The Characteristics and Expression Analysis of the Tomato SlRBOH Gene Family under Exogenous Phytohormone Treatments and Abiotic Stresses
Abstract
:1. Introduction
2. Results
2.1. Identification of SlRBOH Gene Family Members in Tomato and Analysis of Chromosomal Localization
2.2. Analysis of Physicochemical Properties and Subcellular Localization of the Tomato SlRBOH Family Members
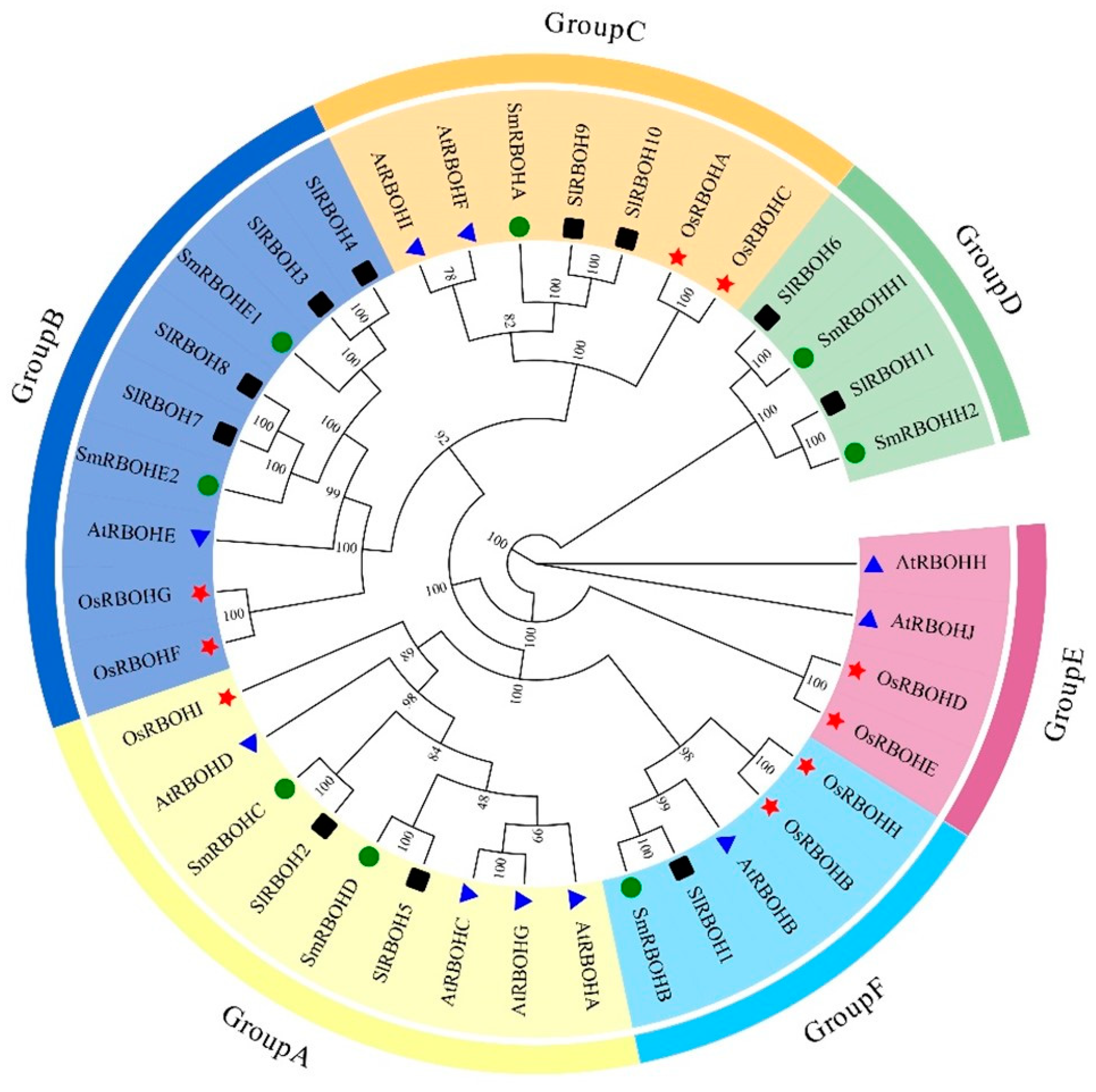
2.3. Structural Analysis and Phylogenetic Tree Analysis of the SlRBOH Gene Family
2.4. Conserved Motifs of the Tomato SlRBOH Family
2.5. Analysis of the Protein Secondary Structure of Tomato SlRBOH Family
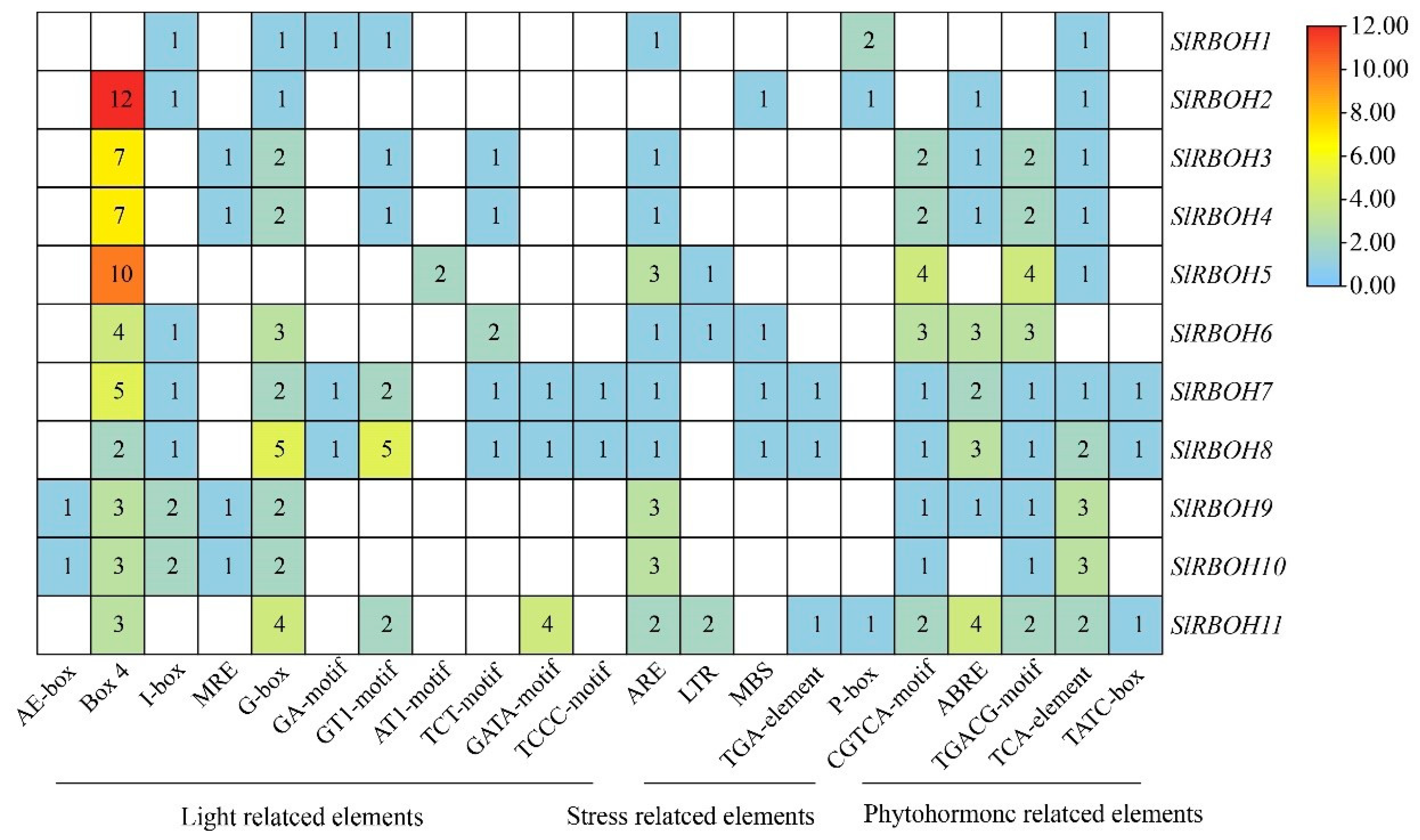
2.6. Analysis of Cis-Acting Elements of Tomato SlRBOH Family Genes
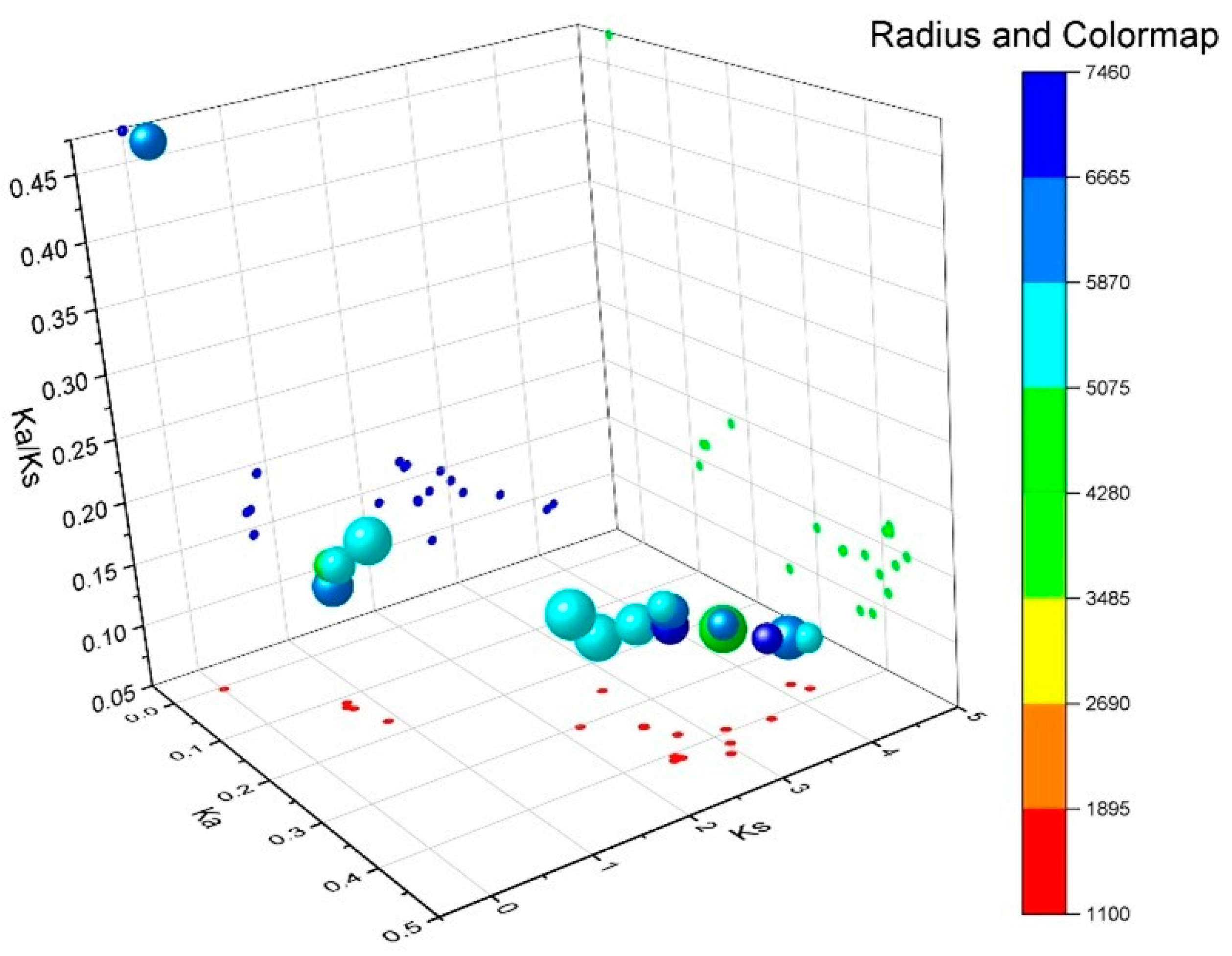
2.7. Evolutionary Selection Pressure Analysis of Tomato SlRBOH Genes
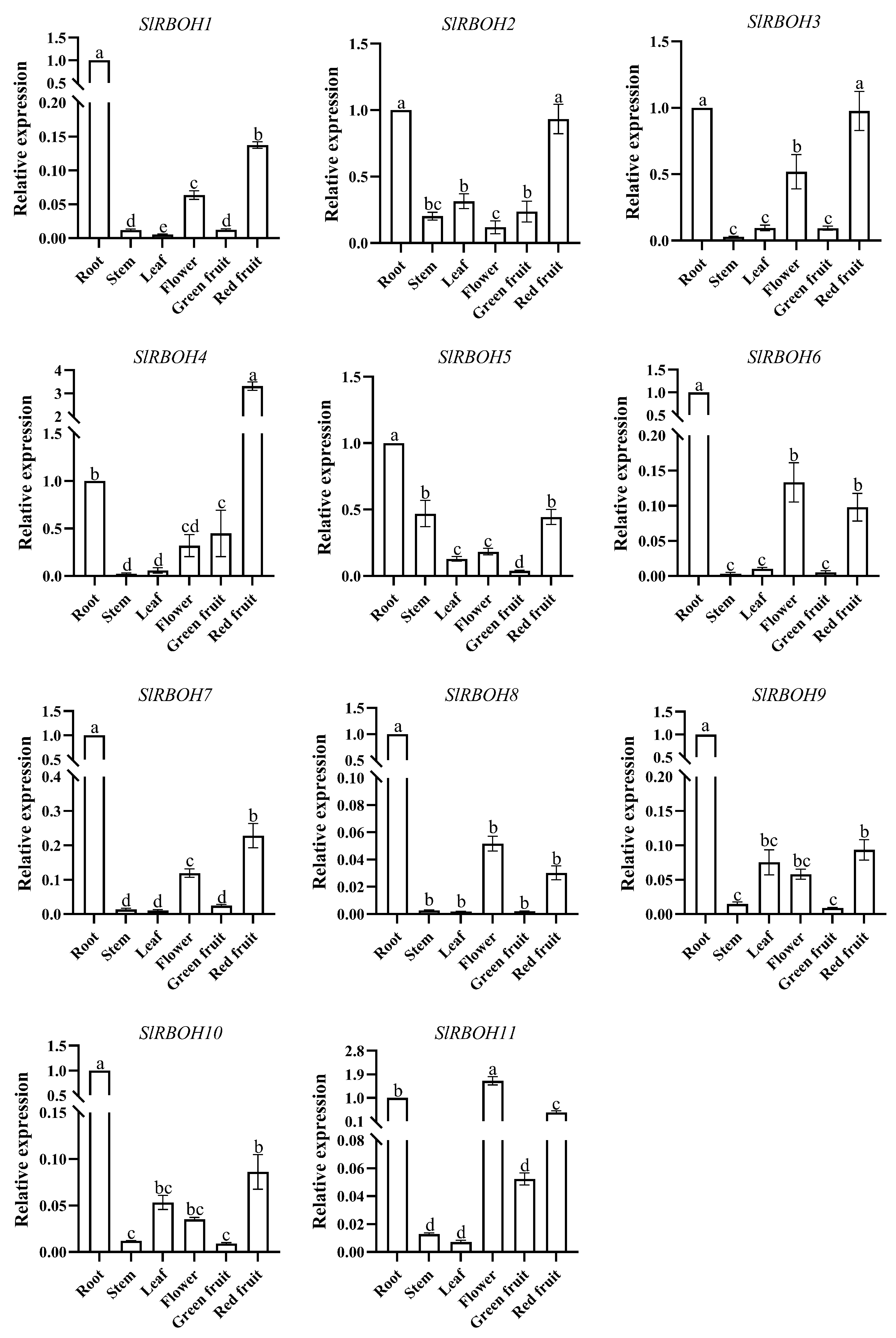
2.8. Tissue-Specific Expression Analysis of Tomato SlRBOH Genes
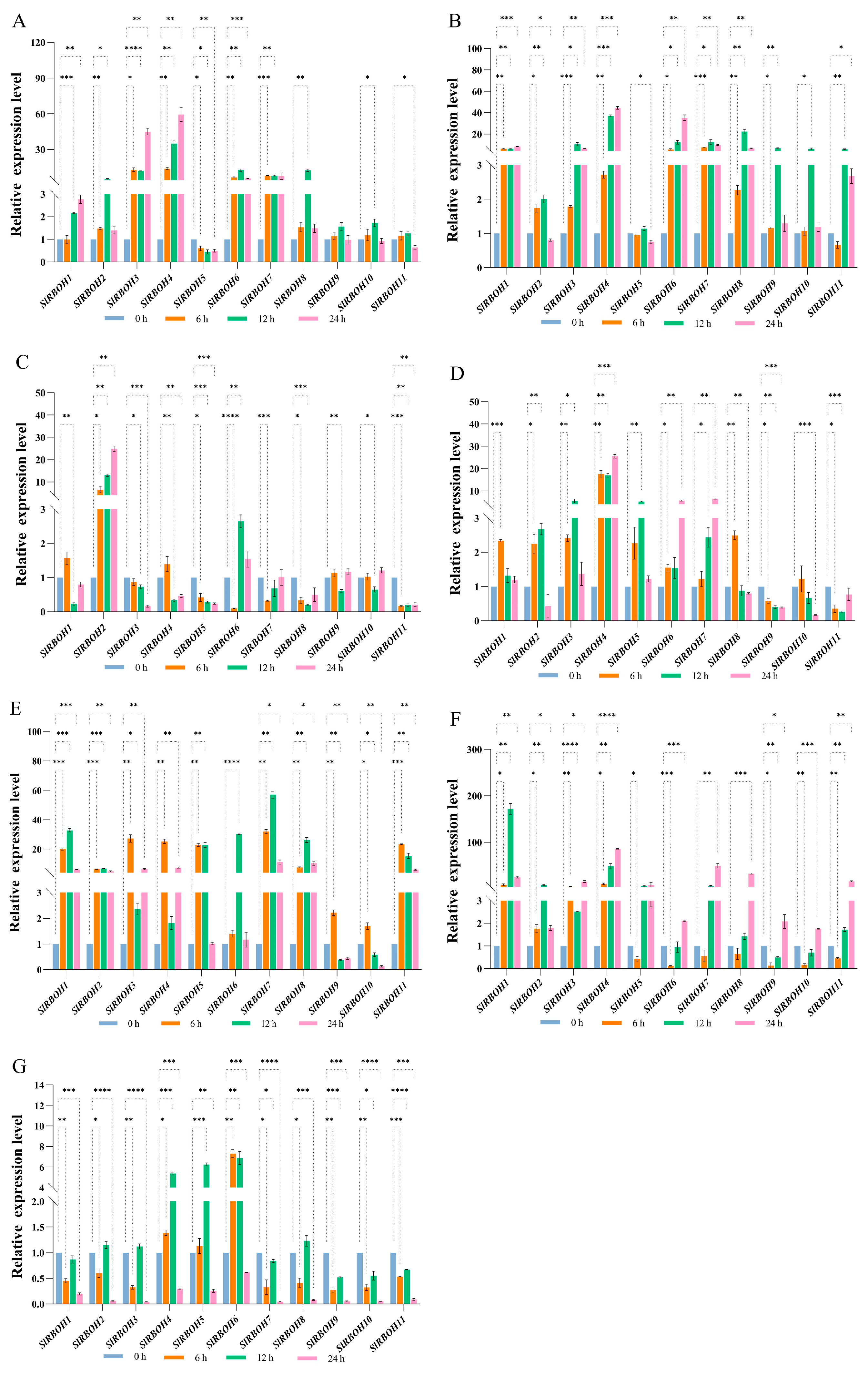
2.9. Expression Analysis of Tomato SlRBOH Genes under Hormonal and Abiotic Stress
3. Discussion
4. Materials and Methods
4.1. Identification of the SlRBOH Family Members in Tomato
4.2. Physicochemical Properties Analysis of the Tomato SlRBOH Gene Family
4.3. Gene location, Ka (Nonsynonymous)/Ks (Synonymous) Analysis, and Gene Structure Analysis
4.4. Conserved Motif and Protein Conserved Domain Analysis
4.5. Phylogenetic Tree and Cis-Acting Elements Analysis
4.6. Collinearity Analysis
4.7. Plant Materials and Growth Conditions
4.8. Stress Treatments and Tissue Expression
4.9. RNA Isolation and qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Chen, D.; Liu, D.; Cheng, Y.; Zhang, X.; Song, L.; Hu, M.; Dong, J.; Shen, F. Comprehensive analysis of the Gossypium hirsutum L. respiratory burst oxidase homolog (Ghrboh) gene family. BMC Genom. 2020, 21, 91. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, Z.; Zhou, Y.; Shen, L.; He, J.; Xia, X.; Zhang, L.; Yang, X. Genome-Wide Identification and Expression Analysis of Respiratory Burst Oxidase Homolog (RBOH) Gene Family in Eggplant (Solanum melongena L.) under Abiotic and Biotic Stress. Genes 2023, 14, 1665. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R.; Graham, D.; Walling, J.G. The barley (Hordeum vulgare ssp. vulgare) respiratory burst oxidase homolog (HvRBOH) gene family and their plausible role on malting quality. Front. Plant Sci. 2021, 12, 608541. [Google Scholar] [PubMed]
