Abstract
Xanthomonas oryzae pv. oryzicola (Xoc) is a notorious plant pathogen. Like most bacterial pathogens, Xoc has evolved a complex regulatory network to modulate the expression of various genes related to pathogenicity. Here, we have identified TfmR, a transcriptional regulator belonging to the TetR family, as a key player in the virulence mechanisms of this phytopathogenic bacterium. We have demonstrated genetically that tfmR is involved in the hypersensitive response (HR), pathogenicity, motility and extracellular polysaccharide production of this phytopathogenic bacterium. Our investigations extended to exploring TfmR’s interaction with RpfG and HrpX, two prominent virulence regulators in Xanthomonas species. We found that TfmR directly binds to the promoter region of RpfG, thereby positively regulating its expression. Notably, constitutive expression of RpfG partly reinstates the pathogenicity compromised by TfmR-deletion mutants. Furthermore, our studies revealed that TfmR also exerts direct positive regulation on the expression of the T3SS regulator HrpX. Similar to RpfG, sustained expression of HrpX partially restores the pathogenicity of TfmR-deletion mutants. These findings underscore TfmR’s multifaceted role as a central regulator governing key virulence pathways in Xoc. Importantly, our research sheds light on the intricate molecular mechanisms underlying the regulation of pathogenicity in this plant pathogen.
1. Introduction
The genus Xanthomonas comprises an important group of Gram-negative phytopathogenic bacteria that cause severe diseases in many important crops [1,2]. Among the most prominent of these pathogens is the species Xanthomonas oryzae pv. oryzicola (Xoc), the causal agent of bacterial leaf streak in rice (BLS), a bacterial disease that is a serious threat to rice productivity [3].
In recent years a large number of Xoc virulence factors have been identified and characterized. Xoc has been shown to produce and secrete a number of factors to promote its pathogenicity, including the extracellular polysaccharide (EPS), adhesion, diffusible signal factor (DSF) cell–cell signals, extracellular enzymes, and type III effectors (T3SEs) secreted through the type III secretion system (T3SS) et al. [3,4,5,6,7,8,9,10,11,12]. However, the molecular mechanisms that underpin the regulation of these virulence factors in Xoc are less explored and understood. Currently, the main pathogenic pathogenesis-related genes that have been identified in Xoc include the rpf gene and the T3SS gene, among others [5,6].
The rpf gene cluster in Xoc mainly encodes the proteins RpfF, RpfC and RpfG. In the genus Xanthomonas they are associated with a regulatory system involving the diffusible signaling factor (DSF) [7]. RpfF is involved in the synthesis of DSF, which is important for intercellular signaling [7,8,9]. RpfC and RpfG constitute a two-component regulatory system, and they are responsible for the perception of DSF signaling molecules and for signaling [7]. When RpfC senses DSF signaling, it undergoes self-phosphorylation and passes the phosphate group to the RpfG [7]. RpfG has a two-component system of a receptive domain and an HD-GYP structural domain [7,10]. Phosphorylation of RpfG activates its HD-GYP structural domain, which possesses the phosphodiesterase activity that hydrolyzes cyclic di-GMP, leading to changes in the intracellular level of cyclic di-GMP [7,11]. In Xoc, RpfG positively regulates pathogenicity and EPS production, and negatively regulates T3SS gene expression [6]. Despite the above studies on the function of RpfG, few transcription factors that directly regulate RpfG have been studied in Xoc.
T3SS plays a crucial role in both plant and animal pathogens successfully infected into the host [12,13]. As with many other bacterial pathogens, T3SS, which is encoded by a cluster of over 20 hypersensitive response and pathogenicity (hrp) genes, is an essential virulence determinant of X. oryzae [14,15]. Mutations in the T3SS gene in X. oryzae result in loss of the ability to elicit its hypersensitive response (HR) in the nonhost plant Nicotiana benthamiana. And the T3SS gene in X. oryzae is essential for the pathogenicity of the host rice plant [14]. In basic culture (XOM2), the T3SS gene is activated [16,17]. Notably the T3SS gene is directly activated by the AraC family transcriptional regulator HrpX in X. oryzae [18]. And the OmpR family regulator HrpG positively regulates HrpX transcription directly[17,19]. Although there are regulatory proteins that directly positively regulate HrpG/HrpX in Xanthomonas spp., there are also other transcription factors that have not yet been identified [20,21].
Several other regulatory proteins have been shown to control the expression of virulence factor genes in other Xanthomonas species. TfmR is a transcriptional regulator belonging to the TetR family of transcriptional regulators (TFRs). In Xanthomonas citri subsp. citri (Xcci) strain 306, TfmR indirectly regulates the expression of T3SS genes through the direct regulation of fatty acid metabolism genes [22]. TfmR plays a positive role in the pathogenicity, HR, motility, and production of EPS in the Xcci strain 306 [22]. TFRs are usually used as transcriptional repressors to regulate the expression of target gene expression [23]. Their regulatory functions vary widely and are now found to be related to bacterial drug resistance, community sensing, catabolism, antibiotic production, and host response [24,25].
Despite the above advances in TfmR in the Xcci 306 strain, little is known about the function of TfmR in Xoc. Too investigate its role in Xoc, we knocked out TfmR in the Xoc GX01 strain. We found that the pathogenicity of the EPS production, motility, HR, and proliferation in the host plant of the deletion mutant ΔtfmR were significantly attenuated.
Interestingly, the expression of rpfG in the ΔtfmR mutant restores motility and EPS yield, and partially restores the pathogenicity and proliferative capacity of the mutant in host plants. In addition, through a series of experiments we showed that TfmR can directly bind to the promoter region of rpfG and positively regulate the expression of the rpfG gene. Our experimental evidence indicates that TfmR can directly bind to the hrpX promoter region and activate its gene expression, and that HrpX restores the mutant’s HR and partially restores pathogenicity and proliferative capacity in the host plant. Based on our evidence, we can show that in Xoc, TfmR regulates a series of cellular processes through the regulation of RpfG and HrpX, respectively, which in turn regulates pathogenicity in the host plant.
2. Results
2.1. TfmR Plays a Role in Xoc Full Virulence
To explore the role of TetR family transcriptional regulators in Xoc, we conducted sequence comparison and protein sequence-identity analysis of all nine TetR family transcriptional regulators in Xoc. The analysis showed low sequence identity of these proteins. (Figure S1A,B), suggesting potential functional diversity in Xoc.
Subsequently, we generated deletion mutants for each of the nine TetR family transcriptional regulators using the homology double-swap method. These mutants were then inoculated into rice (Oryza sativa L. ssp. Japonica cultivar Nipponbare) using the leaf-infiltration method (Figure S1C). Our results indicated a significant reduction in pathogenicity, specifically in the mutant strain ΔXOCgx_1556. Remarkably, the amino acid sequence encoded by XOCgx_1556 (accession number QEO96549.1) shared a high similarity (98.5%) with the Xcci TfmR (strain 306) [22]. Consequently, we designated the protein encoded by XOCgx_1556 as TfmR.
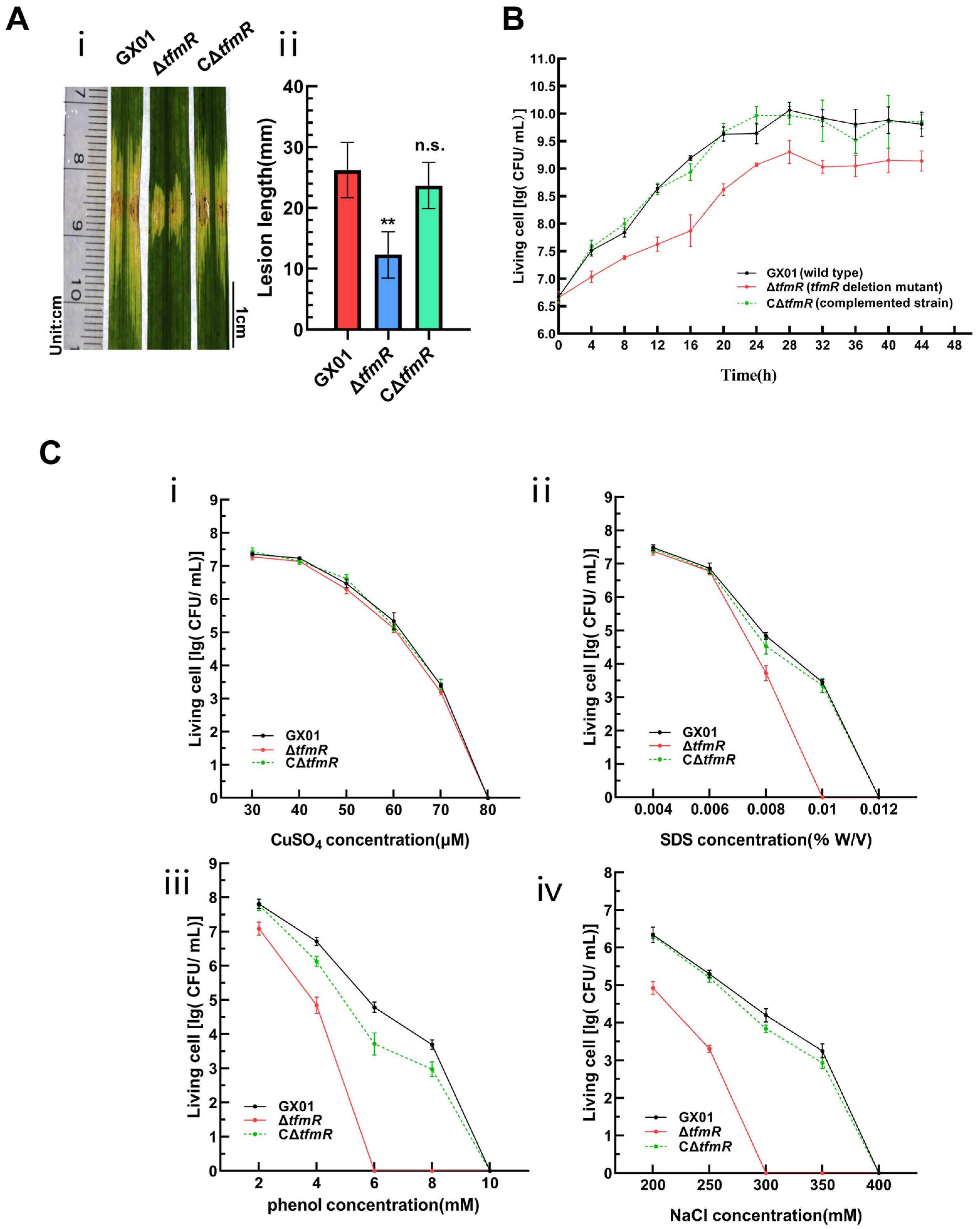
To further elucidate the function of TfmR in Xoc, we utilized the pXUK [26] plasmid to construct the complementary strain CΔtfmR. Pathogenicity assays were performed on wild-type strain GX01, deletion mutant ΔtfmR, and complementation strain CΔtfmR. Lesions induced by ΔtfmR were significantly shorter compared to the wild type (Figure 1A). Notably, the complemented strain CΔtfmR exhibited virulence symptoms (lesion length) similar to those of the wild type (Figure 1A and Figure S3A).
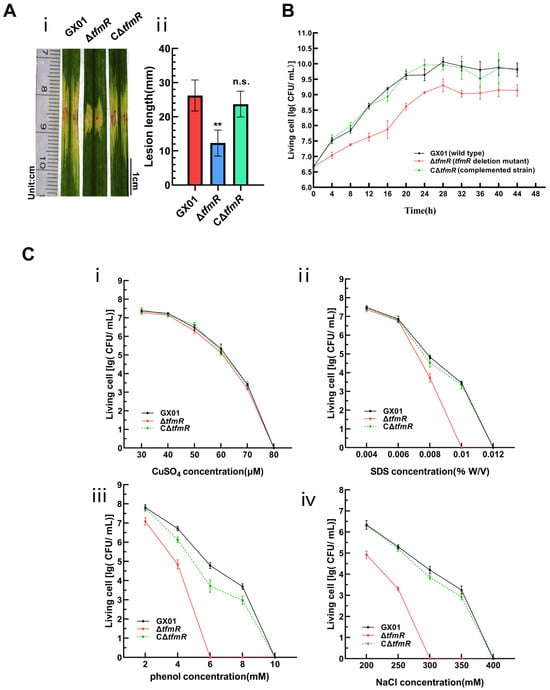
Figure 1.
