Virophages, Satellite Viruses, Virophage Replication and Its Effects and Virophage Defence Mechanisms for Giant Virus Hosts and Giant Virus Defence Systems against Virophages
Abstract
1. Characteristics of Virophages
2. Virophages and Satellite Viruses
3. Virophage Replication and Its Effects
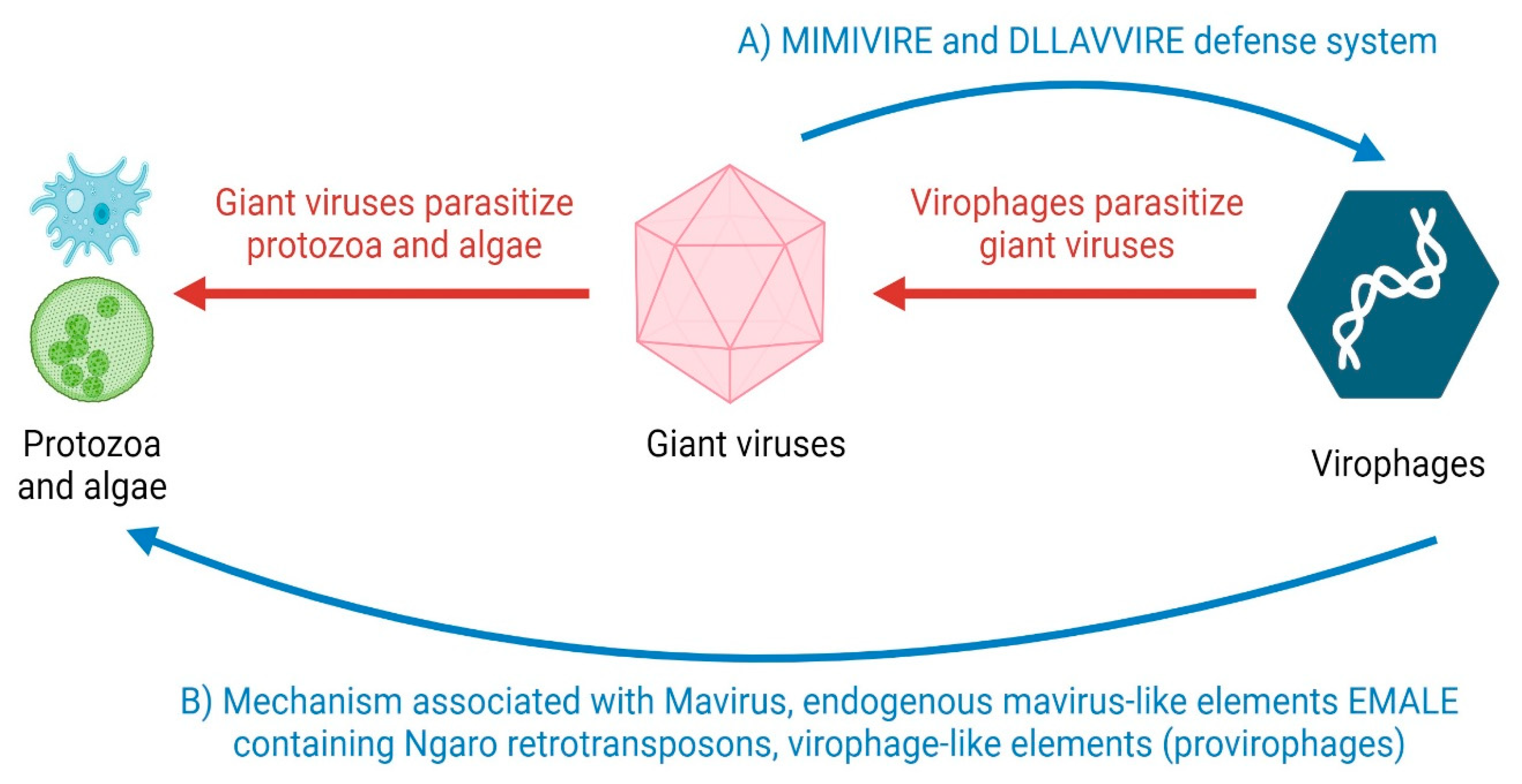
4. Mechanisms of Defensive Action of Virophages for Giant Virus Hosts
5. Defence Systems of Giant Viruses against Virophages
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- La Scola, B.; Desnues, C.; Pagnier, I.; Robert, C.; Barrassi, L.; Fournous, G.; Merchat, M.; Suzan-Monti, M.; Forterre, P.; Koonin, E.; et al. The Virophage as a Unique Parasite of the Giant Mimivirus. Nature 2008, 455, 100–104. [Google Scholar] [CrossRef]
- Mougari, S.; Sahmi-Bounsiar, D.; Levasseur, A.; Colson, P.; La Scola, B. Virophages of Giant Viruses: An Update at Eleven. Viruses 2019, 11, 733. [Google Scholar] [CrossRef]
- Rolland, C.; Andreani, J.; Louazani, A.C.; Aherfi, S.; Francis, R.; Rodrigues, R.; Silva, L.S.; Sahmi, D.; Mougari, S.; Chelkha, N.; et al. Discovery and Further Studies on Giant Viruses at the IHU Mediterranee Infection That Modified the Perception of the Virosphere. Viruses 2019, 11, 312–339. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Niedźwiedzka-Rystwej, P.; Czupryńska, P.; Deptuła, W. Protozoal Giant Viruses: Agents Potentially Infectious to Humans and Animals. Virus Genes 2019, 55, 574–591. [Google Scholar] [CrossRef]
- Schulz, F.; Yutin, N.; Ivanova, N.N.; Ortega, D.R.; Lee, T.K.; Vierhelig, J.; Daims, H.; Horn, M.; Wagner, M.; Jensen, G.J.; et al. Giant Viruses with an Expanded Complement of Translation System Components. Science 2017, 356, 82–85. [Google Scholar] [CrossRef]
- Andrade, A.C.; Arantes, T.S.; Rodrigues, R.A.L.; Machado, T.B.; Dornas, F.P.; Landell, M.F.; Furst, C.; Borges, L.G.A.; Dutra, L.A.L.; Almeida, G.; et al. Ubiquitous Giant: A Plethora of Giant Viruses Found in Brazil and Antarctica. Virol. J. 2018, 15, 22. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bekliz, M.; Colson, P.; La Scola, B. The Expanding Family of Virophages. Viruses 2016, 8, 317. [Google Scholar] [CrossRef]
- Ruiz-Saenz, J.; Rodas, J.D. Viruses, Virophages, and Their Living Nature. Acta Virol. 2010, 54, 85–90. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Czupryńska, P.; Poniewierska-Baran, A.; Deptuła, W. Characteristics of Virophages and Giant Viruses. Acta. Bioch. Pol. 2018, 65, 487–496. [Google Scholar] [CrossRef]
