Harnessing Metformin’s Immunomodulatory Effects on Immune Cells to Combat Breast Cancer
Abstract
1. Introduction
2. Results
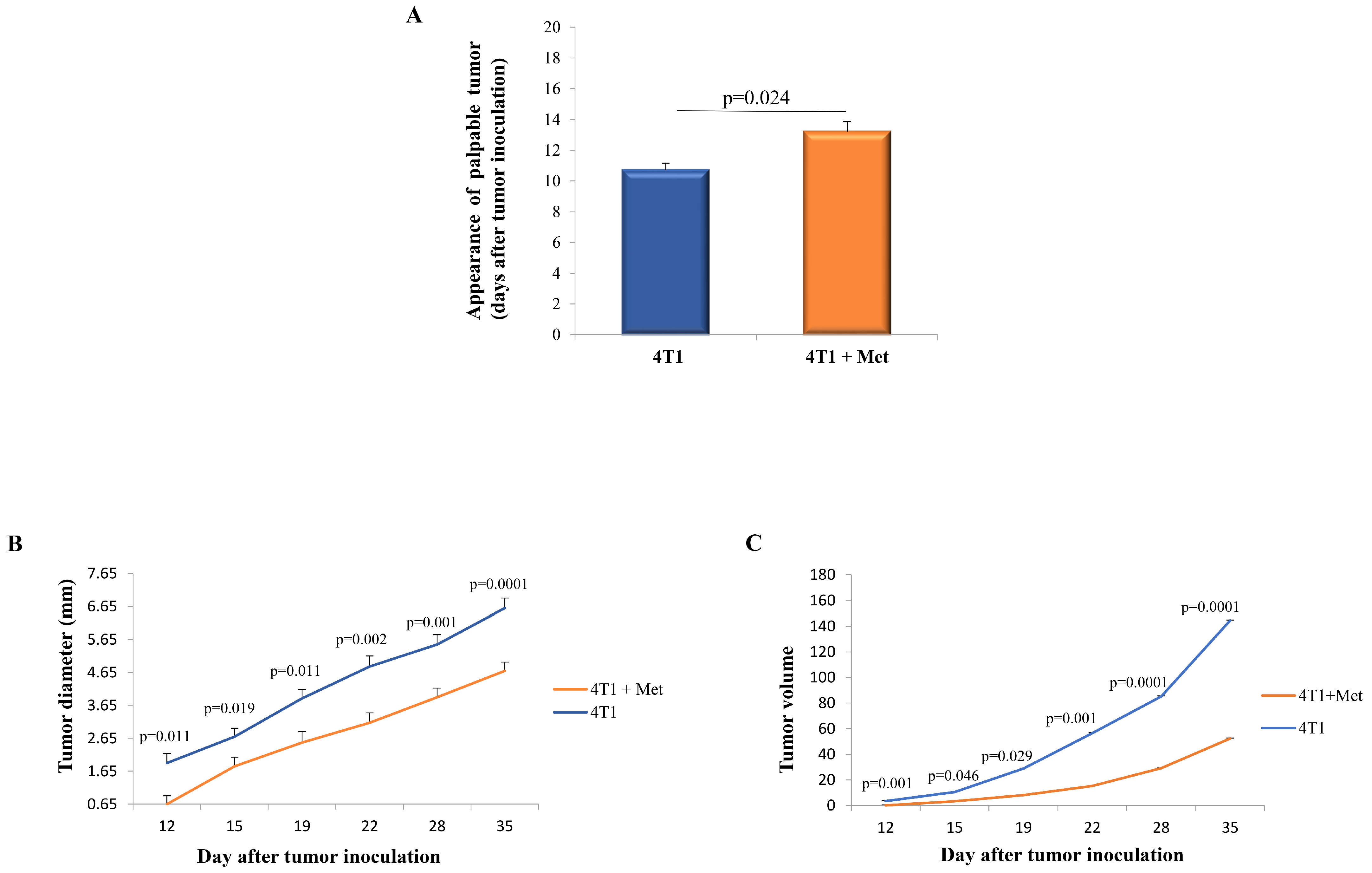
2.1. Metformin Administration Results in Delayed Appearance of the Palpable Tumor and Slower Progression of 4T1 Breast Cancer
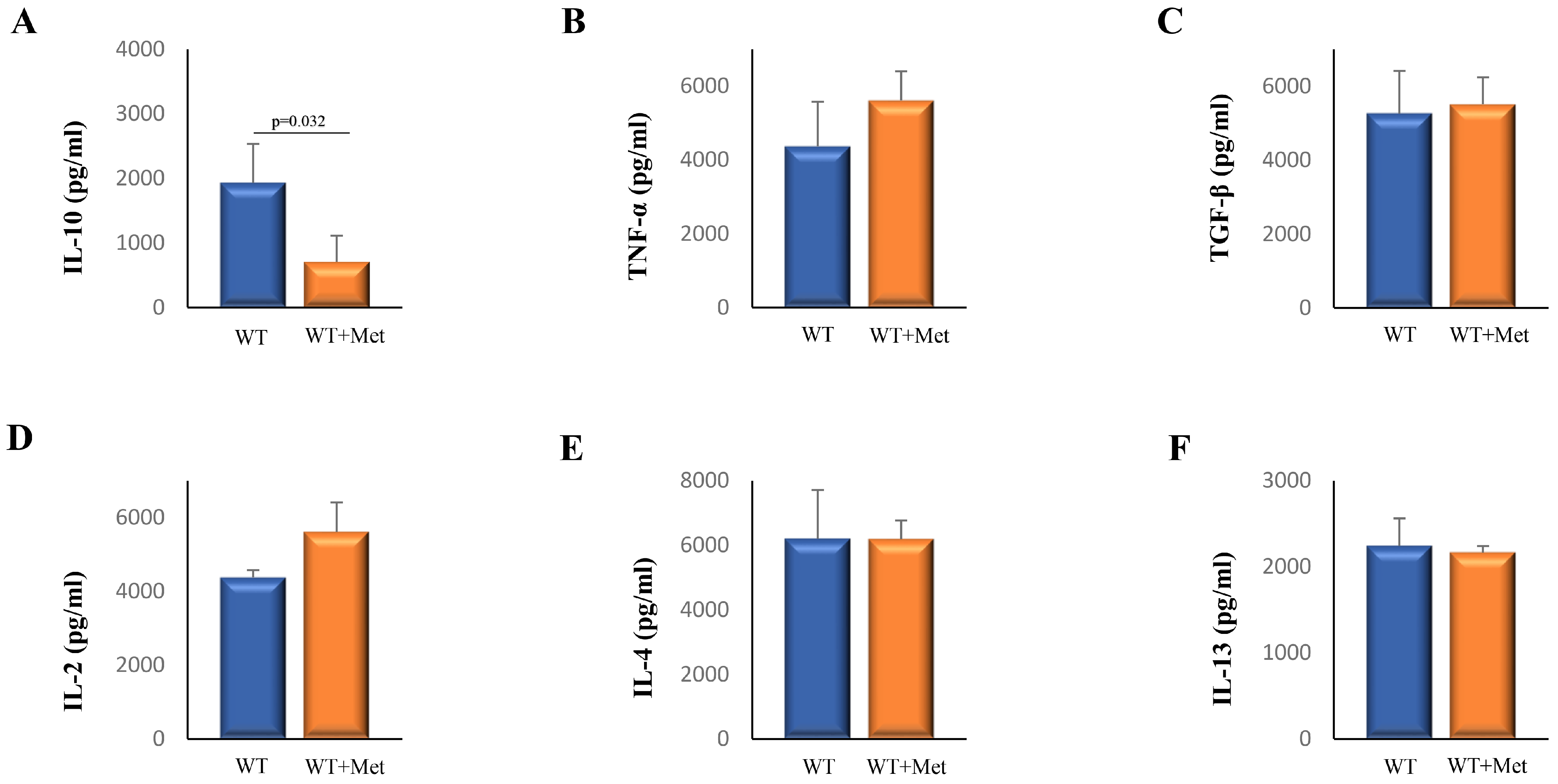
2.2. Metformin Decreases Serum Levels of Interleukin (IL)-10