TfmR is required for full virulence of Xoc. (A) Xoc strain pathogenicity assay. (i) Phenotypic chart of lesion length. (ii) Statistical chart of lesion length, values are means ± SD (n = 30). Significance was determined by ANOVA and Dunnett’s post hoc test to compare with the wild type. ** p < 0.01; n.s., not significant. The experiment was repeated three times with similar results. (B) Growth of Xoc strains in complex medium (NB). The strains were inoculated into NB medium at a final density of 0.01 (OD600). The growth of the strains was recorded every 4 h. (C) Stress tolerance test of Xoc strains. Survival experiments performed by subculturing strains overnight on fresh NA plates supplemented with different concentrations of CuSO4 (i), SDS (ii), phenol (iii), and NaCl (iv). Values given are the means ± SD (n = 3 biological repeats).
To investigate the specific functions of TfmR in manipulating Xoc pathogenesis, we conducted a series of phenotypic tests. Compared to the wild type, the growth rate of the deletion mutant ΔtfmR in nutrient-rich liquid medium (NB) significantly lagged behind during the early exponential phase (Figure 1B). Notably, the growth status of the complementation strain CΔtfmR in NB medium remained consistent with that of the wild type (Figure 1B), suggesting an indispensable role of TfmR in Xoc growth. Furthermore, our results demonstrated that the deletion mutant ΔtfmR exhibited a lower survival rate under stress conditions induced by sodium dodecyl sulfate (SDS), phenol, and NaCl, while the complemented strain responded similarly to the wild type (Figure 1C). Additionally, the secretion of protease and amylase was higher in the deletion mutant ΔtfmR compared to the wild type, and in the complemented strain, the enhanced enzyme activities were restored to wild-type levels (Figure S4).
These findings suggest that in Xoc, TfmR has a key role in bacterial growth and confers resistance to specific stressors, but appears to negatively regulate extracellular enzyme production. However, despite these findings, the regulatory mechanisms of TfmR in Xoc pathogenicity remain unclear.
2.2. TfmR Is a Global Regulator That Regulates the Expression of a Large Number of Genes Involved in Various Cellular Processes
To further elucidate the regulatory role of TfmR in Xoc, we conducted RNA sequencing (RNA-Seq) to determine the transcriptome of the TfmR-deletion mutant strain ΔtfmR. Both the mutant strain and the wild-type strain GX01 were cultured in NB medium until reaching the mid-exponential phase (OD600 = 0.8). Subsequently, bacterial RNA was extracted, library preparation was performed, and the generated data were analyzed for differential gene expression.
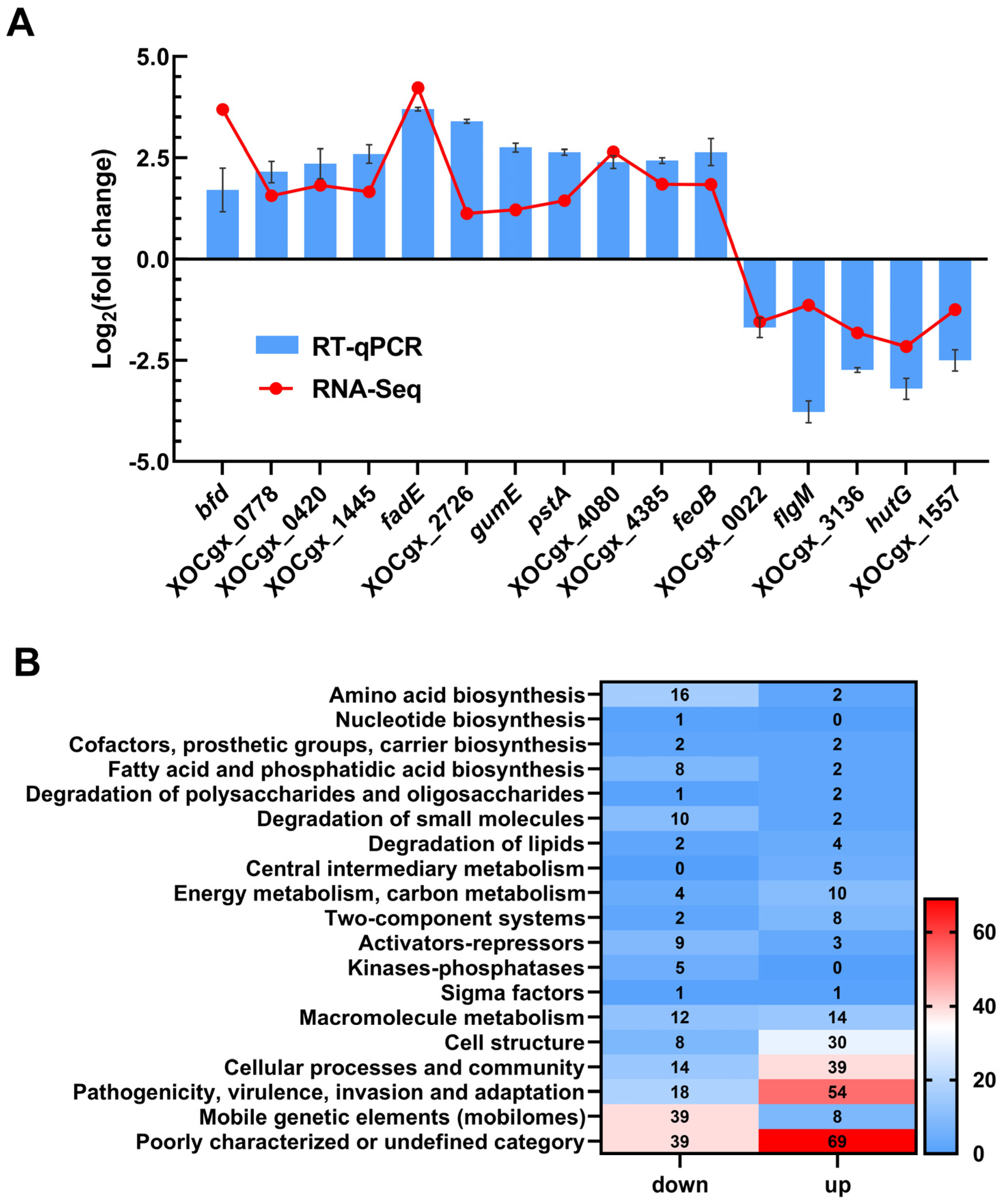
Analysis of the transcriptome data revealed differential expression of 446 genes in the mutant ΔtfmR, with 191 genes significantly down-regulated and 255 genes significantly up-regulated (Table S1). To validate the transcriptome data, we randomly selected 16 genes and assessed their relative expression levels using reverse transcription quantitative real-time PCR (RT-qPCR). The results showed broadly consistent expression patterns with those observed in the transcriptome analysis (Figure 2A).
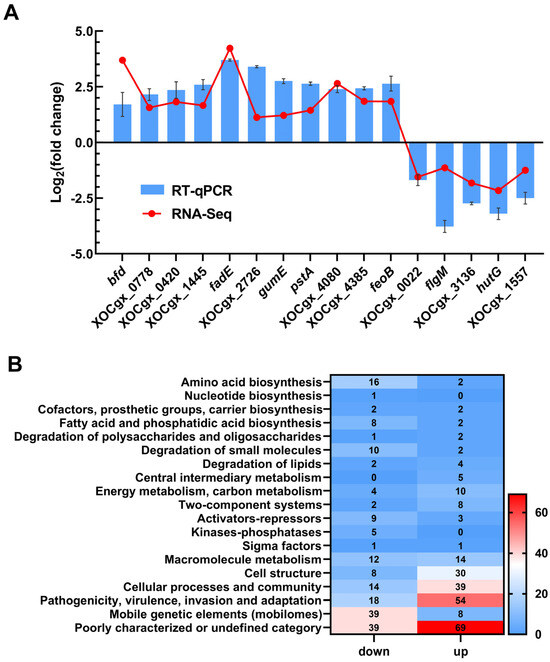
Figure 2.
TfmR is a global regulatory protein that affects the expression of many genes. (A) A total of 16 genes from the transcriptome data were selected to verify the accuracy of the transcriptome by RT–qPCR. Values are the means ± SD (n = 3 biological replicates). (B) Functional categories of differentially expressed genes (DEGs) (|log2(fold change)| ≥ 1) in ΔtfmR mutants. The transcriptomes of Xoc strains cultured in NB medium were investigated by RNA–Seq. In the 446 DEGs of ΔtfmR mutants, 191 genes were significantly down–regulated and 255 genes significantly up–regulated.
Functional clustering analysis of the differentially expressed genes, based on the genome annotation of Xoc strain GX01, revealed that they could be categorized into 19 functional categories. Among these, 108 genes were classified as belonging to the ‘poorly characterized or undefined category’. The majority of the remaining genes were associated with ‘mobile genetic elements (mobilomes)’, ‘pathogenicity, virulence, invasion and adaptation’, ‘cell structure’, ‘cellular processes and community’, ‘degradation of small molecules’, ‘amino acid biosynthesis’, and ‘fatty acid and phosphatidic acid biosynthesis’. Notably, 72 genes were categorized under ‘pathogenicity, virulence, invasion and adaptation’ (Figure 2B). These findings provide valuable insights into the regulatory mechanisms orchestrated by TfmR in Xoc.
2.3. Xoc TfmR Directly Binds to the Promoter of rpfG and Activates Its Transcription
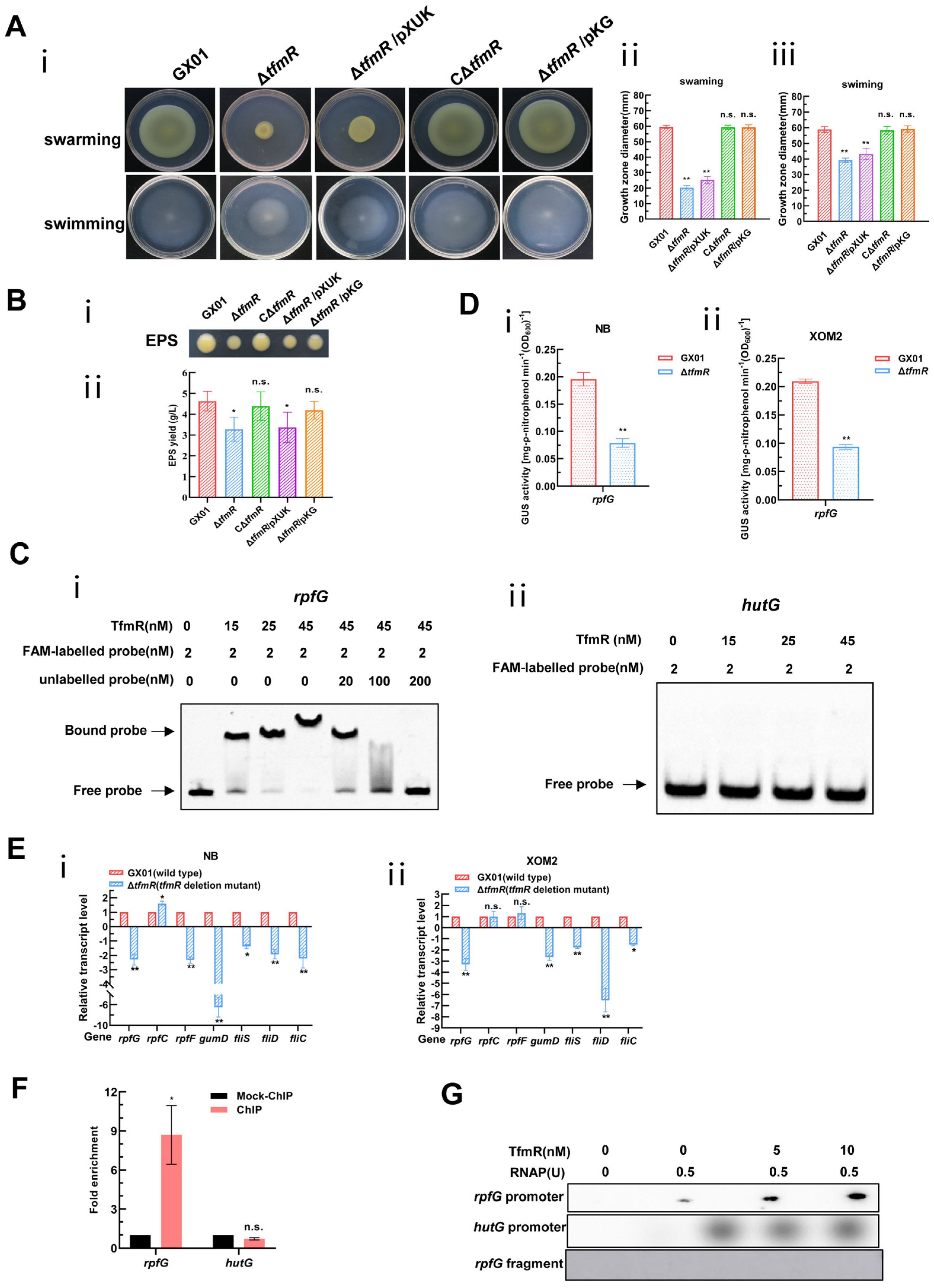
Although our transcriptomic data showed that 191 genes were significantly downregulated in the mutant ΔtfmR, there were few genes with respect to pathogenicity among these, since the mutant ΔtfmR has a significant decrease in pathogenicity compared to the wild type, and the production of extracellular polysaccharides as well as motility is reduced (Figure 1A, Figure S3A and Figure 3A,B). This is very similar to the phenotype after deletion of the rpf gene in Xanthomonas [6,7,27,28,29,30]. We guessed that TfmR is likely to regulate the expression of the rpf gene. Therefore, we performed an electrophoretic mobility shift assay (EMSA) on the promoter regions of rpfF, rpfC and rpfG, and found that TfmR did bind to the promoter region of rpfG (Figure 3C and Figure S6A), but not to the promoter regions of rpfF and rpfC (Figure S5). After that, we investigated in depth the regulatory mechanism of TfmR on RpfG.