- Andreani, J.; Khalil, J.Y.B.; Baptiste, E.; Hasni, I.; Michelle, C.; Raoult, D.; Levasseur, A.; La Scola, B. A New Virus among the Giant Viruses. Front. Microbiol. 2018, 8, 2643. [Google Scholar] [CrossRef]
- Mihara, T.; Koyano, H.; Hingamp, P.; Grimsley, N.; Goto, S.; Ogata, H. Taxon Rihness of „Megaviridae” Exceeds Those of Bacteria and Arhaea in the Ocean. Microbes Environ. 2018, 33, 162–171. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Weinheimer, A.R.; Martinez-Gutierrez, C.A.; Aylward, F.O. Widespread Endogenization of Giant Viruses Shapes Genomes of Green Algae. Nature 2020, 588, 141–145. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Martinez-Gutierrez, C.A.; Weinheimer, A.R.; Aylward, F.O. Dynamic Genome Evolution and Complex Virocell Metabolizm of Globally-Distributed Giant Viruses. Nat. Commun. 2020, 11, 1710. [Google Scholar] [CrossRef]
- del Arco, A.; Fischer, M.G.; Becks, L. Evolution of Virus and Virophage Facilitates Persistence in a Tripartite Microbial System. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nino Barreat, J.G.; Katzourakis, A. Ecological and Evolutionary Dynamics of Cell-Virus-Virophage Systems. PLoS Comput. Biol. 2024, 20. [Google Scholar] [CrossRef]
- Taylor, B.P.; Cortez, M.H.; Weitz, J.S. The Virus of My Virus Is My Friend: Ecological Effects of Virophage with Alternative Modes of Coinfection. J. Theor. Biol. 2014, 354, 124–136. [Google Scholar] [CrossRef]
- Fischer, M.G.; Suttle, C.A. A Virophage at the Origin of Large DNA Transposons. Science 2011, 8, 231–234. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Chrzanowska, S.; Gurgacz, N.; Stosik, M.; Deptuła, W. Virophages—Facts Known and Unknown. Viruses 2023, 15, 1321. [Google Scholar] [CrossRef]
- Colson, P.; Lamballerie, X.; Fournous, G.; Raoult, D. Reclassification of Giant Viruses Composing a Fourth Domain of Life in the New Order Megavirales. Curr. Opin. Microbiol. 2016, 31, 16–24. [Google Scholar] [CrossRef]
- Boughalmi, M.; Pagnier, I.; Aherfi, S.; Colson, P.; Raoult, D.; La Scola, B. First Isolation of a Marseillevirus in the Diptera Syrphidae Eristalis Tenax. Intervirology 2013, 56, 386–394. [Google Scholar] [CrossRef]
- Andrade, K.R.; Borrato, P.P.V.M.; Rodrigues, F.P.; Silva, L.C.F.; Dornas, F.P.; Pilotto, M.R.; La Scola, B.; Almeida, G.M.F.; Kroon, E.G.; Abrahao, J.S. Oysters as Hot Spots for Mimivirus Isolation. Arch. Virol. 2015, 160, 477–482. [Google Scholar] [CrossRef]
