A Combination of Microarray-Based Profiling and Biocomputational Analysis Identified miR331-3p and hsa-let-7d-5p as Potential Biomarkers of Ulcerative Colitis Progression to Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics: Nonrelated IBD-Inflammation, UC, and CRC Patients
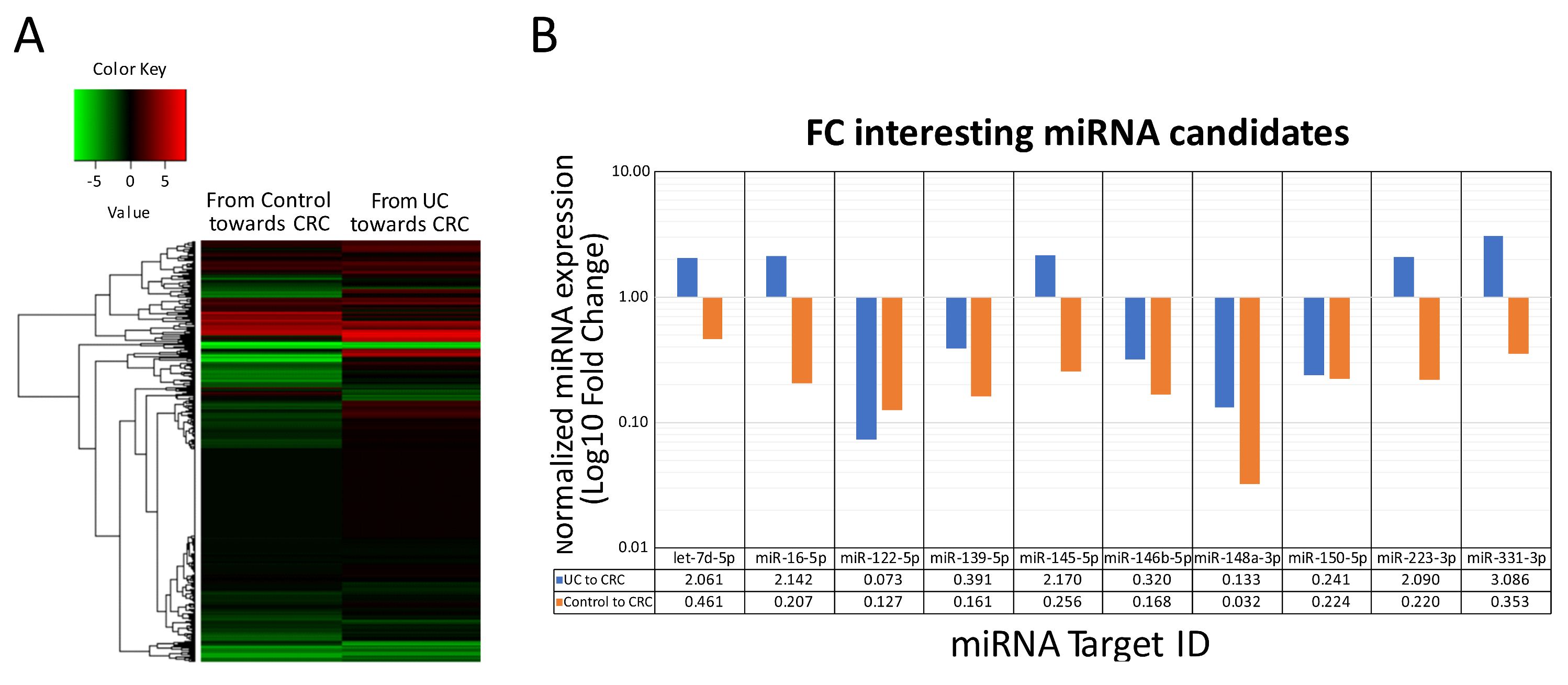
2.2. In Serum Circulating miRNA Expression Profiling of Nonrelated IBD-Inflammation, Ulcerative Colitis, Colorectal Cancer Patients
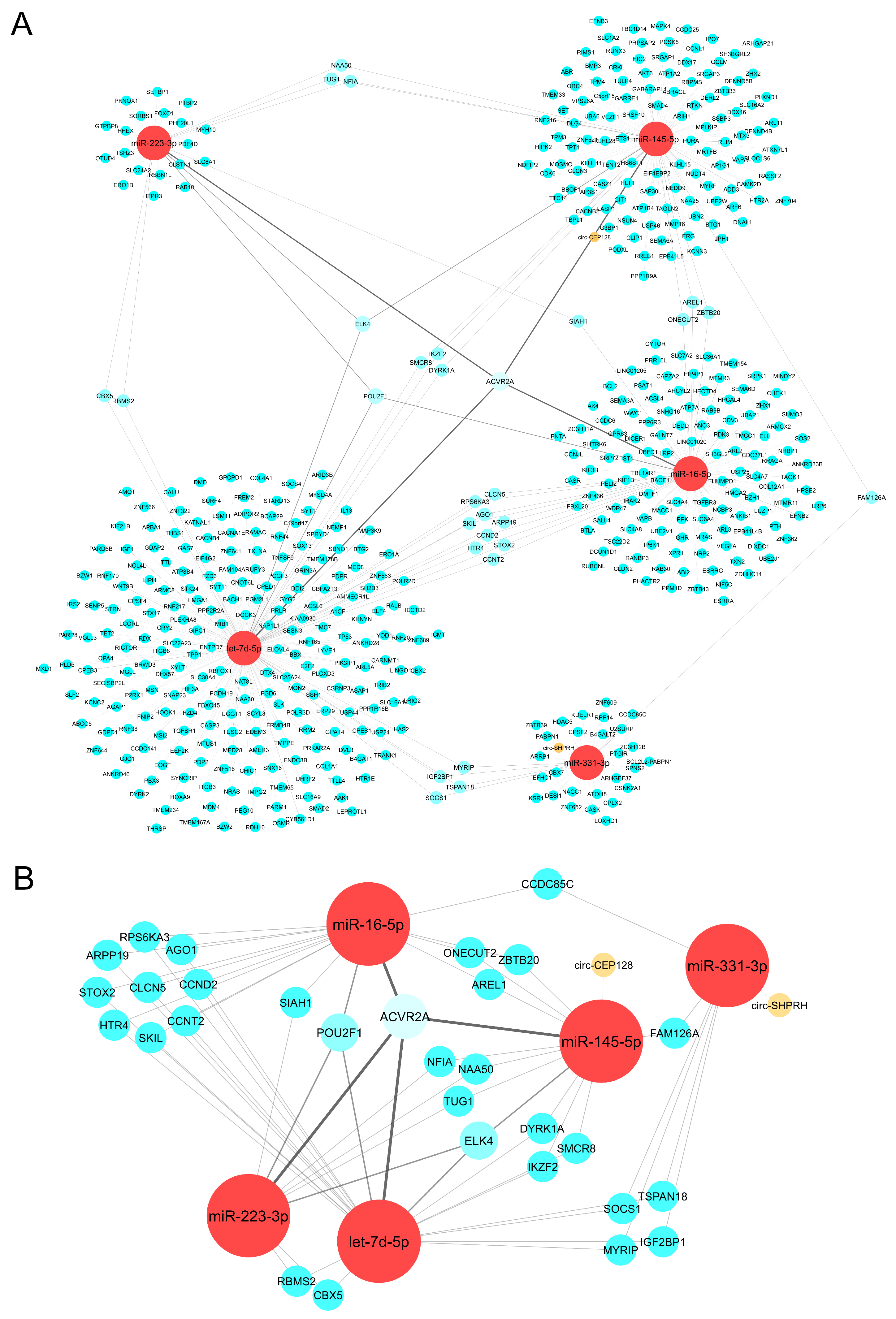
2.3. CeRNA Network Revealed the Crosslink between circRNAs, miRNAs, and mRNAs
2.4. PPI Network Analysis Showed Protein Clusters and Top Hub Genes Involved in the Tumoral Progression Landscape
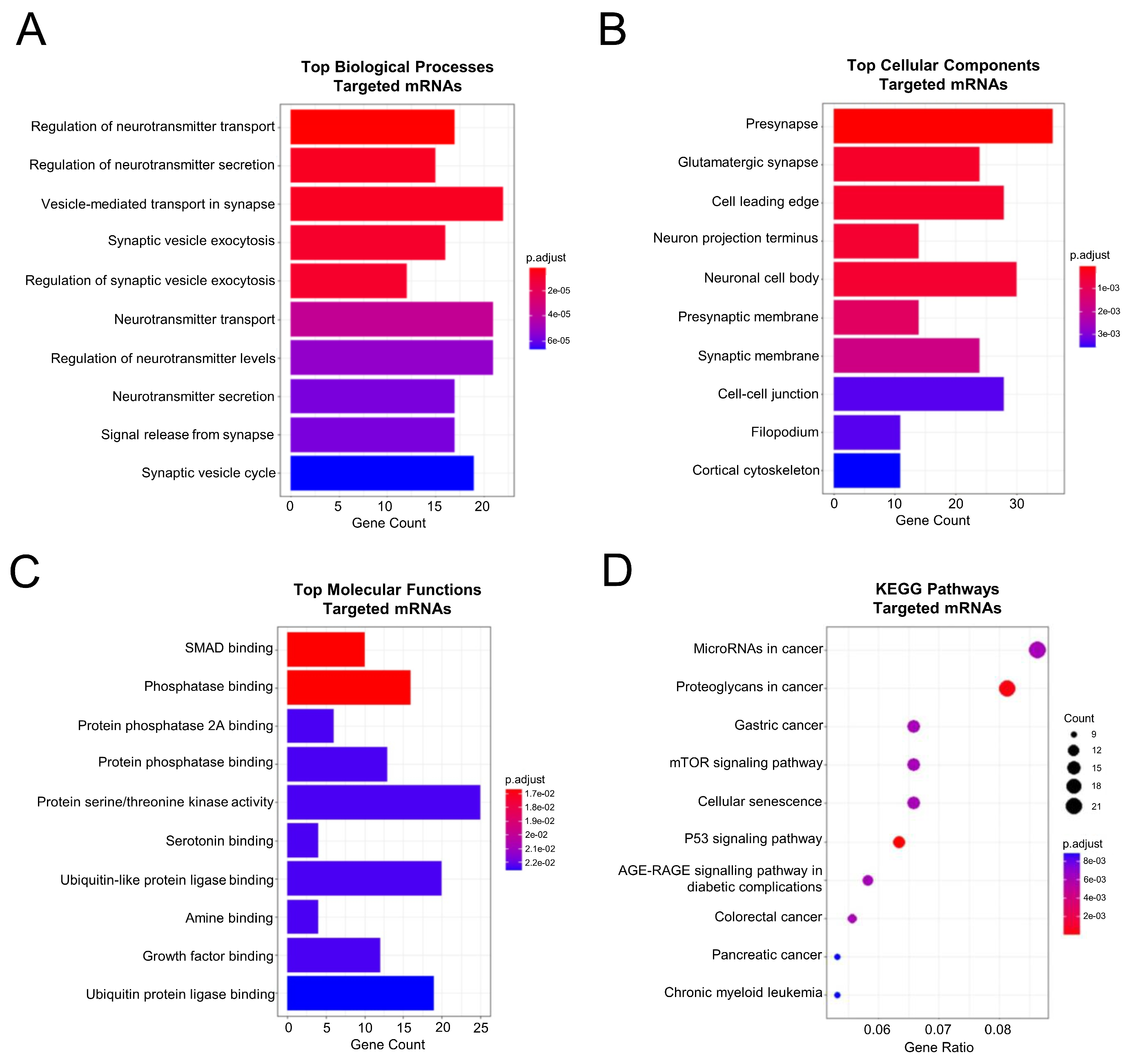
2.5. Functional Enrichment and Pathway Analysis of the PPI Network Members Pointed towards the Classical Colorectal Tumoral Axis and a Neuronal Component
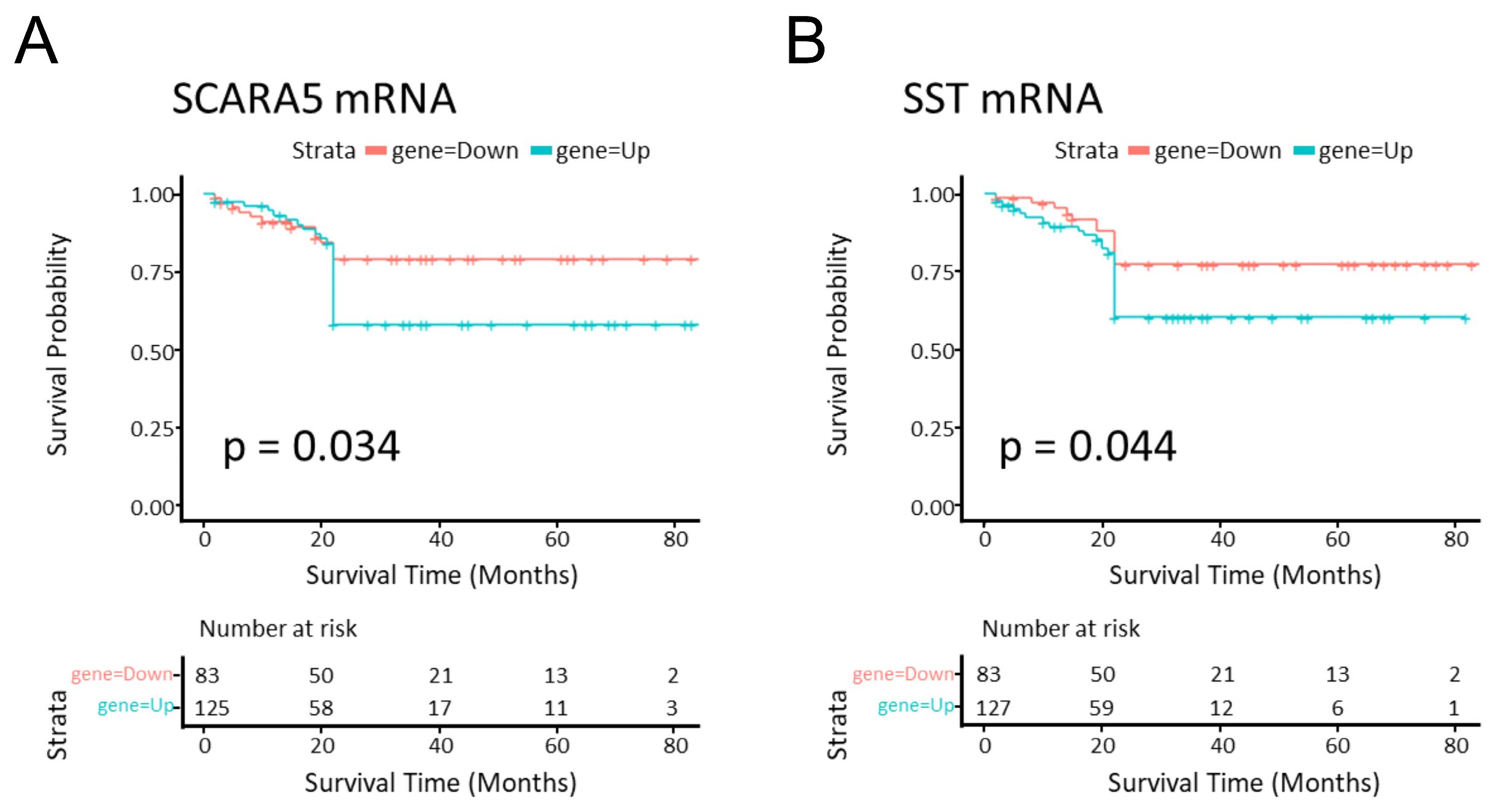
2.6. SCARA5 and SST Genes Were Related to Survival Prognosis in CRC
2.7. Tissue and Serum mRNA and miRNA Database Validation Led to SOCS1 mRNA, hsa-miR-331-3p, and hsa-let-7d-5p Candidates
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. Sample Processing
4.3. RNA Purification
4.4. miRNA Profiling and Real-Time PCR Analysis
4.5. Statistical Analysis
4.6. miRNA Target Prediction
4.7. Competitive Endogenous RNA (ceRNA) ncRNA-miRNA-mRNA Network Establishment
4.8. Protein–Protein Interaction (PPI) Network Construction, Protein Clusters, and Hub Gene Analysis
4.9. Enrichment Analysis of PPI Network
4.10. Gene Expression Dataset
4.11. In Silico Tissue–Serum DEG Profile Comparison
4.12. Identification of DEGs Related to CRC Survival Prognosis
4.13. miRNA Expression Dataset Analysis
4.14. In Silico Tissue–Serum miRNA Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Du, L.; Ha, C. Epidemiology and Pathogenesis of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 643–654. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, P.; Shao, L. Correlation of ulcerative colitis and colorectal cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2021, 12, 2814–2822. [Google Scholar] [CrossRef]