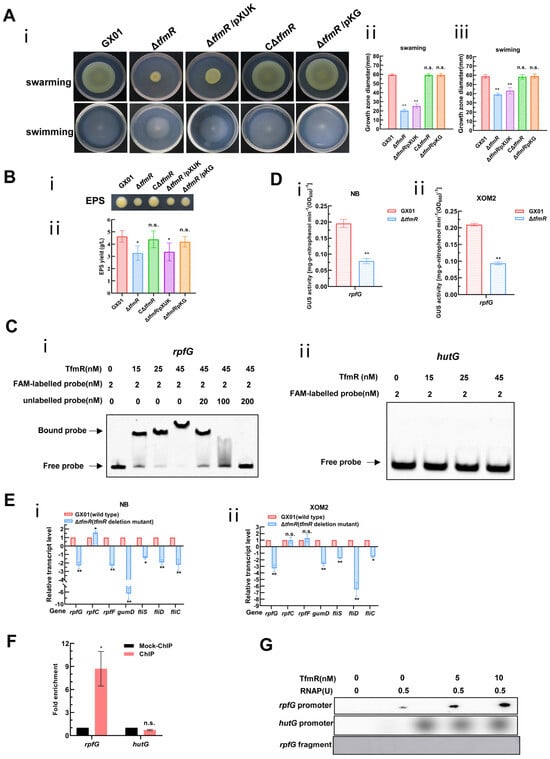
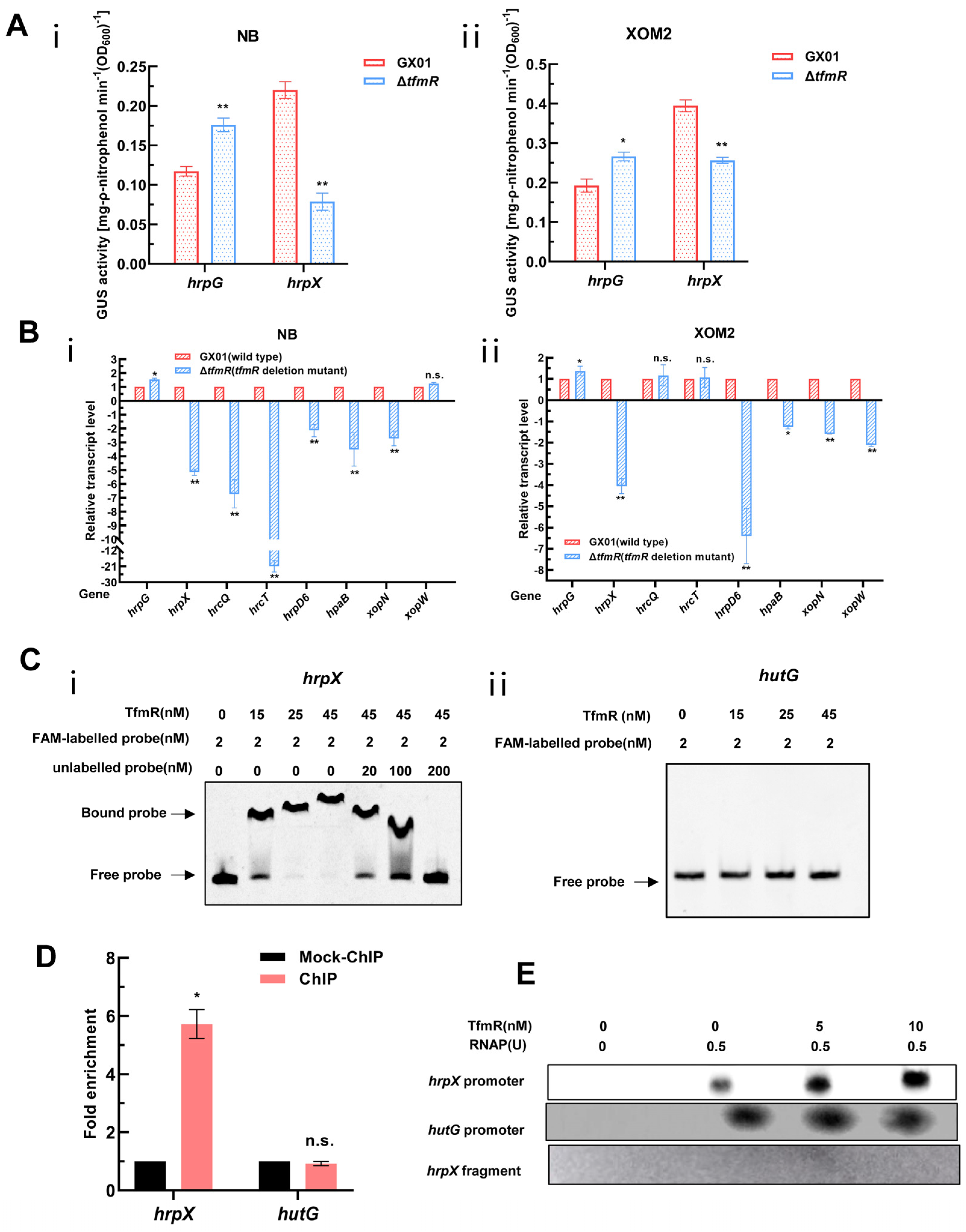
Figure 3.
Constitutive expression of rpfG restores motility and EPS production of the mutant ΔtfmR. And Xoc TfmR binds directly to the promoter of RpfG and activates its transcription. (A) Constitutive expression of rpfG restores motility of the mutant ΔtfmR. (i) Example photo of a bacterial strain. (ii) Mean measurements of colony diameter for each strain on “swarming” plates. (iii) Mean measurements of colony diameter for each strain on “swimming” plates. Data shown are the mean ± SD (n = 10). Significance was determined by ANOVA and Dunnett’s post hoc test for comparison with to the wild type. ** p < 0.01; n.s., not significant. (B) Constitutive expression of rpfG restores the yield of the mutant ΔtfmR EPS. (i) Xoc strains were grown on NA plates supplemented with 2% sucrose for 3 days. (ii) Xoc strains were cultured in NB medium supplemented with 2% sucrose for 3 days and EPS was precipitated from the culture supernatant. Values given are the means ± SD of triplicate measurements from a representative experiment, and significance was determined by analysis of variance (ANOVA) and Dunnett’s post hoc test for comparison with the wild type. * p < 0.05; n.s., not significant. Similar results were obtained in two other independent experiments. (C) Electrophoretic mobility shift and competition assays of TfmR with the promoter region of rpfG (i) and hutG (ii) (negative control); the bound– and free–DNA fragments are marked with the words Bound probe and Free probe, respectively, and the concentrations are indicated at the top of each lane. (D) ß–Glucuronidase (GUS) activity of the gusA reporter of the rpfG gene promoter in the ΔtfmR mutant and the wild type in NB medium (i), or in XOM2 medium (ii). The data shown are the mean and standard deviation of three measurements. The experiment was repeated three times and similar results were obtained. Differences were evaluated by Student’s t-test (** p < 0.01; * p < 0.05; n.s., no significance at p ≤ 0.05). (E) Detection of ΔtfmR mutant and wild–type expression of rpf genes in NB medium (i), or XOM2 medium (ii), revealed by RT–qPCR analysis. Values are the means ± SD (n = 3 biological replicates). Differences were evaluated by Student’s t-test (** p < 0.01; * p < 0.05; n.s., no significance at p ≤ 0.05). (F) Fold enrichment of the promoter region of rpfG in the GX01/TfmR::3 × Flag–ChIP samples compared with the Mock–ChIP samples (with anti–HA antibody), as measured by ChIP–qPCR using hutG as the negative control. Data are presented as means ± SD (n = 3). Differences were evaluated using Student’s t-test (* p < 0.05; n.s., no significance at p ≤ 0.05). (G) In vitro transcription experiments showing TfmR activates the transcription of rpfG. RNA was produced from a 323 bp template DNA fragment containing the rpfG promoter using E. coli RNA polymerase (RNAP) holoenzyme. A 334 bp template DNA fragment containing the hutG promoter and a 150 bp template DNA fragment of the rpfG coding sequence were used as controls. Lane 1, template DNA alone; lane 2, template DNA with RANP; lanes 3–4, template DNA with RANP and 5 and 10 nM TrxA–TfmR.
To further investigate, we engineered a reporter plasmid for RpfG, where its promoter sequence was fused with the gusA gene, enhancing gusA activity. The introduction of this reporter plasmid into both the wild-type and mutant-ΔtfmR strains, followed by cultivation in nutrient-rich (NB) and plant-mimicking (XOM2) media, revealed significantly lower β-glucuronidase (GUS) activity in the mutant ΔtfmR compared to the wild type in both conditions (Figure 3D).
Furthermore, analysis of rpfG, rpfC, and rpfF transcript levels via reverse transcription quantitative real-time PCR (RT-qPCR) corroborated diminished rpfG expression in the mutant ΔtfmR across both media types. Hence, we posit that TfmR activates rpfG expression in Xoc. Consistent with previous findings [6,30], reduced expression of genes gumD, fliS, fliD, and fliC was observed in the deletion mutant of tfmR, akin to the effects of rpfG deletion mutants, possibly due to decreased rpfG expression (Figure 3E).
Based on our previous EMSA experiments using TrxA-tagged TfmR proteins, it was indeed shown that there are mobility shifts in the DNA probes spanning the rpfG promoter region in vitro, which intensified with increasing TrxA-TfmR protein concentration. Notably, no shifted bands were observed in the presence of TrxA protein alone or with a control DNA fragment (Figure 3C and Figure S6A). Chromatin immunoprecipitation-qPCR (ChIP-qPCR) analysis further confirmed direct binding of TfmR to the rpfG promoter in vivo (Figure 3F and Figure S7), while in vitro transcription experiments demonstrated that TfmR induced rpfG transcription in the presence of RNA polymerase allosteric enzyme (RNAP) (Figure 3G). Collectively, these findings strongly support the hypothesis that TfmR regulates rpfG expression by directly binding to its promoter region, thus elucidating a crucial regulatory pathway in Xoc pathogenesis.