- Abrahão, J.S.; Dornas, F.P.; Silva, L.C.F.; Almeida, G.M.; Boratto, P.V.M.; Colson, P.; La Scola, B.; Kroon, E.G. Acanthamoeba Polyphaga Mimivirus and Other Giant Viruses: An Open Field to Outstanding Discoveries. Virol. J. 2014, 11, 120. [Google Scholar] [CrossRef]
- Desnues, C.; Raoult, D. Virophages Question the Existence of Satellites. Nat. Rev. Genet. 2012, 10, 234–235. [Google Scholar] [CrossRef]
- Fischer, M.G. The Virophage Family Lavidaviridae. Curr. Issues Mol. Biol. 2021, 40, 1–24. [Google Scholar] [CrossRef]
- Marie, V.; Lin, J. Cannibalistic Viruses in the Aquatic Environment: Role of Virophages in Manipulating Microbial Communities. Int. J. Environ. Sci. Technol. 2016, 13, 2097–2104. [Google Scholar] [CrossRef]
- Krupovic, M.; Kuhn, J.H.; Fischer, M.G. A Classification System for Virophages and Satellite Viruses. Arch. Virol. 2016, 161, 233–247. [Google Scholar] [CrossRef]
- Aherfi, S.; Colson, P.; La Scola, B.; Raoult, D. Giant Viruses of Amoebas: An Update. Front. Microbiol. 2016, 7, 349. [Google Scholar] [CrossRef]
- Krupovic, M.; Cvirkaite-Krupovic, V. Virophages or Satellite Viruses? Nat. Rev. Microbiol. 2011, 9, 762–764. [Google Scholar] [CrossRef]
- Jeudy, S.; Garcin, E.; Schmitt, A.; Abergel, C. Structures of Two Main Components of the Virophage and Marseilleviridae Virions Extend the Range of Unrelated Viruses Using Fiber Head as Common Receptor Binding Fold. bioRxiv 2023. [Google Scholar] [CrossRef]
- Paez-Espino, D.; Zhou, J.; Roux, S.; Nayfach, S.; Pavlopoulos, G.A.; Schulz, F.; McMahon, K.D.; Walsh, D.; Woyke, T.; Ivanova, N.N.; et al. Diversity, Evolution, and Classification of Virophages Uncovered through Global Metagenomics. Microbiome 2019, 7, 157. [Google Scholar] [CrossRef]
- Yau, S.; Lauro, F.M.; DeMaere, M.Z.; Brown, M.V.; Thomas, T.; Raftery, M.J.; Andrews-Pfannkoch, C.; Lewis, M.; Hoffman, J.M.; Gibson, J.A.; et al. Virophage Control of Antarctic Algal Host–Virus Dynamics. Proc. Natl. Acad. Sci. USA 2011, 108, 6163–6168. [Google Scholar] [CrossRef]
- Fischer, M.G. Sputnik and Mavirus: More than Just Satellite Viruses. Nat. Rev. Microbiol. 2012, 10, 78–79. [Google Scholar] [CrossRef]
- Katzourakis, A.; Aswad, A. The Origins of Giant Viruses, Virophages and Their Relatives in Host Genomes. BMC Biol. 2014, 12, 51–54. [Google Scholar] [CrossRef]
- Desnues, C.; Boyer, M.; Raoult, D. Chapter 3—Sputnik, a Virophage Infecting the Viral Domain of Life. In Bacteriophages, Part A; Łobocka, M., Szybalski, W.T., Eds.; Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2012; Volume 82, pp. 63–89. [Google Scholar]