- Yashiro, M. Ulcerative colitis-associated colorectal cancer. World J. Gastroenterol. 2014, 20, 16389–16397. [Google Scholar] [CrossRef]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J. Crohns Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, T.; Wu, D.; Li, J.; Wang, M.; Sun, Y.; Hou, S. Colorectal Cancer in Ulcerative Colitis: Mechanisms, Surveillance and Chemoprevention. Curr. Oncol. 2022, 29, 6091–6114. [Google Scholar] [CrossRef] [PubMed]
- Low, E.N.D.; Mokhtar, N.M.; Wong, Z.; Ali, R.A.R. Colonic Mucosal Transcriptomic Changes in Patients with Long-Duration Ulcerative Colitis Revealed Colitis-Associated Cancer Pathways. J. Crohns Colitis 2019, 13, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Dong, M.; Dai, C.; Wu, S. Inflammation and Inflammatory Cytokine Contribute to the Initiation and Development of Ulcerative Colitis and Its Associated Cancer. Inflamm. Bowel Dis. 2019, 25, 1595–1602. [Google Scholar] [CrossRef]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Bocchetti, M.; Ferraro, M.G.; Ricciardiello, F.; Ottaiano, A.; Luce, A.; Cossu, A.M.; Scrima, M.; Leung, W.-Y.; Abate, M.; Stiuso, P.; et al. The Role of microRNAs in Development of Colitis-Associated Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 3967. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Z.; Yan, Y.; Ji, L.; He, J.; Xuan, B. Enterotoxigenic Bacteroidesfragilis Promotes Intestinal Inflammation and Malignancy by Inhibiting Exosome-Packaged miR-149-3p. Gastroenterology 2021, 161, 1552–1566. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, W.; Chen, Z.; Li, M.; Li, S.; Wu, B.; Bu, R. Circular RNA CEP128 promotes bladder cancer progression by regulating Mir-145-5p/Myd88 via MAPK signaling pathway. Int. J. Cancer 2019, 145, 2170–2181. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Qiu, C.-L.; Wang, M.; Mao, N.; Wu, S.-F.; Dai, Y.