Expediting Next-Generation Hybrid Technology in Recalcitrant Maize Inbreds through In Vivo Targeted Activity of CRISPR/Cas9
Abstract
1. Introduction
2. Results
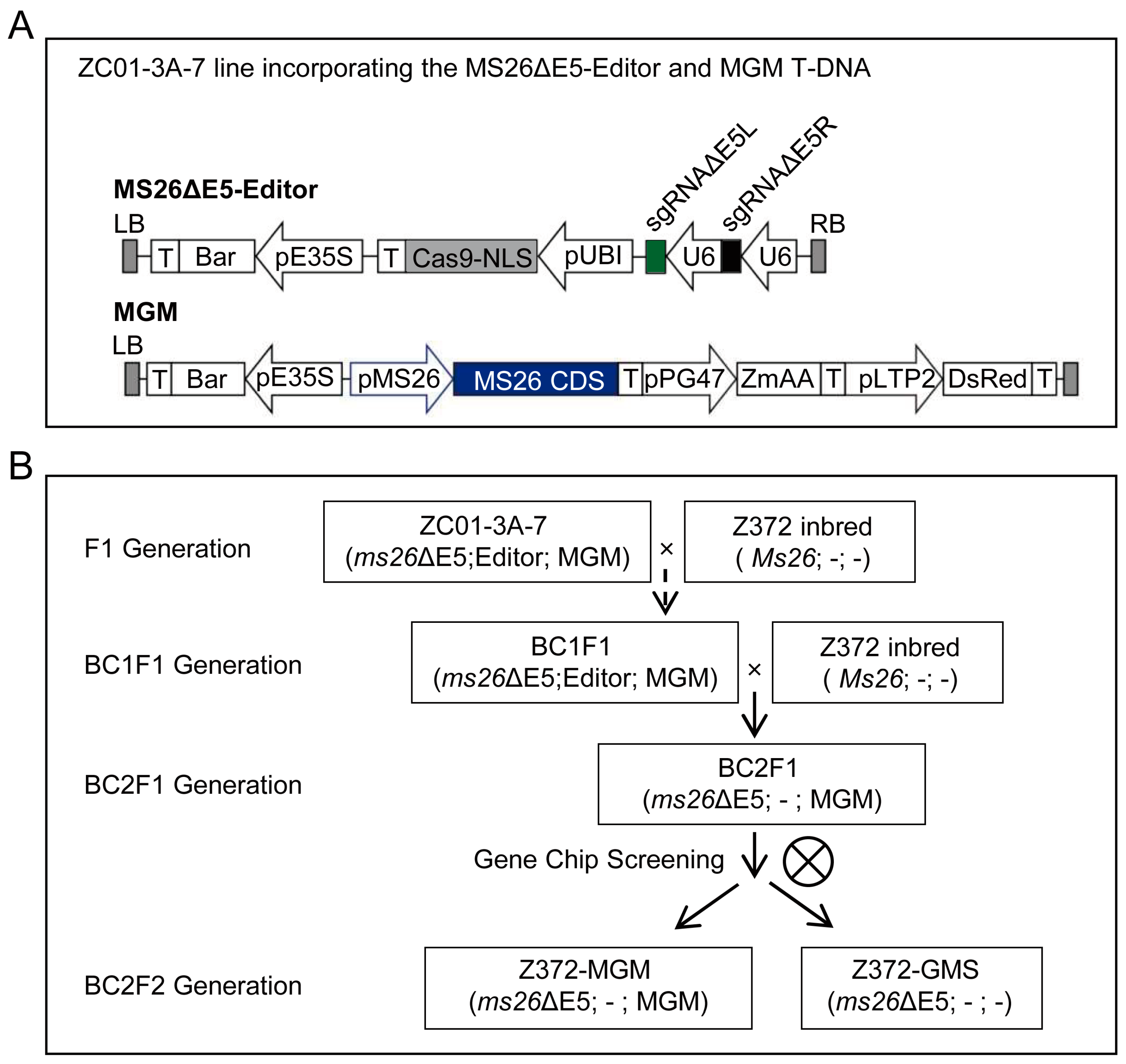
2.1. The Experimental Strategy and Rationale of the Design
2.2. Generation and Characterization of the ms26ΔE5 Mutant in Z372 Inbred Line
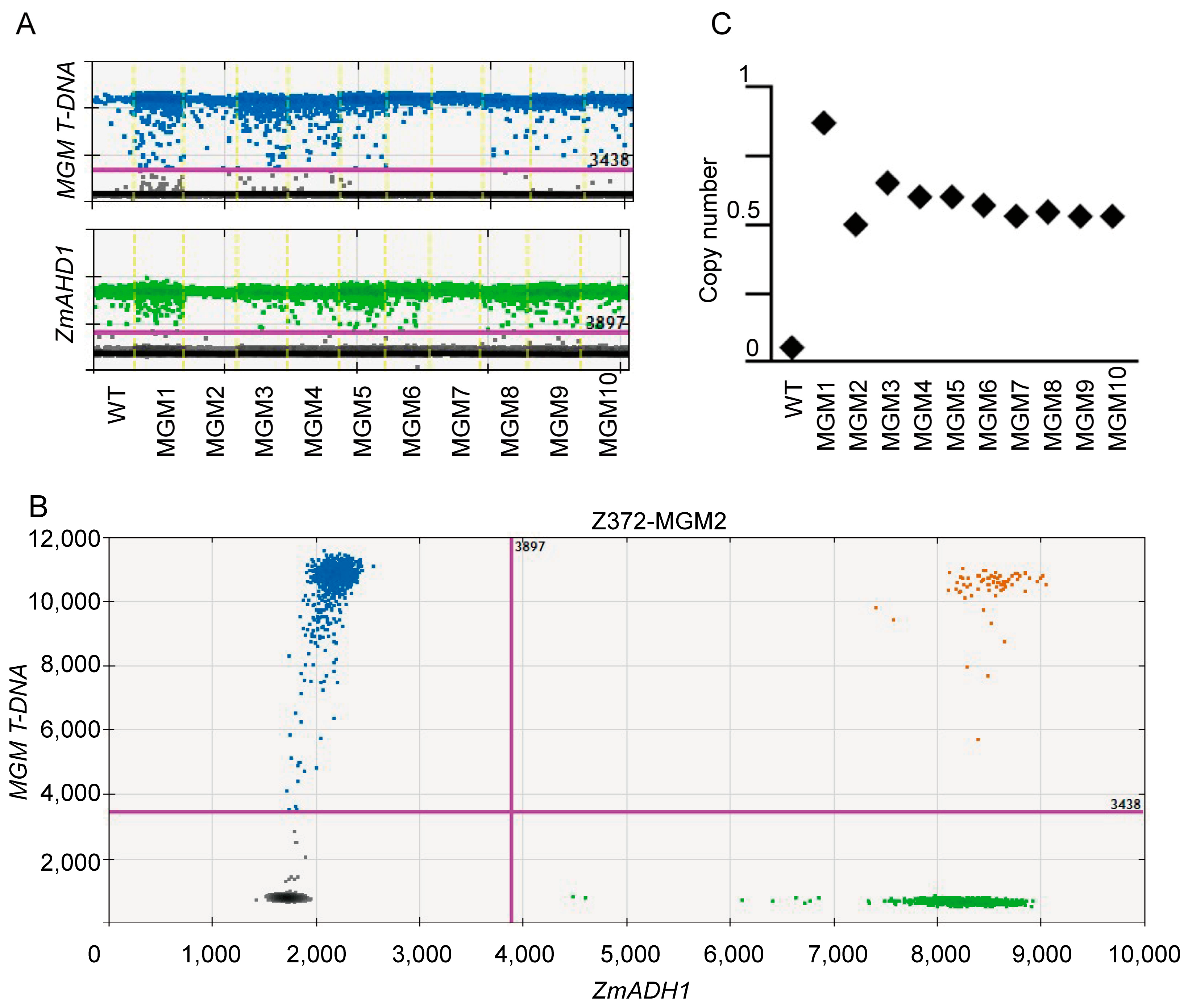
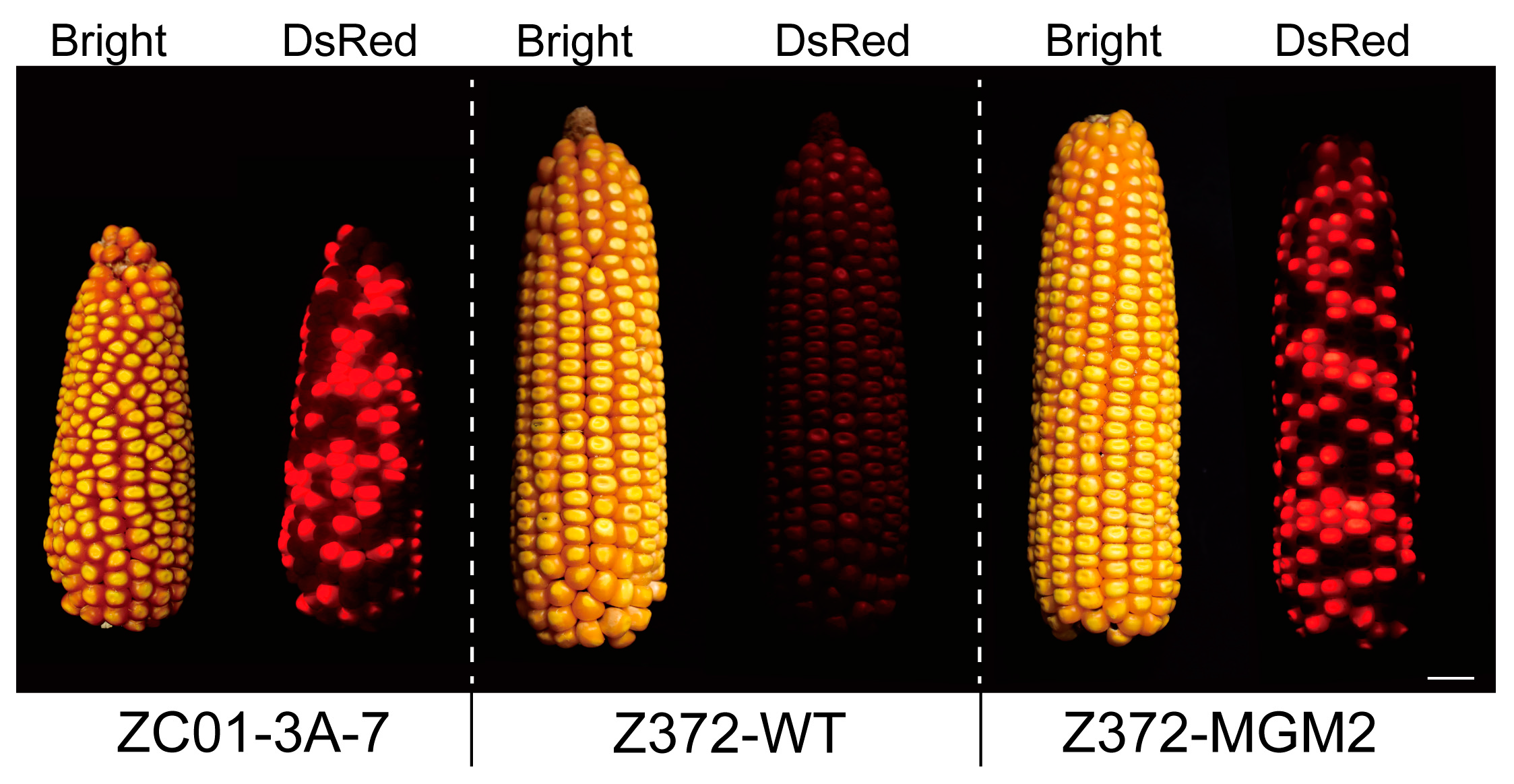
2.3. Generation and Characterization of the MGM Line in Z372
2.4. Whole-Genome SNP Analysis and Selection of Z372-GMS and Z372-MGM Plants
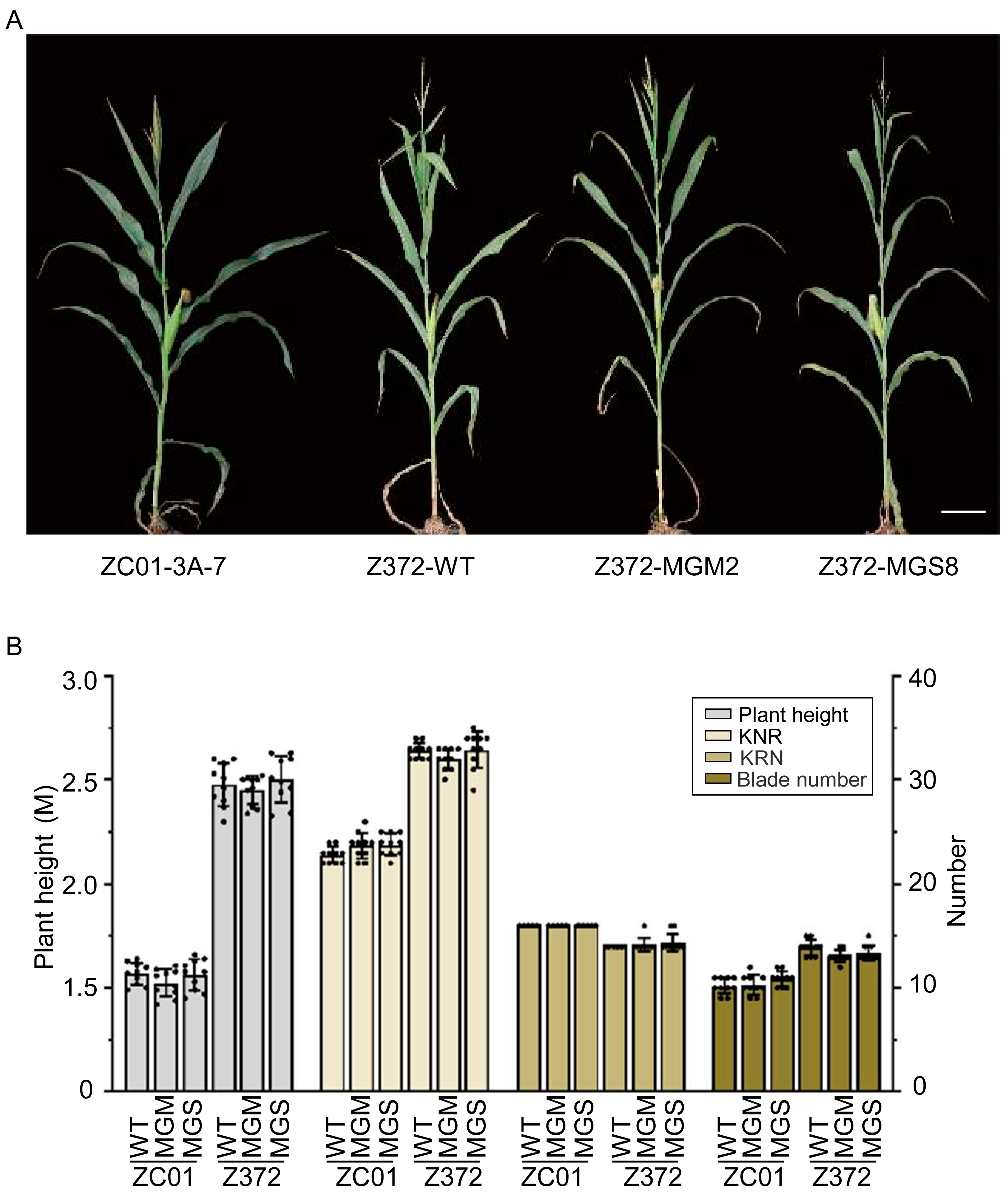
2.5. Phenotypic Evaluation and Stability Assessment of Z372-GMS
2.6. Phenotypic and Molecular Characterization of Z372-MGM
2.7. Assessment of Z372-MGM’s Ability to Propagate Z372-GMS
2.8. Agronomic Trait Assessment of Z372-GMS and Z372-MGM Plants
3. Discussion
3.1. The ms26ΔE5 Mutation in Z372 MGM Was Introduced through Genetic Editing, Rather than Resulting from Genetic Introgression
3.2. The Linked Drag Introduced by MGM Cannot Be Transmitted to Z372 GMS
4. Materials and Methods
4.1. DNA Extraction from Maize Leaf Tissue Using the CTAB Method
4.2. Detection of Genetically Modified Component
4.3. The Identification of MGM Transgenic Copy Number
4.4. Copy Number Calculating
4.5. Whole-Genome SNP Genotyping
4.6. Whole-Genome SNP Analysis and Genotype Correction Using SMOOTH Statistical Method
