Abstract
Bioproducts derived from platelets have been extensively used across various medical fields, with a recent notable surge in their application in dermatology and aesthetic procedures. These products, such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF), play crucial roles in inducing blood vessel proliferation through growth factors derived from peripheral blood. PRP and PRF, in particular, facilitate fibrin polymerization, creating a robust structure that serves as a reservoir for numerous growth factors. These factors contribute to tissue regeneration by promoting cell proliferation, differentiation, and migration and collagen/elastin production. Aesthetic medicine harnesses these effects for diverse purposes, including hair restoration, scar treatment, striae management, and wound healing. Furthermore, these biological products can act as adjuvants with other treatment modalities, such as laser therapy, radiofrequency, and microneedling. This review synthesizes the existing evidence, offering insights into the applications and benefits of biological products in aesthetic medicine.
1. Introduction
Biological products are diverse substances, including vaccines, growth factors, immunomodulators, monoclonal antibodies, and hematological components. Various studies have demonstrated the use of numerous biologics in almost every field of medicine. The use of autologous hematological components, especially platelet-rich plasma (PRP), has become a highly attractive therapeutic tool for various applications since the biological functions of these products go beyond hemostasis [1].
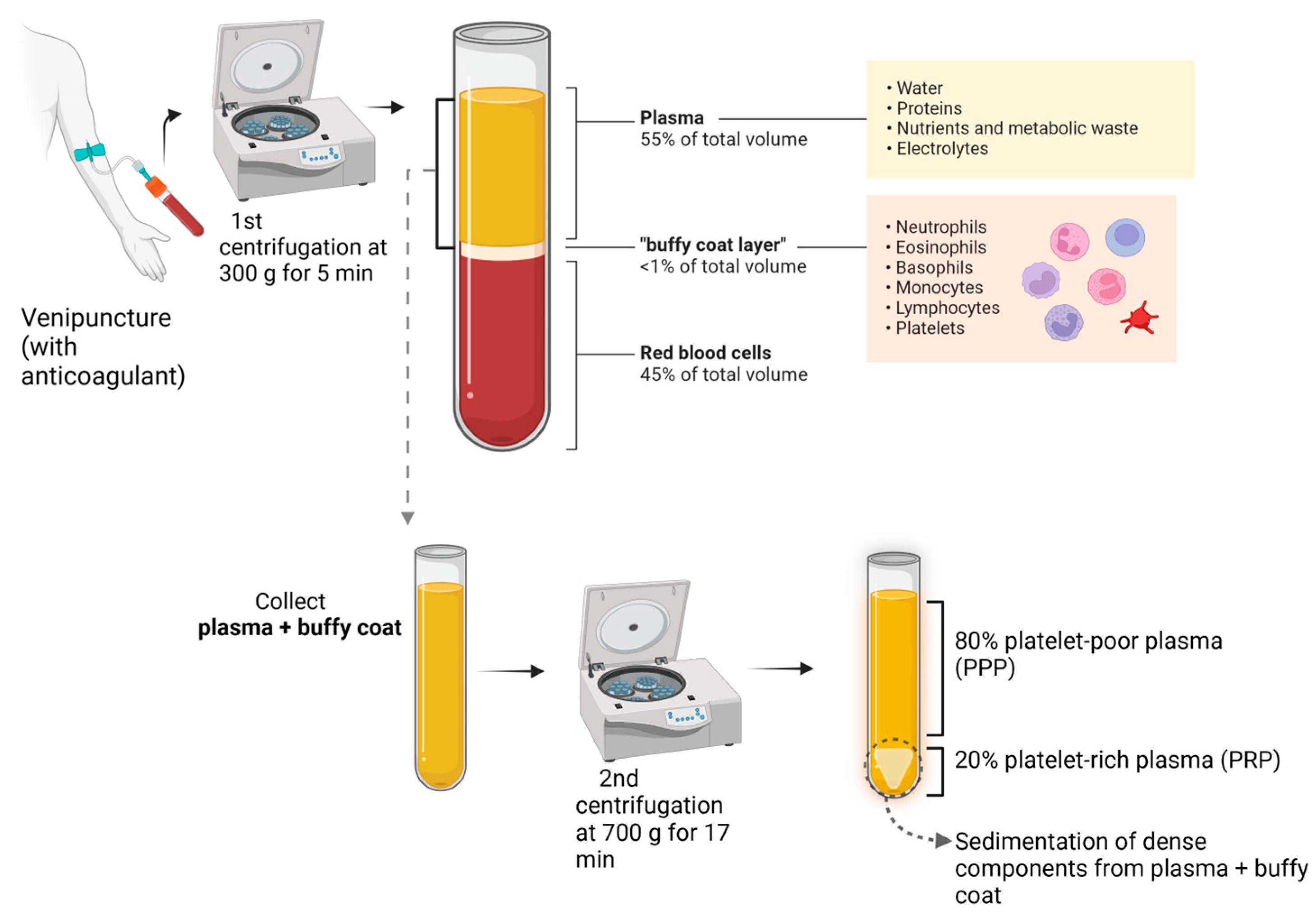
According to the International Olympic Committee, PRP is an autologous preparation derived from whole blood in which platelets are concentrated in a small fraction of the plasma [2] (Figure 1).
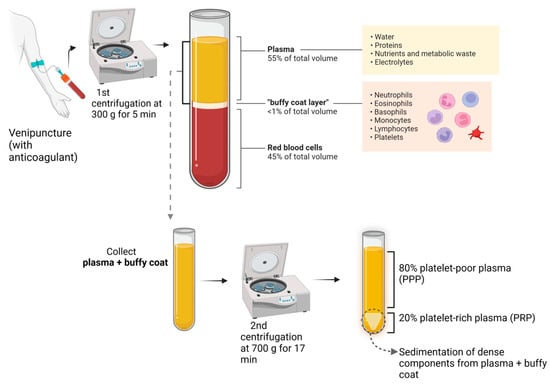
Figure 1.
PRP preparation.
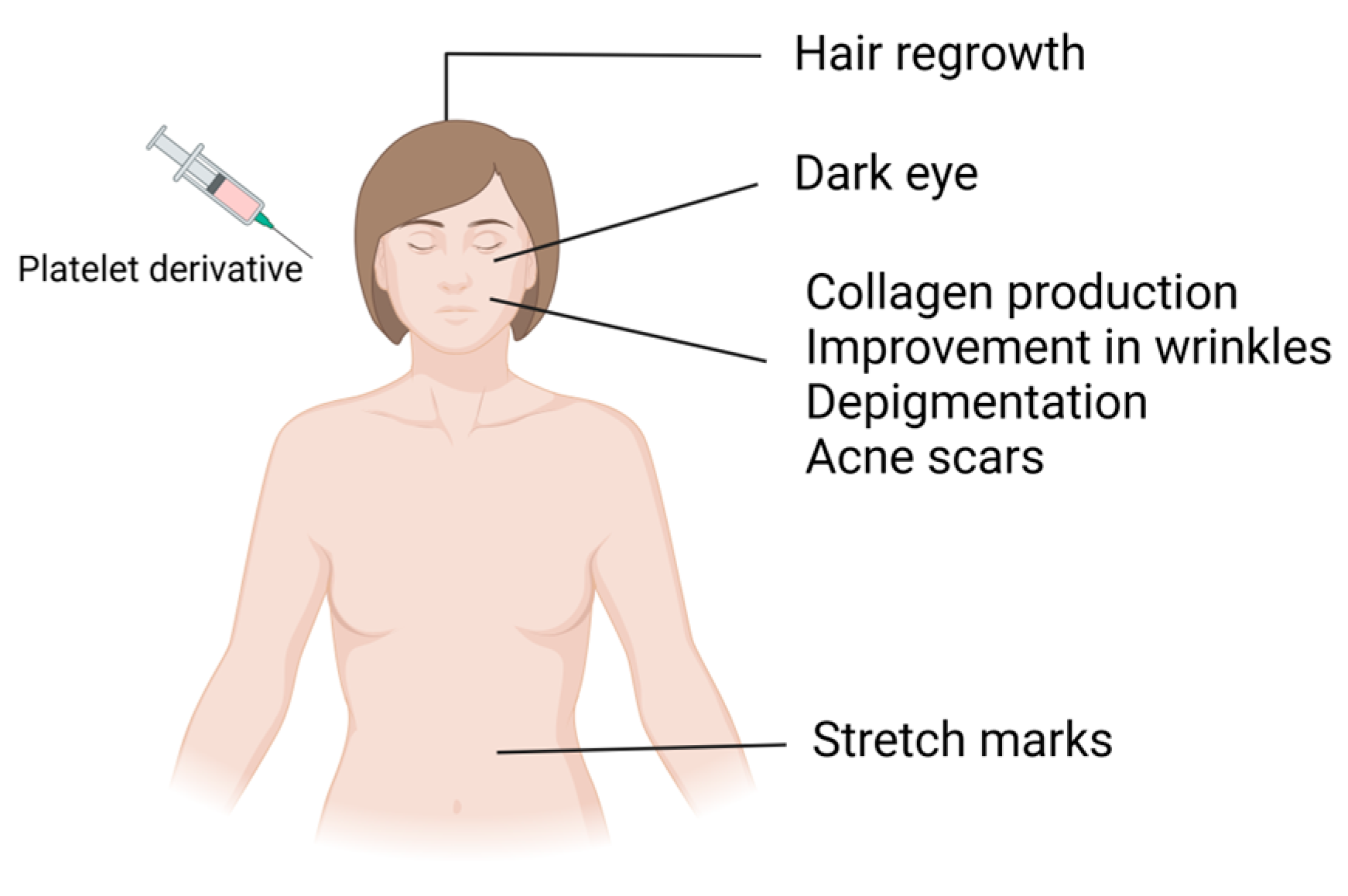
Platelet concentrates carry various growth factors contained in alpha and dense granules. Alpha granules possess seven important growth factors: platelet-derived growth factors (PDGFaa, PDGFbb, and PDGFab), transforming growth factor beta (TGFβ1 and 2), epithelial growth factor (EGF), and vascular endothelial growth factor (VEGF) [1]. These growth factors regulate processes such as cell proliferation, matrix remodeling, differentiation, angiogenesis, and chemotaxis. Dense granules contain bioactive substances such as ADP, ATP, serotonin, and calcium, which, after activation and subsequent platelet degranulation, increase membrane permeability and stimulate regenerative processes, promoting vascular remodeling and immunomodulation through the release of signaling molecules [1,2,3,4]. In addition to these advantages, since platelet-derived bioproducts are autologous products, they do not present the risk of allergic induction and have been extensively used in various medical specialties, including cardiac surgery, oral surgery, orthopedics, sports medicine, and facial plastic surgery. Furthermore, these bioproducts have undergone a significant upsurge in dermatology and aesthetic procedures in recent years [5,6,7]. These biological products have served as regenerative agents for several years, leveraging growth factors derived from peripheral blood to induce vascularization in different tissues. Upon activation, these cells form a fibrin network in a liquid state, releasing a plethora of growth factors that foster tissue regeneration by stimulating cell proliferation, differentiation, and migration and the production of collagen and elastin [8]. These effects have been explored in aesthetic medicine for different purposes, such as alopecia treatment, scar treatment, surgery, and wound healing (Figure 2). These products may also be used as adjuvants in other treatment modalities, including ablative and nonablative laser therapy, bipolar radiofrequency, and microneedling [9,10,11,12,13,14].
