Effect of Cigarette Smoking on Clinical and Molecular Endpoints in COPD Patients
Abstract
1. Introduction
2. Results
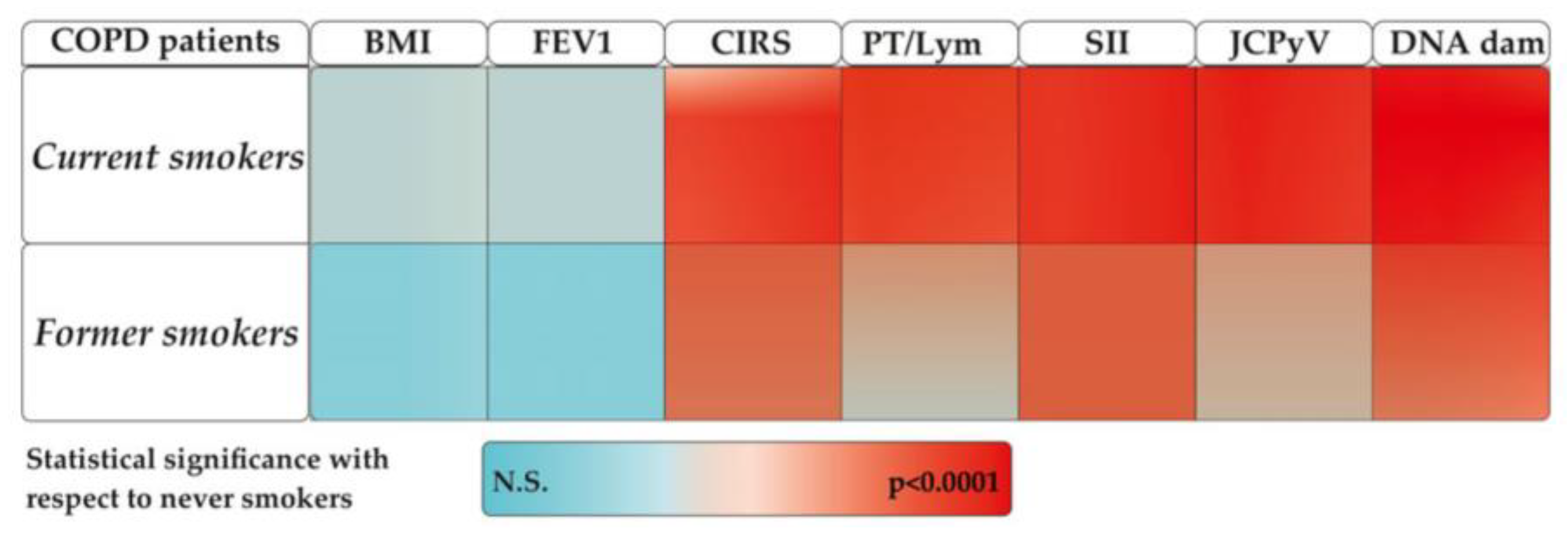
2.1. Demographic and Clinical Characteristics
2.2. Oxidative and Inflammatory Parameters
2.3. DNA Damage
2.4. Identification of Virus in COPD Patients
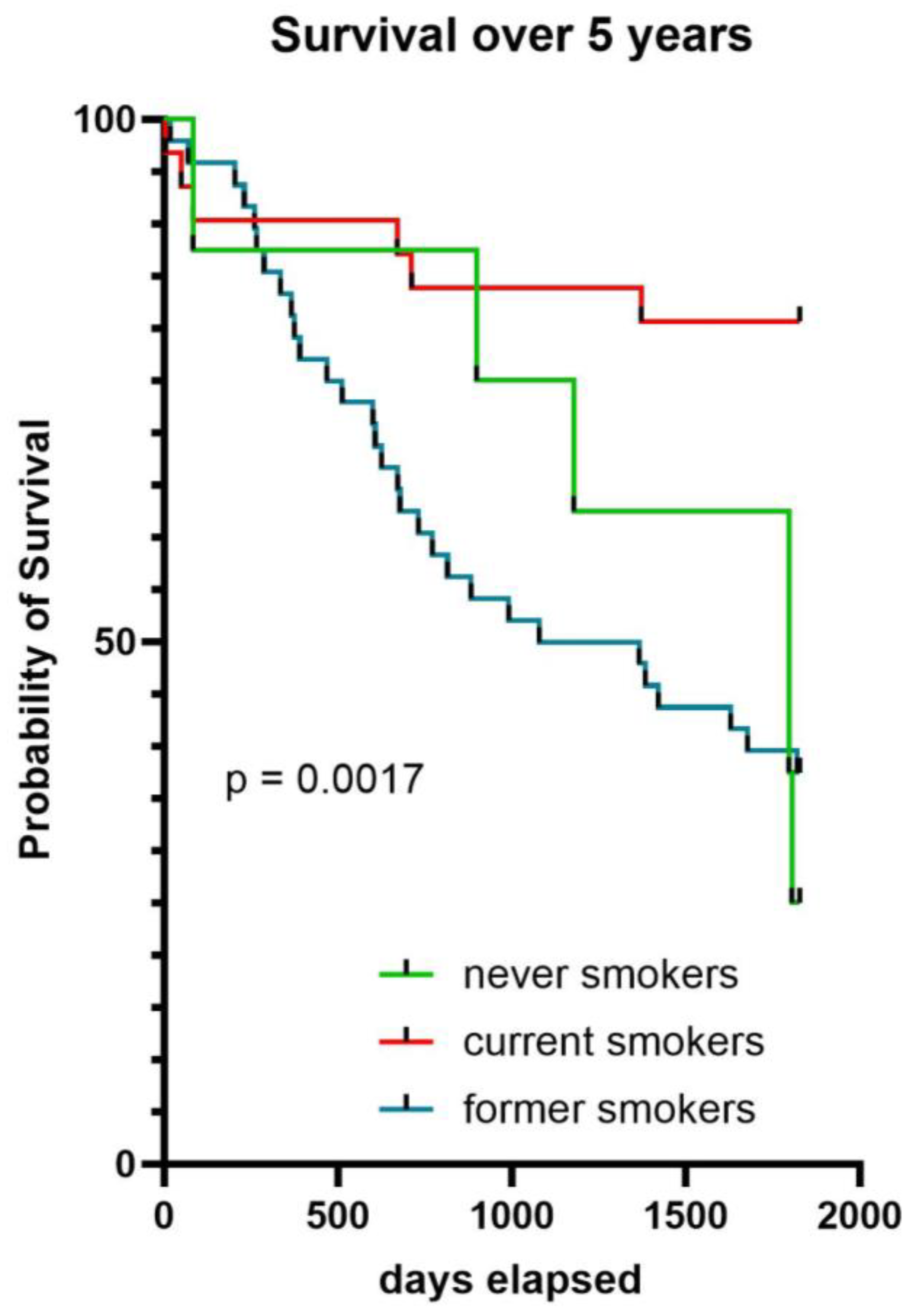
2.5. Survival
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Alkaline Comet Assay
4.3. Markers of Oxidative Stress
4.3.1. Malonaldehyde (MDA) Assay
4.3.2. 8-Hydroxy-2′-deoxyguanosine (8OHdG) Assay
4.4. Markers of Inflammation
4.5. Blood Test
4.6. Virus Detection
4.6.1. JCPyV and BKPyV
4.6.2. Influenza A Virus Subtype H1N1
4.6.3. TTV DNA Detection and Quantification
4.7. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.A.; Gallagher, K.M.; Jajosky, R.A.; Ward, J.; Sharp, P.; Anderson, W.J.; Abellera, J.P.; Aranas, A.E.; Mayes, M.; Wodajo, M.S.; et al. Summary of Notifiable Diseases—United States. 2010. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5953a1.htm (accessed on 22 March 2024).
