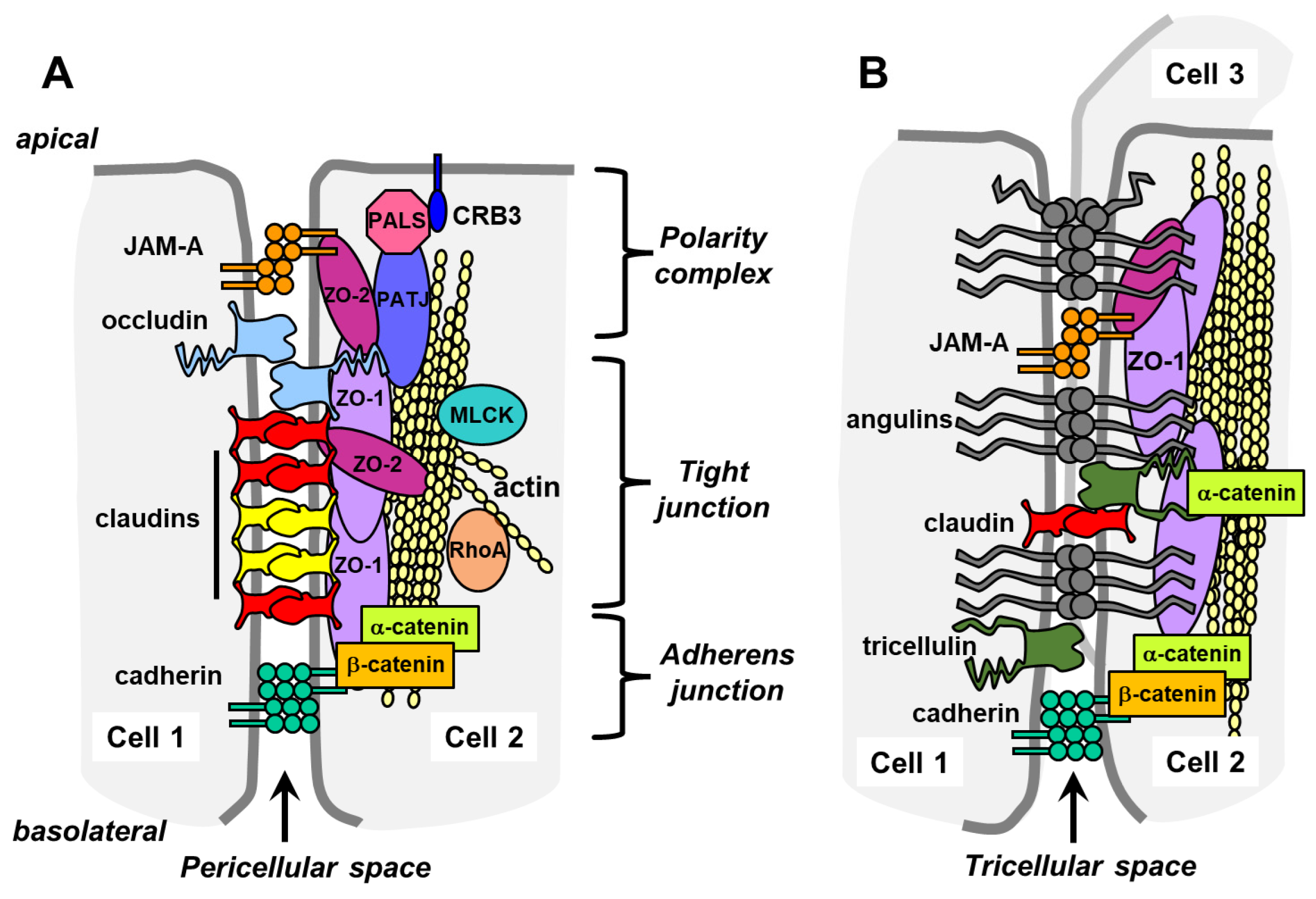
2.1. Transmembrane Tight Junction Proteins
2.2. Tricellular Junctions
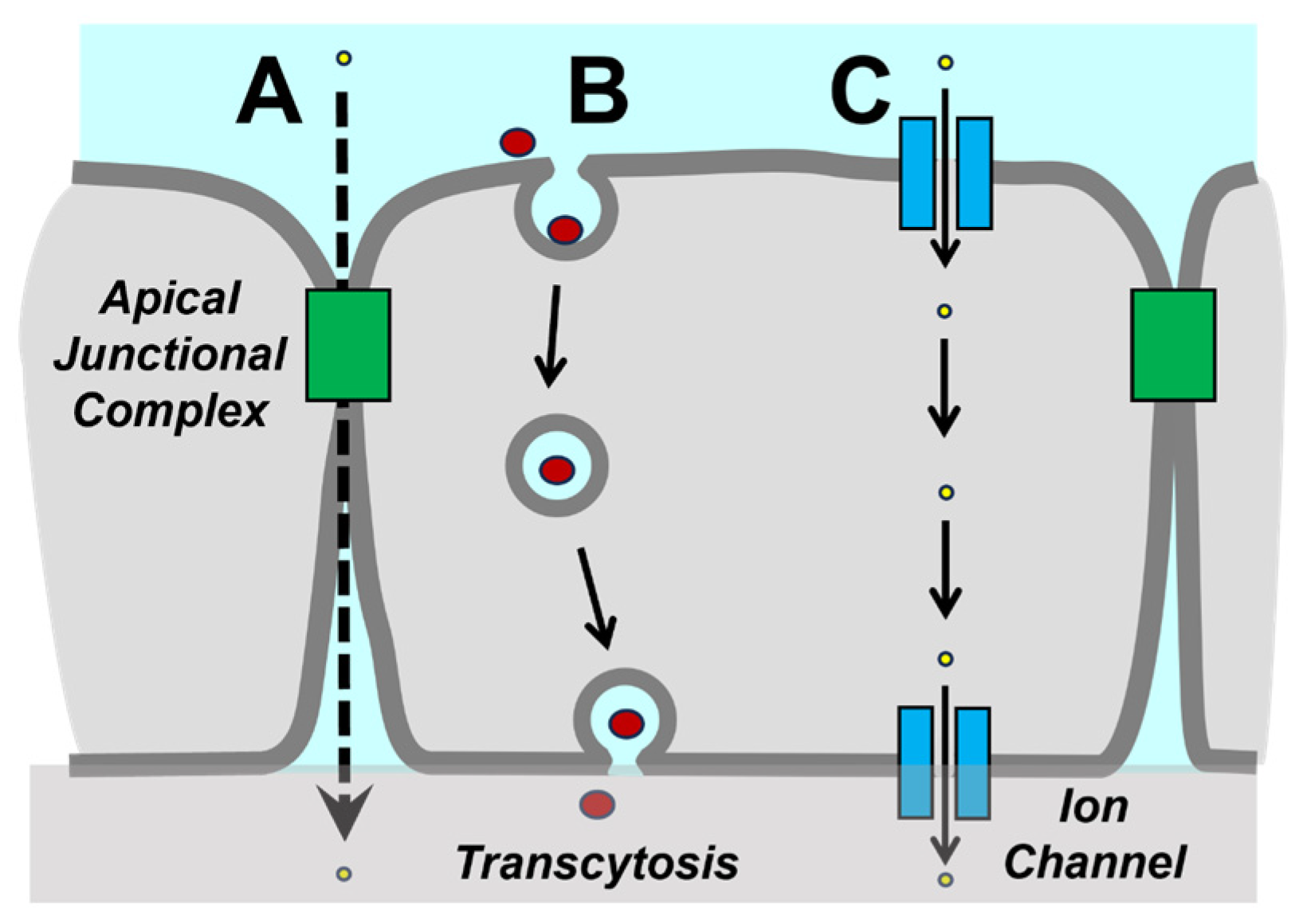
3. Transcytosis
4. Transdermal Drug Delivery
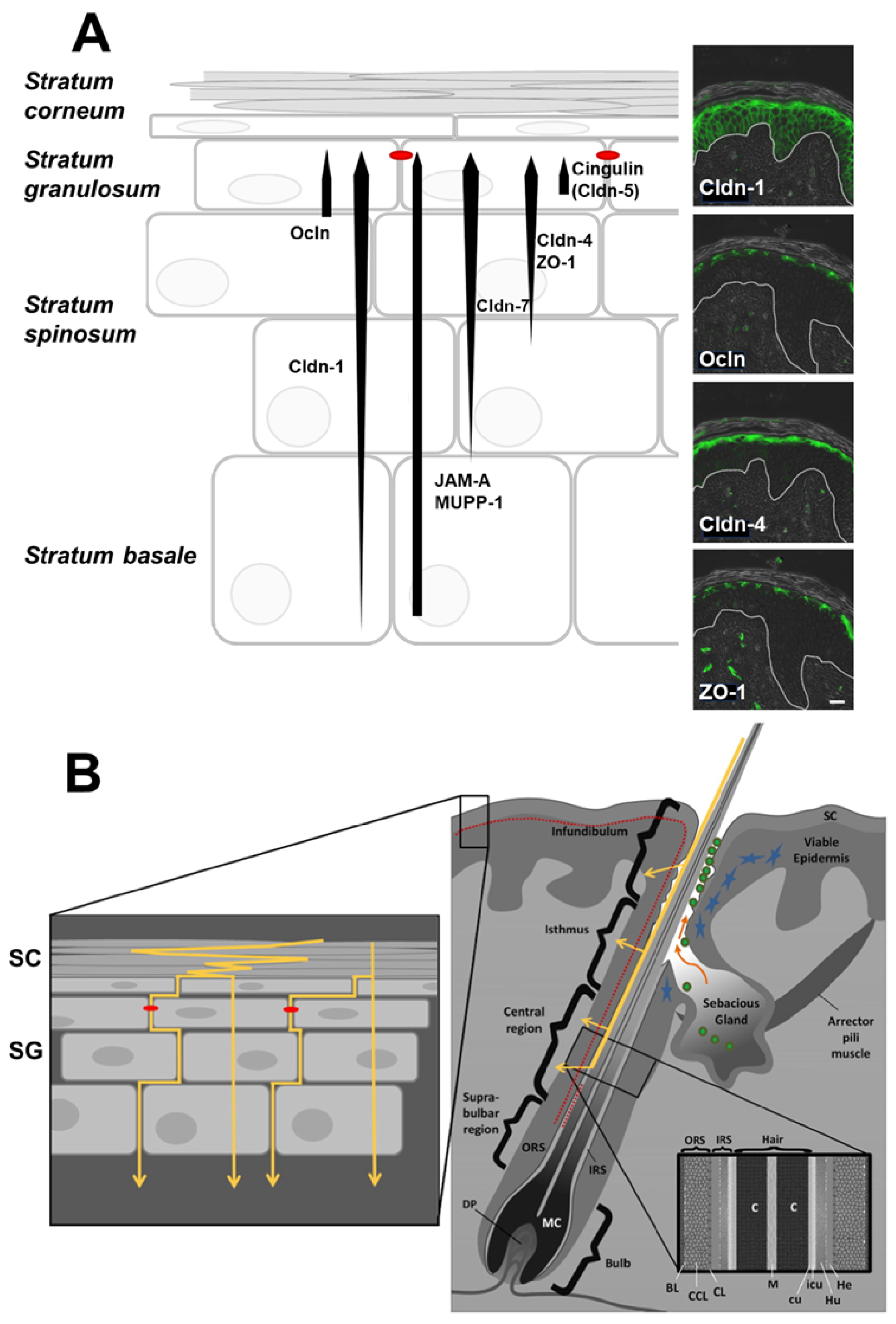
4.1. Topical Agents and Microneedles
4.2. Nanohydrogels
4.3. Inorganic Nanoparticles
4.4. Chitosan-Coated Nanoparticles
4.5. Lipid-Based Nanoparticles
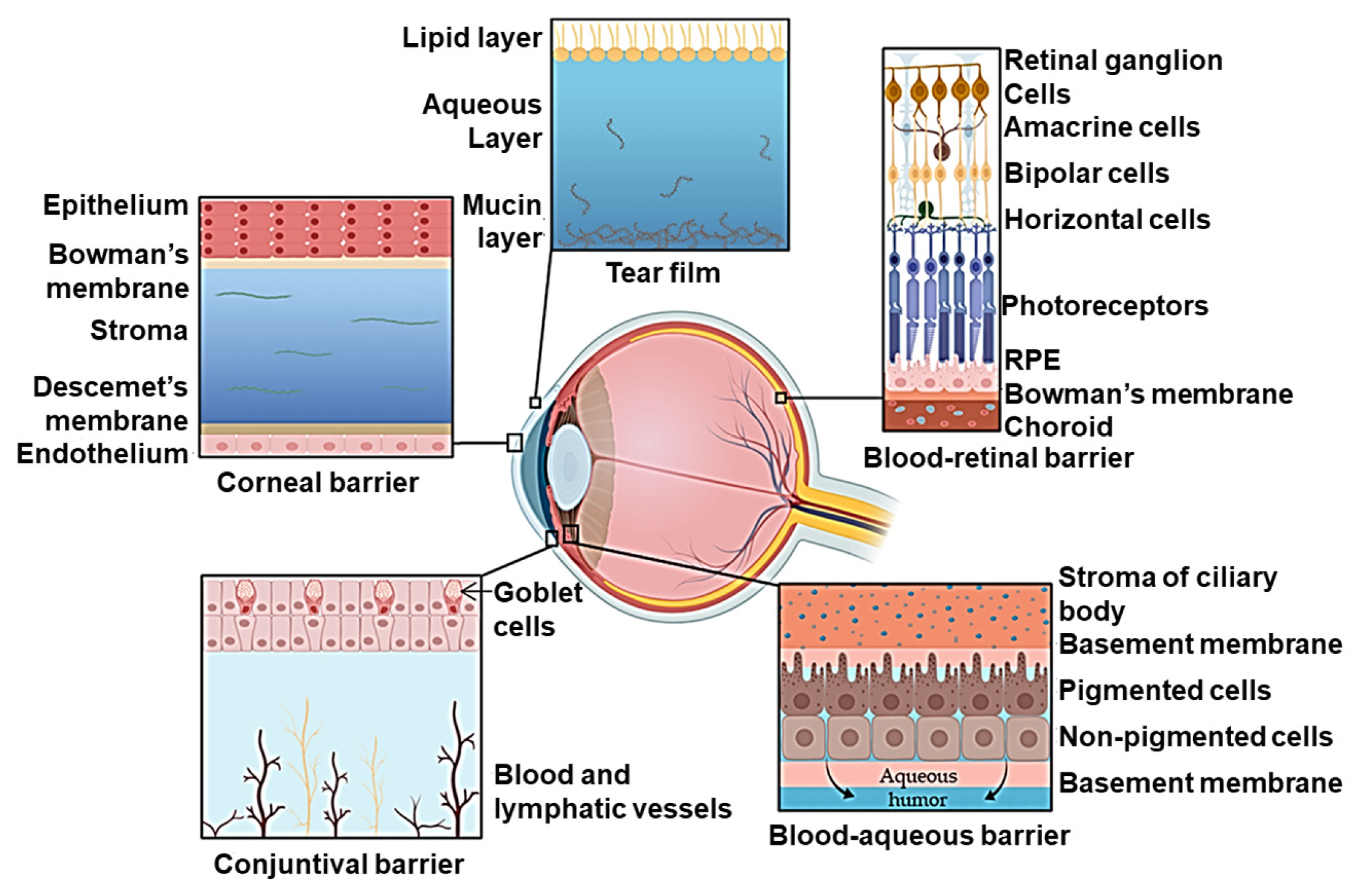
5. Ocular Drug Delivery
5.1. Topical Solutions and Implant-Based Drug Delivery
5.2. Optimizing Nanocarriers for Ocular Drug Delivery
5.3. Charged and Coated Nanomicelles
5.4. Polymeric Nanoparticles
5.5. Inorganic Nanoparticles
5.6. Lipid-Based Nanoparticles
6. Pulmonary Drug Delivery
6.1. Nanoscale Materials for Pulmonary Delivery
6.2. Mesoporous Silica and Calcium Phosphate Nanoparticles
6.3. Nanoliposomes and Nanomicelles
7. Oral Drug Delivery
7.1. Particle Geometry and Physical Characteristics
7.2. Inorganic Nanoparticles
7.3. Chitosan and Polymer Derivatized Nanoparticles
7.4. Targeted Nanoparticles and Dendrimers
7.5. Permeation Enhancers
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Marchiando, A.M.; Graham, W.V.; Turner, J.R. Epithelial barriers in homeostasis and disease. Annu. Rev. Pathol. 2010, 5, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Capaldo, C.T.; Farkas, A.E.; Nusrat, A. Epithelial adhesive junctions. F1000Prime Rep. 2014, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Anderson, J.M. Architecture of tight junctions and principles of molecular composition. Semin. Cell Dev. Biol. 2014, 36, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Vogelmann, R.; Nelson, W.J. Fractionation of the epithelial apical junctional complex: Reassessment of protein distributions in different substructures. Mol. Biol. Cell 2005, 16, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Nusrat, A. RhoGTPases, actomyosin signaling and regulation of the epithelial Apical Junctional Complex. Semin. Cell Dev. Biol. 2014, 36, 194–203. [Google Scholar] [CrossRef]
- Wibbe, N.; Ebnet, K. Cell Adhesion at the Tight Junctions: New Aspects and New Functions. Cells 2023, 12, 2701. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Furuse, M.; Takai, Y. Recent advances in understanding tight junctions. Fac. Rev. 2021, 10, 18. [Google Scholar] [CrossRef]
- Piontek, J.; Krug, S.M.; Protze, J.; Krause, G.; Fromm, M. Molecular architecture and assembly of the tight junction backbone. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183279. [Google Scholar] [CrossRef] [PubMed]
- Mandel, L.J.; Bacallao, R.; Zampighi, G. Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature 1993, 361, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.; Chavez Munguia, B.; Gonzalez-Mariscal, L. ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp. Cell Res. 2007, 313, 1533–1547. [Google Scholar] [CrossRef] [PubMed]
- Koval, M. Junctional Interplay in Lung Epithelial Barrier Function. In Lung Epithelial Biology in the Pathogenesis of Pulmonary Disease; Sidhaye, V.K., Koval, M., Eds.; Academic Press: Oxford, UK, 2017; pp. 1–20. [Google Scholar]
- Furuse, M.; Izumi, Y.; Oda, Y.; Higashi, T.; Iwamoto, N. Molecular organization of tricellular tight junctions. Tissue Barriers 2014, 2, e28960. [Google Scholar] [CrossRef]
- Krug, S.M.; Amasheh, S.; Richter, J.F.; Milatz, S.; Gunzel, D.; Westphal, J.K.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 2009, 20, 3713–3724. [Google Scholar] [CrossRef]
- Citi, S. The mechanobiology of tight junctions. Biophys. Rev. 2019, 11, 783–793. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Lechuga, S.; Marino-Melendez, A.; Naydenov, N.G. Unique and redundant functions of cytoplasmic actins and nonmuscle myosin II isoforms at epithelial junctions. Ann. N. Y. Acad. Sci. 2022, 1515, 61–74. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Rosenthal, R.; Gunzel, D.; Piontek, J.; Krug, S.M.; Ayala-Torres, C.; Hempel, C.; Theune, D.; Fromm, M. Claudin-15 forms a water channel through the tight junction with distinct function compared to claudin-2. Acta Physiol. 2020, 228, e13334. [Google Scholar] [CrossRef]
- Lynn, K.S.; Peterson, R.J.; Koval, M. Ruffles and spikes: Control of tight junction morphology and permeability by claudins. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183339. [Google Scholar] [CrossRef]
- Suarez-Artiles, L.; Breiderhoff, T.; Girardello, R.; Gonschior, H.; Rodius, S.; Lesur, A.; Reimer, U.; Ramberger, E.; Perez-Hernandez, D.; Muller, D.; et al. Pan-claudin family interactome analysis reveals shared and specific interactions. Cell Rep. 2022, 41, 111588. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Koval, M.; Ranganathan, S.; Fanayan, S.; Hancock, W.S.; Lundberg, E.K.; Beavis, R.C.; Lane, L.; Duek, P.; McQuade, L.; et al. Systems Proteomics View of the Endogenous Human Claudin Protein Family. J. Proteome Res. 2016, 15, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hernandez, V.; Quiros, M.; Nusrat, A. Intestinal epithelial claudins: Expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017, 1397, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Gonschior, H.; Schmied, C.; Van der Veen, R.E.; Eichhorst, J.; Himmerkus, N.; Piontek, J.; Gunzel, D.; Bleich, M.; Furuse, M.; Haucke, V.; et al. Nanoscale segregation of channel and barrier claudins enables paracellular ion flux. Nat. Commun. 2022, 13, 4985. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Renigunta, V.; Zhou, Y.; Sunq, A.; Wang, J.; Yang, J.; Renigunta, A.; Baker, L.A.; Hou, J. Biochemical and biophysical analyses of tight junction permeability made of claudin-16 and claudin-19 dimerization. Mol. Biol. Cell 2015, 26, 4333–4346. [Google Scholar] [CrossRef] [PubMed]
