Free Fatty Acid 4 Receptor Activation Attenuates Collagen-Induced Arthritis by Rebalancing Th1/Th17 and Treg Cells
Abstract
1. Introduction
2. Result
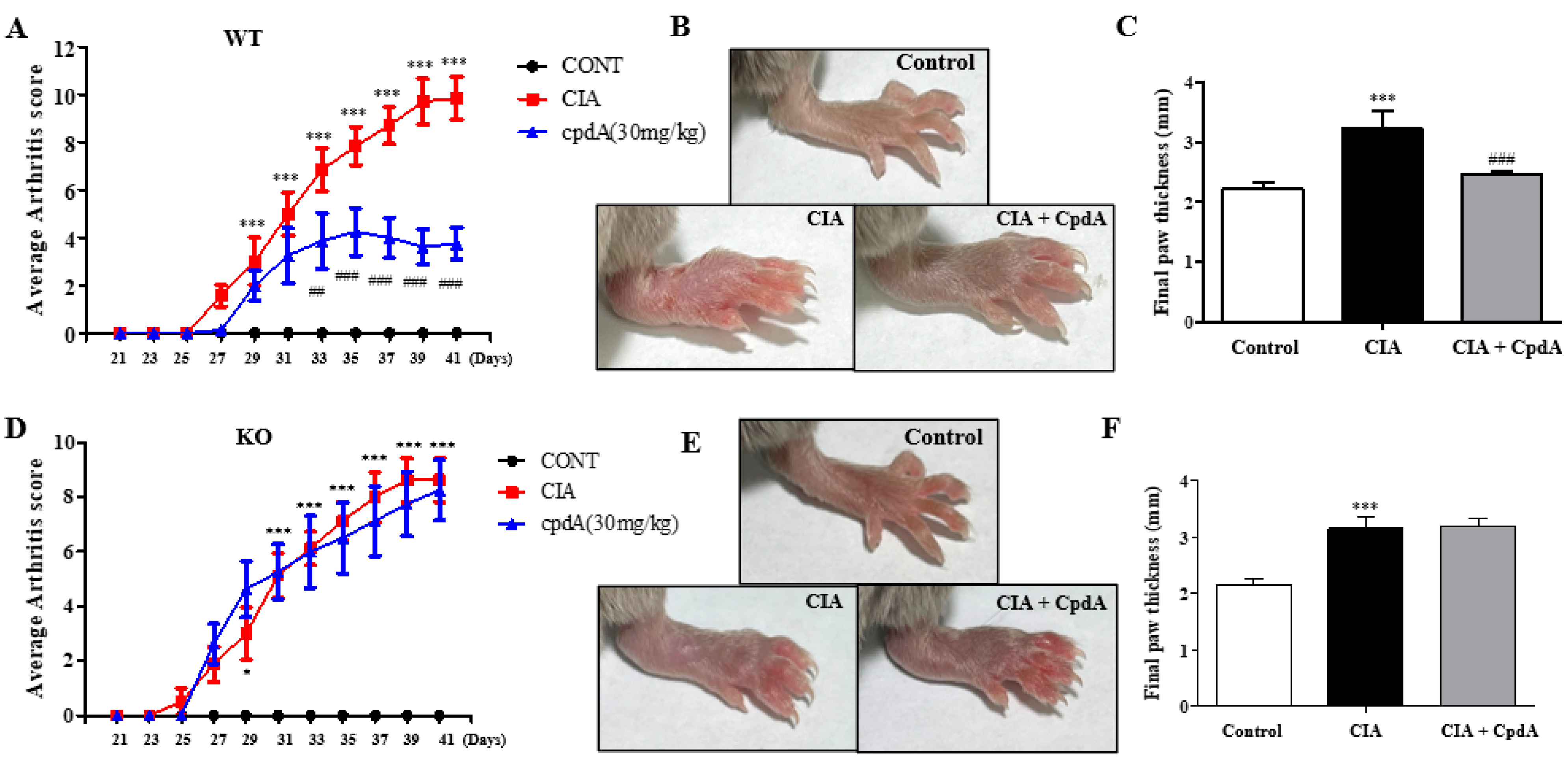
2.1. CpdA Activation of Ffa4 Suppressed Arthritis Development and Thickening of Foots
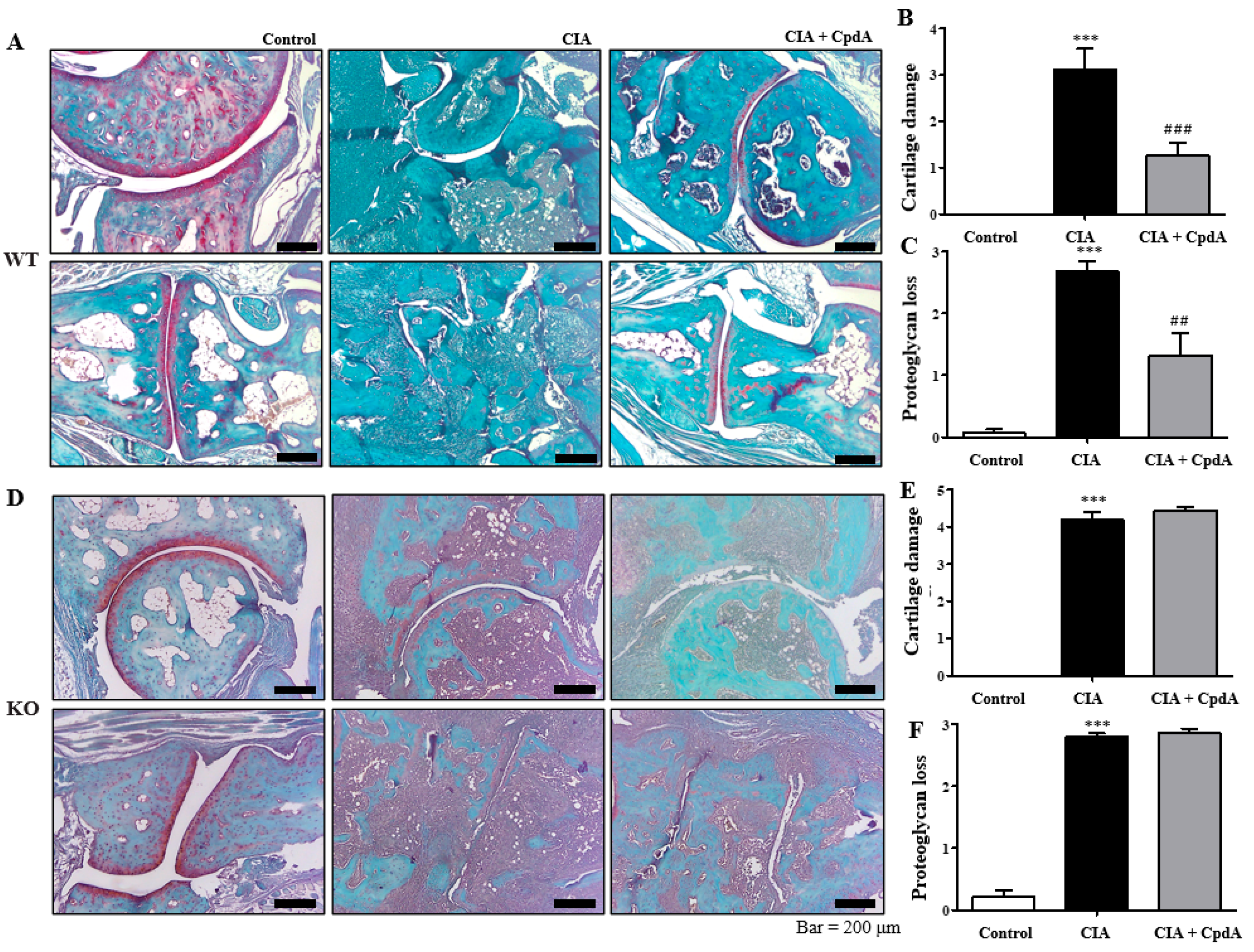
2.2. CpdA Activation of Ffa4 Suppressed Bone Erosion, Inflammation, Cartilage Damage, and Proteoglycan Loss
2.3. CpdA Activation of Ffa4 Suppressed Enlargement of Spleens and Rebalanced Th1/Th17 and Treg Cells in Spleens
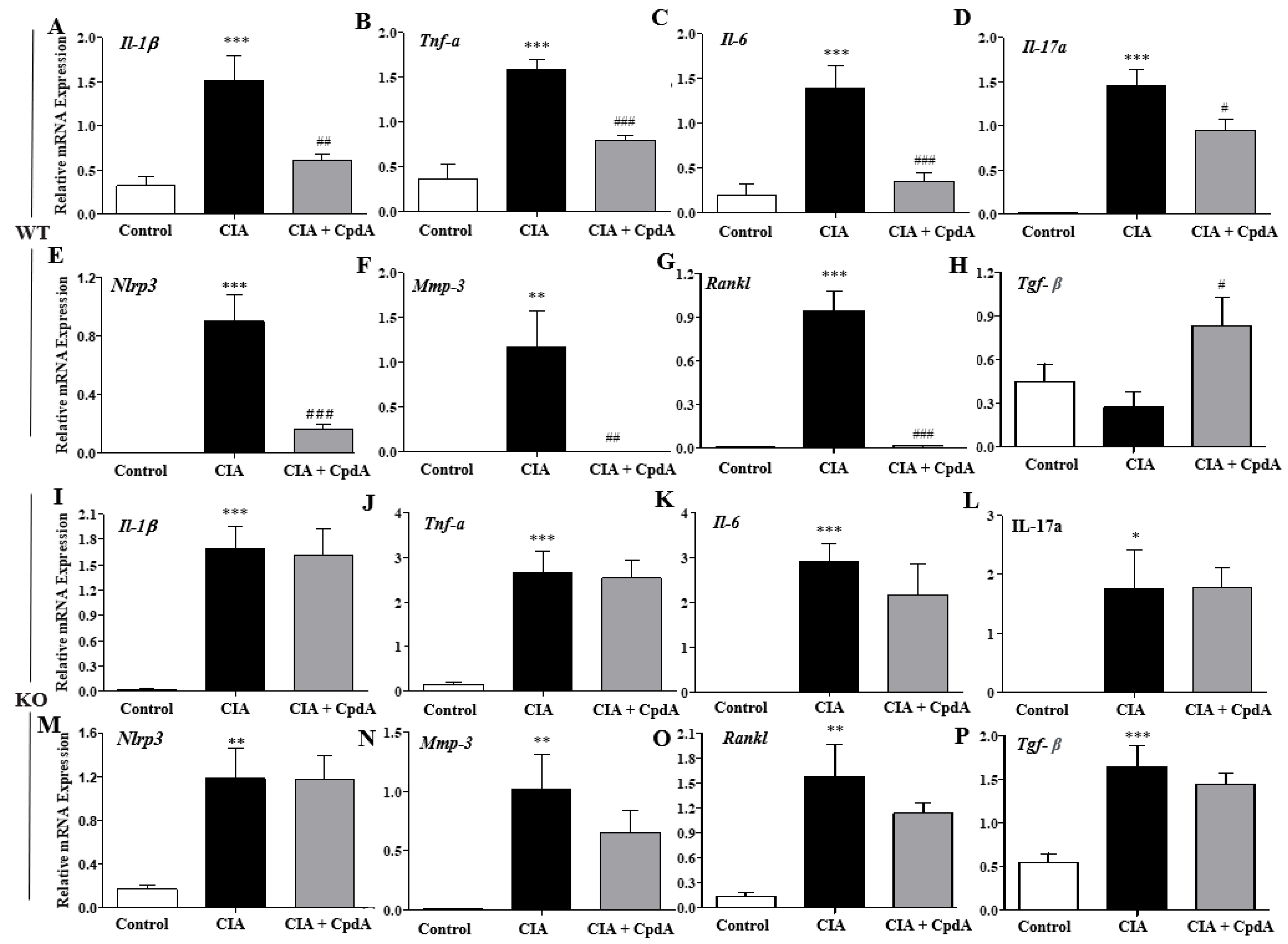
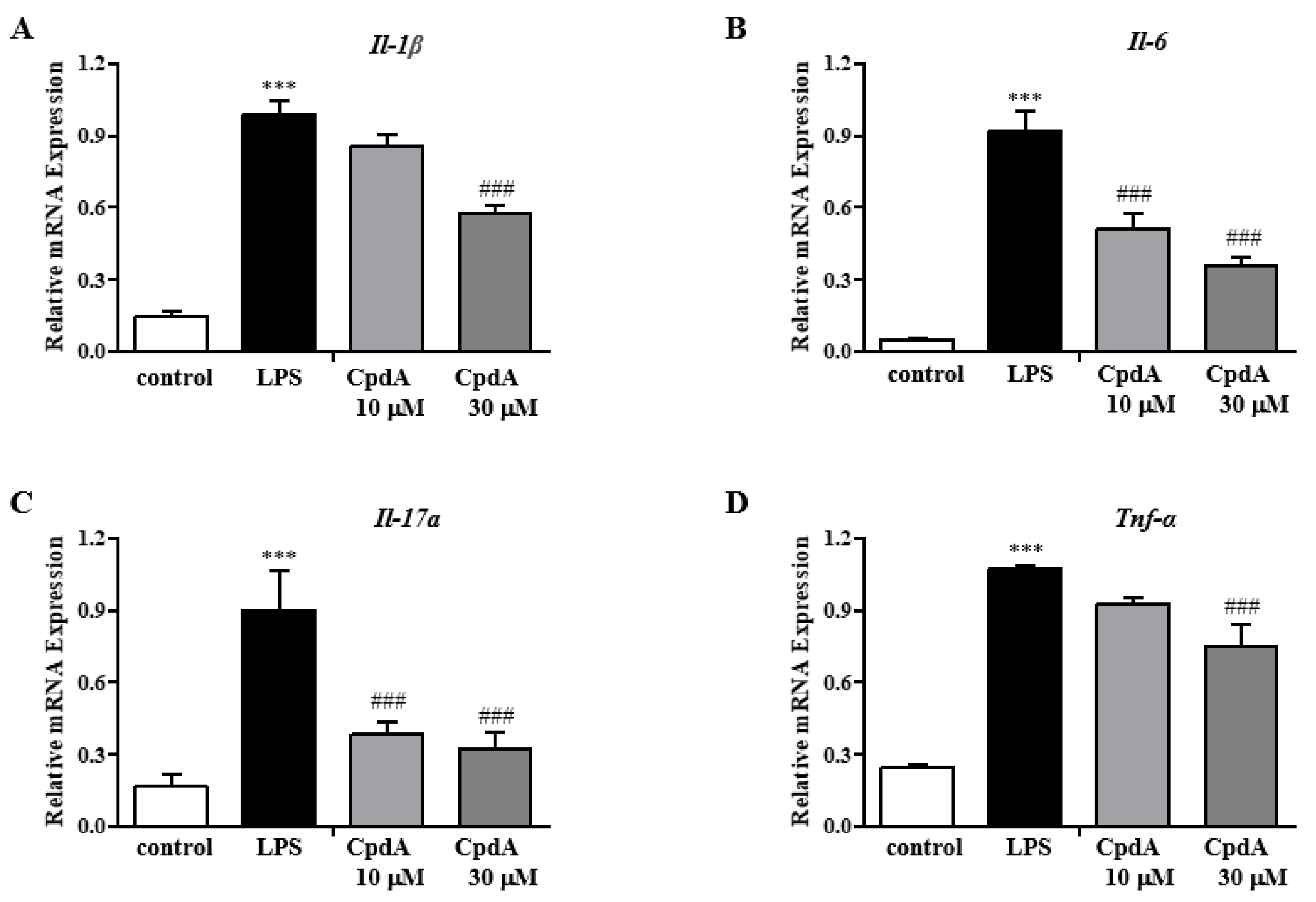
2.4. CpdA Activation of Ffa4 Suppressed mRNA Expression Levels of Pro-Inflammatory Cytokines in Foot Tissues
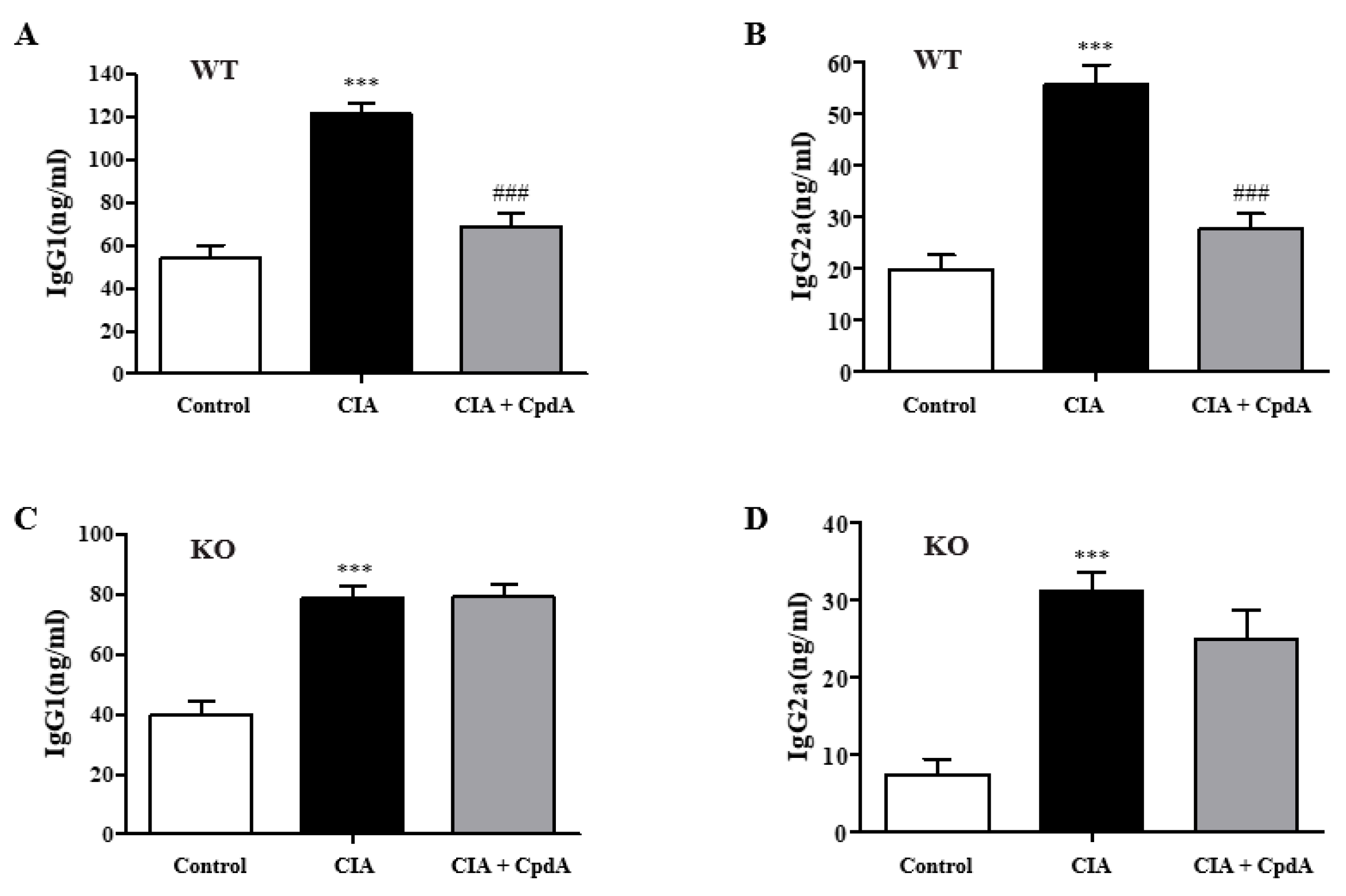
2.5. CpdA Activation of Ffa4 Suppressed Serum IgG Levels
2.6. CpdA Activation of Ffa4 Suppressed mRNA Expression Levels of Inflammatory Cytokines in SW982 Human Synovial Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells
4.3. Mouse Strain
4.4. Treatment of Human Synovial SW982 Cells
4.5. Induction of Rheumatoid Arthritis in DBA/1J Mice and CpdA Administration
4.6. Measurement of the Severity of Arthritis
4.7. Histological Assessment of Arthritis
4.8. Flowcytometric Analysis
4.9. Quantitative Real-Time PCR
4.10. Enzyme-Linked Immunosorbent Assay (ELISA)
4.11. Normality and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Kvien, T.K.; Uhlig, T.; Ødegård, S.; Heiberg, M.S. Epidemiological aspects of rheumatoid arthritis: The sex ratio. Ann. N. Y Acad. Sci. 2006, 1069, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, M.; Bashiri, H.; Khorramdelazad, H.; Barzaman, K.; Hashemi, N.; Sereshki, H.A.; Sahebkar, A.; Karami, J. Destructive Roles of Fibroblast-like Synoviocytes in Chronic Inflammation and Joint Damage in Rheumatoid Arthritis. Inflammation 2021, 44, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, S.; Mauri, C.; Ehrenstein, M.R. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J. Exp. Med. 2007, 204, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Behrens, F.; Himsel, A.; Rehart, S.; Stanczyk, J.; Beutel, B.; Zimmermann, S.Y.; Koehl, U.; Möller, B.; Gay, S.; Kaltwasser, J.P.; et al. Imbalance in distribution of functional autologous regulatory T cells in rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Chabaud, M.; Fossiez, F.; Taupin, J.L.; Miossec, P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J. Immunol. 1998, 161, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Miossec, P.; Korn, T.; Kuchroo, V.K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009, 361, 888–898. [Google Scholar] [CrossRef]
- Yang, P.; Qian, F.Y.; Zhang, M.F.; Xu, A.L.; Wang, X.; Jiang, B.P.; Zhou, L.L. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J. Leukoc. Biol. 2019, 106, 1233–1240. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S. JAK-STAT inhibitors: Immersing therapeutic approach for management of rheumatoid arthritis. Int. Immunopharmacol. 2020, 86, 106731. [Google Scholar] [CrossRef]
- Leslie, C.A.; Gonnerman, W.A.; Ullman, M.D.; Hayes, K.C.; Franzblau, C.; Cathcart, E.S. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J. Exp. Med. 1985, 162, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- Ierna, M.; Kerr, A.; Scales, H.; Berge, K.; Griinari, M. Supplementation of diet with krill oil protects against experimental rheumatoid arthritis. BMC Musculoskelet. Disord. 2010, 11, 136. [Google Scholar] [CrossRef]
- Volker, D.H.; FitzGerald, P.E.; Garg, M.L. The eicosapentaenoic to docosahexaenoic acid ratio of diets affects the pathogenesis of arthritis in Lew/SSN rats. J. Nutr. 2000, 130, 559–565. [Google Scholar] [CrossRef]
- Woo, S.J.; Lim, K.; Park, S.Y.; Jung, M.Y.; Lim, H.S.; Jeon, M.G.; Lee, S.I.; Park, B.H. Endogenous conversion of n-6 to n-3 polyunsaturated fatty acids attenuates K/BxN serum-transfer arthritis in fat-1 mice. J. Nutr. Biochem. 2015, 26, 713–720. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, K.; Kim, K.H.; Kim, J.H.; Choi, J.S.; Shim, S.C. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PLoS ONE 2018, 13, e0194331. [Google Scholar] [CrossRef]