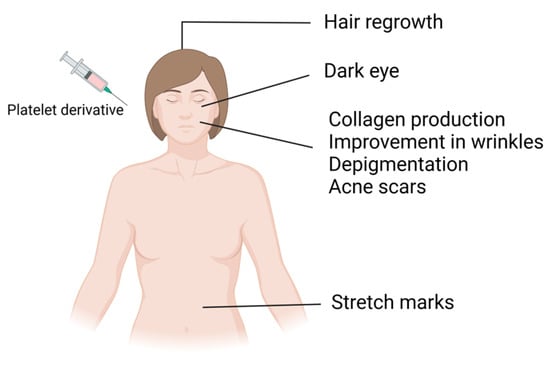
Figure 2.
Application of platelet derivatives in regenerative aesthetics.
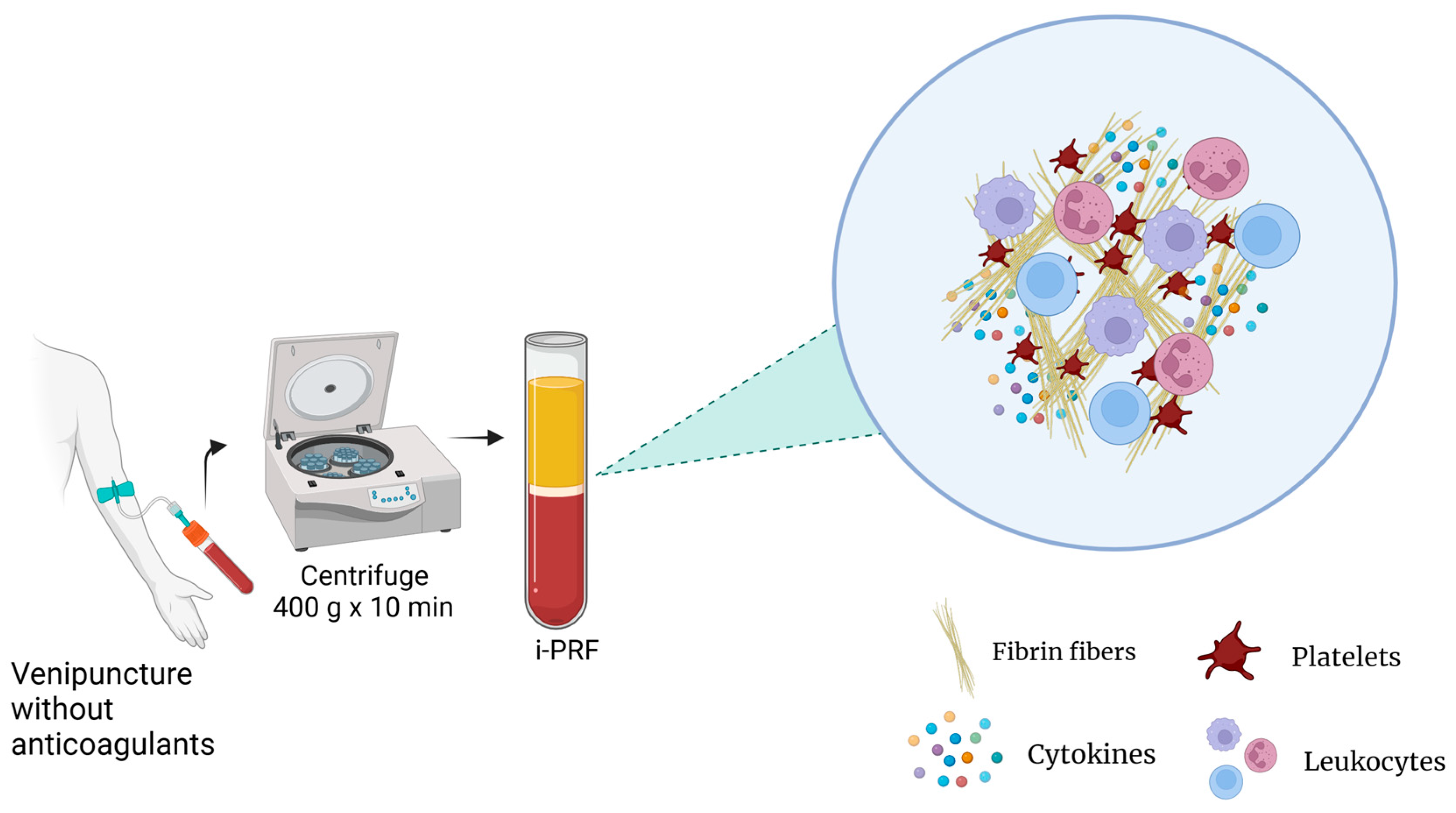
Currently, there are different types of PRP preparation methods for which parameters such as the number of centrifugation rounds, relative centrifugal force (RCF), and time can vary. This influences the platelet integrity, composition, and effectiveness of the product. Additionally, the variance among the methods and contents between PRP products results in different terminologies and results, making standardization and reproducibility challenging [3]. For instance, studies have reported that the anticoagulants used in PRP preparations may also interfere with several processes, such as wound healing [8]. For these reasons, platelet-rich fibrin (PRF), an alternative platelet concentrate, was developed with no additives (anticoagulants) and is produced under lower centrifugal forces (Figure 3). This protocol enhances the content and distribution of the cells and growth factors within PRF matrices. Furthermore, it enables injection in a manner similar to PRP, offering the added benefits of fibrin clot formation shortly after injection into the target tissue. Its feasibility is attributed to its easy handling and a growth factor content relatively comparable to those of certain PRP products [15].
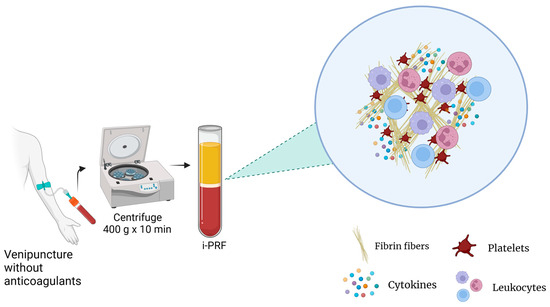
Figure 3.
PRF preparation.
Considering these findings, when new techniques are used, PRP and its derivatives can deliver results that meet patient and physician expectations.
Therefore, the objective of this review was to highlight the roles of PRP and its derivatives, including PRF, in aesthetics. This manuscript will also cover their associated benefits when compared to or associated with conventional treatments.
2. Methods
A thorough literature review was conducted to offer a comprehensive understanding of the emerging applications of PRP and its derivatives in aesthetic medicine and dermatology. The PUBMED database was employed from October to December 2023 to identify relevant reports. The search strategy involved combining the terms “aesthetic use” with variations of platelet-rich plasma (PRP), including platelet-rich fibrin (PRF), injectable platelet-rich fibrin (i-PRF), and PRP itself. This search yielded approximately 700 results (476 for PRP and 210 for PRF). Titles, abstracts, and full texts were meticulously screened and selected by the authors. A total of 69 articles were included in this study based on their relevance and publication years, of which 43 were randomized clinical trials, as listed in Table 1.

Table 1.
Baseline characteristics of the randomized clinical trials included in the studies.
3. Aesthetic Conditions
3.1. Alopecia
Currently, alternatives for treating hair loss focus on promoting cell proliferation and differentiation during the growth cycle. The effective pharmacological treatments are 2–5% topical minoxidil, which works through several mechanisms, including arteriolar vasodilation, anti-inflammatory effects, Wingless and Int-1 gene (Wnt)/β-catenin signaling, and oral finasteride, which is a selective 5α-reductase type II inhibitor. Although these drugs improve patients with androgenetic alopecia (AGA), researchers continue to search for more effective alternatives and limited side effects. For these reasons, due to the significant ability of PRP to promote tissue regeneration, new treatment protocols have been developed using this bioproduct, demonstrating efficacy in the treatment of alopecia, especially in AGA [57].