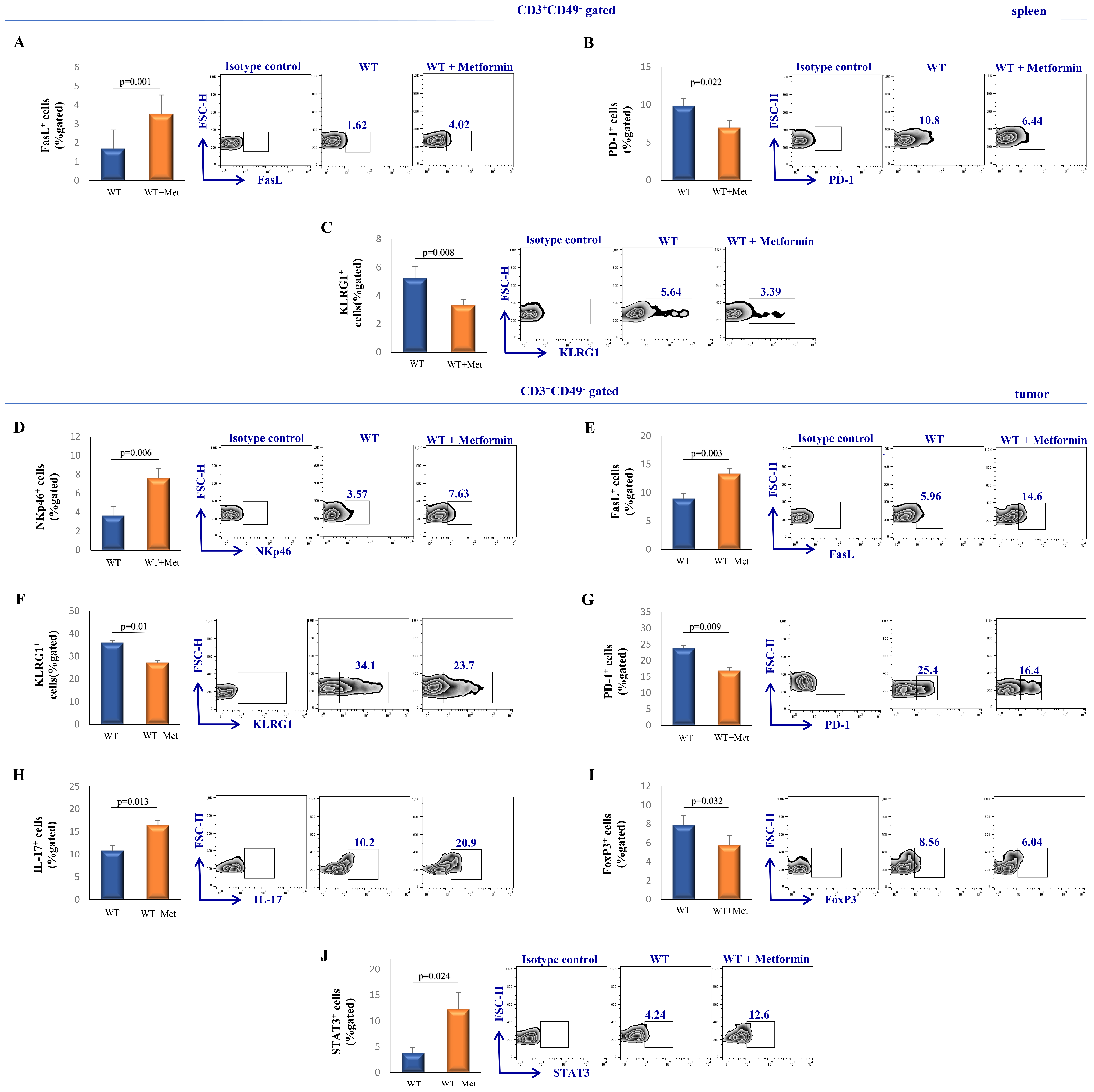
2.3. Metformin Treatment Suppresses the Inhibitory Phenotype of NKT Cells While Enhancing Their Cytotoxic Potential
2.4. Metformin Modulates the Functional Phenotype of Splenic and Tumor-Infiltrating T Cells in Tumor-Bearing Mice
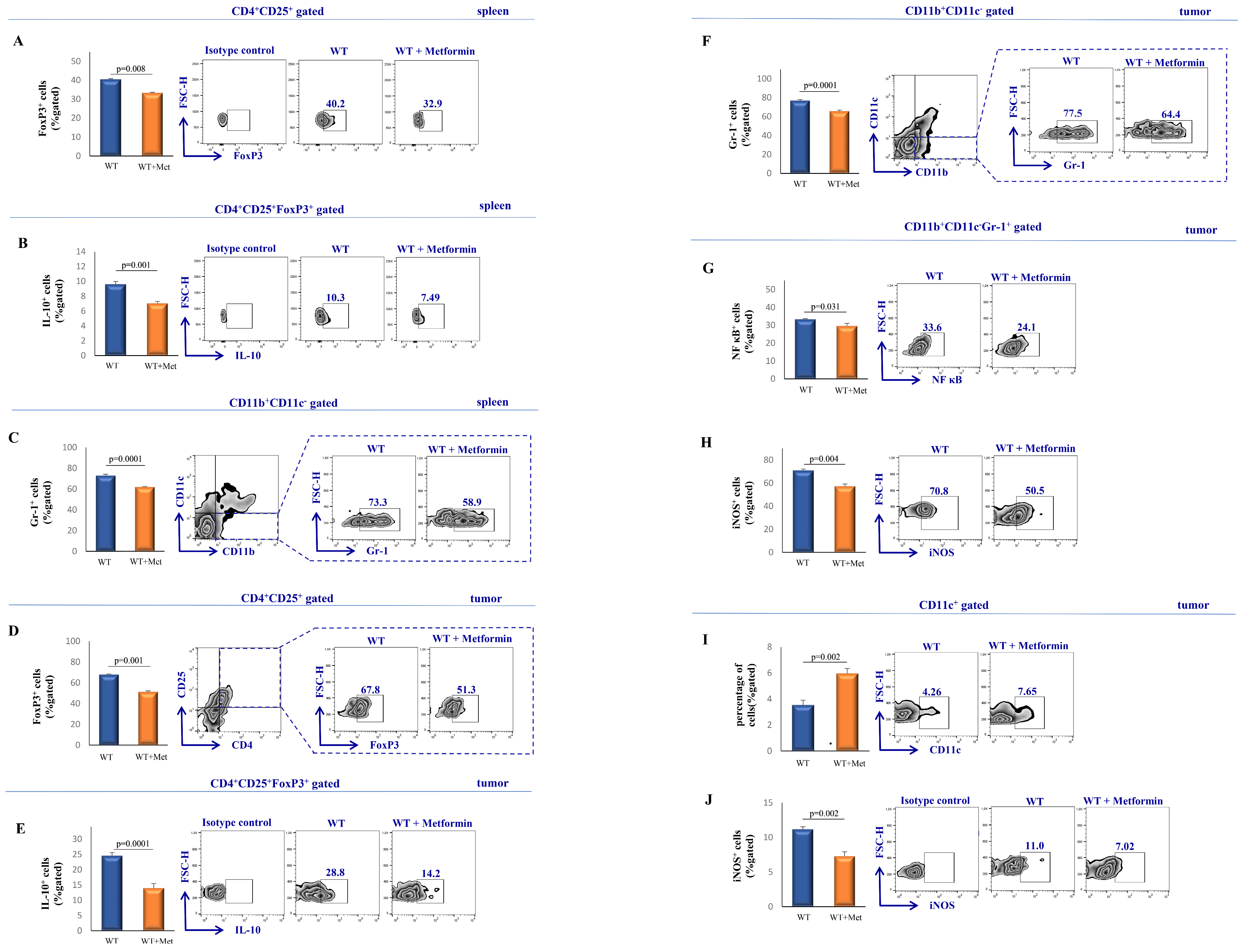
2.5. Administration of Metformin Attenuates the Accumulation and Activation of Immunosuppressive Cell Populations
