Therapeutic Potential of Ginsenosides on Bone Metabolism: A Review of Osteoporosis, Periodontal Disease and Osteoarthritis
Abstract
1. Introduction
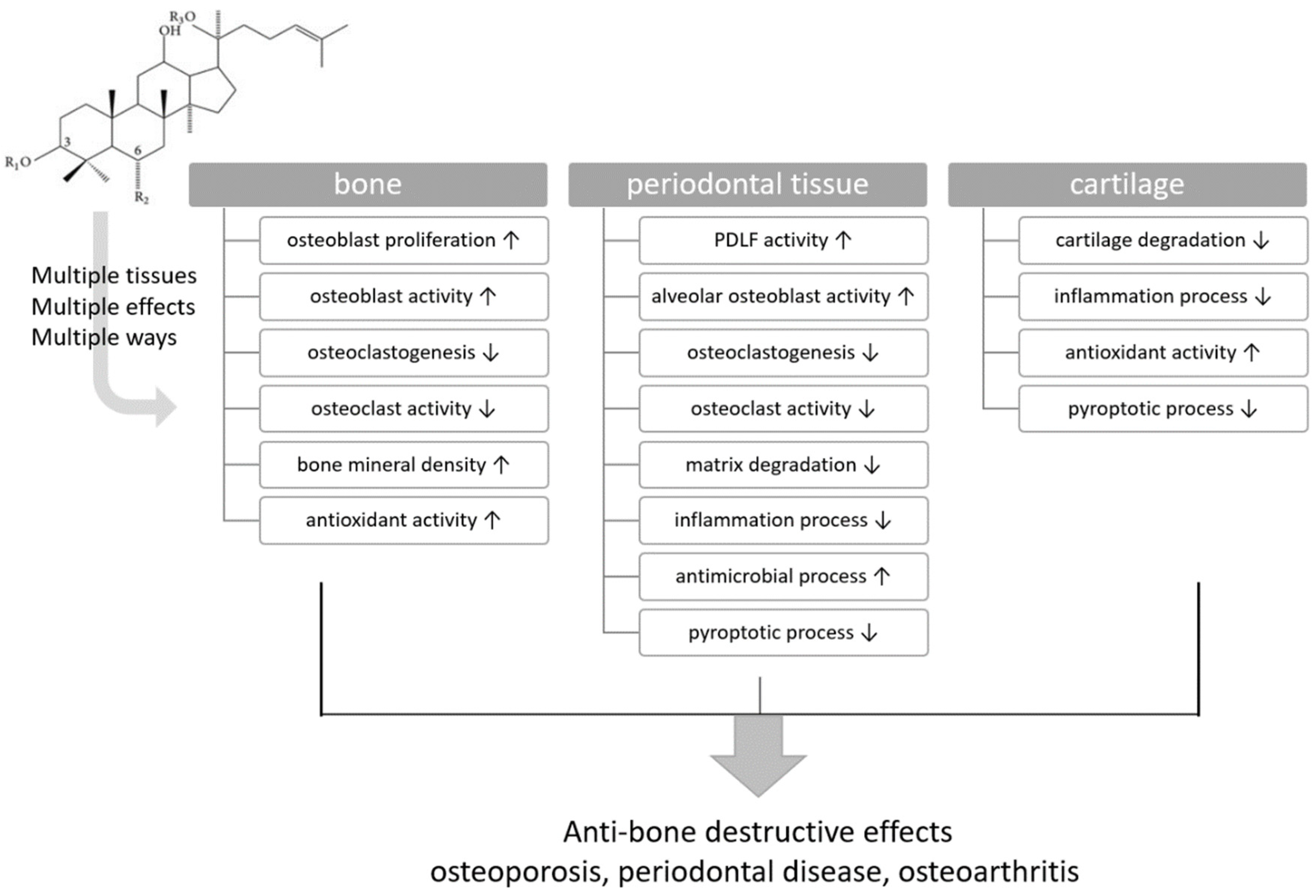

| Target Tissues | Bone | Periodontal Tissue | Cartilage |
|---|---|---|---|
| Ginsenoside | Functions | ||
| G-Rb1 | osteoblast activity ↑ | cartilage degradation ↓ | |
| osteoclastogenesis ↓ | inflammatory process ↓ | ||
| osteoclastic activity ↓ | antioxidant activity ↑ | ||
| bone mineral density ↑ | |||
| G-Rb2 | osteoblastic cell proliferation ↑ | ||
| osteoblast activity ↑ | |||
| osteoclastogenesis ↓ | |||
| osteoclastic activity ↓ | |||
| antioxidant activity ↑ | |||
| G-Rb3 | osteoclastogenesis ↓ | cartilage degradation ↓ | |
| osteoclastic activity ↓ | |||
| matrix degradation ↓ | |||
| inflammatory process ↓ | |||
| gingivitis ↓ | |||
| G-Rc | osteoblastic cell viability ↑ | cartilage degradation ↓ | |
| osteoblast activity ↑ | |||
| bone mineral density ↑ | |||
| G-Rd | osteoblast activity ↑ | osteoclastogenesis ↓ | cartilage degradation ↓ |
| osteoclastic activity ↓ | |||
| matrix degradation ↓ | |||
| inflammatory process ↓ | |||
| antimicrobial process ↑ | |||
| G-Re | osteoblast activity ↑ | periodontal ligament fibroblast activity ↑ | |
| osteoclastogenesis ↓ | inflammatory process ↓ | ||
| osteoclastic activity ↓ | |||
| G-Rf | periodontal ligament fibroblast activity ↑ | cartilage degradation ↓ | |
| inflammatory process ↓ | intestinal inflammatory process ↓ | ||
| antioxidant activity ↑ | |||
| G-Rg1 | osteogenic differentiation from BMSCs ↑ | periodontal ligament fibroblast proliferation ↑ | cartilage degradation ↓ |
| adipogenic differentiation from BMSCs ↓ | periodontal ligament fibroblast activity ↑ | ||
| inflammatory process ↓ | |||
| pyroptotic process ↓ | |||
| G-Rg2 | osteoclastogenesis ↓ | ||
| osteoclastic activity ↓ | |||
| G-Rg3 | osteoblast activity ↑ | cartilage degradation ↓ | |
| osteoclastogenesis ↓ | |||
| osteoclastic activity ↓ | |||
| bone mineral density ↑ | |||
| G-Rh1 | osteoblastic cell proliferation ↑ | ||
| osteoblast activity ↑ | |||
| antioxidant activity ↑ | |||
| G-Rh2 | osteoblast activity ↑ | antimicrobial process ↑ | |
| osteoclastogenesis ↓ | |||
| osteoclastic activity ↓ | |||
| bone mineral density ↑ | |||
| G-Rk1 | inflammatory process ↓ | ||
| CK | osteoblast activity ↑ | cartilage degradation ↓ | |
| osteoclastogenesis ↓ | chondrocyte proliferation ↑ | ||
| osteoclastic activity ↓ | chondrocyte differentiation ↑ | ||
| matrix degradation ↓ | inflammatory process ↓ | ||
| bone mineral density ↑ | pyroptotic process ↓ | ||
| antioxidant activity ↑ | |||
| NGR1 | osteoblastic viability ↑ | alveolar osteoblast activity ↑ | |
| osteoblastic differentiation ↑ | |||
| osteoblast activity ↑ | |||
| osteoclastogenesis ↓ | |||
| osteoclastic activity ↓ | |||
| antioxidant activity ↑ | |||
| PNS | osteoblast activity ↑ | joint destruction ↓ | |
| osteoclastogenesis ↓ | inflammatory process ↓ | ||
| osteoclastic activity ↓ | |||
| bone mineral density ↑ | |||
| Ginseng Extracts | osteoblast activity ↑ | periodontal ligament fibroblast proliferation ↑ | |
| osteoclastogenesis ↓ | periodontal ligament fibroblast activity ↑ | ||
| bone mineral density ↑ | osteoclastogenesis ↓ | ||
| osteoclastic activity ↓ | |||
| matrix degradation ↓ | |||
| alveolar bone protection | |||
| inflammatory process ↓ | |||
| antimicrobial process ↑ | |||
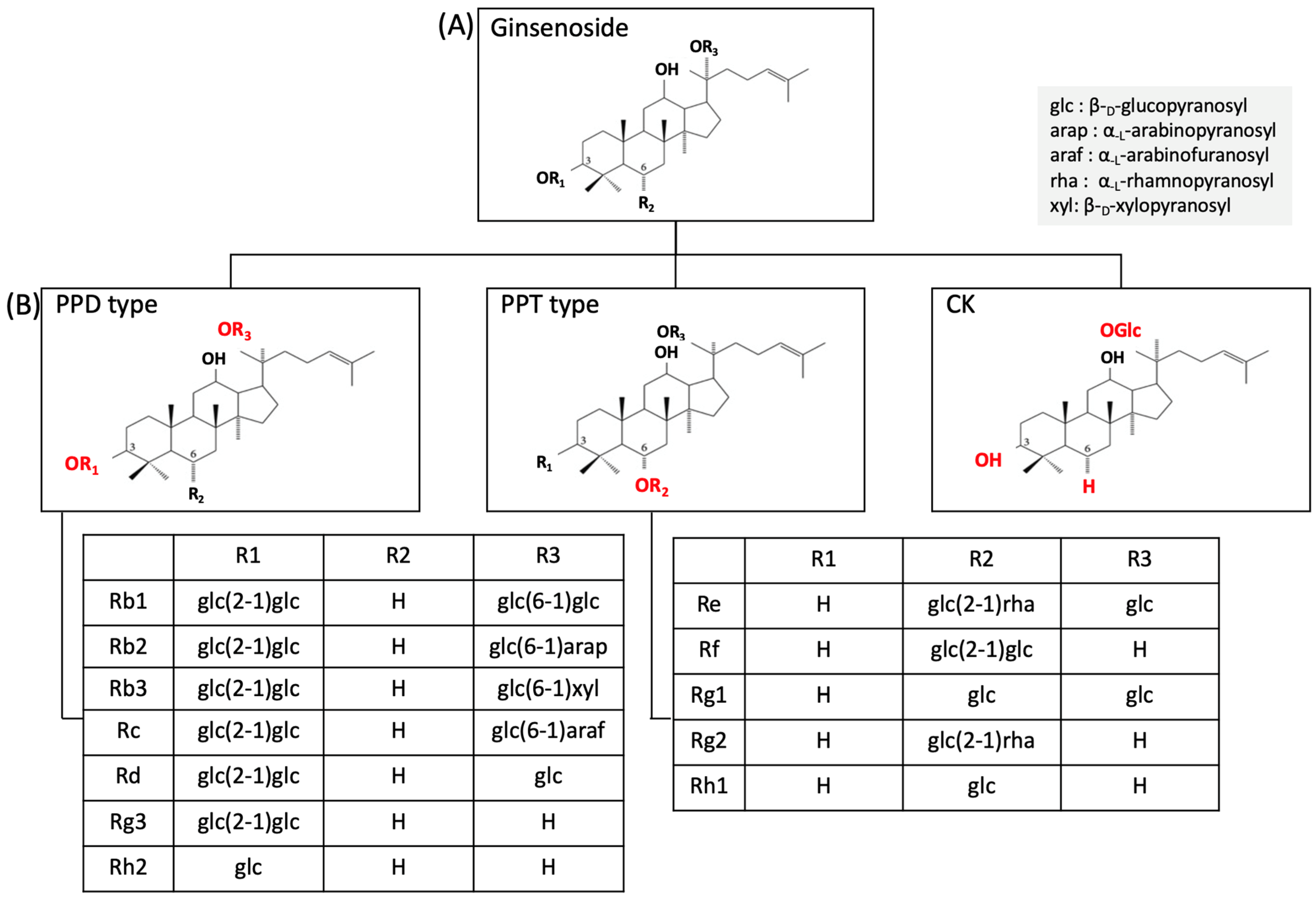
2. Panax Ginseng and Ginsenoside
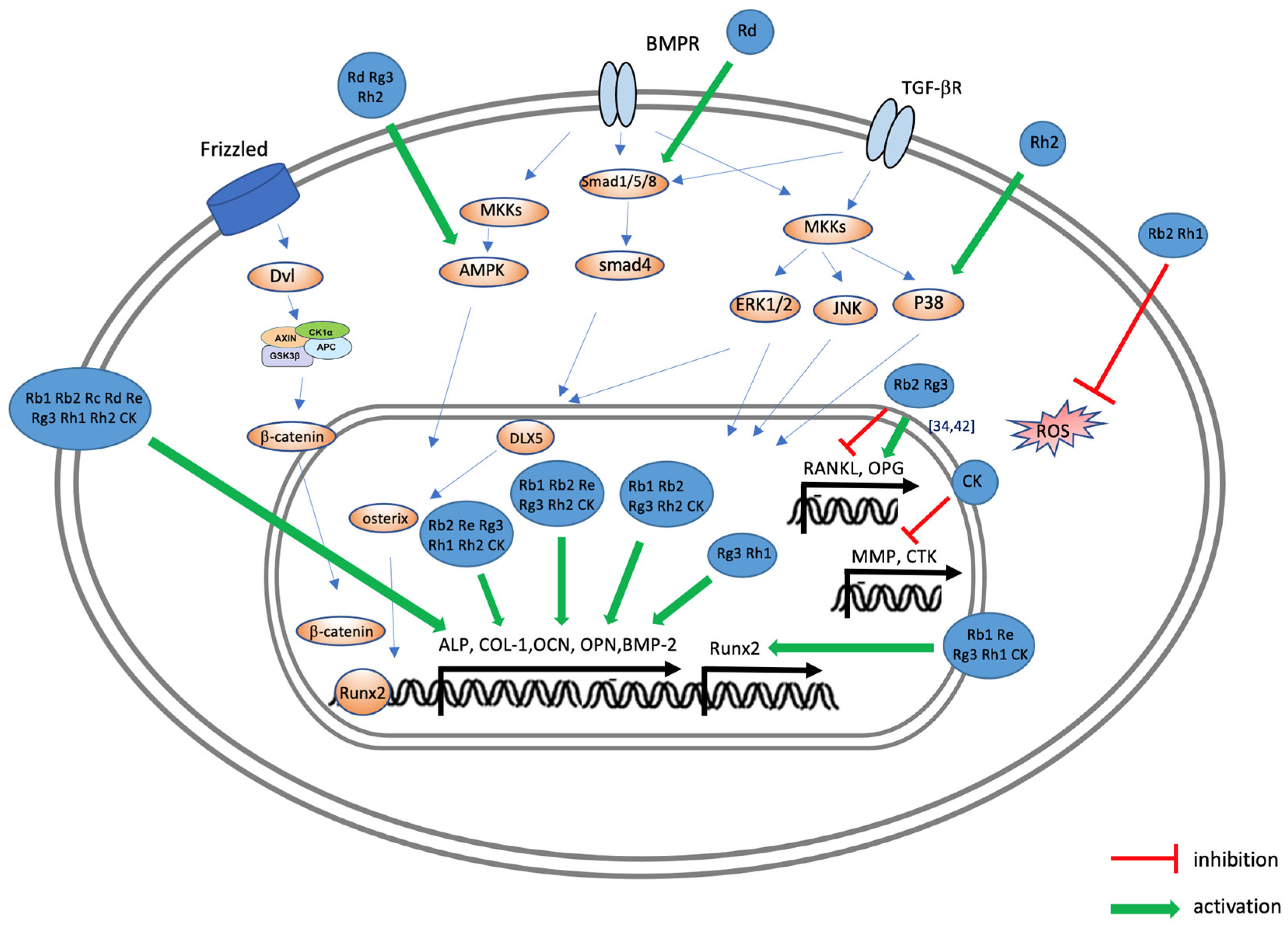
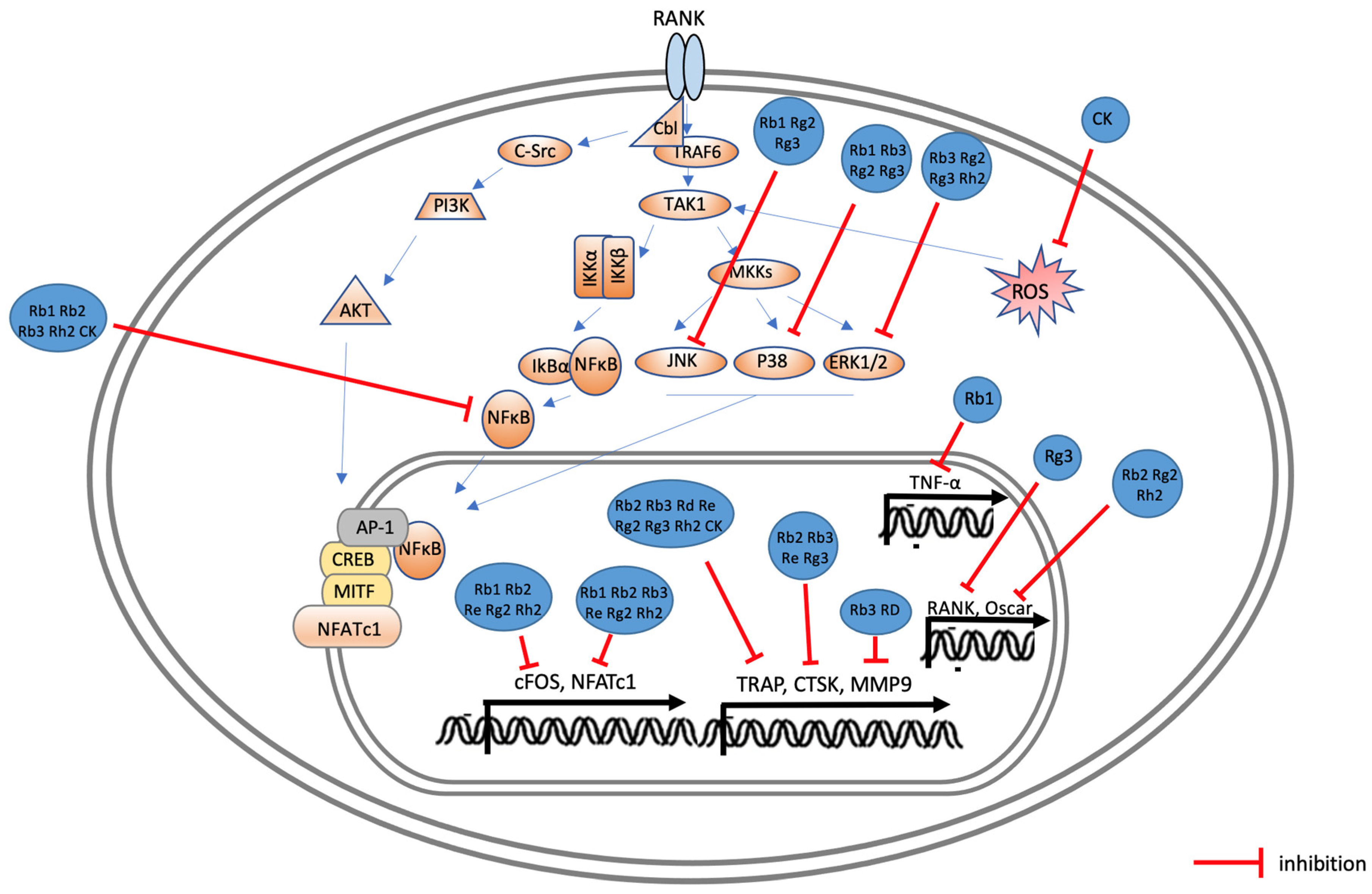
3. Effects of Ginsenoside on Osteoporosis
| Active Compound/Extracts | Properties | In Vitro Model | Activity and Mechanism | In Vivo Model | Activity and Mechanism |
|---|---|---|---|---|---|
| G-Rb1 | osteogenic | isolated osteoblasts from DEX-OP rats | ↑ ALP activity ↑ Runx2, OCN, and OPN mRNA (0.0145 mg/mL) [8] | DEX-OP rats | ↑ BMD and BV/TV ↓ DEX-induced OP through the AHR/PRELP/NF-κB signaling ↑ AHR and PRELP proteins ↓ NF-κB p65 protein (IP 3 and 6 mg/kg/day) [8] |
| anti-osteoclastogenic | RAW264.7 cells | ↓ osteoclast differentiation ↓ TNFα mRNA ↓ c-Fos, NFATc1 mRNA ↓ nucleus translocation and activation of NF-κB ↓ JNK and p38 phosphorylation (0.1, 1, and 10 μM) [9] | |||
| G-Rb2 | osteogenic | MC3T3-E1 cells, H2O2-induced oxidative damage model | ↑ cell proliferation ↑ ALP mRNA ↑ calcium deposition ↑ ALP, COL-1, OCN, and OPN mRNA against oxidative damage induced by H2O2 ↓ RANKL and IL-6 (0.1, 1, and 10 μM) [12] | OVX-OP mice | ↓ blood MDA in OVX mice ↑ GSH activity in OVX mice ↑ BMD in OVX mice (IP 4.6 and 18.5 μmol/kg/day) [12] |
| KD-OP mice | ↑ bone volume fraction ↑ serum BALP ↑ OCN ↓ TRAP, PPAR-γ, and CTSK (IP 18.5 μmol/kg/day) [33] | ||||
| anti-osteoclastogenic | RAW264.7 cells | ↓ TRAP (+) MNC generation and TRAP mRNA ↑ OPG mRNA ↓ bone resorption ↓ NFATc1, c-Fos, OSCAR, CTSK mRNA ↓ NF-κB activation ↓ STAT3 activation (0.1, 1, and 10 μM) [34] | |||
| antioxidant | MC3T3-E1, H2O2-induced oxidative damage model | ↓ H2O2-induced production of ROS ↑ ALP, COL-1, OCN, and OPN mRNA against oxidative damage induced by H2O2 (0.1,1,10 μM) [12] | |||
| G-Rc | osteogenic | MC3T3-E1 cells | ↑ cell viability ↑ ALP staining ↑ calcium deposition ↑ β-catenin, p-GSK-3β, Runx2, ALP, and COL-1 mRNA (25, 50, 100, 200, 400, and 800 μM) [35] | OVX-OP mice | ↑ BMD ↑ trabecular bone number ↑ microstructure of trabecular bone ↑ Runx2, ALP, COL-1, BMP-2, OCN, mRNA, protein (gavage 25 and 50 mg/kg) [35] |
