Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy
Abstract
1. Introduction
2. Mechanistic Study of Noble Metal Nanoparticles for Photothermal Therapy in Cancer Therapy
3. Noble Metal Nanomaterials in Cancer Therapy
3.1. Gold Nanoparticles
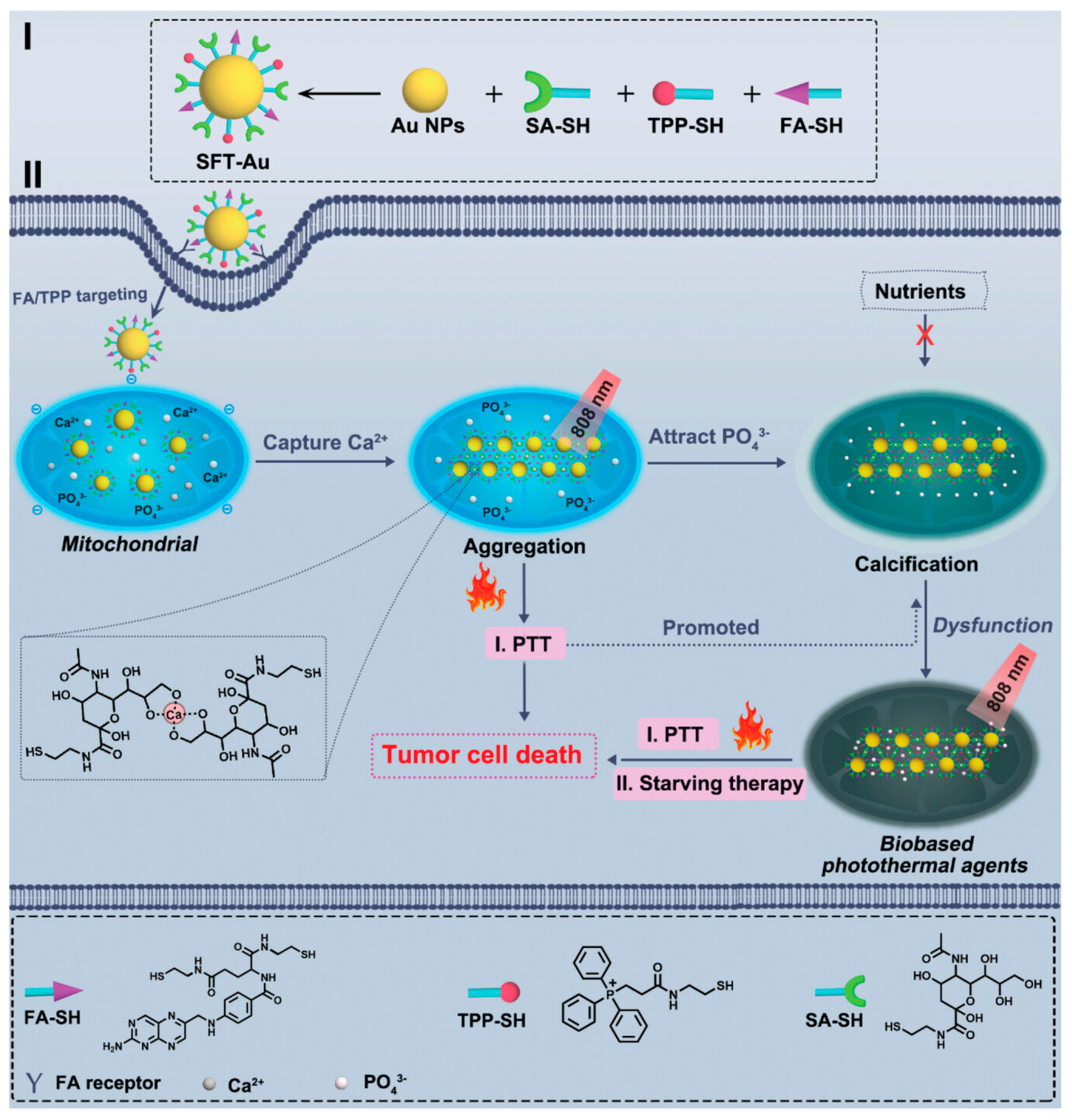
3.1.1. Photothermal Therapy
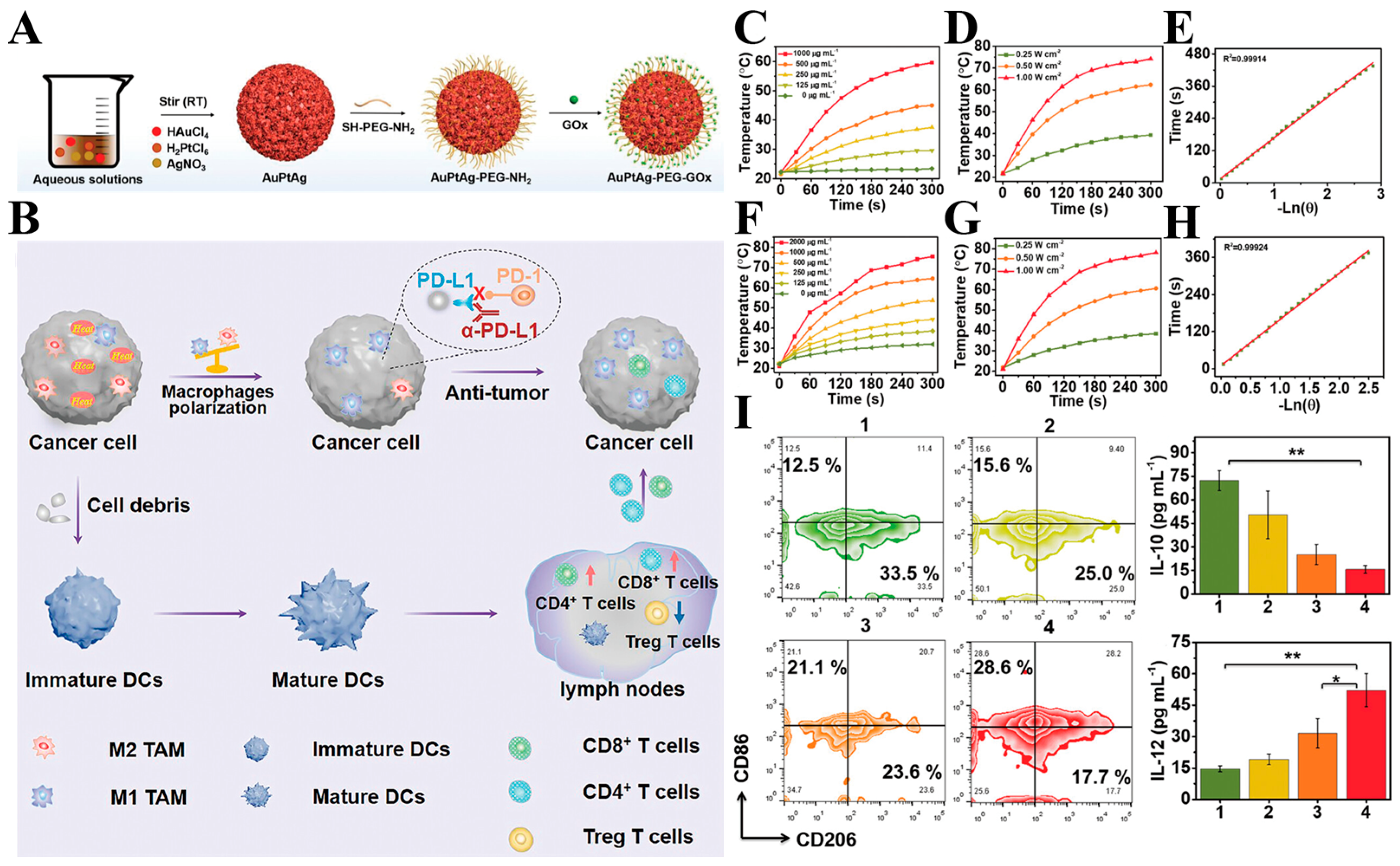
3.1.2. Combined Photothermal and Immunotherapy Therapy
3.1.3. Other Combined Therapies
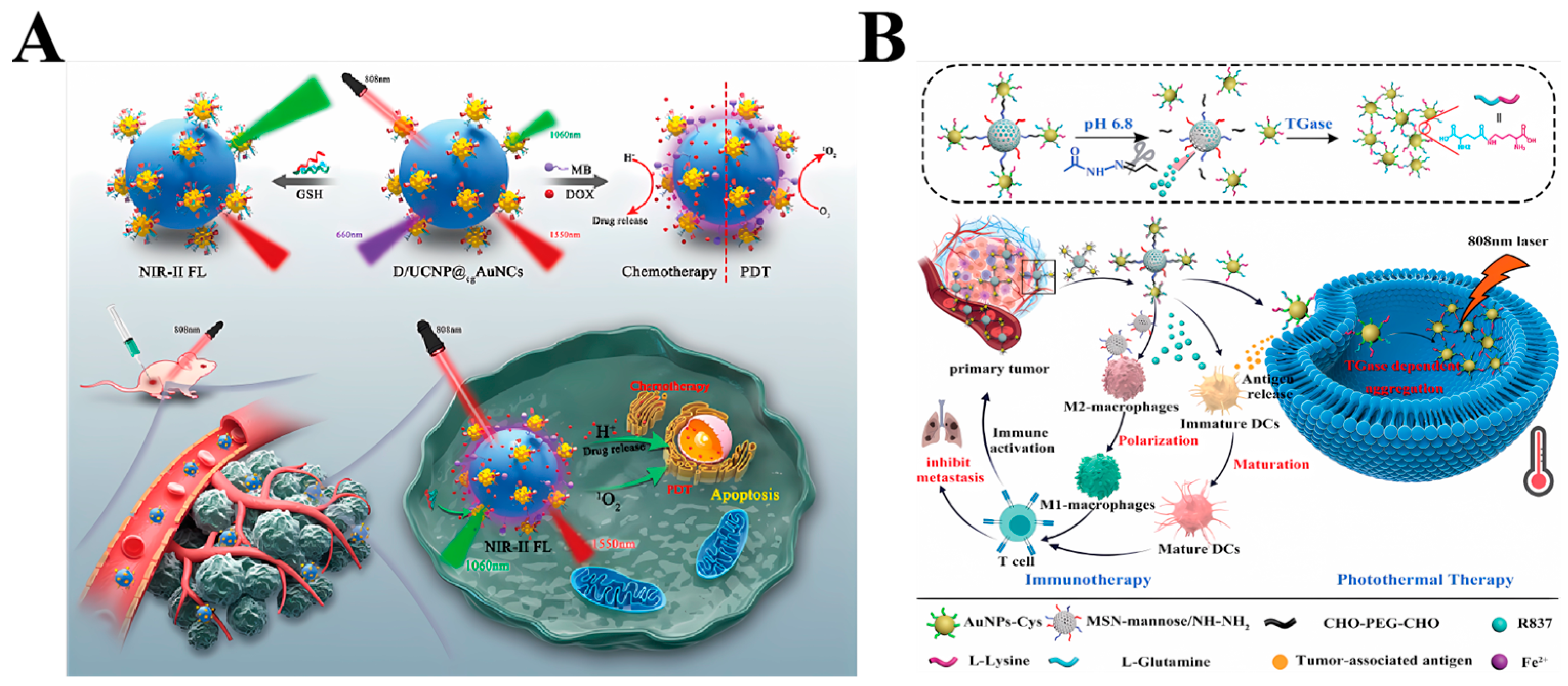
3.1.4. Biological Imaging
3.1.5. Drug Delivery
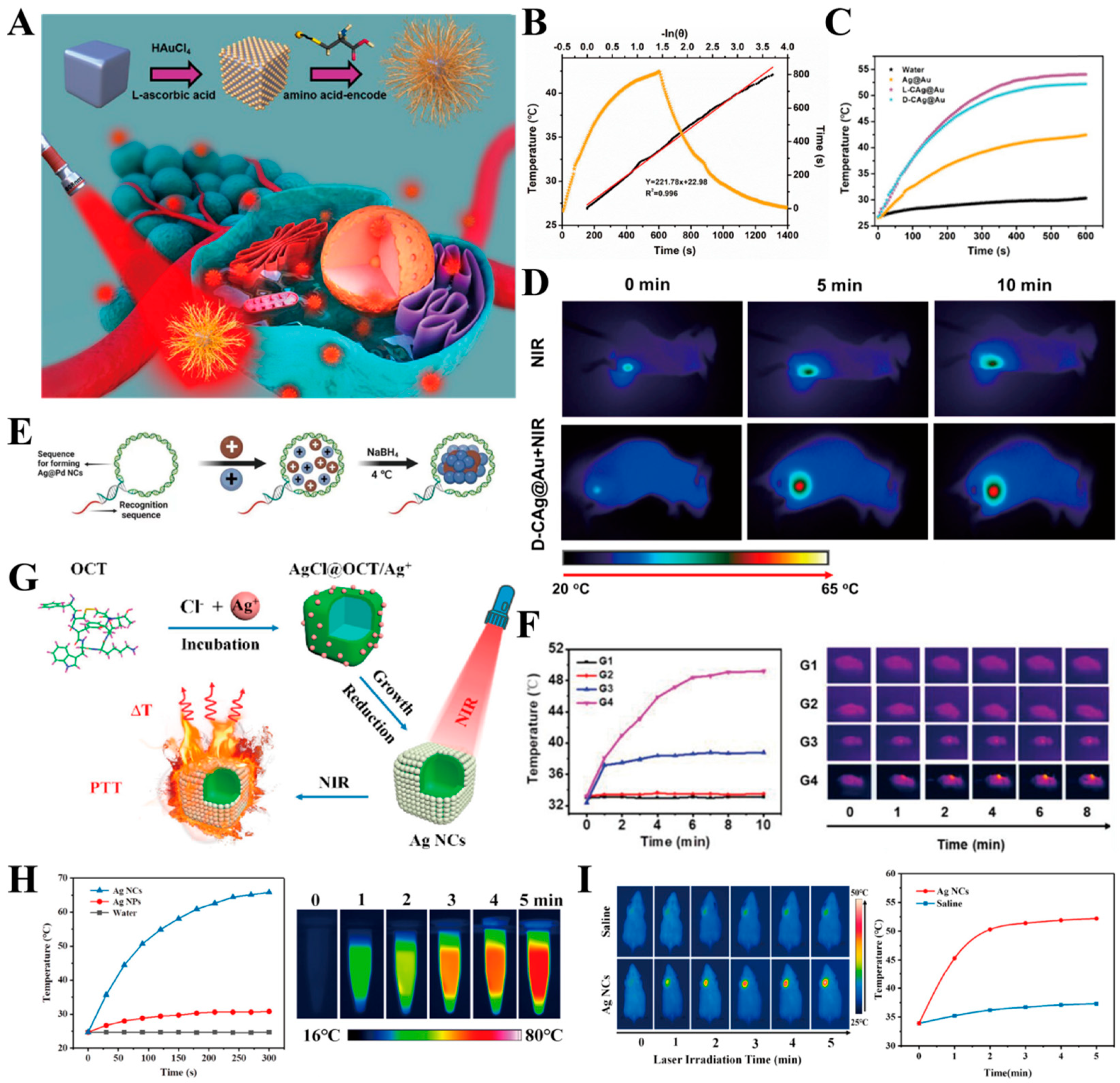
3.2. Silver Nanoparticles
3.2.1. Photothermal Therapy

3.2.2. Combined Photothermal and Immune Therapy
3.2.3. Other Combination Therapies
3.2.4. Biological Imaging
3.2.5. Drug Delivery
3.3. Platinum Nanoparticles
3.4. Palladium Nanoparticles
4. The Advantages and Disadvantages of Noble Metal Nanoparticles
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| •OH | hydroxyl radicals |
| PTT | Photothermal therapy |
| PTA | Photothermal transducer |
| PDT | Photodynamic therapy |
| SDT | Sonodynamic therapy |
| SPR | Surface plasmon resonance |
| LSPR | Localized surface plasmon resonance |
| PNPs | Plasma nanoparticles |
| AuNSs | Gold nanospheres |
| AuNRs | Gold nanorods |
| AuNSTs | Gold nanostars |
| DCs | Dendritic cells |
| ACT | Adoptive cell therapy |
| NCs | nanocomplexes |
| NIPAM | (HPMA)-co-N-(1-vinyl-2-pyrrolidone) |
| ROS | Reactive oxygen species |
| THPP | Tetra(4-hydroxyphenyl)porphyrin |
| NIRF | Near-infrared fluorescence |
| PAI | Photoacoustic imaging |
| PA | Photoacoustic |
| NCT | Nanocatalytic therapy |
| CQDs | Colloidal quantum dots |
| PD-1 | Programmed death receptor |
| PD-L1 | Programmed death ligand 1 |
| CTLA-4 | Cytotoxic t-lymphocyte antigen 4 |
| ICD | Immunogenic cell death |
| SCF | Sliver-containing films |
| MRI | Magnetic resonance imaging |
| CDT | Chemodynamic |
| DC | Dendritic cell |
| SDT | Sonodynamic therapy |
| XPS | X-ray photoelectron spectroscopy |
| MOFs | Metal–organic Frameworks |
| OCT | Octreotide |
| SERS | Surface-Enhanced Raman spectroscopy |
| H2O2 | hydrogen peroxide |
References
- Kamineni, A.; Doria-Rose, V.P.; Chubak, J.; Inadomi, J.M.; Corley, D.A.; Haas, J.S.; Kobrin, S.C.; Winer, R.L.; Elston Lafata, J.; Beaber, E.F.; et al. Evaluation of Harms Reporting in U.S. Cancer Screening Guidelines. Ann. Intern. Med. 2022, 175, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Agatha, C.M.; Wijovi, F.; Putri, H.; Kurniawan, A. Risk of Sarcopenia as the Side Effect of Chemotherapy among Breast Cancer Patients: Preliminary Study. Ann. Oncol. 2018, 29, vii82. [Google Scholar] [CrossRef]
- Tang, W.; Zhen, Z.; Wang, M.; Wang, H.; Chuang, Y.; Zhang, W.; Wang, G.D.; Todd, T.; Cowger, T.; Chen, H.; et al. Red Blood Cell-Facilitated Photodynamic Therapy for Cancer Treatment. Adv. Funct. Mater. 2016, 26, 1757–1768. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Cai, Z.; Huang, K.; Yu, T.; Lan, H.; Zhang, Q.; Wu, L.; Luo, H. Tumor Microenvironment-Sensitive Ca2+ Nanomodulator Combined with the Sonodynamic Process for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2024, 16, 8275–8288. [Google Scholar] [CrossRef]
- Yue, H.; Yuan, L.; Zhang, W.; Zhang, S.; Wei, W.; Ma, G. Macrophage Responses to the Physical Burden of Cell-Sized Particles. J. Mater. Chem. B 2018, 6, 393–400. [Google Scholar] [CrossRef]
- Tang, Z.; Hou, Y.; Huang, S.; Hosmane, N.S.; Cui, M.; Li, X.; Suhail, M.; Zhang, H.; Ge, J.; Iqbal, M.Z.; et al. Dumbbell-Shaped Bimetallic AuPd Nanoenzymes for NIR-II Cascade Catalysis-Photothermal Synergistic Therapy. Acta Biomater. 2024, 177, 431–443. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhang, J.; Han, T.; Hu, X.; Lee, M.M.S.; Wang, D.; Tang, B.Z. A Facile Strategy of Boosting Photothermal Conversion Efficiency through State Transformation for Cancer Therapy. Adv. Mater. 2021, 33, 2105999. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.; Sun, L.; Liu, B. Mesoporous Noble Metal–Metalloid/Nonmetal Alloy Nanomaterials: Designing Highly Efficient Catalysts. ACS Nano 2021, 15, 18661–18670. [Google Scholar] [CrossRef]