- Hunter, D.J.; Reddy, K.S. Noncommunicable Diseases. N. Engl. J. Med. 2013, 369, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General: (590462011-001) [Dataset]; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010. [CrossRef]
- Fletcher, C.; Peto, R. The natural history of chronic airflow obstruction. BMJ 1977, 1, 1645–1648. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Anzueto, A.; Celli, B.R.; Mortimer, K.; Salvi, S.; Vogelmeier, C.F. GOLD 2023 Executive Summary: Responses from the GOLD Scientific Committee. Eur. Respir. J. 2023, 61, 2300616. [Google Scholar] [CrossRef] [PubMed]
- Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 16 April 2024).
- Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 27 February 2024).
- Agusti, A.; Melen, E.; DeMeo, D.L.; Breyer-Kohansal, R.; Faner, R. Pathogenesis of chronic obstructive pulmonary disease: Understanding the contributions of gene–environment interactions across the lifespan. Lancet Respir. Med. 2022, 10, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; Vogelmeier, C.; Faner, R. COPD 2020: Changes and challenges. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L879–L883. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.M.; Kirby, M.; Hoffman, E.A.; Kronmal, R.A.; Aaron, S.D.; Allen, N.B.; Bertoni, A.; Coxson, H.O.; Cooper, C.; Couper, D.J.; et al. Association of Dysanapsis With Chronic Obstructive Pulmonary Disease Among Older Adults. JAMA 2020, 323, 2268–2280. [Google Scholar] [CrossRef]
- Ma, Y.; Long, Y.; Chen, Y. Roles of Inflammasome in Cigarette Smoke-Related Diseases and Physiopathological Disorders: Mechanisms and Therapeutic Opportunities. Front. Immunol. 2021, 12, 720049. [Google Scholar] [CrossRef]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Møller, P. Effect of age and sex on the level of DNA strand breaks and oxidatively damaged DNA in human blood cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 838, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Kocyigit, A.; Gencer, M.; Aksoy, N.; Selek, S. Increased DNA damage in patients with chronic obstructive pulmonary disease who had once smoked or been exposed to biomass. Respir. Med. 2006, 100, 1270–1276. [Google Scholar] [CrossRef]
- Maluf, S.W.; Mergener, M.; Dalcanale, L.; Costa, C.C.; Pollo, T.; Kayser, M.; da Silva, L.B.; Pra, D.; Teixeira, P.J.Z. DNA damage in peripheral blood of patients with chronic obstructive pulmonary disease (COPD). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 626, 180–184. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Wu, T.-C.; Chen, P.-Y.; Hsieh, L.-Y.; Yeh, S.-L. Comparison of Plasma and Intake Levels of Antioxidant Nutrients in Patients with Chronic Obstructive Pulmonary Disease and Healthy People in Taiwan: A Case-control Study. Asia Pac. J. Clin. Nutr. 2010, 19, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.G.D.; Rosa, H.T.D.; Charlier, C.F.; Salvador, M.; Moura, D.J.; Valim, A.R.D.M.; Guecheva, T.N.; Henriques, J.A.P. DNA Damage and Oxidative Stress in Patients with Chronic Obstructive Pulmonary Disease. Open Biomark. J. 2013, 6, 1–8. [Google Scholar] [CrossRef]
- Oit-Wiscombe, I.; Virag, L.; Soomets, U.; Altraja, A. Increased DNA Damage in Progression of COPD: A Response By Poly(ADP-Ribose) Polymerase-1. PLoS ONE 2013, 8, e70333. [Google Scholar] [CrossRef]