Although PRP is widely and effectively used to treat hair loss, the exact mechanism of action of PRP for this purpose has yet to be fully elucidated [58]. It is known that the hair follicle is a self-renewing mini-organ that undergoes metabolic and morphological changes during its cycle. It involves the anagen hair growth phase, the catagen hair growth phase, the return phase of the cycle, the telogen hair growth phase, and the inactive phase. PRP can have various effects on hair, and one of the key factors is its antiapoptotic effect, which is activated by the Bcl-2 protein and Akt signaling. This contributes to hair growth by increasing the longevity of dermal papilla cells throughout the hair cycle. Additionally, research has suggested that PRP treatment upregulates the FGF-7/β-catenin signaling pathway, leading to follicular stem cell differentiation, stimulating hair growth, and prolonging the anagen phase of the growth cycle. PRP also appears to enhance the perifollicular vascular plexus, elevating angiogenic factors such as VEGF and PDGF [18].
A study on AGA treatment with PRP in rats showed that this biologic product accelerated the hair cycle, suggesting that PRP injections could promote hair growth. To verify the therapeutic efficacy of PRP in humans, researchers conducted a randomized, placebo-controlled, double-blinded study with 52 patients with AGA. The study results demonstrated a substantial increase in the hair density after PRP injections within a short timeframe. At the 6-month mark, the PRP group exhibited significant improvements in their hair counts, hair diameters, and proportion of anagen hairs compared to the control group and baseline measurements [13].
Multiple clinical studies provide evidence supporting the efficacy and safety of using PRP for treating AGA in men. In a randomized, placebo-controlled study involving 23 male patients, hair growth parameters were assessed three months after the initial PRP session and compared with the baseline values in the treated and control areas. The study revealed significant increases in the average hair count and total hair density in the PRP-treated area. Microscopic evaluation two weeks after the last PRP treatment indicated increases in the epidermal thickness in the capillary skin compared to the baseline, accompanied by a rise in the number of follicles. Additionally, researchers observed an increase in basal epidermal keratinocytes and follicular bulb cells [18]. These data showed that PRP can be an effective treatment option for hair loss.
Butt and colleagues [21] evaluated the effectiveness of PRP for hair restoration in 30 patients, including men and women, using various parameters, such as the terminal-to-vellus hair ratio, the hair density, the hair-pulling test, photographs, the physician global assessment score, and the patient global assessment score. The authors showed that PRP is an effective treatment option for androgenetic alopecia, resulting in greater hair density and physician and patient global assessment scores and an increase in the terminal-to-vellus hair ratio.
The type of study design directly influences the results. Several split-scalp studies evaluating intradermal PRP versus saline have shown that PRP improves androgenetic alopecia, but other studies have failed to prove this. Gentile and collaborators [20] performed a randomized, placebo-controlled trial comparing the hair regrowth of patients receiving PRP injections via controlled, programmed mechanical injections at a depth of 5 mm using a medical injection gun versus placebo injections. Patients underwent three sessions, resulting in an increase of 33.6 hairs in the target area and an average increase in density of 45.9 hairs per cm3 compared to those in the placebo group. Similarly, Alves and Grimalt [19] conducted a study on twenty-five patients with AGA. They revealed that PRP treatment has a positive effect on AGA and could even be regarded as a promising therapy for this disorder.
Conversely, Shapiro et al. [23] analyzed the effect of the intradermal injection of PRP on hair regrowth and thickness compared with saline. While the hair density significantly increased in the PRP-treated area compared to the baseline, there was also a modest increase in the placebo-treated areas. These findings suggest no significant difference in the hair density changes between the two groups. The variations in the study outcomes may be attributed to differences in the techniques and types of injections used, influencing the diffusion and action of PRP. This “design effect” could explain the inconsistent findings in PRP studies, highlighting the need for additional research to identify factors affecting treatment outcomes.
Most AGA studies are carried out on men or on groups containing men and women; however, the effects in these two groups have been yet to be evaluated separately. According to a meta-analysis assessing the efficacy of PRP separately in men and women, PRP significantly increased the hair diameter in both sexes, but the hair density was significantly increased only in men [59]. Although the efficacy of PRP in men and women differed between the two groups, the administration of PRP was promising in both.
Numerous techniques, including PRP therapy and microneedling, have been employed individually or in combination to promote hair growth and mitigate hair loss. Microneedling is based on the premise that limited and controlled injury to the skin triggers a natural healing cascade, provoking the release of growth factors and, therefore, stimulating the vital dermal structures necessary for hair growth and strengthening. With this in mind, Muhammad and colleagues compared the efficacies of the combined or isolated application of PRP and microneedling in treating AGA. The group that underwent PRP application through microneedling experienced a significantly greater average hair count than the group that received PRP alone. Compared with PRP alone, microneedling + topical PRP resulted in a greater increase in the hair count in individuals experiencing hair loss. Combining these two techniques demonstrated synergistic effects and was proven to be more effective than either method alone, especially in patients with AGA [25].
A study published in 2021 demonstrated the effectiveness of PRP treatment compared to intradermal application in AGA patients treated with microneedling. The hair count, hair density, terminal hair count, and terminal hair density significantly differed between the two groups and before treatment. A statistically significant difference was found between the averages of anagen and telogen hair in addition to the hair length in the groups treated with microneedling [26].
Several authors have combined PRP with other substances to improve the treatment efficacy of AGA. In a recent study, Wu and collaborators [27] used PRP plus fibroblast growth factor (PRPF) and minoxidil. In this prospective, randomized, controlled study, 75 patients with AGA were divided into three groups according to the type of treatment used. In group 1, PRPF was used as a monotherapy through intradermal injections; in group 2, patients received only 5% topical minoxidil twice a day; in group 3, PRPF injections were performed in combination with topical applications of minoxidil. All patients treated with PRPF showed improvements in their hair counts, terminal hair counts, and growth rates compared to those treated with minoxidil monotherapy.
Wei et al. [28] also compared the efficacy and safety of PRP combined with topical minoxidil for treating AGA. Thirty male patients were divided into two treatment groups: the first group received PRP with 5% topical minoxidil, and the second group received PRP with a topical placebo. The hair density/quantity, clinical efficacy, and patient satisfaction were more pronounced in the first group. This study showed that the effects of PRP and minoxidil treatment exceeded those of PRP alone, demonstrating the former as a potentially beneficial treatment strategy for AGA.
Although the application of PRP in hair loss conditions is mainly based on AGA, some studies have reported its benefits in other types of alopecia, such as telogen effluvium. Chronic telogen effluvium is characterized by diffuse scalp hair loss. El-Dawla et al. evaluated the safety and efficacy of different preparation methods of PRP versus a placebo in thirty women with chronic telogen effluvium. They concluded that PRP can be acknowledged as an effective therapeutic alternative for patients with chronic telogen effluvium [29]. Moftah and colleagues [24] studied different PRP preparation protocols regarding the number of spins, centrifugal force, type of centrifuge, and tube size to evaluate which protocol has better clinical efficacy in treating female pattern hair loss (FPHL). Patients were divided into four groups as follows: one spin using a centrifugation force of 1000× g for 10 min; two spins using a centrifugation force of 250× g for the first and 450× g for the second, both for 10 min; two spins with a centrifugation force of 250× g for the first and 1000× g for the second, both for 10 min. All of these groups used small sodium citrate tubes (2 mL VACUTEST tubes, Buffered Citrate 9NC 3.2%, made in Italy) and a non-digital centrifuge (Electronic Centrifuge 80-1; maximum speed: 4000 rpm; timer range: 0–60 min; capacity: 20 mL × 6; made in China). In the last group, PRP was prepared with one spin, using a centrifugation force of 220× g for 10 min, with a large sodium citrate tube (9 mL VACUETTE tube, 9NC coagulation sodium citrate 3.2%, made in Austria) and a digital centrifuge (HERMLE z 326 k centrifuge; maximum speed: 18,000 rpm; maximum capacity: 4 × 100 mL; timer: from 10 s to 99 min; temperature range: from −20 to 40 °C; made in Germany).
This study revealed that the PLT was lower in groups I, II, and III, in which a non-digital centrifuge was used at a high rotation speed with small sodium citrate tubes. Conversely, protocols using a digital centrifuge, large sodium citrate tubes, and a low-speed spin (220× g) were more effective at preparing PRP. These findings support existing research suggesting an inverse relationship between the platelet count and centrifugation speed. In terms of efficacy, a study revealed that PRP is effective and safe for treating female pattern hair loss (FPHL), as all patients exhibited statistically significant increases in their percentages of terminal hairs and average hair widths after treatment [24].
To investigate whether the discrepancies in the results reported in the literature regarding PRP in patients with androgenetic alopecia could be linked to the protocol used and correlated with the platelet and growth factor levels, Rodrigues et al. conducted an analysis of the platelet count and growth factor levels in PRP and their correlation with hair growth. In this study, 26 patients were randomly assigned to receive four subcutaneous injections of either PRP or saline. The authors observed the favorable use of PRP as a therapeutic alternative for treating androgenetic alopecia, but no association was found between the platelet count and the evaluated growth factors. The conclusion was that clinical improvement might be associated with other mechanisms [22].