- Mao, P.; Liu, C.; Niu, Y.; Qin, Y.; Song, F.; Han, M.; Palmer, R.E.; Maier, S.A.; Zhang, S. Disorder-Induced Material-Insensitive Optical Response in Plasmonic Nanostructures: Vibrant Structural Colors from Noble Metals. Adv. Mater. 2021, 33, 2007623. [Google Scholar] [CrossRef]
- Dalmases, M.; Ibáñez, M.; Torruella, P.; Fernàndez-Altable, V.; López-Conesa, L.; Cadavid, D.; Piveteau, L.; Nachtegaal, M.; Llorca, J.; Ruiz-González, M.L.; et al. Synthesis and Thermoelectric Properties of Noble Metal Ternary Chalcogenide Systems of Ag–Au–Se in the Forms of Alloyed Nanoparticles and Colloidal Nanoheterostructures. Chem. Mater. 2016, 28, 7017–7028. [Google Scholar] [CrossRef]
- You, J.; Liu, L.; Huang, W.; Manners, I.; Dou, H. Correction to “Redox-Active Micelle-Based Reaction Platforms for In Situ Preparation of Noble Metal Nanocomposites with Photothermal Conversion Capability”. ACS Appl. Mater. Interfaces 2021, 13, 32599. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Wang, Y.; Li, X.; Qi, K.; Wang, J.; Xu, H. Black Silver Nanocubes@Amino Acid-Encoded Highly Branched Gold Shells with Efficient Photothermal Conversion for Tumor Therapy. ACS Appl. Mater. Interfaces 2023, 15, 236–248. [Google Scholar] [CrossRef]
- Hussein, E.; Zagho, M.; Nasrallah, G.; Elzatahry, A. Recent Advances in Functional Nanostructures as Cancer Photothermal Therapy. Int. J. Nanomed. 2018, 13, 2897–2906. [Google Scholar] [CrossRef]
- Yang, W.; Liang, H.; Ma, S.; Wang, D.; Huang, J. Gold Nanoparticle Based Photothermal Therapy: Development and Application for Effective Cancer Treatment. Sustain. Mater. Technol. 2019, 22, e00109. [Google Scholar] [CrossRef]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, 2001806. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Joshi, R.; Kaur, G.; Garg, V.K.; Sak, K.; Varol, M.; Kaur, J.; Alharbi, S.A.; Alahmadi, T.A.; Aggarwal, D.; et al. Metal Nanoparticles in Cancer: From Synthesis and Metabolism to Cellular Interactions. J. Nanostructure Chem. 2023, 13, 321–348. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, N.; Xu, J.; Zhu, X.; Ling, G.; Zhang, P. Novel Strategies in Melanoma Treatment Using Silver Nanoparticles. Cancer Lett. 2023, 561, 216148. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical Development and Potential of Photothermal and Photodynamic Therapies for Cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Q.; Li, J.; Peng, S.; Wang, X.; Cai, R. Near-Infrared Photoactivated Nanomedicines for Photothermal Synergistic Cancer Therapy. Nano Today 2021, 37, 101073. [Google Scholar] [CrossRef]
- Cullion, K.; Ostertag-Hill, C.A.; Pan, M.; Timko, B.; Boscolo, E.; Kohane, D.S. Ablation of Venous Malformations by Photothermal Therapy with Intravenous Gold Nanoshells. Nano Lett. 2023, 23, 7092–7099. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Baek, Y.; Ha, T.; Choi, D.; Lee, W.J.; Cho, Y.; Park, J.; Kim, S.; Doh, J. Gold Nanoparticle-Carrying T Cells for the Combined Immuno-Photothermal Therapy. Small 2023, 19, 2301377. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, X.; Du, Y.; Han, M.; Wang, Z.; Wang, Y.; Yan, F.; Liu, Y. Gold Nanorod–Mesoporous Silica Core Shell Nanocomposites for NIR-II Photothermal Ablation and Dual PD-L1/VEGF Blockade Therapy in Hepatocellular Carcinoma. Chem. Eng. J. 2023, 459, 141426. [Google Scholar] [CrossRef]