2.4. Constitutive Expression of rpfG Restores Mutant ΔtfmR Motility, EPS Production, Pathogenicity, and In Vivo Plant Growth
The significantly reduced pathogenicity of the Xoc GX01 mutant strain ΔtfmR implies that TfmR may be involved in other cellular processes required for full pathogenicity of Xoc, such as cell motility, and extracellular polysaccharide (EPS) production. To confirm that the effect of TfmR on these cellular processes is due to the regulation of rpfG expression, we constitutively expressed RpfG in the TfmR-deficient mutant ΔtfmR and determined its phenotype. To accomplish this, we utilized the pXUK plasmid to construct the cross-complementary strain ΔtfmR/pKG (Table S2), while we introduced the pXUK null plasmid into the mutant strain ΔtfmR, named ΔtfmR/pXUK (Table S2). The results showed that both the swarming and swimming abilities of the mutant strain ΔtfmR were significantly reduced, relative to that of the wild-type strain GX01 under the tested conditions, and that the complementary strain CΔtfmR could completely restore the motility of mutant strain ΔtfmR (Figure 3A). Whereas the ΔtfmR/pXUK strain had similar motility to the mutant ΔtfmR, the ΔtfmR/pKG strain had similar motility to GX01 and the complementary strain CΔtfmR (Figure 3A). Under the tested conditions, the yield of EPS of the deletion-mutant ΔtfmR was significantly lower than that of the wild-type strain GX01, and the complementary strain CΔtfmR could completely restore its phenotype; the yield of EPS of the ΔtfmR/pXUK strain was similar to that of ΔtfmR, and the yield of EPS of the ΔtfmR/pKG strain was also similar to that of GX01 and CΔtfmR (Figure 3B). The above results suggest that constitutive expression of rpfG can restore motility and EPS production in ΔtfmR strains.
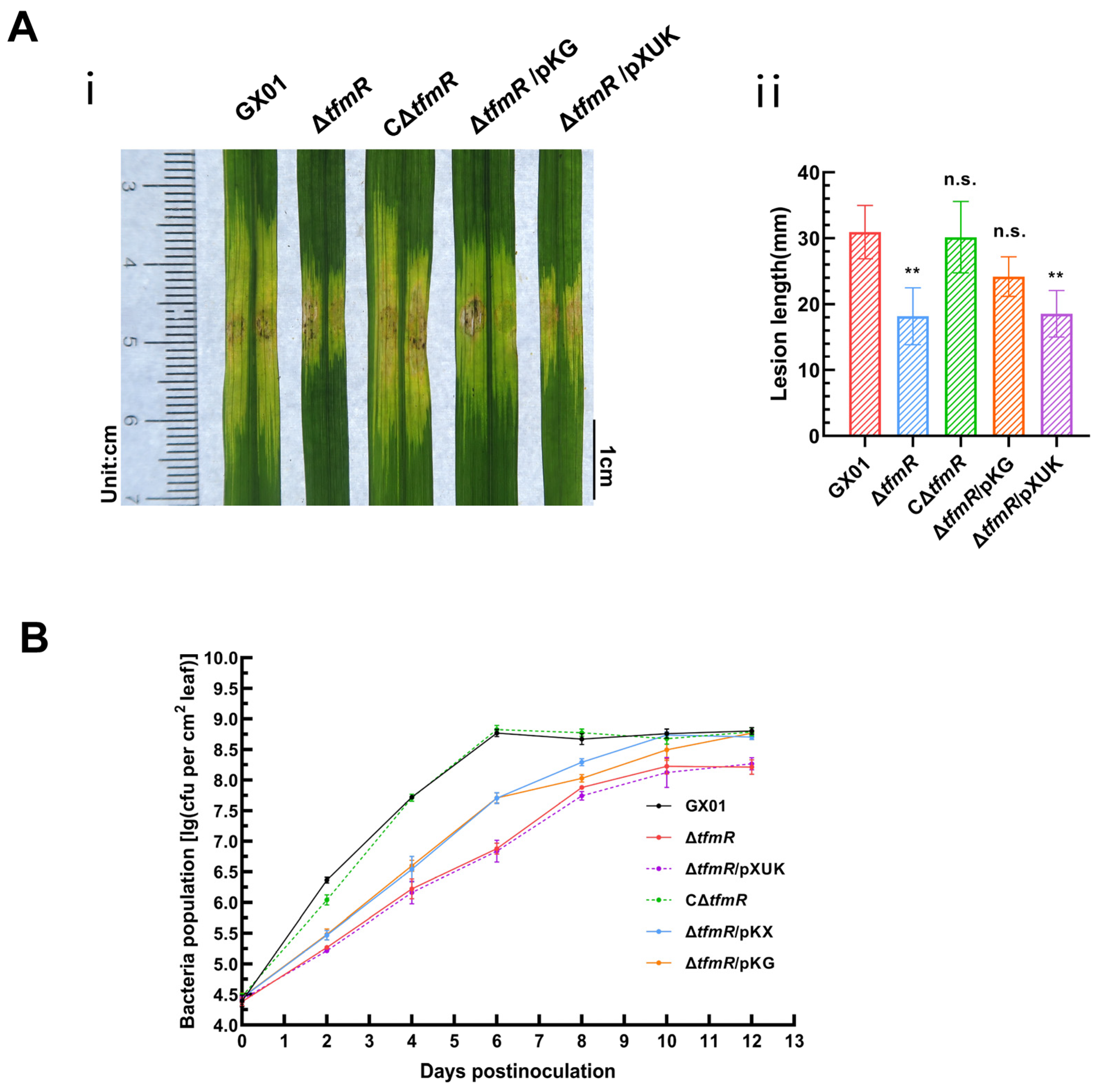
To test the significant reduction in pathogenicity of the mutant strain ΔtfmR in relation to RpfG, we inoculated the wild-type strain GX01, mutant strain ΔtfmR, complementary strain CΔtfmR, constitutively expressed strain ΔtfmR/pKG and ΔtfmR/pXUK strain, respectively, into the host rice plant (Oryza sativa L. ssp. Japonica cultivar Nipponbare) by the leaf-infiltrating method. The results showed that the length of diseased spots was significantly lower than that of the wild-type GX01 in both the mutant strain ΔtfmR and the ΔtfmR/pKG strains; both had similar spot lengths that were significantly lower than that of the wild-type GX01, whereas the complementary strain CΔtfmR could fully recover to wild-type levels and the ΔtfmR/pKG strain produced spots that could mostly recover to wild-type levels (Figure 4A and Figure S3B). In our subsequent experiments to detect the proliferation of the pathogen in host tissues, we could observe that the ability of the ΔtfmR/pKG strain to proliferate in the host plant was also mostly restored to that of the wild type (Figure 4B). These results suggest that constitutive expression of rpfG can largely restore the pathogenicity and growth of the ΔtfmR strain in host plants.
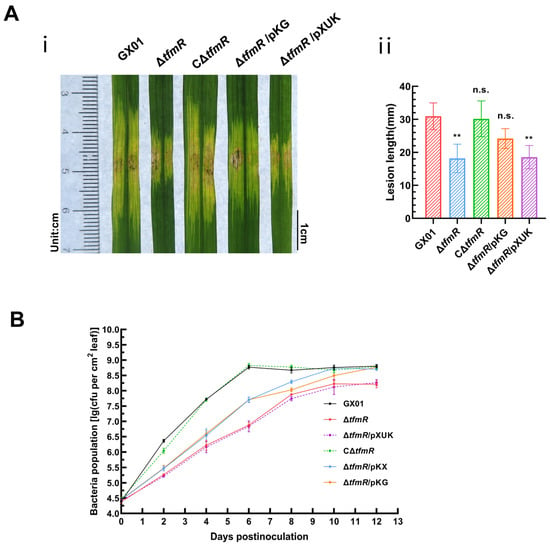
Figure 4.
Constitutive expression of rpfG restores restores ΔtfmR mutant pathogenicity and proliferation in host plants. (A) Constitutive expression of rpfG can partially characterize the pathogenicity of the mutant ΔtfmR. (i) Phenotypic chart of lesion length. (ii) Statistical chart of lesion length, values are means ± SD (n = 30). Significance was determined by ANOVA and Dunnett’s post hoc test to compare with the wild type. ** p < 0.01; n.s., not significant. The experiment was repeated three times, with similar results. (B) Both constitutive expression of hrpX and constitutive expression of rpfG partially restored the proliferative capacity of the mutant ΔtfmR in the host rice plant. Data are shown as the mean ± SD (n = 3 biological repeats).
2.5. Xoc TfmR Directly Binds to the Promoter of hrpX and Activates Its Transcription
According to a previous report by Sun et al., RpfG negatively regulates the expression of hrpG and hrpX genes [6]. Given that TfmR positively regulates RpfG expression, we constructed two GUS reporter plasmids for two key hrp-regulated genes, hrpG and hrpX, as per the previous method, and introduced them into both the wild-type and mutant-ΔtfmR strains.
As anticipated, the GUS enzyme activity of the HrpG reporter in the mutant ΔtfmR was significantly higher than that of the wild type in both NB and XOM2 medium (Figure 5A). Interestingly, however, the GUS enzyme activity of the HrpX reporter in mutant ΔtfmR was significantly lower than that of the wild type in both media (Figure 5A). To validate this result, we conducted RT-qPCR to analyze the expression changes of a set of T3SS or T3SS-related genes in both the wild type and mutant ΔtfmR. The results revealed that while the expression of hrpG in both media of mutant ΔtfmR was significantly higher than that of the wild type, the expression of hrpX and most T3SS or T3SS-related genes in mutant ΔtfmR was significantly lower than that of the wild type in both the NB and XOM2 medium (Figure 5B). These findings are consistent with the results of the GUS reporter system, suggesting a significant decrease in HrpX expression in the mutant ΔtfmR, potentially indicating direct regulation of HrpX expression by TfmR.
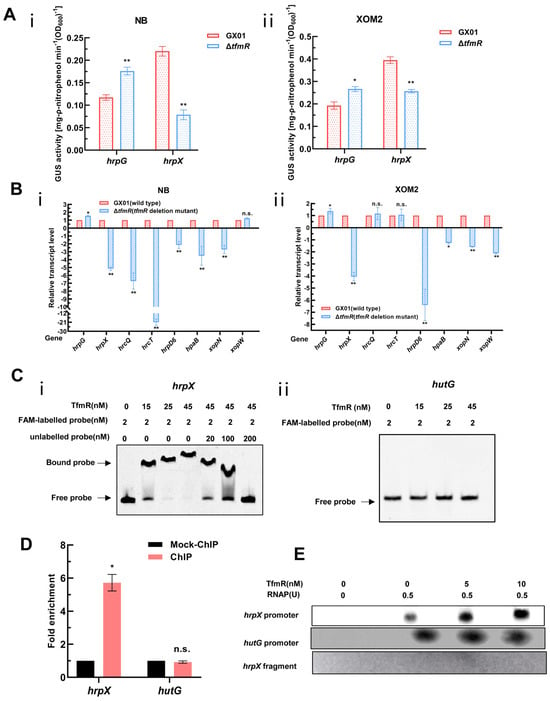
Figure 5.
