Neuromodulatory Responses Elicited by Intermittent versus Continuous Transcranial Focused Ultrasound Stimulation of the Motor Cortex in Rats
Abstract
:1. Introduction
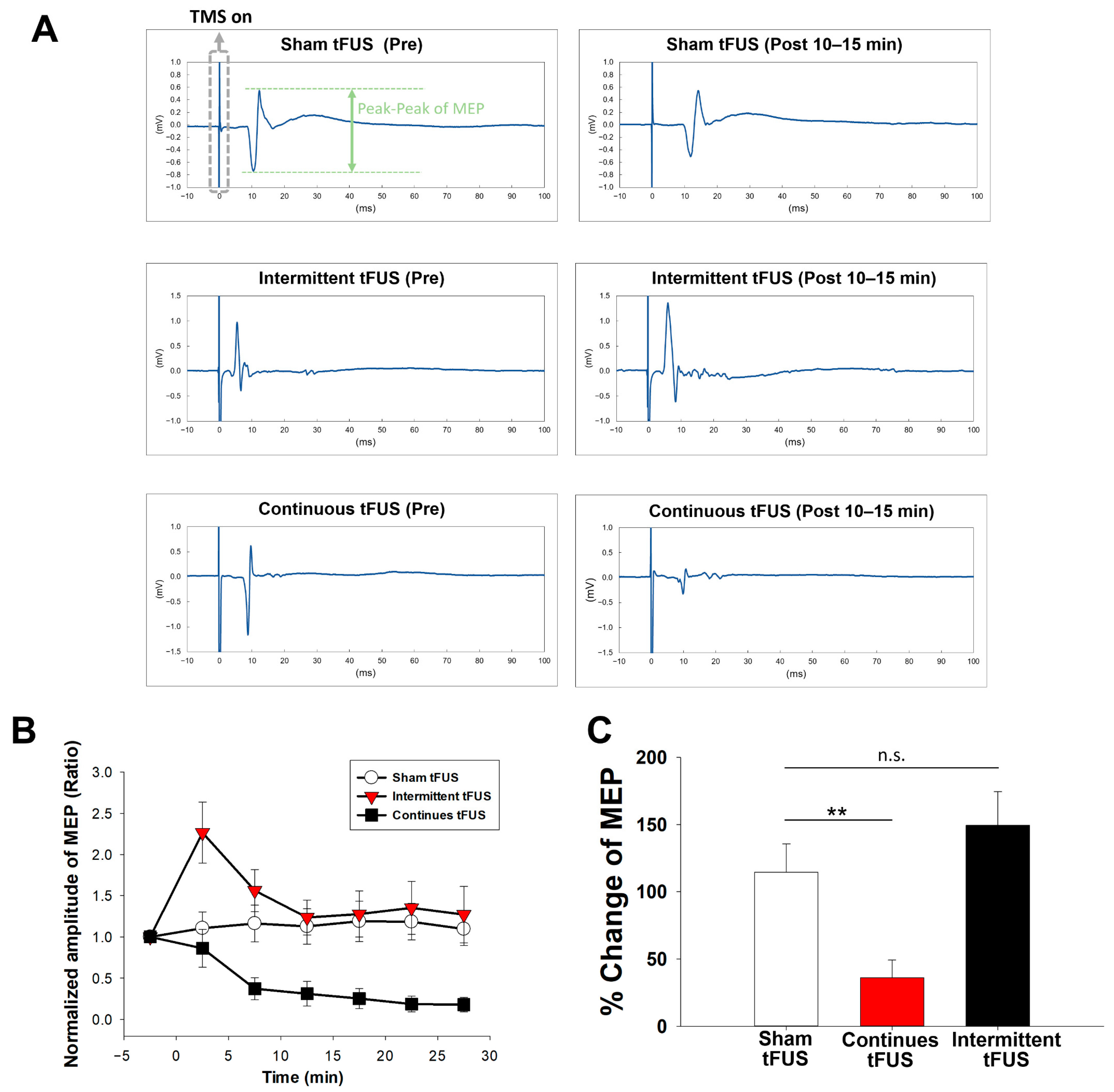
2. Results
2.1. Effects of Intermittent and Continuous tFUS on Motor Cortical Excitability
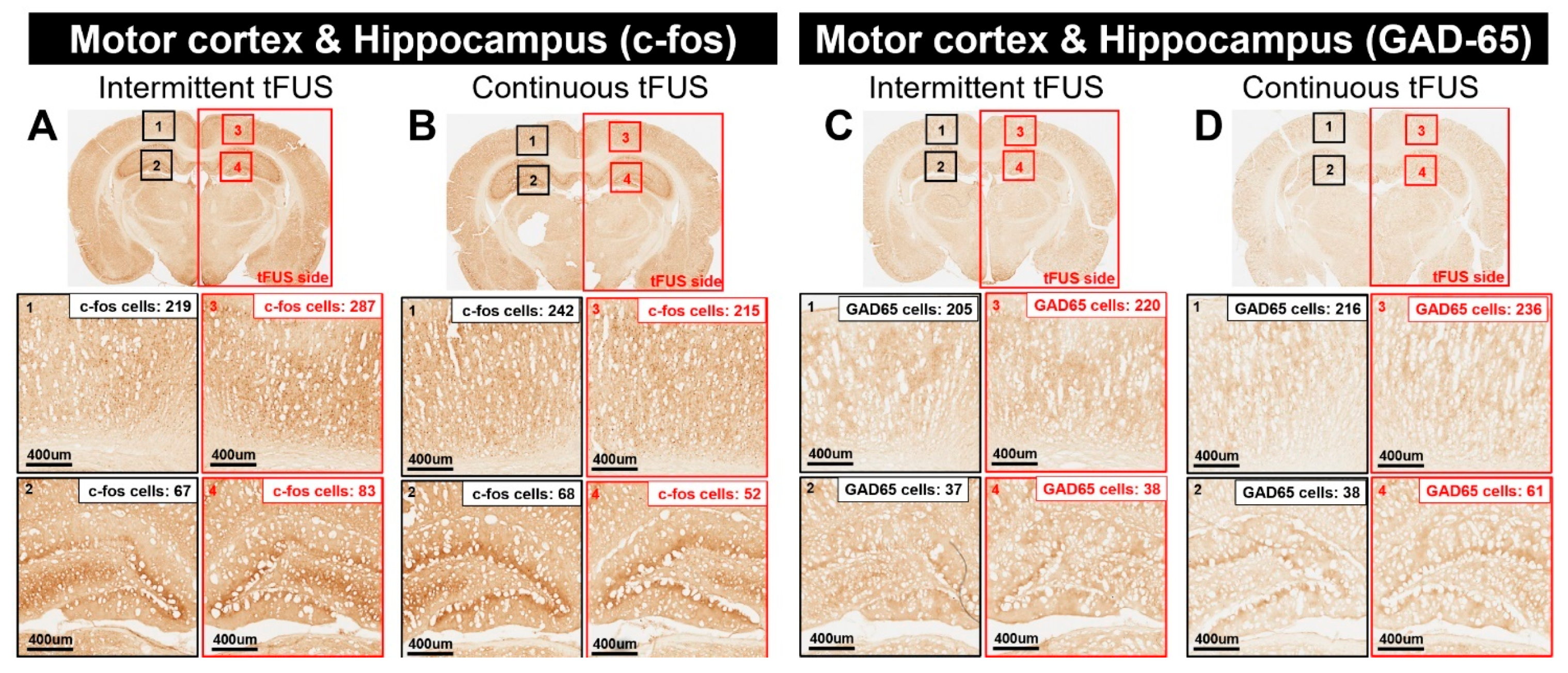
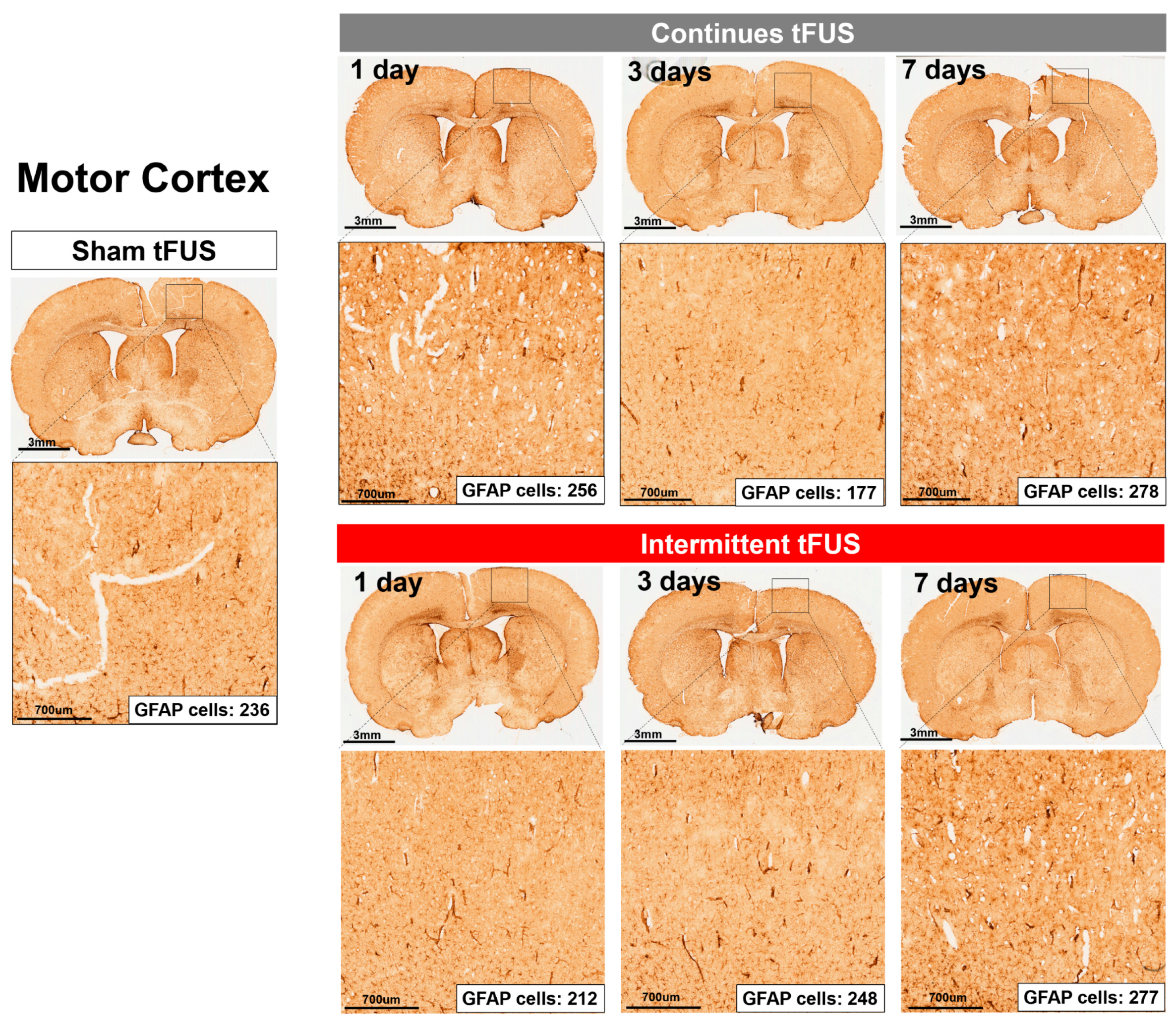
2.2. Histological Assays
3. Discussion
4. Materials and Methods
4.1. Animals and Preparations
4.2. tFUS Procedures
4.3. TMS Assessment
4.4. Immunohistochemical Analysis
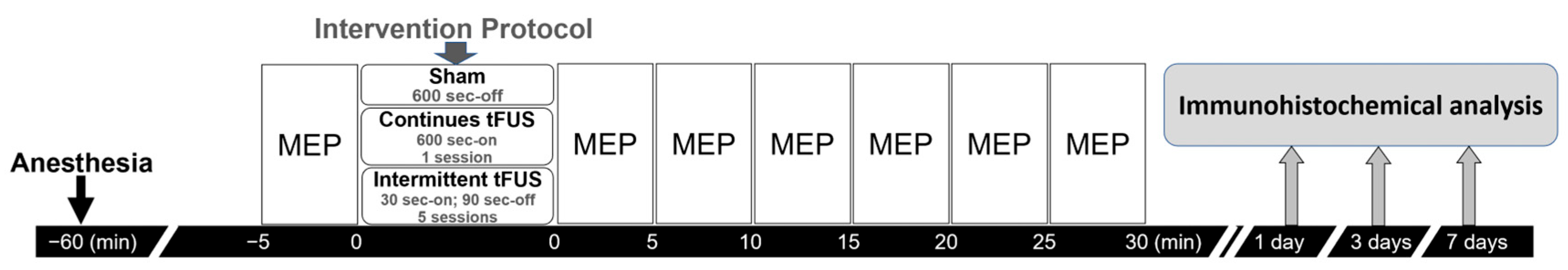
4.5. Experiment Design
4.6. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darmani, G.; Bergmann, T.O.; Butts Pauly, K.; Caskey, C.F.; de Lecea, L.; Fomenko, A.; Fouragnan, E.; Legon, W.; Murphy, K.R.; Nandi, T.; et al. Non-invasive transcranial ultrasound stimulation for neuromodulation. Clin. Neurophysiol. 2022, 135, 51–73. [Google Scholar] [CrossRef] [PubMed]
- Darrow, D.P. Focused Ultrasound for Neuromodulation. Neurotherapeutics 2019, 16, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Bologna, M. Low-Intensity Transcranial Ultrasound Stimulation: Mechanisms of Action and Rationale for Future Applications in Movement Disorders. Brain Sci. 2022, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Pahk, K.J.; Kim, H. A review of low-intensity focused ultrasound for neuromodulation. Biomed. Eng. Lett. 2017, 7, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Tufail, Y.; Finsterwald, M.; Tauchmann, M.L.; Olson, E.J.; Majestic, C. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 2008, 3, e3511. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.P.; Jin, M.; Xia, A.W.; Li, A.S.; Lin, T.T.; Liu, Y.; Kan, R.L.; Zhang, B.B.; Kranz, G.S. The effectiveness and safety of low-intensity transcranial ultrasound stimulation: A systematic review of human and animal studies. Neurosci. Biobehav. Rev. 2024, 156, 105501. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Hill, A.T.; Rogasch, N.C.; Hoy, K.E.; Fitzgerald, P.B. Use of theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 63, 43–64. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta burst stimulation of the human motor cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.C.; Huang, C.S.; Chang, P.K.; Chen, R.S.; Chen, K.T.; Hsieh, T.H.; Liu, H.L. Weak Ultrasound Contributes to Neuromodulatory Effects in the Rat Motor Cortex. Int. J. Mol. Sci. 2023, 24, 2578. [Google Scholar] [CrossRef]
- Legon, W.; Bansal, P.; Tyshynsky, R.; Ai, L.; Mueller, J.K. Transcranial focused ultrasound neuromodulation of the human primary motor cortex. Sci. Rep. 2018, 8, 10007. [Google Scholar] [CrossRef]
- Samuel, N.; Zeng, K.; Harmsen, I.E.; Ding, M.Y.R.; Darmani, G.; Sarica, C.; Santyr, B.; Vetkas, A.; Pancholi, A.; Fomenko, A.; et al. Multi-modal investigation of transcranial ultrasound-induced neuroplasticity of the human motor cortex. Brain Stimul. 2022, 15, 1337–1347. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chang, M.Y.; Liu, H.H.; He, X.K.; Chan, S.Y.; Huang, Y.Z.; Peng, C.W.; Chang, P.K.; Pan, C.Y.; Hsieh, T.H. Cortical Electrical Stimulation Ameliorates Traumatic Brain Injury-Induced Sensorimotor and Cognitive Deficits in Rats. Front. Neural Circuits 2021, 15, 693073. [Google Scholar] [CrossRef]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Krishna, V.; Sammartino, F.; Rezai, A. A Review of the Current Therapies, Challenges, and Future Directions of Transcranial Focused Ultrasound Technology: Advances in Diagnosis and Treatment. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Kubanek, J. Neuromodulation with transcranial focused ultrasound. Neurosurg. Focus. 2018, 44, E14. [Google Scholar] [CrossRef] [PubMed]
- Beisteiner, R.; Hallett, M.; Lozano, A.M. Ultrasound Neuromodulation as a New Brain Therapy. Adv. Sci. 2023, 10, e2205634. [Google Scholar] [CrossRef]