| G-Rd | osteogenic | MC3T3-E1 cells | ↑ ALP, COL-1, OCN, OPN, and OSX mRNA ↑ BMP-2 mRNA ↑ calcium deposition ↑ AMPK ↑ Smad1/5 phosphorylation (10, 20, and 40 μM) [36] | ||
| G-Re | osteogenic | MC3T3-E1 cells | ↑ ALP activity ↑ Runx2, ALP, COL-1, OCN, and mRNA ↑ calcium deposition (5, 10, 25, 50, and 100 μM) [37] | ||
| anti-osteoclastogenic | BMMs | ↓ TRAP (+) MNCs generation ↓ TRAP activity ↓ NFATc1, c-Fos, and TRAP mRNA ↓ ERK phosphorylation (1, 2.5, 5, 10, 25, 50, and 100 μM) [38] | zebrafish model | more narrow distribution of TRAP staining ↓ TRAP and CTSK mRNA [38] | |
| G-Rg1 | osteogenic | BMSCs | ↑ osteogenic differentiation of BMMSCs (5,10,20 μg/mL) [56] | ||
| antioxidant | BMSCs | ↓ adipogenic differentiation by decreasing oxidative stress ↓ adipocyte distribution aging mice (5, 10, 20 μg/mL) [56] | |||
| G-Rg2 | anti-osteoclastogenic | BMMs | ↓ osteoclast differentiation ↓ c-Fos and NFATc1 mRNA ↓ TRAP, Acp5, and Oscar mRNA ↓ p38, ERK, and JNK phosphorylation (1, 5, 10, 20, and 40 μM) [39] | ||
| G-Rg3 | osteogenic | MC3T3-E1 cells | ↑ phosphorylated AMPK and autophagy ↑ Runx2, ALP, COL-1, OCN, and OPN mRNA ↑ calcium deposition ↓ mTOR signaling (10 and 20 μmol/L) [17] | OVX-OP mice | ↓ OVX-induced BW increases, BMD decreases, and histological changes in femur tissues ↑ Runx2, ALP, COL-1, OCN, and OPN ↓ TRAP ↑ autophagy and AMPK signaling ↓ mTOR signaling (IP 20 mg/kg) [17] |
| MC3T3-E1 cells | ↑ ALP, COL-1 mRNA (10 and 100 μg/mL) [41] | ||||
| Primary osteoblasts | ↑ ALP activity ↑ calcium deposition ↓ RANKL mRNA and protein ↑ OPG mRNA and protein (1, 5, 10, 20, and 100 μM) [42] | GC-OP | ↓ DEXA-induced BW increases and BMD decreases ↓ TRACP-5b activity ↓ CTx ↑ BMP-2, BMPR1A, and Runx2 mRNA (gavage 10 and 20 mg/kg) [42] | ||
| anti-osteoclastogenic | RAW264.7 cells | ↓ pit formation ↓ TRAP (+) MNC generation ↓ RANK, TRAP, and CTSK mRNA ↓ p38, ERK, and JNK phosphorylation (0.01, 0.1, 1, 10, and 100 μM) [40] | |||
| G-Rh1 | osteogenic | MC3T3-E1 cells | ↑ cell growth ↑ ALP activity and COL-1 protein ↑ calcium deposition ↑ BMP-2 and Runx2 mRNA (0.01, 0.05, 0.5, and 5 μg/mL) [43] | ||
| antioxidant | AMA presented MC3T3-E1 cells | ↑ glutathione ↓ ROS production enhanced by AMA (0.01, 0.05, 0.5, and 5 μg/mL) [43] | |||
| G-Rh2 | osteogenic | MC3T3-E1 cells | ↑ ALP, COL-1, OCN, and OSX mRNA ↑ calcium deposition ↑ AMPK phosphorylation ↑ p38 phosphorylation [44] | C57BL/6 mice | ↑ BMD (IP 3 mg/kg) [46] |
| MC3T3-E1 cells | ↑ ALP, COL-1 OCN, OPN, and OSX mRNA ↑ calcium deposition ↑ PKD and AMPK phosphorylation [45] | ||||
| anti-osteoclastogenic | BMMs | ↓ TRAP (+) MNC generation ↓ c-Fos, NFATc1, TRAP, and Oscar ↓ ERK phosphorylation ↓ NF-κB (5, 10, and 20 μM) [46] | |||
| CK | osteogenic | MC3T3-E1 | ↑ ALP activity ↑ Runx2, ALP, COL-1, mRNA ↑ OPG mRNA ↑ Wnt10b, Wnt11, Lrp5, β-catenin (1, 2, 4, 8, and 16 μM) [47] | rat open femoral fracture model | ↑ fracture repair (local injection 500 μM) [48] |
| OVX-OP mice | ↓ osteoclast number and surface area ↑ bone structure characteristics ↑ ALP, OCN, and OPN (IHC staining) ↓ MMP-9 and CTSK (IHC staining) (IP, 10 mg/kg) [13] | ||||
| BMSCs | ↑ ALP, OCN, OPN, and OSX mRNA ↑ calcium deposition ↑ nuclear translocation of β-catenin, expression of Runx2 ↑ hUVEC formation (1 and 10 μM) [48] | ||||
| anti-osteoclastogenic | RAW264.7 cells, BMMs | ↓ TRAP (+) MNC generation ↓ NF-κB phosphorylation ↓ bone resorption (1 and 10 μM) [13] | |||
| antioxidant | RAW264.7 cells | ↓ ROS activity (1 and 10 μM) [13] | |||
| NGR1 | osteogenic | hASCs | ↑ cell migration and osteogenic differentiation ↑ VEGF mRNA ↑ adhesion and spreading of hASCs on the bio-inert glass surface ↓ RANKL/OPG expression ratio (0.01, 0.05, 0.5, and 5 μg/mL) [49] | ||
| MC3T3-E1 cells | ↑ ALP activity ↑ ALP, COL-1, and OCN mRNA ↑ calcium deposition (5, 50, 100, 200, and 1000 μg/mL) [51] | ||||
| MC3T3-E1 cells | ↑ Runx2, ALP, and COL-1, OCN ↑ calcium deposition in OS injury model (10, 25, and 50 μM) [52] | ||||
| anti-osteoclastogenic | Raw264.7 cells | ↓ p38, ERK1/2, JNK1/2, and NF-κB phosphorylation ↓ TRAP (+) MNC generation ↓ osteoclast bone resorption (5, 10, and 20 μM) [50] | mouse calvarial osteolysis model | ↓ mouse calvarial osteolysis (IP 10 and20 mg/kg) [50] | |
| antioxidant | MC3T3-E1, H2O2-induced oxidative damage model | ↓ H2O2-induced osteoblast apoptosis ↑ osteoblast viability ↓ H2O2-induced mitochondrial ROS restored mitochondrial membrane potential and blocked JNK activated by H2O2 (10, 25, and 50 μM) [52] | |||
| PNS | osteogenic | MC3T3-E1 cells | ↑ ALP activity and calcium deposition ↑ COL-1 and OCN mRNA (0.05 and 0.5 mg/mL) [53] | ||
| BMSCs | ↑ ALP activity and calcium deposition ↑ ALP, Cbfa1, and bone sialoprotein mRNA ↑ p38 and ERK phosphorylation [54] | ||||
| OVX-OP mice | ↑ restore bone mass ↑ CD31 and OCN ↓ serum NTX (P.O. 40 and 80 mg/kg) [55] | ||||
| Ginseng extracts | osteogenic | MC3T3-E1 cells | ↓ caspase-3 and -9 mRNA ↑ Bcl2, IAPs, and XIAP mRNA ↑ Runx2, ALP, and BMP mRNA ↑ ALP activity ↑ AKT phosphorylation ↓ JNK phosphorylation (250, 500, and 1000 mg/mL) [57] | OVX-OP mice | Pg or Bo alone did not affect OVX-induced bone loss recovered bone weight (Pg:Bo) ↑ BMD (Pg:Bo = 3:1) ↓ OC formation (Pg:Bo = 3:1) ↓ blood glucose level (Pg:Bo = 3:1) (P.O. 500 mg/kg/day) [58] |