Rinaldi and collaborators compared the efficacy and safety of PRP with those of triamcinolone acetonide (TrA) or a placebo in 45 patients with alopecia areata (A.A.). Patients who underwent PRP treatment exhibited significantly greater hair regrowth than those treated with the TrA or placebo. The study revealed that 38% and 71% of patients in the TrA group experienced regression at the 6th and 12th months, respectively. In contrast, no patients in the PRP group experienced recurrence at 6 months, and only 31% did so at 12 months. Overall, 96% of patients in the PRP group achieved the regrowth of fully pigmented hair from the initiation of hair growth. In contrast, only 25% of patients in the TrA group had pigmented hair at the beginning of hair growth [16].
Furthermore, Vazques et al. [17] evaluated PRF for the treatment of A.A. in a 28-year-old patient who developed this condition after a symptomatic COVID-19 infection. He underwent two PRF intradermal sessions, the first in March and the second in May, at which point his A.A. resolved at the 6-month follow-up. The authors concluded that PRF for A.A. is a promising treatment for patients with this autoimmune disease.
PRP and PRF, used alone or in combination with various protocols, techniques, or other agents, such as 5% minoxidil, appear promising for treating different types of alopecia or hair loss in both men and women. However, factors such as the study design, PRP preparation protocols, nature and severity of the disease, PRP diffusivity, and potential associations with other substances can impact the quality of the results. Consequently, further research is needed to establish a standard protocol that optimizes treatment outcomes.
3.2. Skin Rejuvenation
The demand for aesthetic procedures, particularly rejuvenation, has steadily increased in recent years due to the aging global population. Skin aging is influenced by intrinsic factors, such as genetic background and chronological age, as well as extrinsic factors, such as exposure to ultraviolet radiation, trauma, air pollution, alcohol consumption, nutritional issues, smoking, and reactive oxygen species causing DNA damage. These factors contribute to decreased or hyperexpression in melanocytes, reduced fibroblast activity, and diminished collagen and elastin synthesis, leading to dermal disruption and the dysregulation of the stem cell population responsible for tissue repair [60,61]. Subsequent skin changes involve hyper- or hypopigmentation, skin laxity, and the development of both superficial and deep wrinkles, significantly impacting self-image and social acceptance [62]. Regarding rejuvenation, the primary goal is to counteract or minimize the aging process using either surgical or noninvasive methods. As the demand for aesthetic procedures to prevent related changes and enhance skin quality has risen, there has been a notable decline in the number of patients opting for surgical interventions [61]. Among the nonsurgical aesthetic procedures, PRP has been extensively researched and has yielded favorable results with high patient satisfaction and no significant adverse effects [62]. PRP offers numerous benefits in facial rejuvenation, addressing concerns such as atrophic acne scars, pigmentation disorders, wrinkles, folds, loss of elasticity, and tissue volume loss. The exact mechanism of action of PRP and its derivatives in facial rejuvenation has yet to be fully understood. It is hypothesized that the growth factors in PRP may facilitate tissue repair, increase cell proliferation, and influence the expression of differentiation genes. This, in turn, supports angiogenesis and cellular rejuvenation processes, potentially leading to more enduring effects than other procedures [63]. In vitro studies have indicated that PRP may enhance collagen expression, remodel the extracellular matrix, promote fibroblast proliferation, and facilitate fibroblast differentiation into myofibroblasts [64].
The application of PRP and its derivatives is intended to improve the skin quality, texture, and tone via injection or topical application combined with microneedling either individually or in combination with other aesthetic procedures. Banihashemi and colleagues [38] tested two sessions of pure PRP with a 3-month interval for facial rejuvenation in 30 female participants. According to patient reports, they observed significant improvements in periorbital dark circles and nasolabial folds. They concluded that facial rejuvenation with PRP is an effective and noninvasive technique.
Among the different types of wrinkles, periorbital wrinkles are the most common and challenging to rejuvenate, as the periorbital skin is thinner. Considering that PRP and plasma gel have been used for skin rejuvenation, Diab and colleagues compared the use of both products for periorbital wrinkles in 40 female patients. The PRP and plasma gel were injected into each side of the face during two treatment sessions four weeks apart. Patients were followed up 2 weeks after each treatment session and 12 weeks after the last session. The authors showed that after the second session, both modalities had significantly improved periorbital wrinkles, achieving superior results with the plasma gel. However, they were not able to ameliorate periorbital hyperpigmentation [41]. However, Nilforoushzadeh et al. showed that the combination of an erbium-doped yttrium aluminum garnet (Er: YAG) laser and PRP is significantly more effective for periorbital hyperpigmentation and wrinkles than Er: YAG laser monotherapy [39].
Similarly, in a prospective clinical trial, Mahmoodabadi and colleagues verified the effectiveness of PRP and its derivatives in treating periorbital wrinkles [45]. PRF was injected into the periorbital regions of 15 volunteers. The results demonstrated improvements in deep, delicate, and small wrinkles, periorbital hyperpigmentation, and the overall freshness of the skin at the injection site. Participants reported swelling at the injection site lasting up to one day after the procedure, which resolved on its own without any complications.
Based on the evidence that PRP conveys favorable results for hyperpigmentation treatment, Gonzalez-Ojeda and colleagues [43] studied the role of PRP in treating melasma. Twenty female patients with melasma underwent three intradermal PRP sessions at an interval of 15 days, which were evaluated before and after treatment. Comparisons were made regarding the concentration of melanin in the treated area, the severity index score, the degree of patient satisfaction, and the degree of histological changes. Through dermatoscopy examinations, decreases in pigmentation after treatment with PRP and histopathological improvements were reported. Reductions in skin atrophy, solar elastosis, and inflammatory infiltration were also observed.
Several cosmetics and chemical peels, including tranexamic acid, constitute the therapeutic armamentarium for treating melasma with some success. Therefore, Patil and Bubna [42] compared the benefits and safety of tranexamic acid (4 mg/mL) versus PRP for melasma treatment. In total, 40 patients with melasma were randomly distributed into two groups and received either tranexamic acid or PRP. Patients received weekly intradermal injections for one month, totaling five injections. No significant adverse effects were found in either group, and both had rapid or substantial improvements in melasma, demonstrating that these agents are effective and safe therapeutic options for this disease.
Mumtaz et al. compared the effectiveness of PRP to that of tranexamic acid (4 mg/mL) in the treatment of melasma, obtaining superior results with PRP. The authors divided 64 patients with melasma into two groups: one with 1 mL of PRP and one with intradermal tranexamic acid. Treatment was offered every four weeks for twelve weeks, and the final result was observed in the 24th week [40].
Since PRP has potential benefits for facial skin rejuvenation, it has been used in several investigations. A study examined the effectiveness of PRP intradermal injections for facial rejuvenation through biometric parameters and patient satisfaction. Significant reductions in the number and area of blemishes, decreases in the count and depth of wrinkles, and improvements in the redness and firmness of the skin were observed. Regarding the patient satisfaction index, after six months, an average score greater than 90% was achieved [33]. A similar study evaluated the efficacy and safety of intradermal PRP injections for rejuvenation and wrinkle treatment in 20 patients with different types of wrinkles. In this investigation, the intradermal injection of PRP was well tolerated and capable of rejuvenating the face, promoting the significant correction of wrinkles, especially in the nasolabial folds [30].
When combined with other skin-boosting biomolecules, such as hyaluronic acid, PRP has emerged as an effective treatment for enhancing the skin quality, addressing bleeding, and combating signs of aging. An extensive study involving 80 patients with facial aging examined the clinical efficacy, adverse reactions, and durability of PRP + skin booster effects. Over a year of observation, patients reported positive changes in their skin condition, enhanced quality of life, and increased satisfaction with their appearance following treatment. The study concluded that PRP + skin booster was effective and safe at alleviating issues such as coarse pores and wrinkles, contributing to overall facial rejuvenation. These findings offer compelling evidence supporting the clinical application of PRP in skin booster treatments [44].
Hassan et al. focused on testing injectable platelet-rich fibrin (i-PRF) for facial skin rejuvenation. The study reported significant improvements in various skin parameters, including surface spots, pores, wrinkles, ultraviolet spots, skin texture, and porphyrins, at the 3-month follow-up. Patient satisfaction with appearance also showed substantial enhancements, particularly regarding skin quality and overall facial appearance, covering areas such as the cheeks, lower face, jawline, and lips. Notably, the study highlighted the safety and effectiveness of intradermal i-PRF, emphasizing increased patient satisfaction without significant adverse effects [34]. In contrast, Silva and collaborators used lyophilized PRP because the facility needed to obtain numerous samples via single venipuncture, which is helpful for multiple injections. The authors evaluated the effect of this preparation by monthly intradermal injections compared with saline in treating skin aging through a phase II pilot study. They found that applying freeze-dried PRP via mesotherapy did not improve skin aging [37]. A split-face study comparing the benefits of PRP on photoaged skin with those of saline control skin in male and female participants with bilateral cheek rhytids was performed by Alam and colleagues. The average photoaging scores related to fine lines, mottled pigmentation, roughness, and pallor, reported by two dermatologists, showed no significant differences between the PRP and control groups. However, patient satisfaction regarding texture, wrinkles, pigmentation, and telangiectasias was more significant in the PRP-treated group [32]. These controversial studies show that the lack of standardization may interfere with the quality of the results from using PRP in aesthetics.