- Sun, R.; Liu, Y.; Chen, Y.; Jiang, Q.; Chen, P.; Shuai, Q.; Luo, Z.; Yang, X.; Jiang, Y.; Hu, Y.; et al. Efficient Enhancement of Photoluminescence and Second-Harmonic Generation of Few-Layer InSe Coupled with Surface-Plasmonic Ag Prism Array. Sci. China Mater. 2023, 66, 2788–2794. [Google Scholar] [CrossRef]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape Effects of Gold Nanoparticles in Photothermal Cancer Therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, S.; Guo, Y.; Zhang, H.; Ma, K.; Wang, J.; Zhao, Q.; Zhou, L.; Cai, W. Defect Damping-Enhanced Plasmonic Photothermal Conversion. ACS Nano 2023, 17, 10300–10312. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.Y.; Huang, C.Z.; Zhan, L.; Zhou, J. Plasmonic Single Nanoparticle for Resonance Light Scattering Imaging Analysis and Applications. TrAC Trends Anal. Chem. 2023, 164, 117090. [Google Scholar] [CrossRef]
- Zeng, X.; Yan, S.; Di, C.; Lei, M.; Chen, P.; Du, W.; Jin, Y.; Liu, B.-F. “All-in-One” Silver Nanoprism Platform for Targeted Tumor Theranostics. ACS Appl. Mater. Interfaces 2020, 12, 11329–11340. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart Gold Nanostructures for Light Mediated Cancer Theranostics: Combining Optical Diagnostics with Photothermal Therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef]
- Wu, P.; Gao, Y.; Lu, Y.; Zhang, H.; Cai, C. High Specific Detection and Near-Infrared Photothermal Therapy of Lung Cancer Cells with High SERS Active Aptamer–Silver–Gold Shell–Core Nanostructures. Analyst 2013, 138, 6501. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Qiu, Y.; Li, W.; Guo, X.; Li, Q.; Zhang, H.; Zhou, J.; Du, Y.; Yuan, H.; et al. Specific Photothermal Therapy to the Tumors with High EphB4 Receptor Expression. Biomaterials 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Kolovskaya, O.S.; Zamay, T.N.; Belyanina, I.V.; Karlova, E.; Garanzha, I.; Aleksandrovsky, A.S.; Kirichenko, A.; Dubynina, A.V.; Sokolov, A.E.; Zamay, G.S.; et al. Aptamer-Targeted Plasmonic Photothermal Therapy of Cancer. Mol. Ther.—Nucleic Acids 2017, 9, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Tang, X.; Liu, J.; Zhu, Z.; Mu, W.; Tang, W.; Zhang, Y.; Chen, X. Precise Starving Therapy via Physiologically Dependent Photothermal Conversion Promoted Mitochondrial Calcification Based on Multi-Functional Gold Nanoparticles for Effective Tumor Treatment. Adv. Funct. Mater. 2023, 33, 2303596. [Google Scholar] [CrossRef]
- Villar-Alvarez, E.; Golán-Cancela, I.; Pardo, A.; Velasco, B.; Fernández-Vega, J.; Cambón, A.; Al-Modlej, A.; Topete, A.; Barbosa, S.; Costoya, J.A.; et al. Inhibiting HER3 Hyperphosphorylation in HER2-Overexpressing Breast Cancer through Multimodal Therapy with Branched Gold Nanoshells. Small 2023, 19, 2303934. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, T.; He, T.; Li, Y.; Yin, S. Cancer Cell Membrane–Encapsulated Biomimetic Nanoparticles for Tumor Immuno-Photothermal Therapy. Chem. Eng. J. 2023, 463, 142495. [Google Scholar] [CrossRef]
- Hsieh, H.-H.; Chen, C.-L.; Chan, H.-W.; Chi, K.-H.; Wu, C.-Y. Enhanced Antitumour Response of Gold Nanostar-Mediated Photothermal Therapy in Combination with Immunotherapy in a Mouse Model of Colon Carcinoma. Br. J. Cancer 2023, 130, 406–416. [Google Scholar] [CrossRef]
- Xiao, G.; Zhao, Y.; Wang, X.; Zeng, C.; Luo, F.; Jing, J. Photothermally Sensitive Gold Nanocage Augments the Antitumor Efficiency of Immune Checkpoint Blockade in Immune “Cold” Tumors. Front. Immunol. 2023, 14, 1279221. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, M.; Chang, X.; Tang, X.; Yuan, P.; Tian, R.; Zhu, Z.; Zhang, Y.; Chen, X. Tumor-Specific Photothermal-Therapy-Assisted Immunomodulation via Multiresponsive Adjuvant Nanoparticles. Adv. Mater. 2023, 35, 2300086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, J.; Qiu, X.; Liu, Y.; Feng, W.; Li, F. Upconversion Nanocomposite for Programming Combination Cancer Therapy by Precise Control of Microscopic Temperature. Nat. Commun. 2018, 9, 2176. [Google Scholar] [CrossRef]