| GC-OP mice | ↓ bone loss (P.O. 100 mg/kg/d or 500 mg/kg/d) [57] |
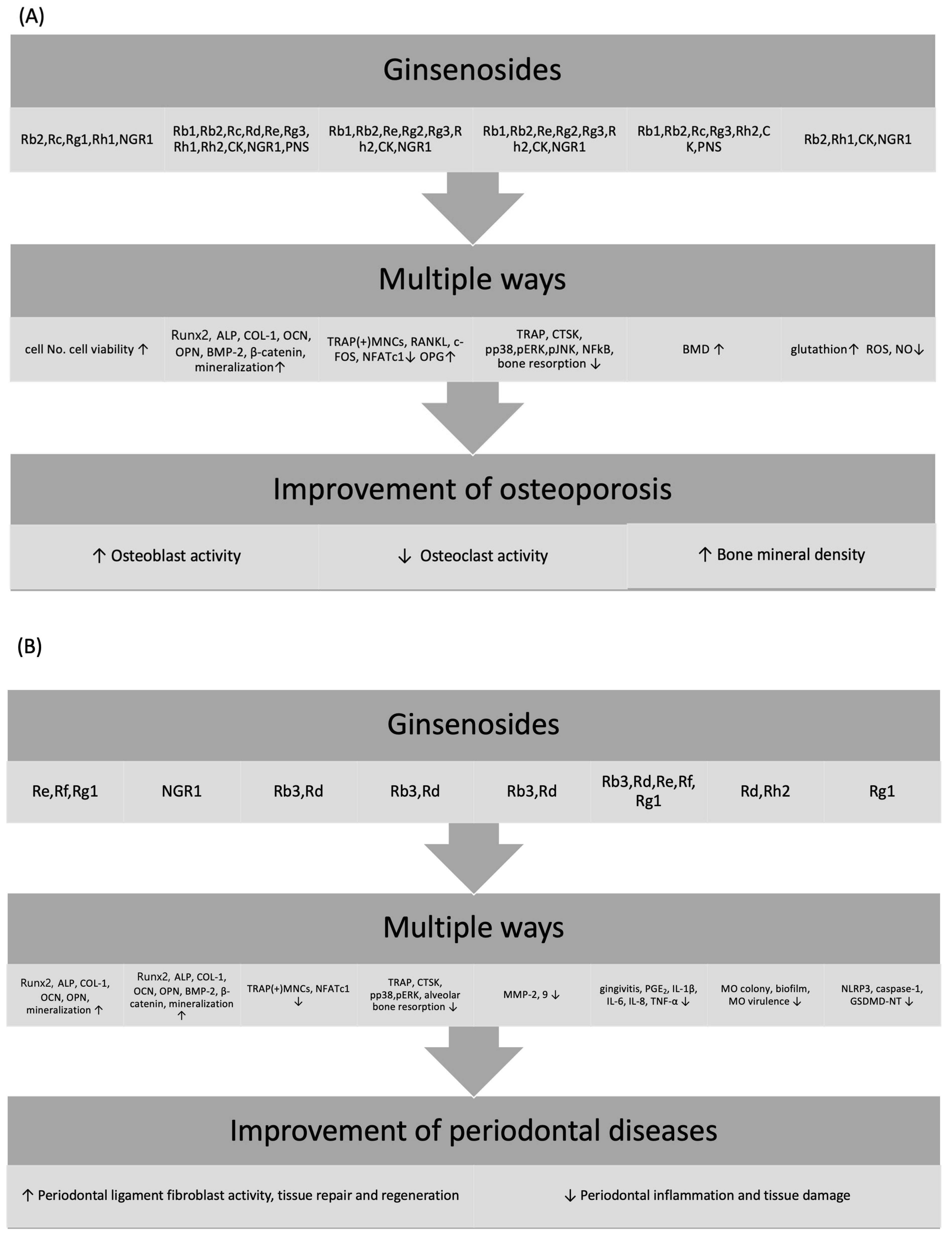
4. Effects of Ginsenosides on Periodontal Disease
| Active Compound/Extracts | Properties | In Vitro Model | Activity and Mechanism | In Vivo Model | Activity and Mechanism |
|---|---|---|---|---|---|
| G-Rb3 | anti-osteoclastogenic | RAW264.7 cells and BMMs | ↓ TRAP (+) MNC generation ↓ NFATc1, MMP-9, CTSK, and ACP mRNA ↓ MMP-9 and CTSK proteins ↓ p38, ERK, and p65 NF-κB phosphorylation (50, 100, and 150 μM) [10] | P. gingivalis -LPS-induced periodontitis in rats | ↓ p-ERK in alveolar bone surface, blood vessels, odontoblasts, and gingival epithelia ↓ gingivitis ↓ alveolar bone resorption (gingival injection, 100 μM) [10] |
| P. gingivalis -LPS-induced periodontitis in rats | ↓ alveolar bone resorption ↓ TRAP (+) MNC generation (gingival injection, 100 μM) [14] | ||||
| anti-inflammatory/antimicrobial/anti-pyroptotic | P. gingivalis-LPS-stimulated hPDLCs | ↓ IL-1β, IL-6, and IL-8 mRNA ↓ p38 and p65 NF-κB, AKT phosphorylation (25, 50, and 100 μM) [14] | |||
| G-Rd | anti-osteoclastogenic | RAW264.7 cells and BMMs | ↓ TRAP (+) MNC generation ↓ RANKL-induced ACP, NFATc1, and MMP-9 mRNA (50 and 100 μM) [72] | ligature-induced periodontitis in mouse | ↓ CEJ–ABC distances ↓ alveolar resorption (gingival injection 300 μM) [72] |
| anti-inflammatory/antimicrobial/anti-pyroptotic | hGFs via LPS stimulation | ↓ LPS-stimulated IL-1β, IL-6, and CXCL8 mRNA ↓ LPS-stimulated IL-1β, IL-6, and IL-8 secretion (100 and 200 μM) [72] | ligature-induced periodontitis in mouse | ↓ bacteria colonies (gingival injection 300 μM) [72] | |
| P. gingivalis | ↓ total biomass of bio films (100 and 200 μM) [72] | ||||
| G-Re, Ra8, Rf | osteogenic | hPDLCs | ↑ calcium deposition ↑ Runx2, ALP, and OPN mRNA (40 μM) [67] | ||
| anti-inflammatory/antimicrobial/anti-pyroptotic | P. gingivalis -LPS-stimulated hPDLCs | ↑ HO-1 protein via the nuclear translocation of Nrf2 ↑ The HO-1 protein is regulated by EGFR ↓ PGE2, NO, and IL-6, TNF-α secretion ↓ COX2 and NOS protein (5, 10, 20, and 40 μM) [67] | |||
| G-Rg1 | osteogenic | hPDLCs | ↑ cell proliferation ↑ Runx2, ALP, COL-1, OCN, and OPN mRNA ↑ calcium deposition (10 μmol/L) [68] | ||
| hDPSCs | ↑ cell proliferation ↑ DSPP, ALP, and OCN mRNA ↑ BMP-2 and FGF-2 protein (5 μmol/L) [70] | ||||
| hDPSCs | ↑ cell proliferation ↑ ALP activity ↑ calcium deposition ↑ DSPP and DMP-1 mRNA (0.5, 2.5, 5, and 10 μmol/L) [18] | ||||
| anti-inflammatory/antimicrobial/anti-pyroptotic | hPDLCs | ↑ cell viability ↓ pyroptosis ↓ lactate dehydrogenase, IL-1β, and IL-18 secretion ↓ aberrant mitochondrial fission and mtROS production ↑ ATP content and mitochondrial membrane potential level ↑ Drp1 phosphorylation ↓ NLRP3, ASC, Caspase-1, and GSDMD-NT mRNA (50, 100, and 200 μM) [69] | |||
| G-Rh2 | anti-inflammatory/antimicrobial/anti-pyroptotic | Streptococcus mutans, Streptococcus sobrinus, and Streptococcus sanguinis | ↓ biomass accumulation ↓ bacterial growth ↓ extracellular polysaccharide synthesis disrupts cell membranes ↓ acetaldehyde/alcohol dehydrogenase mRNA (6.25, 12.5, 25, 50, and100 ng μL−1) [73] | ||
| P. gingivalis | ↑ clearance of P. gingivalis [74] | ||||
| NGR1 | osteogenic | hAOBs | ↑ ALP activity ↑ Runx2, OCN, and OPN ↓ p50 and p-p65 ↓ DKK1 mRNA ↑ AXIN2 and β-catenin mRNA ↑ calcium deposition (2.5, 5, 10, 20, and 40 μmol/L) [71] | ||
| Ginseng extracts | osteogenic | hPDLCs | ↑ Runx2, ALP, COL-1, and OPN mRNA protein ↑ Calcium deposition (50, 100, 150, and 200 μg/mL) [78] | ligature-induced periodontitis in mouse P. gingivalis -LPS-induced periodontitis in rats | ligature-induced periodontitis in mouse ↑ alveolar bone volume after tooth extraction ↑ BMD of the tooth socket P. gingivalis-LPS-induced periodontitis in rats ↓ alveolar bone loss restored BMD loss ↓ inflammatory invasion of periodontal cells (gingival injection 50 mg/kg) [78] |
| hPDLCs | ↑ cell proliferation (0.25 and 2 mg/mL) [79] | P. gingivalis -LPS-induced periodontitis in rats | ↓ alveolar bone loss ↓ MMP-9 around the gingival connective tissue (gingival injection 150, 300, and 360 mg/kg) [80] | ||
| anti-osteoclastogenic | RAW264.7 cells | ↓ LPS-stimulated TRAP(+) MNC generation (0.08, 0.4, and 2 mg/mL) [79] | |||
| anti-inflammatory/antimicrobial/anti-pyroptotic | P. gingivalis -LPS-stimulated hPDLCs | ↓ TNF-α, IL-1β, and IL-6 secretion ↓ PGE2 and NO secretion ↓ NOS and COX2 protein ↑ HO-1 protein (50, 100, 150, and 200 μg/mL) [78] | |||
| hPDLCs, RAW264.7 cells | ↓ LPS-induced MMP-2 in PDLF ↓ LPS-stimulated activation of JNK and ERK in RAW264.7 cells ↓ LPS-stimulated degradation of IKB in RAW264.7 cells ↓ MMP-9 and iNOS in RAW264.7 cells ↓ NOS in RAW264.7 cells (0.08, 0.4, and 2 mg/mL) [79] | ||||
| hGFs and hPDLCs | ↓ TNF-α and IL-6 secretion (0.156, 0.312, and 0.625 mg/mL) [80] | ||||
| P. gingivalis | Symphytum officinale (S), Panax Ginseng (G), and metronidazole (F) S+F: biofilm inhibition (98.7%) G+F: biofilm inhibition (98.2%) [81] |
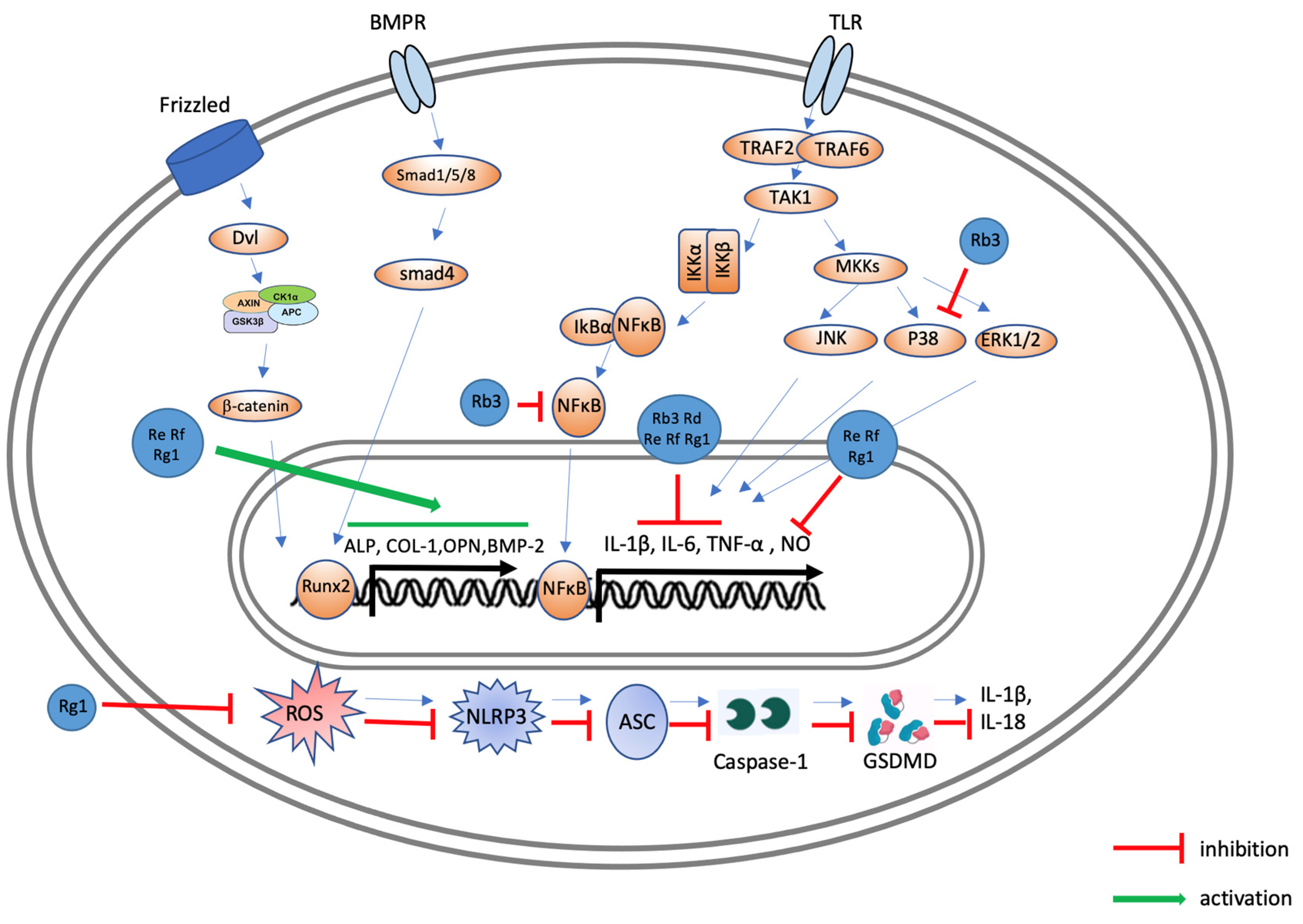
5. Effects of Ginsenosides on Osteoarthritis
| Active Compound/Extracts | Chondroprotective | Anti-Inflammatory/Anti-Pyroptotic | ||
|---|---|---|---|---|