- Baek, H.; Lockwood, D.; Mason, E.J.; Obusez, E.; Poturalski, M.; Rammo, R.; Nagel, S.J.; Jones, S.E. Clinical Intervention Using Focused Ultrasound (FUS) Stimulation of the Brain in Diverse Neurological Disorders. Front. Neurol. 2022, 13, 880814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pan, N.; Wang, Y.; Liu, C.; Hu, S. Transcranial Focused Ultrasound Neuromodulation: A Review of the Excitatory and Inhibitory Effects on Brain Activity in Human and Animals. Front. Human Neurosci. 2021, 15, 749162. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, B.; Zuo, Z.; Long, X.; Hu, S.; Li, S.; Su, X.; Wang, Y.; Liu, C. Excitatory-inhibitory modulation of transcranial focus ultrasound stimulation on human motor cortex. CNS Neurosci. Ther. 2023, 29, 3829–3841. [Google Scholar] [CrossRef]
- Kim, Y.G.; Kim, S.E.; Lee, J.; Hwang, S.; Yoo, S.S.; Lee, H.W. Neuromodulation Using Transcranial Focused Ultrasound on the Bilateral Medial Prefrontal Cortex. J. Clin. Med. 2022, 11, 3809. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, W.; Lee, J.E.; Xu, L.; Croce, P.; Foley, L.; Yoo, S.S. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PLoS ONE 2019, 14, e0224311. [Google Scholar] [CrossRef] [PubMed]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Tillery, S.I.; Tyler, W.J. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Li, G.F.; Zhao, H.X.; Zhou, H.; Yan, F.; Wang, J.Y.; Xu, C.X.; Wang, C.Z.; Niu, L.L.; Meng, L.; Wu, S.; et al. Improved Anatomical Specificity of Non-invasive Neuro-stimulation by High Frequency (5 MHz) Ultrasound. Sci. Rep. 2016, 6, 24738. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.C.; Sanguinetti, J.L.; Badran, B.W.; Yu, A.B.; Klein, E.P.; Abbott, C.C.; Hansberger, J.T.; Clark, V.P. Increased Excitability Induced in the Primary Motor Cortex by Transcranial Ultrasound Stimulation. Front. Neurol. 2018, 9, 1007. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, A.; Neudorfer, C.; Dallapiazza, R.F.; Kalia, S.K.; Lozano, A.M. Low-intensity ultrasound neuromodulation: An overview of mechanisms and emerging human applications. Brain Stimul. 2018, 11, 1209–1217. [Google Scholar] [CrossRef]