- Chen, G.; Du, J.; Gu, L.; Wang, Q.; Qi, Q.; Li, X.; Zhang, R.; Yang, H.; Miao, Y.; Li, Y. Metal-Sensitized Au-Bi2O3 Nanoheterojunction for Immunogenic Cell Death-Boosted Sono-Immuno Cancer Therapy. Chem. Eng. J. 2024, 482, 148953. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, Y.; Yin, C. Gold Nanorod-Chitosan Based Nanocomposites for Photothermal and Chemoembolization Therapy of Breast Cancer. Int. J. Biol. Macromol. 2024, 259, 129197. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, W.; Zhang, R.; Zhang, C.; Yan, J.; Feng, J.; Rosenholm, J.M.; Shi, T.; Shen, X.; Zhang, H. Minimally Invasive Injection of Biomimetic Nano@Microgel for in Situ Ovarian Cancer Treatment through Enhanced Photodynamic Reactions and Photothermal Combined Therapy. Mater. Today Bio 2023, 20, 100663. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, L.; Zhou, L.; Wu, T.; Zhao, S.; Zhang, L. Single-Excitation Triple-Emission Down-/Up-Conversion Nanoassemblies for Tumor Microenvironment-Enhanced Ratiometric NIR-II Fluorescence Imaging and Chemo-/Photodynamic Combination Therapy. Anal. Chem. 2023, 95, 3830–3839. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Qiu, X.; Wang, D.; Yang, H.; Zhao, J.; Qi, Y.; Zhang, L.; Chen, X.; Yang, M.; Gu, W.; et al. Carbon Nanomaze for Biomolecular Detection with Zeptomolar Sensitivity. Adv. Funct. Mater. 2021, 31, 2006521. [Google Scholar] [CrossRef]
- Yang, X.; Huang, S.; Chikkaraddy, R.; Goerlitzer, E.S.A.; Chen, F.; Du, J.; Vogel, N.; Weiss, T.; Baumberg, J.J.; Hou, Y. Chiral Plasmonic Shells: High-Performance Metamaterials for Sensitive Chiral Biomolecule Detection. ACS Appl. Mater. Interfaces 2022, 14, 53183–53192. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhang, Y.; Wu, Z.; Bao, Z.; Lin, L.; Ye, J. Locating Three-Dimensional Position of Deep-Seated SERS Phantom Lesions in Thick Tissues Using Tomographic Transmission Raman Spectroscopy. ACS Appl. Mater. Interfaces 2023, 15, 44665–44675. [Google Scholar] [CrossRef] [PubMed]
- Boguslawski, M.J.; Wall, Z.J.; Vizvary, S.R.; Moore, I.D.; Bareian, M.; Allcock, D.T.C.; Wineland, D.J.; Hudson, E.R.; Campbell, W.C. Raman Scattering Errors in Stimulated-Raman-Induced Logic Gates in Ba + 133. Phys. Rev. Lett. 2023, 131, 063001. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tan, Y.; Tang, J.; He, K.; Zhou, Y.; Liu, J. Transcytosis-Based Renal Tubular Reabsorption of Luminescent Gold Nanoparticles for Enhanced Tumor Imaging. Angew. Chem. Int. Ed. 2024, 63, e202316900. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, T.; Wu, Z.; Zhang, F.; Wang, Y.; Wang, X.; Zhang, Z.; Li, C.; Lv, X.; Chen, D.; et al. Universal Method for Label-Free Detection of Pathogens and Biomolecules by Surface-Enhanced Raman Spectroscopy Based on Gold Nanoparticles. Anal. Chem. 2023, 95, 4050–4058. [Google Scholar] [CrossRef]
- Matthews, J.R.; Shirazinejad, C.R.; Isakson, G.A.; Demers, S.M.E.; Hafner, J.H. Structural Analysis by Enhanced Raman Scattering. Nano Lett. 2017, 17, 2172–2177. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.; Yoo, S.; Jung, I.; Lee, S.; Kim, J.; Son, J.; Kim, J.-E.; Kim, J.-M.; Nam, J.-M.; et al. Enormous Enhancement in Single-Particle Surface-Enhanced Raman Scattering with Size-Controllable Au Double Nanorings. Chem. Mater. 2022, 34, 2197–2205. [Google Scholar] [CrossRef]
- Aliru, M.L.; Aziz, K.; Bodd, M.; Sanders, K.; Mahadevan, L.S.K.; Sahoo, N.; Tailor, R.C.; Krishnan, S. Targeted Gold Nanoparticles Enhance Radiation Effects in Pancreatic Tumor Models. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E574–E575. [Google Scholar] [CrossRef]