- Gallus, S.; Scala, M.; Possenti, I.; Jarach, C.M.; Clancy, L.; Fernandez, E.; Gorini, G.; Carreras, G.; Malevolti, M.C.; Commar, A.; et al. The role of smoking in COVID-19 progression: A comprehensive meta-analysis. Eur. Respir. Rev. 2023, 32, 220191. [Google Scholar] [CrossRef] [PubMed]
- Arcavi, L.; Benowitz, N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004, 164, 2206–2216. [Google Scholar] [CrossRef]
- Corre, F.; Lellouch, J.; Schwartz, D. Smoking and leucocyte-counts: Results of an epidemiological survey. Lancet 1971, 298, 632–634. [Google Scholar] [CrossRef]
- Saetta, M.; Di Stefano, A.; Turato, G.; Facchini, F.M.; Corbino, L.; Mapp, C.E.; Maestrelli, P.; Ciaccia, A.; Fabbri, L.M. CD8+ T-Lymphocytes in Peripheral Airways of Smokers with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 822–826. [Google Scholar] [CrossRef]
- Matsas, S.; Aguiar, P.N., Jr.; Del Giglio, A. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as biomarkers to prognosticate survival in advanced gastric cancer patients in the era of immunotherapy: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2024, 15, 33–51. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, T.C.; Ansari, T.W.; Barnes, N.C.; Jeffery, P.K. Inflammation in bronchial biopsies of subjects with chronic bronchitis: Inverse relationship of CD8+ T lymphocytes with FEV1. Am. J. Respir. Crit. Care Med. 1997, 155, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Zwaans, W.A.R.; Mallia, P.; van Winden, M.E.C.; Rohde, G.G.U. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—A systematic review. J. Clin. Virol. 2014, 61, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; Donaldson, G.C.; Bhowmik, A.; Jeffries, D.J.; Wedzicha, J.A. Time Course and Recovery of Exacerbations in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2000, 161, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Bellettato, C.M.; Braccioni, F.; Romagnoli, M.; Casolari, P.; Caramori, G.; Fabbri, L.M.; Johnston, S.L. Infections and Airway Inflammation in Chronic Obstructive Pulmonary Disease Severe Exacerbations. Am. J. Respir. Crit. Care Med. 2006, 173, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.; Harper-Owen, R.; Bhowmik, A.; Moric, I.; Sanderson, G.; Message, S.; Maccallum, P.; Meade, T.W.; Jeffries, D.J.; Johnston, S.L.; et al. Respiratory Viruses, Symptoms, and Inflammatory Markers in Acute Exacerbations and Stable Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1618–1623. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Dobson, S. BK and JC virus: A review. J. Infect. 2014, 68, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Giacconi, R.; Maggi, F.; Macera, L.; Pistello, M.; Provinciali, M.; Giannecchini, S.; Martelli, F.; Spezia, P.G.; Mariani, E.; Galeazzi, R.; et al. Torquetenovirus (TTV) load is associated with mortality in Italian elderly subjects. Exp. Gerontol. 2018, 112, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mili, M.; Ceppi, M.; Bruzzone, M.; Azqueta, A.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.; Møller, P.; Teixeira, J.P.; et al. The hCOMET project: International database comparison of results with the comet assay in human biomonitoring. Baseline frequency of DNA damage and effect of main confounders. Mutat. Res. Rev. Mol. Mech. Mutagen. 2021, 787, 108371. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Pirera, E.; Pintus, C.; De Rosa, R.; Profita, M.; Musiari, G.; Siscaro, G.; Tuttolomondo, A. The Role of the Cumulative Illness Rating Scale (CIRS) in Estimating the Impact of Comorbidities on Chronic Obstructive Pulmonary Disease (COPD) Outcomes: A Pilot Study of the MACH (Multidimensional Approach for COPD and High Complexity) Study. J. Pers. Med. 2023, 13, 1674. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Celli, B.R.; Agustí, A.; Criner, G.J.; Dransfield, M.T.; Divo, M.; Krishnan, J.K.; Lahousse, L.; Montes De Oca, M.; Salvi, S.S.; et al. COPD and multimorbidity: Recognising and addressing a syndemic occurrence. Lancet Respir. Med. 2023, 11, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Fogarty, C.; Kelsen, S.; Long, W.; Ramsdell, J.; Allison, J.; Mahler, D.; Saadeh, C.; Siler, T.; Snell, P.; et al. The Safety and Efficacy of Infliximab in Moderate to Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Mahler, D.A.; Huang, S.; Tabrizi, M.; Bell, G.M. Efficacy and Safety of a Monoclonal Antibody Recognizing Interleukin-8 in COPD. Chest 2004, 126, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Martinez, F.; Yates, J.; Newby, D.E. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet 2016, 387, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Satici, M.O.; Eroglu, S.E. Unraveling the clinical significance and prognostic value of the neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index, systemic inflammation response index, and delta neutrophil index: An extensive literature review. Turk. J. Emerg. Med. 2024, 24, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Mokry, J.; Barosova, R.; Hanusrichterova, J. Advances in the Use of N-Acetylcysteine in Chronic Respiratory Diseases. Antioxidants 2023, 12, 1713. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Kuo, S.; Lin, L.; Yang, Y. The efficacy of N -acetylcysteine in chronic obstructive pulmonary disease patients: A meta-analysis. Ther. Adv. Respir. Dis. 2023, 17, 175346662311585. [Google Scholar] [CrossRef] [PubMed]
- Kapustina, V.; Ovcharenko, S.; Litvicki, P. High doses of N-acetylcysteine alone or in combination with inhaled corticosteroids and oxidative stress in patients with COPD. Eur. Respir. J. 2011, 38 (Suppl. S55), 3911. Available online: https://erj.ersjournals.com/content/38/Suppl_55/p3911 (accessed on 12 February 2024).
- Henn, S.A.; Succop, P.; Talaska, G.; Anderson, K.; Hecht, S.S.; Gross, M. Carcinogen-DNA adducts are increased in the exfoliated urothelial cells of wives of smokers: Biological monitoring of passive smoke exposure. Polycycl. Aromat. Compd. 2004, 24, 475–485. [Google Scholar] [CrossRef]
- Bonassi, S.; Neri, M.; Lando, C.; Ceppi, M.; Lin, Y.; Chang, W.P.; Holland, N.; Kirsch-Volders, M.; Zeiger, E.; Fenech, M. Effect of smoking habit on the frequency of micronuclei in human lymphocytes: Results from the Human MicroNucleus project. Mutat. Res. Rev. Mutat. Res. 2003, 543, 155–166. [Google Scholar] [CrossRef]
- Hoffmann, H.; Högel, J.; Speit, G. The effect of smoking on DNA effects in the comet assay: A meta-analysis. Mutagenesis 2005, 20, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Yu, T.; Phillips, B.; Schmezer, P. The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1994, 307, 261–271. [Google Scholar] [CrossRef]