- Gaia, M.; Pagnier, I.; Campocasso, A.; Fournous, G.; Raoult, D.; La Scola, B. Broad Spectrum of Mimiviridae Virophage Allows Its Isolation Using a Mimivirus Reporter. PLoS ONE 2013, 8, e61912. [Google Scholar] [CrossRef]
- Azevedo, B.L.D.; Júnior, J.P.A.; João, P.A.; Ullmann, L.S.; Rodrigues, R.A.L.; Abrahão, J.S. The Discovery of a New Mimivirus Isolate in Association with Virophage-Transpoviron Elements in Brazil Highlights the Main Genomic and Evolutionary Features of This Tripartite System. Viruses 2022, 14, 206. [Google Scholar] [CrossRef]
- Dutta, D.; Ravichandiran, V.; Sukla, S. Virophages: Association with Human Diseases and Their Predicted Role as Virus Killers. Pathog. Dis. 2021, 79, ftab049. [Google Scholar] [CrossRef]
- Borges, I.A.; de Assis, F.L.; Silva, L.K.d.S.; Abrahão, J. Rio Negro Virophage: Sequencing of the near Complete Genome and Transmission Electron Microscopy of Viral Factories and Particles. Braz. J. Microbiol. 2018, 49, 260–261. [Google Scholar] [CrossRef]
- Hackl, T.; Duponchel, S.; Barenhoff, K.; Weinmann, A.; Fischer, M.G. Virophages and Retrotransposons Colonize the Genomes of a Heterotrophic Flagellate. Elife 2021, 10, e72674. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.; Weiel, M.; Winkler, A.; Schug, A.; Reinstein, J. The Trimeric Major Capsid Protein of Mavirus Is Stabilized by Its Interlocked N-Termini Enabling Core Flexibility for Capsid Assembly. J. Mol. Biol. 2021, 433, 166859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, D.; Childers, A.; McDermott, T.R.; Wang, Y.; Liles, M.R. Three Novel Virophage Genomes Discovered from Yellowstone Lake Metagenomes. J. Virol. 2015, 89, 1278–1285. [Google Scholar] [CrossRef]
- Gaia, M.; Benamar, S.; Boughalmi, M.; Pagnier, I.; Croce, O.; Colson, P.; Raoult, D.; La Scola, B. Zamilon, a Novel Virophage with Mimiviridae Host Specificity. PLoS ONE 2014, 9, e94923. [Google Scholar] [CrossRef] [PubMed]
- Blanc, G.; Gallot-Lavallée, L.; Maumus, F. Provirophages in the Bigelowiella Genome Bear Testimony to Past Encounters with Giant Viruses. Proc. Natl. Acad. Sci. USA 2015, 112, E5318–E5326. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Zhang, W.; Zhou, X.; Wang, H.; Sun, G.; Xiao, J.; Pan, Y.; Yan, S.; Wang, Y. Novel Virophages Discovered in a Freshwater Lake in China. Front. Microbiol. 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wu, Z.; Xu, S.; Wang, Y. Isolation and Identification of a Large Green Alga Virus (Chlorella Virus XW01) of Mimiviridae and Its Virophage (Chlorella Virus Virophage SW01) by Using Unicellular Green Algal Cultures. J. Virol. 2022, 96, e02114-21. [Google Scholar] [CrossRef] [PubMed]