- Tyler, W.J.; Lani, S.W.; Hwang, G.M. Ultrasonic modulation of neural circuit activity. Curr. Opin. Neurobiol. 2018, 50, 222–231. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Fricke, K.; Seeber, A.A.; Thirugnanasambandam, N.; Paulus, W.; Nitsche, M.A.; Rothwell, J.C. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2011, 105, 1141–1149. [Google Scholar] [CrossRef]
- Labedi, A.; Benali, A.; Mix, A.; Neubacher, U.; Funke, K. Modulation of inhibitory activity markers by intermittent theta-burst stimulation in rat cortex is NMDA-receptor dependent. Brain Stimul. 2014, 7, 394–400. [Google Scholar] [CrossRef]
- Hausmann, A.; Weis, C.; Marksteiner, J.; Hinterhuber, H.; Humpel, C. Chronic repetitive transcranial magnetic stimulation enhances c-fos in the parietal cortex and hippocampus. Brain Res. Mol. Brain Res. 2000, 76, 355–362. [Google Scholar] [CrossRef]
- Lenz, M.; Vlachos, A. Releasing the Cortical Brake by Non-Invasive Electromagnetic Stimulation? rTMS Induces LTD of GABAergic Neurotransmission. Front. Neural Circuits 2016, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Tufail, Y.; Yoshihiro, A.; Pati, S.; Li, M.M.; Tyler, W.J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 2011, 6, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Kubanek, J.; Shukla, P.; Das, A.; Baccus, S.A.; Goodman, M.B. Ultrasound Elicits Behavioral Responses through Mechanical Effects on Neurons and Ion Channels in a Simple Nervous System. J. Neurosci. 2018, 38, 3081–3091. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Wan, H.; Li, J.H.; Li, J.M. Effect of low-intensity pulsed ultrasound on the expression of neurotrophin-3 and brain-derived neurotrophic factor in cultured Schwann cells. Microsurgery 2009, 29, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F. Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985, 8, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.G.; Maysinger, D.; Almazan, G.; Funnel, R.; Cuello, A.C. Analysis of c-Fos and glial fibrillary acidic protein (GFAP) expression following topical application of potassium chloride (KCl) to the brain surface. Brain Res. 1998, 784, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, C.; Hanson, L.G.; Siebner, H.R.; Lee, H.J.; Thielscher, A. Safety of transcranial focused ultrasound stimulation: A systematic review of the state of knowledge from both human and animal studies. Brain Stimul. 2019, 12, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Tsai, C.H.; Lin, C.J.; Lee, C.C.; Yu, H.Y.; Hsieh, T.H.; Liu, H.L. Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo. Brain Stimul. 2020, 13, 35–46. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, L.; Xia, X.; Lin, Z.; Zhou, W.; Pang, N.; Bian, T.; Yuan, T.; Niu, L.; Zheng, H. Transcranial Ultrasound Stimulation Suppresses Neuroinflammation in a Chronic Mouse Model of Parkinson’s Disease. IEEE Trans. Biomed. Eng. 2021, 68, 3375–3387. [Google Scholar] [CrossRef]
- Zhou, H.; Niu, L.; Xia, X.; Lin, Z.; Liu, X.; Su, M.; Guo, R.; Meng, L.; Zheng, H. Wearable Ultrasound Improves Motor Function in an MPTP Mouse Model of Parkinson’s Disease. IEEE Trans. Biomed. Eng. 2019, 66, 3006–3013. [Google Scholar] [CrossRef]
- Folloni, D.; Verhagen, L.; Mars, R.B.; Fouragnan, E.; Constans, C.; Aubry, J.F.; Rushworth, M.F.S.; Sallet, J. Manipulation of Subcortical and Deep Cortical Activity in the Primate Brain Using Transcranial Focused Ultrasound Stimulation. Neuron 2019, 101, 1109–1116.e5. [Google Scholar] [CrossRef]
- Verhagen, L.; Gallea, C.; Folloni, D.; Constans, C.; Jensen, D.E.; Ahnine, H.; Roumazeilles, L.; Santin, M.; Ahmed, B.; Lehericy, S.; et al. Offline impact of transcranial focused ultrasound on cortical activation in primates. Elife 2019, 8, e40541. [Google Scholar] [CrossRef] [PubMed]