| Experimental Model | Activity and Mechanism | Experimental Model | Activity and Mechanism | |
| G-Rb1 | chondrocytes with osteoarthritis | ↓ intracellular ROS production (30 and100 μg/kg) [11] | ||
| hollow trephine on femur trochlea-induced rabbit OA | ↓ PGE2 and MMP-3 serum level ↑ TIMP-1 mRNA ↓ MMP-13-, MMP-3, and MMP-1 mRNA ↓ p-Akt, p-P65, and p-p38 protein ↓ chondrocyte-related irregularities (implant, 30 and 100 μg/kg) [11] | |||
| MIA-induced OA | ↑ histological structure ↓ IL-1β, IL-6, and TNF-α in joint tissues ↓ miR-12-5p levels ↑ FGF-18 (gavage, 5 mg/kg) [15] | |||
| MIA-induced OA in OVX rat | ↑ BMP-2 and COL-2A mRNA ↓ MMP-13, COX2, and TGF-β mRNA ↓ pathological changes in MIA-induced OA in OVX rats ↓ cartilage and GAG degradation (intraarticularly injection, 3 and 10 μg/kg) [87] | MIA-induced OA in OVX rats | ↓ IL-1β, IL-6, MCP-1/CCL-2, COX2, and PGE2 serum level (intra-articular injection, 3 and 10 μg/kg) [87] | |
| G-Rb3 | S12 murine articular cartilage cell line | ↓ MMP-3 secretion (1, 10, and 100 μg/mL) [88] | ||
| G-Rc | chondrocyte (IL-1β-treated SW1353) | ↓ MMP-13 secretion (5, 10, 15, and 20 μM) [89] | ||
| G-Rd | S12 murine articular cartilage cell line | ↓ MMP-3 secretion (1,10,100 μg/mL) [88] | ||
| chondrocyte (IL-1β-treated SW1353) | ↓ MMP-13 secretion (5, 10, 15, and 20 μM) [89] | |||
| G-Rf | chondrocyte (IL-1β-treated SW1353) | ↓ MMP-13 secretion (5, 10, 15, and 20 μM) [89] | TNF-α-stimulated HT-29 cells, RAW264.7 cells | ↓ IL-1β, IL-6, TNF-α, NO, and ROS secretion ↓ TNF-α/LPS-induced NF-κB phosphorylation [90] |
| G-Rg1 | IL-1β-induced chondrocyte | ↓ MMP-13, COX2, and PGE2 mRNA, protein ↓ COL-2 and aggrecan degradation (0.1, 1, and 10 μg/mL) [20] | ||
| ACLT–OA rats | ↓ cartilage degeneration ↓ COL-2 loss and MMP-13 level (0.1, 1, and 10 μg/mL) [20] | |||
| G-Rg3 | chondrocyte (IL-1β-treated SW1353) | ↓ MMP-13 secretion (5, 10, 15, and 20 μM) [89] | ||
| G-Rk1 | LPS-stimulated RAW264.7 cells | ↓ NO, IL-6, IL-1β, TNF-α, and MCP-1 mRNA ↓ NF-κB and Jak2/STAT3 phosphorylation (10, 20, and 40 μmol/L) [91] | ||
| CK | H2O2-stimulated MC3T3-E1 | ↑ ALP and COL-1 activity ↑ calcium deposition ↑ ALP and COL-1 mRNA [92] | H2O2-stimulated MC3T3-E1 cells | ↓ H2O2-induced ROS and NO ↓ IKK and IL-1β [92] |
| PMCs | ↓ MMP-3 and MMP-13, ADAMTS5 secretion ↓ IL-6 secretion ↓ IL-1β protein (10, 20, and 50 μM) [93] | PMCs | ↓ NLRP3, GSDMD-NT, and caspase-1 protein (10, 20, and 50 μM) [93] | |
| immature murine articular chondrocytes (iMACs) | ↑ chondrocyte proliferation ↑ chondrocyte differentiation ↓ cellular senescence and apoptosis-related gene expression [19] | |||
| chondrocytes | ↓ MMP-3, MMP-13, ADAMTS4, and ADAMTS5 mRNA ↑ COL-2A mRNA ↓ IRE1α activation (0.3, 3, and 30 nM) [16] | chondrocytes | ↓ Caspase-1, GSDMD protein (0.3,3,30 nM) [16] | |
| destabilization of the medial meniscus (DMM) of mice | ↓ OARSI score ↑ COL-2 ↓ MMP-13 (diet supplement, 40 mg/kg) [93] | destabilization of the medial meniscus (DMM) of mice | ↓ NLRP3 and GSDMD-NT protein 13 (diet supplement, 40 mg/kg) [93] | |
| destabilization of the medial meniscus (DMM) of mice | ↑ aggrecan, COMP ↓ number of MMP-13-positive cells and TUNEL-positive cells ↓ number of pIkBα-positive cells ↓ AKT1, Annexin A2, and NFkB ↓apoptosis in osteoarthritic cartilage [19] | |||
| MIA-induced rat OA | ↓ OARSI score ↓ MMP-13, IRE1α, and TXNIP level (gavage, 20 and 80 mg/kg/200 mL saline) [16] | MIA-induced rat OA | ↓ IL-1β, IL-18, and TNF-α serum levels ↓ caspase-1 activity and NLRP3 level (gavage, 20 and 80 mg/kg/200 mL saline) [16] | |
| PNS | AIA rabbit | ↓ articular chondrocyte apoptosis ↓ lumbar vertebral and articular bone destruction ↓ arthritic muscular fiber atrophy ↓ inflammatory cell numbers ↑ bone density and microarchitecture (gavage, 75 mg/kg/day) [94] | ||
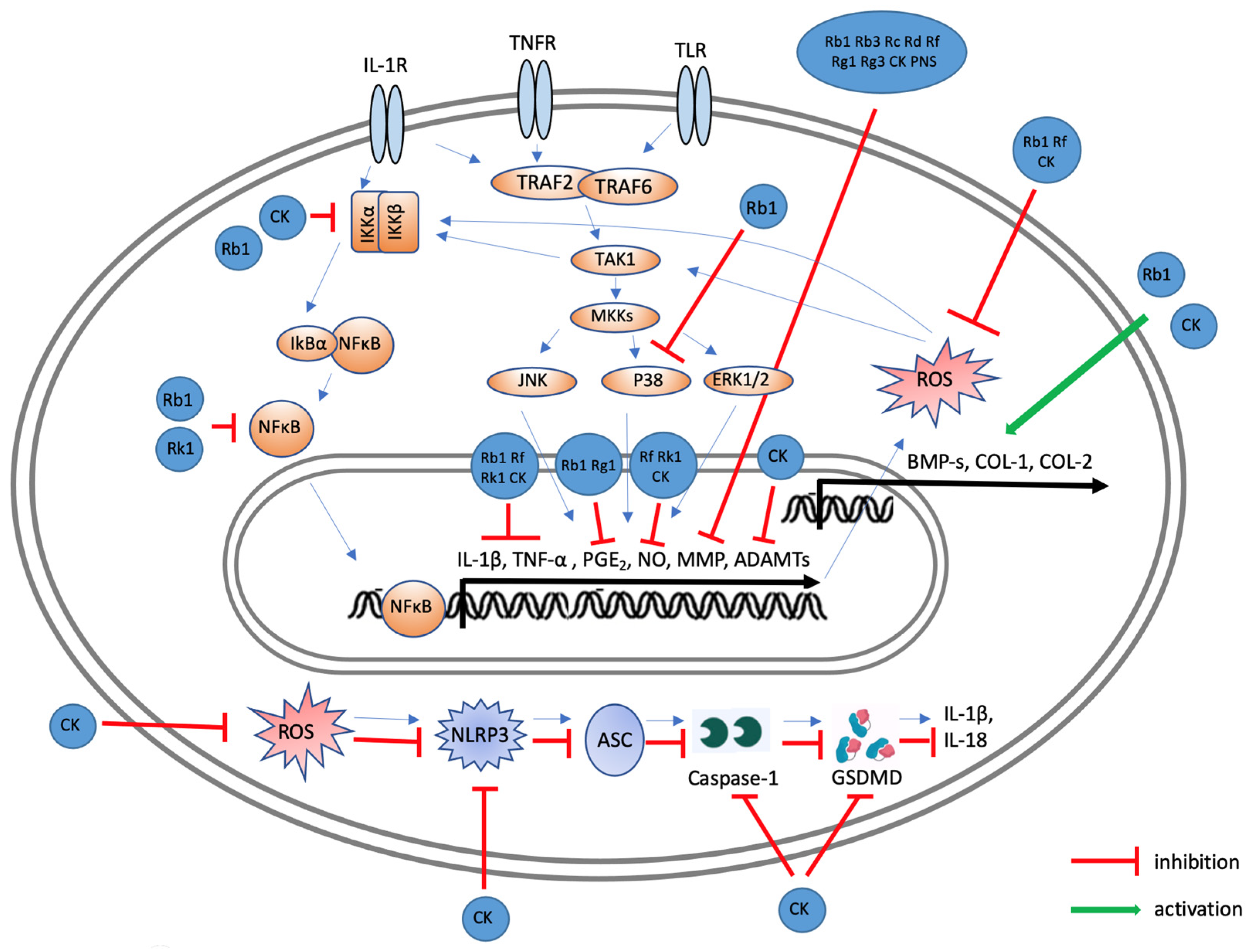
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W. Future developments in osteoporosis therapy. Endocr. Metab. Immune Disord. Drug Targets 2009, 9, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wei, C.; Zhou, L.; Qin, A.; Yang, M.; Tickner, J.; Huang, Y.; Zhao, J.; Xu, J. Luteoloside prevents lipopolysaccharide-induced osteolysis and suppresses RANKL-induced osteoclastogenesis through attenuating RANKL signaling cascades. J. Cell. Physiol. 2018, 233, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Liu, D.; Zhang, X.; Li, J.; Wang, M.; Xu, T.; Liu, Z. Effects of ginsenosides on bone remodelling for novel drug applications: A review. Chin. Med. 2020, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Pan, W.; Cao, X.; Liu, C. Potential Oral Health Benefits of Ginseng and Its Extracts. Int. Dent. J. 2023, 73, 473–480. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Liao, X. Protective effects of ginseng and ginsenosides in the development of osteoarthritis (Review). Exp. Ther. Med. 2023, 26, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jian Du, J.; Yu, M.; Suo, L. Ginsenoside Rb1 prevents osteoporosis via the AHR/PRELP/NF-κB signaling axis. Phytomedicine 2022, 104, 154205. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Li, J.; Du, J.; Lv, X.; Weng, L.; Ling, C. Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB and MAPKs pathways. Food Chem. Toxicol. 2012, 50, 1610–1615. [Google Scholar] [CrossRef]