- Raya-Sandino, A.; Lozada-Soto, K.M.; Rajagopal, N.; Garcia-Hernandez, V.; Luissint, A.C.; Brazil, J.C.; Cui, G.; Koval, M.; Parkos, C.A.; Nangia, S.; et al. Claudin-23 reshapes epithelial tight junction architecture to regulate barrier function. Nat. Commun. 2023, 14, 6214. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Velez, I.; Belardi, B. Storming the gate: New approaches for targeting the dynamic tight junction for improved drug delivery. Adv. Drug Deliv. Rev. 2023, 199, 114905. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.C.; Higashi, T.; Fukazawa, Y.; Otani, T.; Tauchi, M.; Higashi, A.Y.; Furuse, M.; Chiba, H. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell 2021, 32, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Avila-Flores, A.; Betanzos, A. The relationship between structure and function of tight junctions. In Tight Junctions, 2nd ed.; Anderson, J.M., Cereijido, M., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 89–120. [Google Scholar]
- Monteiro, A.C.; Sumagin, R.; Rankin, C.R.; Leoni, G.; Mina, M.J.; Reiter, D.M.; Stehle, T.; Dermody, T.S.; Schaefer, S.A.; Hall, R.A.; et al. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol. Biol. Cell 2013, 24, 2849–2860. [Google Scholar] [CrossRef]
- Otani, T.; Nguyen, T.P.; Tokuda, S.; Sugihara, K.; Sugawara, T.; Furuse, K.; Miura, T.; Ebnet, K.; Furuse, M. Claudins and JAM-A coordinately regulate tight junction formation and epithelial polarity. J. Cell Biol. 2019, 218, 3372–3396. [Google Scholar] [CrossRef]
- Higashi, T.; Tokuda, S.; Kitajiri, S.; Masuda, S.; Nakamura, H.; Oda, Y.; Furuse, M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2--tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J. Cell Sci. 2013, 126, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Torres, C.; Krug, S.M.; Rosenthal, R.; Fromm, M. Angulin-1 (LSR) Affects Paracellular Water Transport, However Only in Tight Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 7827. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Furuse, K.; Otani, T.; Wakayama, T.; Furuse, M. Angulin-1 seals tricellular contacts independently of tricellulin and claudins. J. Cell Biol. 2021, 220, e202005062. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.; Koval, W.T.; Molina, S.A.; Bock, S.M.; Lillard, J.W., Jr.; Ross, R.F.; Desai, T.A.; Koval, M. Calibrated flux measurements reveal a nanostructure-stimulated transcytotic pathway. Exp. Cell Res. 2017, 355, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.Y.; Fairn, G.D.; Lee, W.L. Transcellular vesicular transport in epithelial and endothelial cells: Challenges and opportunities. Traffic 2018, 19, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Kennedy, P.J.; Santos, H.A.; Barrias, C.; Sarmento, B. A comprehensive review of the neonatal Fc receptor and its application in drug delivery. Pharmacol. Ther. 2016, 161, 22–39. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishii-Watabe, A.; Tada, M.; Kobayashi, T.; Kanayasu-Toyoda, T.; Kawanishi, T.; Yamaguchi, T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 2010, 184, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.T.; Aveson, V.G. Neonatal Fc receptor and IgG-based therapeutics. MAbs 2011, 3, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Sleep, D.; Cameron, J.; Evans, L.R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta 2013, 1830, 5526–5534. [Google Scholar] [CrossRef]
- Kitamura, T.; Garofalo, R.P.; Kamijo, A.; Hammond, D.K.; Oka, J.A.; Caflisch, C.R.; Shenoy, M.; Casola, A.; Weigel, P.H.; Goldblum, R.M. Human intestinal epithelial cells express a novel receptor for IgA. J. Immunol. 2000, 164, 5029–5034. [Google Scholar] [CrossRef]
- Wei, H.; Wang, J.Y. Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis. Int. J. Mol. Sci. 2021, 22, 2284. [Google Scholar] [CrossRef] [PubMed]
- Jerdeva, G.V.; Tesar, D.B.; Huey-Tubman, K.E.; Ladinsky, M.S.; Fraser, S.E.; Bjorkman, P.J. Comparison of FcRn- and pIgR-mediated transport in MDCK cells by fluorescence confocal microscopy. Traffic 2010, 11, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Cervenak, J.; Doleschall, M.; Bender, B.; Mayer, B.; Schneider, Z.; Doleschall, Z.; Zhao, Y.; Bosze, Z.; Hammarstrom, L.; Oster, W.; et al. NFkappaB induces overexpression of bovine FcRn: A novel mechanism that further contributes to the enhanced immune response in genetically modified animals carrying extra copies of FcRn. MAbs 2013, 5, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, F.; Qian, S.; Bi, D.; He, Q.; Jin, H.; Luo, R.; Li, S.; Meng, X.; Li, Z. TGEV infection up-regulates FcRn expression via activation of NF-kappaB signaling. Sci. Rep. 2016, 6, 32154. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, R.; Qian, S.; Qiao, C.; Liu, X.; Zhou, Z.; Li, Z. Clostridium butyricum CB1 up-regulates FcRn expression via activation of TLR2/4-NF-kappaB signaling pathway in porcine small intestinal cells. Vet. Immunol. Immunopathol. 2021, 240, 110317. [Google Scholar] [CrossRef] [PubMed]
- Zaid Alkilani, A.; Hamed, R.; Musleh, B.; Sharaire, Z. Breaking boundaries: The advancements in transdermal delivery of antibiotics. Drug Deliv. 2024, 31, 2304251. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Candi, E.; Schmidt, R.; Melino, G. The cornified envelope: A model of cell death in the skin. Nat. Rev. Mol. Cell Biol. 2005, 6, 328–340. [Google Scholar] [CrossRef]
- Kirschner, N.; Brandner, J.M. Barriers and more: Functions of tight junction proteins in the skin. Ann. N. Y. Acad. Sci. 2012, 1257, 158–166. [Google Scholar] [CrossRef]