- Colucci, V.; Strichartz, G.; Jolesz, F.; Vykhodtseva, N.; Hynynen, K. Focused ultrasound effects on nerve action potential in vitro. Ultrasound Med. Biol. 2009, 35, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, F.; Hu, J.; Hu, J.; Xu, Q.; Cong, N.; Zhang, Q.; Liu, L.; Mantini, D.; Zhang, Z.; et al. Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: Evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin. 2019, 21, 101620. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Choi, H.E.; Jung, H.; Lee, B.J.; Lee, K.H.; Lim, Y.J. Comparison of the Effects of 1 Hz and 20 Hz rTMS on Motor Recovery in Subacute Stroke Patients. Ann. Rehabil. Med. 2014, 38, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Rothwell, J.C. Long lasting effects of rTMS and associated peripheral sensory input on MEPs, SEPs and transcortical reflex excitability in humans. J. Physiol. 2002, 540, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Dayal, V.; Limousin, P.; Foltynie, T. Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease: The Effect of Varying Stimulation Parameters. J. Park. Dis. 2017, 7, 235–245. [Google Scholar] [CrossRef]
- Razmkon, A.; Abdollahifard, S.; Taherifard, E.; Roshanshad, A.; Shahrivar, K. Effect of deep brain stimulation on freezing of gait in patients with Parkinson’s disease: A systematic review. Br. J. Neurosurg. 2023, 37, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Hendee, J.; Ruan, J.; Zhirova, A.; Ye, J.; Dima, M. Neuron matters: Neuromodulation with electromagnetic stimulation must consider neurons as dynamic identities. J. Neuroeng. Rehabil. 2022, 19, 116. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Huang, Y.Z.; Rotenberg, A.; Pascual-Leone, A.; Chiang, Y.H.; Wang, J.Y.; Chen, J.J. Functional Dopaminergic Neurons in Substantia Nigra are Required for Transcranial Magnetic Stimulation-Induced Motor Plasticity. Cereb. Cortex 2015, 25, 1806–1814. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Muller, A.; Hynynen, K. Ultrasound insertion loss of rat parietal bone appears to be proportional to animal mass at submegahertz frequencies. Ultrasound Med. Biol. 2011, 37, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Doyal, A.; Schoenherr, J.; Flynn, D. Motor Evoked Potential. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK580548/ (accessed on 3 April 2023).