- Zanjanchi, P.; Asghari, S.M.; Mohabatkar, H.; Shourian, M.; Shafiee Ardestani, M. Conjugation of VEGFR1/R2-Targeting Peptide with Gold Nanoparticles to Enhance Antiangiogenic and Antitumoral Activity. J. Nanobiotechnol. 2022, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, S.; Feng, X.; Li, X.; Yang, J.; Liu, Q.; Li, M.; Chai, Y.; Yang, C.; Lin, S.; et al. Tumor-Targeting Gene-Photothermal Synergistic Therapies Based on Multifunctional Polydopamine Nanoparticles. Chem. Eng. J. 2023, 457, 141315. [Google Scholar] [CrossRef]
- Simón, M.; Jørgensen, J.T.; Norregaard, K.; Henriksen, J.R.; Clergeaud, G.; Andresen, T.L.; Hansen, A.E.; Kjaer, A. Neoadjuvant Gold Nanoshell-Based Photothermal Therapy Combined with Liposomal Doxorubicin in a Mouse Model of Colorectal Cancer. Int. J. Nanomed. 2023, 18, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.C.; Schwass, D.R.; Tompkins, G.R.; Bobbala, S.K.R.; Medlicott, N.J.; Meledandri, C.J. AgNP/Alginate Nanocomposite Hydrogel for Antimicrobial and Antibiofilm Applications. Carbohydr. Polym. 2021, 251, 117017. [Google Scholar] [CrossRef] [PubMed]
- Steckiewicz, K.P.; Cieciórski, P.; Barcińska, E.; Jaśkiewicz, M.; Narajczyk, M.; Bauer, M.; Kamysz, W.; Megiel, E.; Inkielewicz-Stepniak, I. Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: Their Potential Use in Treating Periodontitis. Int. J. Nanomed. 2022, 17, 495–517. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Pal, R.; Ali, M.; Singh, L.M.; Shahidur Rahman, D.; Kumar Ghosh, S.; Sengupta, M. Immunomodulatory Properties of Silver Nanoparticles Contribute to Anticancer Strategy for Murine Fibrosarcoma. Cell Mol. Immunol. 2016, 13, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Jiang, S.; Li, F.; Lin, J.; Wang, T.; Huang, P. Degradable Silver-Based Nanoplatform for Synergistic Cancer Starving-like/Metal Ion Therapy. Mater. Horiz. 2019, 6, 169–175. [Google Scholar] [CrossRef]
- Wu, H.; Lin, J.; Liu, P.; Huang, Z.; Zhao, P.; Jin, H.; Ma, J.; Wen, L.; Gu, N. Reactive Oxygen Species Acts as Executor in Radiation Enhancement and Autophagy Inducing by AgNPs. Biomaterials 2016, 101, 1–9. [Google Scholar] [CrossRef]
- Hsu, J.C.; Barragan, D.; Tward, A.E.; Hajfathalian, M.; Amirshaghaghi, A.; Mossburg, K.J.; Rosario-Berríos, D.N.; Bouché, M.; Andrianov, A.K.; Delikatny, E.J.; et al. A Biodegradable “One-For-All” Nanoparticle for Multimodality Imaging and Enhanced Photothermal Treatment of Breast Cancer. Adv. Healthc. Mater. 2023, 13, 2303018. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Wei, J.-S.; Chen, J.-W.; Cheng, K.; Zhang, F.; Ashraf, G.; Li, Y.; Hou, X.-L.; Zhang, R.-Y.; Hu, Y.-G.; et al. A Nanoplatform of Hollow Ag2S/Ag Nanocomposite Shell for Photothermal and Enhanced Sonodynamic Therapy Mediated by Photoacoustic and CT Imaging. Chem. Eng. J. 2022, 433, 133196. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.; Mu, F.; Wang, J.; Shen, C.; Wang, H.; Huang, F.; Chen, B.; Luo, Z.; et al. DNA-Templated Ag@Pd Nanoclusters for NIR-II Photoacoustic Imaging-Guided Photothermal-Augmented Nanocatalytic Therapy. Adv. Healthc. Mater. 2023, 12, 2300267. [Google Scholar] [CrossRef]
- Yoo, D.; Jeong, S.; Ju, H.M.; Jeong, W.; Kim, K.; Choi, M.-J. Dual-Ligand Surface Passivation Enables Monodisperse Ag2S Colloidal Quantum Dots for Efficient Near-Infrared Photothermal Therapy. ACS Mater. Lett. 2024, 6, 308–313. [Google Scholar] [CrossRef]
- Bian, K.; Zhang, X.; Liu, K.; Yin, T.; Liu, H.; Niu, K.; Cao, W.; Gao, D. Peptide-Directed Hierarchical Mineralized Silver Nanocages for Anti-Tumor Photothermal Therapy. ACS Sustain. Chem. Eng. 2018, 6, 7574–7588. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S. Nanomaterials in Tumor Immunotherapy: New Strategies and Challenges. Mol. Cancer 2023, 22, 94. [Google Scholar] [CrossRef]
- Kong, C.; Chen, X. Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review. Int. J. Nanomed. 2022, 17, 6427–6446. [Google Scholar] [CrossRef]