- Hu, C.-H.; Wang, P.-Q.; Zhang, P.-P.; Nie, X.-M.; Li, B.-B.; Tai, L.; Liu, W.-T.; Li, W.-Q.; Chen, K.-M. NADPH Oxidases: The Vital Performers and Center Hubs during Plant Growth and Signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Numata, N.; Nakajima, K.; Katou, S.; Kawakita, K.; Rowland, O.; Jones, J.D.; Doke, N. Nicotiana benthamiana gp91 phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 2003, 15, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.; Dunand, C.; Puppo, A.; Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pati, P.K. Analysis of cis-acting regulatory elements of Respiratory burst oxidase homolog (Rboh) gene families in Arabidopsis and rice provides clues for their diverse functions. Comput. Biol. Chem. 2016, 62, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawakita, K.; Maeshima, M.; Doke, N.; Yoshioka, H. Subcellular localization of Strboh proteins and NADPH-dependent O2−-generating activity in potato tuber tissues. J. Exp. Bot. 2006, 57, 1373–1379. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Yan, A.; Deng, J.; Xie, Y.; Liu, S.; Liu, D.; He, L.; Weng, J.; Xu, J. Evolutionary Analysis of Respiratory Burst Oxidase Homolog (RBOH) Genes in Plants and Characterization of ZmRBOHs. Int. J. Mol. Sci. 2023, 24, 3858. [Google Scholar] [CrossRef]
- Yu, S.; Kakar, K.U.; Yang, Z.; Nawaz, Z.; Lin, S.; Guo, Y.; Ren, X.L.; Baloch, A.A.; Han, D. Systematic study of the stress-responsive Rboh gene family in Nicotiana tabacum: Genome-wide identification, evolution and role in disease resistance. Genomics 2020, 112, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Xu, X.; Gao, M.; Li, J.; Guo, C.; Song, J.; Wang, X. Genome-wide analysis of respiratory burst oxidase homologs in grape (Vitis vinifera L.). Int. J. Mol. Sci. 2013, 14, 24169–24186. [Google Scholar] [CrossRef] [PubMed]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sun, L.; Zhang, L.; Song, Y.; Hu, P.; Li, C.; Hao, F.S. AtrbohD and AtrbohF negatively regulate lateral root development by changing the localized accumulation of superoxide in primary roots of Arabidopsis. Planta 2015, 241, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, M.M.; Wang, Y.J.; Gao, Y.T.; Li, R.; Wang, G.F.; Li, W.Q.; Liu, W.T.; Chen, K.M. The plasma membrane NADPH oxidase OsRbohA plays a crucial role in developmental regulation and drought-stress response in rice. Physiol. Plant. 