Clinical evidence indicates that PRP can be utilized independently or with lasers, microneedling agents, or other substances, such as hyaluronic acid. Combining hyaluronic acid with PRP may amplify the release and retention of growth factors, enhancing collagen synthesis and stimulating fibroblast activation, thereby contributing to skin rejuvenation [31]. A prospective, open-label study by Hersant et al. aimed to showcase the clinical advantages of combining PRP and hyaluronic acid. The study, utilizing FACE-Q scores and biophysical measurements, revealed a substantial improvement at the 6-month mark compared to the baseline [36].
A study carried out in Korea showed that PRP combined with a fractional laser increased patient satisfaction and skin elasticity and decreased the rate of erythema. It also increases the cohesion of the dermal epidermis, the amount of collagen fibers, and the number of fibroblasts [65]. Gawdat et al. compared fractionated radiofrequency (fr-RF) microneedling alone and in combination with PRP for rejuvenating the neck region. The combined treatment showed an overall improvement in the appearance of the neck, as evidenced by the statistically significant increase in the dermal thickness, moderate-to-excellent results according to a medical evaluation by the Global Aesthetic Improvement Scale (GAIS), and great patient satisfaction [14].
PRP and its derivatives associated with other rejuvenation techniques optimize the results and reduce the side effects of some methods, accelerating the regeneration process. One of these associations was demonstrated by Cai et al. [35], who attempted to evaluate whether PRP could improve the restorative effects of erbium fractional laser treatments. This procedure is widely used because it considerably improves skin aging, but it is associated with several side effects, such as erythema and pigmentation. They concluded that erbium fractional laser irradiation combined with PRP application is an effective and safe option for improving aging facial skin, with minimal side effects, suggesting the use of the combination instead of the laser alone.
Atrophic acne scarring presents a therapeutic challenge in aesthetic medicine, but various modalities are available to address this concern. These include microneedling, radiofrequency, fractional lasers, punch excision, suturing, subcision, and dermabrasion, as well as autologous fat grafting, autologous dermal grafts, and autologous PRP and PRF [66]. Cho and colleagues [67] evaluated the effects of PRP in vitro on the activation of dermal fibroblasts through extracellular matrix remodeling. Cell proliferation and migration assays, an ELISA, and Western blotting revealed that PRP elevated the expression of type I collagen, elastin, and matrix metalloproteinases 1 and 2, thereby expediting the wound-healing process. These results offer valuable insights into the potential mechanisms by which PRP facilitates tissue remodeling, suggesting its applicability in addressing aesthetic dysfunctions such as acne scars.
PRP and microneedling have gained popularity as off-label treatments for rejuvenation and body repair. In aesthetic medicine, they represent comprehensive and informed resources. A recent study involving 40 patients with atrophic acne scars compared the efficacy of autologous i-PRF + microneedling to that of microneedling alone. The area treated with i-PRF exhibited a significant reduction in acne scarring, and the mean patient satisfaction score was notably greater [48]. Asif and colleagues [46] conducted a split-face comparative study to treat atrophic acne scars, comparing PRP + microneedling versus microneedling + distilled water. In the PRP + microneedling group, 40% of patients reported “excellent” results, while 60% rated the results as “good”. The study concluded that the combination of PRP and microneedling is significantly more effective than microneedling alone.
Acne scars are among the most common aesthetic dysfunctions that can affect self-esteem and quality of life. Therefore, there is a rising demand for treatment alternatives that can minimize or ameliorate this disorder. An experimental analytical study was conducted on 40 patients to compare PRP administration after subcision for acne scars. The right side of the face was injected with autologous PRP into each scar after performing subcision. The left side of the face was the control, where only subcision was performed. PRP + subcision led to superior results in post-acne scars compared to subcision alone. They concluded that PRP and subcision act synergistically to improve the appearance of acne scars [47].
Diab et al. performed a comparative study using PRF versus PRP and intradermal application and microneedling to treat atrophic acne scars. The participants were allocated into two groups, with each receiving treatments on each side of the face. The left side of the face received the intradermal injection of PRP (group 1) or PRF (group 2), and the right side received topical PRP or PRF followed by microneedling. No significant differences in the acne scar severity were observed on either side of the face. However, the improvement in the PRF group, either alone or combined with microneedling, was significantly greater than that in the PRP-only group [49].
The CO2 dot matrix laser is a widely used method for treating acne scars, and its effects have been reported in several studies. However, one of the limitations of this technique is its high cost. Furthermore, the effectiveness of this method depends mainly on the penetration capacity of the laser, the homogeneous energy distribution, and the excessive thermal coagulation, which can cause adverse reactions, such as thermal damage and pigmentation of the patient’s skin [68]. Guo and colleagues studied the clinical efficacy and safety of a CO2 dot matrix laser combined with PRP. They concluded that combining the two techniques can strongly improve the scar repair efficacy and psychosocial health and quality of life in patients with acne scars [50].
4. Striae
Striae distensae (S.D.) are commonly referred to as stretch marks. S.D. manifest clinically as parallel striae perpendicular to the skin’s tension lines. These tumors resemble dermal scars histologically and are often associated with factors such as pregnancy, obesity, hormonal changes, and genetic predisposition, primarily affecting females. There are two types of stretch marks based on their clinical and histopathological features and categorized by their maturation stages. In the initial stage, stretch marks known as “striae rubra” (S.R.) manifest as immature, tense, and erythematous lesions due to the reorganization of and reduction in elastin and fibrillin fibers and the structural changes in collagen. Over time, they progress to the atrophic and hypopigmented stage and are then referred to as “striae alba” (S.A.), characterized by the local breakdown of elastin and collagen with mast cell enzymes released in the mid-dermal tract [69,70].
Various treatment modalities, including different types of lasers (pulsed-dye, fractional CO2, Nd YAG, Er Glass, Er YAG, and diode lasers), radiofrequency, microdermal abrasion, carboxytherapy, chemical peels, and topical cosmetics such as sodium ascorbate and tretinoin, have been explored in numerous studies, yielding variable results for the treatment of stretch marks. However, these methods have not yet been proven to be sufficiently compelling and have minimal adverse effects. Leveraging the well-established biological effects of PRP, this biological tool has emerged as a promising option for treating stretch marks, either as a standalone therapy or in combination with other alternatives [70]. To confirm this hypothesis, de Castro and colleagues [55] subjected patients with abdominal striae to intralesional PRP injections to characterize and compare the structural changes in the collagen and elastic fibers in these areas. Furthermore, the authors suggested that this treatment’s possible mechanisms of action were related to signaling pathways involving Toll-like receptors (TLRs) and growth factors. Biopsies of the treated areas were conducted at the initiation of treatment and at weeks 6 and 12 posttreatment, revealing the effectiveness of PRP in reducing the area of stretch marks. This reduction was accompanied by the stimulation of collagen and elastic-fiber synthesis and remodeling. Moreover, PRP increased the immunoreactivities of TLR2 and TLR4, subsequently increasing the TNF-α, VEGF, and IGF-1 levels. These findings underscore PRP’s promising therapeutic potential for treating stretch marks.
Another recent study described and analyzed stretch-marked-derived fibroblasts (SMFs) subjected to two in vitro treatments: sodium ascorbate and PRP. The type I collagen expression was measured before and after adding different concentrations of PRP and sodium ascorbate to the culture medium. This study demonstrated that SMFs treated with both substances exhibited increases in type I collagen expression and cell proliferation. After 24 h of incubation with 1% PRP or 5% PRP + sodium ascorbate, the cell viability increased by 140% and 151%, respectively, and it increased by 156% and 178%, respectively, after 48 h. These results showed that both treatments were effective and suggested that the improvement in stretch marks mediated by the metabolic activity of SMF was viable [71].
In light of the information above, it is evident that the utilization of PRP can play a crucial role in treating stretch marks. This process appears to be mediated by the release of growth factors that act on the proliferation of fibroblasts, accounting for tissue repair and the production of collagen and elastic fibers. Based on these hypotheses, Abdel-Motaleb [53] assessed whether incorporating PRP would enhance the efficacy of microneedling in addressing stretch marks. Forty individuals with stretch marks were separated into two groups: those receiving microneedling alone or those receiving microneedling in conjunction with PRP. The study findings indicated that the combined therapy resulted in the superior enhancement of skin lesions, improved collagen and elastic-fiber deposition, heightened fibroblast proliferative activity, and the reduced expression of the caspase-3 protein in the epidermis.
Various kinds of lasers, such as fractional CO2 lasers, are effective for S.D. To evaluate the synergistic effect of this laser alone with PRP in S.D. treatment, Sayed et al. [56] compared the efficacy of a fractional CO2 laser alone versus a CO2 laser + PRP in S.D. treatment. They studied this effect in thirty adult female patients with S.D. divided into two groups: laser monotherapy or laser + PRP. Skin biopsies were taken from the lesions before and after treatment for histopathological evaluation. The authors observed that the combined treatment was more effective than the fractional CO2 laser alone. Similarly, Neinaa and colleagues [51] evaluated the synergistic effect of a fractional CO2 and pulsed-dye laser (PDL) with PRP in thirty S.D. patients. Patients received an intradermal injection of PRP on both sides, followed by a fractional CO2 laser on the right side and a PDL on the left side. The patients received three treatment sessions at 6-week intervals. The authors observed that both treatment sides had significantly improved clinical S.D. lesion outcomes; a significantly greater degree of clinical improvement with better outcomes and fewer side effects were observed in response to the PRP + fractional CO2 laser irradiation than in response to the PRP + PDL.
