Rethinking the Esterquats: Synthesis, Stability, Ecotoxicity and Applications of Esterquats Incorporating Analogs of Betaine or Choline as the Cation in Their Structure
Abstract
:1. Introduction
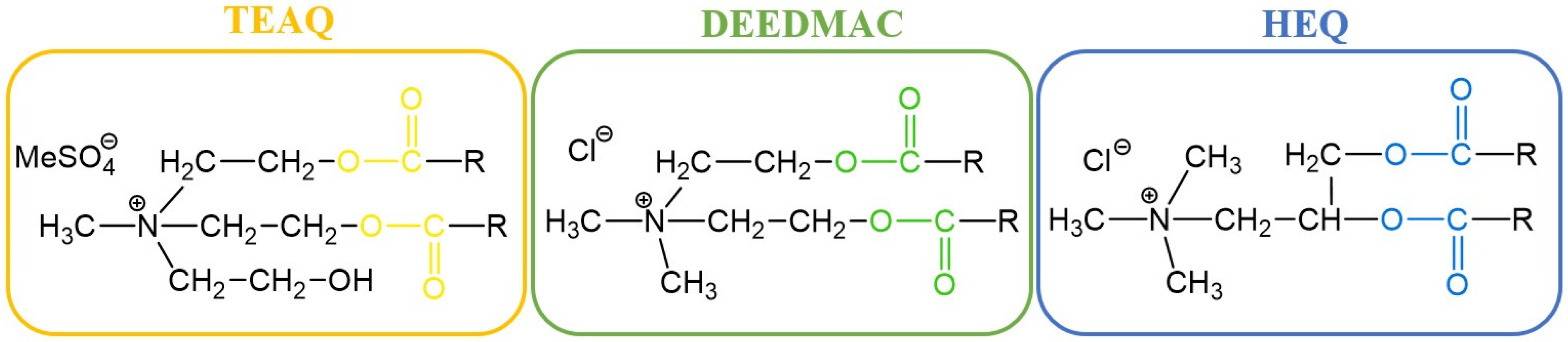
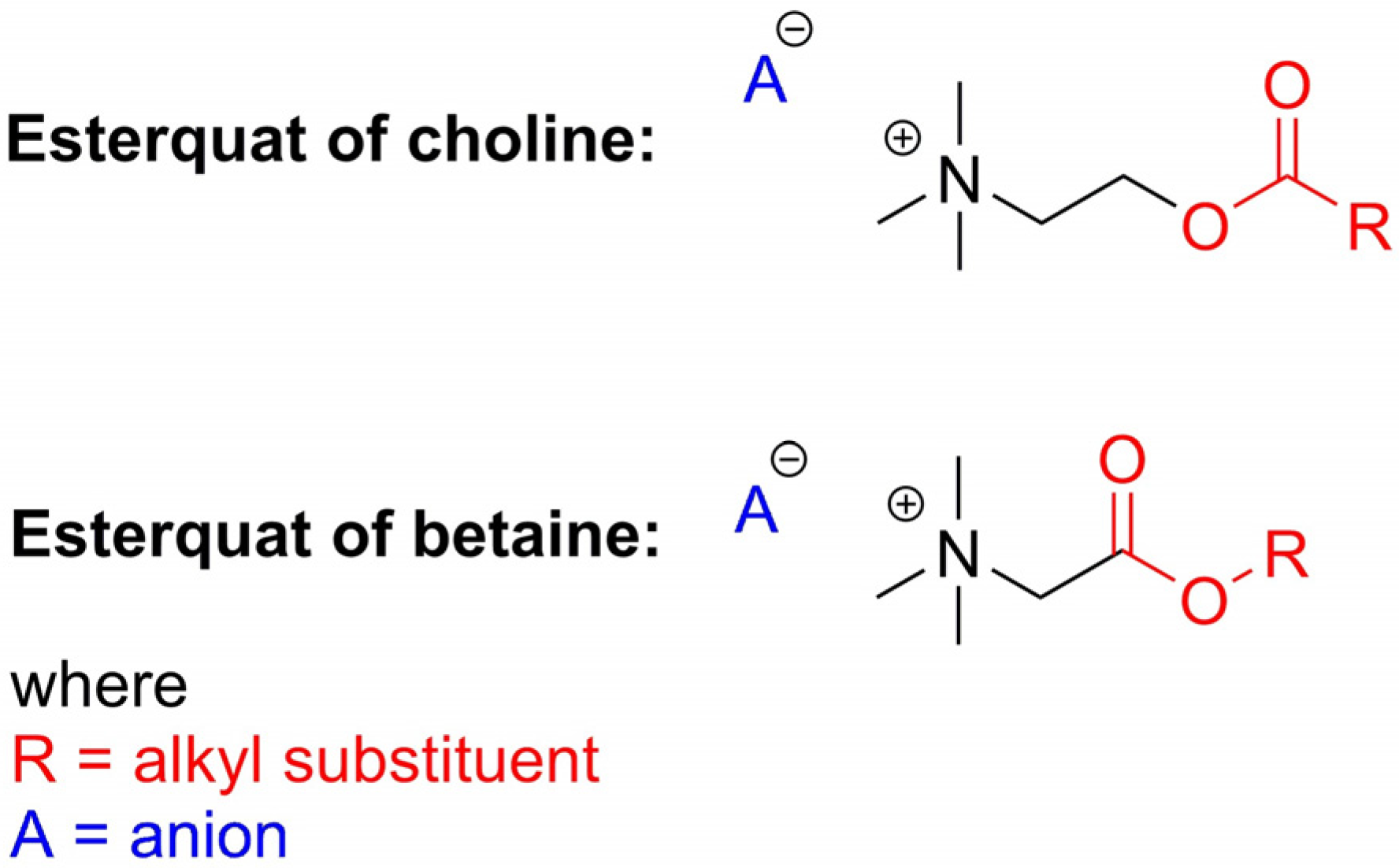
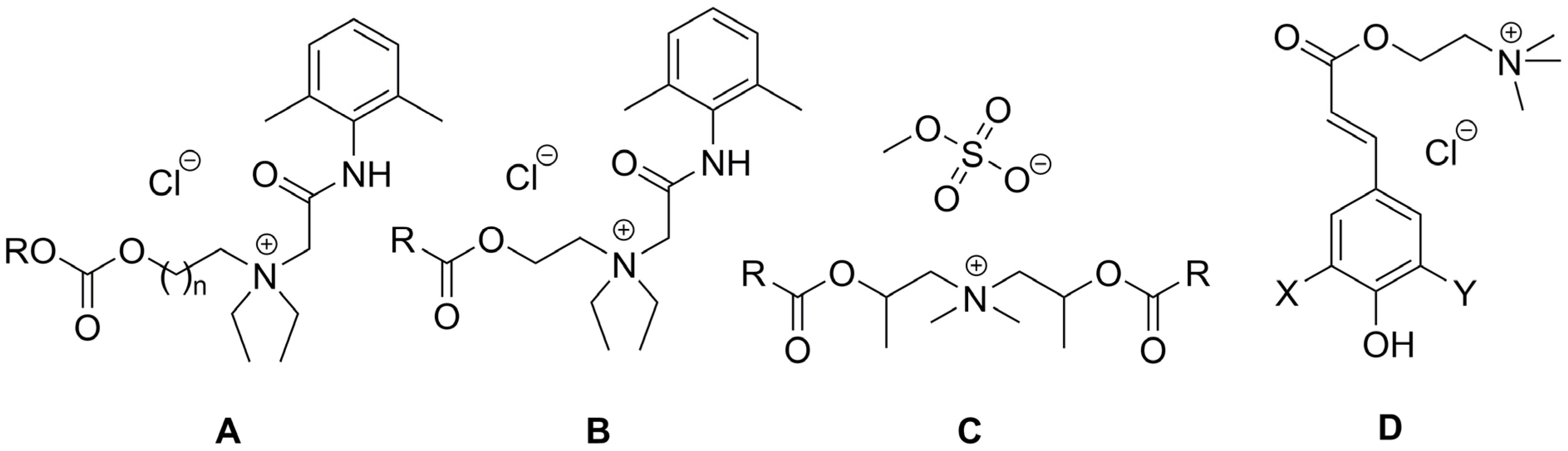
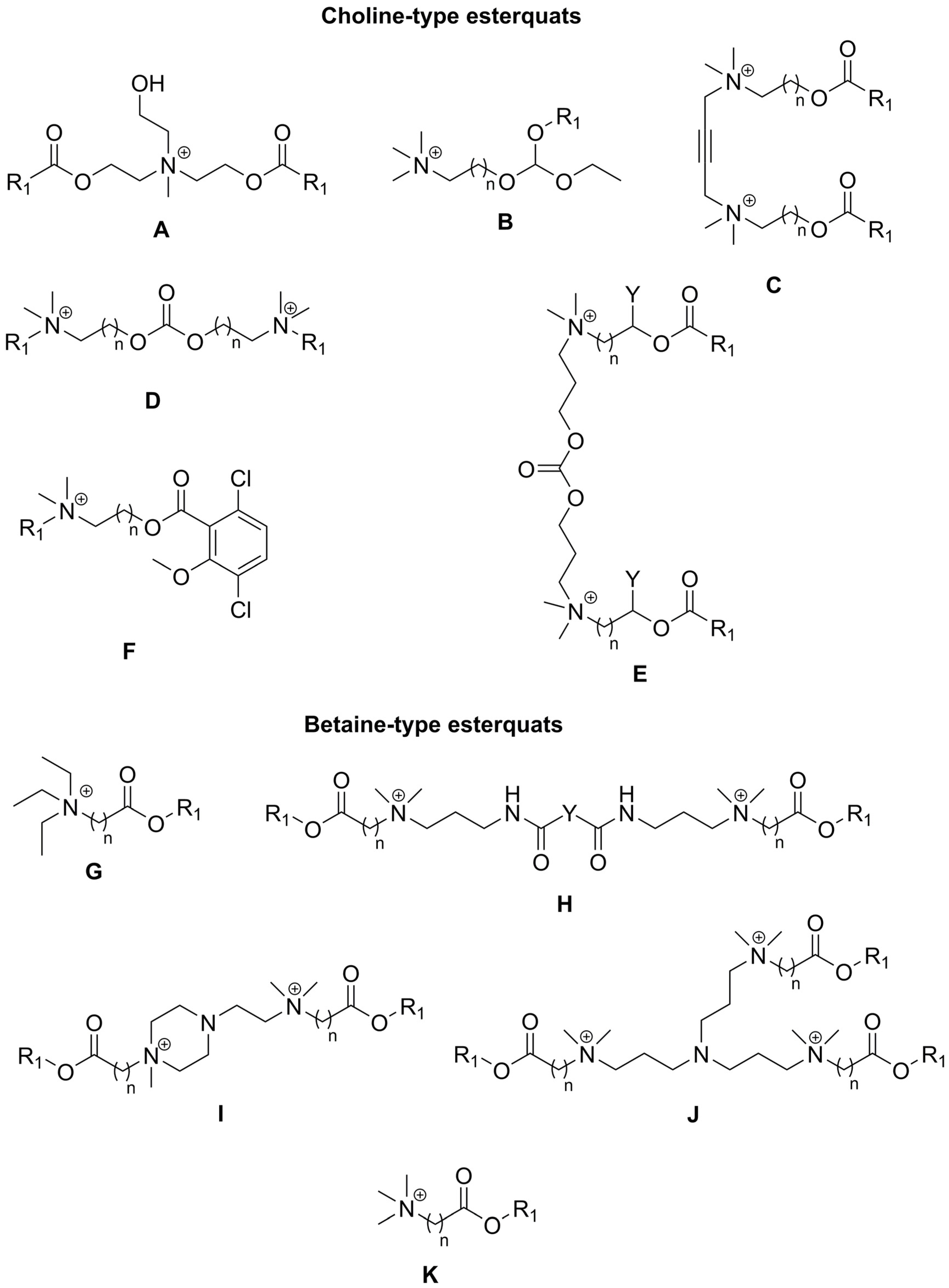
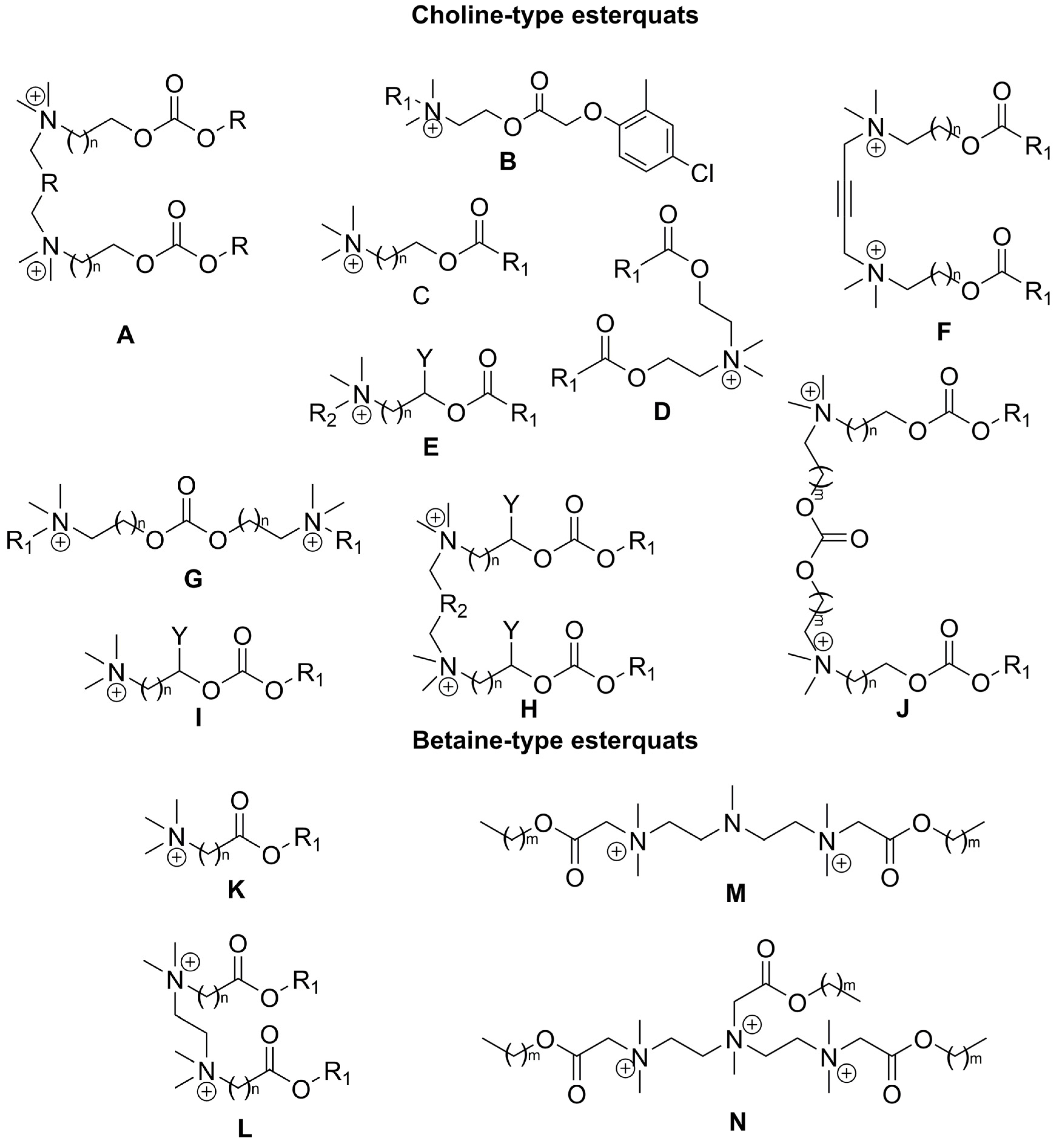
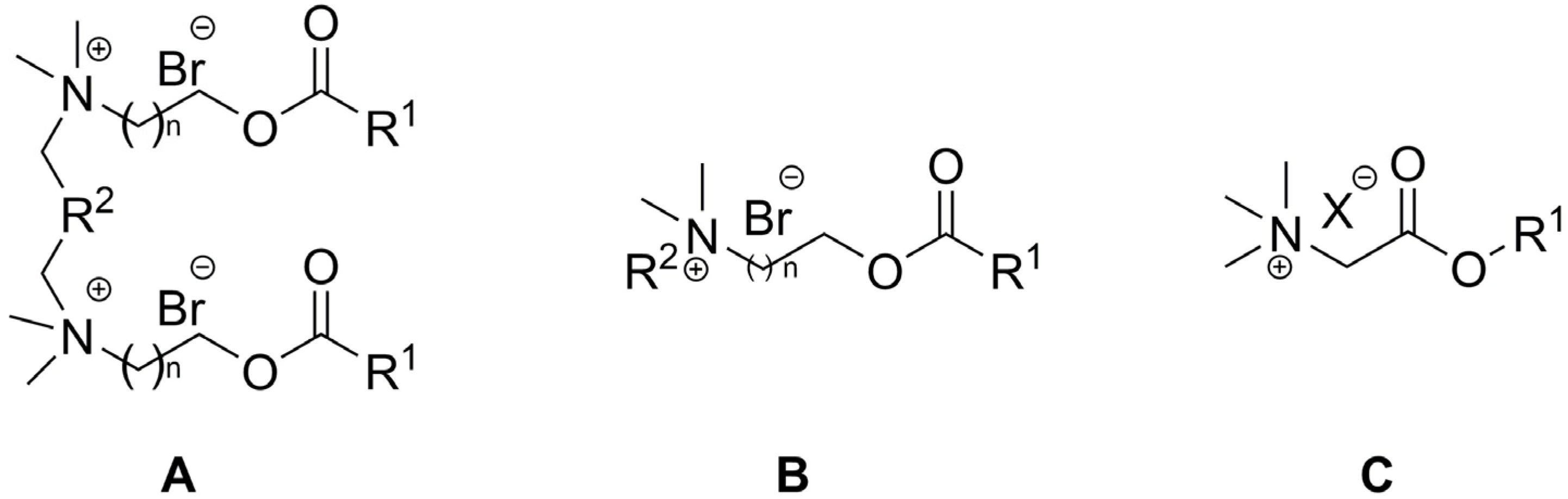
2. Types of Esterquats
2.1. Synthesis of Choline-Type Esterquats
2.2. Potential of Choline-Based Esterquats Synthesized from Biologically Active Compounds
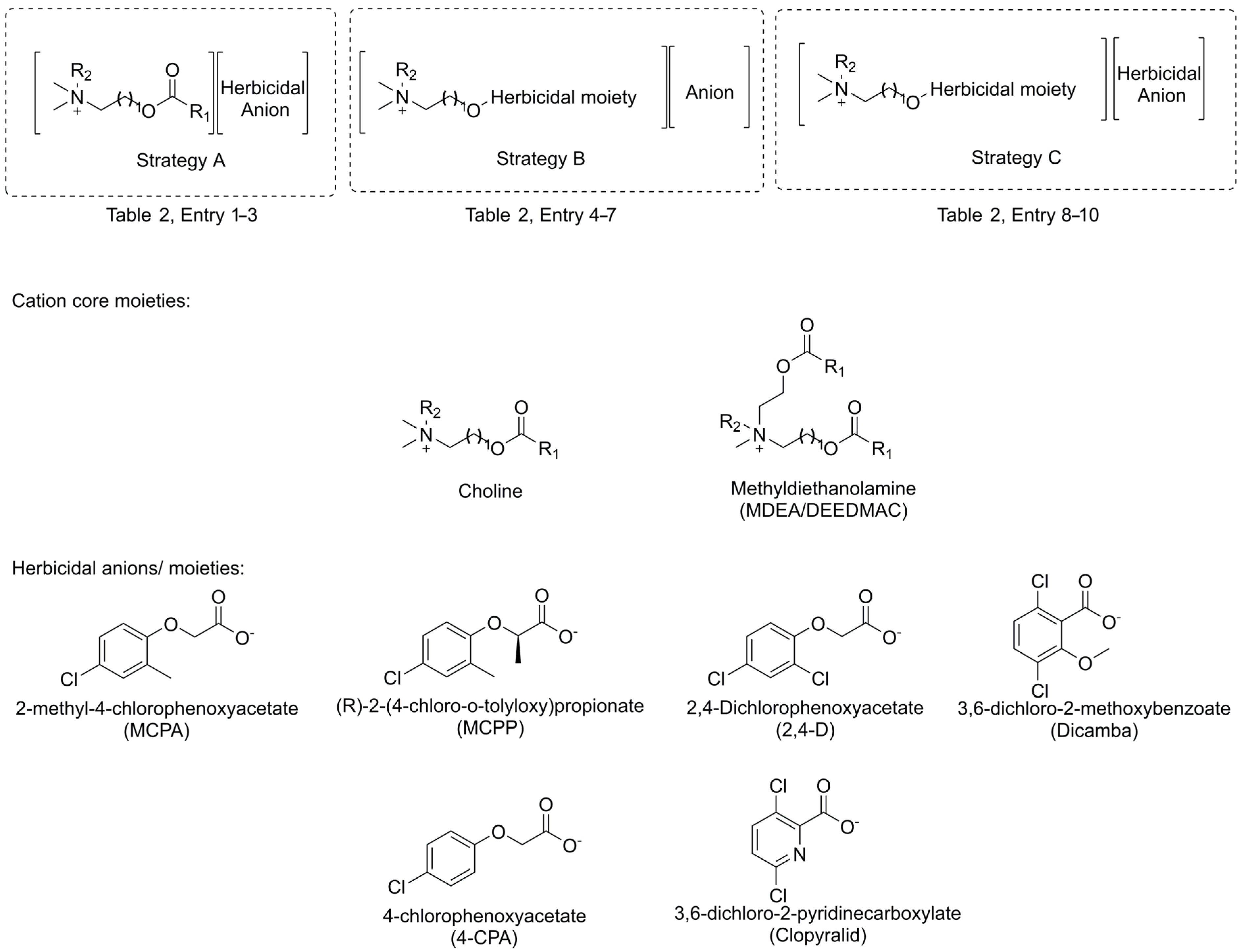
2.2.1. Herbicidal Formulations
2.2.2. Other Biologically Active Systems
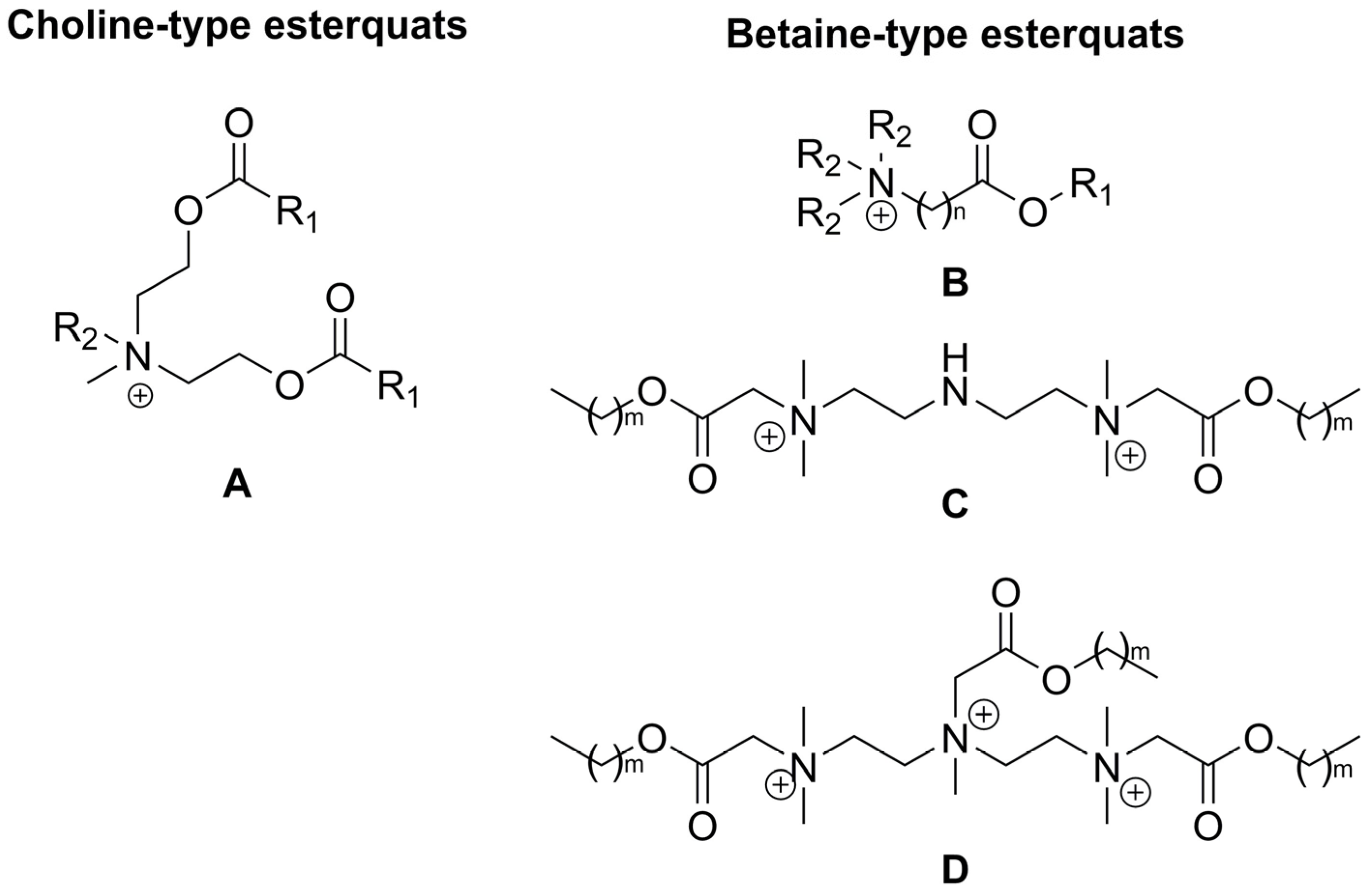
2.3. Synthesis of Betaine-Type Esterquats
2.4. Potential of Betaine-Based Esterquats Synthesized from Biologically Active Compounds
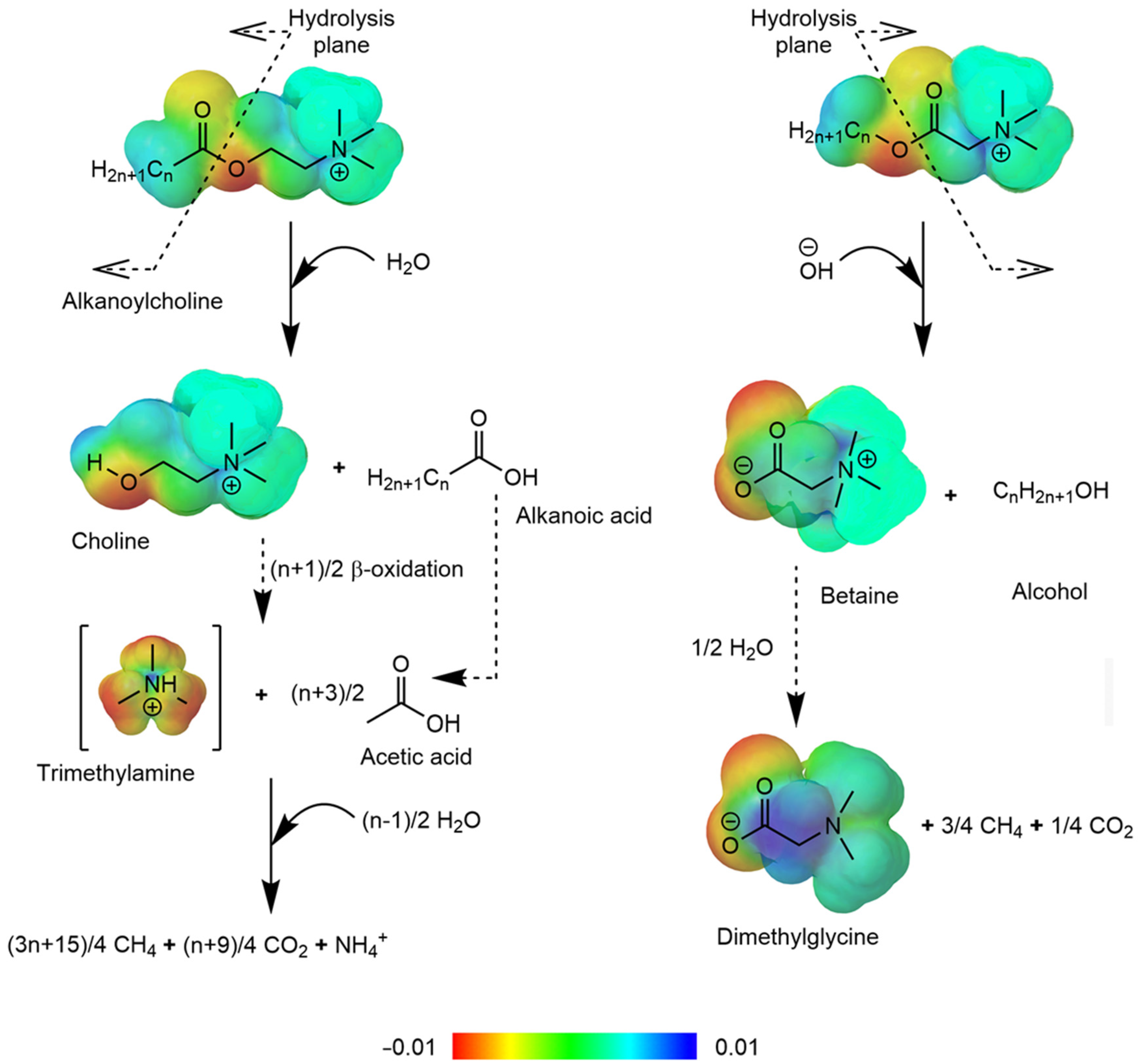
3. Susceptibility to Hydrolysis of Choline-Type and Betaine-Type Esterquats
4. Biological Tests
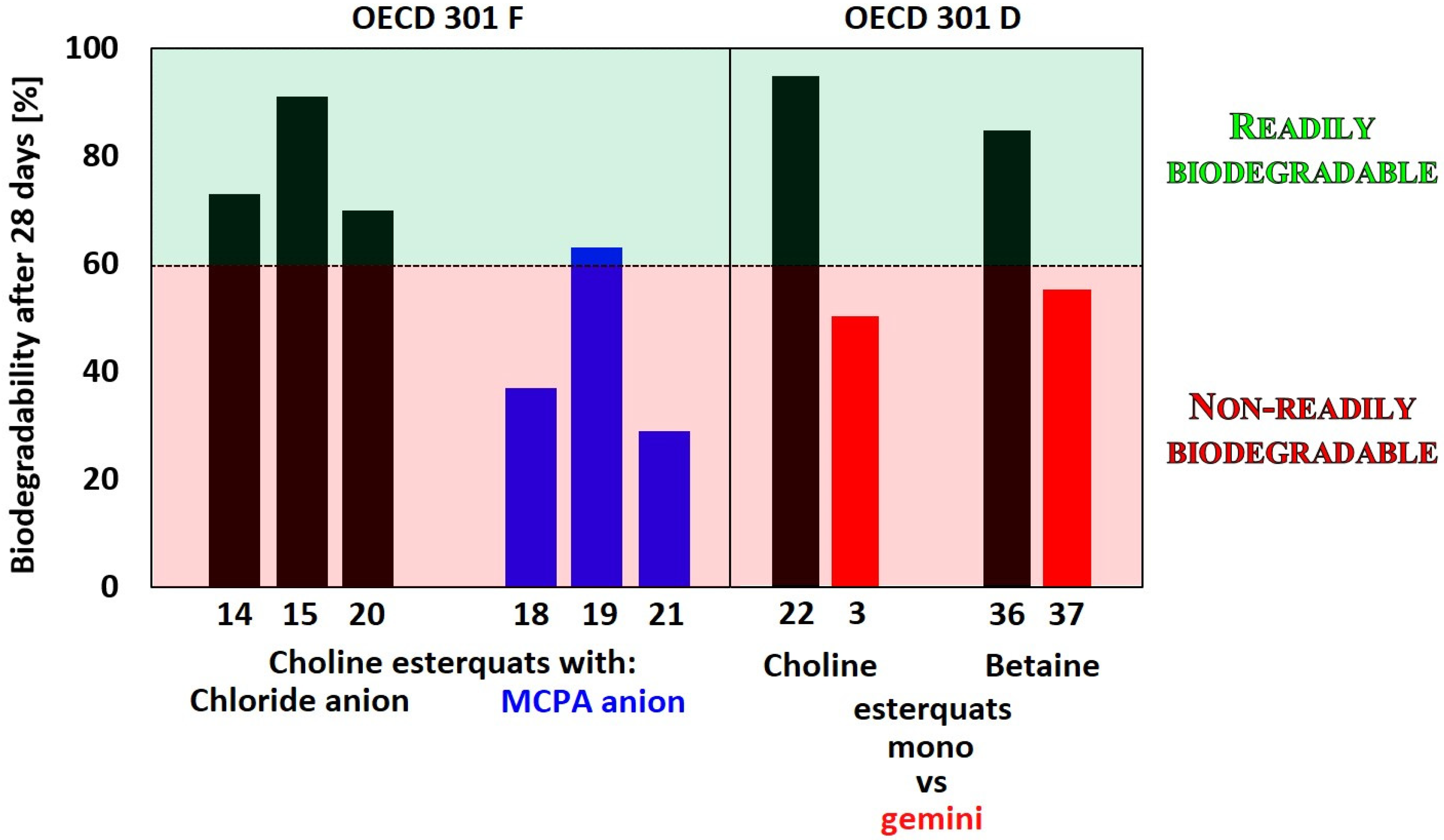
4.1. Biodegradation of Esterquats
4.2. Toxicity of Esterquats
4.2.1. Antimicrobial Activity of Esterquats
4.2.2. Toxicity of Esterquats toward Human Cells
4.2.3. Toxicity of Esterquats toward Mammalian and Aquatic Life