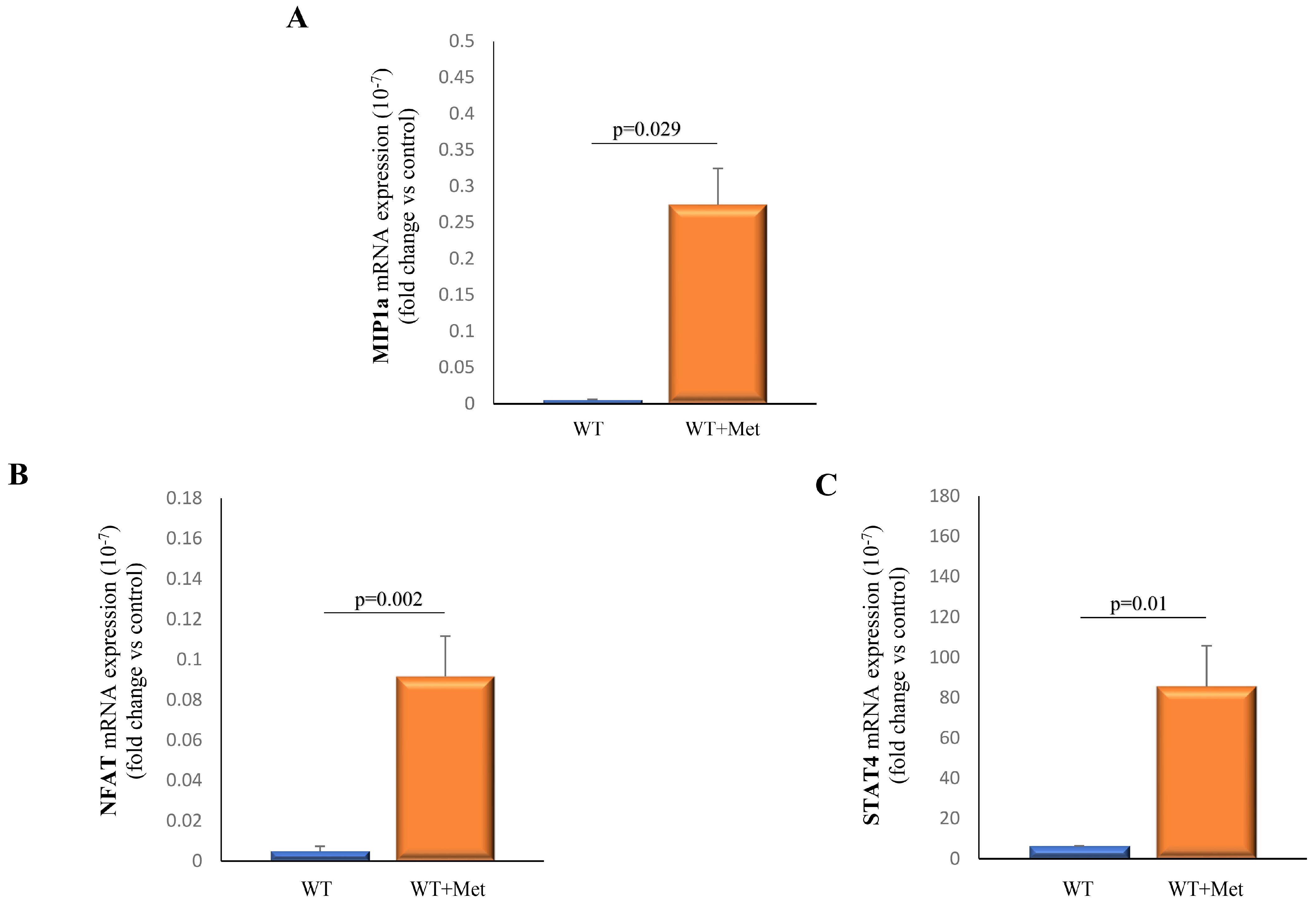
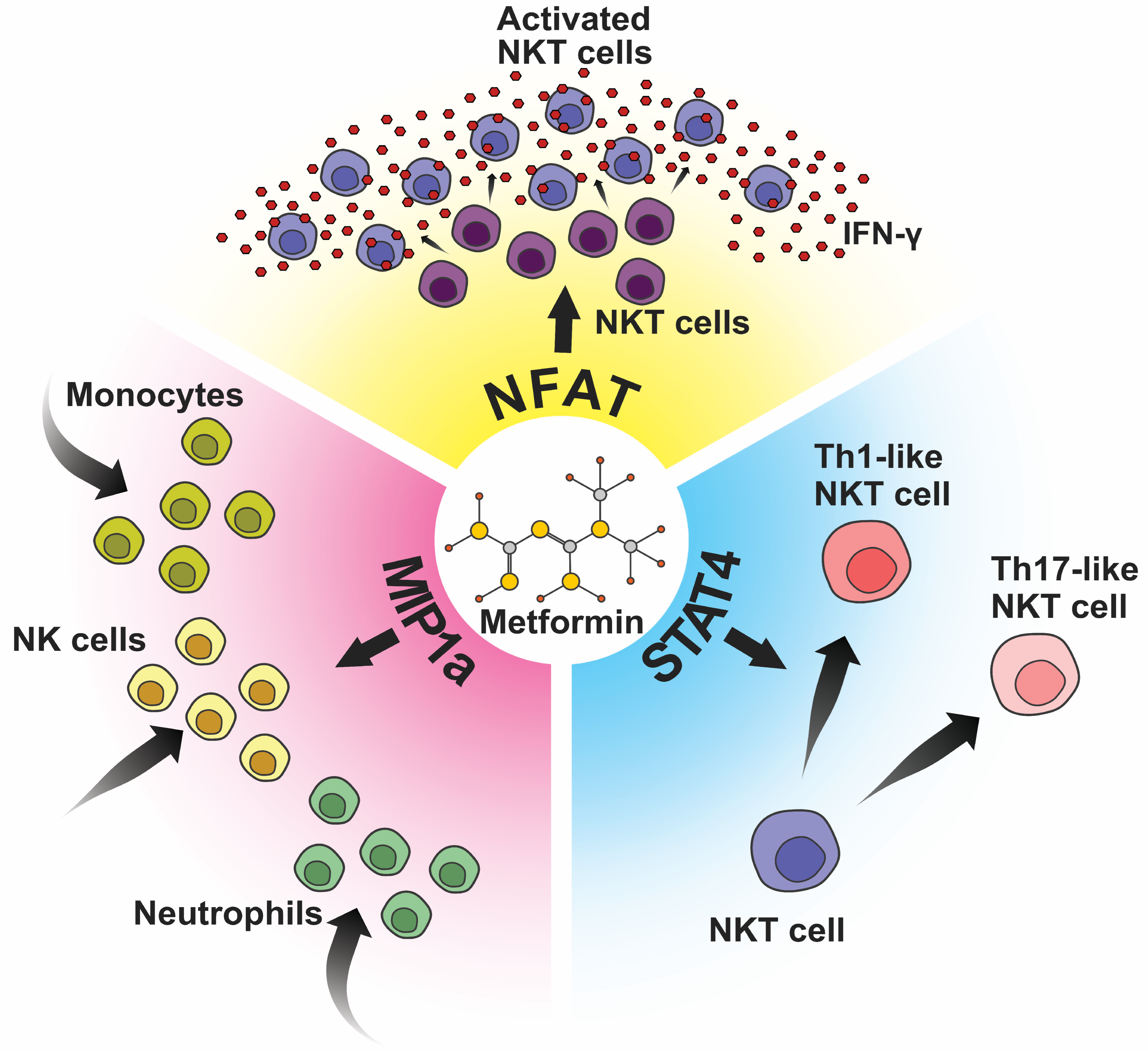
2.6. Metformin Administration Demonstrates a Significant Impact on the Expression Profiles of Transcriptional Factors and Chemokines within Splenocytes
3. Discussion
3.1. Metformin’s Multifaceted Role in Cancer Treatment
3.2. Metformin and Immune Modulation
3.3. Metformin’s Role in Amplifying NK Cell Response to Cancer
3.4. NKT Cells: Orchestrators of Immune Equilibrium in the Tumor Microenvironment
3.5. Metformin and NKT Cells: Bridging Innate and Adaptive Immunity in Cancer Therapy
3.6. Metformin’s Modulation of T Cell Phenotypes: Enhancing the Adaptive Immune Response against Cancer
3.7. Metformin’s Immunomodulatory Effects: Diminishing Immunosuppression and Enhancing Tumor Immunity
3.8. Metformin’s Impact on NKT Cell Activation Markers
4. Materials and Methods
4.1. Experimental Design, Tumor Induction, and Metformin Administration
- (1)
- wild-type BALB/C mice with induced mammary tumors which were given 200 mg/kg of metformin diluted in 100 μL of distilled water daily;
- (2)
- wild-type BALB/C mice which were induced mammary tumors and given 100 μL of distilled water daily.