- Yoshida, K.; Yokouchi, M.; Nagao, K.; Ishii, K.; Amagai, M.; Kubo, A. Functional tight junction barrier localizes in the second layer of the stratum granulosum of human epidermis. J. Dermatol. Sci. 2013, 71, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Iwamoto, N.; Akashi, M.; Kojima, T.; Hisatsune, J.; Sugai, M.; Furuse, M. Tight junction dysfunction in the stratum granulosum leads to aberrant stratum corneum barrier function in claudin-1-deficient mice. J. Dermatol. Sci. 2013, 70, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Huber, M.; Fellmann, F.; Beckmann, J.S.; Frenk, E.; Hohl, D. Confirmation of the origin of NISCH syndrome. Hum. Mutat. 2006, 27, 408–410. [Google Scholar] [CrossRef] [PubMed]
- Basler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The role of tight junctions in skin barrier function and dermal absorption. J. Control. Release 2016, 242, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zorn-Kruppa, M.; Vidal, Y.S.S.; Houdek, P.; Wladykowski, E.; Grzybowski, S.; Gruber, R.; Gorzelanny, C.; Harcup, J.; Schneider, S.W.; Majumdar, A.; et al. Tight Junction barriers in human hair follicles—Role of claudin-1. Sci. Rep. 2018, 8, 12800. [Google Scholar] [CrossRef] [PubMed]
- Mathes, C.; Brandner, J.M.; Laue, M.; Raesch, S.S.; Hansen, S.; Failla, A.V.; Vidal, S.; Moll, I.; Schaefer, U.F.; Lehr, C.M. Tight junctions form a barrier in porcine hair follicles. Eur. J. Cell Biol. 2016, 95, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Basler, K.; Brandner, J.M. Tight junctions in skin inflammation. Pflugers Arch. 2017, 469, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Suresh, P.; Patnaik, S. Physical means of stratum corneum barrier manipulation to enhance transdermal drug delivery. Curr. Drug Deliv. 2015, 12, 122–138. [Google Scholar] [CrossRef]
- Del Rio-Sancho, S.; Pan Delgado, D.; de la Fuente, G.F.; Garcia-Caballero, T.; Taboada-Suarez, A.; Csaba, N.; Bao-Varela, C.; Jose Alonso, M. Laser-induced transient skin disruption to enhance cutaneous drug delivery. Eur. J. Pharm. Biopharm. 2020, 156, 165–175. [Google Scholar] [CrossRef]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Velasquez, F.C.; Kwon, S.; Azhdarinia, A.; Pinkston, K.; Harvey, B.R.; Chan, W.; Rasmussen, J.C.; Ross, R.F.; Fife, C.E.; et al. Lymphatic delivery of etanercept via nanotopography improves response to collagen-induced arthritis. Arthritis Res. Ther. 2017, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Velasquez, F.C.; Rasmussen, J.C.; Greives, M.R.; Turner, K.D.; Morrow, J.R.; Hwu, W.J.; Ross, R.F.; Zhang, S.; Sevick-Muraca, E.M. Nanotopography-based lymphatic delivery for improved anti-tumor responses to checkpoint blockade immunotherapy. Theranostics 2019, 9, 8332–8343. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.; Ryu, J.; Bock, S.; Koval, M.; Mauro, T.; Ross, R.; Desai, T. Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism. Nano Lett. 2015, 15, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shi, X.; Hansen, M.E.; Setiady, I.; Nemeth, C.L.; Celli, A.; Huang, B.; Mauro, T.; Koval, M.; Desai, T.A. Nanotopography Enhances Dynamic Remodeling of Tight Junction Proteins through Cytosolic Liquid Complexes. ACS Nano 2020, 14, 13192–13202. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.R.; Vishwas, S.; Khursheed, R.; Harish, V.; Sravani, A.B.; Khan, F.; Alotaibi, B.; Binshaya, A.; Disouza, J.; Kumbhar, P.S.; et al. Unravelling the role of microneedles in drug delivery: Principle, perspectives, and practices. Drug Deliv. Transl. Res. 2023, 14, 1393–1431. [Google Scholar] [CrossRef]
- Wang, J.; Viola, M.; Migliorini, C.; Paoletti, L.; Arpicco, S.; Di Meo, C.; Matricardi, P. Polysaccharide-Based Nanogels to Overcome Mucus, Skin, Cornea, and Blood-Brain Barriers: A Review. Pharmaceutics 2023, 15, 2508. [Google Scholar] [CrossRef] [PubMed]
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943. [Google Scholar] [CrossRef]
- Anumolu, S.S.; Menjoge, A.R.; Deshmukh, M.; Gerecke, D.; Stein, S.; Laskin, J.; Sinko, P.J. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials 2011, 32, 1204–1217. [Google Scholar] [CrossRef]
- Oh, J.K.; Siegwart, D.J.; Lee, H.I.; Sherwood, G.; Peteanu, L.; Hollinger, J.O.; Kataoka, K.; Matyjaszewski, K. Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: Synthesis, biodegradation, in vitro release, and bioconjugation. J. Am. Chem. Soc. 2007, 129, 5939–5945. [Google Scholar] [CrossRef]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Alvarez Igarzabal, C.I.; Calderon, M. Crossing biological barriers with nanogels to improve drug delivery performance. J. Control. Release 2019, 307, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of gellan-cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur. J. Pharm. Biopharm. 2018, 127, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Chiou, W.F.; Chou, Y.C.; Chen, C.F. Mechanisms in mediating the anti-inflammatory effects of baicalin and baicalein in human leukocytes. Eur. J. Pharmacol. 2003, 465, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.; Carmona, T.; Zacconi, F.C.; Venegas-Yazigi, D.; Cabello-Verrugio, C.; Il Choi, W.; Vilos, C. The Human Dermis as a Target of Nanoparticles for Treating Skin Conditions. Pharmaceutics 2022, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Sano, A.; Ohshima, H.; Terada, H.; Makino, K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids Surf. B Biointerfaces 2008, 65, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.; Katiyar, N.; Vadukumpully, S.; Shankarappa, S.A. Penetration of gold nanoparticles across the stratum corneum layer of thick-Skin. J. Dermatol. Sci. 2018, 89, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Jin, X.; Liu, Q.S.; Zhou, Q.; Jiang, G. Epidermal Penetration of Gold Nanoparticles and Its Underlying Mechanism Based on Human Reconstructed 3D Episkin Model. ACS Appl. Mater. Interfaces 2017, 9, 42577–42588. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, Y.; Gao, J.; Zhang, Z.; Wang, L.; Chen, X.; Mi, J.; Yao, Y.; Guan, D.; Chen, B.; et al. Transdermal Vascular Endothelial Growth Factor Delivery with Surface Engineered Gold Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 5173–5180. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; el-Khordagui, L.K.; Kraus, T.; Schneider, M. Mechanism and determinants of nanoparticle penetration through human skin. Nanoscale 2011, 3, 4989–4999. [Google Scholar] [CrossRef]