5. Applications
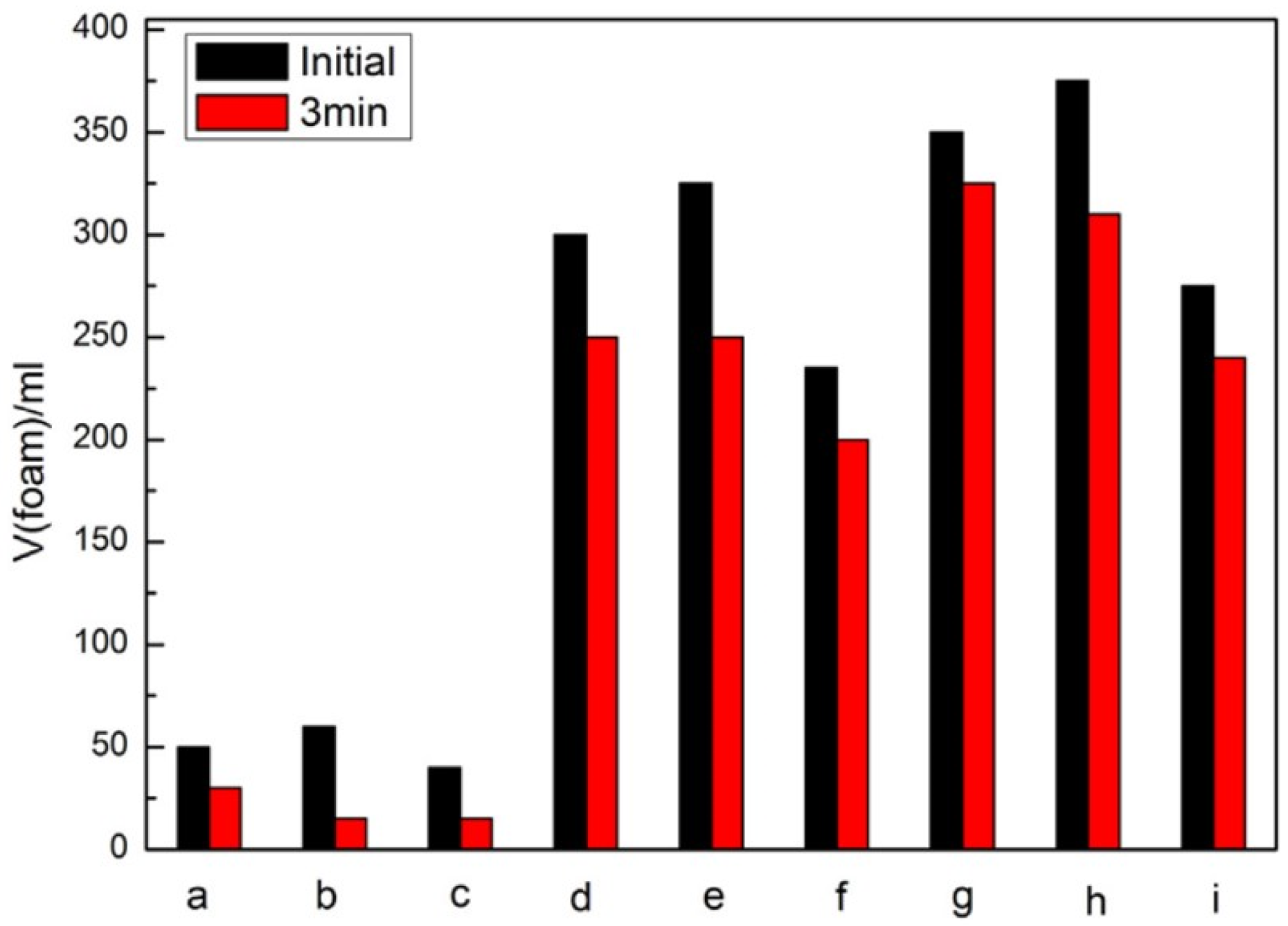
5.1. Surfactants, Emulsifiers and Foam Stabilizers
5.2. Flocculants, Flotation Collectors and Fabric Softeners
5.3. Pharmaceuticals and Antiseptics
5.4. Agrochemicals
5.5. Materials in Electrochemistry
6. Conclusions and Perspectives
- Simplification of synthetic procedures that comply with the concept of ‘green chemistry’ (e.g., utilization of nontoxic solvents, high atom economy, use of sources of natural origin, minimization of unit operations);
- Elucidation of standards for physicochemical characterization of newly synthesized esterquats. The proposed set should include tests focused on assessing their susceptibility to hydrolysis;
- Standardization of the toxicity assay is required to ease the comparison of collected results from various experiments. Esterquats should be assessed on an appropriately selected group of organisms with the use of one unified protocol or a group of protocols adapted to their respective application;
- The biodegradation of both ions of esterquats in soil as well as in water should be determined separately to understand their persistence in various environments. In effect, the standard activated sludge and bacteria isolated from soils should be used as a consistent set of experiments.