- Ellahueñe, M.F.; Pérez-Alzola, L.P.; Farfán-Urzua, M.; González-Hormazabal, P.; Garay, M.; Olmedo, M.I.; Last, J.A. Preliminary Evaluation of DNA Damage Related with the Smoking Habit Measured by the Comet Assay in Whole Blood Cells. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1223–1229. [Google Scholar] [CrossRef]
- Şardaş, S.; Aygün, N.; Karakaya, A.E. Genotoxicity studies on professional hair colorists exposed to oxidation hair dyes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1997, 394, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Maluf, S.W.; Erdtmann, B. Follow-up study of the genetic damage in lymphocytes of pharmacists and nurses handling antineoplastic drugs evaluated by cytokinesis-block micronuclei analysis and single cell gel electrophoresis assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 471, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Lamonaca, P.; Milic, M.; Rojas, E.; Prinzi, G.; Cardaci, V.; Vitiello, L.; Proietti, S.; Santoro, A.; Tomino, C.; et al. Biomarkers of DNA damage in COPD patients undergoing pulmonary rehabilitation: Integrating clinical parameters with genomic profiling. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019, 843, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Doorenbos, C.S.E.; Jonker, J.; Hao, J.; Gore, E.J.; Kremer, D.; Knobbe, T.J.; De Joode, A.A.E.; Sanders, J.S.F.; Thaunat, O.; Niesters, H.G.M.; et al. Smoking, Alcohol Intake and Torque Teno Virus in Stable Kidney Transplant Recipients. Viruses 2023, 15, 2387. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.O.; Lange, P.; Hilberg, O.; Farver-Vestergaard, I.; Ibsen, R.; Løkke, A. COPD and Smoking Status—It Does Matter: Characteristics and Prognosis of COPD According to Smoking Status. Chronic Obstr. Pulm. Dis. J. COPD Found. 2024, 11, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Saint-AndrÉ, V.; Charbit, B.; Biton, A.; Rouilly, V.; Possémé, C.; Bertrand, A.; Rotival, M.; Bergstedt, J.; Patin, E.; Albert, M.L.; et al. Smoking changes adaptive immunity with persistent effects. Nature 2024, 626, 827–835. [Google Scholar] [CrossRef]
- Papi, A.; Faner, R.; Pavord, I.; Baraldi, F.; McDonald, V.M.; Thomas, M.; Miravitlles, M.; Roche, N.; Agustí, A. From treatable traits to GETomics in airway disease: Moving towards clinical practice. Eur. Respir. Rev. 2024, 33, 230143. [Google Scholar] [CrossRef]
- Melén, E.; Faner, R.; Allinson, J.P.; Bui, D.; Bush, A.; Custovic, A.; Garcia-Aymerich, J.; Guerra, S.; Breyer-Kohansal, R.; Hallberg, J.; et al. Lung-function trajectories: Relevance and implementation in clinical practice. Lancet 2024, 403, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, A.; Li, Y. Hypoxia-inducible factor 1-alpha is a driving mechanism linking chronic obstructive pulmonary disease to lung cancer. Front. Oncol. 2022, 12, 984525. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Giunta, S.; Xia, S. Hypoxia in Aging and Aging-Related Diseases: Mechanism and Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 8165. [Google Scholar] [CrossRef] [PubMed]
- Asanov, M.; Bonassi, S.; Proietti, S.; Minina, V.I.; Tomino, C.; El-Zein, R. Genomic instability in chronic obstructive pulmonary disease and lung cancer: A systematic review and meta-analysis of studies using the micronucleus assay. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108344. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ec.europa.eu/eurostat/documents/203647/203710/EHIS_wave_1_guidelines.pdf/ffbeb62c-8f64-4151-938c-9ef171d148e0 (accessed on 5 March 2024).
