Evaluating the Impact of Probiotic Therapy on the Endocannabinoid System, Pain, Sleep and Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial in Dancers
Abstract
:1. Introduction
2. Results
2.1. Anthropometric Profile, Dietary Composition
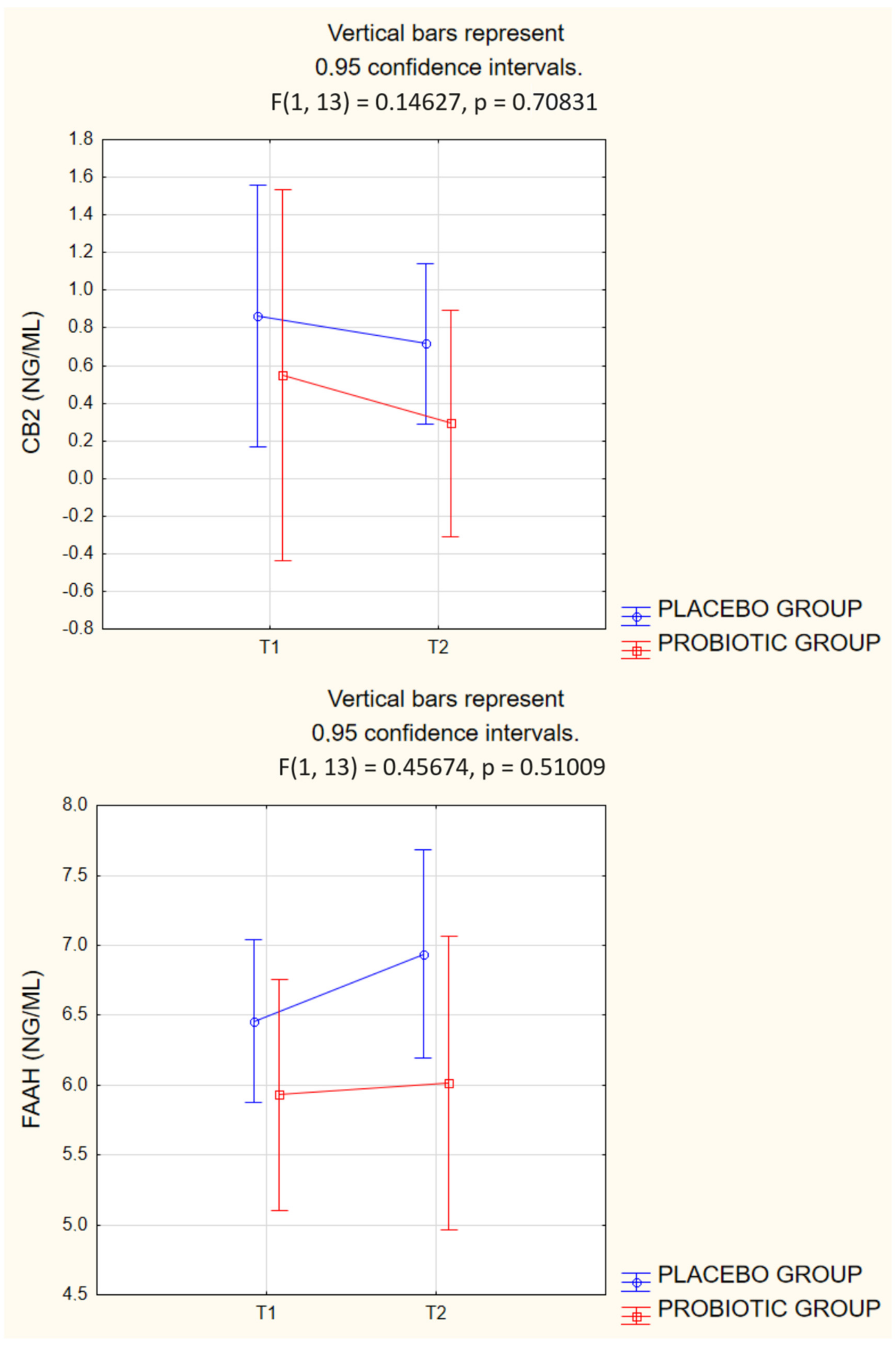
2.2. Endocannabinoid System, Pain, Sleep and Fatigue
3. Discussion
3.1. Endocannabinoid System
3.2. Pain, Sleep and Fatigue
3.3. Limitations
4. Conclusions
5. Materials and Methods
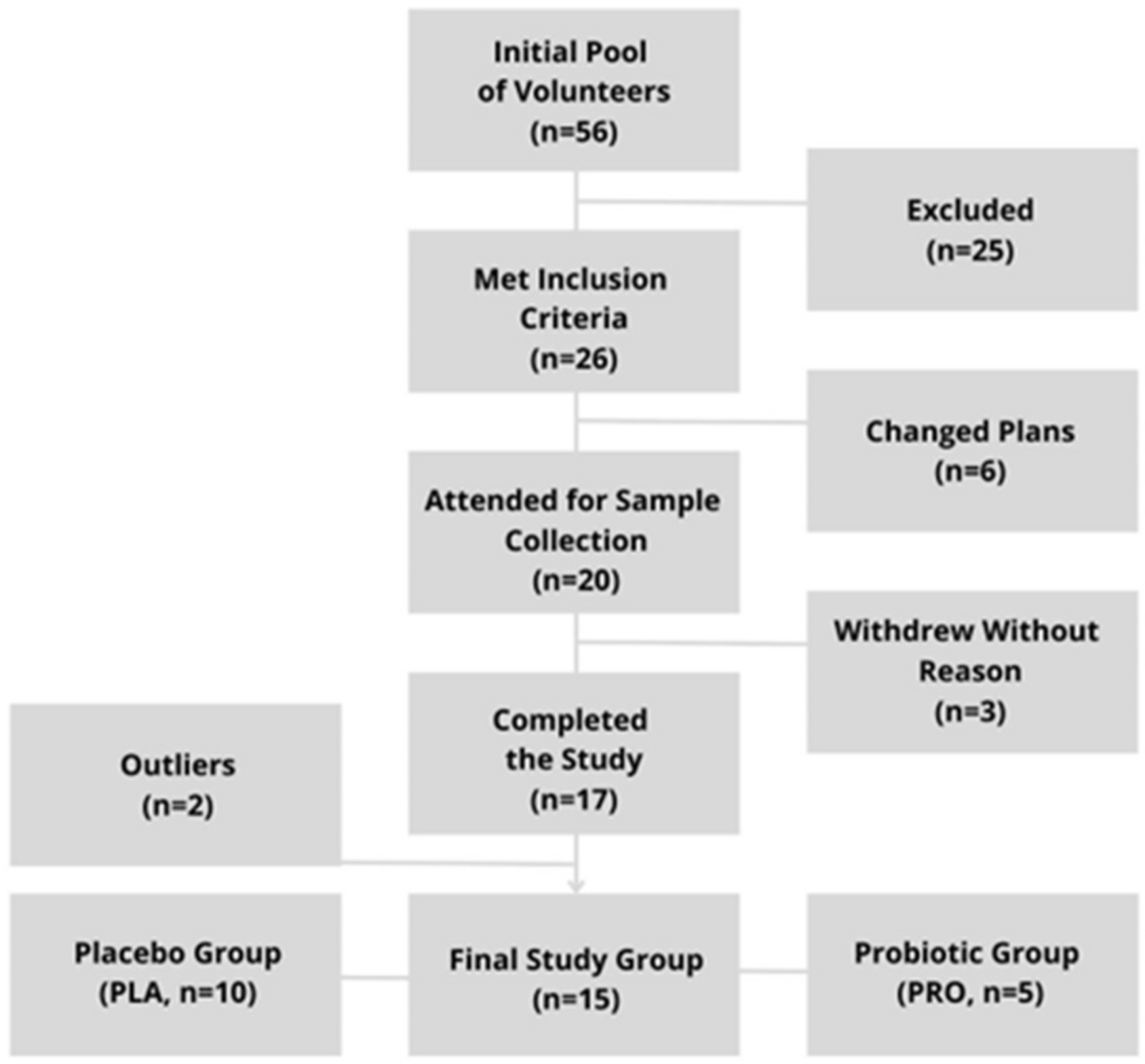
5.1. Study Group
5.2. Study Protocol
5.3. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Alger, B.E.; Kim, J. Supply and Demand for Endocannabinoids. Trends Neurosci. 2011, 34, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.I.; Nicoll, R.A. Endocannabinoid signaling in the brain. Science 2002, 296, 678–682. [Google Scholar] [CrossRef] [PubMed]
- LoVerme, J.; La Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The search for the palmitoylethanolamide receptor. Life Sci. 2005, 77, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Jonsson, K.O.; Tiger, G. Fatty acid amide hydrolase: Biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2-arachidonoylglycerol, palmitoylethanolamide, and oleamide. Biochem. Pharmacol. 2001, 62, 517–526. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Nitulescu, G.; Mihai, D.P.; Nitulescu, G.M. Identifying FAAH Inhibitors as New Therapeutic Options for the Treatment of Chronic Pain through Drug Repurposing. Pharmaceuticals 2021, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids--at the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, R.; De Meirleir, K. Exercise and brain neurotransmission. Sports Med. 1995, 20, 160–188. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Armstrong, L.E.; VanHeest, J.L. The unknown mechanism of the overtraining syndrome: Clues from depression and psychoneuroimmunology. Sports Med. 2002, 32, 185–209. [Google Scholar] [CrossRef] [PubMed]
- JanssenDuijghuijsen, L.M.; Mensink, M.; Lenaerts, K.; Fiedorowicz, E.; Protégé study group; van Dartel, D.A.; Mes, J.J.; Luiking, Y.C.; Keijer, J.; Wichers, H.J.; et al. The effect of endurance exercise on intestinal integrity in well-trained healthy men. Physiol. Rep. 2016, 4, e12994. [Google Scholar] [CrossRef] [PubMed]
- Van Winden, D.; van Rijn, R.M.; Savelsbergh, G.J.P.; Oudejans, R.R.D.; Stubbe, J.H. The Association Between Stress and Injury: A Prospective Cohort Study Among 186 First-Year Contemporary Dance Students. Front. Psychol. 2021, 12, 770494. [Google Scholar] [CrossRef] [PubMed]