- Tak, Y.K.; Pal, S.; Naoghare, P.K.; Rangasamy, S.; Song, J.M. Shape-Dependent Skin Penetration of Silver Nanoparticles: Does It Really Matter? Sci. Rep. 2015, 5, 16908. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Quan, G.; Sun, Y.; Yang, D.; Pan, X.; Wu, C. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. J. Control. Release 2020, 325, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, M.; Zheng, L.; Yang, Y.; Cui, X.; Xu, T.; Zhang, W.; Wang, C. Noninvasive transdermal delivery of mesoporous silica nanoparticles using deep eutectic solvent. J. Control. Release 2022, 343, 43–56. [Google Scholar] [CrossRef]
- Mohamed, A.L.; Elmotasem, H.; Salama, A.A.A. Colchicine mesoporous silica nanoparticles/hydrogel composite loaded cotton patches as a new encapsulator system for transdermal osteoarthritis management. Int. J. Biol. Macromol. 2020, 164, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Lu, R. Mechanism and Application of Chitosan and Its Derivatives in Promoting Permeation in Transdermal Drug Delivery Systems: A Review. Pharmaceuticals 2022, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wei, T.; Xu, Y.; Jin, Q.; Chao, Y.; Lu, J.; Xu, J.; Zhu, J.; Yan, X.; Chen, M.; et al. Non-invasive transdermal delivery of biomacromolecules with fluorocarbon-modified chitosan for melanoma immunotherapy and viral vaccines. Nat. Commun. 2024, 15, 820. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, Y.; Yu, F.S. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4093–4100. [Google Scholar]
- Leong, Y.Y.; Tong, L. Barrier function in the ocular surface: From conventional paradigms to new opportunities. Ocul. Surf. 2015, 13, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Schulze, U.; Garcia-Posadas, L.; Arranz-Valsero, I.; Lopez-Garcia, A.; Paulsen, F.; Diebold, Y. Structural and functional alteration of corneal epithelial barrier under inflammatory conditions. Curr. Eye Res. 2012, 37, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Massaro-Giordano, M.; Macchi, I.; Lambiase, A.; Bonini, S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J. Cell. Physiol. 2013, 228, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, L.; Espina, M.; Severino, P.; Cano, A.; Ettcheto, M.; Camins, A.; Garcia, M.L.; Souto, E.B.; Sanchez-Lopez, E. Lipid Nanoparticles for the Posterior Eye Segment. Pharmaceutics 2021, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Assi, L.; Chamseddine, F.; Ibrahim, P.; Sabbagh, H.; Rosman, L.; Congdon, N.; Evans, J.; Ramke, J.; Kuper, H.; Burton, M.J.; et al. A Global Assessment of Eye Health and Quality of Life: A Systematic Review of Systematic Reviews. JAMA Ophthalmol. 2021, 139, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Schlesinger, E.B.; Desai, T.A. Nanostructured materials for ocular delivery: Nanodesign for enhanced bioadhesion, transepithelial permeability and sustained delivery. Ther. Deliv. 2015, 6, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Vaneev, A.; Tikhomirova, V.; Chesnokova, N.; Popova, E.; Beznos, O.; Kost, O.; Klyachko, N. Nanotechnology for Topical Drug Delivery to the Anterior Segment of the Eye. Int. J. Mol. Sci. 2021, 22, 12368. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, X.; Shao, M.; Chang, W.; Zhang, H.; Liu, Z. Eyedrop delivery of therapeutic proteins with zwitterionic polymers to treat dry age-related macular degeneration. Biomaterials 2024, 305, 122429. [Google Scholar] [CrossRef] [PubMed]
- Edward, A.; Prausnitz, M.R. Predicted permeability of the cornea to topical drugs. Pharm. Res. 2001, 18, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Abla, K.K.; Mehanna, M.M. Lipid-based nanocarriers challenging the ocular biological barriers: Current paradigm and future perspectives. J. Control. Release 2023, 362, 70–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luan, F.; Yue, H.; Song, C.; Wang, S.; Feng, J.; Zhang, X.; Yang, W.; Li, Y.; Wei, W.; et al. Recent advances of smart materials for ocular drug delivery. Adv. Drug Deliv. Rev. 2023, 200, 115006. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.; Beg, S.; Kohli, K. Target strategies for drug delivery bypassing ocular barriers. J. Drug Deliv. Sci. Technol. 2020, 55, 101389. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, J.; Niu, M.; Gao, X.; Zhang, G.; Yu, H.; Yang, X.; Liu, L. Ten Years of Knowledge of Nano-Carrier Based Drug Delivery Systems in Ophthalmology: Current Evidence, Challenges, and Future Prospective. Int. J. Nanomed. 2021, 16, 6497–6530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, M.; Shen, W.; Xu, Y.; Shao, A.; Xu, P.; Yao, K.; Han, H.; Ye, J. Recent Advances in Nanomedicine for Ocular Fundus Neovascularization Disease Management. Adv. Healthc. Mater. 2024, e2304626. [Google Scholar] [CrossRef] [PubMed]
- Bernards, D.A.; Ma, C.J.; Zhang, Y.; Rodriguez, T.M.; Dickson, J.; Kharbikar, B.N.; Bhisitkul, R.B.; Desai, T.A. Injectable Devices for Delivery of Liquid or Solid Protein Formulations. ACS Mater. Au 2023, 3, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Samy, K.E.; Bernards, D.A.; Desai, T.A. Recent advances in intraocular sustained-release drug delivery devices. Drug Discov. Today 2019, 24, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jia, F.; Wang, Y.; Liu, J.; Tian, Y.; Sun, X.; Lei, Y.; Ji, J. Trans-corneal drug delivery strategies in the treatment of ocular diseases. Adv. Drug Deliv. Rev. 2023, 198, 114868. [Google Scholar] [CrossRef]