Xoc TfmR binds directly to the promoter of HrpX and activates its transcription. (A) ß–Glucuronidase (GUS) activity of the gusA reporter of the hrpG and hrpX gene promoter in the ΔtfmR mutant and the wild type in NB medium (i), or in XOM2 medium (ii). The data shown are the mean and standard deviation of three measurements. The experiment was repeated three times and similar results were obtained. Differences were evaluated by Student’s t-test (** p < 0.01; * p < 0.05; n.s., no significance at p ≤ 0.05). (B) Detection of ΔtfmR–mutant and wild-type expression of T3SS genes in NB medium (i), or XOM2 medium (ii) revealed by RT–qPCR analysis. Values are the means ± SD (n = 3 biological replicates). Differences were evaluated by Student’s t-test (** p < 0.01; * p < 0.05; n.s., no significance at p ≤ 0.05). (C) Electrophoretic mobility shift and competition assays of TfmR with the promoter region of hrpX (i) and hutG (ii) (negative control); the bound– and free–DNA fragments are marked with the words Bound probe and Free probe, respectively, and the concentrations are indicated at the top of each lane. (D) Fold enrichment of the promoter region of hrpX in the GX01/TfmR::3 × Flag–ChIP samples compared with the Mock–ChIP samples (with anti–HA antibody), as measured by ChIP–qPCR using hutG as the negative control. Data are presented as means ± SD (n = 3). Differences were evaluated using Student’s t–test (* p < 0.05; n.s., no significance at p ≤ 0.05). (E) In vitro transcription experiments showing TfmR activates the transcription of hrpX. RNA was produced from a 371 bp template DNA fragment containing the hrpX promoter using E. coli RNA polymerase (RNAP) holoenzyme. A 334 bp template DNA fragment containing the hutG promoter and a 161 bp template DNA fragment of the hrpX coding sequence were used as controls. Lane 1, template DNA alone; lane 2, template DNA with RANP; lanes 3–4, template DNA with RANP and 5 and 10 nM TrxA–TfmR.
To investigate whether TfmR directly binds to the HrpX promoter to activate its expression, we conducted EMSA experiments in vitro. The results demonstrated that TrxA-TfmR protein could bind to the promoter region of HrpX under experimental conditions, while TrxA protein did not exhibit binding under the same conditions (Figure 5C and Figure S6B). Notably, under the same conditions, the TrxA-TfmR protein also failed to bind to the promoter region of HutG (negative control) (Figure 5C). Subsequently, ChIP-qPCR experiments conducted in vivo in bacteria revealed significant enrichment of the HrpX promoter region in the TfmR-ChIP samples compared to mock ChIP control samples, confirming the direct binding of TfmR to the HrpX promoter region in vivo. Conversely, no binding was detected in the negative control hutG promoter region (Figure 5D and Figure S7). These experiments conclusively demonstrated that TfmR directly binds to the promoter of HrpX.
Following this, we conducted in vitro transcription experiments, which showed that the transcription products of hrpX increased with the concentration of TfmR protein, while the transcription products of hutG remained unchanged (Figure 5E). In summary, these experiments confirm that TfmR directly binds to the promoter of HrpX and activates its transcription.
2.6. Constitutive Expression of hrpX Restores ΔtfmR Mutant HR, Pathogenicity, and Proliferation in Host Plants
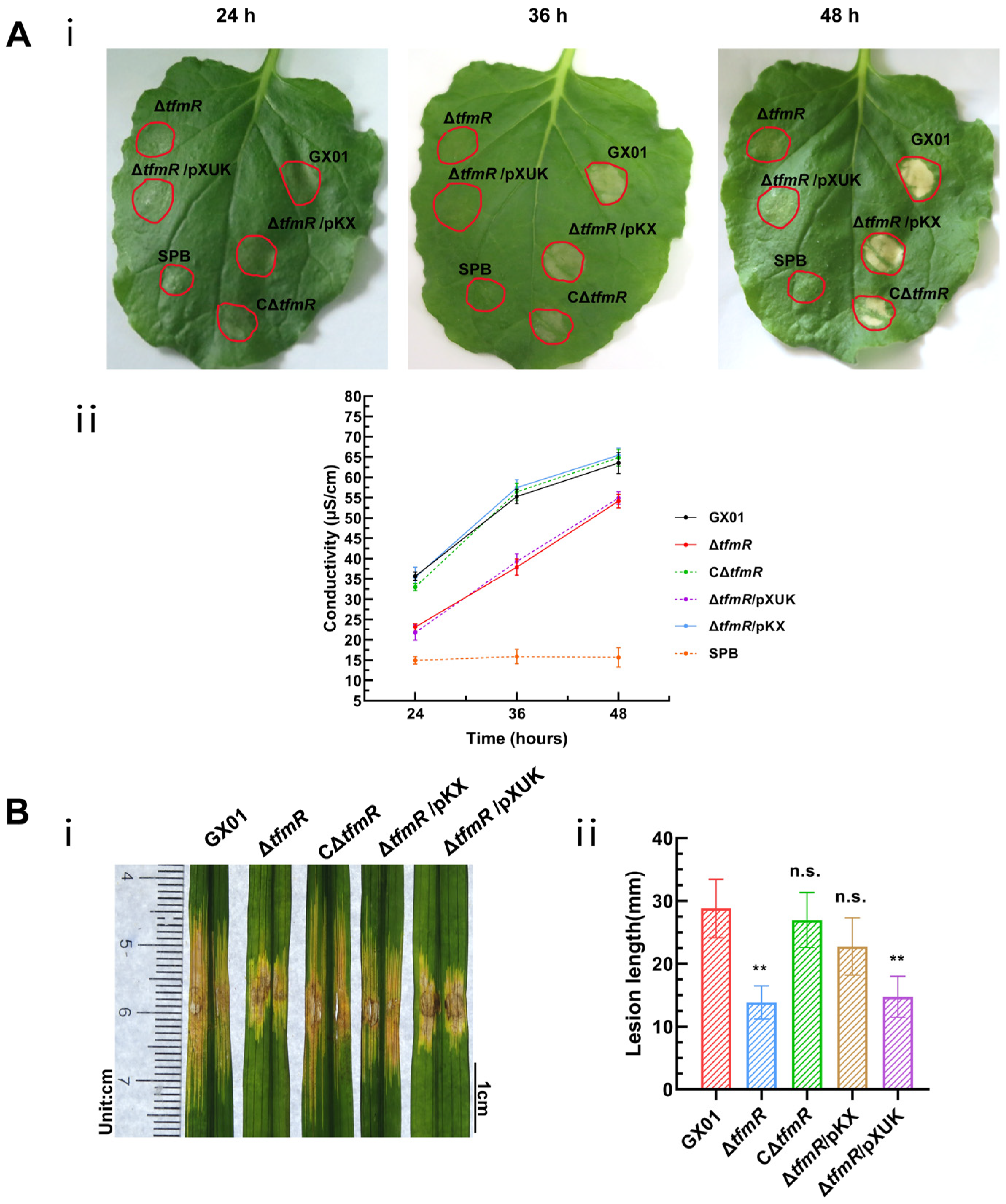
The impact of TfmR mutation on the expression of the T3SS gene prompted an evaluation of its effect on HR induction using an osmotic assay. While the wild-type strain and the complementary strain CΔtfmR exhibited pronounced HR symptoms at 24 h, the mutant strain ΔtfmR showed observable HR symptoms only at 36 h, indicating delayed and attenuated HR (Figure 6A). To confirm that TfmR’s effect on plant T3SS is mediated by the modulation of hrpX expression, we tested a TfmR mutant constitutively expressing hrpX in various HR-induced assays.
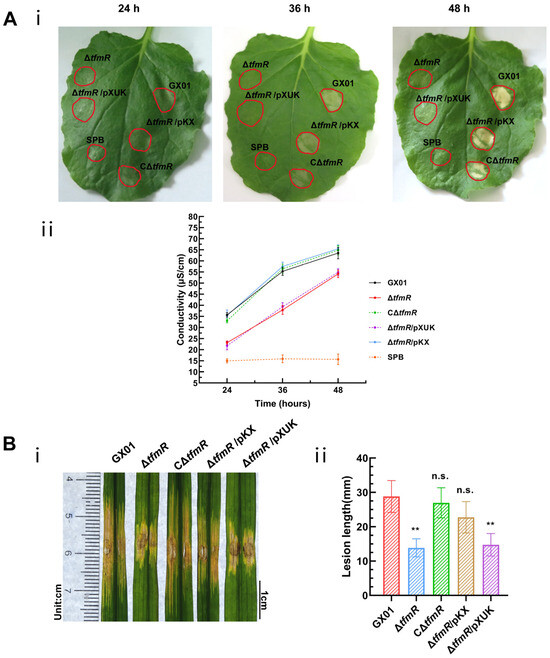
Figure 6.
Constitutive expression of hrpX restores ΔtfmR mutant HR and pathogenicity. (A) Constitutively expressing hrpX in the ΔtfmR mutant restores its ability for hypersensitive response (HR) induction in nonhost plant N. benthamiana. (i) The HR symptoms recorded at 24, 36, and 48 hours post-inoculation (hpi). Three replications were performed in each experiment, and the experiment was repeated three times. The results presented are from a representative experiment, and similar results were obtained in all other independent experiments. (ii) The electrolyte leakage from N. benthamiana leaves inoculated with Xoc strains. Three samples were taken for each measurement in each experiment. Data are shown as mean and standard deviation. (B) Constitutive expression of hrpX partially restores the pathogenicity of mutant ΔtfmR. (i) Phenotypic chart of lesion length. (ii) Statistical chart of lesion length, values are means ± SD (n = 30). Significance was determined by ANOVA and Dunnett’s post hoc test to compare with the wild type. ** p < 0.01; n.s., not significant. The experiment was repeated three times with similar results.
A plasmid constitutively expressing hrpX was constructed and introduced into the mutant ΔtfmR, designated as ΔtfmR/pKX (Table S2). This strain exhibited HR comparable to the wild-type strain GX01. Quantitative assessment of HR induction using an electrolyte leakage assay revealed that both the mutant-ΔtfmR and ΔtfmR/pXUK strains had significantly lower HR than GX01 at 24 h, 36 h, and 48 h. Conversely, both the complementary strain CΔtfmR and the constitutively expressed strain ΔtfmR/pKX showed HR levels similar to the wild-type strain (Figure 6A).
To examine the relationship between the decrease in pathogenicity of mutant ΔtfmR and the T3SS gene, pathogenicity tests were conducted. Results mirrored those of the HR experiments, with both mutant strains ΔtfmR and ΔtfmR/pXUK exhibiting significantly reduced pathogenicity compared to GX01. However, the complementary strain CΔtfmR fully restored pathogenicity, while the constitutively expressed strain ΔtfmR/pKX partially restored it (Figure 6B and Figure S3C). Additionally, the in-plant growth curve demonstrated that the mutant strain ΔtfmR exhibited significantly weaker growth, while the constitutively expressed strain ΔtfmR/pKX partially restored proliferation in plants (Figure 4B).
These results collectively indicate that constitutive expression of hrpX can restore HR, pathogenicity, and proliferation of the ΔtfmR strain in plants, suggesting that TfmR regulates the T3SS gene by modulating hrpX expression.