4.2. Palpable Tumor Appearance and Growth
4.3. Flow Cytometric Analyses of Splenocytes and Tumor-Infiltrating Leukocytes
4.4. Serum Level of Cytokines
4.5. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Yates, L.R.; Knappskog, S.; Wedge, D.; Farmery, J.H.R.; Gonzalez, S.; Martincorena, I.; Alexandrov, L.B.; Van Loo, P.; Haugland, H.K.; Lilleng, P.K.; et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell 2017, 32, 169–184.e167. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner, G.; Schernthaner, G.H. The right place for metformin today. Diabetes Res. Clin. Pract. 2020, 159, 107946. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an adjuvant treatment for cancer: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Lukacs, J.D.; Johnston, B. The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead. Cancers 2021, 13, 5174. [Google Scholar] [CrossRef] [PubMed]
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells: Bridging innate and adaptive immunity. Cell Tissue Res. 2011, 343, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Van Kaer, L. NKT cells: What’s in a name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Courtney, A.N.; Tian, G.; Metelitsa, L.S. Natural killer T cells and other innate-like T lymphocytes as emerging platforms for allogeneic cancer cell therapy. Blood 2023, 141, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef]
- Skuli, S.J.; Alomari, S.; Gaitsch, H.; Bakayoko, A.; Skuli, N.; Tyler, B.M. Metformin and Cancer, an Ambiguanidous Relationship. Pharmaceuticals 2022, 15, 626. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.M.; Walter, P.; Jackson, A.; Clark, L.; Franasiak, J.; Bolac, C.; Havrilesky, L.J.; Secord, A.A.; Moore, D.T.; Gehrig, P.A.; et al. Metformin is associated with improved survival in endometrial cancer. Gynecol. Oncol. 2014, 132, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.L.; Qin, X. Metformin and cancer-specific survival among breast, colorectal, or endometrial cancer patients: A nationwide data linkage study. Diabetes Res. Clin. Pract. 2021, 175, 108755. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, T.T.; Wang, F.; Cui, B.; Zhao, C.X.; Yu, J.J.; Lv, X.X.; Zhang, X.W.; Yang, Z.N.; Huang, B.; et al. Metformin suppresses melanoma progression by inhibiting KAT5-mediated SMAD3 acetylation, transcriptional activity and TRIB3 expression. Oncogene 2018, 37, 2967–2981. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Bahrambeigi, S.; Shafiei-Irannejad, V. Immune-mediated anti-tumor effects of metformin; targeting metabolic reprogramming of T cells as a new possible mechanism for anti-cancer effects of metformin. Biochem. Pharmacol. 2020, 174, 113787. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Martin-Castillo, B.; Menendez, J.A. Metformin as an archetype immuno-metabolic adjuvant for cancer immunotherapy. Oncoimmunology 2019, 8, e1633235. [Google Scholar] [CrossRef]
- Pearce, E.L.; Walsh, M.C.; Cejas, P.J.; Harms, G.M.; Shen, H.; Wang, L.-S.; Jones, R.G.; Choi, Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 2009, 460, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, I.; Radosavljevic, G.; Mitrovic, M.; Juranic, V.L.; McKenzie, A.N.; Arsenijevic, N.; Jonjic, S.; Lukic, M.L. ST2 deletion enhances innate and acquired immunity to murine mammary carcinoma. Eur. J. Immunol. 2011, 41, 1902–1912. [Google Scholar] [CrossRef]
- Jovanovic, I.P.; Pejnovic, N.N.; Radosavljevic, G.D.; Pantic, J.M.; Milovanovic, M.Z.; Arsenijevic, N.N.; Lukic, M.L. Interleukin-33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int. J. Cancer 2014, 134, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, A.R.; Jovanovic, I.P.; Jurisevic, M.M.; Jovanovic, M.Z.; Jovanovic, M.M.; Pavlovic, S.P.; Arsenijevic, N.N.; Supic, G.M.; Vojvodic, D.V.; Jovanovic, M.M.; et al. Metformin promotes antitumor activity of NK cells via overexpression of miRNA-150 and miRNA-155. Am. J. Transl. Res. 2023, 15, 2727–2737. [Google Scholar] [PubMed]
- Xia, W.; Qi, X.; Li, M.; Wu, Y.; Sun, L.; Fan, X.; Yuan, Y.; Li, J. Metformin promotes anticancer activity of NK cells in a p38 MAPK dependent manner. Oncoimmunology 2021, 10, 1995999. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Yang, F.; He, Z.; Cai, Y. iTRAQ-based quantitative proteomic analysis of the inhibition of cervical cancer cell invasion and migration by metformin. Biomed. Pharmacother. 2020, 123, 109762. [Google Scholar] [CrossRef] [PubMed]