- Jia, F.; Li, L.; Fang, Y.; Song, M.; Man, J.; Jin, Q.; Lei, Y.; Ji, J. Macromolecular Platform with Super-Cation Enhanced Trans-Cornea Infiltration for Noninvasive Nitric Oxide Delivery in Ocular Therapy. ACS Nano 2020, 14, 16929–16938. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Pepic, I.; Hafner, A.; Lovric, J.; Pirkic, B.; Filipovic-Grcic, J. A nonionic surfactant/chitosan micelle system in an innovative eye drop formulation. J. Pharm. Sci. 2010, 99, 4317–4325. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Seah, I.; Xue, K.; Wong, W.; Tan, Q.S.W.; Ma, X.; Lin, Q.; Lim, J.Y.C.; Liu, Z.; Parikh, B.H.; et al. Antiangiogenic Nanomicelles for the Topical Delivery of Aflibercept to Treat Retinal Neovascular Disease. Adv. Mater. 2022, 34, e2108360. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Zorzi, G.K.; Hileeto, D.; Lopez-Garcia, A.; Calonge, M.; Seijo, B.; Sanchez, A.; Diebold, Y. A nanomedicine to treat ocular surface inflammation: Performance on an experimental dry eye murine model. Gene Ther. 2013, 20, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Gao, H.; Chen, L.; Jiang, Y.; Li, S.; Chao, Y.; Liu, N.; Wang, Y.; Wei, T.; Liu, Y.; et al. Eyedrop-based macromolecular ophthalmic drug delivery for ocular fundus disease treatment. Sci. Adv. 2023, 9, eabq3104. [Google Scholar] [CrossRef]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wei, Y.; Wu, Q.; Zhou, K.; Liu, T.; Zhang, Y.; Jiang, N.; Xiao, W.; Chen, J.; Liu, Q.; et al. Liposomes for effective drug delivery to the ocular posterior chamber. J. Nanobiotechnol. 2019, 17, 64. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, J.; Liu, S.; Li, B.; Wang, J.; Tang, J.; Pu, X.; Huang, Z.; Liao, X.; Yin, G. Dendritic Oligoethylenimine Decorated Liposome with Augmented Corneal Retention and Permeation for Efficient Topical Delivery of Antiglaucoma Drugs. Nano Lett. 2023, 23, 11193–11202. [Google Scholar] [CrossRef]
- Bohley, M.; Dillinger, A.E.; Schweda, F.; Ohlmann, A.; Braunger, B.M.; Tamm, E.R.; Goepferich, A. A single intravenous injection of cyclosporin A-loaded lipid nanocapsules prevents retinopathy of prematurity. Sci. Adv. 2022, 8, eabo6638. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.F. The structure and function of the lung. Int. J. Tuberc. Lung Dis. 2010, 14, 391–396. [Google Scholar]
- Tam, A.; Wadsworth, S.; Dorscheid, D.; Man, S.F.; Sin, D.D. The airway epithelium: More than just a structural barrier. Ther. Adv. Respir. Dis. 2011, 5, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Selo, M.A.; Sake, J.A.; Kim, K.J.; Ehrhardt, C. In vitro and ex vivo models in inhalation biopharmaceutical research—Advances, challenges and future perspectives. Adv. Drug Deliv. Rev. 2021, 177, 113862. [Google Scholar] [CrossRef]
- Guagliardo, R.; Perez-Gil, J.; De Smedt, S.; Raemdonck, K. Pulmonary surfactant and drug delivery: Focusing on the role of surfactant proteins. J. Control. Release 2018, 291, 116–126. [Google Scholar] [CrossRef]
- Santos Cavaiola, T.; Edelman, S. Inhaled insulin: A breath of fresh air? A review of inhaled insulin. Clin. Ther. 2014, 36, 1275–1289. [Google Scholar] [CrossRef]
- Newman, S.P. Drug delivery to the lungs: Challenges and opportunities. Ther. Deliv. 2017, 8, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Ivey, J.W.; Vehring, R.; Finlay, W.H. Understanding pressurized metered dose inhaler performance. Expert Opin. Drug Deliv. 2015, 12, 901–916. [Google Scholar] [CrossRef]
- Chandel, A.; Goyal, A.K.; Ghosh, G.; Rath, G. Recent advances in aerosolised drug delivery. Biomed. Pharmacother. 2019, 112, 108601. [Google Scholar] [CrossRef]
- Peng, S.; Wang, W.; Zhang, R.; Wu, C.; Pan, X.; Huang, Z. Nano-Formulations for Pulmonary Delivery: Past, Present, and Future Perspectives. Pharmaceutics 2024, 16, 161. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Gao, Q.; Wu, K.; Ouyang, J.; Guo, W.; Liang, X.J. Harnessing inhaled nanoparticles to overcome the pulmonary barrier for respiratory disease therapy. Adv. Drug Deliv. Rev. 2023, 202, 115111. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.H.; Moharram, A.A. Airway Surgery for Laryngotracheal Stenosis During the COVID-19 Pandemic: Institutional Guidelines. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3652–3658. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Marazioti, A.; Kannavou, M.; Natsaridis, E.; Gkartziou, F.; Kogkos, G.; Mourtas, S. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 2021, 174, 53–86. [Google Scholar] [CrossRef] [PubMed]
- Forest, V.; Pourchez, J. Nano-delivery to the lung—By inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Zhou, W.; Huang, G.; Lei, F.; Chen, Y.; Ma, Z.; Chen, L.; Yang, M. Nanocrystals based pulmonary inhalation delivery system: Advance and challenge. Drug Deliv. 2022, 29, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fernandez, A.; Sancenon, F.; Martinez-Manez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Narang, R.K.; Rath, G.; Goyal, A.K. Advances in pulmonary delivery of nanoparticles. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 75–96. [Google Scholar] [CrossRef]
- Patel, B.G.N.; Ahsan, F. Barriers that inhaled particles encounter. In ISAM Textbook of Aerosol Medicine; Dhand, R., Ed.; International Society for Aerosols in Medicine: Werne, Germany, 2015; pp. 707–727. [Google Scholar]