4.7. Pollens Staining with Iodine–Potassium Iodide (KI/I2)
4.8. Imaging and Fluorescence Observation
4.9. Chi-Square Analysis
4.10. Field Experiment and Trait Measurements
4.11. Planting and Management of Materials across Generations
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duvick, D.N. Biotechnology in the 1930s: The development of hybrid maize. Nat. Rev. Genet. 2001, 2, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Horner, H.T.; Palmer, R.G. Mechanisms of genic male sterility. Crop Sci. 1995, 35, 1527–1535. [Google Scholar] [CrossRef]
- Rogers, J.S.; Edwardson, J.R. The utilization of cytoplasmic male-sterile inbreds in the production of corn hybrids. Agron. J. 1952, 35, 8–13. [Google Scholar] [CrossRef]
- Ullstrup, A.J. The impacts of the southern corn leaf blight epidemics of 1970–1971. Annu. Rev. Phytopathol. 1972, 10, 37–50. [Google Scholar] [CrossRef]
- Williams, M.E. Genetic engineering for pollination control. Trends Biotechnol. 1995, 13, 344–349. [Google Scholar] [CrossRef]
- Wu, Y.; Fox, T.W. Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnol. J. 2016, 14, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, S. Construction of a multicontrol sterility system for a maize male-sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD-finger transcription factor. Plant Biotechnol. J. 2018, 16, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, C. RNA-guided Cas9 as an in vivo desired-target mutator in maize. Plant Biotechnol. J. 2017, 15, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Svitashev, S.; Young, J.K. Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol. 2015, 169, 931–945. [Google Scholar] [CrossRef]
- Svitashev, S.; Schwartz, C. Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Dong, L.; Qi, X. Supersweet and waxy: Meeting the diverse demands for specialty maize by genome editing. Plant Biotechnol. J. 2019, 17, 1853–1855. [Google Scholar] [CrossRef]
- Dong, L.; Li, L. Genome editing and double-fluorescence proteins enable robust maternal haploid induction and identification in maize. Mol. Plant 2018, 11, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, C. Genome editing enables next-generation hybrid seed production technology. Mol. Plant 2020, 13, 1262–1269. [Google Scholar] [CrossRef]
- Birch, R.G. Plant transformation: Problems and strategies for practical application. Annu. Rev. Plant Biol. 1997, 48, 297–326. [Google Scholar] [CrossRef]
- Qi, X.; Wu, H. Conversion of a normal maize hybrid into a waxy version using in vivo CRISPR/Cas9 targeted mutation activity. Crop J. 2020, 8, 440–448. [Google Scholar] [CrossRef]
- Doyle, J. DNA protocols for plants. Mol. Tech. Taxon. 1991, 57, 283–293. [Google Scholar]
- Hindson, B.J.; Ness, K.D. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, Q. Development of high-resolution multiple-SNP arrays for genetic analyses and molecular breeding through genotyping by target sequencing and liquid chip. Plant Commun. 2021, 2, 100230. [Google Scholar] [CrossRef]
- Van Os, H.; Stam, P. SMOOTH: A statistical method for successful removal of genoty** errors from high-density genetic linkage data. Theor. Appl. Genet. 2005, 11, 187–194. [Google Scholar] [CrossRef]
- Hunt, H.V.; Moots, H.M. Waxy phenotype evolution in the allotetraploid cereal broomcorn millet: Mutations at the GBSSI locus in their functional and phylogenetic context. Mol. Biol. Evol. 2013, 30, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Franke, T.M.; Ho, T. The chi-square test: Often used and more often misinterpreted. Am. J. Eval. 2012, 33, 448–458. [Google Scholar] [CrossRef]


| Sample Name | Sequence (5′ to 3′) 1 |
|---|---|
| Z372 | CCTCCGCGATGCAACGCATATGTGGC-//-CCGCTTAATCCACGACAAATAACG |
| ZC01-3A-7 | CCTCCGGTCCGCGA---------------------------//-------------AATCCACGACAAATAACG |
| Z372-MGM1 | CCTCCGGTCCG-GA---------------------------//--------------AATCCACGACAAATAACG |
| Z372-MGM2 | CCTCCGGTCCG-GA---------------------------//--------------AATCCACGACAAATAACG |
| Z372-MGM3 | CCTCCGGTCCGCGA--------------------------//-----------------------------GACAAATAACG |
| Z372-MGM4 | CCTCCGGTCCGCGA-------------------------//------------------------------GACAAATAACG |
| Z372-MGM5 | CCT--------------------------------------------------//-----------------------------GACAAATAACG |