Supplementary Materials
Funding
Conflicts of Interest
References
- Mishra, S.; Tyagi, V.K. Ester Quats: The Novel Class of Cationic Fabric Softeners. J. Oleo Sci. 2007, 56, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Javadian, S.; Aghdastinat, H.; Tehrani-Bagha, A.; Gharibi, H. Self-Assembled Nano Structures of Cationic Ester-Containing Gemini Surfactants: The Surfactant Structure and Salt Effects. J. Chem. Thermodyn. 2013, 62, 201–210. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Oskarsson, H.; van Ginkel, C.G.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Chemical Hydrolysis and Biodegradation. J. Colloid Interface Sci. 2007, 312, 444–452. [Google Scholar] [CrossRef]
- Gaida, B.; Brzęczek-Szafran, A. Insights into the Properties and Potential Applications of Renewable Carbohydrate-Based Ionic Liquids: A Review. Molecules 2020, 25, 3285. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.; Stjerndahl, M.; Holmberg, K. Ester-based Surfactants: Are They Stable Enough? J. Surfact Deterg. 2022, 26, 229–236. [Google Scholar] [CrossRef]
- Para, G.; Łuczyński, J.; Palus, J.; Jarek, E.; Wilk, K.A.; Warszyński, P. Hydrolysis Driven Surface Activity of Esterquat Surfactants. J. Colloid Interface Sci. 2016, 465, 174–182. [Google Scholar] [CrossRef]
- Watson, M.K.; Tezel, U.; Pavlostathis, S.G. Biotransformation of Alkanoylcholines under Methanogenic Conditions. Water Res. 2012, 46, 2947–2956. [Google Scholar] [CrossRef]
- Schowanek, D.; Borsboom-Patel, T.; Bouvy, A.; Colling, J.; de Ferrer, J.A.; Eggers, D.; Groenke, K.; Gruenenwald, T.; Martinsson, J.; Mckeown, P.; et al. New and Updated Life Cycle Inventories for Surfactants Used in European Detergents: Summary of the ERASM Surfactant Life Cycle and Ecofootprinting Project. Int. J. Life Cycle Assess 2018, 23, 867–886. [Google Scholar] [CrossRef]
- Syguda, A.; Gielnik, A.; Borkowski, A.; Woźniak-Karczewska, M.; Parus, A.; Piechalak, A.; Olejnik, A.; Marecik, R.; Ławniczak, Ł.; Chrzanowski, Ł. Esterquat Herbicidal Ionic Liquids (HILs) with Two Different Herbicides: Evaluation of Activity and Phytotoxicity. New J. Chem. 2018, 42, 9819–9827. [Google Scholar] [CrossRef]
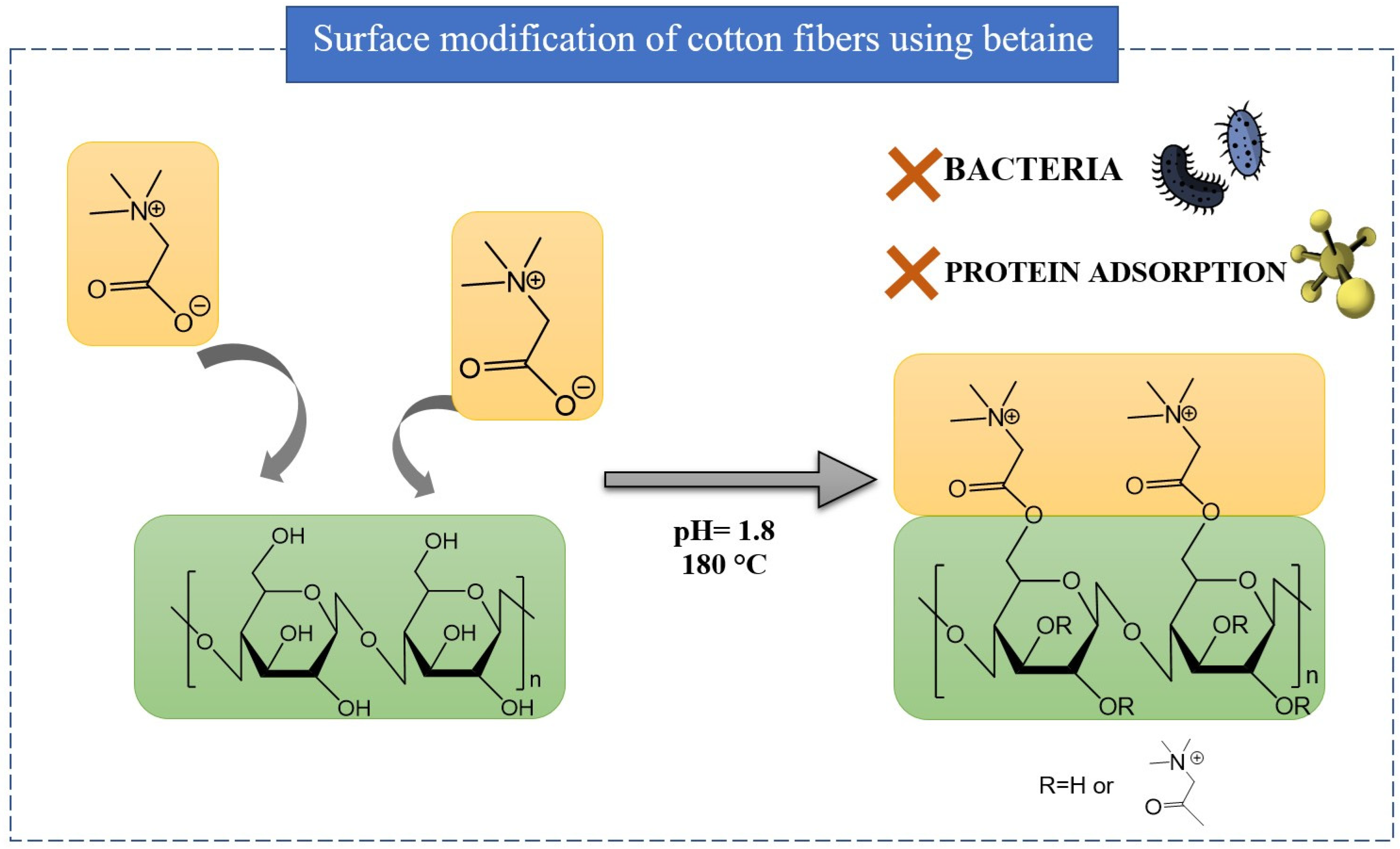
- Duan, P.; Xu, Q.; Zhang, X.; Chen, J.; Zheng, W.; Li, L.; Yang, J.; Fu, F.; Diao, H.; Liu, X. Naturally Occurring Betaine Grafted on Cotton Fabric for Achieving Antibacterial and Anti-Protein Adsorption Functions. Cellulose 2020, 27, 6603–6615. [Google Scholar] [CrossRef]
- Niemczak, M.; Sobiech, Ł.; Grzanka, M. Iodosulfuron-Methyl-Based Herbicidal Ionic Liquids Comprising Alkyl Betainate Cation as Novel Active Ingredients with Reduced Environmental Impact and Excellent Efficacy. J. Agric. Food Chem. 2020, 68, 13661–13671. [Google Scholar] [CrossRef] [PubMed]
- Pernak, J.; Czerniak, K.; Niemczak, M.; Chrzanowski, Ł.; Ławniczak, Ł.; Fochtman, P.; Marcinkowska, K.; Praczyk, T. Herbicidal Ionic Liquids Based on Esterquats. New J. Chem. 2015, 39, 5715–5724. [Google Scholar] [CrossRef]
- Wilms, W.; Woźniak-Karczewska, M.; Syguda, A.; Niemczak, M.; Ławniczak, Ł.; Pernak, J.; Rogers, R.D.; Chrzanowski, Ł. Herbicidal Ionic Liquids: A Promising Future for Old Herbicides? Review on Synthesis, Toxicity, Biodegradation, and Efficacy Studies. J. Agric. Food Chem. 2020, 68, 10456–10488. [Google Scholar] [CrossRef]
- Stachowiak, W.; Smolibowski, M.; Kaczmarek, D.K.; Rzemieniecki, T.; Niemczak, M. Toward Revealing the Role of the Cation in the Phytotoxicity of the Betaine-Based Esterquats Comprising Dicamba Herbicide. Sci. Total Environ. 2022, 845, 157181. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Mezzetta, A.; Nowicki, J.; Łuczak, J.; Guazzelli, L. Betaine and L-Carnitine Ester Bromides: Synthesis and Comparative Study of Their Thermal Behaviour and Surface Activity. J. Mol. Liq. 2021, 334, 115988. [Google Scholar] [CrossRef]
- Lindstedt, M.; Allenmark, S.; Thompson, R.A.; Edebo, L. Antimicrobial Activity of Betaine Esters, Quaternary Ammonium Amphiphiles Which Spontaneously Hydrolyze into Nontoxic Components. Antimicrob. Agents Chemother. 1990, 34, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Han, J.; Wu, X.; Wang, G.; Jiang, D. Betaine Ester-Shell Functionalized Hyperbranched Polymers for Potential Antimicrobial Usage: Guest Loading Capability, pH Controlled Release and Adjustable Compatibility. Polymer 2014, 55, 6261–6270. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Shi, D.; Yang, Y.; Zhang, Y.; Zhu, W. Cationic Gemini Surfactants Containing Both Amide and Ester Groups: Synthesis, Surface Properties and Antibacterial Activity. J. Mol. Liq. 2020, 299, 112248. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Cationic Ester-Containing Gemini Surfactants: Physical−Chemical Properties. Langmuir 2010, 26, 9276–9282. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Kabir-ud-Din; Beg, M. Ester-Bonded Cationic Gemini Surfactants: Assessment of Their Cytotoxicity and Antimicrobial Activity. J. Mol. Liq. 2016, 222, 390–394. [Google Scholar] [CrossRef]
- Parajó, J.J.; Macário, I.P.E.; De Gaetano, Y.; Dupont, L.; Salgado, J.; Pereira, J.L.; Gonçalves, F.J.M.; Mohamadou, A.; Ventura, S.P.M. Glycine-Betaine-Derived Ionic Liquids: Synthesis, Characterization and Ecotoxicological Evaluation. Ecotoxicol. Environ. Saf. 2019, 184, 109580. [Google Scholar] [CrossRef] [PubMed]
- Czerniak, K.; Dwiecki, K.; Majchrzycki, Ł.; Czerniak, A.; Białas, W. Synthesis and Application of Ammonium-Based Poly(Ionic Liquids) as Novel Cationic Flocculants. Chem. Pap. 2017, 71, 639–646. [Google Scholar] [CrossRef]
- Lundberg, D.; Ljusberg-Wahren, H.; Norlin, A.; Holmberg, K. Studies on Dodecyl Betainate in Combination with Its Degradation Products or with Phosphatidyl Choline–Phase Behavior and Hemolytic Activity. J. Colloid Interface Sci. 2004, 278, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, M.; Prochaska, K. The Effect of Electrolyte and Temperature on Adsorption Properties of Esterquats. Fluid Phase Equilibria 2014, 364, 95–103. [Google Scholar] [CrossRef]
- Cyboran-Mikołajczyk, S.; Bonarska-Kujawa, D.; Kleszczyńska, H.; Łuczyński, J. Effects of Interaction of Gemini Ester Quat Surfactants with Biological Membranes. Tenside Surfactants Deterg. 2016, 53, 20–28. [Google Scholar] [CrossRef]
- Piotrowska, A.; Syguda, A.; Chrzanowski, Ł.; Heipieper, H.J. Toxicity of Synthetic Herbicides Containing 2,4-D and MCPA Moieties towards Pseudomonas Putida Mt-2 and Its Response at the Level of Membrane Fatty Acid Composition. Chemosphere 2016, 144, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.N.; Hou, J.; Liu, L.; Yang, P.X.; Zhang, J.Q.; An, M.Z.; Li, N. A Piperidinium-Based Ester-Functionalized Ionic Liquid as Electrolytes in Li/LiFePO4 Batteries. Ionics 2017, 23, 3151–3161. [Google Scholar] [CrossRef]
- Esterquats Market Size & Analysis|Global Industry Report, 2016–2024. Available online: https://www.grandviewresearch.com/industry-analysis/esterquats-market (accessed on 24 September 2022).
- HERA Esterquats Environmental Risk Assessment Report; HERA: Paris, France, 2008; p. 42. Available online: https://www.heraproject.com/files/17-E-01-03-2008%20%20HERA%20EQ%20Environment%20Final%20Draft.pdf (accessed on 1 April 2024).
- Spekreijse, J.; Lammens, T.; Parisi, C.; Ronzon, T.; Vis, M. Insights into the European Market for Bio-Based Chemicals; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Oikonomou, E.K.; Grandisson, C.; Golemanov, K.; Ahuja, R.; Berret, J.-F. Silicone Incorporation into an Esterquat Based Fabric Softener in Presence of Guar Polymers. Colloids Surf. A Physicochem. Eng. Asp. 2021, 615, 126175. [Google Scholar] [CrossRef]
- Abdul Aziz, H.; Yusoff, R.; Cheng, N.G.; Idris, Z.; Ramli, N.A.S. Production of N-methyldiethanolamine Di-ester via Heterogeneous Transesterification of Palm Methyl Ester over Modified Calcium Oxide Catalyst by Metal Oxides. J. Surfactants Deterg. 2023, 26, 477–490. [Google Scholar] [CrossRef]
- Abdul Aziz, H.; Kheireddine Aroua, M.; Yusoff, R.; Azeerah Abas, N.; Idris, Z. Optimization of Transesterification of Palm-Based Methyl Palmitate and Triethanolamine towards Maximum Di-Esteramine Content. Biocatal. Agric. Biotechnol. 2017, 10, 352–359. [Google Scholar] [CrossRef]
- Sikarskie, M.; Pulukkody, R.; Bauer, P.; Leal, L.M.; Partani, E.M.; Miller, D.S.; Todd, J.P. Fabric Care Formulation. WO2022203868A1, 29 September 2022. [Google Scholar]
- Weng, X.; Mei, G.; Zhao, T.; Zhu, Y. Utilization of Novel Ester-Containing Quaternary Ammonium Surfactant as Cationic Collector for Iron Ore Flotation. Sep. Purif. Technol. 2013, 103, 187–194. [Google Scholar] [CrossRef]
- Obiols, O.P.; Bonastre, N.; Llosas, J.B. Esterquats. U.S. Patent 5880299A, 9 March 1999. [Google Scholar]
- Faunce, J.A.; Butikas, R.A.; Bernhardt, R.J.; Kovach, S.E.; Germain, T.; Wolfe, P.S.; Zaporowski, L.F.; Dameshek, A.A. Esterquat Compositions. U.S. Patent 20210128432A1, 6 May 2021. [Google Scholar]
- Bigorra, L.J.D.; Pi, S.R.D.; Prat, Q.E.D. Kosmetische Zubereitungen und Verfahren zu ihrer Herstellung. DE19635195C1, 29 January 1998. [Google Scholar]
- Schröder, T.; Scheele, S.; Mette, M. Glanz-Conditioner. DE102018202803A1, 29 August 2019. [Google Scholar]
- Koehle, H.-J.; Seidel, K.; Schwab, P.; Westerholt, U. Formulation Comprising Ester Quats Based on Isopropanolamine. U.S. Patent 9801797B2, 31 October 2017. [Google Scholar]
- Natoli, S.N.; Panandiker, R.K.; Miracle, G.S.; Hoover, J.M. Pro-Benefit-Agent Compounds with Carbon/Nitrogen Bonds. U.S. Patent 20220403297A1, 22 December 2022. [Google Scholar]
- Popova, P.; Notabi, M.K.; Code, C.; Arnspang, E.C.; Andersen, M.Ø. Co-Delivery of siRNA and Etoposide to Cancer Cells Using an MDEA Esterquat Based Drug Delivery System. Eur. J. Pharm. Sci. 2019, 127, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Wang, C.-C. Cationic Solid Lipid Nanoparticles with Primary and Quaternary Amines for Release of Saquinavir and Biocompatibility with Endothelia. Colloids Surf. B Biointerfaces 2013, 101, 101–105. [Google Scholar] [CrossRef] [PubMed]
- OECD Work on Pesticides and Sustainable Pest Management. Available online: https://www.oecd.org/chemicalsafety/pesticides-biocides/OECD-Pest-Vision-Final.pdf (accessed on 19 February 2023).
- Emergen Research Herbicides Market, By Type (Synthetic, Bio-Based), By Application (Pulses & Oilseeds, Grains & Cereals, Commercial Crops, Fruits & Vegetables, Turf & Ornamentals), By Mode of Action (Selective, Non-Selective), and By Region Forecast to 2030. Available online: https://www.emergenresearch.com/amp/industry-report/herbicides-market (accessed on 19 February 2023).