However, Preclaro and colleagues [52] also studied the synergistic effect of a fractional CO2 laser with PRP, albeit in striae gravidarum (S.G.), a connective-tissue dysfunction commonly observed in primigravidae. They evaluated 16 patients with S.G. treated with a fractional CO2 laser followed by PRP on one side of the abdomen and with a fractional CO2 laser followed by normal saline on the other. The study was performed in three sessions at 4-week intervals and showed that the combination of an ablative fractional CO2 laser and autologous PRP was superior regarding the clinical improvement and patient satisfaction score, but the outcome measures were not significantly different. These findings may suggest that differences in the protocols or types of striae may interfere with the synergistic effects of PRP and other treatments.
Ebrahim et al. [54] compared the efficacy and safety of PRP monotherapy versus PRP + subcision or medium-depth peeling (70% glycolic acid followed by 35% trichloroacetic acid) in seventy-five female patients with S.D. They concluded that using PRP in combination with subcision or peeling was more effective than using PRP alone.
5. Authors’ Note: Choosing between PRP and i-PRF for Aesthetic Procedures
Physicians often face the dilemma of selecting the most suitable platelet derivative for aesthetic procedures. Recent studies have shed light on the comparative effectiveness of PRP and i-PRF in promoting skin rejuvenation and enhancing aesthetic outcomes.
One study [72] demonstrated that i-PRF stimulates greater dermal skin fibroblast cell migration and proliferation and collagen synthesis compared to PRP. The study found that all platelet concentrates were non-toxic to cells and promoted high cell survival rates. Fluid-PRF showed significant advantages over the PRP and control groups: skin fibroblasts migrated over 350% more compared to the control and 200% more compared to the PRP group, induced greater cell proliferation at 5 days, and resulted in significantly higher mRNA levels of TGF-beta, collagen 1, and fibronectin compared to the PRP group. Additionally, fluid-PRF demonstrated a significantly greater ability to induce collagen matrix synthesis than PRP. This finding underscores the potential superiority of i-PRF in promoting tissue regeneration and enhancing aesthetic results.
Moreover, a comprehensive review [7] highlights an important consideration regarding the diffusibility of PRP and the durable scaffolding effect of fibrin from PRF. The therapeutic value of PRP, known for its diffusible nature, may sometimes be hindered depending on the injection site. In contrast, i-PRF offers a natural product with no anticoagulant needed, minimizing the interference with natural biochemistry and lowering costs. However, i-PRF does require a more experienced operator due to the technical complexities in its preparation. The operator must collect blood quickly and centrifuge it immediately to avoid premature coagulation, ideally within 2 min and 30 s [7]. Despite these challenges, i-PRF presents a more feasible treatment alternative, particularly in procedures requiring enhanced tissue regeneration and longevity.
In light of these findings, physicians should carefully consider the specific requirements of each aesthetic procedure and the desired outcomes when choosing between PRP and i-PRF. While PRP may offer potent signaling effects, i-PRF’s durable scaffolding effect and superior cell stimulation properties, along with its feasibility and cost-effectiveness, make it a promising option for achieving optimal aesthetic results.
Ultimately, the decision between PRP and i-PRF should be guided by a thorough understanding of their properties, mechanisms of action, and clinical evidence, in conjunction with the individual needs and goals of each patient.
6. Conclusions
While the literature underscores the potential benefits of PRP and PRF in addressing various aesthetic concerns, such as wrinkles, skin texture, hair loss, acne scars, and hyperpigmentation, challenges persist within the field. The lack of standardized protocols for PRP and PRF preparation remains a significant impediment, leading to variability in the methods and concentrations across studies and hindering consistency and efficacy.
The absence of an official PRP protocol for precise goals is due to various factors, primarily the lack of standardization in PRP preparation methods. Variability in techniques, such as centrifugation and anticoagulants, leads to diverse platelet concentrations and growth factor compositions, hindering uniform treatment outcomes. Additionally, the diverse applications of PRP across medical specialties require tailored protocols. Different treatment objectives, such as orthopedic versus dermatological applications, necessitate specialized formulations and delivery methods. However, consensus on optimal PRP formulations remains elusive due to the complex interplay of variables.
Despite these hurdles, PRP and PRF offer promising, minimally invasive treatments for aesthetic concerns. However, the reliance on small sample sizes in some studies underscores the challenges in drawing robust conclusions from the available evidence. While the evidence suggests a promising role for PRP and PRF, it is essential to acknowledge the limitations inherent in the current body of literature. Moving forward, efforts to address these challenges will be crucial in advancing the field and ensuring the reproducibility and reliability of outcomes. To establish standardized PRP protocols, collaborative efforts among stakeholders are crucial. Consensus guidelines, informed by empirical evidence from clinical trials, are needed to address this challenge. Technological advancements, such as automated processing systems, may offer potential solutions to enhance standardization.
In conclusion, while PRP and its derivatives hold significant promise as minimally invasive treatments for various aesthetic concerns, addressing the current challenges and uncertainties surrounding their use is paramount. By fostering collaboration and driving innovation, the aesthetic medicine community can overcome these challenges and realize the full potential of PRP and i-PRF in enhancing patient outcomes and satisfaction.
Author Contributions
Conceptualization, L.C.S., G.S.S. and B.L.R.; methodology, G.S.S. and N.S.; validation, S.B.C.V., R.J.B. and G.L.L.; investigation, A.d.C.S.R. and R.S.; data curation, C.H.T.; writing—original draft preparation, G.S.S.; writing—review and editing, B.L.R.; visualization, S.F.; supervision, S.F.; project administration, J.F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dos Santos, R.G.; Santos, G.S.; Alkass, N.; Chiesa, T.L.; Azzini, G.O.; da Fonseca, L.F.; Dos Santos, A.F.; Rodrigues, B.L.; Mosaner, T.; Lana, J.F. The regenerative mechanisms of platelet-rich plasma: A review. Cytokine 2021, 144, 155560. [Google Scholar] [CrossRef] [PubMed]
- Engebretsen, L.; Steffen, K.; Alsousou, J.; Anitua, E.; Bachl, N.; Devilee, R.; Everts, P.; Hamilton, B.; Huard, J.; Jenoure, P.; et al. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br. J. Sports Med. 2010, 44, 1072–1081. [Google Scholar] [CrossRef]
- Perez, A.G.; Lana, J.F.; Rodrigues, A.A.; Luzo, A.C.; Belangero, W.D.; Santana, M.H. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014, 2014, 176060. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.S.; Kumar, A.S.; Kirit, R.; Konathan, R.; Sivamani, R.K. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J. Cosmet. Dermatol. 2015, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Acebes-Huerta, A.; Arias-Fernández, T.; Bernardo, Á.; Muñoz-Turrillas, M.C.; Fernández-Fuertes, J.; Seghatchian, J.; Gutiérrez, L. Platelet-derived bioproducts: Classification update, applications, concerns and new perspectives. Transfus. Apher. Sci. 2020, 59, 102716. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aurora, J.K.; Dubey, K.N.; Tandon, P.; Tiwari, S. A comparative analysis between intra articular injections of injectable platelet rich fibrin versus platelet rich plasma in the management of temporomandibular disorders: A randomized control trial. Natl. J. Maxillofac. Surg. 2023, 14, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Purita, J.; Everts, P.A.; De Mendonça Neto, P.A.T.; de Moraes Ferreira Jorge, D.; Mosaner, T.; Huber, S.C.; Azzini, G.O.M.; da Fonseca, L.F.; Jeyaraman, M.; et al. Platelet-Rich Plasma Power-Mix Gel (ppm)—An Orthobiologic Optimization Protocol Rich in Growth Factors and Fibrin. Gels 2023, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Skin. Therapy Lett. 2019, 24, 1–6. [Google Scholar]