- Iyoda, T.; Yamasaki, S.; Ueda, S.; Shimizu, K.; Fujii, S.I. Natural Killer T and Natural Killer Cell-Based Immunotherapy Strategies Targeting Cancer. Biomolecules 2023, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Terabe, M.; Berzofsky, J.A. The role of NKT cells in tumor immunity. Adv. Cancer Res. 2008, 101, 277–348. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Harada, M.; Dashtsoodol, N.; Kojo, S. Discovery of NKT cells and development of NKT cell-targeted anti-tumor immunotherapy. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 2015, 91, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Ugolini, S.; Blaise, D.; Chabannon, C.; Brossay, L. Targeting natural killer cells and natural killer T cells in cancer. Nat. Rev. Immunol. 2012, 12, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Krijgsman, D.; Hokland, M.; Kuppen, P.J.K. The Role of Natural Killer T Cells in Cancer-A Phenotypical and Functional Approach. Front. Immunol. 2018, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, eaan5931. [Google Scholar] [CrossRef] [PubMed]
- Kmieciak, M.; Basu, D.; Payne, K.; Toor, A.; Yacoub, A.; Wang, X.Y.; Smith, L.; Bear, H.D.; Manjili, M.H. Activated NKT cells and NK cells render T cells resistant to myeloid-derived suppressor cells and result in an effective adoptive cellular therapy against breast cancer in the FVBN202 transgenic mouse. J. Immunol. 2011, 187, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Sack, B.; Baldwin, M.; Vaughan, A.; Kappe, S. Interferon-Mediated Innate Immune Responses against Malaria Parasite Liver Stages. Cell Rep. 2014, 7, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Buschard, K.; Hansen, A.K.; Jensen, K.; Lindenbergh-Kortleve, D.J.; de Ruiter, L.F.; Krohn, T.C.; Hufeldt, M.R.; Vogensen, F.K.; Aasted, B.; Osterbye, T.; et al. Alcohol facilitates CD1d loading, subsequent activation of NKT cells, and reduces the incidence of diabetes in NOD mice. PLoS ONE 2011, 6, e17931. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rhost, S.; Löfbom, L.; Cardell, S.L. Defining a novel subset of CD1d-dependent type II natural killer T cells using natural killer cell-associated markers. Scand. J. Immunol. 2019, 90, e12794. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bloushtain, N.; Qimron, U.; Bar-Ilan, A.; Hershkovitz, O.; Gazit, R.; Fima, E.; Korc, M.; Vlodavsky, I.; Bovin, N.V.; Porgador, A. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J. Immunol. 2004, 173, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Wingender, G.; Krebs, P.; Beutler, B.; Kronenberg, M. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178-dependent and is correlated with antigenic potency. J. Immunol. 2010, 185, 2721–2729. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Molling, J.W.; von Mensdorff-Pouilly, S.; Verheijen, R.H.; Hooijberg, E.; Kramer, D.; Reurs, A.W.; van den Eertwegh, A.J.; von Blomberg, B.M.; Scheper, R.J.; et al. IFN-gamma-producing human invariant NKT cells promote tumor-associated antigen-specific cytotoxic T cell responses. J. Immunol. 2008, 181, 2446–2454. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, K.; Smith, C.; Bonifaz, L.; Steinman, R.M. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 2003, 198, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Yamashita, N.; Ogawa, T.; Koseki, K.; Warabi, E.; Ohue, T.; Komatsu, M.; Matsushita, H.; Kakimi, K.; Kawakami, E.; et al. Mitochondrial reactive oxygen species trigger metformin-dependent antitumor immunity via activation of Nrf2/mTORC1/p62 axis in tumor-infiltrating CD8T lymphocytes. J. Immunother. Cancer 2021, 9, e002954. [Google Scholar] [CrossRef]
- Engelmann, P.; Farkas, K.; Kis, J.; Richman, G.; Zhang, Z.; Liew, C.W.; Borowiec, M.; Niewczas, M.A.; Jalahej, H.; Orbán, T. Characterization of human invariant natural killer T cells expressing FoxP3. Int. Immunol. 2011, 8, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Nemunaitis, J.; Fong, T.; Shabe, P.; Martineau, D.; Ando, D. Comparison of serum interleukin-10 (IL-10) levels between normal volunteers and patients with advanced melanoma. Cancer Investig. 2001, 19, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Snyder-Cappione, J.E.; Nixon, D.F.; Chi, J.C.; Nguyen, M.L.; Kirby, C.K.; Milush, J.M.; Koth, L.L. Invariant natural killer T (iNKT) cell exhaustion in sarcoidosis. Eur. J. Immunol. 2013, 43, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Maruyama, T.; Saito, N.; Koganei, S.; Yamamoto, K.; Matsumoto, N. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J. Exp. Med. 2006, 203, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Amin, D.; Richa, T.; Mollaee, M.; Zhan, T.; Tassone, P.; Johnson, J.; Luginbuhl, A.; Cognetti, D.; Martinez-Outschoorn, U.; Stapp, R.; et al. Metformin Effects on FOXP3(+) and CD8(+) T Cell Infiltrates of Head and Neck Squamous Cell Carcinoma. Laryngoscope 2020, 130, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Croudace, J.E.; Curbishley, S.M.; Mura, M.; Willcox, C.R.; Illarionov, P.A.; Besra, G.S.; Adams, D.H.; Lammas, D.A. Identification of distinct human invariant natural killer T-cell response phenotypes to alpha-galactosylceramide. BMC Immunol. 2008, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, F.; Tian, Y.; Cao, L.; Gao, Q.; Zhang, C.; Zhang, K.; Shen, C.; Ping, Y.; Maimela, N.R.; et al. Metformin Enhances the Antitumor Activity of CD8(+) T Lymphocytes via the AMPK-miR-107-Eomes-PD-1 Pathway. J. Immunol. 