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Instant Cotinine Testing Kit|Williams Medical. Available online: https://www.wms.co.uk/Diagnostics/Drug-and-Alcohol/Cotinine-Testing-Casette/p/D1069 (accessed on 16 April 2024).
- Ilari, S.; Vitiello, L.; Russo, P.; Proietti, S.; Milić, M.; Muscoli, C.; Cardaci, V.; Tomino, C.; Bonassi, G.; Bonassi, S. Daily Vegetables Intake and Response to COPD Rehabilitation. The Role of Oxidative Stress, Inflammation and DNA Damage. Nutrients 2021, 13, 2787. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Ceppi, M.; Møller, P.; Azqueta, A.; Milić, M.; Neri, M.; Brunborg, G.; Godschalk, R.; Koppen, G.; Langie, S.A.S.; et al. DNA damage in circulating leukocytes measured with the comet assay may predict the risk of death. Sci. Rep. 2021, 11, 16793. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Ai, X.; Liao, Z.; You, C.; Cheng, Y. The prognostic values of neutrophil to lymphocyte ratio for outcomes in chronic obstructive pulmonary disease. Medicine 2019, 98, e16371. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, H.; Chen, L.; Ma, X.; Li, X.; Gao, Y.; Zhang, Y.; Xie, Y.; Zhang, X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 2016, 7, 31926–31942. [Google Scholar] [CrossRef]
- Zhou, X.; Du, Y.; Huang, Z.; Xu, J.; Qiu, T.; Wang, J.; Wang, T.; Zhu, W.; Liu, P. Prognostic Value of PLR in Various Cancers: A Meta-Analysis. PLoS ONE 2014, 9, e101119. [Google Scholar] [CrossRef]
- Takahashia, M.; Asabea, S.; Okamoto, H.; Gotandab, Y.; Kishimotoc, J.; Tsudad, F. TT virus is distributed in various leukocyte subpopulations at distinct levels, with the highest viral load in granulocytes. Biochem. Biophys. Res. Commun. 2002, 290, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.J.; Brar, A.K.; Herter, B.N.; Rankin, A.; Wylie, K.M. The plasma virome in longitudinal samples from pregnant patients. Front. Cell. Infect. Microbiol. 2023, 13, 1061230. [Google Scholar] [CrossRef]
- Goel, N.; Singh, B.; Arora, N.; Kumar, R. Effect of smoking on atopic predisposition and sensitization to allergens. Indian J. Chest Dis. 2008, 50, 329–333. [Google Scholar]
- Misson, P.; Van Den Brûle, S.; Barbarin, V.; Lison, D.; Huaux, F. Markers of macrophage differentiation in experimental silicosis. J. Leukoc. Biol. 2004, 76, 926–932. [Google Scholar] [CrossRef] [PubMed]

| Variables | Total n = 87 | Never- Smokers n = 10 (11.5%) | Current Smokers n = 32 (36.78%) | Former Smokers n = 45 (51.72%) | p-Value |
|---|---|---|---|---|---|
| Sex | NS | ||||
| Male | 40 (44.90) | 5 (50) | 13 (40.62) | 23 (51.11) | |
| Female | 47 (55.10) | 5 (50) | 19 (59.37) | 22 (48.88) | |
| Age (years) | 72.38 ± 8.74 | 75.50 ± 8.78 | 71.86 ± 9.15 | 71.82 ± 8.36 | NS |
| Education | NS | ||||
| ≤8 years | 52 (59.77) | 7 (70.0) | 10 (31.25) | 30 (66.66) | |
| >9 years | 29 (33.30) | -- | 5 (15.62) | 15 (33.34) | |
| BMI | 27.33 ± 7.33 | 25.17 ± 6.59 | 26.55 ± 4.65 | 28.77 ± 9.32 | NS |
| Clinical Features | |||||
| Six minutes walking test (6 MWT, meter) | 87.47 ± 87.46 | 82.71 ± 85.60 | 63.75 ± 74.25 | 101.00 ± 92.75 | NS |
| Barthel Index | 70.34 ± 21.77 | 64.63 ± 23.05 | 68.46 ± 24.45 | 72.23 ± 20.36 | NS |
| Borg Scale Dyspnea | 7.87 ± 0.92 | 8.25 ± 1.03 | 7.87 ± 0.95 | 7.81 ± 0.89 | NS |
| Forced Expiratory Volume in 1 s (FEV) | 48.57 ± 24.87 | 42.23 ± 26.12 | 51.69 ± 23.85 | 48.58 ± 25.75 | NS |