- Asim, M.; Shah, R.; Sharif, S.; Ouellette, S.; Shah, A.; Rao, B. A Randomized Control Trial Comparing the Efficacy of Platelet-Rich Plasma and 5% Topical Minoxidil for the Treatment of Androgenetic Alopecia. J. Drugs Dermatol. 2023, 22, 905–909. [Google Scholar]
- Amelia, K.; Hausauer, D.H.J. PRP and Microneedling in Aesthetic Medicine; Georg Thieme Verlag: New York, NY, USA, 2019; Volume 1. [Google Scholar]
- Asilian, A.; Amiri, A.; Mokhtari, F.; Faghihi, G.; Iraji, F.; Mozafarpoor, S. Platelet-rich plasma versus carboxytherapy for the treatment of periocular hyperpigmentation; which approach is superior? Dermatol. Ther. 2021, 34, e14980. [Google Scholar] [CrossRef]
- Cabrera-Ramírez, J.O.; Puebla-Mora, A.G.; González-Ojeda, A.; García-Martínez, D.; Cortés-Lares, J.A.; Márquez-Valdés, A.R.; Contreras-Hernández, G.I.; Bracamontes-Blanco, J.; Saucedo Ortiz, J.A.; Fuentes-Orozco, C. Platelet-Rich Plasma for the Treatment of Photodamage of the Skin of the Hands. Actas Dermo-Sifiliográficas 2017, 108, 746–751. [Google Scholar] [CrossRef]
- Qu, Q.; Zhou, Y.; Shi, P.; Du, L.; Fan, Z.; Wang, J.; Li, X.; Chen, J.; Zhu, D.; Ye, K.; et al. Platelet-rich plasma for androgenic alopecia: A randomized, placebo-controlled, double-blind study and combined mice model experiment. J. Cosmet. Dermatol. 2021, 20, 3227–3235. [Google Scholar] [CrossRef]
- Gawdat, H.; Allam, R.S.H.M.; Hegazy, R.; Sameh, B.; Ragab, N. Comparison of the efficacy of Fractional Radiofrequency Microneedling alone and in combination with platelet-rich plasma in neck rejuvenation: A clinical and optical coherence tomography study. J. Cosmet. Dermatol. 2022, 21, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Frautschi, R.S.; Hashem, A.M.; Halasa, B.; Cakmakoglu, C.; Zins, J.E. Current Evidence for Clinical Efficacy of Platelet Rich Plasma in Aesthetic Surgery: A Systematic Review. Aesthet. Surg. J. 2017, 37, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Trink, A.; Sorbellini, E.; Bezzola, P.; Rodella, L.; Rezzani, R.; Ramot, Y.; Rinaldi, F. A randomized, double-blind, placebo- and active-controlled, half-head study to evaluate the effects of platelet-rich plasma on alopecia areata. Br. J. Dermatol. 2013, 169, 690–694. [Google Scholar] [CrossRef]
- Vazques, O.A.; Safeek, R.H.; Becker, H. Alopecia Areata Treated with Advanced Platelet-rich Fibrin Using Micronization. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4032. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Bielli, A.; Scioli, M.G.; Orlandi, A.; Cervelli, V. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl. Med. 2015, 4, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Grimalt, R. Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol. Surg. 2016, 42, 491–497. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S.; Scioli, M.G.; Bielli, A.; Orlandi, A.; Cervelli, V. Mechanical and Controlled PRP Injections in Patients Affected by Androgenetic Alopecia. J. Vis. Exp. 2018, 131, e56406. [Google Scholar]
- Butt, G.; Hussain, I.; Ahmed, F.J.; Choudhery, M.S. Efficacy of platelet-rich plasma in androgenetic alopecia patients. J. Cosmet. Dermatol. 2019, 18, 996–1001. [Google Scholar]
- Rodrigues, B.L.; Montalvão, S.A.L.; Cancela, R.B.B.; Silva, F.A.R.; Urban, A.; Huber, S.C.; Júnior, J.L.R.C.; Lana, J.F.S.D.; Annichinno-Bizzacchi, J.M. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J. Am. Acad. Dermatol. 2019, 80, 694–700. [Google Scholar] [CrossRef]
- Shapiro, J.; Ho, A.; Sukhdeo, K.; Yin, L.; Lo Sicco, K. Evaluation of platelet-rich plasma as a treatment for androgenetic alopecia: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1298–1303. [Google Scholar] [CrossRef]
- Moftah, N.H.; Taha, N.E.; Alhabibi, A.M.; Hamdino, M. Different platelet-rich plasma preparation protocols in Female pattern hair loss: Does it affect the outcome? A pilot study. J. Cosmet. Dermatol. 2022, 21, 3316–3326. [Google Scholar] [CrossRef]
- Muhammad, A.; Iftikhar, N.; Mashhood, A.; Saleem, Z.; Sundus, M.; Khalid, A.A.; Khan, S.; Naveed, S.; Shahid, W.; Ajmal, U.; et al. Comparison of Efficacy of Platelet-Rich Plasma (PRP) With PRP Micro needling in Androgenetic Alopecia. Cureus 2022, 14, e30418. [Google Scholar] [PubMed]
- Ozcan, K.N.; Sener, S.; Altunisik, N.; Turkmen, D. Platelet rich plasma application by derma pen micro needling and intradermal point-by-point injection methods, and their comparison with clinical findings and trichoscan in patients with androgenetic alopecia. Dermatol. Ther. 2022, 35, e15182. [Google Scholar] [CrossRef]
- Wu, S.; Liu, S.; Chen, J.; Dai, D.; Liu, W.; Le, D.; Guan, Q.; Miao, Y.; Hu, Z.; Qu, Q. Evaluation of platelet-rich plasma plus basic fibroblast growth factor combined with minoxidil in the treatment of androgenetic alopecia: A randomized controlled trial. J. Cosmet. Dermatol. 2023, 22, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, Y.; Long, B.; Zhang, Y.; Zhang, C.; Zhang, S. Injections of platelet-rich plasma prepared by automatic blood cell separator combined with topical 5% minoxidil in the treatment of male androgenetic alopecia. Skin Res. Technol. 2023, 29, e13315. [Google Scholar] [CrossRef] [PubMed]
- El-Dawla, R.E.; Abdelhaleem, M.; Abdelhamed, A. Evaluation of the safety and efficacy of platelet-rich plasma in the treatment of female patients with chronic telogen effluvium: A randomized, controlled, double-blind, pilot clinical trial. Indian J. Dermatol. Venereol. Leprol. 2023, 89, 195–203. [Google Scholar] [CrossRef]
- Elnehrawy, N.Y.; Ibrahim, Z.A.; Eltoukhy, A.M.; Nagy, H.M. Assessment of the efficacy and safety of single platelet-rich plasma injection on different types and grades of facial wrinkles. J. Cosmet. Dermatol. 2017, 16, 103–111. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Niddam, J.; La Padula, S.; Noel, W.; Ezzedine, K.; Rodriguez, A.M.; Meningaud, J.P. Efficacy of autologous platelet-rich plasma combined with hyaluronic acid on skin facial rejuvenation: A prospective study. J. Am. Acad. Dermatol. 2017, 77, 584–586. [Google Scholar] [CrossRef]
- Alam, M.; Hughart, R.; Champlain, A.; Geisler, A.; Paghdal, K.; Whiting, D.; Hammel, J.A.; Maisel, A.; Rapcan, M.J.; West, D.P.; et al. Effect of Platelet-Rich Plasma Injection for Rejuvenation of Photoaged Facial Skin: A Randomized Clinical Trial. JAMA Dermatol. 2018, 154, 1447–1452. [Google Scholar] [CrossRef]
- Everts, P.A.; Pinto, P.C.; Girao, L. Autologous pure platelet-rich plasma injections for facial skin rejuvenation: Biometric instrumental evaluations and patient-reported outcomes to support antiaging effects. J. Cosmet. Dermatol. 2019, 18, 985–995. [Google Scholar] [CrossRef]
- Hassan, H.; Quinlan, D.J.; Ghanem, A. Injectable platelet-rich fibrin for facial rejuvenation: A prospective, single-center study. J. Cosmet. Dermatol. 2020, 19, 3213–3221. [Google Scholar] [CrossRef]
- Cai, J.; Tian, J.; Chen, K.; Cheng, L.H.; Xuan, M.; Cheng, B. Erbium fractional laser irradiation combined with autologous platelet-rich plasma and platelet-poor plasma application for facial rejuvenation. J. Cosmet. Dermatol. 2020, 19, 1975–1979. [Google Scholar] [CrossRef]
- Hersant, B.; SidAhmed-Mezi, M.; Aboud, C.; Niddam, J.; Levy, S.; Mernier, T.; La Padula, S.; Meningaud, J.P. Synergistic Effects of Autologous Platelet-Rich Plasma and Hyaluronic Acid Injections on Facial Skin Rejuvenation. Aesthet. Surg. J. 2021, 41, NP854–NP865. [Google Scholar] [CrossRef]
- da Silva, L.Q.; Cancela, R.B.B.; de Lima Montalvão, S.A.; Huber, S.C.; Vieira-Damiani, G.; Triglia, R.M.; Annichino-Bizzacchi, J.M. The effect of lyophilized platelet rich-plasma on skin aging: A nonrandomized, controlled, pilot trial. Arch. Dermatol. Res. 2021, 313, 863–871. [Google Scholar] [CrossRef]
- Banihashemi, M.; Zabolinejad, N.; Salehi, M.; Hamidi Alamdari, D.; Nakhaizadeh, S. Platelet-rich Plasma use for facial rejuvenation: A clinical trial and review of current literature. Acta Biomed. 2021, 92, e2021187. [Google Scholar]
- Nilforoushzadeh, M.A.; Heidari-Kharaji, M.; Alavi, S.; Mahmoudbeyk, M.; Torkamaniha, E.; Peyrovan, A.; Nouri, M.; Zare, S. Assessing the effectiveness of the combination therapy with fractional Er-YAG laser and platelet-rich plasma in treatment of periorbital dark circles patients: A clinical trial. J. Cosmet. Dermatol. 2021, 20, 3526–3536. [Google Scholar] [CrossRef]