- Sun, M.; Ji, Y.; Zhou, S.; Chen, R.; Yao, H.; Du, M. Ginsenoside Rb3 inhibits osteoclastogenesis via ERK/NF-κB signaling pathway in vitro and in vivo. Oral. Dis. 2023, 29, 3460–3471. [Google Scholar] [CrossRef]
- Hossain, M.A.; Alam, M.J.; Bumseok Kim, B.; Kang, C.W.; Kim, J.H. Ginsenoside-Rb1 prevents bone cartilage destruction through down-regulation of p-Akt, p-P38, and p-P65 signaling in rabbit. Phytomedicine 2022, 100, 154039. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Gao, B.; Jie, Q.; Wei, B.-Y.; Fan, J.; Zhang, H.-Y.; Zhang, J.-K.; Li, X.-J.; Shi, J.; Luo, Z.-J.; et al. Ginsenoside-Rb2 displays anti-osteoporosis effects through reducing oxidative damage and bone-resorbing cytokines during osteogenesis. Bone 2014, 66, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gao, Z.; Wu, S.; Chen, C.; Liu, Y.; Wang, M.; Zhang, Y.; Li, L.; Zou, H.; Zhao, G.; et al. Ginsenoside compound-K attenuates OVX-induced osteoporosis via the suppression of RANKL-induced osteoclastogenesis and oxidative stress. Nat. Prod. Bioprospect. 2023, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ji, Y.; Li, Z.; Chen, R.; Zhou, S.; Liu, C.; Du, M. Ginsenoside Rb3 Inhibits Pro-Inflammatory Cytokines via MAPK/AKT/NF-κB Pathways and Attenuates Rat Alveolar Bone Resorption in Response to Porphyromonas gingivalis LPS. Molecules 2020, 25, 4815. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Che, G.; Man, G.; Xiao, F. Ginsenoside Rb1 from Panax ginseng attenuates monoiodoacetate-induced osteoarthritis by inhibiting miR-21-5p/FGF18-mediated inflammation. J. Food Biochem. 2022, 46, e14340. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Feng, X.; Zhou, Z.; Qin, S.; Chen, S.; Zhao, J.; Hou, J.; Liu, D. Ginsenoside Compound K Ameliorates Osteoarthritis by Inhibiting the Chondrocyte Endoplasmic Reticulum Stress-Mediated IRE1α-TXNIP-NLRP3 Axis and Pyroptosis. J. Agric. Food Chem. 2023, 71, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, F.; Chen, X.; Wu, X.; Zhu, J. Ginsenoside Rg3 attenuates ovariectomy-induced osteoporosis via AMPK/mTOR signaling pathway. Drug Dev. Res. 2020, 81, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, X.; Zhou, Y.; Wang, Y.; Yang, K.; Zhang, F.; Jiang, R. Effect of ginsenoside Rg1 on proliferation and differentiation of human dental pulp cells in vitro. Aust. Dent. J. 2012, 57, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Shin, H.H.; Kim, D.H.; Ryu, J.H.; Jin, E.J. Adhesive ginsenoside compound K patches for cartilage tissue regeneration. Regen. Biomater. 2023, 10, rbad077. [Google Scholar] [CrossRef]
- Cheng, W.; Jing, J.; Wang, Z.; Wu, D.; Huang, Y. Chondroprotective Effects of Ginsenoside Rg1 in Human Osteoarthritis Chondrocytes and a Rat Model of Anterior Cruciate Ligament Transection. Nutrients 2017, 9, 263. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Lai, X.D.; Ouyang, J.; Yang, J.D. Effects of Ginsenoside Rg3 on fatigue resistance and SIRT1 in aged rats. Toxicology 2018, 1, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Petkov, W. Pharmacological studies of the drug P. ginseng C.A. Meyer. Arzneim. Forsch. 1959, 9, 305–311. [Google Scholar]
- Lee, M.S.; Hwang, J.T.; Kim, S.H.; Yoon, S.; Kim, M.S.; Yang, H.J.; Kwon, D.Y. Ginsenoside Rc, an active component of Panax ginseng, stimulates glucose uptake in C2C12 myotubes through an AMPK-dependent mechanism. J. Ethnopharmacol. 2010, 127, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Lim, H.J.; Jun, J.H.; Choi, J.; Lee, M.S. Ginseng for treating hypertension: A systematic review and meta-analysis of double blind, randomized, placebo-controlled trials. Curr. Vasc. Pharmacol. 2017, 15, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Rhee, M.H.; Lee, J.; Kim, S.H.; Yang, Y.; Kim, H.G.; Kim, Y.; Kim, C.; Kwak, Y.-S.; Kim, J.-H.; et al. Ginsenoside Rc from Korean red ginseng (Panax ginseng, C.A. Meyer) attenuates inflammatory symptoms of gastritis, hepatitis and arthritis. Am. J. Chin. Med. 2016, 44, 595–615. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Yang, Y.; Kwak, Y.S.; Song, G.G.; Kim, M.Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J. Ginseng Res. 2017, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Smith, E.; Yeo, K.; Tomita, Y.; Price, T.J.; Yool, A.; Townsend, A.R.; Hardingham, J.E. Differential antiangiogenic and anticancer activities of the active metabolites of ginsenoside Rg3. J. Ginseng Res. 2024, 48, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Ru, W.; Wang, D.; Xu, Y.; He, X.; Sun, Y.E.; Qian, L.; Zhou, X.; Qin, Y. Chemical constituents and bioactivities of Panax ginseng (CA Mey). Drug Discov. Ther. 2015, 9, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rodan, G.A. Introduction to bone biology. Bone 1992, 13 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Jilka, R.L. Bone marrow, cytokines and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N. Engl. J. Med. 1995, 332, 305–311. [Google Scholar] [CrossRef]