2016, 156, 421–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Luo, L.; Lu, C.; Kong, W.; Cheng, L.; Xu, X.; Liu, J. Genome Wide Identification of Respiratory Burst Oxidase Homolog (Rboh) Genes in Citrus sinensis and Functional Analysis of CsRbohD in Cold Tolerance. Int. J. Mol. Sci. 2022, 23, 648. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Li, Y.; Li, S.; Shi, A.; Zhou, M.; Ren, H.; Yan, Y.; He, C.; Wang, J.; Sun, M.; et al. Photosynthesis Mediated by RBOH-Dependent Signaling Is Essential for Cold Stress Memory. Antioxidants 2022, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chang, Y.L.; Wu, H.T.; Shalmani, A.; Liu, W.T.; Li, W.Q.; Xu, J.W.; Chen, K.M. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020, 39, 1767–1784. [Google Scholar] [CrossRef] [PubMed]
- Kiyono, H.; Katano, K.; Suzuki, N. Links between Regulatory Systems of ROS and Carbohydrates in Reproductive Development. Plants 2021, 10, 1652. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, G. Signal function studies of ROS, especially RBOH-dependent ROS, in plant growth, development and environmental stress. J. Plant Growth Regul. 2020, 39, 157–171. [Google Scholar] [CrossRef]
- Xing, Y.; Lee, C. Assessing the application of Ka/Ks ratio test to alternatively spliced exons. Bioinformatics 2005, 21, 3701–3703. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lu, S.; Liu, T.; Nai, G.; Ren, J.; Gou, H.; Chen, B.; Mao, J. Genome-Wide Identification and Abiotic Stress Response Analysis of PP2C Gene Family in Woodland and Pineapple Strawberries. Int. J. Mol. Sci. 2023, 24, 4049. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Miller, G.; Morales, J.; Shulaev, V.; Torres, M.A.; Mittler, R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011, 14, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.H.; Wei, X.Y.; Yuan, B.; Yao, L.B.; Ma, T.T.; Zhang, P.P.; Wang, X.; Wang, P.Q.; Liu, W.T.; Li, W.Q.; et al. Genome-Wide Identification and Functional Analysis of NADPH Oxidase Family Genes in Wheat During Development and Environmental Stress Responses. Front. Plant Sci. 2018, 9, 906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; He, Y.; Hu, W.; Zhang, Y.; Wang, X.; Tang, H. Identification of NADPH oxidase family members associated with cold stress in strawberry. FEBS Open Bio 2018, 8, 593–605. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Li, W.Q.; Li, W.Y.; Wu, G.L.; Zhou, C.Y.; Chen, K.M. Characterization of Rice NADPH oxidase genes and their expression under various environmental conditions. Int. J. Mol. Sci. 2013, 14, 9440–9458. [Google Scholar] [CrossRef]
- Gao, R.; Luo, Y.; Pan, X.; Wang, C.; Liao, W. Genome-wide identification of SHMT family genes in cucumber (Cucumis sativus L.) and functional analyses of CsSHMTs in response to hormones and abiotic stresses. 3 Biotech. 2022, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Z.; Pan, X.; Yao, K.; Wang, Y.; Yang, T.; Huang, G.; Liao, W.; Wang, C. Genome-Wide Identification and Characterization of Tomato Fatty Acid β-Oxidase Family Genes KAT and MFP. Int. J. Mol. Sci. 2024, 25, 2273. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Koroleva, O.A.; Tomlinson, M.L.; Leader, D.; Shaw, P.; Doonan, J.H. High-throughput protein localization in Arabidopsis using Agrobacterium-mediated transient expression of GFP-ORF fusions. Plant J. Cell Mol. Biol. 2005, 41, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, H.; Ouyang, B.; Shi, C.; Demidchik, V.; Hao, Z.; Yu, M.; Shabala, S. NADPH oxidases and the evolution of plant salinity tolerance. Plant Cell Environ. 2020, 43, 2957–2968. [Google Scholar] [CrossRef] [PubMed]
- Kapli, P.; Yang, Z.; Telford, M.J. Phylogenetic tree building in the genomic age. Nat. Rev. Genet. 2020, 21, 428–444. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.; Finkelstein, A. Theory of protein secondary structure and algorithm of its prediction. Biopolym. Orig. Res. Biomol. 1983, 22, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Rose, G.D. A physical basis for protein secondary structure. Proc. Natl. Acad. Sci. USA 1999, 96, 14258–14263. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Y.; Pan, T.; Li, Y.; Hong, Y.; Chen, W.; Yang, Y.; Zhao, G.; Shabala, S.; Yu, M. Genome-wide analysis of respiratory burst oxidase homolog gene family in pea (Pisum sativum L.). Front. Plant Sci. 2023, 14, 1321952. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Xu, S.; Han, B.; Wu, M.Z.; Yuan, X.X.; Han, Y.; Gu, Q.; Xu, D.K.; Yang, Q.; Shen, W.B. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis. Plant J. Cell Mol. Biol. 2011, 66, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Duan, Z.Q.; Bai, L.; Zhao, Z.G.; Zhang, G.P.; Cheng, F.M.; Jiang, L.X.; Chen, K.M. Drought-stimulated activity of plasma membrane nicotinamide adenine dinucleotide phosphate oxidase and its catalytic properties in rice. J. Integr. Plant Biol. 2009, 51, 1104–1115. [Google Scholar] [CrossRef]
- Sagi, M.; Davydov, O.; Orazova, S.; Yesbergenova, Z.; Ophir, R.; Stratmann, J.W.; Fluhr, R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 2004, 16, 616–628. [Google Scholar] [CrossRef]
- Keller, T.; Damude, H.G.; Werner, D.; Doerner, P.; Dixon, R.A.; Lamb, C. A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 1998, 10, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Li, B.; Shi, Q.; Geng, R.; Geng, S.; Liu, J.; Zhang, Y.; Cai, Y. Comprehensive Analysis of Respiratory Burst Oxidase Homologs (Rboh) Gene Family and Function of GbRboh5/18 on Verticillium Wilt Resistance in Gossypium barbadense. Front. Genet. 2020, 11, 788. [Google Scholar] [CrossRef]
- Kawarazaki, T.; Kimura, S.; Iizuka, A.; Hanamata, S.; Nibori, H.; Michikawa, M.; Imai, A.; Abe, M.; Kaya, H.; Kuchitsu, K. A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. Biochim. Et. Biophys. Acta 2013, 1833, 2775–2780. [Google Scholar] [CrossRef]
- Kyozuka, J.; McElroy, D.; Hayakawa, T.; Xie, Y.; Wu, R.; Shimamoto, K. Light-regulated and cell-specific expression of tomato rbcS-gusA and rice rbcS-gusA fusion genes in transgenic rice. Plant Physiol. 1993, 102, 991–1000. [Google Scholar] [CrossRef]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH Oxidase RBOH Functions in Rice Roots during Lysigenous Aerenchyma Formation under Oxygen-Deficient Conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Gisbert, C.; Bishop, G.J.; Dixon, M.S.; García-Martínez, J.L. Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 2006, 57, 2037–2047. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, C.; Liu, Z.; Gao, R.; Feng, L.; Li, A.; Yao, K.; Liao, W. Identification of ABF/AREB gene family in tomato (Solanum lycopersicum L.) and functional analysis of ABF/AREB in response to ABA and abiotic stresses. PeerJ 2023, 11, e15310. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, X.; Wang, C.; Yun, F.; Huang, D.; Yao, Y.; Gao, R.; Ye, F.; Liu, X.; Liao, W. Genome-wide identification and expression analysis of serine hydroxymethyltransferase (SHMT) gene family in tomato (Solanum lycopersicum). PeerJ 2022, 10, e12943. [Google Scholar] [CrossRef]
- Yao, K.; Yao, Y.; Ding, Z.; Pan, X.; Zheng, Y.; Huang, Y.; Zhang, Z.; Li, A.; Wang, C.; Li, C.; et al. Characterization of the FLA Gene Family in Tomato (Solanum lycopersicum L.) and the Expression Analysis of SlFLAs in Response to Hormone and Abiotic Stresses. Int. J. Mol. Sci. 2023, 24, 16063. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]






| Gene | Gene ID | Gene Locus | ORF (bp) | Amino Acid | Instability Index | Molecular Weight/kDa | pI | Aliphatic Index | Signal Peptide | Transmembrane Domain | Grand Average of Hydropathicity (GRAVY) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SlRBOH1 | XM_004230184.4 | Chr01 | 2598 | 865 | 39.6 | 98,730.42 | 8.92 | 84.28 | No | Yes | −0.266 |
| SlRBOH2 | NM_001247342.2 | Chr03 | 2817 | 938 | 37.49 | 105,307.48 | 9.13 | 81.88 | No | Yes | −0.311 |
| SlRBOH3 | XM_004239534.4 | Chr05 | 2643 | 880 | 49.74 | 100,465.8 | 8.71 | 90.55 | No | Yes | −0.154 |
| SlRBOH4 | XM_019213765.2 | Chr05 | 2571 | 856 | 50.71 | 97,500.21 | 8.58 | 91.04 | No | Yes | −0.155 |
| SlRBOH5 | XM_004241593.4 | Chr06 | 2574 | 857 | 45.03 | 97,729.55 | 9.09 | 85.01 | No | Yes | −0.294 |
| SlRBOH6 | XM_019214404.2 | Chr06 | 2493 | 830 | 41 | 95,392.74 | 9.11 | 80.37 | No | Yes | −0.267 |
| SlRBOH7 | XM_026032004.1 | Chr07 | 2856 | 951 | 46.71 | 107,414.41 | 8.91 | 88.63 | No | Yes | −0.182 |
| SlRBOH8 | XM_019214972.2 | Chr07 | 2841 | 946 | 46.82 | 106,813.63 | 8.75 | 89.1 | No | Yes | −0.172 |
| SlRBOH9 | NM_001374505.1 | Chr08 | 2892 | 963 | 49.59 | 109,078.14 | 9.14 | 88.13 | No | Yes | −0.215 |
| SlRBOH10 | NM_001247197.3 | Chr08 | 2970 | 989 | 50.43 | 112,288.95 | 9.28 | 89.26 | No | Yes | −0.212 |
| SlRBOH11 | XM_004251404.4 | Chr11 | 2331 | 776 | 41.32 | 89,066.78 | 8.82 | 83.44 | No | Yes | −0.196 |
| Gene | Plasma Membrane | Endoplasmic Reticulum | Nucleus | Peroxisome | Chloroplast | Mitochondrion | Cytosol |
|---|---|---|---|---|---|---|---|