- Kremer, J.M.; Bigauoette, J.; Michalek, A.V.; Timchalk, M.A.; Lininger, L.; Rynes, R.I.; Huyck, C.; Zieminski, J.; Bartholomew, L.E. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet 1985, 1, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Veselinovic, M.; Vasiljevic, D.; Vucic, V.; Arsic, A.; Petrovic, S.; Tomic-Lucic, A.; Savic, M.; Zivanovic, S.; Stojic, V.; Jakovljevic, V. Clinical Benefits of n-3 PUFA and ɤ-Linolenic Acid in Patients with Rheumatoid Arthritis. Nutrients 2017, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Lourdudoss, C.; Wolk, A.; Nise, L.; Alfredsson, L.; Vollenhoven, R.V. Are dietary vitamin D, omega-3 fatty acids and folate associated with treatment results in patients with early rheumatoid arthritis? Data from a Swedish population-based prospective study. BMJ Open 2017, 7, e016154. [Google Scholar] [CrossRef]
- Bahadori, B.; Uitz, E.; Thonhofer, R.; Trummer, M.; Pestemer-Lach, I.; McCarty, M.; Krejs, G.J. omega-3 Fatty acids infusions as adjuvant therapy in rheumatoid arthritis. JPEN J. Parenter. Enteral Nutr. 2010, 34, 151–155. [Google Scholar] [CrossRef]
- Beyer, K.; Lie, S.A.; Kjellevold, M.; Dahl, L.; Brun, J.G.; Bolstad, A.I. Marine ω-3, vitamin D levels, disease outcome and periodontal status in rheumatoid arthritis outpatients. Nutrition 2018, 55–56, 116–124. [Google Scholar] [CrossRef]
- Jeffery, L.; Fisk, H.L.; Calder, P.C.; Filer, A.; Raza, K.; Buckley, C.D.; McInnes, I.; Taylor, P.C.; Fisher, B.A. Plasma Levels of Eicosapentaenoic Acid Are Associated with Anti-TNF Responsiveness in Rheumatoid Arthritis and Inhibit the Etanercept-driven Rise in Th17 Cell Differentiation in Vitro. J. Rheumatol. 2017, 44, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, G.; Zhang, X.; Xing, G.; Hu, X.; Yang, L.; Li, D. Lipid extract from hard-shelled mussel (Mytilus coruscus) improves clinical conditions of patients with rheumatoid arthritis: A randomized controlled trial. Nutrients 2015, 7, 625–645. [Google Scholar] [CrossRef] [PubMed]