- Wieder, T.; Eigentler, T.; Brenner, E.; Röcken, M. Immune Checkpoint Blockade Therapy. J. Allergy Clin. Immunol. 2018, 142, 1403–1414. [Google Scholar] [CrossRef]
- He, M.; Yang, T.; Wang, Y.; Wang, M.; Chen, X.; Ding, D.; Zheng, Y.; Chen, H. Immune Checkpoint Inhibitor-Based Strategies for Synergistic Cancer Therapy. Adv. Healthc. Mater. 2021, 10, 2002104. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, Y.; Zheng, Y.; Kang, X. Advancing Immune Checkpoint Blockade in Colorectal Cancer Therapy with Nanotechnology. Front. Immunol. 2022, 13, 1027124. [Google Scholar] [CrossRef]
- Wang, M.; Chang, M.; Zheng, P.; Sun, Q.; Wang, G.; Lin, J.; Li, C. A Noble AuPtAg-GOx Nanozyme for Synergistic Tumor Immunotherapy Induced by Starvation Therapy-Augmented Mild Photothermal Therapy. Adv. Sci. 2022, 9, 2202332. [Google Scholar] [CrossRef]
- Jin, L.; Shen, S.; Huang, Y.; Li, D.; Yang, X. Corn-like Au/Ag Nanorod-Mediated NIR-II Photothermal/Photodynamic Therapy Potentiates Immune Checkpoint Antibody Efficacy by Reprogramming the Cold Tumor Microenvironment. Biomaterials 2021, 268, 120582. [Google Scholar] [CrossRef]
- Bai, Y.; Hua, J.; Zhao, J.; Wang, S.; Huang, M.; Wang, Y.; Luo, Y.; Zhao, S.; Liang, H. A Silver-Induced Absorption Red-Shifted Dual-Targeted Nanodiagnosis-Treatment Agent for NIR-II Photoacoustic Imaging-Guided Photothermal and ROS Simultaneously Enhanced Immune Checkpoint Blockade Antitumor Therapy. Adv. Sci. 2023, 11, 2306375. [Google Scholar] [CrossRef]
- Yi, X.; Duan, Q.-Y.; Wu, F.-G. Low-Temperature Photothermal Therapy: Strategies and Applications. Research 2021, 2021, 9816594. [Google Scholar] [CrossRef]
- Gupta, N.; Malviya, R. Understanding and Advancement in Gold Nanoparticle Targeted Photothermal Therapy of Cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Bian, J.; Zhang, X.; Fu, Y.; Li, Z.; Wei, S.; Xu, Z.; Liu, X.; Liu, Z.; et al. Ag/Pd Bimetal Nanozyme with Enhanced Catalytic and Photothermal Effects for ROS/Hyperthermia/Chemotherapy Triple-Modality Antitumor Therapy. Chem. Eng. J. 2020, 397, 125438. [Google Scholar] [CrossRef]
- Gong, P.; Li, C.; Wang, D.; Song, S.; Wu, W.; Liu, B.; Shen, J.; Liu, J.; Liu, Z. Enzyme Coordination Conferring Stable Monodispersity of Diverse Metal–Organic Frameworks for Photothermal/Starvation Therapy. J. Colloid. Interface Sci. 2023, 642, 612–622. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, Q.; Sun, B.; Chu, X.; Zhang, M.; She, Z.; Li, Z.; Zhou, N.; Wang, J.; Li, A. MoO3-x Nanosheets-Based Platform for Single NIR Laser Induced Efficient PDT/PTT of Cancer. J. Control. Release 2021, 338, 46–55. [Google Scholar] [CrossRef]
- Lee, D.; Song, J.; Song, G.; Pang, Y. Metal-Enhanced Fluorescence of Dyes with Quadrupole Surface Plasmon Resonance of Silver Nanoparticles. Nanoscale Adv. 2022, 4, 2794–2805. [Google Scholar] [CrossRef]
- Saul, P.; Schröder, L.; Schmidt, A.B.; Hövener, J. Nanomaterials for Hyperpolarized Nuclear Magnetic Resonance and Magnetic Resonance Imaging. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1879. [Google Scholar] [CrossRef]
- Sun, I.-C.; Dumani, D.S.; Emelianov, S.Y. Applications of the Photocatalytic and Photoacoustic Properties of Gold Nanorods in Contrast-Enhanced Ultrasound and Photoacoustic Imaging. ACS Nano 2024, 18, 3575–3582. [Google Scholar] [CrossRef]
- Moonshi, S.S.; Vazquez-Prada, K.X.; Tang, J.; Westra van Holthe, N.J.; Cowin, G.; Wu, Y.; Tran, H.D.N.; Mckinnon, R.; Bulmer, A.C.; Ta, H.T. Spiky Silver-Iron Oxide Nanohybrid for Effective Dual-Imaging and Synergistic Thermo-Chemotherapy. ACS Appl. Mater. Interfaces 2023, 15, 42153–42169. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Belova, M.M.; Kotelnikova, P.A.; Shilova, O.N.; Mirkasymov, A.B.; Danilova, N.V.; Komedchikova, E.N.; Popovtzer, R.; Deyev, S.M.; Nikitin, M.P. Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission. Pharmaceutics 2022, 14, 1013. [Google Scholar] [CrossRef]