-H. Hsa_circ_0001649: A circular RNA and potential novel biomarker for colorectal cancer. Biochem. Biophys. Res. Commun. 2018, 497, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 2020, 395, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Shahnazari, M.; Afshar, S.; Emami, M.H.; Amini, R.; Jalali, A. Novel biomarkers for neoplastic progression from ulcerative colitis to colorectal cancer: A systems biology approach. Sci. Rep. 2023, 13, 3413. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, L.; Dong, Z.; Li, J.; Yu, Y.; Chen, X. Expression patterns and prognostic value of m(6)A-related genes in colorectal cancer. Am. J. Transl. Res. 2019, 11, 3972–3991. [Google Scholar] [PubMed]
- Lukas, M. Inflammatory bowel disease as a risk factor for colorectal cancer. Dig. Dis. 2010, 28, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, H.; Wang, R.; Huang, M.; Wu, Q.; Wang, Y.; Zhang, W.; Liu, Y. Hsa-let-7d-5p Promotes Gastric Cancer Progression by Targeting PRDM5. J. Oncol. 2022, 2022, 2700651. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Silva, A.P.; Pereira, K.S.; Tolentino-Araujo, G.T.; Nicolau, E.d.S.; Silva-Ferreira, C.M.; Teixeira, A.L.; Diniz, B.S. Shared Biologic Pathways Between Alzheimer Disease and Major Depression: A Systematic Review of MicroRNA Expression Studies. Am. J. Geriatr. Psychiatry 2016, 24, 903–912. [Google Scholar] [CrossRef]
- Cunningham, C.C.; Wade, S.; Floudas, A.; Orr, C.; McGarry, T.; Wade, S.; Cregan, S. Serum miRNA Signature in Rheumatoid Arthritis and ‘At-Risk Individuals’. Front. Immunol. 2021, 12, 633201. [Google Scholar] [CrossRef]
- Ni, Y.; Lu, C.; Wang, W.; Gao, W.; Yu, C. circBANP promotes colorectal cancer growth and metastasis via sponging let-7d-5p to modulate HMGA1/Wnt/β-catenin signaling. Mol. Ther. Oncolytics 2021, 21, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Khoshbakht, T.; Hussen, B.M.; Abdullah, S.T.; Taheri, M.; Samadian, M. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int. 2022, 22, 342. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, S.; Wang, Y.; Tang, H.; Wang, B.; Sun, X.; Qu, J.; Rao, B. A Novel Defined Necroptosis-Related miRNAs Signature for Predicting the Prognosis of Colon Cancer. Int. J. Gen. Med. 2022, 15, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cui, L.; Lin, C.; Wang, Y.; Liu, Z.; Miao, X. LncRNA CDKN2B-AS1 relieved inflammation of ulcerative colitis via sponging miR-16 and miR-195. Int. Immunopharmacol. 2020, 88, 106970. [Google Scholar] [CrossRef]
- Zhou, R.; Qiu, P.; Wang, H.; Yang, H.; Yang, X.; Ye, M.; Wang, F.; Zhao, Q. Identification of microRNA-16-5p and microRNA-21-5p in feces as potential noninvasive biomarkers for inflammatory bowel disease. Aging 2021, 13, 4634–4646. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, L.; Ye, X.; Tao, M.; Wu, J. miR-145-5p suppresses proliferation, metastasis and EMT of colorectal cancer by targeting CDCA3. Pathol. Res. Pract. 2020, 216, 152872. [Google Scholar] [CrossRef] [PubMed]
- Polytarchou, C.; Oikonomopoulos, A.; Mahurkar, S.; Touroutoglou, A.; Koukos, G.; Hommes, D.W.; Iliopoulos, D. Assessment of Circulating MicroRNAs for the Diagnosis and Disease Activity Evaluation in Patients with Ulcerative Colitis by Using the Nanostring Technology. Inflamm. Bowel Dis. 2015, 21, 2533–2539. [Google Scholar] [CrossRef]
- Ranjbaran, J.; Safarpour, H.; Nomiri, S.; Tavakoli, T.; Rezaei, Z.; Salmani, F.; Larki, P.; Chamani, E. Experimental validation of in silico analysis estimated the reverse effect of upregulated hsa-miR-106a-5p and hsa-miR-223-3p on SLC4A4 gene expression in Iranian patients with colorectal adenocarcinoma by RT-qPCR. Cancer Med. 2023, 12, 7005–7018. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lei, C. Neuroprotective effects of miR-331-3p through improved cell viability and inflammatory marker expression: Correlation of serum miR-331-3p levels with diagnosis and severity of Alzheimer’s disease. Exp. Gerontol. 2021, 144, 111187. [Google Scholar] [CrossRef]