- Stough, J.M.A.; Yutin, N.; Chaban, Y.V.; Moniruzzaman, M.; Gann, E.R.; Pound, H.L.; Steffen, M.M.; Black, J.N.; Koonin, E.V.; Wilhelm, S.W.; et al. Genome and Environmental Activity of a Chrysochromulina Parva Virus and Its Virophages. Front. Microbiol. 2019, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Chan, L.-K.; Egan, R.; Malmstrom, R.R.; McMahon, K.D.; Sullivan, M.B. Ecogenomics of Virophages and Their Giant Virus Hosts Assessed through Time Series Metagenomics. Nat. Commun. 2017, 8, 858. [Google Scholar] [CrossRef] [PubMed]
- Bekliz, M.; Verneau, J.; Benamar, S.; Raoult, D.; La Scola, B.; Colson, P. A New Zamilon-like Virophage Partial Genome Assembled from a Bioreactor Metagenome. Front. Microbiol. 2015, 6, 1308. [Google Scholar] [CrossRef] [PubMed]
- Hauroder, B.W.C. New Giant Virus in Free-Living Amoeba, Wiley Analytical Science. Available online: https://analyticalscience.wiley.com/content/article-do/new-giant-virus-free-living-amoeba (accessed on 1 January 2024).
- Xu, S.; Zhou, L.; Liang, X.; Zhou, Y.; Chen, H.; Yan, S.; Wang, Y. Novel Cell-Virus-Virophage Tripartite Infection Systems Discovered in the Freshwater Lake Dishui Lake in Shanghai, China. J. Virol. 2020, 94, e00149-20. [Google Scholar] [CrossRef]
- Bäckström, D.; Yutin, N.; Jørgensen, S.L.; Dharamshi, J.; Homa, F.; Zaremba-Niedwiedzka, K.; Spang, A.; Wolf, Y.I.; Koonin, E.V.; Ettema, T.J.G. Virus Genomes from Deep Sea Sediments Expand the Ocean Megavirome and Support Independent Origins of Viral Gigantism. mBio 2019, 10, e02497-18. [Google Scholar] [CrossRef]
- Mougari, S.; Bekliz, M.; Abrahao, J.; Di Pinto, F.; Levasseur, A.; La Scola, B. Guarani Virophage, a New Sputnik-Like Isolate From a Brazilian Lake. Front. Microbiol. 2019, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Bellas, C.M.; Sommaruga, R. Polinton-like Viruses Are Abundant in Aquatic Ecosystems. Microbiome 2021, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Potapov, S.A.; Belykh, O.I. Virophages Found in Viromes from Lake Baikal. Biomolecules 2023, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Nino Barreat, J.G.; Katzourakis, A. A billion years arms-race between viruses, virophages, and eukaryotes. Elife 2023, 12, RP86617. [Google Scholar] [CrossRef]
- Virus Taxonomy—ICTV. 2022. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 1 January 2024).