- Mustonen, A.M.; Käkelä, R.; Lehenkari, P.; Huhtakangas, J.; Turunen, S.; Joukainen, A.; Kääriäinen, T.; Paakkonen, T.; Kröger, H.; Nieminen, P. Distinct fatty acid signatures in infrapatellar fat pad and synovial fluid of patients with osteoarthritis versus rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124.e4. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2005, 11, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Huang, J.; Lee, B.K.; Jung, Y.S.; Im, E.; Koh, J.M.; Im, D.S. Omega-3 polyunsaturated fatty acids protect human hepatoma cells from developing steatosis through FFA4 (GPR120). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Park, S.J.; Koh, J.M.; Im, D.S. Free fatty acid receptor 4 (FFA4) activation ameliorates 2,4-dinitrochlorobenzene-induced atopic dermatitis by increasing regulatory T cells in mice. Acta Pharmacol. Sin. 2020, 41, 1337–1347. [Google Scholar] [CrossRef]
- Son, S.E.; Koh, J.M.; Im, D.S. Activation of Free Fatty Acid Receptor 4 (FFA4) Ameliorates Ovalbumin-Induced Allergic Asthma by Suppressing Activation of Dendritic and Mast Cells in Mice. Int. J. Mol. Sci. 2022, 23, 5270. [Google Scholar] [CrossRef]
- Lee, K.P.; Park, S.J.; Kang, S.; Koh, J.M.; Sato, K.; Chung, H.Y.; Okajima, F.; Im, D.S. ω-3 Polyunsaturated fatty acids accelerate airway repair by activating FFA4 in club cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L835–Ll844. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Park, S.Y.; Baek, J.E.; Lee, S.Y.; Baek, W.Y.; Lee, S.Y.; Lee, Y.S.; Yoo, H.J.; Kim, H.; Lee, S.H.; et al. Free Fatty Acid Receptor 4 (GPR120) Stimulates Bone Formation and Suppresses Bone Resorption in the Presence of Elevated n-3 Fatty Acid Levels. Endocrinology 2016, 157, 2621–2635. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef]