- Li, R.; Li, X.; Huang, Y.; Qiu, H.; Li, L.; Bi, Z. LncRNA SOX2OT Knockdown Alleviates Lipopolysaccharide-Induced Damage of PC12 Cells by Regulating miR-331-3p/Neurod1 Axis. World Neurosurg. 2021, 147, 293–305. [Google Scholar] [CrossRef]
- Chen, M.-L.; Hong, C.-G.; Yue, T.; Li, H.-M.; Duan, R.; Hu, W.-B.; Cao, J.; Wang, Z.-X.; Chen, C.-Y.; Hu, X.-K.; et al. Inhibition of miR-331-3p and miR-9-5p ameliorates Alzheimer’s disease by enhancing autophagy. Theranostics 2021, 11, 2395–2409. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Kataoka, S.; Nakayama, M.; Ali, M.A.E.; Oshima, H.; Yamamoto, D.; Park, J.-W.; Takegami, Y.; An, T.; Jenkins, N.A.; et al. CRISPR-Cas9-mediated gene knockout in intestinal tumor organoids provides functional validation for colorectal cancer driver genes. Proc. Natl. Acad. Sci. USA 2019, 116, 15635–15644. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Li, J.; Zhu, J.; Zuo, S.; Wang, X.; Liu, Y.; Liu, J.; Liu, X.; Wang, P.; Chen, S. Hydrogen Sulfide Creates a Favorable Immune Microenvironment for Colon Cancer. Cancer Res. 2023, 83, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Arreguín, K.; Bensard, C.; Schell, J.C.; Swanson, E.; Chen, X.; Rutter, J.; Tantin, D. Oct1/Pou2f1 is selectively required for colon regeneration and regulates colon malignancy. PLoS Genet. 2019, 15, e1007687. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Nevala, S.; Ukwattage, S.; Wirta, E.-V.; Ahtiainen, M.; Ristimäki, A.; Seppälä, T.T.; Lepistö, A.; Mecklin, J.-P.; Peltomäki, P. Immunoprofiles and DNA Methylation of Inflammatory Marker Genes in Ulcerative Colitis-Associated Colorectal Tumorigenesis. Biomolecules 2021, 11, 1440. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Lin, C.C.; Chen, W.S.; Jiang, J.K.; Yang, S.H.; Chang, S.C.; Ho, C.-L.; Yang, C.-C.; Huang, S.-C.; Chao, Y.; et al. Insulin-Like Growth Factor 2 mRNA-Binding Protein 1 (IGF2BP1) Is a Prognostic Biomarker and Associated with Chemotherapy Responsiveness in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6940. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Luo, J.; Fu, X.; Liu, P.; Liu, S.; Pan, Y. FAM126A interacted with ENO1 mediates proliferation and metastasis in pancreatic cancer via PI3K/AKT signaling pathway. Cell Death Discov. 2022, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-T.; Chen, T.; Lu, S.; Hu, W.; Zhang, Q.; Tan, J.; Sun, D.; Li, L.; Sun, X.; Xu, C.; et al. Identification of Significant Modules and Targets of Xian-Lian-Jie-Du Decoction Based on the Analysis of Transcriptomics, Proteomics and Single-Cell Transcriptomics in Colorectal Tumor. J. Inflamm. Res. 2022, 15, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, W.C.; McAlister, V.; Zhou, Q.; Zheng, X. Circular RNA in colorectal cancer. J. Cell Mol. Med. 2021, 25, 3667–3679. [Google Scholar] [CrossRef]
- Chin, C.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Chu, Q.; Lu, S.; Zhou, L.; Lu, X.; Liu, C.; Mao, L.; Ye, C.; Timko, M.P.; et al. The Circular RNA Profiles of Colorectal Tumor Metastatic Cells. Front. Genet. 2018, 9, 34. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, W.; Wang, X.; Wang, T.; Chen, Y.; Chen, B.; Liu, R.; Bai, P.; Xing, J. Circular RNA CEP128 acts as a sponge of miR-145-5p in promoting the bladder cancer progression via regulating SOX11. Mol. Med. 2018, 24, 40. [Google Scholar] [CrossRef]
- Hao, S.; Cong, L.; Qu, R.; Liu, R.; Zhang, G.; Li, Y. Emerging roles of circular RNAs in colorectal cancer. OncoTargets Ther. 2019, 12, 4765–4777. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Ren, X.; Xin, S.; Lan, X.; Lu, G.; Lin, Y.; Yang, S.; Zeng, Z.; Liao, W.; Ding, Y.-Q.; et al. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget 2016, 7, 26680–26691. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-T.; Shen, L.; Zuo, L.; Shashikanth, N.; Ong, M.L.D.M.; Wu, L.; Zha, J.; Edelblum, K.L.; Wang, Y.; Wang, Y.; et al. Inflammation-induced Occludin Downregulation Limits Epithelial Apoptosis by Suppressing Caspase-3 Expression. Gastroenterology 2019, 157, 1323–1337. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Chen, J.; Chen, S.; Li, Z.; Liu, H.; Bai, Y.; Zhi, F. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics 2020, 10, 12204–12222. [Google Scholar] [CrossRef] [PubMed]