- Hendry, D.; Campbell, A.; Smith, A.; Hopper, L.; Straker, L.; O’Sullivan, P. Movement quantity and quality: How do they relate to pain and disability in dancers? PLoS ONE 2022, 17, e0268444. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.; Thuru, X.; Gelot, A.; Barnich, N.; Neut, C.; Dubuquoy, L.; Dubuquoy, C.; Merour, E.; Geboes, K.; Chamaillard, M.; et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Greenwood-Van Meerveld, B.; Sarnelli, G.; Sharkey, K.A.; Storr, M.; Tack, J. Targeting the endocannabinoid system for the treatment of abdominal pain in irritable bowel syndrome. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Zielińska, A.; Scott, R.J.; Słomski, R.; Pławski, A. Endocannabinoid System as a Promising Therapeutic Target in Inflammatory Bowel Disease—A Systematic Review. Front. Immunol. 2021, 12, 790803. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Clinical endocannabinoid deficiency (CECD): Can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol. Lett. 2004, 25, 31–39. [Google Scholar]
- Ringel-Kulka, T.; Goldsmith, J.R.; Carroll, I.M.; Barros, S.P.; Palsson, O.; Jobin, C.; Ringel, Y. Lactobacillus acidophilus NCFM affects colonic mucosal opioid receptor expression in patients with functional abdominal pain—A randomised clinical study. Aliment. Pharmacol. Ther. 2014, 40, 200–207. [Google Scholar] [CrossRef]
- Liu, M.; Tandorost, A.; Moludi, J.; Dey, P. Prebiotics Plus Probiotics May Favorably Impact on Gut Permeability, Endocannabinoid Receptors, and Inflammatory Biomarkers in Patients with Coronary Artery Diseases: A Clinical Trial. Food Sci. Nutr. 2023, 12, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Gioacchini, G.; Pengo, G.; Suchodolski, J.S.; Jergens, A.E.; Allenspach, K.; Gavazza, A.; Scarpona, S.; Berardi, S.; Galosi, L.; et al. Enterocolic increase of cannabinoid receptor type 1 and type 2 and clinical improvement after probiotic administration in dogs with chronic signs of colonic dysmotility without mucosal inflammatory changes. Neurogastroenterol. Motil. 2020, 32, e13717. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, M.; Cerdà-Cuéllar, M.; Martínez, V. Antibiotic-induced dysbiosis alters host-bacterial interactions and leads to colonic sensory and motor changes in mice. Gut Microbes 2015, 6, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, J.T.; Harui, A.; Kiertscher, S.M.; Roth, J.D.; Roth, M.D. Differential expression of intracellular and extracellular CB(2) cannabinoid receptor protein by human peripheral blood leukocytes. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2013, 8, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Barbetti, M.; Mancabelli, L.; Vacondio, F.; Longhi, G.; Ferlenghi, F.; Viglioli, M.; Turroni, F.; Carnevali, L.; Mor, M.; Ventura, M.; et al. Social stress-induced depressive-like symptoms and changes in gut microbial and lipidomic profiles are prevented by pharmacological inhibition of FAAH activity in male rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 131, 110963. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, F.; Sotoodehnejadnematalahi, F.; Hajebrahimi, Z.; Fateh, A.; Siadat, S.D. Effects of active, inactive, and derivatives of Akkermansia muciniphila on the expression of the endocannabinoid system and PPARs genes. Sci. Rep. 2022, 12, 10031. [Google Scholar] [CrossRef] [PubMed]
- Steve, P.H. Alexander, Fatty Acid Amide Hydrolase (FAAH). In Bylund, xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., David, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–7. ISBN 9780080552323. [Google Scholar] [CrossRef]
- Atamaniuk, J.; Vidotto, C.; Tschan, H.; Bachl, N.; Stuhlmeier, K.M.; Müller, M.M. Increased concentrations of cell-free plasma DNA after exhaustive exercise. Clin. Chem. 2004, 50, 1668–1670. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Destouni, A.; Margonis, K.; Jamurtas, A.Z.; Vrettou, C.; Kouretas, D.; Mastorakos, G.; Mitrakou, A.; Taxildaris, K.; Kanavakis, E.; et al. Cell-free plasma DNA as a novel marker of aseptic inflammation severity related to exercise overtraining. Clin. Chem. 2006, 52, 1820–1824. [Google Scholar] [CrossRef] [PubMed]
- Fatahi, S.; Hosseini, A.; Sohouli, M.H.; Sayyari, A.; Khatami, K.; Farsani, Z.F.; Amiri, H.; Dara, N.; de Souza, I.G.O.; Santos, H.O. Effects of probiotic supplementation on abdominal pain severity in pediatric patients with irritable bowel syndrome: A systematic review and meta-analysis of randomized clinical trials. World J. Pediatr. 2022, 18, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.; Gordon, M.; Sinopoulou, V.; Akobeng, A.K. Probiotics for management of functional abdominal pain disorders in children. Cochrane Database Syst. Rev. 2023, 2, CD012849. [Google Scholar] [CrossRef] [PubMed]
- Didari, T.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J. Gastroenterol. 2015, 21, 3072–3084. [Google Scholar] [CrossRef] [PubMed]