- Chami, R.; Bensajjay, F.; Alehyen, S.; El Achouri, M.; Boudalia, M.; Marrakchi, K.; Bellaouchou, A.; Guenbour, A. Electrochemical Investigation and Quantum Chemicalstudies on Corrosion Inhibition for Ester-Quats Surfactants on Iron in Hydrochloricacid. J. Mater. Environ. 2016, 8, 3034–3045. [Google Scholar]
- Syguda, A.; Wojcieszak, M.; Materna, K.; Woźniak-Karczewska, M.; Parus, A.; Ławniczak, Ł.; Chrzanowski, Ł. Double-Action Herbicidal Ionic Liquids Based on Dicamba Esterquats with 4-CPA, 2,4-D, MCPA, MCPP, and Clopyralid Anions. ACS Sustain. Chem. Eng. 2020, 8, 14584–14594. [Google Scholar] [CrossRef]
- Parus, A.; Homa, J.; Radoński, D.; Framski, G.; Woźniak-Karczewska, M.; Syguda, A.; Ławniczak, Ł.; Chrzanowski, Ł. Novel Esterquat-Based Herbicidal Ionic Liquids Incorporating MCPA and MCPP for Simultaneous Stimulation of Maize Growth and Fighting Cornflower. Ecotoxicol. Environ. Saf. 2021, 208, 111595. [Google Scholar] [CrossRef] [PubMed]
- Fahri, F.; Bacha, K.; Chiki, F.F.; Mbakidi, J.-P.; Panda, S.; Bouquillon, S.; Fourmentin, S. Air Pollution: New Bio-Based Ionic Liquids Absorb Both Hydrophobic and Hydrophilic Volatile Organic Compounds with High Efficiency. Environ. Chem. Lett. 2020, 18, 1403–1411. [Google Scholar] [CrossRef]
- Mbakidi, J.-P.; Kerkache, A.; Lazar, F.; Bouquillon, S. Dissolution of Cellulose and Lignin with Biobased Ionic Liquids. J. Solut. Chem. 2022, 51, 345–356. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Ecotoxicity of Surfactants in the Terrestrial Environment. Fresenius Environ. Bull. 2009, 18, 1666–1673. [Google Scholar]
- Czuryszkiewicz, D.; Maćkowiak, A.; Marcinkowska, K.; Borkowski, A.; Chrzanowski, Ł.; Pernak, J. Herbicidal Ionic Liquids Containing the Acetylcholine Cation. ChemPlusChem 2019, 84, 268–276. [Google Scholar] [CrossRef]
- Coates, P.M.; Betz, J.M.; Blackman, M.R.; Cragg, G.M.; Levine, M.; Moss, J.; White, J.D. (Eds.) Encyclopedia of Dietary Supplements; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-351-23630-0. [Google Scholar]
- Zeisel, S.H.; Corbin, K.D. Choline. In Present Knowledge in Nutrition; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 405–418. ISBN 978-0-470-95917-6. [Google Scholar]
- Han, B.; Geng, T.; Jiang, Y.; Ju, H. Synthesis and Properties of Di-Chain Esterquat Surfactants. J. Surfact. Deterg. 2015, 18, 91–95. [Google Scholar] [CrossRef]
- Lozano, P.; Daz, M.; De Diego, T.; Iborra, J.L. Ester Synthesis from Trimethylammonium Alcohols in Dry Organic Media Catalyzed by immobilizedCandida Antarctica Lipase B. Biotechnol. Bioeng. 2003, 82, 352–358. [Google Scholar] [CrossRef]
- Mouterde, L.M.M.; Peru, A.A.M.; Mention, M.M.; Brunissen, F.; Allais, F. Sustainable Straightforward Synthesis and Evaluation of the Antioxidant and Antimicrobial Activity of Sinapine and Analogues. J. Agric. Food Chem. 2020, 68, 6998–7004. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.D.; Mezzetta, A.; Guazzelli, L.; Scanlan, E.M. Radical-Mediated Thiol–Ene ‘Click’ Reactions in Deep Eutectic Solvents for Bioconjugation. Green Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- González-Rivera, J.; Mero, A.; Husanu, E.; Mezzetta, A.; Ferrari, C.; D’Andrea, F.; Bramanti, E.; Pomelli, C.S.; Guazzelli, L. Combining Acid-Based Deep Eutectic Solvents and Microwave Irradiation for Improved Chestnut Shell Waste Valorization. Green Chem. 2021, 23, 10101–10115. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Rivera, J.G.; Mezzetta, A.; Ruiz, J.C.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Tatsumi, T.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ono, D.; Takeda, T.; Ikeda, I. Novel Hydrolyzable and Biodegradable Cationic Gemini Surfactants: 1,3-Bis[(Acyloxyalkyl)-Dimethylammonio]-2-Hydroxypropane Dichloride. J. Surfactants Deterg. 2000, 3, 167–172. [Google Scholar] [CrossRef]
- Tammelin, L.-E.; Gmelin, R.; Jensen, R.B.; Stenhagen, E.; Thorell, B. Syntheses of Choline Esters of Monobasic Carbonic Acids. Acta Chem. Scand. 1956, 10, 145–146. [Google Scholar] [CrossRef]
- Pernak, J.; Syguda, A.; Janiszewska, D.; Materna, K.; Praczyk, T. Ionic Liquids with Herbicidal Anions. Tetrahedron 2011, 67, 4838–4844. [Google Scholar] [CrossRef]
- Chung, Y.; Park, S. Reversed Phase High Performance Liquid Chromatographic Separation of Esterquats with Indirect Spectrophotometric Detection. Microchem. J. 1998, 60, 42–50. [Google Scholar] [CrossRef]
- Steiger, P.; Friedli, F.E. A Study on the Long-Term Stability and Removal of Quats on Treated Fabric. J. Surfactants Deterg. 2002, 5, 207–210. [Google Scholar] [CrossRef]
- Gunjan; Tyagi, V.K. Synthesis of Rice Bran Fatty Acids (RBFAs) Based Cationic Surfactants and Evaluation of Their Performance Properties in Combination with Nonionic Surfactant. Tenside Surfactants Deterg. 2014, 51, 497–505. [Google Scholar] [CrossRef]
- Narayanan, V.L.; Umapathy, M.J. Synthesis, Characterization and Antimicrobial Evaluation of Three New Cationic Surfactants. Tenside Surfactants Deterg. 2015, 52, 170–178. [Google Scholar] [CrossRef]
- Umapathy, M.J.; Narayanan, V.L.; Magesan, P.; Chiranjeevi, P.; Jemima, S.W. Synthesis and Characterization of Biodegradable Cationic Esterquat Surfactants and the Evaluation of Its Physico-Chemical Properties. Tenside Surfactants Deterg. 2016, 53, 249–258. [Google Scholar] [CrossRef]
- Tatsumi, T.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ono, D.; Takeda, T.; Ikeda, I. Novel Hydrolyzable and Biodegradable Cationic Gemini Surfactants: Bis(Ester-Ammonium) Dichloride Having a Butenylene or a Butynylene Spacer. J. Surfactants Deterg. 2001, 4, 279–285. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, L.; Xing, F. Synthesis and Properties of Dissymmetric Gemini Surfactants. J. Surfactants Deterg. 2011, 14, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Zhao, X.; Wei, W.; Chang, H. Equilibrium and Dynamic Surface Tension Properties of Gemini Quaternary Ammonium Salt Surfactants with Ester Groups. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 130–139. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Ermanson, L.V.; Godovikov, N.N. Synthesis of Choline Esters of Carboranecarboxylic Acids. Russ. Chem. Bull. 1976, 25, 2425–2427. [Google Scholar] [CrossRef]
- Węgrzyńska, J.; Chlebicki, J.; Maliszewska, I. Preparation, Surface-Active Properties and Antimicrobial Activities of Bis(Ester Quaternary Ammonium) Salts. J. Surfactants Deterg. 2007, 10, 109–116. [Google Scholar] [CrossRef]
- Migahed, M.A.; Negm, N.A.; Shaban, M.M.; Ali, T.A.; Fadda, A.A. Synthesis, Characterization, Surface and Biological Activity of Diquaternary Cationic Surfactants Containing Ester Linkage. J. Surfactants Deterg. 2016, 19, 119–128. [Google Scholar] [CrossRef]
- Liu, D.; Yang, X.; Liu, P.; Mao, T.; Shang, X.; Wang, L. Synthesis and Characterization of Gemini Ester Surfactant and Its Application in Efficient Fabric Softening. J. Mol. Liq. 2020, 299, 112236. [Google Scholar] [CrossRef]
- Topuzyan, V.O.; Gerasimyan, D.A.; Bagdasaryan, A.L.; Mndzhoyan, O.L. Synthesis and Biological Properties of Choline Esters of Amino Acids. Pharm. Chem. J. 1983, 17, 87–91. [Google Scholar] [CrossRef]
- Hellberg, P.-E. Ortho Ester-Based Cleavable Cationic Surfactants. J. Surfactants Deterg. 2002, 5, 217–227. [Google Scholar] [CrossRef]
- Gilbert, E.A.; Guastavino, J.F.; Murguía, M.C. Synthesis and Surface-active Properties of Novel Cleavable Gemini Surfactants. J. Surfactants Deterg. 2021, 25, 27–35. [Google Scholar] [CrossRef]
- Banno, T.; Toshima, K.; Kawada, K.; Matsumura, S. Synthesis and Properties of Biodegradable and Chemically Recyclable Cationic Surfactants Containing Carbonate Linkages. J. Oleo Sci. 2007, 56, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Topuzyan, V.O.; Karapetyan, I.R.; Alebyan, G.P. Synthesis and Anticholinesterase Properties of Choline Esters of α-Amino Acids. Pharm. Chem. J. 2014, 48, 163–165. [Google Scholar] [CrossRef]
- Banno, T.; Toshima, K.; Kawada, K.; Matsumura, S. Synthesis and Properties of Gemini-Type Cationic Surfactants Containing Carbonate Linkages in the Linker Moiety Directed Toward Green and Sustainable Chemistry. J. Surfactants Deterg. 2009, 12, 249–259. [Google Scholar] [CrossRef]
- Banno, T.; Kawada, K.; Matsumura, S. Creation of Novel Green and Sustainable Gemini-Type Cationics Containing Carbonate Linkages. J. Surfactants Deterg. 2010, 13, 387–398. [Google Scholar] [CrossRef]
- Stachowiak, W.; Wysocki, M.; Niemczak, M. “Bitter” Results: Toward Sustainable Synthesis of the Most Bitter Substances, Denatonium Saccharinate and Denatonium Benzoate, Starting from a Popular Anesthetic, Lidocaine. J. Chem. Educ. 2022, 99, 1604–1611. [Google Scholar] [CrossRef]
- Tang, L.; Yang, J.; Yin, Q.; Yang, L.; Gong, D.; Qin, F.; Liu, J.; Fan, Q.; Li, J.; Zhao, W.; et al. Janus Particles Self-Assembled from a Small Organic Atypical Asymmetric Gemini Surfactant. Chem. Commun. 2017, 53, 8675–8678. [Google Scholar] [CrossRef]
- Zhuang, W.; Hachem, K.; Bokov, D.; Javed Ansari, M.; Taghvaie Nakhjiri, A. Ionic Liquids in Pharmaceutical Industry: A Systematic Review on Applications and Future Perspectives. J. Mol. Liq. 2022, 349, 118145. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Q.; Chen, Z.; Wu, W.; Lu, Y.; Qi, J. Ionic Liquids as a Useful Tool for Tailoring Active Pharmaceutical Ingredients. J. Control. Release 2021, 338, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Lattuada, M.; Hatton, T.A. Synthesis, Properties and Applications of Janus Nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Häckl, K.; Mühlbauer, A.; Ontiveros, J.F.; Marinkovic, S.; Estrine, B.; Kunz, W.; Nardello-Rataj, V. Carnitine Alkyl Ester Bromides as Novel Biosourced Ionic Liquids, Cationic Hydrotropes and Surfactants. J. Colloid Interface Sci. 2018, 511, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Cayley, S.; Lewis, B.A.; Record, M.T. Origins of the Osmoprotective Properties of Betaine and Proline in Escherichia Coli K-12. J. Bacteriol. 1992, 174, 1586–1595. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.J.; Roussel, E.G.; Parkes, R.J.; Sass, H. Glycine Betaine as a Direct Substrate for Methanogens (Methanococcoides Spp.). Appl. Environ. Microbiol. 2014, 80, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Abranches, D.O.; Silva, L.P.; Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Understanding the Formation of Deep Eutectic Solvents: Betaine as a Universal Hydrogen Bond Acceptor. ChemSusChem 2020, 13, 4916–4921. [Google Scholar] [CrossRef] [PubMed]
- Colombo Dugoni, G.; Mezzetta, A.; Guazzelli, L.; Chiappe, C.; Ferro, M.; Mele, A. Purification of Kraft Cellulose under Mild Conditions Using Choline Acetate Based Deep Eutectic Solvents. Green Chem. 2020, 22, 8680–8691. [Google Scholar] [CrossRef]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are Natural Deep Eutectic Solvents Always a Sustainable Option? A Bioassay-Based Study. Environ. Sci. Pollut. Res. 2022, 30, 17268–17279. [Google Scholar] [CrossRef]
- Muntz, J.A. The Inability of Choline to Transfer A Methyl Group Directly to Homocysteine for Methionine Formation. J. Biol. Chem. 1950, 182, 489–499. [Google Scholar] [CrossRef]
- Mäkelä, P. Agro-Industrial Uses of Glycinebetaine. Sugar Tech. 2004, 6, 207–212. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.; Honarvar, M.; Zarei, A.R.; Mashhadi Akbar Boojar, M.; Bakhoda, H. A New Approach for Separation and Recovery of Betaine from Beet Molasses Based on Cloud Point Extraction Technique. J. Food Sci. Technol. 2018, 55, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Registration Dossier—ECHA. Available online: https://echa.europa.eu/pl/registration-dossier/-/registered-dossier/15954/7/3/2 (accessed on 9 September 2022).
- Holden, D.A. Synthesis and Spreading Behaviour of Some Reactive Derivatives of Long-Chain Alcohols and Carboxylic Acids. Can. J. Chem. 1984, 62, 574–579. [Google Scholar] [CrossRef]
- Nederberg, F.; Bowden, T.; Hilborn, J. Synthesis, Characterization, and Properties of Phosphoryl Choline Functionalized Poly ε-Caprolactone and Charged Phospholipid Analogues. Macromolecules 2004, 37, 954–965. [Google Scholar] [CrossRef]
- Lundberg, D.; Holmberg, K. Nuclear Magnetic Resonance Studies on Hydrolysis Kinetics and Micellar Growth in Solutions of Surface-Active Betaine Esters. J. Surfact. Deterg. 2004, 7, 239–246. [Google Scholar] [CrossRef]
- Fatma, N.; Ansari, W.H.; Panda, M.; ud-Din, K. Mixed Micellization Behavior of Gemini (Cationic Ester-Bonded) Surfactants with Conventional (Cationic, Anionic and Nonionic) Surfactants in Aqueous Medium. Z. Für Phys. Chem. 2013, 227, 121–132. [Google Scholar] [CrossRef]
- Liu, X.-G.; Xing, X.-J.; Gao, Z.-N. Synthesis and Physicochemical Properties of Star-like Cationic Trimeric Surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 374–381. [Google Scholar] [CrossRef]
- Thompson, R.A.; Allenmark, S.; Larsen, S.; Teuber, L.; Lucanska, B.; Krätsmar-Smogrovic, J.; Valent, A.; Alminger, T.; Erickson, M.; Grundevik, I.; et al. Effects of Molecular Association on the Rates of Hydrolysis of Long-Chain Alkyl Betainates (Alkoxycarbonyl-N,N,N-Trialkylmethanaminium Halides). Acta Chem. Scand. 1989, 43, 690–693. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, G.; Carr, L.R.; Vaisocherová, H.; Chen, S.; Jiang, S. The Hydrolysis of Cationic Polycarboxybetaine Esters to Zwitterionic Polycarboxybetaines with Controlled Properties. Biomaterials 2008, 29, 4719–4725. [Google Scholar] [CrossRef]
- Messadi, A.; Mohamadou, A.; Boudesocque, S.; Dupont, L.; Fricoteaux, P.; Nguyen-Van-Nhien, A.; Courty, M. Syntheses and Characterisation of Hydrophobic Ionic Liquids Containing Trialkyl(2-Ethoxy-2-Oxoethyl)Ammonium or N-(1-Methylpyrrolidyl-2-Ethoxy-2-Oxoethyl)Ammonium Cations. J. Mol. Liq. 2013, 184, 68–72. [Google Scholar] [CrossRef]
- Kim, J.-H.; Woo, H.-S.; Jin, S.-J.; Lee, J.S.; Kim, W.; Ryu, K.; Kim, D.-W. Lithium–Oxygen Batteries with Ester-Functionalized Ionic Liquid-Based Electrolytes. RSC Adv. 2015, 5, 80014–80021. [Google Scholar] [CrossRef]
- Allen, R.A.; Jennings, M.C.; Mitchell, M.A.; Al-Khalifa, S.E.; Wuest, W.M.; Minbiole, K.P.C. Ester- and Amide-Containing multiQACs: Exploring Multicationic Soft Antimicrobial Agents. Bioorganic Med. Chem. Lett. 2017, 27, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Yasa, S.R.; Poornachandra, Y.; Kumar, C.G.; Penumarthy, V. Synthesis, Characterization, Antimicrobial and Anti-Biofilm Activity of a New Class of 11-Bromoundecanoic Acid-Based Betaines. Med. Chem. Res. 2017, 26, 2592–2601. [Google Scholar] [CrossRef]
- Bhadani, A.; Endo, T.; Sakai, K.; Sakai, H.; Abe, M. Synthesis and Dilute Aqueous Solution Properties of Ester Functionalized Cationic Gemini Surfactants Having Different Ethylene Oxide Units as Spacer. Colloid Polym. Sci. 2014, 292, 1685–1692. [Google Scholar] [CrossRef]
- Wen, Y.; Ge, X.; Gao, W.; Wei, W.; Qiao, Y.; Chang, H. Synthesis and Aggregation Properties of Ethylene Glycol Ester-Based Cationic Gemini Surfactants. Colloid Interface Sci. Commun. 2020, 37, 100274. [Google Scholar] [CrossRef]
- Garcia, M.T.; Ribosa, I.; Kowalczyk, I.; Pakiet, M.; Brycki, B. Biodegradability and Aquatic Toxicity of New Cleavable Betainate Cationic Oligomeric Surfactants. J. Hazard. Mater. 2019, 371, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Yasa, S.R.; Kaki, S.S.; Poornachandra, Y.; Kumar, C.G.; Penumarthy, V. Synthesis, Characterization, Antimicrobial and Biofilm Inhibitory Studies of New Esterquats. Bioorganic Med. Chem. Lett. 2016, 26, 1978–1982. [Google Scholar] [CrossRef] [PubMed]
- Granö, H.; Yli-Kauhaluoma, J.; Suortti, T.; Käki, J.; Nurmi, K. Preparation of Starch Betainate: A Novel Cationic Starch Derivative. Carbohydr. Polym. 2000, 41, 277–283. [Google Scholar] [CrossRef]
- Sievänen, K.; Kavakka, J.; Hirsilä, P.; Vainio, P.; Karisalmi, K.; Fiskari, J.; Kilpeläinen, I. Cationic Cellulose Betainate for Wastewater Treatment. Cellulose 2015, 22, 1861–1872. [Google Scholar] [CrossRef]
- Vassel, B.; Skelly, W.G. N-Chlorobetainyl Chloride: Ammonium Chloride, [(Chloroformyl)Methyl]Trimethyl-. In Organic Syntheses; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; p. 28. ISBN 978-0-471-26422-4. [Google Scholar]
- Goursaud, F.; Berchel, M.; Guilbot, J.; Legros, N.; Lemiègre, L.; Marcilloux, J.; Plusquellec, D.; Benvegnu, T. Glycine Betaine as a Renewable Raw Material to “Greener” New Cationic Surfactants. Green Chem. 2008, 10, 310. [Google Scholar] [CrossRef]
- De Gaetano, Y.; Mohamadou, A.; Boudesocque, S.; Hubert, J.; Plantier-Royon, R.; Dupont, L. Ionic Liquids Derived from Esters of Glycine Betaine: Synthesis and Characterization. J. Mol. Liq. 2015, 207, 60–66. [Google Scholar] [CrossRef]
- Pérusse, D.; Guégan, J.P.; Rolland, H.; Guilbot, J.; Benvegnu, T. Efficient Solvent-Free Cationization of Alkylpolyglycoside Based Surfactant Compositions Using Natural Glycine Betaine. Green Chem. 2016, 18, 1664–1673. [Google Scholar] [CrossRef]
- Journoux-Lapp, C.; De Oliveira Vigier, K.; Bachmann, C.; Marinkovic, S.; Estrine, B.; Frapper, G.; Jérôme, F. Elucidation of the Role of Betaine Hydrochloride in Glycerol Esterification: Towards Bio-Based Ionic Building Blocks. Green Chem. 2017, 19, 5647–5652. [Google Scholar] [CrossRef]
- Sharma, M.; Aguado, R.; Murtinho, D.; Valente, A.J.M.; Ferreira, P.J.T. Novel Approach on the Synthesis of Starch Betainate by Transesterification. Int. J. Biol. Macromol. 2021, 182, 1681–1689. [Google Scholar] [CrossRef]
- Webb, R.G.; Haskell, M.W.; Stammer, C.H. A Nuclear Magnetic Resonance Method for Distinguishing .Alpha.-Amino Acids from .Beta. and .Gamma. Isomers. J. Org. Chem. 1969, 34, 576–580. [Google Scholar] [CrossRef]
- Holmberg, K. Novel Surfactants: Preparation, Applications, and Biodegradability, 2nd ed.; rev.expanded.; M. Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-5621-5. [Google Scholar]
- Belda, E.; Van Heck, R.G.A.; José Lopez-Sanchez, M.; Cruveiller, S.; Barbe, V.; Fraser, C.; Klenk, H.; Petersen, J.; Morgat, A.; Nikel, P.I.; et al. The Revisited Genome of Pseudomonas Putida KT2440 Enlightens Its Value as a Robust Metabolic Chassis. Environ. Microbiol. 2016, 18, 3403–3424. [Google Scholar] [CrossRef]
- Parekh, T.; Tsai, M.; Spiro, S. Choline Degradation in Paracoccus Denitrificans: Identification of Sources of Formaldehyde. J. Bacteriol. 2024, 206, e0008124. [Google Scholar] [CrossRef] [PubMed]
- Shieh, H.S. Aerobic Degradation of Choline: I. Fermentation of Choline by A Marine Bacterium, Achromobacter cholinophagum N. sp. Can. J. Microbiol. 1964, 10, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Mausz, M.A.; Chen, Y. Microbiology and Ecology of Methylated Amine Metabolism in Marine Ecosystems. In Methylotrophs and Methylotroph Communities; Caister Academic Press: Poole, UK, 2019; ISBN 978-1-912530-04-5. [Google Scholar]
- Kayashima, T.; Taruki, M.; Katagiri, K.; Nabeoka, R.; Yoshida, T.; Tsuji, T. Comparison of Biodegradation Performance of OECD Test Guideline 301C with That of Other Ready Biodegradability Tests: Comparison of Performance of Ready Biodegradability Tests. Environ. Toxicol. Chem. 2014, 33, 328–333. [Google Scholar] [CrossRef]
- OECD. Test No. 310: Ready Biodegradability—CO2 in Sealed Vessels (Headspace Test); OECD Guidelines for the Testing of Chemicals, Section 3; OECD: Paris, France, 2014; ISBN 978-92-64-22450-6.