- Koval, M.; Preiter, K.; Adles, C.; Stahl, P.D.; Steinberg, T.H. Size of IgG-opsonized particles determines macrophage response during internalization. Exp. Cell Res. 1998, 242, 265–273. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W.; et al. Pulmonary delivery nanomedicines towards circumventing physiological barriers: Strategies and characterization approaches. Adv. Drug Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.S.; Suk, J.S.; Woodworth, G.F.; Hanes, J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials 2013, 34, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shi, Y.; Zhang, Y.; Lei, F.; Ren, R.; Tang, X. Opportunities and Challenges for Inhalable Nanomedicine Formulations in Respiratory Diseases: A Review. Int. J. Nanomed. 2024, 19, 1509–1538. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, K.; Deng, G.; Liu, X.; Wu, X.; Hu, H.; Zhang, Y.; Gao, W.; Li, Q. Mitochondria-Modulating Porous Se@SiO2 Nanoparticles Provide Resistance to Oxidative Injury in Airway Epithelial Cells: Implications for Acute Lung Injury. Int. J. Nanomed. 2020, 15, 2287–2302. [Google Scholar] [CrossRef] [PubMed]
- Detampel, P.; Tehranian, S.; Mukherjee, P.; Foret, M.; Fuerstenhaupt, T.; Darbandi, A.; Bogari, N.; Hlasny, M.; Jeje, A.; Olszewski, M.A.; et al. Caveolin-initiated macropinocytosis is required for efficient silica nanoparticles’ transcytosis across the alveolar epithelial barrier. Sci. Rep. 2022, 12, 9474. [Google Scholar] [CrossRef]
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodriguez, G.B.; Patricio, T.; Esposti, L.D.; et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hamedinasab, H.; Rezayan, A.H.; Mellat, M.; Mashreghi, M.; Jaafari, M.R. Development of chitosan-coated liposome for pulmonary delivery of N-acetylcysteine. Int. J. Biol. Macromol. 2020, 156, 1455–1463. [Google Scholar] [CrossRef]
- Murata, M.; Nakano, K.; Tahara, K.; Tozuka, Y.; Takeuchi, H. Pulmonary delivery of elcatonin using surface-modified liposomes to improve systemic absorption: Polyvinyl alcohol with a hydrophobic anchor and chitosan oligosaccharide as effective surface modifiers. Eur. J. Pharm. Biopharm. 2012, 80, 340–346. [Google Scholar] [CrossRef]
- Yu, Y.; Ni, M.; Zheng, Y.; Huang, Y. Airway epithelial-targeted nanoparticle reverses asthma in inhalation therapy. J. Control. Release 2024, 367, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Alexander, C.; Garnett, M.; Eaton, M.; Stolnik, S. Fc-mediated transport of nanoparticles across airway epithelial cell layers. J. Control. Release 2012, 158, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, S.; Yao, Y.; Shen, X.; Li, L.; Huang, Y. Increasing stiffness promotes pulmonary retention of ligand-directed dexamethasone-loaded nanoparticle for enhanced acute lung inflammation therapy. Bioact. Mater. 2023, 20, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Cao, Y.; Nemeth, C.L.; Chirra, H.D.; Chevalier, R.W.; Xu, A.M.; Melosh, N.A.; Desai, T.A. Fabrication of Sealed Nanostraw Microdevices for Oral Drug Delivery. ACS Nano 2016, 10, 5873–5881. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, A.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Duran-Lobato, M.; Niu, Z.; Alonso, M.J. Oral Delivery of Biologics for Precision Medicine. Adv. Mater. 2020, 32, e1901935. [Google Scholar] [CrossRef]
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635. [Google Scholar] [CrossRef]
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020, 5, 127–148. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Zhu, C.; Guo, S.; Gan, Y. Advances in the transepithelial transport of nanoparticles. Drug Discov. Today 2016, 21, 1155–1161. [Google Scholar] [CrossRef]
- El-Sayed, M.; Rhodes, C.A.; Ginski, M.; Ghandehari, H. Transport mechanism(s) of poly (amidoamine) dendrimers across Caco-2 cell monolayers. Int. J. Pharm. 2003, 265, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.L.; Lock, J.Y.; Carrier, R.L. Engineering the Mucus Barrier. Annu. Rev. Biomed. Eng. 2018, 20, 197–220. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Sousa, F.; Stevens, M.M.; Desai, T.A. Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Adv. Drug Deliv. Rev. 2020, 167, 89–108. [Google Scholar] [CrossRef]
- Netsomboon, K.; Bernkop-Schnurch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Erstling, J.A.; Bag, N.; Gardinier, T.C.; Kohle, F.F.E.; DomNwachukwu, N.; Butler, S.D.; Kao, T.; Ma, K.; Turker, M.Z.; Feuer, G.B.; et al. Overcoming Barriers Associated with Oral Delivery of Differently Sized Fluorescent Core-Shell Silica Nanoparticles. Adv. Mater. 2024, 36, e2305937. [Google Scholar] [CrossRef]
- Li, D.; Zhuang, J.; He, H.; Jiang, S.; Banerjee, A.; Lu, Y.; Wu, W.; Mitragotri, S.; Gan, L.; Qi, J. Influence of Particle Geometry on Gastrointestinal Transit and Absorption following Oral Administration. ACS Appl. Mater. Interfaces 2017, 9, 42492–42502. [Google Scholar] [CrossRef]
- Zheng, N.; Li, J.; Xu, C.; Xu, L.; Li, S.; Xu, L. Mesoporous silica nanorods for improved oral drug absorption. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1132–1140. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, J.; Guo, X.; Gou, K.; Sang, Z.; Wang, Y.; Bian, Y.; Li, S.; Li, H. Chiral mesoporous silica nano-screws as an efficient biomimetic oral drug delivery platform through multiple topological mechanisms. Acta Pharm. Sin. B 2022, 12, 1432–1446. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, L.; Li, L.; Wu, J.; He, J.; Huang, Y. Coordination of rigidity modulation and targeting ligand modification on orally-delivered nanoparticles for the treatment of liver fibrosis. J. Control. Release 2022, 341, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xing, L.; Chen, L.; Zhou, R.; Wu, J.; Zhu, X.; Li, L.; Xiang, Y.; Wu, R.; Zhang, L.; et al. Tailored elasticity combined with biomimetic surface promotes nanoparticle transcytosis to overcome mucosal epithelial barrier. Biomaterials 2020, 262, 120323. [Google Scholar] [CrossRef] [PubMed]