| St. George’s Respiratory Questionnaire (SGRQ) | 48.68 ± 15.90 | 57.80 ± 20.02 | 49.30 ± 15.09 | 47.36 ± 15.82 | NS |
| Maugeri Respiratory Failure (MRF-26) | 72.70 ± 14.65 | 72.21 ± 9.82 | 75.71 ± 15.13 | 71.15 ± 15.12 | NS |
| CIRS comorbidity | 2.86 ± 1.35 | 1.71 ± 0.76 | 3.58 ± 1.02 | 2.65 ± 1.39 | 0.0009 |
| Type 2 Diabetes (N.%) | 28 (32.18) | 3 (30.00) | 7 (21.90) | 18 (40.00) | NS |
| Cardiovascular diseases (N. %) | 38 (46.34) | 4 (25.00) | 16 (45.00) | 18 (40.00) | NS |
| Oxygen Supplementation | NS | ||||
| Yes | 29 (33.30) | 4 (40) | 10 (31.25) | 15 (33.30) | |
| No | 58 (66.70) | 6 (60) | 22 (68.75) | 30 (66.70) | |
| Inflammatory Parameters | |||||
| Lymphocytes/Monocytes | 2.77 ± 198 | 3.28 ± 1.20 | 2.55 ± 2.19 | 2.79 ± 2.03 | NS |
| Neutrophils/Lymphocytes | 5.24 ± 3.43 | 4.04 ± 2.05 | 5.95 ± 4.05 | 5.10 ± 3.27 | NS |
| Platelets/Lymphocytes | 203.40 ± 152.9 | 132.20 ± 58.21 | 289.60 ± 200.2 | 166.10 ± 106.5 | 0.0009 |
| Systemic Immune-inflammation Index (SII) | 1511 ± 849.80 | 549.30 ± 196.70 | 1678 ± 816.40 | 1350 ± 1133 | 0.0258 |
| PCR | 5.89 ± 16.19 | 0.67 ± 0.06 | 0.77 ± 0.87 | 9.83 ± 21.00 | NS |
| Oxidative parameters | |||||
| MDA (µM) | 42.44 ± 13.05 | 39.08 ± 11.32 | 42.39 ± 14.00 | 42.96 ± 13.30 | NS |
| 8-Oxo-dG (pg/mL) | 25.33 ± 11.15 | 20.69 ± 4.07 | 28.16 ± 15.69 | 25.18 ± 10.35 | NS |
| IL6 (pg/mL) | 72.94 ± 113.7 | 67.86 ± 78.76 | 53.57 ± 136.00 | 82.70 ± 107.00 | NS |
| DNA damage (% of Tail intensity) | |||||
| At admission | 19.47 ± 7.37 | 13.50 ± 3.07 | 25.14 ± 7.31 | 16.31 ± 4.48 | <0.001 |
| After 3 weeks | 21.79 ± 7.26 | 15.02 ± 3.55 | 25.84 ± 5.30 | 20.35 ± 7.57 | <0.001 |
| COPD patients positive for viruses | |||||
| H1N1 | 1 (1.10%) | -- | 1 (2.80%) | -- | |
| JCPyV | 41/79 (51.90%) | 8/11 (72.70%) | 21/32 (65.60%) | 12/36 (33.30%) | 0.010 |
| BKPyV | 27/78 (34.60%) | 5/11 (45.50%) | 7/32 (21.90%) | 15/35 (42.90%) | NS |
| TTV | 51/87 (58.6%) | 6/8 (75.0%) | 21/33 (63.3%) | 24/48 (50.06%) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, P.; Milani, F.; De Iure, A.; Proietti, S.; Limongi, D.; Prezioso, C.; Checconi, P.; Zagà, V.; Novazzi, F.; Maggi, F.; et al. Effect of Cigarette Smoking on Clinical and Molecular Endpoints in COPD Patients. Int. J. Mol. Sci. 2024, 25, 5834. https://doi.org/10.3390/ijms25115834
Russo P, Milani F, De Iure A, Proietti S, Limongi D, Prezioso C, Checconi P, Zagà V, Novazzi F, Maggi F, et al. Effect of Cigarette Smoking on Clinical and Molecular Endpoints in COPD Patients. International Journal of Molecular Sciences. 2024; 25(11):5834. https://doi.org/10.3390/ijms25115834
Chicago/Turabian StyleRusso, Patrizia, Francesca Milani, Antonio De Iure, Stefania Proietti, Dolores Limongi, Carla Prezioso, Paola Checconi, Vincenzo Zagà, Federica Novazzi, Fabrizio Maggi, and et al. 2024. "Effect of Cigarette Smoking on Clinical and Molecular Endpoints in COPD Patients" International Journal of Molecular Sciences 25, no. 11: 5834. https://doi.org/10.3390/ijms25115834
APA StyleRusso, P., Milani, F., De Iure, A., Proietti, S., Limongi, D., Prezioso, C., Checconi, P., Zagà, V., Novazzi, F., Maggi, F., Antonelli, G., & Bonassi, S. (2024). Effect of Cigarette Smoking on Clinical and Molecular Endpoints in COPD Patients. International Journal of Molecular Sciences, 25(11), 5834. https://doi.org/10.3390/ijms25115834