- OECD. Test No. 301: Ready Biodegradability; OECD Guidelines for the Testing of Chemicals, Section 3; OECD: Paris, France, 1992; ISBN 978-92-64-07034-9.
- Wilms, W.; Woźniak-Karczewska, M.; Niemczak, M.; Lisiecki, P.; Zgoła-Grześkowiak, A.; Ławniczak, Ł.; Framski, G.; Pernak, J.; Owsianiak, M.; Vogt, C.; et al. Quantifying the Mineralization of 13C-Labeled Cations and Anions Reveals Differences in Microbial Biodegradation of Herbicidal Ionic Liquids between Water and Soil. ACS Sustain. Chem. Eng. 2020, 8, 3412–3426. [Google Scholar] [CrossRef]
- Pernak, J.; Niemczak, M.; Chrzanowski, Ł.; Ławniczak, Ł.; Fochtman, P.; Marcinkowska, K.; Praczyk, T. Betaine and Carnitine Derivatives as Herbicidal Ionic Liquids. Chem. Eur. J. 2016, 22, 12012–12021. [Google Scholar] [CrossRef] [PubMed]
- Mbakidi, J.-P.; Barjhoux, I.; Aguibi, K.; Geffard, A.; Rioult, D.; Palos Ladeiro, M.; Bouquillon, S. Synthesis of New Betaine-Based Ionic Liquids by Using a “One-Pot” Amidation Process and Evaluation of Their Ecotoxicity through a New Method Involving a Hemocyte-Based Bioassay. ACS Sustain. Chem. Eng. 2021, 9, 15427–15441. [Google Scholar] [CrossRef]
- Homa, J.; Stachowiak, W.; Olejniczak, A.; Chrzanowski, Ł.; Niemczak, M. Ecotoxicity Studies Reveal That Organic Cations in Dicamba-Derived Ionic Liquids Can Pose a Greater Environmental Risk than the Herbicide Itself. Sci. Total Environ. 2024, 922, 171062. [Google Scholar] [CrossRef] [PubMed]
- Feder-Kubis, J.; Wnętrzak, A.; Suchodolski, J.; Tomasz Mitkowski, P.; Krasowska, A. Imidazolium Room-Temperature Ionic Liquids with Alkoxymethyl Substituent: A Quest for Improved Microbiological Selectivity. Chem. Eng. J. 2022, 442, 136062. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Radošević, K.; Radojčić Redovniković, I.; Halambek, J.; Gaurina Srček, V. A Brief Overview of the Potential Environmental Hazards of Ionic Liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Stolte, S.; Steudte, S.; Igartua, A.; Stepnowski, P. The Biodegradation of Ionic Liquids—The View from a Chemical Structure Perspective. Curr. Org. Chem. 2011, 15, 1946–1973. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-Microbial Activities of Ionic Liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Chauhan, V.; Kumar, M.; Soni, I.; Shandilya, P.; Singh, S. Synthesis, Physical Properties and Cytotoxic Assessment of Ester-Terminated Gemini Imidazolium Surfactants. J. Mol. Liq. 2023, 387, 122645. [Google Scholar] [CrossRef]
- Dimethylbis [2-[(1-Oxooctadecyl)Oxy]Ethyl]Ammonium Chloride 100.061.237|4aeb06d5-Fd94-41fa-8ff6-Bb79ff37ff94—ECHA CHEM. Available online: https://chem.echa.europa.eu/100.061.237/dossier-view/4aeb06d5-fd94-41fa-8ff6-bb79ff37ff94/IUC5-426f0393-99aa-4a36-afc0-e5a2880fe2e9_677a74f0-18db-4afb-b038-c02f010bbb4b?searchText=267-382-0 (accessed on 16 May 2024).
- TEA-Esterquat 100.313.340|20435d81-78a8-4eb9-9cdf-19905f6f9f68—ECHA CHEM. Available online: https://chem.echa.europa.eu/100.313.340/dossier-view/20435d81-78a8-4eb9-9cdf-19905f6f9f68/d640bbfa-54f4-4f29-987b-b2dd0f73a793_900e5447-76a7-4181-8037-d159f9a50a97?searchText=esterquat (accessed on 16 May 2024).
- Behler, A.D.; Fabry, B.D.; Pi, R.D.; Bigorra, L.J.D.; Prat, Q.E.D. Verfahren zur Herstellung Fester Esterquats Mit Verbessertem Emulgiervermögen. DE4335782C1, 28 July 1994. [Google Scholar]
- Agbley, E.K.; Zuniga, A.; Hernandez, E.H. Silicone-Free Personal Care Compositions and Methods for the Same. U.S. Patent 20220202673A1, 30 June 2022. [Google Scholar]
- Guimares, J.P.D.; Carvalho, L.V.D. Hair Treatment Compositions Comprising an Esterquat. WO2020061658A1, 2 April 2020. [Google Scholar]
- Barberan, P.C.; Costero, J.R. Hair Conditioner. U.S. Patent 10617619B2, 14 April 2020. [Google Scholar]
- Rub, M.A.; Khan, F.; Asiri, A.M. The Influence of Various Solvents on the Interaction between Gemini Surfactant (Ester-Bonded) and Imipramine Hydrochloride: An Aggregational, Interfacial, and Thermodynamic Study. J. Mol. Liq. 2021, 334, 116524. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Q.; Fei, Y.; Zhou, F.; Wang, P.; Deng, Y. Biodegradable Betaine-Based Aprotic Task-Specific Ionic Liquids and Their Application in Efficient SO 2 Absorption. Green Chem. 2015, 17, 3798–3805. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, J.; Wang, J.; Chong, Y. Corrosion Inhibition Effect of Betaine Type Quaternary Ammonium Salt on AA2024-T3 in 0.01 mol·L−1 NaOH: Experimental and Theoretical Research. J. Mol. Struct. 2023, 1274, 134395. [Google Scholar] [CrossRef]








| Synthetic Procedures | Location | References |
|---|---|---|
| Fischer esterification of aminoalcohols | pp. S4–S8, ESI | [29,49,55,61,64,65,66,67,68,69,70,71] |
| Acylation and subsequent quaternization of amino alcohols | pp. S8–S11, ESI | [3,6,9,47,63,72,73,74,75] |
| Other methods of derivatization of carboxylic acids | pp. S11–S16, ESI | [56,57,62,76,77,78,79,80,81,82] |
| Utilization of biologically active compounds | pp. S17–S20, ESI | [9,47,57,83,84] |
| Strategy | Cation | Anion | Activity | Benefits | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Core | R1 (Strategy A) or Herbicidal Moiety (Strategies B and C) | R2 | ||||||
| 1 | A | Choline | -CH=CH2 | CH3 | MCPA 1 | Herbicidal | Replacement of conventional tetraalkylammonium cation in salt-type herbicides with more environmentally-friendly esterquat | [12] |
| A | Choline | -CH=CH2 | CH3 | MCPP 2 | ||||
| A | Choline | -CH=CH2 | CH3 | 2,4-D 3 | ||||
| A | Choline | -CH=CH2 | CH3 | Dicamba 4 | ||||
| 2 | A | Choline | -C(CH3)=CH2 | CH3 | MCPA | Herbicidal | [12] | |
| A | Choline | -C(CH3)=CH2 | CH3 | MCPP | ||||
| A | Choline | -C(CH3)=CH2 | CH3 | 2,4-D | ||||
| A | Choline | -C(CH3)=CH2 | CH3 | Dicamba | ||||
| 3 | A | MDEA | Tallow 5 | CH3 | MCPA | [12] | ||
| A | MDEA | Tallow | CH3 | MCPP | Herbicidal | |||
| A | MDEA | Tallow | CH3 | 2,4-D | ||||
| 4 | B | Choline | 2,4-D | C6H13 | Br | Herbicidal | Esterified form of herbicide exhibits higher activity and is non-volatile due to presence of ions | [26] |
| B | Choline | 2,4-D | C10H21 | Br | ||||
| 5 | B | Choline | MCPA | C6H13 | Br | Herbicidal | [26] | |
| B | Choline | MCPA | C10H21 | Br | ||||
| 6 | B | Choline | MCPA | C10H21 | Br | Herbicidal | Synthesized as precursors to esterquats with two active ingredients within the molecule | [9] |
| 7 | B | Choline | Dicamba | C10H21 | Br | Herbicidal | [47] | |
| B | Choline | Dicamba | C12H25 | Br | ||||
| B | Choline | Dicamba | C14H29 | Br | ||||
| 8 | C | Choline | 2,4-D | C10H21 | MCPA | Herbicidal | Introduction of additional active ingredient into the molecule, which contributes to enhancement of efficacy, broadening spectrum of activity and minimization of resistance issue | [9] |
| C | Choline | 2,4-D | C10H21 | MCPP | ||||
| C | Choline | 2,4-D | C10H21 | 4-CPA 6 | ||||
| C | Choline | MCPA | C10H21 | 2,4-D | ||||
| C | Choline | MCPA | C10H21 | MCPP | ||||
| C | Choline | MCPA | C10H21 | 4-CPA | ||||
| C | Choline | MCPP | C10H21 | 2,4-D | ||||
| C | Choline | MCPP | C10H21 | 2,4-D | ||||
| C | Choline | MCPP | C10H21 | MCPA | ||||
| C | Choline | 4-CPA | C10H21 | MCPP | ||||
| C | Choline | MCPA | C10H21 | Clopyralid 7 | ||||
| C | Choline | MCPA | C10H21 | Dicamba | ||||
| C | Choline | 4-CPA | C10H21 | Clopyralid | ||||
| C | Choline | 4-CPA | C10H21 | Dicamba | ||||
| 9 | C | Choline | MCPA | C8H17 | MCPP | Herbicidal | Introduction of additional active ingredient into the molecule, which contributes to enhancement of efficacy, broadening spectrum of activity and minimization of resistance issue | [48] |
| C | Choline | MCPA | C9H19 | MCPP | ||||
| C | Choline | MCPA | C10H21 | MCPP | ||||
| C | Choline | MCPA | C11H23 | MCPP | ||||
| C | Choline | MCPA | C12H25 | MCPP | ||||
| C | Choline | MCPA | C14H29 | MCPP | ||||
| 10 | C | Choline | Dicamba | C10H21 | 4-CPA | Herbicidal | Introduction of additional active ingredient into the molecule, which contributes to enhancement of efficacy, broadening spectrum of activity and minimization of resistance issue | [47] |
| C | Choline | Dicamba | C12H25 | 4-CPA | ||||
| C | Choline | Dicamba | C14H29 | 4-CPA | ||||
| C | Choline | Dicamba | C10H21 | 2,4-D | ||||
| C | Choline | Dicamba | C12H25 | 2,4-D | ||||
| C | Choline | Dicamba | C14H29 | 2,4-D | ||||
| C | Choline | Dicamba | C10H21 | MCPA | ||||
| C | Choline | Dicamba | C12H25 | MCPA | ||||
| C | Choline | Dicamba | C14H29 | MCPA | ||||
| C | Choline | Dicamba | C10H21 | MCPP | ||||
| C | Choline | Dicamba | C12H25 | MCPP | ||||
| C | Choline | Dicamba | C14H29 | MCPP | ||||
| C | Choline | Dicamba | C10H21 | Clopyralid | ||||
| C | Choline | Dicamba | C12H25 | Clopyralid | ||||
| C | Choline | Dicamba | C14H29 | Clopyralid | ||||
| Core | Moiety | n | R1 (Figure 6) | Anion | Activity | Available Metrics | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|
| Lidocaine-derived choline | ||||||||
| A | Lidocaine-derived choline | 1 | C5H11 | Cl | Analgesic and surface activity | ND 1 | Self-assembled JPs 6 | [84] |
| A | Lidocaine-derived choline | 1 | C7H15 | Cl | REC50 2 3.5 mmol/L CMC 3 2.51 mM | |||
| A | Lidocaine-derived choline | 1 | C9H19 | Cl | - | |||
| A | Lidocaine-derived choline | 1 | C12H25 | Cl | - | |||
| A | Lidocaine-derived choline | 1 | C7H15 | Cl | - | |||
| B | Lidocaine-derived choline | - | C8H17 | Cl | - | EC50 (DPPH) 4,5 3.8 mmol/L CMC 1.27 mM | ||
| MDEA derivative | ||||||||
| C | MDEA esterquat for delivery of cytostatic drugs | - | - | - | - | - | Delivery of cytostatic drugs and siRNA | [42] |
| Sinapine derivative | X | Y | Anion | |||||
| D | Sinapine | OCH3 | OCH3 | Cl | Antimicrobial and antioxidant/Food preservative | EC50 (E. coli) 4 0.0056 µg/mL EC50 (DPPH) 4.5 18.1 nmol/L | Antioxidant antimicrobial, anti-UV, anti-inflammatory, anticancer properties | [57] |
| D | Coumaric acid | H | H | Cl | EC50 (E. coli) 4 > 0.0375 µg/mL EC50 (DPPH) 4,5 > 150 nmol/L | |||
| D | Caffeic acid | OH | H | Cl | EC50 (E. coli) 4 0.0017 µg/mL EC50 (DPPH) 4,5 6.26 nmol/L | |||
| D | Ferulic acid | OCH3 | H | Cl | EC50 (E. coli) 4 0.0103 µg/mL EC50 (DPPH) 4,5 36.73 nmol/L |
| Synthetic Procedures | Location | References |
|---|---|---|
| Synthesis from haloacyl halides | pp. S29–S32, ESI | [3,16,17,18,98,99,100,101,102,103] |
| Synthesis from haloesters | pp. S32–S37, ESI | [104,105,106,107,108,109,110,111,112] |
| Synthesis from chlorobetainyl chloride | pp. S37–S38, ESI | [113,114,115] |
| Synthesis of glycine betaine by classic esterification | pp. S38–S41, ESI | [10,116,117,118,119,120,121] |
| Synthesis by O-alkylation of betaines | pp. S41–S42, ESI | [11,88] |
| Synthesis by anion exchange | p. S42, ESI | [11,14] |
| No | Cation | Anion | Moiety Introduced | Activity/Potential Application | Available Metrics | Notes | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | R1 = from C2H5 to C18H37 | IS-M 1 | IS-M | herbicidal/agrochemical | Application dose–10 g a.i./ha 2 | All compounds were as effective as commercial product without addition of adjuvant | [11] |
| 2 | R1 = from C2H5 to C18H37 | Dicamba 3 | Dicamba | herbicidal/agrochemical | Application dose–200 g a.i./ha | All compounds were more effective than commercial active ingredient (sodium salt of dicamba) product without addition of adjuvant | [14] |
| Entry | Cation | Anion | Hydrolysis Rate Time to Complete Hydrolysis | Conditions | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Core | R1 | n | Y | |||||
| Choline-type esterquats | ||||||||
| 1 | A | Tallow 1 | - | - | CH3SO4− | 0.5% per month | Atmospheric | [65] |
| 2 | B | C8H17 | 1 | - | Cl | Complete/200 min * | 21 °C, pH = 4 | [77] |
| 3 | B | C10H21 | 1 | - | Cl | >300 min * | 21 °C, pH = 4 | |
| 4 | B | C10H21 | 1 | - | Cl | 160 days * | 21 °C, pH = 8 | |
| 5 | B | C10H21 | 1 | - | Cl | Stable 2, >>365 days * | 21 °C, pH = 10 | |
| 6 | B | C12H25 | 1 | - | Cl | >19 h * | 21 °C, pH = 3 | |
| 7 | B | C12H25 | 1 | - | Cl | >22.5 h * | 21 °C, pH = 4 | |