- Fischer, M.G. Virophages go nuclear in the marine alga Bigelowiella natans. Proc. Natl. Acad. Sci. USA 2015, 112, 11750–11751. [Google Scholar] [CrossRef]
- Nasrin, T.; Hoque, M.; Ali, S. Microsatellite Signature Analysis of Twenty-One Virophage Genomes of the Family Lavidaviridae. Gene 2023, 851, 147037. [Google Scholar] [CrossRef]
- Roitman, S.; Rozenberg, A.; Lavy, T.; Brussaard, C.P.D.; Kleifeld, O.; Béjà, O. Isolation and Infection Cycle of a Polinton-like Virus Virophage in an Abundant Marine Alga. Nat. Microbiol. 2023, 8, 332–346. [Google Scholar] [CrossRef]
- Kalafati, E.; Papanikolaou, E.; Marinos, E.; Anagnou, N.P.; Pappa, K.I. Mimiviruses: Giant Viruses with Novel and Intriguing Features. Mol. Med. Rep. 2022, 25, 207. [Google Scholar] [CrossRef]
- Roux, S.; Fischer, M.G.; Hackl, T.; Katz, L.A.; Schulz, F.; Yutin, N. Updated Virophage Taxonomy and Distinction from Polinton-like Viruses. Biomolecules 2023, 13, 204. [Google Scholar] [CrossRef]
- Tokarz-Deptuła, B.; Śliwa-Dominiak, J.; Kubiś, M.; Deptuła, W. Mimiwirus APMV, APMV, Mimivirus Mamavirus and Its Virophage—Structure and Characteristic. Postępy Mikrobiol. 2013, 52, 105–109. (In Polish) [Google Scholar]
- Tokarz-Deptuła, B.; Śliwa-Dominiak, J.; Adamski, M.; Kubiś, M.; Ogórkiewicz, A.; Deptuła, W. Virophages—New Biological Elements. Postępy Mikrobiol. 2015, 54, 217–223. (In Polish) [Google Scholar]
- Duponchel, S.; Fischer, M.G. Viva Lavidaviruses! Five Features of Virophages That Parasitize Giant DNA Viruses. PLoS Pathog. 2019, 21, e1007592. [Google Scholar] [CrossRef] [PubMed]
- Born, D.; Reuter, L.; Mersdorf, U.; Mueller, M.; Fischer, M.G.; Meinhart, A.; Reinstein, J. Capsid Protein Structure, Self-Assembly, and Processing Reveal Morphogenesis of the Marine Virophage Mavirus. Proc. Natl. Acad. Sci. USA 2018, 115, 7332–7337. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.G.; Hackl, T. Host Genome Integration and Giant Virus-Induced Reactivation of the Virophage Mavirus. Nature 2016, 540, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Azza, S.; Barrassi, L.; Klose, T.; Campocasso, A.; Pagnier, I.; Fournous, G.; Borg, A.; Robert, C.; Zhang, X.; et al. Mimivirus Shows Dramatic Genome Reduction after Intraamoebal Culture. Proc. Natl. Acad. Sci. USA 2011, 108, 10296–10301. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Krupovic, M. Polintons, Virophages and Transpovirons: A Tangled Web Linking Viruses, Transposons and Immunity. Curr. Opin. Virol. 2017, 25, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, A.; Bekliz, M.; Chabriele, E.; Pontarotti, P.; La Scola, B.; Raoult, D. MIMIVIRE Is a Defence System in Mimivirus That Confers Resistance to Virophage. Nature 2016, 531, 249–252. [Google Scholar] [CrossRef]
- Mortensen, K.; Lam, T.J.; Ye, Y. Comparison of CRISPR-Cas Immune Systems in Healthcare-Related Pathogens. Front. Micriobiol. 2021, 12, 758782. [Google Scholar] [CrossRef]
| Virophages with the Described “Host” and Its Host Cell | |||||