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.J.; Preat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Lamson, N.G.; Berger, A.; Fein, K.C.; Whitehead, K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. 2020, 4, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Leve, F.; Bonfim, D.P.; Fontes, G.; Morgado-Diaz, J.A. Gold nanoparticles regulate tight junctions and improve cetuximab effect in colon cancer cells. Nanomedicine 2019, 14, 1565–1578. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, M.; Ni, X.; Wang, J.; Rong, H.; Su, Y.; Yu, S.; Mohammad, I.S.; Leung, S.S.Y.; Hu, H. Virus-Mimicking Mesoporous Silica Nanoparticles with an Electrically Neutral and Hydrophilic Surface to Improve the Oral Absorption of Insulin by Breaking Through Dual Barriers of the Mucus Layer and the Intestinal Epithelium. ACS Appl. Mater. Interfaces 2021, 13, 18077–18088. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50 (Suppl. S1), S91–S101. [Google Scholar] [CrossRef]
- Takeuchi, H.; Thongborisute, J.; Matsui, Y.; Sugihara, H.; Yamamoto, H.; Kawashima, Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 1583–1594. [Google Scholar] [CrossRef]
- Hsu, L.W.; Lee, P.L.; Chen, C.T.; Mi, F.L.; Juang, J.H.; Hwang, S.M.; Ho, Y.C.; Sung, H.W. Elucidating the signaling mechanism of an epithelial tight-junction opening induced by chitosan. Biomaterials 2012, 33, 6254–6263. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.H.; Hsu, L.W.; Tseng, M.T.; Lee, P.L.; Sonjae, K.; Ho, Y.C.; Sung, H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Hill, T.A.; Fairlie, D.P.; Maher, S.; Mrsny, R.J. Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 2020, 157, 2–36. [Google Scholar] [CrossRef] [PubMed]
- van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N-trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441. [Google Scholar] [CrossRef] [PubMed]
- Pratap-Singh, A.; Guo, Y.; Baldelli, A.; Singh, A. Mercaptonicotinic acid activated thiolated chitosan (MNA-TG-chitosan) to enable peptide oral delivery by opening cell tight junctions and enhancing transepithelial transport. Sci. Rep. 2023, 13, 17343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chao, Y.; Jin, Q.; Chen, L.; Shen, J.J.; Zhu, J.; Chai, Y.; Lu, P.; Yang, N.; Chen, M.; et al. Oral Delivery of Therapeutic Antibodies with a Transmucosal Polymeric Carrier. ACS Nano 2023, 17, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiang, H.; Yang, W.; Xu, Y.; Feng, T.; Cai, H.; Wang, S.; Liu, Z.; Zhang, Z.; Zhang, J. Oral insulin delivery by epithelium microenvironment-adaptive nanoparticles. J. Control. Release 2022, 341, 31–43. [Google Scholar] [CrossRef]
- Lee, J.H.; Sahu, A.; Choi, W.I.; Lee, J.Y.; Tae, G. ZOT-derived peptide and chitosan functionalized nanocarrier for oral delivery of protein drug. Biomaterials 2016, 103, 160–169. [Google Scholar] [CrossRef]
- Pinto, S.; Hosseini, M.; Buckley, S.T.; Yin, W.; Garousi, J.; Graslund, T.; van Ijzendoorn, S.; Santos, H.A.; Sarmento, B. Nanoparticles targeting the intestinal Fc receptor enhance intestinal cellular trafficking of semaglutide. J. Control. Release 2024, 366, 621–636. [Google Scholar] [CrossRef]
- Yang, T.; Wang, A.; Nie, D.; Fan, W.; Jiang, X.; Yu, M.; Guo, S.; Zhu, C.; Wei, G.; Gan, Y. Ligand-switchable nanoparticles resembling viral surface for sequential drug delivery and improved oral insulin therapy. Nat. Commun. 2022, 13, 6649. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, K.M.; Kolhatkar, R.B.; Swaan, P.W.; Eddington, N.D.; Ghandehari, H. Transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers: Influence of size, charge and fluorescent labeling. Pharm. Res. 2006, 23, 2818–2826. [Google Scholar] [CrossRef]
- El-Sayed, M.; Ginski, M.; Rhodes, C.; Ghandehari, H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J. Control. Release 2002, 81, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.S.; Vijayalakshmi, N.; Swaan, P.W.; Ghandehari, H. G3.5 PAMAM dendrimers enhance transepithelial transport of SN38 while minimizing gastrointestinal toxicity. J. Control. Release 2011, 150, 318–325. [Google Scholar] [CrossRef]
- Abramson, A.; Caffarel-Salvador, E.; Soares, V.; Minahan, D.; Tian, R.Y.; Lu, X.; Dellal, D.; Gao, Y.; Kim, S.; Wainer, J.; et al. A luminal unfolding microneedle injector for oral delivery of macromolecules. Nat. Med. 2019, 25, 1512–1518. [Google Scholar] [CrossRef]
- Abramson, A.; Caffarel-Salvador, E.; Khang, M.; Dellal, D.; Silverstein, D.; Gao, Y.; Frederiksen, M.R.; Vegge, A.; Hubalek, F.; Water, J.J.; et al. An ingestible self-orienting system for oral delivery of macromolecules. Science 2019, 363, 611–615. [Google Scholar] [CrossRef]
- Fein, K.C.; Lamson, N.G.; Whitehead, K.A. Structure-Function Analysis of Phenylpiperazine Derivatives as Intestinal Permeation Enhancers. Pharm. Res. 2017, 34, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Lamson, N.G.; Fein, K.C.; Gleeson, J.P.; Newby, A.N.; Xian, S.; Cochran, K.; Chaudhary, N.; Melamed, J.R.; Ball, R.L.; Suri, K.; et al. The strawberry-derived permeation enhancer pelargonidin enables oral protein delivery. Proc. Natl. Acad. Sci. USA 2022, 119, e2207829119. [Google Scholar] [CrossRef]
- Kam, K.R.; Walsh, L.A.; Bock, S.M.; Koval, M.; Fischer, K.E.; Ross, R.F.; Desai, T.A. Nanostructure-mediated transport of biologics across epithelial tissue: Enhancing permeability via nanotopography. Nano Lett. 2013, 13, 164–171. [Google Scholar] [CrossRef]
- Samy, K.E.; Cao, Y.; Kim, J.; Konichi da Silva, N.R.; Phone, A.; Bloomer, M.M.; Bhisitkul, R.B.; Desai, T.A. Co-Delivery of Timolol and Brimonidine with a Polymer Thin-Film Intraocular Device. J. Ocul. Pharmacol. Ther. 2019, 35, 124–131. [Google Scholar] [CrossRef]