| 8 | B | C12H25 | 1 | - | Cl | 120 min * | 50 °C, pH = 4 | |
| 9 | B | C14H29 | 1 | - | Cl | 270 min * | 50 °C, pH = 4 | |
| 10 | B | C16H33 | 1 | - | Cl | 110 min * | 50 °C, pH = 4 | |
| 11 | C | C10H21 | 1 | - | 2Cl | 43% in 20 h | 20 °C, 5 eq. NaOH | [78] |
| 12 | C | C10H21 | 1 | - | 2Cl | 62% in 20 h | 20 °C, 10 eq. NaOH | |
| 13 | C | C14H29 | 1 | - | 2Cl | 10% in 20 h | 80 °C, 75% (v/v) AcOH | |
| 14 | C | C14H29 | 1 | - | 2Cl | 12% in 20 h | 80 °C, 10 eq. HCl | |
| 15 | C | C14H29 | 1 | - | 2Cl | 92% in 20 h | 80 °C, 10 eq. NaOH | |
| 16 | C | C14H29 | 1 | - | 2Cl | 2% in 20 h | 80 °C, water | |
| 17 | D | C12H25 | 1 | - | 2I | 9% in 10 days | 25 °C, pH = 7 (non-buffered water) | [81] |
| 18 | D | C12H25 | 1 | - | 2I | 50% in 6 days | 25 °C, pH = 7 (phosphate-buffered water) | |
| 19 | D | C12H25 | 1 | - | 2I | Unstable ** 3 | 40 °C, pH = 7 (non-buffered water) | |
| 20 | D | C12H25 | 2 | - | 2I | 1% in 10 days | 25 °C, pH = 7 (non-buffered water) | |
| 21 | D | C12H25 | 2 | - | 2I | 5% in 10 days | 25 °C, pH = 7 (phosphate-buffered water) | |
| 22 | D | C12H25 | 2 | H | 2I | 40% in 8 days | 40 °C, pH = 7 (phosphate-buffered water) | |
| 23 | E | C12H25 | 2 | H | 2I | 2% in 20 days | 25 °C, pH = 7 (non-buffered water) | [82] |
| 24 | E | C12H25 | 2 | H | 2I | 2% in 20 days | 25 °C, pH = 4 (acetate-buffered water) | |
| 25 | E | C12H25 | 2 | H | 2I | 4% in 20 days | 25 °C, pH = 7 (phosphate-buffered water) | |
| 26 | E | C12H25 | 1 | CH3 | 2I | 3% in 20 days | 25 °C, pH = 7 (non-buffered water) | |
| 27 | E | C12H25 | 1 | CH3 | 2I | 10% in 20 days | 25 °C, pH = 4 (acetate-buffered water) | |
| 28 | E | C12H25 | 1 | CH3 | 2I | 20% in 20 days | 25 °C, pH = 7 (phosphate-buffered water) | |
| 29 | F | C14H29 | 1 | - | Br | Stable ** 2 | 0.4 M HCl | [47] |
| 30 | F | C14H29 | 1 | - | Br | 30 min | 0.4 M NaOH | |
| 31 | F | C14H29 | 1 | - | Dicamba 4 | Stable ** 2 | 0.4 M HCl | |
| 32 | F | C14H29 | 1 | - | Dicamba | 30 min | 0.4 M NaOH | |
| Betaine-type esterquats | ||||||||
| 33 | G | C14H29 | 1 | - | Cl | Stable ** 2 | 30 °C, pH = 3 | [16] |
| 34 | G | C14H29 | 1 | - | Cl | 90% in 24 h | 30 °C, pH = 5 | |
| 35 | G | C14H29 | 1 | - | Cl | 15% in 18 h | 30 °C, pH = 6 | |
| 36 | G | C14H29 | 1 | - | Cl | 100% in 20 h | 25 °C, pH = 7 | |
| 37 | G | C14H29 | 1 | - | Cl | 90% in 4 h | 30 °C, pH = 8 | |
| 38 | G | C14H29 | 1 | - | Cl | 90% in 2 h | 30 °C, pH = 9 | |
| 39 | G | C14H29 | 1 | - | Cl | 100% in 5 h | 25 °C, pH = 7.9 (phosphate buffer) | |
| 40 | G | C14H29 | 1 | - | Cl | 100% in 5 h | 25 °C, pH = 7.9 (phosphate buffer), 0.1 M NaCl | |
| 41 | G | C14H29 | 1 | - | Cl | 25% in 5 h | 25 °C, pH = 7.9 (phosphate buffer), 0.5 M NaCl | |
| 42 | G | C14H29 | 1 | - | Cl | 15% in 5 h | 25 °C, pH = 7.9 (phosphate buffer), 1 M NaCl | |
| 43 | H | C8H17 | 1 | (CH2)2 | 2Cl | 1 min | 0.025 M H2SO4 | [18] |
| 44 | H | C8H17 | 1 | (CH2)2 | 2Cl | 10 s | 0.025 M NaOH | |
| 45 | H | C10H21 | 1 | (CH2)2 | 2Cl | 34 min | 0.025 M H2SO4 | |
| 46 | H | C10H21 | 1 | (CH2)2 | 2Cl | 15 s | 0.025 M NaOH | |
| 47 | H | C12H25 | 1 | (CH2)2 | 2Cl | 210 min | 0.025 M H2SO4 | |
| 48 | H | C12H25 | 1 | (CH2)2 | 2Cl | 20 s | 0.025 M NaOH | |
| 49 | H | C8H17 | 1 | (CH2)3 | 2Cl | 1 min | 0.025 M H2SO4 | |
| 50 | H | C8H17 | 1 | (CH2)3 | 2Cl | 10 s | 0.025 M NaOH | |
| 51 | H | C10H21 | 1 | (CH2)3 | 2Cl | 41 min | 0.025 M H2SO4 | |
| 52 | H | C10H21 | 1 | (CH2)3 | 2Cl | 10 s | 0.025 M NaOH | |
| 53 | H | C12H25 | 1 | (CH2)3 | 2Cl | 255 min | 0.025 M H2SO4 | |
| 54 | H | C12H25 | 1 | (CH2)3 | 2Cl | 20 s | 0.025 M NaOH | |
| 55 | H | C8H17 | 1 | (CH2)4 | 2Cl | 1 min | 0.025 M H2SO4 | |
| 56 | H | C8H17 | 1 | (CH2)4 | 2Cl | 10 s | 0.025 M NaOH | |
| 57 | H | C10H21 | 1 | (CH2)4 | 2Cl | 46 min | 0.025 M H2SO4 | |
| 58 | H | C10H21 | 1 | (CH2)4 | 2Cl | 15 s | 0.025 M NaOH | |
| 59 | H | C12H25 | 1 | (CH2)4 | 2Cl | 340 min | 0.025 M H2SO4 | |
| 60 | H | C12H25 | 1 | (CH2)4 | 2Cl | 25 s | 0.025 M NaOH | |
| 61 | I | C10H21 | 1 | - | 2Br | Unstable ** 3 | pH = 4 | [107] |
| 62 | I | C10H21 | 1 | - | 2Br | 16 h | pH = 7 | |
| 63 | I | C10H21 | 1 | - | 2Br | Unstable ** 3 | pH = 10 | |
| 64 | J | C10H21 | 1 | - | 2Br | Unstable ** 3 | pH = 4 | [107] |
| 65 | J | C10H21 | 1 | - | 2Br | 6 h | pH = 7 | |
| 66 | J | C10H21 | 1 | - | 2Br | Unstable ** 3 | pH = 10 | |
| 67 | K | C18H37 | 1 | - | CH3SO4 | Stable ** 2 | RT, pH = 3.5 | [116] |
| 68 | K | C18H37 | 1 | - | CH3SO4 | Stable ** 2 | RT, pH = 4.5 | |
| 69 | K | C18H37 | 1 | - | CH3SO4 | Stable ** 2 | RT, pH = 5.6 | |
| 70 | K | C18H37 | 1 | - | CH3SO4 | 80% in 20 days | RT, pH = 6.6 | |
| 71 | K | C18H37 | 1 | - | CH3SO4 | 90% in 20 days | RT, pH = 7.5 | |
| 72 | K | C18H37 | 1 | - | CH3SO4 | 100% in 15 days | RT, pH = 8.1 | |
| Entry | Core | Structural Features of the Cation | Anion | Test | Biodegradation [%] | Ref. |
|---|---|---|---|---|---|---|
| Choline-type esterquats | ||||||
| 1 | A | n = 1, R1 = C12H25, R2 = C2H4 | 2Cl | OECD 301C | 58 * | [69] |
| 2 | A | n = 1, R1 = C9H19, R2 = CH2 | 2Br | OECD 301D | 45 ** | [19] |
| 3 | A | n = 1, R1 = C11H23, R2 = CH2 | 2Br | OECD 301D | 50 ** | [3] |
| 4 | A | n = 1, R1 = C9H19, R2 = C4H8 | 2Br | OECD 301D | 40 ** | [19] |
| 5 | A | n = 1, R1 = C9H19, R2 = CH2OH | 2Cl | OECD 301C | 58 * | [61] |
| 6 | A | n = 1, R1 = C11H23, R2 = CH2OH | 2Cl | OECD 301C | 58 * | |
| 7 | A | n = 1, R1 = C13H27, R2 = CH2OH | 2Cl | OECD 301C | 58 * | |
| 8 | A | n = 1, R1 = C12H25, R2 = trans-CH=CH | 2Cl | OECD 301C | 52 * | [69] |
| 9 | A | n = 1, R1 = C12H25, R2 = cis-CH=CH | 2Cl | OECD 301C | 55 * | |
| 10 | A | n = 2, R1 = C12H25, R2 = CH2OH | 2Cl | OECD 301C | 59 * | |
| 11 | A | n = 3, R1 = C9H19, R2 = CH2OH | 2Cl | OECD 301C | 50 * | [61] |
| 12 | A | n = 5, R1 = C8H17, R2 = trans-CH=CH | 2Cl | OECD 301C | 52 * | [69] |
| 13 | A | n = 5, R1 = C7H15, R2 = CH2OH | 2Cl | OECD 301C | 40 * | [61] |
| 14 | B | R1 = C8H17 | MCPP 1 | OECD 301F | 12 ** | [48] |
| 15 | B | R1 = C9H19 | MCPP | OECD 301F | 11 ** | |
| 16 | B | R1 = C10H21 | MCPP | OECD 301F | 9 ** | |
| 17 | B | R1 = C11H23 | MCPP | OECD 301F | 6 ** | |
| 18 | B | R1 = C12H25 | MCPP | OECD 301F | 4 ** | |
| 19 | B | R1 = C14H29 | MCPP | OECD 301F | 2 ** | |
| 20 | C | n = 1, R1 = CHCH2 | Cl | OECD 301F | 73 ** | [12] |
| 21 | C | n = 1, R1 = C(CH3)CH2 | Cl | OECD 301F | 70 ** | |
| 22 | C | n = 1, R1 = C11H23 | Cl | OECD 301C | 78 * | [61] |
| 23 | C | n = 1, R1 = C9H19 | Cl | OECD 301D | 90 ** | [19] |
| 24 | C | n = 1, R1 = CHCH2 | MCPA 2 | OECD 301F | 37 ** | [12] |
| 25 | C | n = 1, R1 = C(CH3)CH2 | MCPA | OECD 301F | 29 ** | |
| 26 | D | R1 = tallow | Cl | OECD 301F | 91 ** | [12] |
| 27 | D | R1 = tallow | MCPA | OECD 301F | 63 ** | |
| 28 | E | n = 1, Y = H, R1 = C11H23, R2 = CH3 | Br | OECD 301D | 95 ** | [3] |
| 29 | F | n = 1, R1 = C12H25 | 2Cl | OECD 301C | 52 * | [69] |
| 30 | G | n = 1, R1 = C10H21 | 2I | OECD 301C | 60 ** | [81] |
| 31 | G | n = 1, R1 = C12H25 | 2I | OECD 301C | 70 ** | |
| 32 | G | n = 1, R1 = C14H29 | 2I | OECD 301C | 60 ** | |
| 33 | G | n = 2, R1 = C10H21 | 2I | OECD 301C | 30 ** | |
| 34 | G | n = 2, R1 = C12H25 | 2I | OECD 301C | 45 ** | |
| 35 | G | n = 2, R1 = C14H29 | 2I | OECD 301C | 20 ** | |
| 36 | H | n = 1, Y = CH3, R1 = C12H25 R2 = CH2 | 2I | OECD 301C | 31 ** | [82] |
| 37 | H | n = 2, Y = H, R1 = C12H25, R2 = CH2 | 2I | OECD 301C | 25 ** | |
| 38 | I | n = 1, Y = H, R1 = C12H25 | I | OECD 301C | 60 ** | [82] |
| 39 | I | n = 1, Y = CH3, R1 = C12H25 | I | OECD 301C | 35 ** | |
| 40 | J | n = 2, m = 1, R1 = C12H25 | 2I | OECD 301C | 60 ** | [82] |
| 41 | J | n = 2, m = 2, R1 = C12H25 | 2I | OECD 301C | 29 ** | |
| Betaine-type esterquats | ||||||
| 42 | K | n = 1, R1 = C12H25 | Br | OECD 301D | 85 ** | [3] |
| 43 | L | n = 1, R1 = C12H25 | 2Br | OECD 301D | 55 ** | |
| 44 | M | m = 7 | 2Cl | OECD 310 | 31 ** | [111] |
| 45 | M | m = 9 | 2Cl | OECD 310 | 39 ** | |
| 46 | N | m = 7 | 3Cl | OECD 310 | 41 ** | |
| 47 | N | m = 9 | 3Cl | OECD 310 | 42 ** | |
| 48 | N | m = 11 | 3Cl | OECD 310 | 52 ** | |
| No | Core; Cation | Anion | Parameter (Units) | Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|
| 1 | A; R1 = C11H23, R2 = C8H17 | Br | IZD 1 (mm) (at 1000 µg/mL) | C. tropicalis: 15; E. coli: 10; S. aureus: 9 | [67] |
| 2 | A; R1 = C13H27, R2 = C8H17 | Br | IZD (mm) (at 1000 µg/mL) | C. tropicalis: 13; E. coli: 11; S. aureus: 12 | |
| 3 | A; R1 = C15H31, R2 = C8H17 | Br | IZD (mm) (at 1000 µg/mL) | C. tropicalis: 13; E. coli: 13; S. aureus: 15 | |
| 4 | B; n = 1, Y = H, R1 = C11H23, R2 = C5H11 | 2Cl | MIC 2 (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | [73] |
| 5 | B; n = 1, Y = H, R1 = C11H23, R2 = C6H13 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | |
| 6 | B; n = 1, Y = H, R1 = C11H23, R2 = C8H17 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | |
| 7 | B; n = 2, Y = H, R1 = C9H19, R2 = C4H9 | 2Cl | IZD (mm) (at 1000 µg/mL) | A. Fumigatus: 18.6; B. subtilis: 21.3; E. coli: 15.9; S. pneumonia: 19.6 | [74] |
| 8 | B; n = 2, Y = H, R1 = C9H19, R2 = C5H11 | 2Cl | IZD (mm) (at 1000 µg/mL) | A. Fumigatus: 15.7; B. subtilis: 19.8; E. coli: 15.3; S. pneumonia: 16.2 | |
| 9 | B; n = 2, Y = H, R1 = C9H19, R2 = C6H13 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: 512; S. marcescens: >512 | [73] |
| 10 | B; n = 2, Y = H, R1 = C9H19, R2 = C6H13 | 2Cl | IZD (mm) (at 1000 µg/mL) | A. Fumigatus: 12.9; B. subtilis: 15.9; E. coli: 12.9; S. pneumonia: 15.1 | [74] |
| 11 | B; n = 2, Y = CH3, R1 = C9H19, R2 = C6H13 | 2Cl | MIC (µg/mL) | B. subtilis: 512; E. coli: >512; R. rubra: >512; S. aureus: 256; S. marcescens: >512 | [73] |
| 12 | B; n = 2, Y = H, R1 = C9H19, R2 = C8H17 | 2Cl | MIC (µg/mL) | B. subtilis: >256; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | |
| 13 | B; n = 2, Y = CH3, R1 = C9H19, R2 = C8H17 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: 256; S. marcescens: >512 | |
| 14 | B; n = 2, Y = H, R1 = C11H23, R2 = C4H9 | 2Cl | IZD (mm) (at 1000 µg/mL) | A. Fumigatus: 17. 8; B. subtilis: 22. 4; E. coli: 16.9; S. pneumonia: 20.6 | [74] |
| 15 | B; n = 2, Y = H, R1 = C11H23, R2 = C5H11 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: 512; S. marcescens: >512 | [73] |
| 16 | B; n = 2, Y = H, R1 = C11H23, R2 = C6H13 | 2Cl | MIC (µg/mL) | B. subtilis: 128; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | |
| 17 | B; n = 2, Y = H, R1 = C11H23, R2 = C5H11 | 2Cl | IZD (mm) (at 1000 µg/mL) | A. Fumigatus: 17.6; B. subtilis: 19.9; E. coli: 16.9; S. pneumonia: 18.4 | [74] |
| 18 | B; n = 2, Y = CH3, R1 = C11H23, R2 = C5H11 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: 256; S. marcescens: >512 | [73] |
| 19 | B; n = 2, Y = H, R1 = C11H23, R2 = C6H13 | 2Cl | IZD (mm) (at 1000 µg/mL) | A. Fumigatus: 16.3; B. subtilis: 18.3; E. coli: 15.9; S. pneumonia: 17.3 | [74] |
| 20 | B; n = 2, Y = CH3, R1 = C11H23, R2 = C6H13 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: 256; S. marcescens: >512 | [73] |
| 21 | B; n = 2, Y = H, R1 = C11H23, R2 = C8H17 | 2Cl | MIC (µg/mL) | A. Fumigatus: 3.9; B. subtilis: 0.49; E. coli: 15.63; S. pneumonia: 0.98 | [74] |
| IZD (mm) (at 1000 µg/mL) | A. Fumigatus: 12.6; B. subtilis: 15.4; E. coli: 12.7; S. pneumonia: 14.6 | ||||
| 22 | B; n = 2, Y = H, R1 = C11H23, R2 = C8H17 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: 512; S. marcescens: >512; | [73] |
| 23 | B; n = 2, Y = CH3, R1 = C11H23, R2 = C8H17 | 2Cl | MIC (µg/mL) | B. subtilis: 256; E. coli: >512; R. rubra: >512; S. aureus: 256; S. marcescens: >512 | |
| 24 | B; n = 2, Y = CH3, R1 = C13H27, R2 = C6H13 | 2Cl | MIC (µg/mL) | B. subtilis: 512; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | |
| 25 | B; n = 2, Y = H, R1 = C13H27, R2 = C8H17 | 2Cl | MIC (µg/mL) | B. subtilis: >512; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | |
| 26 | B; n = 2, Y = CH3, R1 = C13H27, R2 = C8H17 | 2Cl | MIC (µg/mL) | B. subtilis: >512; E. coli: >512; R. rubra: >512; S. aureus: >512; S. marcescens: >512 | |