- Dardmeh, F.; Nielsen, H.I.; Alipour, H.; Kjærgaard, B.; Brandsborg, E.; Gazerani, P. Potential Nociceptive Regulatory Effect of Probiotic Lactobacillus rhamnosus PB01 (DSM 14870) on Mechanical Sensitivity in Diet-Induced Obesity Model. Pain Res. Manag. 2016, 2016, 5080438. [Google Scholar] [CrossRef] [PubMed]
- Alipour, H.; Gazerani, P.; Heidari, M.; Dardmeh, F. Modulatory Effect of Probiotic Lactobacillus rhamnosus PB01 on Mechanical Sensitivity in a Female Diet-Induced Obesity Model. Pain Res. Manag. 2021, 2021, 5563959. [Google Scholar] [CrossRef] [PubMed]
- Gil-Hernández, E.; Ruiz-González, C.; Rodriguez-Arrastia, M.; Ropero-Padilla, C.; Rueda-Ruzafa, L.; Sánchez-Labraca, N.; Roman, P. Effect of gut microbiota modulation on sleep: A systematic review and meta-analysis of clinical trials. Nutr. Rev. 2023, 81, 1556–1570. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Moon, J.M.; Walden, K.E.; Hagele, A.M.; Allen, L.E.; Gaige, C.J.; Krieger, J.M.; Jäger, R.; Pane, M.; Mumford, P. Multi-strain probiotic improves subjective sleep quality with no impact on body composition, hemodynamics, and physical activity. Benef. Microbes 2024, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Lu, J.; Qiao, G.; Mao, X.; Zhao, J.; Wang, G.; Tian, P.; Chen, W. Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial. Nutrients 2023, 15, 4700. [Google Scholar] [CrossRef]
- Aslan Çİn, N.N.; Açik, M.; Tertemİz, O.F.; Aktan, Ç.; Akçali, D.T.; Çakiroğlu, F.P.; Özçelİk, A.Ö. Effect of prebiotic and probiotic supplementation on reduced pain in patients with fibromyalgia syndrome: A double-blind, placebo-controlled randomized clinical trial. Psychol. Health Med. 2024, 29, 528–541. [Google Scholar] [CrossRef] [PubMed]
- McBride, C.; Bronner, S. Injury characteristics in professional modern dancers: A 15-year analysis of work-related injury rates and patterns. J. Sports Sci. 2022, 40, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.C.; Mendes, L.A.; Mota, L.A.; Lima, P.O.; Almeida, G.P. Training Load, Pain Intensity, and Functioning Can Explain Injuries in Dancers: A Classification and Regression Tree (CART) Analysis. Med. Probl. Perform. Artist. 2022, 37, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Xu, F.; Liu, G.; Pang, B.; Liao, N.; Li, H.; Shi, J. Gut microbiota as a potential target for developing anti-fatigue foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 3065–3080. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M. Intestinal bacteria associated with irritable bowel syndrome and chronic fatigue. Neurogastroenterol. Motil. 2023, 35, e14621. [Google Scholar] [CrossRef] [PubMed]