- Isomura, H.; Fujie, K.; Shibata, K.; Inoue, N.; Iizuka, T.; Takebe, G.; Takahashi, K.; Nishihira, J.; Izumi, H.; Sakamoto, W. Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology 2004, 197, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, P.; Di Silvio, L. Osteoblasts in bone tissue engineering. Proc. Inst. Mech. Eng. H 2010, 224, 1415–1440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, J.; Yang, Z.; Xie, C.; Huang, Y.; Ling, L.; Cao, Y.; Hu, H.; Hua, Y. The Ginsenoside Exhibits Antiosteoporosis Effects in Ketogenic-Diet-Induced Osteoporosis via Rebalancing Bone Turnover. Front. Pharmacol. 2021, 11, 593820. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.; Liu, J.; Wang, C.; Yuan, Z.; Bi, L.; Liang, J.; Su, K.; Qiu, Y.; Song, T.; Fan, J.; et al. Ginsenoside Rb2 inhibits osteoclast differentiation through nuclear factor-kappaB and signal transducer and activator of transcription protein 3 signaling pathway. Biomed. Pharmacother. 2017, 92, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, X.; Li, L.; Xu, T.; Li, M.; Zhao, Q.; Yu, J.; Wang, J.; Liu, Z. Ginsenoside Rc Promotes Bone Formation in Ovariectomy-Induced Osteoporosis In Vivo and Osteogenic Differentiation In Vitro. Int. J. Mol. Sci. 2022, 23, 6187. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, Y.G.; Quan, H.Y.; Kim, S.J.; Jung, M.S.; Chung, S.H. Ginsenoside Rd stimulates the differentiation and mineralization ofosteoblastic MC3T3-E1 cells by activating AMP-activated protein kinase via the BMP-2 signalling pathway. Fitoterapia 2012, 83, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-M.; Kim, D.H.; Han, H.-J.; Park, C.-M.; Ganipisetti, S.R.; Valan Arasu, M.; Kim, Y.O.; Park, C.G.; Kim, B.-Y.; Soung, N.-K. Ginsenoside Re Promotes Osteoblast Differentiation in Mouse Osteoblast Precursor MC3T3-E1 Cells and a Zebrafish Model. Molecules 2016, 22, 42. [Google Scholar] [CrossRef]
- Park, C.M.; Kim, H.M.; Kim, D.H.; Han, H.J.; Noh, H.; Jang, J.H.; Park, S.-H.; Chae, H.-J.; Chae, S.-W.; Ryu, E.K.; et al. Ginsenoside Re inhibits osteoclast differentiation in mouse bone marrow-derived macrophages and zebrafifish scale model. Mol. Cells. 2016, 39, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, S.Y.; Kim, J.H.; Kim, N.; Lee, J. Ginsenoside Rg2 inhibits osteoclastogenesis by downregulating the NFATc1, c-Fos, and MAPK pathways. BMB Rep. 2023, 56, 551–556. [Google Scholar] [CrossRef]
- Siddiqi, M.H.; Siddiqi, M.Z.; Kang, S.; Noh, H.Y.; Ahn, S.; Simu, S.Y.; Simu, S.Y.; Aziz, M.A.; Sathishkumar, N.; Pérez, Z.E.J.; et al. Inhibition of Osteoclast Differentiation by Ginsenoside Rg3 in RAW264.7 Cells via RANKL, JNK and p38 MAPK Pathways through a Modulation of Cathepsin K: An In Silico and In Vitro Study. Phytother. Res. 2015, 29, 1286–1294. [Google Scholar] [CrossRef]
- Siddiqi, M.Z.; Siddiqi, M.H.; Kim, Y.J.; Jin, Y.; Huq, M.A.; Yang, D.C. Effect of Fermented Red Ginseng Extract Enriched in Ginsenoside Rg3 on the Differentiation and Mineralization of Preosteoblastic MC3T3-E1 Cells. J. Med. Food 2015, 18, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, K.; Wei, B.; Liu, X.; Lei, Z.; Bai, X. Ginsenosides Rg3 attenuates glucocorticoid-induced osteoporosis through regulating BMP-2/BMPR1A/Runx2 signaling pathway. Chem. Biol. Interact. 2016, 256, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.H.; Siddiqi, M.Z.; Ahn, S.; Kim, Y.J.; Yang, D.C. Ginsenoside Rh1 induces mouse osteoblast growth and differentiation through the bone morphogenetic protein 2/runt-related gene 2 signalling pathway. J. Pharm. Pharmacol. 2014, 66, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Jung, M.S.; Park, Y.G.; Yuan, H.D.; Quan, H.Y.; Chung, S.H. Ginsenoside Rh2(S) induces the differentiation and mineralization of osteoblastic MC3T3-E1 cells through activation of PKD and p38 MAPK pathways. BMB Rep. 2011, 44, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Park, K.H.; Jung, M.S.; Huang, B.; Yuan, H.D.; Quan, H.Y.; Chung, S.H. Ginsenoside Rh2(S) induces differentiation and mineralization of MC3T3-E1 cells through activation of the PKD/AMPK signalling pathways. Int. J. Mol. Med. 2011, 28, 753–759. [Google Scholar] [PubMed]
- He, L.; Lee, J.; Jang, J.H.; Lee, S.H.; Nan, M.H.; Oh, B.C.; Lee, S.G.; Kim, H.H.; Soung, N.K.; Ahn, J.S.; et al. Ginsenoside Rh2 inhibits osteoclastogenesis through down-regulation of NF-κB, NFATc1 and c-Fos. Bone 2012, 50, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, H.; Zhu, H.; Zhou, P.; Shi, X. New metabolites from the biotransformation of ginsenoside Rb1 by Paecilomyces bainier sp.229 and activities in inducing osteogenic differentiation by Wnt/β-catenin signaling activation. J. Ginseng Res. 2018, 42, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gu, S.; Zhou, B.; Wang, M.; Zhang, Y.; Wu, S.; Cao, Z.; Xu, L. Ginsenoside Compound K Enhances Fracture Healing via Promoting Osteogenesis and Angiogenesis. Front. Pharmacol. 2022, 13, 855393. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Y.; Lan, H.; Wei, N.; Zheng, Z.; Wu, L.; Jaspers, R.T.; Wu, G.; Pathak, J.L. Notoginsenoside R1 Promotes Migration, Adhesin, Spreading, and Osteogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stromal Cells. Molecules 2022, 27, 3403. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, L.; Li, X.; Zhang, Z.; Sun, Y.; Wang, J. Notoginsenoside R1 suppresses wear particle-induced osteolysis and RANKL mediated osteoclastogenesis in vivo and in vitro. Int. Immunopharmacol. 2017, 47, 118–125. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Z.; Guo, J.; Xu, G.; Li, Y.; Xu, T.; Lv, H.; Chen, J.; Wu, G. Notoginsenoside R1 significantly promotes in vitro osteoblastogenesis. Int. J. Mol. Med. 2016, 38, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, H.; Zhang, X.; Jaspers, R.T.; Yu, Q.; Ji, Y.; Forouzanfar, T.; Wang, D.; Huang, S.; Wu, G. Notoginsenoside R1 attenuates oxidative stress-induced osteoblast dysfunction through JNK signalling pathway. J. Cell Mol. Med. 2021, 25, 11278–11289. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Cheng, Y.; Yuan, P.; Dang, X.; Guo, X.; Wang, W. Panax notoginseng stimulates alkaline phosphatase activity, collagen synthesis, and mineralization in osteoblastic MC3T3-E1 cells. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Liu, Z.-Y.; Chang, B.; Liu, D.-X.; Chen, B.; Guo, C.; Wang, Y.-G.; Xu, J.-K.; Huang, D.-Y.; Du, S.-X. Panax notoginseng saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK and P38 MAPK signaling pathways. Cell Physiol. Biochem. 2011, 28, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, Y.; Zou, Z.; Li, L.; Wei, F.; Liu, C.; Ling, Z.; Zou, X. Panax Notoginseng Saponins Prevent Bone Loss by Promoting Angiogenesis in an Osteoporotic Mouse Model. Biomed. Res. Int. 2020, 2020, 8412468. [Google Scholar] [CrossRef]
- Hou, J.; Wang, L.; Wang, C.; Ma, R.; Wang, Z.; Xiao, H.; Zeng, D.; Ling, L.; Wang, Y. Ginsenoside Rg1 Reduces Oxidative Stress Via Nrf2 Activation to Regulate Age-Related Mesenchymal Stem Cells Fate Switch Between Osteoblasts and Adipocytes. Evid. Based Complement. Alternat. Med. 2022, 2022, 1411354. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.; Kang, K.S.; Chun, K.H.; Hwang, G.S. Protective effect of Korean Red Ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. J. Ginseng Res. 2015, 39, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.S.; Agidigbi, T.S.; Kwon, Y.M.; Kim, D.-G.; Kim, R.I.; In, G.; Lee, M.-H.; Kim, C. Effect of Co-Administration of Panax ginseng and Brassica oleracea on Postmenopausal Osteoporosis in Ovariectomized Mice. Nutrients 2020, 12, 2415. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, M.Z.; Ahn, S.; Kang, S.; Kim, Y.-J.; Sathishkumar, N.; Yang, D.-U.; Yang, D.-C. Ginseng saponins and the treatment of osteoporosis: Mini literature review. J. Ginseng Res. 2013, 37, 261–268. [Google Scholar] [CrossRef]
- Papapanou, P.N. The prevalence of periodontitis in the US: Forget what you were told. J. Dent. Res. 2012, 91, 907–908. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Seymour, R.A.; Heasman, P.A. Current concepts in periodontal pathogenesis. Dent. Update 2004, 31, 570–572, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Benakanakere, M.; Kinane, D.F. Innate cellular responses to the periodontal biofilm. Front. Oral. Biol. 2012, 15, 41–55. [Google Scholar]
- Teles, R.; Teles, F.; Frias-Lopez, J.; Paster, B.; Haffajee, A. Lessons learned and unlearned in periodontal microbiology. Periodontol. 2000 2013, 62, 95–162. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.; Trombelli, L.; Heitz, F.; Needleman, I.; Moles, D. A systematic review of the effect of surgical debridement vs. non-surgical debridement for the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 92–102. [Google Scholar] [CrossRef]
- Kirkwood, K.L.; Cirelli, J.A.; Rogers, J.E.; Giannobile, W.V. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol. 2000 2007, 43, 294–315. [Google Scholar] [CrossRef]
- Paquette, D.W.; Williams, R.C. Modulation of host inflammatory mediators as a treatment strategy for periodontal diseases. Periodontol. 2000 2000, 24, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Kaygusuz, O.; Lee, H.S.; Jeong, G.S. Simultaneous quantitative analysis of ginsenosides isolated from the fruit of Panax ginseng C.A. Meyer and regulation of HO-1 expression through EGFR signaling has anti-inflammatory and osteogenic induction effects in HPDL cells. Molecules 2021, 26, 2092. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.H.; Cheng, W.X.; Qin, Z.S.; Sun, K.M.; Zhong, M.; Wang, J.K.; Gao, W.; Yu, Z. Effects of ginsenoside Rg-1 on the proliferation and osteogenic differentiation of human periodontal ligament stem cells. Chin. J. Integr. Med. 2015, 21, 676–681. [Google Scholar] [CrossRef]
- Chu, K.; Zhang, Z.; Chu, Y.; Xu, Y.; Yang, W.; Guo, L. Ginsenoside Rg1 alleviates lipopolysaccharide-induced pyroptosis in human periodontal ligament cells via inhibiting Drp1-mediated mitochondrial fission. Arch. Oral. Biol. 2023, 147, 105632. [Google Scholar] [CrossRef]
- Wang, P.; Wei, X.; Zhang, F.; Yang, K.; Qu, C.; Luo, H.; He, L. Ginsenoside Rg1 of Panax ginseng stimulates the proliferation, odontogenic/osteogenic differentiation and gene expression profiles of human dental pulp stem cells. Phytomedicine 2014, 21, 177–183. [Google Scholar] [CrossRef]
- Huang, L.; Li, Q. Notoginsenoside R1 promotes differentiation of human alveolar osteoblasts in inflammatory microenvironment through inhibiting NF-κB pathway and activating Wnt/β-catenin pathway. Mol. Med. Rep. 2020, 22, 4754–4762. [Google Scholar] [CrossRef]
- Zhou, S.; Ji, Y.; Yao, H.; Guo, H.; Zhang, Z.; Wang, Z.; Du, M. Application of Ginsenoside Rd in Periodontitis with Inhibitory Effects on Pathogenicity, Inflammation, and Bone Resorption. Front. Cell Infect. Microbiol. 2022, 12, 813953. [Google Scholar] [CrossRef]