|---|---|---|---|---|---|
| No. | Name of the Virophage | Name of the Giant Virus and/or Its Family and Genus | Eucaryotic Host of the Giant Virus | Year of Statement and/or Description | References |
| 1. | Sputnik | Mamavirus ACMV (Acanthaomeba castellanii mamavirus) | Acanthamoeba (A.) castellani | 2008 | [35] |
| Mimivirus APMV (Acanthamoeba polyphaga mimivirus) | A. polyphaga | [35] | |||
| 2. | Sputnik 2 | Lentille virus | A. polyphaga | 2012 | [35] |
| 3. | Sputnik 3 | Mimiviridae mainly of the C lineage or virophage is “free” of the giant virus | A. polyphaga for viruses of the genus Mimivirus | 2013 | [36] |
| 4. | Sputnik argentum | Mimivirus argentum | Probably genus amoebas A. castellani | 2022 | [37] |
| 5. | Mavirus | Cafeteria roenbergensis virus (CroV) | Flagellate Cafeteria roenbergensis | 2010 | [38] |
| 6. | Organic Lake Virophage (OLV) | Viruses of the Phycodnaviridae family | Phototrophic marine algae—unnamed | 2011 | [32] |
| 7. | Rio Negro Virophage (RNV) | Samba virus | A. castellanii | 2011 | [18,39] |
| 8. | Phaeocystis Globosa Virus Virophage (PGVV) | PgV-16T virus (Phaeocystis globose virus) | Algae of the genus Phaeocystis | 2013 | [40] |
| 9. | Ace Lake Mavirus (ALM) | Probably viruses from the Mimiviridae family | Protozoa unspecified | 2013 | [41] |
| 10. | Yellowstone Lake Virophages 1 (YSLV 1) | Probably viruses from the Phycodnaviridae or Mimiviridae families | Unnamed algae or unspecified amoebas | 2013 | [41,42] |
| 11. | YSLV 2 | ||||
| 12. | YSLV 3 | ||||
| 13. | YSLV 4 | ||||
| 14. | Zamilon | Mont1 virus | A. polyphaga | 2014 | [43] |
| 15. | Rumen virophage (RVP) | Probably viruses from the Mimiviridae family | Indeterminate eukaryotic host—protists | 2015 | [21] |
| 16. | (Dishui Lake Virophage 1 (DSLV 1) | Probably viruses from the family Phycodnaviridae | Freshwater algae, unspecified | 2016 | [44] |
| 17. | Qinghai Lake Virophage (QLV) | Probably viruses from the family Phycodnaviridae | Freshwater algae, unspecified | 2016 | [45] |
| 18. | Platanovirus saccamoebae virophage “Comedo” | KSLT virus probably belongs to the Mimiviridae family | Saccamoeba lacustris | 2018 | [46] |
| 19. | CpV-PLV Curly | CpV-BQ2 virus | Fresh water algae—Chrysochromulina parva | 2019 | [47] |
| 20. | CpV-PLV Moe | ||||
| 21. | CpV-PLV Larry | ||||
| 22. | Chlorella virus virophage (CVV-SW01) | Chlorella virus—CV-XW01 | Freshwater algae of the genus Chlorella | 2022 | [48] |
| Virophages with an undescribed or probable “host” and their possible host cell | |||||
| 1. | YSLV 5 | Undefined | Undefined | 2013 | [42] |
| 2. | YSLV 6 | ||||
| 3. | YSLV 7 | ||||
| 4. | Zamilon 2 | Probably giant viruses—unspecified | Probably amoebas of the genus Acanthamoeba | 2015 | [49] |
| 5. | Virophages from Lake Mendota and Trout Bog fen | Undefined | Undefined | 2017 | [50] |
| 6. | Dishui Lake virophages 2 (DSLV 2) | Probably giant viruses of the family Phycodnaviridae and possible viruses of the genus Mimivirus | Freshwater algae unspecified and/or amoebas | 2018 | [51] |