- Obermoser, K.; Brigo, N.; Schroll, A.; Monfort-Lanzas, P.; Gostner, J.M.; Engl, S.; Geisler, S.; Knoll, M.; Schennach, H.; Weiss, G.; et al. Positive Effects of Probiotic Therapy in Patients with Post-Infectious Fatigue. Metabolites 2023, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Roman, P.; Carrillo-Trabalón, F.; Sánchez-Labraca, N.; Cañadas, F.; Estévez, A.F.; Cardona, D. Are probiotic treatments useful on fibromyalgia syndrome or chronic fatigue syndrome patients? A systematic review. Benef. Microbes 2018, 9, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, A.; Nord, C.E.; Evengård, B. Effect of supplement with lactic-acid producing bacteria on fatigue and physical activity in patients with chronic fatigue syndrome. Nutr. J. 2009, 8, 4. [Google Scholar] [CrossRef] [PubMed]
| PLA (n = 11) | PRO (n = 5) | Total (n = 16) | PLA vs. PRO | |
|---|---|---|---|---|
| Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | t-Test/U Mann-Whitney (p Value) | |
| Age [years] | 20.55 ± 1.04 (19–22) | 20.00 ± 1.30 (19–22) | 20.44 ± 1.09 (19–22) | 0.55 |
| Body mass [kg] | 58.07 ± 6.95 (49.40–68.70) | 60.10 ± 7.31 (48.60–68.30) | 58.08 ± 6.81 (48.60–68.70) | 0.99 |
| BMI (body mass index) [kg/m2] | 21.05 ± 2.18 (17.70–23.40) | 20.80 ± 2.29 (18.10–25.10) | 21.02 ± 2.13 (17.70–25.10) | 0.93 |
| Fat [% body mass] | 27 ± 4 (21–31) | 27 ± 3 (25–31) | 27 ± 3 (21–31) | 0.84 |
| Physical activity level [hours per week] | 17 h 7 min ± 6 h 59 min (8 h–33 h) | 16 h ± 9 h 46 min (9 h 30 min–29 h) | 16 h 46 min ± 7 h 38 min (8 h–33 h) | 0.69 |
| Hand-grip test [kg] | 28.13 ± 4.67 (20.07–38.13) | 26.16 ± 5.33 (17.5–30.2) | 27.51 ± 4.80 (17.5–38.13) | 0.73 |
| PLA (n = 11) | PRO (n = 5) | Total (n = 16) | PLA vs. PRO | |
|---|---|---|---|---|
| Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | Mean ± SD (Min–Max) | t-Test/U Mann-Whitney (p Value) | |
| Energy [kcal] | 1999.23 ± 279.81 (1588.93–2578.45) | 2325.54 ± 425.00 (1835.0–2842.6) | 2101.20 ± 353.22 (1588.93–2842.6) | 0.26 |
| Protein [g] | 85.29 ± 30.13 (49.52–154.26) | 100.47 ± 21.09 (79.42–130.92) | 90.03 ± 27.87 (49.52–154.26) | 0.51 |
| Fat [g] | 74.56 ± 13.93 (57.69–95.74) | 90.47 ± 18.72 (67.02–115.73) | 79.54 ± 16.76 (57.69–115.73) | 0.41 |
| Carbohydrates [g] | 271.12 ± 53.14 (194.89–359.42) | 298.79 ± 57.98 (240.54–376.75) | 279.77 ± 54.36 (194.89–376.75) | 0.75 |
| Fiber [g] | 28.96 ± 15.13 (16.69–47.68) | 21.36 ± 12.67 (15.03–27.63) | 26.59 ± 14.44 (15.03–47.68) | 0.91 |
| PLA (n = 10) Mean ± SD (Min–Max) | PRO (n = 5) Mean ± SD (Min–Max) | 2-Way ANOVA p-Value; (η2) | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | GROUP | TIME | GROUP × TIME | |