| SlRBOH1 | 12 | 2 | |||||
| SlRBOH2 | 14 | ||||||
| SlRBOH3 | 11 | 1 | 2 | ||||
| SlRBOH4 | 10 | 1 | 2 | 2 | |||
| SlRBOH5 | 13 | 1 | |||||
| SlRBOH6 | 10 | 1 | 3 | ||||
| SlRBOH7 | 8 | 1 | 1 | 2 | 2 | ||
| SlRBOH8 | 8 | 1 | 2 | 3 | |||
| SlRBOH9 | 9 | 2 | 1 | 1 | 1 | ||
| SlRBOH10 | 9 | 2 | 1 | 1 | 1 | ||
| SlRBOH11 | 13 | 1 |
| Motif | Width (aa) | Motif Sequence |
|---|---|---|
| Motif 1 | 50 | AYFYWVTREQGSFDWFKGVMNEVAEMDHKGVIEMHNYLTSVYEEGDARSA |
| Motif 2 | 50 | KPPGFKYKSGQYIFVQCPAISSFEWHPFSITSAPGDDYLSVHIRTLGDWT |
| Motif 3 | 50 | LTGFNAFWYSHHLLIIVYILLIVHGTFLYLVHEWYKKTTWMYJAVPLJLY |
| Motif 4 | 50 | LFTWKFLQYKQKAAFQVMGYCLATAKGAAETLKFNMALILLPVCRNTJTW |
| Motif 5 | 50 | VBQRGLPKLLIDGPYGAPAQDYKKYDVLLLVGLGIGATPFISILKDLLNN |
| Motif 6 | 50 | MVQALNHAKNGVDIVSGTRVRTHFARPNWREVFKRIALKHPYSRIGVFYC |
| Motif 7 | 50 | HEFWEQISDQSFDARLQIFFDMVDKNGDGKITEEEVKEJIMLSASANKLS |
| Motif 8 | 50 | TRLGLJIPFDDNINFHKIIAYAIAVGIJJHALNHLACDFPRLINASPEKF |
| Motif 9 | 50 | DFDEQKPSYIDLVKGVEGVTGIVMVILMAIAFTLATRWFRRNVIKLPKPF |
| Motif 10 | 41 | KLKEQAAEYAALIMEELDPENLGYIEJWQLETLLLQRDNYM |
| Protein | Alpha Helix (%) | Extended Strand (%) | Beta Turn (%) | Random Coil (%) | Distribution of Secondary Structure Elements |
|---|---|---|---|---|---|
| SlRBOH1 | 44.74 | 16.76 | 5.32 | 33.18 |  |
| SlRBOH2 | 45.95 | 13.11 | 4.26 | 36.67 |  |
| SlRBOH3 | 45.62 | 15.64 | 5.37 | 33.37 |  |
| SlRBOH4 | 44.65 | 14.64 | 4.98 | 35.72 |  |
| SlRBOH5 | 44.19 | 14.16 | 4.95 | 36.70 |  |
| SlRBOH6 | 45.85 | 12.93 | 4.52 | 36.70 |  |
| SlRBOH7 | 43.23 | 13.85 | 4.02 | 38.90 | 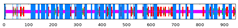 |
| SlRBOH8 | 44.89 | 15.34 | 5.11 | 34.66 |  |
| SlRBOH9 | 44.34 | 14.10 | 5.42 | 36.14 |  |
| SlRBOH10 | 50.90 | 14.05 | 2.71 | 32.35 | 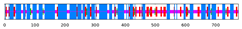 |
| SLRBOH11 | 43.36 | 15.07 | 5.26 | 36.21 |  |
| Cis-Element | Number of Genes | Sequence of Cis-Element | Functions of Cis-Elements |
|---|---|---|---|
| ABRE | 16 | ACGTG | cis-acting element involved in abscisic acid responsiveness |
| CGTCA-motif | 2 | CGTCA | cis-acting regulatory element involved in MeJA-responsiveness |
| MRE | 6 | AACCTAA | MYB binding site involved in light responsiveness |
| I-box | 2 | GGATAAGGTG | part of a light-responsive element |
| AE-box | 56 | AGAAACTT | part of a module for light responsiveness |