2020, 204, 2575–2588. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Lian, J.; Huang, L.; Zhao, Q.; Liu, S.; Zhang, Z.; Chen, X.; Yue, D.; Li, L.; Li, F.; et al. Metformin blocks myeloid-derived suppressor cell accumulation through AMPK-DACH1-CXCL1 axis. Oncoimmunology 2018, 7, e1442167. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, S.; Clattenburg, D.R.; Slauenwhite, D.; Lobert, L.; Johnston, B. Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology 2015, 4, e995562. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Shimizu, K.; Okamoto, Y.; Kunii, N.; Nakayama, T.; Motohashi, S.; Taniguchi, M. NKT cells as an ideal anti-tumor immunotherapeutic. Front. Immunol. 2013, 4, 409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, X. Cross Talk Between Natural Killer T and Dendritic Cells and Its Impact on T Cell Responses in Infections. Front. Immunol. 2022, 13, 837767. [Google Scholar] [CrossRef] [PubMed]
- Glosson-Byers, N.L.; Sehra, S.; Kaplan, M.H. STAT4 is required for IL-23 responsiveness in Th17 memory cells and NKT cells. Jakstat 2014, 3, e955393. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, V.; Zullo, A.J.; Schweitzer, M.N.; Staton, T.L.; Gallo, E.M.; Crabtree, G.R.; Glimcher, L.H. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat. Immunol. 2009, 10, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Huang, J.R.; Tsai, Y.C.; Hung, J.T.; Wu, D.; Fujio, M.; Wong, C.H.; Yu, A.L. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc. Natl. Acad. Sci. USA 2007, 104, 10299–10304. [Google Scholar] [CrossRef] [PubMed]


| Cell Marker Combination | Cell Type Identified | Role in Tumor Development | Reference |
|---|---|---|---|
| CD11b+CD11− | Myeloid-Derived Suppressor cells (MDSCs) | MDSCs are important in establishing an immunosuppressive tumor microenvironment. They inhibit the cytotoxic functions of T cells and NKT cells and recruit Tregs, which collectively contribute to tumor immune evasion and progression. | [30] |
| CD4+CD25+ | Regulatory T cells (Tregs) | Tregs maintain immunological tolerance and suppress anti-tumor immune responses by inhibiting effector T cells and NKT cells through the secretion of inhibitory cytokines like IL-10 and TGF-β. Their suppressive activities are associated with a reduction in overall survival rates in various cancers. | [28] |
| CD3+CD49− | T cells | T cells are essential for adaptive immunity and can directly attack tumor cells. Their activity can be suppressed by Tregs and MDSCs within the immunosuppressive tumor microenvironment. Enhancing their activation and persistence can be pivotal for effective immunotherapy. | [39] |
| CD3+CD49+ | Natural Killer T cells (NKT) cells | NKT cells can function both as tumor-promoters or tumor-inhibitors depending on their subtype and the cytokines they are exposed to. They interact dynamically with MDSCs, Tregs, and dendritic cells, playing a complex role in balancing immune surveillance and tumor promotion. | [29] |
| Gene | Primer | Sequences (5′-3′) |
|---|---|---|
| GAPDH | Forward | GTCTCCTCTGACTTCAACAGCG |
| GAPDH | Reverse | ACCACCCTGTTGCTGTAGCCAA |
| MIP-1a | Forward | CTCACCTGCTGCTACTCATTC |
| MIP-1a | Reverse | CATGATGTTGAGCAGGTGACAGA |
| NFAT | Forward | GGTGCCTTTTGCGAGCAGTATC |
| NFAT | Reverse | CGTATGGACCAGAATGTGACGG |
| STAT4 | Forward | GCTGAATGACGGTGCAAACGG |
| STAT4 | Reverse | GACAGTGGGAGTGGCACCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovic, A.; Jovanovic, I.; Stojanovic, B.; Dimitrijevic Stojanovic, M.; Stojanovic, B.S.; Jurisevic, M.; Simovic Markovic, B.; Jovanovic, M.; Jovanovic, M.; Jovanovic, M.; et al. Harnessing Metformin’s Immunomodulatory Effects on Immune Cells to Combat Breast Cancer. Int. J. Mol. Sci. 2024, 25, 5869. https://doi.org/10.3390/ijms25115869
Petrovic A, Jovanovic I, Stojanovic B, Dimitrijevic Stojanovic M, Stojanovic BS, Jurisevic M, Simovic Markovic B, Jovanovic M, Jovanovic M, Jovanovic M, et al. Harnessing Metformin’s Immunomodulatory Effects on Immune Cells to Combat Breast Cancer. International Journal of Molecular Sciences. 2024; 25(11):5869. https://doi.org/10.3390/ijms25115869
Chicago/Turabian StylePetrovic, Andjela, Ivan Jovanovic, Bojan Stojanovic, Milica Dimitrijevic Stojanovic, Bojana S. Stojanovic, Milena Jurisevic, Bojana Simovic Markovic, Marina Jovanovic, Milan Jovanovic, Mihailo Jovanovic, and et al. 2024. "Harnessing Metformin’s Immunomodulatory Effects on Immune Cells to Combat Breast Cancer" International Journal of Molecular Sciences 25, no. 11: 5869. https://doi.org/10.3390/ijms25115869
APA StylePetrovic, A., Jovanovic, I., Stojanovic, B., Dimitrijevic Stojanovic, M., Stojanovic, B. S., Jurisevic, M., Simovic Markovic, B., Jovanovic, M., Jovanovic, M., Jovanovic, M., & Gajovic, N. (2024). Harnessing Metformin’s Immunomodulatory Effects on Immune Cells to Combat Breast Cancer. International Journal of Molecular Sciences, 25(11), 5869. https://doi.org/10.3390/ijms25115869