| Z372-MGM6 | CCT------------------------------------------------//-------------------------------GACAAATAACG |
| Z372-MGM7 | CCT------------------------------------------------//-------------------------------GACAAATAACG |
| Z372-MGM8 | CCTCCGGTCCGCGA-----------------------//----------------------CCACGACAAATAACG |
| Z372-MGM9 | CCTCCGGTCCGCGA-----------------------//----------------------CCACGACAAATAACG |
| Z372-MGM10 | CCTCCGGTCCGCGA-----------------------//----------------------CCACGACAAATAACG |
| Z372-GMS1 | CCTCCGGTCCG-GA------------------------//-----------------AATCCACGACAAATAACG |
| Z372-GMS2 | CCTCCGGTCCG-GA------------------------//-----------------AATCCACGACAAATAACG |
| Z372-GMS3 | CCTCCGGTCCGCGA-----------------------//-------------------------------GACAAATAACG |
| Z372-GMS4 | CCTCCGGTCCGCGA------------------------//------------------------------GACAAATAACG |
| Z372-GMS5 | CCTCCGGTCCGCGA------------------------//------------------------------GACAAATAACG |
| Z372-GMS6 | CCT------------------------------------------------//-------------------------------GACAAATAACG |
| Z372-GMS7 | CCT-----------------------------------------------//--------------------------------GACAAATAACG |
| Z372-GMS8 | CCT-----------------------------------------------//--------------------------------GACAAATAACG |
| Z372-GMS9 | CCT------------------------------------------------//--------------------------------GACAAATAACG |