- Ouyang, L.; Dan, Y.; Hua, W.; Shao, Z.; Duan, D. Therapeutic effect of omega-3 fatty acids on T cell-mediated autoimmune diseases. Microbiol. Immunol. 2020, 64, 563–569. [Google Scholar] [CrossRef]
- Espersen, G.T.; Grunnet, N.; Lervang, H.H.; Nielsen, G.L.; Thomsen, B.S.; Faarvang, K.L.; Dyerberg, J.; Ernst, E. Decreased interleukin-1 beta levels in plasma from rheumatoid arthritis patients after dietary supplementation with n-3 polyunsaturated fatty acids. Clin. Rheumatol. 1992, 11, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.E.; Mantzioris, E.; Gibson, R.A.; Cleland, L.G.; James, M.J. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996, 63, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi Sözener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Koh, J.M.; Im, D.S. Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice. Int. J. Mol. Sci. 2022, 23, 4482. [Google Scholar] [CrossRef]
- Qin, Z.; Song, J.; Lin, A.; Yang, W.; Zhang, W.; Zhong, F.; Huang, L.; Lü, Y.; Yu, W. GPR120 modulates epileptic seizure and neuroinflammation mediated by NLRP3 inflammasome. J. Neuroinflammation 2022, 19, 121. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Zainal, Z.; Longman, A.J.; Hurst, S.; Duggan, K.; Caterson, B.; Hughes, C.E.; Harwood, J.L. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthr. Cartil. 2009, 17, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Li, L.; Wang, K.; Wu, X.; Chen, H.; Shi, J.; Zhou, C.; Zhang, W.; Hang, K.; et al. Eicosapentaenoic acid supplementation modulates the osteoblast/osteoclast balance in inflammatory environments and protects against estrogen deficiency-induced bone loss in mice. Clin. Nutr. 2023, 42, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kim, W.; Zhou, L.; Wang, N.; Ly, L.H.; McMurray, D.N.; Chapkin, R.S. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J. Nutr. 2006, 136, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Fan, Y.Y.; Barhoumi, R.; Smith, R.