- Cao, X.; Ye, Q.; Fan, M.; Liu, C. Antimicrobial Effects of the Ginsenoside Rh2 on Monospecies and Multispecies Cariogenic Biofilms. J. Appl. Microbiol. 2019, 126, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ji, Y.; Li, T.; Zhao, B.; Guo, H.; Wang, Z.; Yao, H.; Zhang, Z.; Liu, C.; Du, M. Anti-Porphyromonas gingivalis nanotherapy for maintaining bacterial homeostasis in periodontitis. Int. J. Antimicrob. Agents 2023, 61, 106801. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Dixit, V.M. Inflammasomes and their roles in health and disease. Annu. Rev. Cell Dev. Biol. 2012, 28, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Kim, T.Y.; Park, E.K.; Kim, J.Y.; Jeong, G.S. Panax ginseng fruit has anti-inflammatory effect and induces osteogenic differentiation by regulating Nrf2/HO-1 signaling pathway in in vitro and in vivo models of periodontitis. Antioxidants 2020, 9, 1221. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, M.E.; Ko, S.Y. n-Butanol extracts of Panax notoginseng suppress LPS-induced MMP-2 expression in periodontal ligament fibroblasts and inhibit osteoclastogenesis by suppressing MAPK in LPS-activated RAW264.7 cells. Arch. Oral. Biol. 2011, 56, 1319–1327. [Google Scholar] [CrossRef]
- Lee BALee, H.S.; Jung, Y.S.; Kim, S.W.; Lee, Y.W.; Chang, S.H.; Chung, H.-J.; Kim, O.-S.; Kim, Y.-J. The effects of a novel botanical agent on lipopolysaccharide-induced alveolar bone loss in rats. J. Periodontol. 2013, 84, 1221–1229. [Google Scholar]
- Ibrahim, S.M.; Al-Mizraqchi, A.S.; Haider, J. Metronidazole Potentiation by Panax Ginseng and Symphytum officinale: A New Strategy for P. gingivalis Infection Control. Antibiotics 2023, 12, 1288. [Google Scholar]
- Yi, Y.S. Ameliorative effects of ginseng and ginsenosides on rheumatic diseases. J. Ginseng Res. 2019, 43, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Dilley, J.E.; Bello, M.A.; Roman, N.; McKinley, T.; Sankar, U. Post-traumatic osteoarthritis: A review of pathogenic mechanisms and novel targets for mitigation. Bone Rep. 2023, 18, 101658. [Google Scholar] [CrossRef] [PubMed]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis. Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jiang, L.; Wang, Q.; Chen, H.; Xu, G. Traditional Chinese medicine for knee osteoarthritis: An overview of systematic review. PLoS ONE 2017, 12, e0189884. [Google Scholar] [CrossRef] [PubMed]
- Aravinthan, A.; Hossain, M.A.; Kim, B.; Kang, C.W.; Kim, N.S.; Hwang, K.C.; Kim, J.H. Ginsenoside Rb1 inhibits monoiodoacetate-induced osteoarthritis in postmenopausal rats through prevention of cartilage degradation. J. Ginseng Res. 2021, 45, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Park, N.; Ra, J.; Kim, Y.; Shin, M.; Hong, M.; Kim, S.-H.; Kwon, H.-J.; Hong, S.-P.; Kim, J.; et al. Panax ginseng, C.A. Meyer modulates the levels of MMP3 in S12 murine articular cartilage cell line. J. Ethnopharmacol. 2009, 124, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lim, H.; Shehzad, O.; Kim, Y.S.; Kim, H.P. Ginsenosides from Korean red ginseng inhibit matrix metalloproteinase-13 expression in articular chondrocytes and prevent cartilage degradation. Eur. J. Pharmacol. 2014, 724, 145–151. [Google Scholar] [CrossRef]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Yang, D.C. Suppression of MAPKs/NF-κB Activation Induces Intestinal Anti-Inflammatory Action of Ginsenoside Rf in HT-29 and RAW264.7 Cells. Immunol. Investig. 2016, 45, 439–449. [Google Scholar] [CrossRef]
- Yu, Q.; Zeng, K.W.; Ma, X.L.; Jiang, Y.; Tu, P.F.; Wang, X.M. Ginsenoside Rk1 suppresses pro-inflammatory responses in lipopolysaccharide-stimulated RAW264.7 cells by inhibiting the Jak2/Stat3 pathway. Chin. J. Nat. Med. 2017, 15, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Siddiqi, M.H.; Yoon, S.J.; Ahn, S.; Noh, H.-Y.; Kumar, N.S.; Kim, Y.-J.; Yang, D.-C. Therapeutic potential of compound K as an IKK inhibitor with implications for osteoarthritis prevention: An in silico and in vitro study. Vitro Cell Dev. Biol. Anim. 2016, 52, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, J.; Zhuo, N. Ginsenoside compound K alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. Exp. Ther. Med. 2023, 26, 406. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Yue, L.F.; You, F.T.; Tao, C. Panax notoginseng saponins alleviate osteoporosis and joint destruction in rabbits with antigen-induced arthritis. Exp. Ther. Med. 2021, 22, 1302. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, C.Z.; Mohammadi, S.; Sawadogo, W.R.; Ma, Q.; Yuan, C.S. Pharmacological Effects of Ginseng: Multiple Constituents and Multiple Actions on Humans. Am. J. Chin. Med. 2023, 51, 1085–1104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-Y. Therapeutic Potential of Ginsenosides on Bone Metabolism: A Review of Osteoporosis, Periodontal Disease and Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 5828. https://doi.org/10.3390/ijms25115828
Ko S-Y. Therapeutic Potential of Ginsenosides on Bone Metabolism: A Review of Osteoporosis, Periodontal Disease and Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(11):5828. https://doi.org/10.3390/ijms25115828
Chicago/Turabian StyleKo, Seon-Yle. 2024. "Therapeutic Potential of Ginsenosides on Bone Metabolism: A Review of Osteoporosis, Periodontal Disease and Osteoarthritis" International Journal of Molecular Sciences 25, no. 11: 5828. https://doi.org/10.3390/ijms25115828
APA StyleKo, S.-Y. (2024). Therapeutic Potential of Ginsenosides on Bone Metabolism: A Review of Osteoporosis, Periodontal Disease and Osteoarthritis. International Journal of Molecular Sciences, 25(11), 5828. https://doi.org/10.3390/ijms25115828