| CB2 [ng/mL] | 0.86 ± 1.21 | 0.72 ± 0.75 | 0.55 ± 0.32 | 0.29 ± 0.11 | 0.4168 (0.0513) | 0.1865 (0.1302) | 0.7083 (0.0111) |
| FAAH [ng/mL] | 6.46 ± 0.68 | 6.94 ± 1.13 | 5.93 ± 1.15 | 6.02 ± 0.97 | 0.1293 (0.1679) | 0.3500 (0.0674) | 0.5100 (0.0341) |
| PLA (n = 10) Mean ± SD (Min–Max) | PRO (n = 5) Mean ± SD (Min–Max) | 2-Way ANOVA p-Value; (η2) | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | GROUP | TIME | GROUP × TIME | |
| Abdominal pain (ROME IV) [0 (never)–10 (always)] | 4.1 ± 1.60 | 3.5 ± 1.43 | 3 ± 1.58 | 2.8 ± 1.79 | 0.2896 (0.0857) | 0.1710 (0.1391) | 0.4815 (0.0388) |
| Pressure–pain test [N] | 26.4 ± 15.04 | 22.5 ± 7.04 | 20.9 ± 5.95 | 19.9 ± 5.46 | 0.4317 (0.0482) | 0.3696 (0.0623) | 0.5781 (0.0244) |
| Sleep quality (PSQI) [0 (best)–3 (worst)] | 0.8 ± 0.63 | 1.2 ± 0.42 | 1.4 ± 0.55 | 1 ± 1.23 | 0.5365 (0.0301) | 1.000 (0.0000) | 0.0784 (0.2192) |
| Sleep latency (PSQI) [min] | 17.6 ± 22.58 | 27.6 ± 36.24 | 15.2 ± 13.52 | 15 ± 10.60 | 0.5920 (0.0227) | 0.2511 (0.1000) | 0.2333 (0.1073) |
| Fatigue (FAS) (0–32) | 15.9 ± 8.03 | 13.1 ± 5.41 | 18.6 ± 2.70 | 12.4 ± 4.62 | 0.7387 (0.0089) | 0.0130 (0.3888) | 0.2970 (0.0832) |
| Active strategies (Mini-COPE) [0 (never)–4 (always)] | 3.2 ± 0.75 | 2.9 ± 0.74 | 3.1 ± 0.89 | 2.8 ± 1.10 | 0.8171 (0.0043) | 0.0977 (0.1967) | 1.0000 (0.0000) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiącek, J.; Podgórski, T.; Kusy, K.; Łoniewski, I.; Skonieczna-Żydecka, K.; Karolkiewicz, J. Evaluating the Impact of Probiotic Therapy on the Endocannabinoid System, Pain, Sleep and Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial in Dancers. Int. J. Mol. Sci. 2024, 25, 5611. https://doi.org/10.3390/ijms25115611
Wiącek J, Podgórski T, Kusy K, Łoniewski I, Skonieczna-Żydecka K, Karolkiewicz J. Evaluating the Impact of Probiotic Therapy on the Endocannabinoid System, Pain, Sleep and Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial in Dancers. International Journal of Molecular Sciences. 2024; 25(11):5611. https://doi.org/10.3390/ijms25115611
Chicago/Turabian StyleWiącek, Jakub, Tomasz Podgórski, Krzysztof Kusy, Igor Łoniewski, Karolina Skonieczna-Żydecka, and Joanna Karolkiewicz. 2024. "Evaluating the Impact of Probiotic Therapy on the Endocannabinoid System, Pain, Sleep and Fatigue: A Randomized, Double-Blind, Placebo-Controlled Trial in Dancers" International Journal of Molecular Sciences 25, no. 11: 5611. https://doi.org/10.3390/ijms25115611