| Z372-GMS10 | CCT------------------------------------------------//--------------------------------GACAAATAACG |
| Sample Name | Total SNPs | Z372 SNPs (%) | ZC01 SNPs (%) | Heterozygous SNPs (%) | Background Restoration Rate (%) |
|---|---|---|---|---|---|
| Z372-MGM1 | 25,727 | 89.67 | 0.23 | 10.09 | 94.71 |
| Z372-MGM2 | 25,728 | 92.89 | 0.23 | 6.88 | 96.32 |
| Z372-MGM3 | 25,705 | 88.08 | 0.41 | 11.51 | 93.82 |
| Z372-MGM4 | 25,723 | 89.90 | 0.27 | 9.82 | 94.80 |
| Z372-MGM5 | 25,722 | 89.01 | 0.26 | 10.73 | 94.36 |
| Z372-MGM6 | 25,719 | 91.64 | 0.28 | 8.08 | 95.66 |
| Z372-MGM7 | 25,724 | 92.89 | 0.23 | 6.88 | 96.32 |
| Z372-MGM8 | 25,725 | 92.24 | 0.19 | 7.57 | 96.02 |
| Z372-MGM9 | 25,727 | 88.22 | 0.23 | 11.54 | 93.99 |
| Z372-MGM10 | 25,730 | 92.03 | 0.20 | 7.77 | 95.92 |
| Z372-GMS1 | 25,729 | 91.29 | 0.25 | 8.46 | 95.52 |
| Z372-GMS2 | 25,726 | 90.43 | 0.30 | 9.27 | 95.06 |
| Z372-GMS3 | 25,728 | 92.25 | 0.29 | 7.46 | 95.98 |
| Z372-GMS4 | 25,725 | 93.04 | 0.43 | 6.53 | 96.30 |
| Z372-GMS5 | 25,728 | 96.04 | 0.24 | 3.72 | 97.89 |
| Z372-GMS6 | 25,730 | 97.33 | 0.21 | 2.46 | 98.56 |
| Z372-GMS7 | 25,730 | 90.43 | 0.22 | 9.35 | 95.10 |
| Z372-GMS8 | 25,730 | 97.64 | 0.17 | 2.19 | 98.74 |
| Z372-GMS9 | 25,730 | 96.23 | 0.17 | 3.60 | 98.03 |
| Z372-GMS10 | 25,727 | 95.76 | 0.14 | 4.10 | 97.80 |
| Pollen | Observed | Expected | χ2 (1:1) | χ2 0.05, 1 (1:1) |