| 27 | C; n = 1, R1 = C12H25 | 2I | MIC (µg/mL) | A. niger: 400; B. subtilis: 10; C. albicans: 400; E. coli: 25; M. gypseum: 25; M. luteus: 50; P. aeruginosa: 400; P. chrysogenum: 400; S. aureus: 25; S. cerevisiae: 400; S. typhimurium: 100; T. mentagrophytes: 50 | [81] |
| 28 | C; n = 2, R1 = C12H25 | 2I | MIC (µg/mL) | A. niger: >400; B. subtilis: 5; C. albicans: 400; E. coli: 10; M. gypseum: 400; M. luteus: 5; P. aeruginosa: 200; P. chrysogenum: >400; S. aureus: 5; S. cerevisiae: >400; S. typhimurium: 100; T. mentagrophytes: 200 | |
| 29 | D; n = 2, Y = H, R1 = C12H25, R2 = CH2 | 2I | MIC (µg/mL) | A. niger: >400; B. subtilis: 200; C. albicans: >400; E. coli: 400; M. gypseum: 400; M. luteus: 400; P. aeruginosa: 400; P. chrysogenum: >400; S. aureus: 200; S. cerevisiae: >400; S. typhimurium: >400; T. mentagrophytes: >400 | [82] |
| 30 | D; n = 1, Y = CH3, R1 = C12H25, R2 = CH2 | I | MIC (µg/mL) | A. niger: >400; B. subtilis: 100; C. albicans: >400; E. coli: 200; M. gypseum: 400; M. luteus: 100; P. aeruginosa: >400; P. chrysogenum: >400; S. aureus: 100; S. cerevisiae: >400; S. typhimurium: >400; T. mentagrophytes: 200 | |
| 31 | E; n = 1, Y = H, R1 = C12H25 | I | MIC (µg/mL) | A. niger: >400; B. subtilis: 2.5; C. albicans: >400; E. coli: 10; M. gypseum: 10; M. luteus: 5; P. aeruginosa: 100; P. chrysogenum: 200; S. aureus: 2.5; S. cerevisiae: 400; S. typhimurium: 200; T. mentagrophytes: 50 | |
| 32 | E; n = 1, Y = CH3, R1 = C12H25 | I | MIC (µg/mL) | A. niger: >400; B. subtilis: 5; C. albicans: 400; E. coli: >400; M. gypseum: 5; M. luteus: 2.5; P. aeruginosa: >400; P. chrysogenum: 100; S. aureus: 2.5; S. cerevisiae: 400; S. typhimurium: >400; T. mentagrophytes: 400 | |
| 33 | F; n = 2, m = 1, R1 = C12H25 | 2I | MIC (µg/mL) | A. niger: >400; B. subtilis: 10; C. albicans: 400; E. coli: 200; M. gypseum: 25; M. luteus: 25; P. aeruginosa: 200; P. chrysogenum: 400; S. aureus: 5; S. cerevisiae: 400; S. typhimurium: 400; T. mentagrophytes: 100 | |
| 34 | F; n = 2, m = 2, R1 = C12H25 | 2I | MIC (µg/mL) | A. niger: >200; B. subtilis: 50; C. albicans: >200; E. coli: 200; M. gypseum: 100; M. luteus: 100; P. aeruginosa: >200; P. chrysogenum: >200; S. aureus: 100; S. cerevisiae: >200; S. typhimurium: >200; T. mentagrophytes: >200 | |
| 35 | G; R2 = C10H21 | Br | MIC (µg/mL) | E. coli: 6.42; P. putida: 12.83 | [47] |
| MBC 3 (µg/mL) | E. coli: 12.83; P. putida: 25.67 | ||||
| EC50 4 (µg/mL) | E. coli: 8.73; P. putida: 14.89 | ||||
| 36 | G; R1 = C12H25 | Br | MIC (µg/mL) | E. coli: 6.77; P. putida: 13.53 | |
| MBC (µg/mL) | E. coli: 54.14; P. putida: >54.14 | ||||
| EC50 (µg/mL) | E. coli: 10.83; P. putida: 16.78 | ||||
| 37 | G; R2 = C14H29 | Br | MIC (µg/mL) | E. coli: 14.24; P. putida: 284.72 | |
| MBC (µg/mL) | E. coli: 56.94; P. putida: >56.94 | ||||
| EC50 (µg/mL) | E. coli: 53.53; P. putida: 48.97 | ||||
| 38 | H; R2 = C8H17 | MCPP 5 | MIC (µg/mL) | B. subtilis: 74.83; C. albicans: 149.67; P. putida: 299.33 | [48] |
| MBC (µg/mL) | B. subtilis: 299.33; C. albicans: 299.33; P. putida: 598.67 | ||||
| 39 | H; R2 = C9H19 | MCPP | MIC (µg/mL) | B. subtilis: 76.59; C. albicans: 153.18; P. putida: 306.35 | |
| MBC (µg/mL) | B. subtilis: 306.35; C. albicans: 306.35; P. putida: 612.7 | ||||
| 40 | H; R2 = C10H21 | MCPP | MIC (µg/mL) | B. subtilis: 78.34; C. albicans: 78.34; P. putida: 313.37 | |
| MBC (µg/mL) | B. subtilis: 313.37; C. albicans: 156.68; P. putida: 626.73 | ||||
| 41 | H; R2 = C11H23 | MCPP | MIC (µg/mL) | B. subtilis: 78.34; C. albicans: 78.34; P. putida: 78.34 | |
| MBC (µg/mL) | B. subtilis: 313.37; C. albicans: 156.68; P. putida: 626.73 | ||||
| 42 | H; R2 = C12H25 | MCPP | MIC (µg/mL) | B. subtilis: 20.49; C. albicans: 81.85; P. putida: 81.85 | |
| MBC (µg/mL) | B. subtilis: 163.7; C. albicans: 163.7; P. putida: 327.39 | ||||
| 43 | H; R2 = C14H29 | MCPP | MIC (µg/mL) | B. subtilis: 5.33; C. albicans: 8.54; P. putida: 34.14 | |
| MBC (µg/mL) | B. subtilis: 21.37; C. albicans: 17.07; P. putida: 85.36 | ||||
| 44 | I; R2 = C10H21 | 4-CPA 6 | MIC (µg/mL) | E. coli: 15.48; P. putida: 15.48 | [47] |
| MBC (µg/mL) | E. coli: 30.95; P. putida: 30.95 | ||||
| EC50 (µg/mL) | E. coli: 16.71; P. putida: 22.9 | ||||
| 45 | I; R2 = C12H25 | 4-CPA | MIC (µg/mL) | E. coli: 8.09; P. putida: 16.18 | |
| MBC (µg/mL) | E. coli: 32.35; P. putida: 32.35 | ||||
| EC50 (µg/mL) | E. coli: 20.71; P. putida: 20.71 | ||||
| 46 | I; R2 = C14H29 | 4-CPA | MIC (µg/mL) | E. coli: 33.76; P. putida: 33.76 | |
| MBC (µg/mL) | E. coli: 67.51; P. putida: >67.51 | ||||
| EC50 (µg/mL) | E. coli: 62.11; P. putida: 56.71 | ||||
| 47 | I; R2 = C10H21 | 2,4-D 7 | MIC (µg/mL) | E. coli: 16.34; P. putida: 32.67 | |
| MBC (µg/mL) | E. coli: 32.67; P. putida: 65.35 | ||||
| EC50 (µg/mL) | E. coli: 18.3; P. putida: 33.98 | ||||
| 48 | I; R2 = C12H25 | 2,4-D | MIC (µg/mL) | E. coli: 8.52; P. putida: 17.04 | |
| MBC (µg/mL) | E. coli: 17.04; P. putida: 34.08 | ||||
| EC50 (µg/mL) | E. coli: 15.67; P. putida: 31.35 | ||||
| 49 | I; R2 = C14H29 | 2,4-D | MIC (µg/mL) | E. coli: 35.48; P. putida: 35.48 | |
| MBC (µg/mL) | E. coli: 70.96; P. putida: >70.96 | ||||
| EC50 (µg/mL) | E. coli: 45.41; P. putida: 84.44 | ||||
| 50 | I; R2 = C10H21 | MCPA 8 | MIC (µg/mL) | E. coli: 15.83; P. putida: 15.83 | |
| MBC (µg/mL) | E. coli: 31.65; P. putida: 31.65 | ||||
| EC50 (µg/mL) | E. coli: 13.29; P. putida: 21.52 | ||||
| 51 | I; R2 = C12H25 | MCPA | MIC (µg/mL) | E. coli: 8.26; P. putida: 16.53 | |
| MBC (µg/mL) | E. coli: 16.53; P. putida: 16.53 | ||||
| EC50 (µg/mL) | E. coli: 13.22; P. putida: 20.49 | ||||
| 52 | I; R2 = C14H29 | MCPA | MIC (µg/mL) | E. coli: 34.46; P. putida: 34.46 | |
| MBC (µg/mL) | E. coli: 68.92; P. putida: >68.92 | ||||
| EC50 (µg/mL) | E. coli: 60.65; P. putida: 78.56 | ||||
| 53 | I; R2 = C10H21 | MCPP | MIC (µg/mL) | E. coli: 16.18; P. putida: 16.18 | |
| MBC (µg/mL) | E. coli: 32.35; P. putida: 32.35 | ||||
| EC50 (µg/mL) | E. coli: 31.06; P. putida: 26.53 | ||||
| 54 | I; R2 = C12H25 | MCPP | MIC (µg/mL) | E. coli: 8.44; P. putida: 16.88 | |
| MBC (µg/mL) | E. coli: 16.88; P. putida: 33.76 | ||||
| EC50 (µg/mL) | E. coli: 14.18; P. putida: 16.2 | ||||
| 55 | I; R2 = C14H29 | MCPP | MIC (µg/mL) | E. coli: 17.58; P. putida: 35.16 | |
| MBC (µg/mL) | E. coli: 70.32; P. putida: >70.32 | ||||
| EC50 (µg/mL) | E. coli: 67.51; P. putida: 170.87 | ||||
| 56 | I; R2 = C10H21 | Clopyralid 9 | MIC (µg/mL) | E. coli: 7.81; P. putida: 15.61 | |
| MBC (µg/mL) | E. coli: 31.22; P. putida: 31.22 | ||||
| EC50 (µg/mL) | E. coli: 21.85; P. putida: 23.1 | ||||
| 57 | I; R2 = C12H25 | Clopyralid | MIC (µg/mL) | E. coli: 8.16; P. putida: 16.31 | |
| MBC (µg/mL) | E. coli: 16.31; P. putida: 32.62 | ||||
| EC50 (µg/mL) | E. coli: 16.31; P. putida: 11.09 | ||||
| 58 | I; R2 = C14H29 | Clopyralid | MIC (µg/mL) | E. coli: 17.01; P. putida: 34.03 | |
| MBC (µg/mL) | E. coli: 68.05; P. putida: >68.05 | ||||
| EC50 (µg/mL) | E. coli: 41.51; P. putida: 115.69 | ||||
| 59 | J; X = OCH3, Y = OCH3 | Cl | EC50 (µg/mL) | E. coli: 0.0056 | [57] |
| 60 | J; X = H, Y = H | Cl | EC50 (µg/mL) | E. coli: >0.0375 | |
| 61 | J; X = OH, Y = H | Cl | EC50 (µg/mL) | E. coli: 0.0017 | |
| 62 | J; X = OCH3, Y = H | Cl | EC50 (µg/mL) | E. coli: 0.0103 | |
| Betaine-type esterquats | |||||
| 63 | K; n = 1, m = 2, R1 = C8H17 | 2Cl | MIC (µg/mL) | E. coli: 512; S. aureus: 512 | [18] |
| 64 | K; n = 1, m = 2, R1 = C10H21 | 2Cl | MIC (µg/mL) | E. coli: 128; S. aureus: 64 | |
| 65 | K; n = 1, m = 2, R1 = C12H25 | 2Cl | MIC (µg/mL) | E. coli: 256; S. aureus: 128 | |
| 66 | K; n = 1, m = 3, R1 = C8H17 | 2Cl | MIC (µg/mL) | E. coli: 512; S. aureus: 512 | |
| 67 | K; n = 1, m = 3, R1 = C10H21 | 2Cl | MIC (µg/mL) | E. coli: 256; S. aureus: 128 | |
| 68 | K; n = 1, m = 3, R1 = C12H25 | 2Cl | MIC (µg/mL) | E. coli: 256; S. aureus: 256 | |
| 69 | K; n = 1, m = 4, R1 = C8H17 | 2Cl | MIC (µg/mL) | E. coli: 256; S. aureus: 256 | |
| 70 | K; n = 1, m = 4, R1 = C10H21 | 2Cl | MIC (µg/mL) | E. coli: 64; S. aureus: 32 | |
| 71 | K; n = 1, m = 4, R1 = C12H25 | 2Cl | MIC (µg/mL) | E. coli: 128; S. aureus: 64 | |
| 72 | L; n = 1, R1 = C12H25 | 2Cl | IZD (mm) (at 400 µg/mL) | B. subtilis: 16; C. albicans: 21; E. coli: 10; S. aureus: 19 | [20] |
| 73 | L; n = 1, R1 = C14H29 | 2Cl | IZD (mm) (at 400 µg/mL) | B. subtilis: 14; C. albicans: 18; E. coli: 11; S. aureus: 16 | |
| 74 | L; n = 1, R1 = C16H33 | 2Cl | IZD (mm) (at 400 µg/mL) | B. subtilis: 13; C. albicans: 20; E. coli: 11; S. aureus: 11 | |
| 75 | M; n = 1; R1 = C12H25 | Doc 10 | EC50 (µg/mL) | Aliivibrio fischeri: 4.61 (5 min); 3.47 (15 min); 3.19 (30 min); Raphidocelis subcapitata: 0.58 | [21] |
| 76 | M; n = 1; R1 = C12H25 | SCN | EC50 (µg/mL) | Aliivibrio fischeri: 1.41 (5 min); 0.77 (15 min); 0.61 (30 min); Raphidocelis subcapitata: 0.27 | |
| 77 | M; n = 1; R1 = C14H29 | Doc | EC50 (µg/mL) | Aliivibrio fischeri: 3.19 (5 min); 1.39 (15 min); 1.37 (30 min); Raphidocelis subcapitata: 0.74 | |
| 78 | M; n = 1; R1 = C14H29 | SCN | EC50 (µg/mL) | Aliivibrio fischeri: 1.61 (5 min); 1.10 (15 min); 0.90 (30 min); Raphidocelis subcapitata: 0.54 | |
| 79 | N; n = 1, R1 = C9H19 | 2Br | MIC (µg/mL) | E. coli: >161.52; E. faecalis: >161.52; P. aeruginosa: >161.52; S. aureus (ATCC33592): >161.52; S. aureus (USA300-0114): 161.52; S. aureus: >161.52 | [107] |
| 80 | N; n = 1, R1 = C10H21 | 2Br | MIC (µg/mL) | E. coli: >168.52; E. faecalis: >168.52; P. aeruginosa: >168.52; S. aureus (ATCC33592): 168.52; S. aureus (USA300-0114): 84.26; S. aureus: 168.52 | |
| 81 | N; n = 1, R1 = C11H23 | 2Br | MIC (µg/mL) | E. coli: 44.23; E. faecalis: 44.23; P. aeruginosa: 87.76; S. aureus (ATCC33592): 22.47; S. aureus (USA300-0114): 11.23; S. aureus: 22.47 | |
| 82 | N; n = 1, R1 = C12H25 | 2Br | MIC (µg/mL) | E. coli: 11.68; E. faecalis: 11.68; P. aeruginosa: 23.36; S. aureus (ATCC33592): 5.84; S. aureus (USA300-0114): 2.92; S. aureus: 1.46 | |
| 83 | N; n = 1, R1 = C13H27 | 2Br | MIC (µg/mL) | E. coli: 6.06; E. faecalis: 12.13; P. aeruginosa: 24.26; S. aureus (ATCC33592): 6.06; S. aureus (USA300-0114): 6.06; S. aureus: 3.03 | |
| 84 | N; n = 1, R1 = C15H31 | 2Br | MIC (µg/mL) | E. coli: 51.29; E. faecalis: 26.05; P. aeruginosa: 101.76; S. aureus (ATCC33592): 26.05; S. aureus (USA300-0114): 13.03; S. aureus: 13.03 | |
| 85 | N; n = 1, R1 = C17H35 | 2Br | MIC (µg/mL) | E. coli: 217.52; E. faecalis: 27.84; P. aeruginosa: >217.52; S. aureus (ATCC33592): 27.84; S. aureus (USA300-0114): 13.92; S. aureus: 13.92 | |
| 86 | O; n = 1, m = 8, R1 = C6H5CH2 | Br | MIC (µg/mL) | E. coli: >100.04; E. faecalis: >100.04; P. aeruginosa: >100.04; S. aureus (ATCC33592): >100.04; S. aureus (USA300-0114): >100.04; S. aureus: >100.04 | |
| 87 | O; n = 1, m = 9, R1 = C6H5CH2 | Br | MIC (µg/mL) | E. coli: >103.54; E. faecalis: >103.54; P. aeruginosa: >103.54; S. aureus (ATCC33592): >103.54; S. aureus (USA300-0114): >103.54; S. aureus: >103.54 | |
| 88 | O; n = 1, m = 10, R1 = C6H5CH2 | Br | MIC (µg/mL) | E. coli: 107.04; E. faecalis: 107.04; P. aeruginosa: >107.04; S. aureus (ATCC33592): 53.52; S. aureus (USA300-0114): 107.04; S. aureus: 53.52 | |
| 89 | O; n = 1, m = 11, R1 = C6H5CH2 | Br | MIC (µg/mL) | E. coli: 55.27; E. faecalis: 55.27; P. aeruginosa: >110.54; S. aureus (ATCC33592): 27.86; S. aureus (USA300-0114): 14.15; S. aureus: 14.15 | |
| 90 | O; n = 1, m = 12, R1 = C6H5CH2 | Br | MIC (µg/mL) | E. coli: 28.74; E. faecalis: 14.6; P. aeruginosa: >114.04; S. aureus (ATCC33592): 14.6; S. aureus (USA300-0114): 7.3; S. aureus: 14.6 | |
| 91 | O; n = 1, m = 14, R1 = C6H5CH2 | Br | MIC (µg/mL) | E. coli: 7.75; E. faecalis: 1.94; P. aeruginosa: 30.5; S. aureus (ATCC33592): 0.97; S. aureus (USA300-0114): 0.97; S. aureus: 0.97 | |
| 92 | O; n = 1, m = 16, R1 = C6H5CH2 | Br | MIC (µg/mL) | E. coli: 4.1; E. faecalis: 2.05; P. aeruginosa: >128.04; S. aureus (ATCC33592): 0.51; S. aureus (USA300-0114): 1.02; S. aureus: 0.51 | |
| 93 | O; n = 2, m = 1, R1 = C4H9 | Sac 11 | EC50 (µg/mL) | A. fischeri: 248.48 (5 min); 103.51 (15 min); 68.06 (30 min); R. subcapitata: 4.71 | [21] |