- Lu, R. Egg White-Mediated Green Synthesis of Silver Nanoparticles with Excellent Biocompatibility and Enhanced Radiation Effects on Cancer Cells. Int. J. Nanomed. 2012, 7, 2101–2107. [Google Scholar] [CrossRef]
- Kayani, Z.; Islami, N.; Behzadpour, N.; Zahraie, N.; Imanlou, S.; Tamaddon, P.; Salehi, F.; Daneshvar, F.; Perota, G.; Sorati, E.; et al. Combating Cancer by Utilizing Noble Metallic Nanostructures in Combination with Laser Photothermal and X-Ray Radiotherapy. J. Drug Deliv. Sci. Technol. 2021, 65, 102689. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Lu, J.; Xu, H.; Zhao, S.; Li, X.; Yang, C.; Kong, L.; Guo, Y.; Zhang, Z. Chemodynamic/Photothermal Synergistic Cancer Immunotherapy Based on Yeast Microcapsule-Derived Au/Pt Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 24134–24148. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Gong, F.; Yin, K.; Zhu, W.; Yang, N.; Bai, S.; Liao, F.; Shao, M.; Cheng, L. Silicon Nanowires Decorated with Platinum Nanoparticles Were Applied for Photothermal-Enhanced Sonodynamic Therapy. Theranostics 2021, 11, 9234–9242. [Google Scholar] [CrossRef]
- Zhao, L.; Ge, X.; Yan, G.; Wang, X.; Hu, P.; Shi, L.; Wolfbeis, O.S.; Zhang, H.; Sun, L. Double-Mesoporous Core–Shell Nanosystems Based on Platinum Nanoparticles Functionalized with Lanthanide Complexes for in Vivo Magnetic Resonance Imaging and Photothermal Therapy. Nanoscale 2017, 9, 16012–16023. [Google Scholar] [CrossRef] [PubMed]
- Villalobos Gutiérrez, P.; Muñoz Carrillo, J.; Sandoval Salazar, C.; Viveros Paredes, J.; Gutiérrez Coronado, O. Functionalized Metal Nanoparticles in Cancer Therapy. Pharmaceutics 2023, 15, 1932. [Google Scholar] [CrossRef]
- Singh, P.; Haloi, P.; Singh, K.; Roy, S.; Sarkar, A.; Choudhary, R.; Mohite, C.; Chawla, S.; Konkimalla, V.B.; Sanpui, P.; et al. Palladium Nanocapsules for Photothermal Therapy in the Near-Infrared II Biological Window. ACS Appl. Mater. Interfaces 2023, 15, 39081–39098. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Pan, P.; Zhang, Q.; Ye, J.-J.; Zhang, X.-Z. Engineered Living Bacteriophage-Enabled Self-Adjuvanting Hydrogel for Remodeling Tumor Microenvironment and Cancer Therapy. Nano Lett. 2023, 23, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, L.; Wang, Z.; Xi, W.; Dibaba, S.T.; Wang, S.; Shi, L.; Sun, L. Heterogeneous Growth of Palladium Nanocrystals on Upconversion Nanoparticles for Multimodal Imaging and Photothermal Therapy. J. Mater. Chem. B 2019, 7, 3652–3660. [Google Scholar] [CrossRef]
- Ten, A.; West, C.A.; Jeong, S.; Hopper, E.R.; Wang, Y.; Zhu, B.; Ramasse, Q.M.; Ye, X.; Ringe, E. Bimetallic Copper Palladium Nanorods: Plasmonic Properties and Palladium Content Effects. Nanoscale Adv. 2023, 5, 6524–6532. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, Q.; Tian, P.; Zhang, P.; Zhang, Z.; Voyles, P.M.; Wang, X. Ionic Layer Epitaxy of Nanometer-Thick Palladium Nanosheets with Enhanced Electrocatalytic Properties. Chem. Mater. 2018, 30, 3308–3314. [Google Scholar] [CrossRef]
- Kumaravel, S.; Saravanan, K.K.; Evangeline, B.E.; Niharika, V.; Jayakumar, R.; Kundu, S. DNA-Based Low Resistance Palladium Nano-Spheres for Effective Hydrogen Evolution Reaction. Catal. Sci. Technol. 2021, 11, 5868–5880. [Google Scholar] [CrossRef]
- Li, R.; He, M.; Cui, Y.; Ji, X.; Zhang, L.; Lan, X.; Wang, L.; Han, Z.; Xiao, H. Silver-Palladium Bimetallic Nanoparticles Stabilized by Elm Pod Polysaccharide with Peroxidase-like Properties for Glutathione Detection and Photothermal Anti-Tumor Ability. Int. J. Biol. Macromol. 2024, 264, 130673. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Zheng, W.; Hu, S.; Peng, X.; Luo, Y.; Lee, J.; Chen, H. Multifunctional DNA Scaffold Mediated Gap Plasmon Resonance: Application to Sensitive PD-L1 Sensor. Biosens. Bioelectron. 2024, 247, 115938. [Google Scholar] [CrossRef]