| TGACG-motif | 2 | TGACG | cis-acting regulatory element involved in MeJA-responsiveness |
| G-box | 2 | TACGTG | cis-acting regulatory element involved in light responsiveness |
| ARE | 3 | AAACCA | cis-acting regulatory element essential for anaerobic induction |
| TCA-element | 18 | CCATCTTTTT | cis-acting element involved in salicylic acid responsiveness |
| Box 4 | 12 | ATTAAT | part of a conserved DNA module involved in light responsiveness |
| P-box | 9 | CCTTTTG | gibberellin-responsive element |
| MBS | 4 | CAACTG | MYB binding site involved in drought inducibility |
| GA-motif | 4 | ATAGATAA | part of a light-responsive element |
| GT1-motif | 4 | GGTTAAT | light-responsive element |
| TCT-motif | 2 | TCTTAC | part of a light-responsive element |
| AT1-motif | 3 | AATTATTTTTTATT | part of a light-responsive module |
| LTR | 16 | CCGAAA | cis-acting element involved in low-temperature responsiveness |
| GATA-motif | 2 | AAGATAAGATT | part of a light-responsive element |
| TATC-box | 6 | TATCCCA | cis-acting element involved in gibberellin responsiveness |
| TGA-element | 17 | AACGAC | auxin-responsive element |
| TCCC-motif | 2 | TCTCCCT | part of a light-responsive element |
| G-Box | 6 | CACGTT | cis-acting regulatory element involved in light responsiveness |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Z.; Li, L.; Pan, X.; Yao, K.; Wei, W.; Liao, W.; Wang, C. The Characteristics and Expression Analysis of the Tomato SlRBOH Gene Family under Exogenous Phytohormone Treatments and Abiotic Stresses. Int. J. Mol. Sci. 2024, 25, 5780. https://doi.org/10.3390/ijms25115780
Wang Y, Liu Z, Li L, Pan X, Yao K, Wei W, Liao W, Wang C. The Characteristics and Expression Analysis of the Tomato SlRBOH Gene Family under Exogenous Phytohormone Treatments and Abiotic Stresses. International Journal of Molecular Sciences. 2024; 25(11):5780. https://doi.org/10.3390/ijms25115780
Chicago/Turabian StyleWang, Yuanhui, Zesheng Liu, Long Li, Xuejuan Pan, Kangding Yao, Wenying Wei, Weibiao Liao, and Chunlei Wang. 2024. "The Characteristics and Expression Analysis of the Tomato SlRBOH Gene Family under Exogenous Phytohormone Treatments and Abiotic Stresses" International Journal of Molecular Sciences 25, no. 11: 5780. https://doi.org/10.3390/ijms25115780