| 94 | O; n = 4, m = 1, R1 = C4H9 | Sac | EC50 (µg/mL) | A. fischeri: 1904.43 (5 min); 628.04 (15 min); 401.14 (30 min); R. subcapitata: 12.83 | |
| 95 | P; n = 1, m = 5 | 2Br | MIC (µg/mL) | E. coli: >175.29; E. faecalis: >175.29; P. aeruginosa: >175.29; S. aureus (ATCC33592): >175.29; S. aureus (USA300-0114): >175.29; S. aureus: >175.29 | [107] |
| 96 | P; n = 1, m = 6 | 2Br | MIC (µg/mL) | E. coli: >182.29; E. faecalis: >182.29; P. aeruginosa: >182.29; S. aureus (ATCC33592): >182.29; S. aureus (USA300-0114): 182.29; S. aureus: >182.29 | |
| 97 | P; n = 1, m = 7 | 2Br | MIC (µg/mL) | E. coli: 189.29; E. faecalis: 47.7; P. aeruginosa: >189.29; S. aureus (ATCC33592): 47.7; S. aureus (USA300-0114): 24.23; S. aureus: >189.29 | |
| 98 | P; n = 1, m = 8 | 2Br | MIC (µg/mL) | E. coli: 12.56; E. faecalis: 6.28; P. aeruginosa: 98.14; S. aureus (ATCC33592): 3.14; S. aureus (USA300-0114): 3.14; S. aureus: 3.14 | |
| 99 | P; n = 1, m = 10 | 2Br | MIC (µg/mL) | E. coli: 1.63; E. faecalis: 3.25; P. aeruginosa: 13.01; S. aureus (ATCC33592): 1.63; S. aureus (USA300-0114): 1.63; S. aureus: 1.63 | |
| 100 | P; n = 1, m = 12 | 2Br | MIC (µg/mL) | E. coli: 13.91; E. faecalis: 27.81; P. aeruginosa: 27.81; S. aureus (ATCC33592): 6.95; S. aureus (USA300-0114): 6.95; S. aureus: 6.95 | |
| 101 | P; n = 1, m = 14 | 2Br | MIC (µg/mL) | E. coli: >231.29; E. faecalis: >231.29; P. aeruginosa: >231.29; S. aureus (ATCC33592): >231.29; S. aureus (USA300-0114): 231.29; S. aureus: >231.29 | |
| 102 | R; n = 1; m = 8 | 3Br | MIC (µg/mL) | E. coli: >245.04; E. faecalis: 245.04; P. aeruginosa: >245.04; S. aureus (ATCC33592): 245.04; S. aureus (USA300-0114): 122.52; S. aureus: 245.04 | |
| 103 | R; n = 1; m = 9 | 3Br | MIC (µg/mL) | E. coli: 127.77; E. faecalis: 32.71; P. aeruginosa: 127.77; S. aureus (ATCC33592): 32.71; S. aureus (USA300-0114): 16.35; S. aureus: 32.71 | |
| 104 | R; n = 1; m = 10 | 3Br | MIC (µg/mL) | E. coli: 17.03; E. faecalis: 17.03; P. aeruginosa: 34.05; S. aureus (ATCC33592): 8.51; S. aureus (USA300-0114): 8.51; S. aureus: 8.51 | |
| 105 | R; n = 1; m = 11 | 3Br | MIC (µg/mL) | E. coli: 8.85; E. faecalis: 17.7; P. aeruginosa: 35.4; S. aureus (ATCC33592): 8.85; S. aureus (USA300-0114): 8.85; S. aureus: 4.42 | |
| 106 | R; n = 1; m = 12 | 3Br | MIC (µg/mL) | E. coli: 9.19; E. faecalis: 9.19; P. aeruginosa: 18.37; S. aureus (ATCC33592): 4.59; S. aureus (USA300-0114): 4.59; S. aureus: 4.59 | |
| 107 | R; n = 1; m = 14 | 3Br | MIC (µg/mL) | E. coli: 9.86; E. faecalis: 9.86; P. aeruginosa: 19.71; S. aureus (ATCC33592): 9.86; S. aureus (USA300-0114): 9.86; S. aureus: 4.93 | |
| 108 | R; n = 1; m = 16 | 3Br | MIC (µg/mL) | E. coli: 82.92; E. faecalis: 42.12; P. aeruginosa: 329.04; S. aureus (ATCC33592): 42.12; S. aureus (USA300-0114): 21.06; S. aureus: 82.92 | |
| 109 | S; n = 1, m = 5 | 3Br | MIC (µg/mL) | E. coli: 266.82; E. faecalis: 266.82; P. aeruginosa: >266.82; S. aureus (ATCC33592): 266.82; S. aureus (USA300-0114): 67.24; S. aureus: 67.24 | |
| 110 | S; n = 1, m = 6 | 3Br | MIC (µg/mL) | E. coli: 69.89; E. faecalis: 35.5; P. aeruginosa: >277.32; S. aureus (ATCC33592): 35.5; S. aureus (USA300-0114): 8.87; S. aureus: 17.75 | |
| 111 | S; n = 1, m = 7 | 3Br | MIC (µg/mL) | E. coli: 9.21; E. faecalis: 9.21; P. aeruginosa: 72.53; S. aureus (ATCC33592): 4.61; S. aureus (USA300-0114): 4.61; S. aureus: 2.3 | |
| 112 | S; n = 1, m = 8 | 3Br | MIC (µg/mL) | E. coli: 4.77; E. faecalis: 9.55; P. aeruginosa: 19.09; S. aureus (ATCC33592): 4.77; S. aureus (USA300-0114): 4.77; S. aureus: 2.39 | |
| 113 | S; n = 1, m = 9 | 3Br | MIC (µg/mL) | E. coli: 9.88; E. faecalis: 9.88; P. aeruginosa: 19.76; S. aureus (ATCC33592): 4.94; S. aureus (USA300-0114): 4.94; S. aureus: 2.47 | |
| 114 | S; n = 1, m = 10 | 3Br | MIC (µg/mL) | E. coli: 10.55; E. faecalis: 10.55; P. aeruginosa: >329.82; S. aureus (ATCC33592): 10.55; S. aureus (USA300-0114): 10.55; S. aureus: 5.28 | |
| 115 | S; n = 1, m = 10 | 3Br | MIC (µg/mL) | E. coli: 44.91; E. faecalis: 22.45; P. aeruginosa: 350.82; S. aureus (ATCC33592): 44.91; S. aureus (USA300-0114): 22.45; S. aureus: 175.41 | |
| 116 | T; R1 = CH3, R2 = C6H13, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): 7.8; C. aaseri (MTCC1962): 31.2; C. albicans (MTCC1637): 31.2; C. albicans (MTCC183): 15.6; C. albicans (MTCC277): 31.2; C. albicans (MTCC3017): 15.6; C. albicans (MTCC3018): 15.6; C. albicans (MTCC3958): 31.2; C. albicans (MTCC4748): 15.6; C. albicans (MTCC7315): 15.6; C. albicans (MTCC854): 15.6; C. glabrata (MTCC3019): 15.6; C. krusei (MTCC3020): 15.6; C. parapsilosis (MTCC1744): 15.6; E. coli (MTCC739): 31.2; Issatchenikiahanoiensis (MTCC4755): 15.6; K. planticola (MTCC530): 15.6; M. luteus (MTCC2470): 3.9; P. aeruginosa (MTCC2453): >125; S. aureus (MTCC96): 3.9; S. aureus (mLS16MTCC2940): 7.8 | [112] |
| 117 | T; R1 = CH3, R2 = C12H25, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): 15.6; C. aaseri (MTCC1962): 31.2; C. albicans (MTCC1637): 62.5; C. albicans (MTCC183): 31.2; C. albicans (MTCC277): 31.2; C. albicans (MTCC3017): 31.2; C. albicans (MTCC3018): 62.5; C. albicans (MTCC3958): 62.5; C. albicans (MTCC4748): 15.6; C. albicans (MTCC7315): 31.2; C. albicans (MTCC854): 31.2; C. glabrata (MTCC3019): 31.2; C. krusei (MTCC3020): 62.5; C. parapsilosis (MTCC1744): 62.5; E. coli (MTCC739): 15.6; Issatchenikiahanoiensis (MTCC4755): 31.2; K. planticola (MTCC530): 7.8; M. luteus (MTCC2470): 7.8; P. aeruginosa (MTCC2453): >125; S. aureus (MTCC96): 15.6; S. aureus (mLS16MTCC2940): 7.8 | |
| 118 | T; R1 = CH3, R2 = C18H37, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): >125; C. albicans (MTCC3017): >125; E. coli (MTCC739): >125; K. planticola (MTCC530): >125; M. luteus (MTCC2470): >125; P. aeruginosa (MTCC2453): >125; S. aureus (MTCC96): >125; S. aureus (mLS16MTCC2940): >125 | |
| 119 | T; R1 = C10H20COOCH3, R2 = C6H13, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): 7.8; C. aaseri (MTCC1962): 7.8; C. albicans (MTCC1637): 15.6; C. albicans (MTCC183): 15.6; C. albicans (MTCC277): 15.6; C. albicans (MTCC3017): 7.8; C. albicans (MTCC3018): 15.6; C. albicans (MTCC3958): 15.6; C. albicans (MTCC4748): 7.8; C. albicans (MTCC7315): 15.6; C. albicans (MTCC854): 31.2; C. glabrata (MTCC3019): 15.6; C. krusei (MTCC3020): 31.2; C. parapsilosis (MTCC1744): 15.6; E. coli (MTCC739): >125; Issatchenikiahanoiensis (MTCC4755): 15.6; K. planticola (MTCC530): >125; M. luteus (MTCC2470): 1.9; P. aeruginosa (MTCC2453): >125; S. aureus (MTCC96): 3.9; S. aureus (mLS16MTCC2940): 3.9 | |
| 120 | T; R1 = C10H20COOCH3, R2 = C12H25, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): >125; C. albicans (MTCC3017): >125; E. coli (MTCC739): >125; K. planticola (MTCC530): >125; M. luteus (MTCC2470): >125; P. aeruginosa (MTCC2453): >125; S. aureus (MTCC96): >125; S. aureus (mLS16MTCC2940): >125 | |
| 121 | T; R1 = C10H20COOCH3, R2 = C18H37, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): >125; C. albicans (MTCC3017): >125; E. coli (MTCC739): >125; K. planticola (MTCC530): >125; M. luteus (MTCC2470): >125; P. aeruginosa (MTCC2453): >125; S. aureus (MTCC96): >125; S. aureus (mLS16MTCC2940): >125 | |
| 122 | T; R1 = C6H11, R2 = C6H11, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): 7.8; C. aaseri (MTCC1962): 15.6; C. albicans (MTCC1637): 31.2; C. albicans (MTCC183): 15.6; C. albicans (MTCC277): 15.6; C. albicans (MTCC3017): 15.6; C. albicans (MTCC3018): 15.6; C. albicans (MTCC3958): 15.6; C. albicans (MTCC4748): 15.6; C. albicans (MTCC7315): 15.6; C. albicans (MTCC854): 31.2; C. glabrata (MTCC3019): 31.2; C. krusei (MTCC3020): 31.2; C. parapsilosis (MTCC1744): 15.6; E. coli (MTCC739): >125; Issatchenikiahanoiensis (MTCC4755): 15.6; K. planticola (MTCC530): >125; M. luteus (MTCC2470): 7.8; P. aeruginosa (MTCC2453): >125; S. aureus(MTCC96): 3.9; S. aureus (mLS16MTCC2940): 3.9 | |
| 123 | T; R1 = C8H17, R2 = C8H17, R3 = CH3, R4 = CH3, n = 10 | Cl | MIC (µg/mL) | B. subtilis (MTCC121): 7.8; C. aaseri (MTCC1962): 15.6; C. albicans (MTCC1637): 31.2; C. albicans (MTCC183): 31.2; C. albicans (MTCC277): 15.6; C. albicans (MTCC3017): 15.6; C. albicans (MTCC3018): 31.2; C. albicans (MTCC3958): 31.2; C. albicans (MTCC4748): 15.6; C. albicans (MTCC7315): 15.6; C. albicans (MTCC854): 15.6; C. glabrata (MTCC3019): 31.2; C. krusei (MTCC3020): 31.2; C. parapsilosis (MTCC1744): 62.5; E. coli (MTCC739): >125; Issatchenikiahanoiensis (MTCC4755): 31.2; K. planticola (MTCC530): 31.2; M. luteus (MTCC2470): 3.9; P. aeruginosa (MTCC2453): >125; S. aureus (MTCC96): 3.9; S. aureus (mLS16MTCC2940): 7.8 | |
| No | Core; Cation | Anion | Parameter (Units) | Value | Ref. |
|---|---|---|---|---|---|
| 1 | A; R1 = tallow, R2 = CH3 | Cl | LD50 rat (oral) (mg/kg) | >10,000 | [139] |
| LD50 rabbit (dermal) (mg/kg) | >2000 | ||||
| 96-h LD50 zebrafish (mg/L) | 5.2 | ||||
| 24-h EC50 for D. magna (immobilization) (mg/L) | 14.8 | ||||
| 72-h EC50 for R. Subcapitata (growth) (mg/L) | 1.2 | ||||
| 2 | A; R1 = tallow, R2 = CH3 | MCPA 1 | LD50 rat (oral) (mg/kg) | 2000 | [12] |
| 96-h LD50 rainbow trout (mg/L) | 7–17 | ||||
| 48-h EC50 for D. magna (immobilization) (mg/L) | 0.2–0.5 | ||||
| 72-h EC50 for R. Subcapitata (growth) (mg/L) | 1.6–1.9 | ||||
| 3 | A; R1= tallow, R2 = C2H5OH | MeSO3 | LD50 rat (oral) (mg/kg) | >4480 | [140] |
| LD50 rat (dermal) (mg/kg) | >2000 | ||||
| 96-h LD50 rainbow trout | 1.91 | ||||
| 48-h EC50 for D. magna (immobilization) (mg/L) | 2.23 | ||||
| 72-h EC50 for S. Subcapitatus (growth) (mg/L) | 2.14 | ||||
| 4 | B; n = 1, R1 = C2H5, R2 = CH3 | Dic 2 | 48-h EC50 for D. magna (immobilization) (mg/L) | 395 | [133] |
| 48-h EC50 for A. fransiscana (immobilization) (mg/L) | >1000 | ||||
| 72-h EC50 for C. Vulgaris (growth) (mg/L) | >1000 | ||||
| 5 | B; n = 1, R1 = C10H21, R2 = CH3 | Dic | 48-h EC50 for D. magna (immobilization) (mg/L) | 7.11 | |
| 48-h EC50 for A. fransiscana (immobilization) (mg/L) | 12.1 | ||||
| 72-h EC50 for C. Vulgaris (growth) (mg/L) | 88.9 | ||||
| 6 | B; n = 1, R1 = C16H33, R2 = CH3 | Dic | 48-h EC50 for D. magna (immobilization) (mg/L) | 0.4 | |
| 48-h EC50 for A. fransiscana (immobilization) (mg/L) | 263 | ||||
| 72-h EC50 for C. Vulgaris (growth) (mg/L) | 0.96 | ||||
| 7 | B; n = 1, R1 = C12H25, R2 = CH3 | SCN | 72-h EC50 for R. Subcapitata (growth) (mg/L) | 0.27 | [21] |
| 8 | B; n = 1, R1 = C12H25, R2 = CH3 | Doc 3 | 0.58 | ||
| 9 | B; n = 1, R1 = C14H29, R2 = CH3 | SCN | 0.54 | ||
| 10 | B; n = 1, R1 = C14H29, R2 = CH3 | Doc | 0.74 | ||
| 11 | B; n = 1, R1 = C2H5, R2 = C4H9 | Sac 4 | 4.71 | ||
| 12 | B; n = 3, R1 = C2H5, R2 = C4H9 | Sac | 12.83 | ||
| 13 | C; m = 7 | 2Cl | 48-h EC50 for D. magna (immobilization) (mg/L) | 50 | [111] |
| 14 | C; m = 9 | 2Cl | 7.5 | ||
| 15 | D; m = 7 | 3Cl | 37 | ||
| 16 | D; m = 9 | 3Cl | 8.1 | ||
| 17 | D; m = 11 | 3Cl | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocki, M.; Stachowiak, W.; Smolibowski, M.; Olejniczak, A.; Niemczak, M.; Shamshina, J.L. Rethinking the Esterquats: Synthesis, Stability, Ecotoxicity and Applications of Esterquats Incorporating Analogs of Betaine or Choline as the Cation in Their Structure. Int. J. Mol. Sci. 2024, 25, 5761. https://doi.org/10.3390/ijms25115761
Wysocki M, Stachowiak W, Smolibowski M, Olejniczak A, Niemczak M, Shamshina JL. Rethinking the Esterquats: Synthesis, Stability, Ecotoxicity and Applications of Esterquats Incorporating Analogs of Betaine or Choline as the Cation in Their Structure. International Journal of Molecular Sciences. 2024; 25(11):5761. https://doi.org/10.3390/ijms25115761
Chicago/Turabian StyleWysocki, Marcin, Witold Stachowiak, Mikołaj Smolibowski, Adriana Olejniczak, Michał Niemczak, and Julia L. Shamshina. 2024. "Rethinking the Esterquats: Synthesis, Stability, Ecotoxicity and Applications of Esterquats Incorporating Analogs of Betaine or Choline as the Cation in Their Structure" International Journal of Molecular Sciences 25, no. 11: 5761. https://doi.org/10.3390/ijms25115761