- Mumtaz, M.; Chandio, T.H.; Shahzad, M.K.; Hanif, N.; Anwar, S.; Rafique, S. Comparing the Efficacy of Patelet-rich Plasma (PRP) versus Tranexamic Acid (4 mg/mL) as Intradermal Treatments of Melasma. J. Coll. Physicians Surg. Pak. 2021, 30, 502–505. [Google Scholar]
- Elfar, N.N.; Hasby, E.A. Efficacy and safety of plasma gel versus platelet-rich plasma in periorbital rejuvenation: A comparative split-face clinical and Antera 3D camera study. Arch. Dermatol. Res. 2022, 314, 661–671. [Google Scholar]
- Patil, N.K.; Bubna, A.K. A comparative evaluation of the efficacy of intralesional tranexamic acid versus platelet rich plasma in the treatment of melasma. Dermatol. Ther. 2022, 35, e15534. [Google Scholar] [CrossRef]
- González-Ojeda, A.; Cervantes-Guevara, G.; Chejfec-Ciociano, J.M.; Cervantes-Cardona, G.A.; Acevedo-Guzman, D.; Puebla-Mora, A.G.; Cortés-Lares, J.A.; Chávez-Tostado, M.; Álvarez-Villaseñor, A.S.; Cervantes-Pérez, E.; et al. Treatment of melasma with platelet-rich plasma: A self-controlled clinical trial. Dermatol. Ther. 2022, 35, e15703. [Google Scholar] [CrossRef]
- Shen, J.; Yu, X.; Zhuang, Y.; Kong, Y.; Zhao, J.; Yao, Z. Clinical efficacy and safety of targeted injection of PRP with skin booster in the treatment of aging face. Biotechnol. Genet. Eng. Rev. 2023, 1–17. [Google Scholar] [CrossRef]
- Mahmoodabadi, R.A.; Golafshan, H.A.; Pezeshkian, F.; Shahriarirad, R.; Namazi, M.R. Evaluation of the Effect of Platelet-Rich Fibrin Matrix in the Correction of Periorbital Wrinkles: An Experimental Clinical Trial. Dermatol. Pract. Concept. 2023, 13, e2023050. [Google Scholar] [CrossRef]
- Asif, M.; Kanodia, S.; Singh, K. Combined autologous platelet-rich plasma with micro needling verses micro needling with distilled water in the treatment of atrophic acne scars: A concurrent split-face study. J. Cosmet. Dermatol. 2016, 15, 434–443. [Google Scholar] [CrossRef]
- Deshmukh, N.S.; Belgaumkar, V.A. Platelet-Rich Plasma Augments Subcision in Atrophic Acne Scars: A Split-Face Comparative Study. Dermatol. Surg. 2019, 45, 90–98. [Google Scholar] [CrossRef]
- Krishnegowda, R.; Pradhan, S.N.; Belgaumkar, V.A. A Split-Face Study to Evaluate Efficacy of Autologous Injectable Platelet-Rich Fibrin with Microneedling Against Microneedling with Normal Saline (Placebo Control) in Atrophic Acne Scars. Dermatol. Surg. 2023, 49, 938–942. [Google Scholar] [CrossRef]
- Diab, N.A.F.; Ibrahim, A.M.; Abdallah, A.M. Fluid Platelet-Rich Fibrin (PRF) versus Platelet-Rich Plasma (PRP) in the Treatment of Atrophic Acne Scars: A Comparative Study. Arch. Dermatol. Res. 2023, 315, 1249–1255. [Google Scholar] [CrossRef]
- Guo, R.; Xuan, W.; He, X.; Xu, K. Safety and efficacy of CO2 dot matrix laser combined with platelet-rich plasma on depressed scar after acne vulgaris and influencing factors of its repair effect: A retrospective analysis. J. Cosmet. Dermatol. 2023, 22, 850–861. [Google Scholar] [CrossRef]
- Neinaa, Y.M.E.; Gheida, S.F.; Mohamed, D.A.E. Synergistic effect of platelet-rich plasma in combination with fractional carbon dioxide laser versus its combination with pulsed dye laser in striae distensae: A comparative study. Photodermatol. Photoimmunol. Photomed. 2021, 37, 214–223. [Google Scholar] [CrossRef]
- Preclaro, I.A.C.; Tianco, E.A.V.; Buenviaje-Beloso, M. Efficacy of ablative fractional carbon dioxide laser combined with autologous platelet-rich plasma versus ablative fractional carbon dioxide laser and placebo in the treatment of striae gravidarum: A randomized clinical trial. J. Cosmet. Dermatol. 2022, 21, 4354–4364. [Google Scholar] [CrossRef]
- Abdel-Motaleb, A.A.; Zedan, H.; Mostafa, M.M.; Abu-Dief, E.E.; Gebril, S.M.; Abdelwahed Hussein, M.R. Combined micro needling with topical application of platelet-rich plasma versus micro needling alone in the treatment of stria distensae: Clinicopathological analysis. J. Dermatol. Treat. 2022, 33, 836–847. [Google Scholar] [CrossRef]
- Ebrahim, H.M.; Salem, A.; Salah, T.; Eldesoky, F.; Morsi, H.M. Subcision, chemical peels, and platelet-rich plasma: Combination approaches for the treatment of striae distensae. Dermatol. Ther. 2022, 35, e15245. [Google Scholar] [CrossRef]
- de Castro Roston, J.R.; Reis, I.B.; Luzo, Â.C.M.; Roston, M.O.; Durán, N.; Fávaro, W.J. Evaluation of the tissue repair process and immunomodulatory action of Platelet-Rich Plasma (PRP) in the treatment of abdominal stretch marks. Tissue Cell 2023, 83, 102132. [Google Scholar] [CrossRef]
- Sayed, D.S.; Badary, D.M.; Ali, R.A.; Abou-Taleb, D.A.E. Combined Fractional CO2 Laser with Intradermal Platelet-Rich Plasma versus Fractional CO2 Laser Alone in the Treatment of Striae Distensae. Dermatol. Surg. 2023, 49, 552–558. [Google Scholar] [CrossRef]
- Panchaprateep, R. Medical Treatment for Androgenetic Alopecia. Facial Plast. Surg. 2023, 40, 252–266. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Dev. Ther. 2022, 16, 635–645. [Google Scholar] [CrossRef]
- Gupta, A.K.; Renaud, H.J.; Bamimore, M. Platelet-rich plasma for androgenetic alopecia: Efficacy differences between men and women. Dermatol. Ther. 2020, 33, e14143. [Google Scholar] [CrossRef]
- Beltrán, B.; Sánchez, M.A.R.; Melamed, G.; Pinto, H. Efficacy and safety of photothermal-bioactivated platelet-rich plasma for facial rejuvenation. J. Cosmet. Dermatol. 2023, 22, 671–673. [Google Scholar] [CrossRef]
- Xiao, H.; Xu, D.; Mao, R.; Xiao, M.; Fang, Y.; Liu, Y. Platelet-Rich Plasma in Facial Rejuvenation: A Systematic Appraisal of the Available Clinical Evidence. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1697–1724. [Google Scholar] [CrossRef]
- Atsu, N.; Ekinci-Aslanoglu, C.; Kantarci-Demirkiran, B.; Caf, N.; Nuhoglu, F. The Comparison of Platelet-Rich Plasma versus Injectable Platelet Rich Fibrin in Facial Skin Rejuvenation. Dermatol. Ther. 2023, 2023, 3096698. [Google Scholar] [CrossRef]
- Popescu, M.N.; Iliescu, M.G.; Beiu, C.; Popa, L.G.; Mihai, M.M.; Berteanu, M.; Ionescu, A.M. Autologous Platelet-Rich Plasma Efficacy in the Field of Regenerative Medicine: Product and Quality Control. Biomed. Res. Int. 2021, 2021, 4672959. [Google Scholar] [CrossRef]
- Lee, Z.H.; Sinno, S.; Poudrier, G.; Motosko, C.C.; Chiodo, M.; Saia, W.; Gothard, D.; Thomson, J.E.; Hazen, A. Platelet rich plasma for photodamaged skin: A pilot study. J. Cosmet. Dermatol. 2019, 18, 77–83. [Google Scholar] [CrossRef]
- Shin, M.K.; Lee, J.H.; Lee, S.J.; Kim, N.I. Platelet-rich plasma combined with fractional laser therapy for skin rejuvenation. Dermatol. Surg. 2012, 38, 623–630. [Google Scholar] [CrossRef]
- Bhatt, M.; Jamale, V.; Kale, M.; Hussain, A.A.; Nikam, B.P. Monotherapy of Biofiller for Atrophic Acne Scars: A Prospective Nonrandomized Study. J. Cutan. Aesthet. Surg. 2022, 15, 260–266. [Google Scholar]
- Cho, E.B.; Park, G.S.; Park, S.S.; Jang, Y.J.; Kim, K.H.; Kim, K.J.; Park, E.J. Effect of platelet-rich plasma on proliferation and migration in human dermal fibroblasts. J. Cosmet. Dermatol. 2019, 18, 1105–1112. [Google Scholar] [CrossRef]
- Zhang, D.D.; Zhao, W.Y.; Fang, Q.Q.; Wang, Z.C.; Wang, X.F.; Zhang, M.X.; Hu, Y.Y.; Zheng, B.; Tan, W.Q. The efficacy of fractional CO2 laser in acne scar treatment: A meta-analysis. Dermatol. Ther. 2021, 34, e14539. [Google Scholar] [CrossRef]
- Sawetz, I.; Lebo, P.B.; Nischwitz, S.P.; Winter, R.; Schaunig, C.; Brinskelle, P.; Kamolz, L.P.; Gualdi, A.; Lumenta, D.B. Platelet-rich plasma for striae distensae: What do we know about processed autologous blood contents for treating skin stretchmarks?—A systematic review. Int. Wound J. 2021, 18, 387–395. [Google Scholar] [CrossRef]
- Sany, I.; Mohamed Sobhi, R.; Badawi, A.; Mohamed Elmaadawi, Z.; Mostafa PI, N. Comparative Study between the Efficacy of Fractional CO2 Laser/Radiofrequency, PRP and a Combination of Both in the Treatment of Striae Distensae: A Pilot Study. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1687–1694. [Google Scholar] [CrossRef]
- La Padula, S.; Hersant, B.; Pizza, C.; Chesné, C.; Jamin, A.; Ben Mosbah, I.; Errico, C.; D’Andrea, F.; Rega, U.; Persichetti, P.; et al. Striae Distensae: In Vitro Study and Assessment of a Combined Treatment with Sodium Ascorbate and Platelet Rich Plasma on Fibroblasts. Aesthetic Plast. Surg. 2022, 46, 561–562. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhang, Y.; Miron, R.J. Fluid Platelet-Rich Fibrin Stimulates Greater Dermal Skin Fibroblast Cell Migration, Proliferation, and Collagen Synthesis When Compared to Platelet-Rich Plasma. J. Cosmet. Dermatol. 2019, 18, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).