|---|---|---|---|---|
| Yellow pollens | 1480 | 1518 | 0.618 | 3.84 |
| Purple pollens | 1556 | 1518 | ||
| Total number | 3036 | 3036 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Xiao, B.; Zhu, J.; Liu, C.; Wu, Q.; Xie, C.; Zou, H.; Qi, X. Expediting Next-Generation Hybrid Technology in Recalcitrant Maize Inbreds through In Vivo Targeted Activity of CRISPR/Cas9. Int. J. Mol. Sci. 2024, 25, 5832. https://doi.org/10.3390/ijms25115832
Hou L, Xiao B, Zhu J, Liu C, Wu Q, Xie C, Zou H, Qi X. Expediting Next-Generation Hybrid Technology in Recalcitrant Maize Inbreds through In Vivo Targeted Activity of CRISPR/Cas9. International Journal of Molecular Sciences. 2024; 25(11):5832. https://doi.org/10.3390/ijms25115832
Chicago/Turabian StyleHou, Liudi, Bing Xiao, Jinjie Zhu, Changlin Liu, Qingyu Wu, Chuanxiao Xie, Huawen Zou, and Xiantao Qi. 2024. "Expediting Next-Generation Hybrid Technology in Recalcitrant Maize Inbreds through In Vivo Targeted Activity of CRISPR/Cas9" International Journal of Molecular Sciences 25, no. 11: 5832. https://doi.org/10.3390/ijms25115832
APA StyleHou, L., Xiao, B., Zhu, J., Liu, C., Wu, Q., Xie, C., Zou, H., & Qi, X. (2024). Expediting Next-Generation Hybrid Technology in Recalcitrant Maize Inbreds through In Vivo Targeted Activity of CRISPR/Cas9. International Journal of Molecular Sciences, 25(11), 5832. https://doi.org/10.3390/ijms25115832