| Categories | Material | Barrier | Reduced Results Related to Transepithelial Transit or Barrier Function |
|---|---|---|---|
| Nanoscale modifications of bulk material | Nanostructured surfaces: polypropylene, PEEK | Dermal | Reversible enhanced permeation, tight junction (TJ) rearrangement, and actin cytoskeleton rearrangement [64,65,66] |
| Oral | Reversible enhanced permeation, TJ rearrangement, actin cytoskeleton rearrangement, transcytosis, and paracellular enhancement [36,67,202] | ||
| Nanoporosity | Ocular | Prolonged and controlled topical release [203] | |
| Inorganic nanoparticles (NP) | Silica NP | Oral | Smaller and more negatively charged NP increased drug permeation and modulated barrier function [178] |
| Mesoporous silica NP | Oral | Shape impacted uptake, internalization [172], and adhesion [173], virus-inspired hydrophilic, neutrally charged NP transited mucus and transcytosed barrier [180] | |
| Pulmonary | Surface modification with PEI and PEG facilitated reach of distal lungs and alleviation of inflammatory response [149] | ||
| Gold NP | Dermal | Size-dependent transdermal permeation [78,79], charge-modified permeation [80,81], and shape-modified permeation [82] | |
| Oral | Citrate-capped gold NP reversibly increased paracellular permeation [179] | ||
| Calcium phosphate NP | Pulmonary | Transcytosis through lung epithelium and successful cardiac targeting [151] | |
| Chitosan ceria NP | Ocular | Led to disruption of TJs and drug permeation [120] | |
| Chitosan meso-porous silica NP | Dermal | Paired with chemical permeation enhancers [85] or composite system such as a gel [86] to facilitate skin permeation | |
| Polymeric NP and complexes | Chitosan NP, including nanocomplexes and nanomicelles | Dermal | Delivery of multiple classes of drug cargos, reversible drop in TER, paracellular delivery, and opening of TJs [88] |
| Oral | Permeation enhancement [182,183,187], mucoadhesion [182,183], enhanced transport [176], enhanced paracellular permeability [184,185,186,189], and TJ rearrangement [190] | ||
| Ocular | Zwitterionic chitosan nanocomplexes transiently opened TJs, delivery of high molecular weight therapeutics to the retina and choroid [103,119] | ||
| Polystyrene NP | Oral | Particle size modified particle uptake [169] and transit [170], particle shape altered transit [169,171] | |
| PLGA NP | Oral | Particle stiffness and receptor binding altered transcytosis [174], targeting can be further refined such as ligand switchable system [194] | |
| Zwitterionic hydrogel NP | Oral | Increase in elasticity increased transcytosis, bioavailability of insulin, and increased likelihood of secretion rather than degradation pathways [175] | |
| PEG surface modification of polymeric NP | Oral | Increases intestinal epithelial cell uptake [145,176], hydrophilicity enhances transport | |
| Ocular | Nanomicelles of PEG, poly(propylene glycol), and poly(ɛ-caprolactone) successful retinal delivery likely via transcorneal transcytosis [117] | ||
| Chitosan surface modification of polymeric NP | Oral | Enhanced permeation, mucoadhesion [182,183], hydrophilicity enhances transport [176] and NP uptake [188], enhanced paracellular permeability [184,185,188] | |
| Ocular | Surface modification with chitosan and peptide transporter-1 targeting elements facilitates transit to the posterior region of the eye [115,116] | ||
| Cationic gelatin NP | Ocular | Plasmid delivery via particles restored corneal epithelial barrier integrity [118] | |
| PAMAM dendrimers | Oral | Disrupted TJs, increased drug permeability associated with size increase and charged dendrimers [195], dendrimer composition altered transepithelial path [197] | |
| Lipid-based NP | Liposomes | Dermal | Modified liposomes, such as niosomes, reached the epidermis and the dermis [89,90] |
| Pulmonary | Coating with chitosan or hydrophobic anchors prolonged retention, opened TJs, and enhanced paracellular delivery [152,153]. Fc receptor functionalization increased transcytosis [154,155], increased stiffness, and increased endo and exocytosis [154]. | ||
| Ocular | PAMAM dendrimer-coated liposome demonstrated transcorneal permeability and posterior chamber therapeutic response [121,122], observed to transit via the transcellular and paracellular routes with disruption of TJs [122] | ||
| SLNs and NLCs | Dermal | Increased permeation by creating occlusive film at surface, enhancing hydration [92] | |
| Lipid nanocapsules | Ocular | cRGD decorated nanocapsules traversed the choroidal endothelial barrier and the retinal pigment epithelial barrier to achieve therapeutic results in retina [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, M.E.; Ibrahim, Y.; Desai, T.A.; Koval, M. Nanostructure-Mediated Transport of Therapeutics through Epithelial Barriers. Int. J. Mol. Sci. 2024, 25, 7098. https://doi.org/10.3390/ijms25137098
Hansen ME, Ibrahim Y, Desai TA, Koval M. Nanostructure-Mediated Transport of Therapeutics through Epithelial Barriers. International Journal of Molecular Sciences. 2024; 25(13):7098. https://doi.org/10.3390/ijms25137098
Chicago/Turabian StyleHansen, M. Eva, Yasmin Ibrahim, Tejal A. Desai, and Michael Koval. 2024. "Nanostructure-Mediated Transport of Therapeutics through Epithelial Barriers" International Journal of Molecular Sciences 25, no. 13: 7098. https://doi.org/10.3390/ijms25137098
APA StyleHansen, M. E., Ibrahim, Y., Desai, T. A., & Koval, M. (2024). Nanostructure-Mediated Transport of Therapeutics through Epithelial Barriers. International Journal of Molecular Sciences, 25(13), 7098. https://doi.org/10.3390/ijms25137098