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 2008, 181, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, J.; Meng, Y.; Shi, N.; Wang, X.; Zhou, M.; Li, G.; Yang, Y. DHA Sensor GPR120 in Host Defense Exhibits the Dual Characteristics of Regulating Dendritic Cell Function and Skewing the Balance of Th17/Tregs. Int. J. Biol. Sci. 2020, 16, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Li, J.; Wang, S.; Xie, A.; Sun, W.; Xia, J. Eicosapentaenoic acid disrupts the balance between Tregs and IL-17+ T cells through PPARγ nuclear receptor activation and protects cardiac allografts. J. Surg. Res. 2012, 173, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; Weeks, B.; Wu, C.; McMurray, D.N.; Chapkin, R.S. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS ONE 2012, 7, e49739. [Google Scholar] [CrossRef]
- Monk, J.M.; Jia, Q.; Callaway, E.; Weeks, B.; Alaniz, R.C.; McMurray, D.N.; Chapkin, R.S. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J. Nutr. 2012, 142, 117–124. [Google Scholar] [CrossRef]
- Teague, H.; Rockett, B.D.; Harris, M.; Brown, D.A.; Shaikh, S.R. Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids. Immunology 2013, 139, 386–394. [Google Scholar] [CrossRef]
- Feng, C.; Li, L.; Li, Q.; Switzer, K.; Liu, M.; Han, S.; Zheng, B. Docosahexaenoic acid ameliorates autoimmune inflammation by activating GPR120 signaling pathway in dendritic cells. Int. Immunopharmacol. 2021, 97, 107698. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yen, J.H.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, A.; Plé, A.; Moutairou, K.; Hichami, A.; Khan, N.A. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J. Lipid Res. 2009, 50, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.A.; Wold, A.E.; Sandberg, A.S.; Östman, S.M. The Polyunsaturated Fatty Acids Arachidonic Acid and Docosahexaenoic Acid Induce Mouse Dendritic Cells Maturation but Reduce T-Cell Responses In Vitro. PLoS ONE 2015, 10, e0143741. [Google Scholar] [CrossRef] [PubMed]