3. Discussion
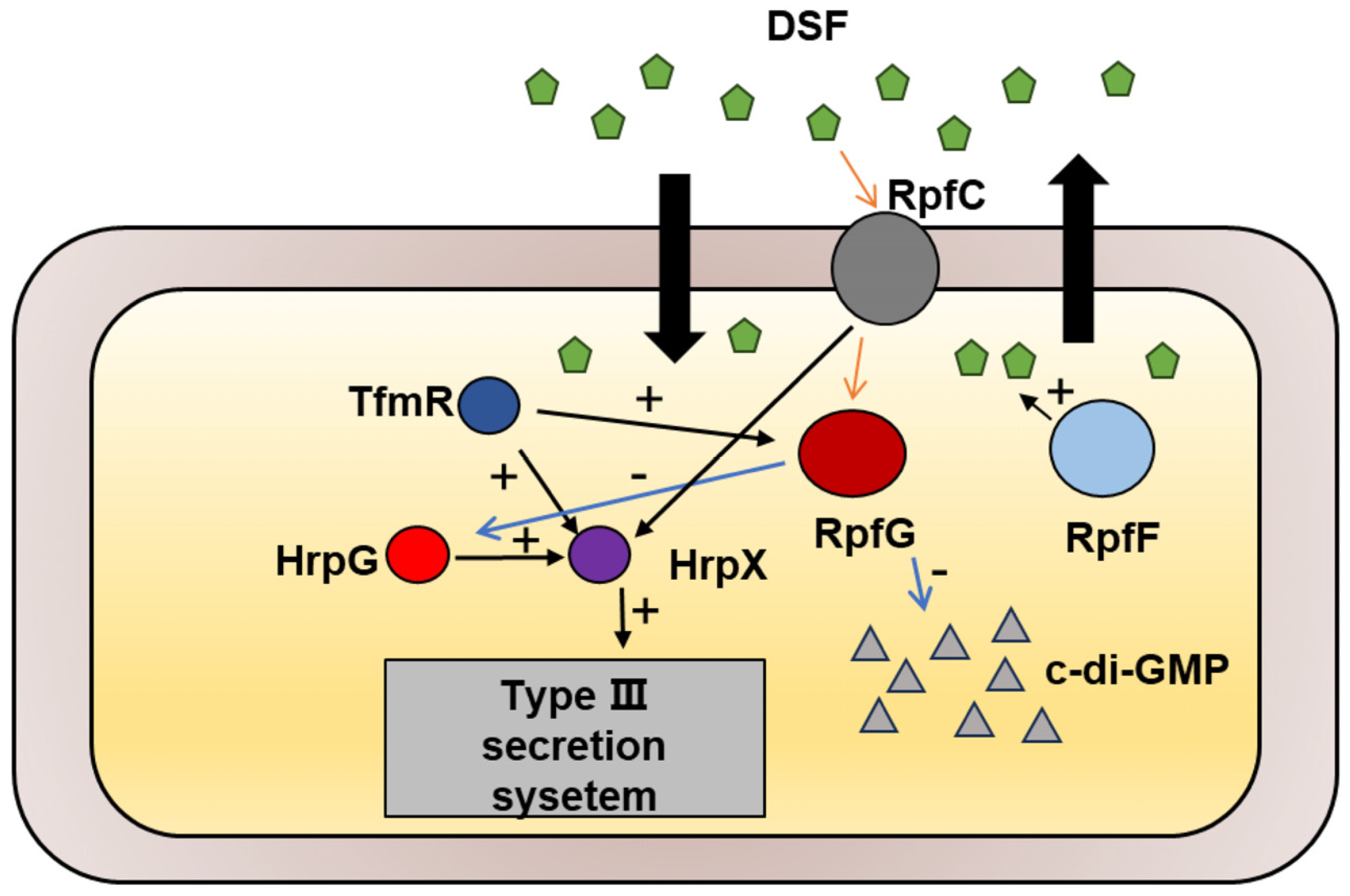
The TetR family of transcriptional regulators governs various bacterial processes [22], and our study has identified TfmR as a significant regulator in the Xoc GX01 strain. Through our investigations, we have demonstrated that Xoc TfmR acts as a global regulator, influencing diverse cellular processes including motility, extracellular enzymes, EPS production, bacterial proliferation, and T3SS gene expression. Specifically, we have identified TfmR as a key regulator of two pathogenesis-related pathways in Xoc. Deletion of TfmR resulted in reduced expression of rpfG and T3SS genes. Our findings indicate that TfmR directly promotes the expression of rpfG and hrpX genes, underscoring its crucial role in positively regulating pathogenicity in Xoc (Figure 7).
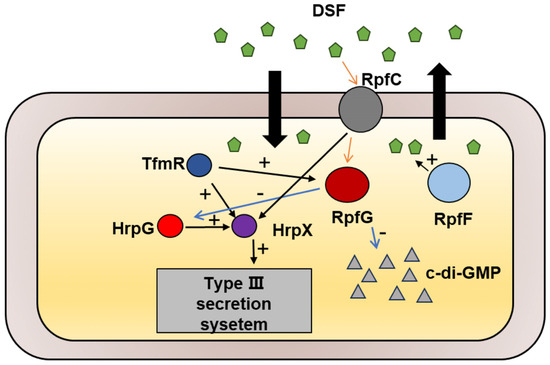
Figure 7.
Schematic representation of the genetic regulation between TfmR and its target genes in Xoc. TfmR is involved in activating the expression of RpfG and HrpX.
The rpf genes cluster in the Xanthomonas spp. plays a pivotal role in pathogenicity, community sensing, and various cellular processes [7]. In the Xanthomonas spp., the two-component system comprising RpfG and the sensor kinase RpfC responds to the cell–cell signaling molecule DSF to regulate the synthesis of virulence factors [7,11,29]. RpfG, with its HD-GYP cyclic di-GMP phosphodiesterase domain, is a well-studied regulator within the rpf gene cluster. Studies across different Xanthomonas species have highlighted its importance in pathogenicity, motility, and virulence-factor production [6,7,27,28,31,32,33,34,35]. RpfG was critical for pathogenicity and colonization of rice in the Xoc strain BLS256, and deletion of the rpfG resulted in a significant decrease in the yield of EPS in the mutant [6]. In Xanthomonas oryzae pv. oryzae (Xoo), RpfG was positively correlated with pathogenicity and proliferation in the host plant, but did not affect Xoo growth in enriched medium [31,32]. In Xanthomonas campestris pv. campestris (Xcc), knockout of rpfG resulted in a significant reduction in pathogenicity, reduced cellulase, reduced EPS production, and reduced motility [7,27,28]. In Xanthomonas albilineans (Xal), single deletion of the rpfG and rpfC genes resulted in mutants with no change in virulence or swimming ability, but double deletion of the rpfG and rpfC genes resulted in a slight decrease in virulence and severely impaired swimming ability [35]. Since the contribution of the rpfG gene to pathogenicity in Xanthomonas is particularly important, a number of genes have also been identified that can regulate the expression of the rpfG gene. In the Xoc RS105 strain, deletion of the zwf gene leads to an increase in RpfG at the transcriptional level [34]. The expression of two genes, rpfC and rpfG, was down-regulated in Xoo with the deletion of the thiG gene [33]. Importantly, to the best of our knowledge, no transcriptional regulator that can directly regulate RpfG in Xanthomonas has been reported yet. Our experimental results for EMSA and ChIP-qPCR indicate that TfmR can directly bind the promoter region of RpfG (Figure 3). And, according to our in vitro transcription, RT-qPCR and GUS enzyme activity experiments, TfmR is a positive transcriptional regulator for the rpfG gene in the Xoc GX01 strain (Figure 3). However, the regulation of the rpfG gene is very complex. Therefore, the next direction of research should focus on the regulatory network of regulatory factors for the rpfG gene.
Moreover, our findings regarding TfmR’s regulation of hrpX expression elucidate a previously unexplored aspect of Xoc pathogenesis. T3SS is essential for Gram-negative bacterial pathogens [12,13]. In Xanthomonas, virulence is dependent on the secretion and transport of T3SS effectors [12]. These effectors are regulated by two master transcription regulators, HrpG and HrpX [17]. Great efforts have been made to reveal the regulatory network of the HrpG/HrpX regulon [17]. The GntR-family transcriptional regulators HpaR1/YtrA from Xcc and Xcci negatively regulate hrpG expression in plant minimal medium, and positively regulate gene expression in plants [36,37]. In Xcc, HpaS is a membrane-bound histidine kinase sensor that forms a two-component signal transduction system with HrpG and activates HrpG activity by phosphorylation [38]. In the Xoc RS105 strain, there is a cross-talk between CheA/VemR and HpaS/HrpG-mediated signaling events that coordinate the hrp gene expression [39]. In addition to HrpG, regulatory factors have been identified in other Xanthomonas spp. that can directly regulate HrpX. In Xoo, the Sigma factor 70 RpoD directly binds to the promoters of hrpG and hrpX to activate T3SS gene expression [40]. And the GntR-family transcriptional regulator Sar can positively regulate the expression of the T3SS gene by directing the promoter of hrpG and hrpX in Xoo [20]. In Xcc, the fis-type transcriptional regulator Flp interacts with the hrpX promoter and positively regulates its expression [41]. It is noteworthy that no proteins (except for HrpG) have been reported in Xoc that directly positively regulate the HrpX. Through EMSA, ChIP-qPCR, and in vitro transcription experiments, we have confirmed in the Xoc GX01 strain TfmR’s direct binding to the hrpX promoter, thereby activating its transcription (Figure 5). Although in previous studies TfmR could indirectly regulate the expression of T3SS genes in Xcci 306 by regulating fatty acid metabolism, TfmR could not directly bind to the promoters of hrpX and hrpG in the Xcci 306 strain [22]. Further studies are needed regarding the differences between the two proteins, Xoc TfmR and Xcci TfmR, in regulating T3SS gene expression.
In addition, differences in culture environments lead to changes in the expression of hrpG/hrpX [17]. According to previous studies, in Xanthomonas, hrpG/hrpX gene expression was repressed in nutrient-rich medium but induced in plants and minimal medium [16,17]. Although many proteins regulating hrpG/hrpX have been identified, the exact mechanism is currently unknown. We performed the transcriptome analysis in nutrient-rich medium, which may be the reason why we did not find significant changes in hrpX expression in our transcriptome. According to Sun et al., in Xoc, RpfG negatively regulates hrpG expression [6], whereas TfmR positively regulates RpfG expression. The slightly higher expression of hrpG in the mutant compared to the wild type in minimal culture is likely to be caused by the decreased expression of RpfG. Our results demonstrate that TfmR positively regulates hrpX and T3SS gene expression while concurrently activating rpfG gene expression. This discovery underscores the intricate regulatory mechanisms governing Xoc virulence.
In conclusion, our work highlights the multifaceted signaling pathways underlying Xoc virulence and describes TfmR as a pivotal regulator orchestrating T3SS gene expression, HR induction, and pathogenesis. By elucidating the regulatory mechanisms involving TfmR, RpfG, and HrpX, we provide valuable insights for developing strategies to combat plant pathogens such as Xoc.
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains and plasmids used in this work are enumerated in Table S2. E. coli (Escherichia coli) strains were cultured in LB (Luria Bertani) medium (containing 5 g yeast extract, 10 g NaCl, and 10 g tryptone per liter) or on LB agar plates (LB supplemented with 30 g agar per liter) at 37 °C. Xoc strains were cultured in NB medium (comprising 1 g yeast extract, 3 g beef extract, 5 g polypeptone, and 10 g sucrose per liter), NA (NB with 30 g agar per liter), or the minimal medium XOM2 [42]. Antibiotics were used, according to the concentrations as required: kanamycin at 25 µg/mL, rifampicin at 50 µg/mL, ampicillin at 100 µg/mL, spectinomycin at 50 µg/mL and Tet at 5 µg/mL for Xoc strains and 15 µg/mL for E. coli strains.
4.2. Nucleic Acid Manipulations
The procedure for nucleic acid manipulation was conducted according to the method outlined by Sambrook et al. [43]. Conjugation between Xoc and E. coli strains was executed as detailed by Turner et al. [37]. The necessary enzymes—restriction endonuclease, T4 DNA ligase, and Pfu polymerase—were supplied by Promega (Shanghai, China). Total RNA was extracted from Xoc strain cultures utilizing the Total RNA Extraction Kit (Invitrogen, Carlsbad, CA, USA), while cDNA synthesis was carried out using the cDNA Synthesis Kit (Invitrogen). For RT-qPCR, the obtained cDNA was diluted and utilized as a template with selected primers targeting specific genes. RT-qPCR was performed in the qPCR thermocycler (Analytik jena qTOWER2.0, Jena, Germany). Each reaction comprised ChamQ universal SYBR qPCR master mix (Vazyme Nanjing, China), appropriate primers, and cDNA. The relative mRNA levels were normalized relative to those of the target genes in the wild-type strain GX01 (equal to 1). The expression levels of the 16S rRNA genes were used as an internal standard. Triplicate RT-qPCR assays were conducted, and all primer sequences applied in this study are listed in Table S3.
4.3. Construction of Mutant Strains
The deletion mutant in Xoc was constructed using the previously employed homology double-swap method. As an example, the mutant ΔtfmR (XOCgx_1556) was constructed as follows [4]. PCR was used to amplify 425 bp of the upstream sequence and 309 bp of the downstream sequence of the tfmR gene, utilizing the appropriate primers. The two fragments were fused with the suicide plasmid pK18mobsacB [44] and transformed into E. coli DH5α. The recombinant plasmid obtained was introduced into Xoc using triparental conjugation with the help of plasmid pRK2073 [45]. Screening for triparental conjugates was carried out using NA plates containing Rif with Kan antibiotics. The single colonies that could grow on the double-resistant plates were all those in which the recombinant plasmid was integrated into the Xoc genome by the first homologous exchange, called a single exchanger. Since the sacB gene in the pK18mobsacB plasmid is a sucrose-lethal gene, the high concentrations of sucrose are utilized to force a second homologous exchange of the single exchanger. This resulted in the deletion mutant ΔtfmR.