- Spampinato, D.A.; Ibanez, J.; Rocchi, L.; Rothwell, J. Motor potentials evoked by transcranial magnetic stimulation: Interpreting a simple measure of a complex system. J. Physiol. 2023, 601, 2827–2851. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Krakauer, J.W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, A.; Muller, P.A.; Vahabzadeh-Hagh, A.M.; Navarro, X.; Lopez-Vales, R.; Pascual-Leone, A.; Jensen, F. Lateralization of forelimb motor evoked potentials by transcranial magnetic stimulation in rats. Clin. Neurophysiol. 2010, 121, 104–108. [Google Scholar] [CrossRef]
- Vahabzadeh-Hagh, A.M.; Muller, P.A.; Pascual-Leone, A.; Jensen, F.E.; Rotenberg, A. Measures of cortical inhibition by paired-pulse transcranial magnetic stimulation in anesthetized rats. J. Neurophysiol. 2011, 105, 615–624. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, T.-H.; Chu, P.-C.; Nguyen, T.X.D.; Kuo, C.-W.; Chang, P.-K.; Chen, K.-H.S.; Liu, H.-L. Neuromodulatory Responses Elicited by Intermittent versus Continuous Transcranial Focused Ultrasound Stimulation of the Motor Cortex in Rats. Int. J. Mol. Sci. 2024, 25, 5687. https://doi.org/10.3390/ijms25115687
Hsieh T-H, Chu P-C, Nguyen TXD, Kuo C-W, Chang P-K, Chen K-HS, Liu H-L. Neuromodulatory Responses Elicited by Intermittent versus Continuous Transcranial Focused Ultrasound Stimulation of the Motor Cortex in Rats. International Journal of Molecular Sciences. 2024; 25(11):5687. https://doi.org/10.3390/ijms25115687
Chicago/Turabian StyleHsieh, Tsung-Hsun, Po-Chun Chu, Thi Xuan Dieu Nguyen, Chi-Wei Kuo, Pi-Kai Chang, Kai-Hsiang Stanley Chen, and Hao-Li Liu. 2024. "Neuromodulatory Responses Elicited by Intermittent versus Continuous Transcranial Focused Ultrasound Stimulation of the Motor Cortex in Rats" International Journal of Molecular Sciences 25, no. 11: 5687. https://doi.org/10.3390/ijms25115687