- Wannick, M.; Bezdek, S.; Guillen, N.; Thieme, M.; Meshrkey, F.; Mousavi, S.; Seeling, M.; Nimmerjahn, F.; Mócsai, A.; Zillikens, D.; et al. Oral administration of the selective GPR120/FFA4 agonist compound A is not effective in alleviating tissue inflammation in mouse models of prototypical autoimmune diseases. Pharmacol. Res. Perspect. 2018, 6, e00438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, D.; Ho, K.W.; Lin, S.; Suen, W.C.; Zhang, H.; Zha, Z.; Li, G.; Leung, P.S. GPR120 is an important inflammatory regulator in the development of osteoarthritis. Arthritis Res. Ther. 2018, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ke, T.; Zhang, Y.; Fu, C.; He, W. Agonism of GPR120 prevented IL-1β-induced reduction of extracellular matrix through SOX-9. Aging (Albany NY) 2020, 12, 12074–12085. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Lee, M.; Kim, S.D.; Jeong, Y.S.; Kim, J.C.; Yang, S.; Kim, H.Y.; Bae, Y.S. Activation of formyl peptide receptor 1 elicits therapeutic effects against collagen-induced arthritis. J. Cell Mol. Med. 2021, 25, 8936–8946. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Zu, B.; Wang, J.; Sheng, K.; Zhao, L.; Xu, W. Acacetin regulated the reciprocal differentiation of Th17 cells and Treg cells and mitigated the symptoms of collagen-induced arthritis in mice. Scand. J. Immunol. 2018, 88, e12712. [Google Scholar] [CrossRef]
- Deng, Y.; Luo, H.; Shu, J.; Shu, H.; Lu, C.; Zhao, N.; Geng, Y.; He, X.; Lu, A. Pien Tze Huang alleviate the joint inflammation in collagen-induced arthritis mice. Chin. Med. 2020, 15, 30. [Google Scholar] [CrossRef]
- Hayer, S.; Vervoordeldonk, M.J.; Denis, M.C.; Armaka, M.; Hoffmann, M.; Bäcklund, J.; Nandakumar, K.S.; Niederreiter, B.; Geka, C.; Fischer, A.; et al. ‘SMASH’ recommendations for standardised microscopic arthritis scoring of histological sections from inflammatory arthritis animal models. Ann. Rheum. Dis. 2021, 80, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Corr, M.; Boyle, D.L.; Ronacher, L.M.; Lew, B.R.; van Baarsen, L.G.; Tak, P.P.; Firestein, G.S. Interleukin 1 receptor antagonist mediates the beneficial effects of systemic interferon beta in mice: Implications for rheumatoid arthritis. Ann. Rheum. Dis. 2011, 70, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Kyburz, D.; Corr, M. The KRN mouse model of inflammatory arthritis. Springer Semin. Immunopathol. 