- Kellermann, L.; Riis, L.B. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig. Med. Res. 2021, 4, 3. [Google Scholar] [CrossRef]
- Fleming, N.I.; Jorissen, R.N.; Mouradov, D.; Christie, M.; Sakthianandeswaren, A.; Palmieri, M.; Day, F.; Li, S.; Tsui, C.; Lipton, L.; et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013, 73, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Asati, V.; Bharti, S.K.; Mahapatra, D.K.; Asati, V.; Budhwani, A.K. Triggering PIK3CA Mutations in PI3K/Akt/mTOR Axis: Exploration of Newer Inhibitors and Rational Preventive Strategies. Curr. Pharm. Des. 2016, 22, 6039–6054. [Google Scholar] [CrossRef]
- Ma, H.-P.; Chang, H.-L.; Bamodu, O.A.; Yadav, V.K.; Huang, T.-Y.; Wu, A.T.H.; Yeh, C.-T.; Tsai, S.-H.; Lee, W.-H. Collagen 1A1 (COL1A1) Is a Reliable Biomarker and Putative Therapeutic Target for Hepatocellular Carcinogenesis and Metastasis. Cancers 2019, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- You, K.; Su, F.; Liu, L.; Lv, X.; Zhang, J.; Zhang, Y.; Liu, B. SCARA5 plays a critical role in the progression and metastasis of breast cancer by inactivating the ERK1/2, STAT3, and AKT signaling pathways. Mol. Cell. Biochem. 2017, 435, 47–58. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Control | UC | CRC | Overall |
|---|---|---|---|---|
| (N = 10) | (N = 10) | (N = 10) | (N = 30) | |
| Age (y) | 5 (50%) | 5 (50%) | 5 (50%) | 15 (50%) |
| <60 y | 5 (50%) | 5 (50%) | 5 (50%) | 15 (50%) |
| ≥60 y | 60.5 | 55.5 | 59.5 | 59.5 |
| Overall * | (40–64.5) | (41.25–64.25) | (57–63.25) | (49.25–64) |
| Sex | ||||
| Female | 5 (50%) | 5 (50%) | 5 (50%) | 15 (50%) |
| Male | 5 (50%) | 5 (50%) | 5 (50%) | 15 (50%) |
| Disease Duration (UC) * | N/A | 20 | N/A | N/A |
| (10.75–32.5) | ||||