| 7. | Dishui Lake virophages 3 (DSLV 3) | ||||
| 8. | Dishui Lake virophages 4 (DSLV 4) | ||||
| 9. | Dishui Lake virophages 5 (DSLV 5) | ||||
| 10. | Dishui Lake virophages 6 (DSLV 6) | ||||
| 11. | Dishui Lake virophages 7 (DSLV 7) | ||||
| 12. | Dishui Lake virophages 8 (DSLV 8) | ||||
| 13. | Loki’s Castle Virophage 1 (LCV 1) | Viruses of the genus Mimivirus, and maybe Pitoviruses | Undefined | 2019 | [52] |
| 14. | Loki’s Castle Virophage 2 (LCV 2) | ||||
| 15. | Guarani | The virophage is “free” of the giant virus, or they are viruses from the Mimiviridae family | For viruses of the genus Mimivirus, possibly unnamed amoebas and/or marine protists | 2019 | [53] |
| 16. | Sisivirophage | Undefined | Undefined | 2019 | [25] |
| 17. | Virophages from Lake Gossenköllesee | Undefined | Undefined | 2021 | [54] |
| 18. | Virophages from Lake Baikal and strait Maloye More | Probably giant viruses of the family Mimiviridae | Undefined | 2023 | [55] |
| Features and Types of Microorganisms | Genetic Material | Defence Mechanisms and Systems |
|---|---|---|
| Virophages and virophage-like elements | dsDNA | Mechanism related with:
|
| Satellite viruses | ssDNa and ssRNA | None |
| Giant viruses | dsDNA | MIMIVIRE and DLLAVVIRE system |
| Bacteria and archaeons | DNA and RNA | CRISPR/Cas system |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarz-Deptuła, B.; Chrzanowska, S.; Baraniecki, Ł.; Gurgacz, N.; Stosik, M.; Sobolewski, J.; Deptuła, W. Virophages, Satellite Viruses, Virophage Replication and Its Effects and Virophage Defence Mechanisms for Giant Virus Hosts and Giant Virus Defence Systems against Virophages. Int. J. Mol. Sci. 2024, 25, 5878. https://doi.org/10.3390/ijms25115878
Tokarz-Deptuła B, Chrzanowska S, Baraniecki Ł, Gurgacz N, Stosik M, Sobolewski J, Deptuła W. Virophages, Satellite Viruses, Virophage Replication and Its Effects and Virophage Defence Mechanisms for Giant Virus Hosts and Giant Virus Defence Systems against Virophages. International Journal of Molecular Sciences. 2024; 25(11):5878. https://doi.org/10.3390/ijms25115878
Chicago/Turabian StyleTokarz-Deptuła, Beata, Sara Chrzanowska, Łukasz Baraniecki, Natalia Gurgacz, Michał Stosik, Jarosław Sobolewski, and Wiesław Deptuła. 2024. "Virophages, Satellite Viruses, Virophage Replication and Its Effects and Virophage Defence Mechanisms for Giant Virus Hosts and Giant Virus Defence Systems against Virophages" International Journal of Molecular Sciences 25, no. 11: 5878. https://doi.org/10.3390/ijms25115878
APA StyleTokarz-Deptuła, B., Chrzanowska, S., Baraniecki, Ł., Gurgacz, N., Stosik, M., Sobolewski, J., & Deptuła, W. (2024). Virophages, Satellite Viruses, Virophage Replication and Its Effects and Virophage Defence Mechanisms for Giant Virus Hosts and Giant Virus Defence Systems against Virophages. International Journal of Molecular Sciences, 25(11), 5878. https://doi.org/10.3390/ijms25115878