2003, 25, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.Y.; Crain, B.; Wu, S.R.; Corr, M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J. Exp. Med. 2003, 197, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Douni, E.; Sfikakis, P.P.; Haralambous, S.; Fernandes, P.; Kollias, G. Attenuation of inflammatory polyarthritis in TNF transgenic mice by diacerein: Comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis Res. Ther. 2004, 6, R65–R72. [Google Scholar] [CrossRef]
- Pettit, A.R.; Ji, H.; von Stechow, D.; Müller, R.; Goldring, S.R.; Choi, Y.; Benoist, C.; Gravallese, E.M. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 2001, 159, 1689–1699. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-E.; Lee, J.-H.; Koh, J.-M.; Im, D.-S. Free Fatty Acid 4 Receptor Activation Attenuates Collagen-Induced Arthritis by Rebalancing Th1/Th17 and Treg Cells. Int. J. Mol. Sci. 2024, 25, 5866. https://doi.org/10.3390/ijms25115866
Lee J-E, Lee J-H, Koh J-M, Im D-S. Free Fatty Acid 4 Receptor Activation Attenuates Collagen-Induced Arthritis by Rebalancing Th1/Th17 and Treg Cells. International Journal of Molecular Sciences. 2024; 25(11):5866. https://doi.org/10.3390/ijms25115866
Chicago/Turabian StyleLee, Jung-Eun, Ju-Hyun Lee, Jung-Min Koh, and Dong-Soon Im. 2024. "Free Fatty Acid 4 Receptor Activation Attenuates Collagen-Induced Arthritis by Rebalancing Th1/Th17 and Treg Cells" International Journal of Molecular Sciences 25, no. 11: 5866. https://doi.org/10.3390/ijms25115866
APA StyleLee, J.-E., Lee, J.-H., Koh, J.-M., & Im, D.-S. (2024). Free Fatty Acid 4 Receptor Activation Attenuates Collagen-Induced Arthritis by Rebalancing Th1/Th17 and Treg Cells. International Journal of Molecular Sciences, 25(11), 5866. https://doi.org/10.3390/ijms25115866