| Disease Stage | N/A | N/A | N/A | |
| I | 3 (30%) | |||
| II | 3 (30%) | |||
| III | 2 (20%) | |||
| IV | 2 (20%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chacon-Millan, P.; Lama, S.; Del Gaudio, N.; Gravina, A.G.; Federico, A.; Pellegrino, R.; Luce, A.; Altucci, L.; Facchiano, A.; Caraglia, M.; et al. A Combination of Microarray-Based Profiling and Biocomputational Analysis Identified miR331-3p and hsa-let-7d-5p as Potential Biomarkers of Ulcerative Colitis Progression to Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 5699. https://doi.org/10.3390/ijms25115699
Chacon-Millan P, Lama S, Del Gaudio N, Gravina AG, Federico A, Pellegrino R, Luce A, Altucci L, Facchiano A, Caraglia M, et al. A Combination of Microarray-Based Profiling and Biocomputational Analysis Identified miR331-3p and hsa-let-7d-5p as Potential Biomarkers of Ulcerative Colitis Progression to Colorectal Cancer. International Journal of Molecular Sciences. 2024; 25(11):5699. https://doi.org/10.3390/ijms25115699
Chicago/Turabian StyleChacon-Millan, Pilar, Stefania Lama, Nunzio Del Gaudio, Antonietta Gerarda Gravina, Alessandro Federico, Raffaele Pellegrino, Amalia Luce, Lucia Altucci, Angelo Facchiano, Michele Caraglia, and et al. 2024. "A Combination of Microarray-Based Profiling and Biocomputational Analysis Identified miR331-3p and hsa-let-7d-5p as Potential Biomarkers of Ulcerative Colitis Progression to Colorectal Cancer" International Journal of Molecular Sciences 25, no. 11: 5699. https://doi.org/10.3390/ijms25115699
APA StyleChacon-Millan, P., Lama, S., Del Gaudio, N., Gravina, A. G., Federico, A., Pellegrino, R., Luce, A., Altucci, L., Facchiano, A., Caraglia, M., & Stiuso, P. (2024). A Combination of Microarray-Based Profiling and Biocomputational Analysis Identified miR331-3p and hsa-let-7d-5p as Potential Biomarkers of Ulcerative Colitis Progression to Colorectal Cancer. International Journal of Molecular Sciences, 25(11), 5699. https://doi.org/10.3390/ijms25115699