- Tong, W.; Tao, G.; Wu, Y.; Chen, X.; Leng, Y.; Huang, X.; Xiong, Y. Aggregation of Noble Metal Nanoparticles: A Versatile Sensing Strategy for Food Safety Monitoring. Trends Food Sci. Technol. 2023, 142, 104243. [Google Scholar] [CrossRef]
- Quinson, J.; Kunz, S.; Arenz, M. Surfactant-Free Colloidal Syntheses of Precious Metal Nanoparticles for Improved Catalysts. ACS Catal. 2023, 13, 4903–4937. [Google Scholar] [CrossRef]
- Velasco, L.; Ouyang, T.; Reinhard, B.M. Two-Color iSCAT Imaging of Ag Nanoparticles Resolves Size and Ambient Refractive Index Changes. Nano Lett. 2023, 23, 4642–4647. [Google Scholar] [CrossRef]
- Liu, T.; Jin, R.; Yuan, P.; Bai, Y.; Cai, B.; Chen, X. Intracellular Enzyme-Triggered Assembly of Amino Acid-Modified Gold Nanoparticles for Accurate Cancer Therapy with Multimode. ACS Appl. Mater. Interfaces 2019, 11, 28621–28630. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luo, N.; Wang, Y.; Li, H.; Zhang, T.; Tang, X.; Hu, Z. Construction of Photocatalyst by Modification of Argentum Noble Metal and Hydrogen Doping into WO3 Nanoparticles: Enhancing the Solar Energy Utilization. Fuel 2023, 352, 128966. [Google Scholar] [CrossRef]
- Qin, J.; Sui, M.; Yuan, B.; Wang, J.; Yuan, Z.; Xu, G. The Decay of Silver Nanoparticles in Preoxidation Process. Sci. Total Environ. 2018, 619–620, 1618–1627. [Google Scholar] [CrossRef]
- Qi, M.; Wang, X.; Chen, J.; Liu, Y.; Liu, Y.; Jia, J.; Li, L.; Yue, T.; Gao, L.; Yan, B.; et al. Transformation, Absorption and Toxicological Mechanisms of Silver Nanoparticles in the Gastrointestinal Tract Following Oral Exposure. ACS Nano 2023, 17, 8851–8865. [Google Scholar] [CrossRef] [PubMed]


| Types | Properties | Shapes | Combination Therapy | Biological Imaging | Drug Delivery |
|---|---|---|---|---|---|
| Gold nanoparticles | LSPR/stability/ catalytic activity | Gold nanorods/ gold nanocage/ gold nanostars/ gold nanospheres | PTT/ IMT/chemotherapy/PDT | Raman spectroscopy | peptide modification/ Localized heating |
| Silver nanoparticles | LSPR/stability/ catalytic activity | quasi-spherical silver nanoparticles/ silver nanorods/ silver nanocubes/ Ag-Rh core-framework nanocubes/ silver prismatic nanocubes | PTT/ IMT/bimetallic/ chemotherapy | MRI/PAI | targeted modification/ functionalized |
| Platinum nanoparticles | LSPR/stability | Platinum Nanorods/ Platinum Nanosheets/ platinum nanospheres | SDT/CDT/ IMT | MRI | functionalized |
| Palladium nanoparticles | LSPR/stability/ catalytic activity/ adjustable optical response | Palladium Nanorods /Palladium Nanosheets/ Palladium nanospheres | bimetallic/ bioactive gel system/ IMT | MRI | functionalized |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Xia, G.; Yang, N.; Yuan, L.; Li, J.; Wang, Q.; Li, D.; Ding, L.; Fan, Z.; Li, J. Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 5632. https://doi.org/10.3390/ijms25115632
Yu S, Xia G, Yang N, Yuan L, Li J, Wang Q, Li D, Ding L, Fan Z, Li J. Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. International Journal of Molecular Sciences. 2024; 25(11):5632. https://doi.org/10.3390/ijms25115632
Chicago/Turabian StyleYu, Shujie, Guoyu Xia, Nan Yang, Longlong Yuan, Jianmin Li, Qingluo Wang, Dingyang Li, Lijun Ding, Zhongxiong Fan, and Jinyao Li. 2024. "Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy" International Journal of Molecular Sciences 25, no. 11: 5632. https://doi.org/10.3390/ijms25115632
APA StyleYu, S., Xia, G., Yang, N., Yuan, L., Li, J., Wang, Q., Li, D., Ding, L., Fan, Z., & Li, J. (2024). Noble Metal Nanoparticle-Based Photothermal Therapy: Development and Application in Effective Cancer Therapy. International Journal of Molecular Sciences, 25(11), 5632. https://doi.org/10.3390/ijms25115632