For complementation of the ΔtfmR, the full length (606 bp) of tfmR was amplified by PCR from the total DNA of the Xoc strain GX01 and inserted into the pXUK vector [26] (adapted from an endogenous plasmid in the Xoc strain GX01), creating the plasmid pKCtfmR. For constitutive expression of RpfG or HrpX in ΔtfmR, we inserted the full-length (1137 bp) of rpfG or the full length (1431 bp) of hrpX into the pXUK vector, named pKG or pKX, respectively. These plasmids, including the pXUK null vector, were introduced into the ΔtfmR by triparental mating, respectively.
Xoc strains chromosomally encoding the proteins fused with a 3 × Flag-tag at the C-terminus were constructed using the method previously described [4]. Using the genomic DNA of strain GX01 as a template and the corresponding primers, a 1069 bp DNA fragment consisting of a 425 bp sequence upstream of the tfmR start codon, a 606 bp coding sequence for tfmR, and a 38 bp coding sequence for Flag was obtained by PCR amplification. Meanwhile, PCR amplification using the corresponding primers resulted in a 362 bp DNA fragment comprising 50 bp of the Flag coding sequence, 3 bp of the stop codon of tfmR and 309 bp downstream of the tfmR stop codon. The two fragments were jointed using overlap extension PCR, and the resulting recombinant fragment was cloned into the suicide plasmid pK18mobsacB. The plasmid was further introduced into the Xoc strain GX01 by conjugation. The transconjugants were screened on selective agar plates and were confirmed by DNA sequencing. This constructed variant strain was named as GX01/TfmR::3 × Flag.
4.4. Pathogenicity Tests, HR Assays, Leakage Assays and in-Plant Growth Curve
To assess the pathogenicity of Xoc in the host rice plants (Oryza sativa L. ssp. Japonica cultivar Nipponbare) the infiltration method was employed, as previously outlined [4]. Rice seedlings were cultivated in the greenhouse for 6 weeks before being inoculated with Xoc strains on their leaves. The Xoc strains, collected from overnight cultures, were washed and adjusted to a consistent final density (OD600 of 0.3, approximately 1 × 108 CFU/mL). These resuspended bacterial cells were inoculated into 6-week-old rice leaves under relevant conditions, and the resulting lesions and symptoms were evaluated 14 days post-inoculation.
HR was tested on Nicotiana benthamiana leaves, as previously described [5]. Following a similar procedure, Xoc cells from cultures were washed and resuspended in sodium phosphate buffer (SPB, 5.8 mM Na2HPO4 and 4.2 mM NaH2PO4, pH 7.0) to an OD600 of 0.5 (5 × 108 CFU/mL). Resuspended bacterial cells were infiltrated into the N. benthamiana leaves. Symptoms were monitored, and conductivity measurements were taken at 24, 36, and 48 h post-inoculation. For conductivity measurements, samples (four 0.4 cm2 leaf disks) were collected using a punch. These obtained leaf discs were immersed in 10 mL of ultrapure water and shaken at 200 rpm for 30 min. Then the leaf disk was removed and the conductivity of the liquid was measured with a DDS-307A conductivity meter.
Quantification of bacterial growth in plants was carried out as per prior procedures [4]. To elaborate, the Xoc bacterial solution adjusted to a certain concentration was inoculated into the rice leaves, and one infiltrated leaf per group of inoculated plants was sampled and homogenized every 48 h. The homogenates were serially diluted with NB medium and then coated onto selective NA plates with appropriate antibiotics, and after a 3-day incubation period, colony-forming units (CFUs) were counted.
4.5. Stress Tolerance Assay
To investigate the susceptibility of the Xoc strain to various environmental stresses, such as heavy metal salts (CuSO4), sodium dodecyl sulfate (SDS), phenol, and hyperosmotic challenge (NaCl), the minimum inhibitory concentration (MIC) method was employed [4]. In brief, the Xoc strain was cultured overnight and subsequently diluted to an OD600 of 1, and the diluted cultures were further diluted and incubated onto corresponding NA plates supplemented with different reagents. After 3 days of incubation at 28 °C, the number of surviving colonies was counted.
4.6. Extracellular-Polysaccharide and -Enzyme Assays
Extracellular-polysaccharide (EPS) and extracellular-enzyme assays were conducted, following previously established protocols [4,5]. For EPS production analysis, the Xoc strain was spotted on NA plates supplemented with 2% sucrose and incubated for 3 days. To quantify EPS production, the Xoc strain was cultured in NB medium containing 2% sucrose at 28 °C with shaking at 220 rpm for 3 days. The EPS was then precipitated from the culture supernatant using anhydrous ethanol.
To assess protease and amylase activities, Xoc strains were spotted on NA plates containing skim milk (for protease) or soluble starch (for amylase) and incubated for 2 days. For quantification of enzymes, bacterial cells were cultured in NB medium for 24 h and adjusted to the same concentration.
4.7. Motility Assay
The method for detecting cell motility followed the previously described protocol [4]. To begin, the Xoc strain culture that had been incubated overnight was rewashed and resuspended to achieve the same concentration (OD600 of 0.5). Subsequently, 3 µL of the resuspended bacterial suspension was stabbed into 0.28% agar plates containing 0.03% bacterial peptone and 0.03% yeast extract for swimming, or spotted onto NA plates consisting of 2% sucrose and 0.6% agar for swarming. After a resting incubation at 28 °C for 5 days, the diameter of the area occupied by the bacterial cells was measured.
4.8. GUS Activity Assays
The measurement of GUS activity in Xoc strains followed the previously described protocol [41]. Both wild-type and mutant strains carrying the reporter plasmid were cultured at 28 °C in the appropriate medium. Bacterial cells were then collected and resuspended in 375 μL 1 mM p-nitrophenyl-β-d-glucuronide extraction buffer (comprising 50 mM sodium dihydrogen phosphate, 0.1% Triton X-100, and 10 mM β-mercaptoethanol, pH 7.2), and incubated for 10 min at 37 °C. This was followed by incubation with 200 mL of 2.5 M 2-amino-2-methyl-1,3-propylene glycol to terminate the reaction. GUS activity assays were performed in triplicate.
4.9. Overexpression and Purification of TrxA-TfmR Protein
To obtain purified TrxA-TfmR protein, the tfmR gene was first cloned and introduced into the expression vector pET32a. Subsequently, the recombinant plasmid was transformed into E. coli strain BL21. The protein expression purification process followed a well-established method, involving induction of the recombinant strain with isopropyl β-D-thiogalactopyranoside (IPTG) and purification of the fusion protein using Ni-NTA resin (Qiagen, Alameda, CA, USA).
4.10. Electrophoretic Mobility Shift Assay (EMSA)
The EMSA procedure followed a previously described protocol [4]. Briefly, DNA fragments labeled with FAM at the 5′ terminal were amplified using the appropriate primers. The purified protein was then incubated with the FAM-tagged DNA fragment in binding buffer [20 mm Tris-HCl, 10 mm NaCl, 1 mm ethylenediminetetraacetic acid (EDTA) and 1 mm dithiothreitol, pH 8.0] containing 1 μg of sonicated salmon sperm DNA and 3 μg of bovine serum albumin, incubated at 28 °C for 30 min, Samples were then loaded onto a 6% polyacrylamide-Tris-borate-EDTA (TBE) gel, and visualized.
4.11. In Vitro Transcription Assays
The in vitro transcription assays were conducted following a previously established protocol [4]. First, the corresponding DNA fragments (including the promoter region and a portion of the coding region of the target gene) were amplified using the appropriate primers. The TrxA-TfmR protein and DNA fragments were incubated in transcription buffer at 28 °C for 30 min. Then, the NTP mixture (250 μM each of ATP, CTP, and GTP; 250 μM biotin 16-UTP) and 0.5 U of E. coli RNA polymerase holoenzyme (New England BioLabs, Ipswich, MA, USA) were added and the reaction was carried out for 1 h at 37 °C. Transcription products were analyzed by electrophoresis at the end of the reaction. The obtained transcripts were visualized using a fluorescence imager screen (GE AI600, Boston, MA, USA).
4.12. Chromatin Immunoprecipitation–Quantitative PCR (ChIP-qPCR)
We performed ChIP-qPCR experiments as previously described, with only minor modifications [4]. Briefly, GX01/TfmR::3 × Flag reporter strains were cultured in medium to the corresponding OD600 values, collected and crosslinked using formaldehyde for cross-linking. Then sonication was performed and, for each ChIP sample, 50 μL of Flag antibody (agarose-conjugated) was added to 3.5 mL of bacterial lysate and incubated at 4 °C overnight. Unbound-DNA fragments were washed and bound-DNA fragments and proteins were eluted with 0.25 M glycine (pH 2.5). RNA was removed from the DNA samples by incubation with RNaseA for 2 h at 37 °C, and then proteins were removed by incubation with proteinase K for 2 h at 55 °C. The DNA samples were subsequently purified using a PCR purification kit (Qiagen, Alameda, CA, USA). To measure the enrichment of RpfG, HrpX, and HutG (negative control) in the ChIP DNA samples, relative-abundance quantitative PCR (qPCR) was performed, using the ChamQ Universal SYBR-qPCR Master Mix (Vazyme) and a real-time PCR thermocycler (Analytik jena qTOWER2.0; jena). The quantity of IP DNA (eluted DNA) was calculated as the percentage of the DNA present in the input DNA (10 μL sample taken prior to IP) using the ΔΔCt method to calculate the relative enrichment as the fold change [46]. The relative IP was calculated by normalizing IP to a mock ChIP using an anti-HA-tag monoclonal antibody (Solarbio, Beijing, China).
4.13. Transcriptome Analysis of the TfmR Mutant
Transcriptome analysis was conducted following the previously described protocol [5]. In brief, RNA was extracted from cell cultures with an OD600 of 0.8. To eliminate any contamination from genomic DNA, RNase-free DNase I was used. After quantification and quality assessment, the total-RNA samples were sent to Novogene (Beijing, China) for library construction and strand-specific RNA sequencing. The resulting clean reads were mapped to the genome of the Xoc strain GX01 (assembly ID 4726371), and gene expression levels were calculated using the RPKM (reads-per-kilobase per million-mapped-reads) method. Differentially expressed genes (DEGs) were identified based on a false discovery rate (FDR) of ≤0.05 and a |log2FC| (log2 of fold change) ≥ 1 threshold. The details of the DEGs can be found in Table S1.
4.14. Western Blotting
Western blotting was performed using the method previously used by Li et al. [47]. First, bacterial proteins separated by SDS-PAGE gels were electro-transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membrane was then blocked with 1% milk for 1 h at room temperature, after which it was incubated with a 1:2500 dilution of anti-Flag-tagged mouse monoclonal antibody (Abmart) as the primary antibody, and then washed with Tris-buffer saline and Tween buffer (Tris 20 mM, NaCl 0.3 M, Tween 20 0.08%). Goat anti-mouse immunoglobulin G (IgG) (Beyotime Biotechnology) coupled with diluted 1:2500 horseradish peroxidase (HRP) was used as secondary antibody. Finally, the luminescent signal was detected according to the manufacturer’s instructions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25115887/s1.
Author Contributions
G.L. and R.L. conceived and designed the project. Z.C. carried out the experiments. Z.M., Q.S., X.X. and W.Y. provided the resources and analyzed the data. Z.C. and R.L. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32360649, 32160617 and 32260501.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The RNA sequencing data generated in this study are available in the NCBI SRA database under the accession codes PRJNA1098699. Other data are presented within the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.A.X.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Vorhölter, F.-J.; Potnis, N.; Jones, J.B.; Van Sluys, M.-A.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding bacterium–plant interactions. Nat. Rev. Microbiol. 2011, 9, 344–355. [Google Scholar] [CrossRef] [PubMed]
- NiÑO-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-L.; Shi, J.-F.; Ma, Z.-F.; Zhou, X.-L.; Ye, W.-X.; Su, Q.; Zhu, G.-N.; Tang, J.-L.; Li, R.-F.; Lu, G.-T. XrvB regulates the type III secretion system by directly repressing hrpG transcription in Xanthomonas oryzae pv. oryzicola. Phytopathol. Res. 2023, 5, 43. [Google Scholar] [CrossRef]
- Li, R.-F.; Ren, P.-D.; Zhang, D.-P.; Cui, P.; Zhu, G.-N.; Xian, X.-Y.; Tang, J.-L.; Lu, G.-T. HpaP divergently regulates the expression of hrp genes in Xanthomonas oryzae pathovars oryzae and oryzicola. Mol. Plant Pathol. 2023, 24, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, C.; Jiang, W.; Wang, L.; Li, C.; Wang, Y.; Dow, J.M.; Sun, W. The HD-GYP domain protein RpfG of Xanthomonas oryzae pv. oryzicola regulates synthesis of extracellular polysaccharides that contribute to biofilm formation and virulence on rice. PLoS ONE 2013, 8, e59428. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; McCarthy, Y.; Andrade, M.; Farah, C.S.; Armitage, J.P.; Dow, J.M. Cell–cell signal-dependent dynamic interactions between HD-GYP and GGDEF domain proteins mediate virulence in Xanthomonas campestris. Proc. Natl. Acad. Sci. USA 2010, 107, 5989–5994. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.M.; Crossman, L.; Findlay, K.; He, Y.-Q.; Feng, J.-X.; Tang, J.-L. Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10995–11000. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.E.; Tang, J.L.; Feng, J.X.; Pan, M.Q.; Wilson, T.J.G.; Slater, H.; Dow, J.M.; Williams, P.; Daniels, M.J. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 1997, 24, 555–566. [Google Scholar] [CrossRef]
- Dow, J.M.; Fouhy, Y.; Lucey, J.F.; Ryan, R.P. The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants. Mol. Plant-Microbe Interact. 2006, 19, 1378–1384. [Google Scholar] [CrossRef]
- Ryan, R.P.; Fouhy, Y.; Lucey, J.F.; Jiang, B.-L.; He, Y.-Q.; Feng, J.-X.; Tang, J.-L.; Dow, J.M. Cyclic di-GMP signalling in the virulence and environmental adaptation of Xanthomonas campestris. Mol. Microbiol. 2007, 63, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef]
- McCann, H.C.; Guttman, D.S. Evolution of the type III secretion system and its effectors in plant–microbe interactions. New Phytol. 2008, 177, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.-F.; Wang, X.-P.; Xiang, Y.; Zhang, B.; Li, Y.-R.; Xiao, Y.-L.; Wang, J.-S.; Walmsley Adrian, R.; Chen, G.-Y. Elucidation of the hrp clusters of Xanthomonas oryzae pv. oryzicola that control the hypersensitive response in nonhost tobacco and pathogenicity in susceptible host rice. Appl. Environ. Microbiol. 2006, 72, 6212–6224. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.-N.; Li, Y.; Carpenter, S.C.D.; Dan, X.; Li, T.; Wu, Q.; Wang, L.; Jiang, W.; Huang, S.; Tang, J.-L.; et al. Complete genome resource of Xanthomonas oryzae pv. oryzicola GX01 isolated in south China. Mol. Plant-Microbe Interact. 2022, 35, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Büttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Teper, D.; Pandey, S.S.; Wang, N. The HrpG/HrpX Regulon of Xanthomonads—An insight to the complexity of regulation of virulence traits in phytopathogenic bacteria. Microorganisms 2021, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Koebnik, R.; Krüger, A.; Thieme, F.; Urban, A.; Bonas, U. Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes. J. Bacteriol. 2006, 188, 7652–7660. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Figueiredo, F.; Jones, J.; Wang, N. HrpG and HrpX play global roles in coordinating different virulence traits of Xanthomonas axonopodis pv. citri. Mol. Plant-Microbe Interact. 2011, 24, 649–661. [Google Scholar] [CrossRef]
- Shao, Y.; Tang, G.; Huang, Y.; Ke, W.; Wang, S.; Zheng, D.; Ruan, L. Transcriptional regulator Sar regulates the multiple secretion systems in Xanthomonas oryzae. Mol. Plant Pathol. 2023, 24, 16–27. [Google Scholar] [CrossRef]
- Huang, D.-L.; Tang, D.-J.; Liao, Q.; Li, H.-C.; Chen, Q.; He, Y.-Q.; Feng, J.-X.; Jiang, B.-L.; Lu, G.-T.; Chen, B.; et al. The Zur of Xanthomonas campestris functions as a repressor and an activator of putative zinc homeostasis genes via recognizing two distinct sequences within its target promoters. Nucleic Acids Res. 2008, 36, 4295–4309. [Google Scholar] [CrossRef] [PubMed]
- Teper, D.; Zhang, Y.; Wang, N. TfmR, a novel TetR-family transcriptional regulator, modulates the virulence of Xanthomonas citri in response to fatty acids. Mol. Plant Pathol. 2019, 20, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, L.; Nodwell Justin, R. The TetR family of regulators. Microbiol. Mol. Biol. Rev. 2013, 77, 440–475. [Google Scholar] [CrossRef] [PubMed]
- Ramos Juan, L.; Martínez-Bueno, M.; Molina-Henares Antonio, J.; Terán, W.; Watanabe, K.; Zhang, X.; Gallegos María, T.; Brennan, R.; Tobes, R. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 2005, 69, 326–356. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, C.; Xie, J. The underling mechanism of bacterial TetR/AcrR family transcriptional repressors. Cell. Signal. 2013, 25, 1608–1613. [Google Scholar] [CrossRef]
- Zhu, P.-C.; Li, Y.-M.; Yang, X.; Zou, H.-F.; Zhu, X.-L.; Niu, X.-N.; Xu, L.-H.; Jiang, W.; Huang, S.; Tang, J.-L.; et al. Type VI secretion system is not required for virulence on rice but for inter-bacterial competition in Xanthomonas oryzae pv. oryzicola. Res. Microbiol. 2020, 171, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Dow, J.M. Intermolecular interactions between HD-GYP and GGDEF domain proteins mediate virulence-related signal transduction in Xanthomonas campestris. Virulence 2010, 1, 404–408. [Google Scholar] [CrossRef] [PubMed]
- An, S.-Q.; Febrer, M.; McCarthy, Y.; Tang, D.-J.; Clissold, L.; Kaithakottil, G.; Swarbreck, D.; Tang, J.-L.; Rogers, J.; Dow, J.M.; et al. High-resolution transcriptional analysis of the regulatory influence of cell-to-cell signalling reveals novel genes that contribute to Xanthomonas phytopathogenesis. Mol. Microbiol. 2013, 88, 1058–1069. [Google Scholar] [CrossRef]
- He, Y.-W.; Ng, A.Y.-J.; Xu, M.; Lin, K.; Wang, L.-H.; Dong, Y.-H.; Zhang, L.-H. Xanthomonas campestris cell–cell communication involves a putative nucleotide receptor protein Clp and a hierarchical signalling network. Mol. Microbiol. 2007, 64, 281–292. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Li, J.-L.; Wang, N. Diffusible Signal Factor-Mediated Quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri. Mol. Plant-Microbe Interact. 2011, 25, 165–179. [Google Scholar] [CrossRef]
- Cho, J.H.; Yoon, J.M.; Lee, S.W.; Noh, Y.H.; Cha, J.S. Xanthomonas oryzae pv. oryzae RpfE regulates virulence and carbon source utilization without change of the DSF production. Plant Pathol. J. 2013, 29, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Lee, M.A.; Yoo, Y.; Cho, M.H.; Lee, S.W. Genome-wide screening to identify responsive regulators involved in the virulence of Xanthomonas oryzae pv. oryzae. Plant Pathol. J. 2019, 35, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liang, X.; Liu, K.; Dong, W.; Wang, J.; Zhou, M.-G. The thiG gene is required for full virulence of Xanthomonas oryzae pv. oryzae by preventing cell aggregation. PLoS ONE 2015, 10, e0134237. [Google Scholar] [CrossRef]
- Guo, W.; Zou, L.-F.; Cai, L.-L.; Chen, G.-Y. Glucose-6-phosphate dehydrogenase is required for extracellular polysaccharide production, cell motility and the full virulence of Xanthomonas oryzae pv. oryzicola. Microb. Pathog. 2015, 78, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rott, P.; Fleites, L.A.; Mensi, I.; Sheppard, L.; Daugrois, J.-H.; Dow, J.M.; Gabriel, D.W. The RpfCG two-component system negatively regulates the colonization of sugar cane stalks by Xanthomonas albilineans. Microbiology 2013, 159, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- An, S.-Q.; Lu, G.-T.; Su, H.-Z.; Li, R.-F.; He, Y.-Q.; Jiang, B.-L.; Tang, D.-J.; Tang, J.-L. Systematic mutagenesis of all predicted gntR genes in Xanthomonas campestris pv. campestris reveals a GntR family transcriptional regulator controlling hypersensitive response and virulence. Mol. Plant-Microbe Interact. 2011, 24, 1027–1039. [Google Scholar] [CrossRef]
- Zhou, X.; Yan, Q.; Wang, N. Deciphering the regulon of a GntR family regulator via transcriptome and ChIP-exo analyses and its contribution to virulence in Xanthomonas citri. Mol. Plant Pathol. 2017, 18, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-F.; Lu, G.-T.; Li, L.; Su, H.-Z.; Feng, G.-f.; Chen, Y.; He, Y.-Q.; Jiang, B.-L.; Tang, D.-J.; Tang, J.-L. Identification of a putative cognate sensor kinase for the two-component response regulator HrpG, a key regulator controlling the expression of the hrp genes in Xanthomonas campestris pv. campestris. Environ. Microbiol. 2014, 16, 2053–2071. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ma, W.; Zou, L.; Xu, X.; Xu, Z.; Deng, C.; Qian, W.; Chen, X.; Chen, G. Xanthomonas oryzae pv. oryzicola response regulator VemR is co-opted by the sensor kinase CheA for phosphorylation of multiple pathogenicity-related targets. Front. Microbiol. 2022, 13, 928551. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Wu, G.-C.; Wang, B.; Guo, B.-D.; Sheng, C.; Zhao, Y.-Y.; Tang, B.; Zhao, Y.-C.; Liu, F.-Q. Sigma factor 70 RpoD contributes to virulence by regulating cell motility, oxidative stress tolerance, and manipulating the expression of hrpG and hrpX in Xanthomonas oryzae pv. oryzae. J. Integr. Agric. 2023. [Google Scholar] [CrossRef]
- Leng, M.; Lu, Z.-J.; Qin, Z.-S.; Qi, Y.-H.; Lu, G.-T.; Tang, J.-L. Flp, a Fis-like protein, contributes to the regulation of type III secretion and virulence processes in the phytopathogen Xanthomonas campestris pv. campestris. Mol. Plant Pathol. 2019, 20, 1119–1133. [Google Scholar] [CrossRef]
- Tsuge, S.; Furutani, A.; Fukunaka, R.; Oku, T.; Tsuno, K.; Ochiai, H.; Inoue, Y.; Kaku, H.; Kubo, Y. Expression of Xanthomonas oryzae pv. oryzae hrp genes in XOM2, a novel synthetic medium. J. Gen. Plant Pathol. 2002, 68, 363–371. [Google Scholar] [CrossRef]
- Sambrook, J.; Maniatis, T.; Fritsch, E. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratories: Cold Spring Harbor, NY, USA, 1989; Volume 1. [Google Scholar]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Leong, S.A.; Ditta, G.S.; Helinski, D.R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J. Biol. Chem. 1982, 257, 8724–8730. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-F.; Peng, J.-L.; Liu, Q.-Q.; Chang, Z.; Huang, Y.-X.; Tang, J.-L.; Lu, G.-T. Xanthomonas campestris VemR enhances the transcription of the T3SS key regulator HrpX via physical interaction with HrpG. Mol. Plant Pathol. 2023, 24, 232–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).