Different Drug Mobilities in Hydrophobic Cavities of Host–Guest Complexes between β-Cyclodextrin and 5-Fluorouracil at Different Stoichiometries: A Molecular Dynamics Study in Water
Abstract
:1. Introduction
2. Results and Discussion
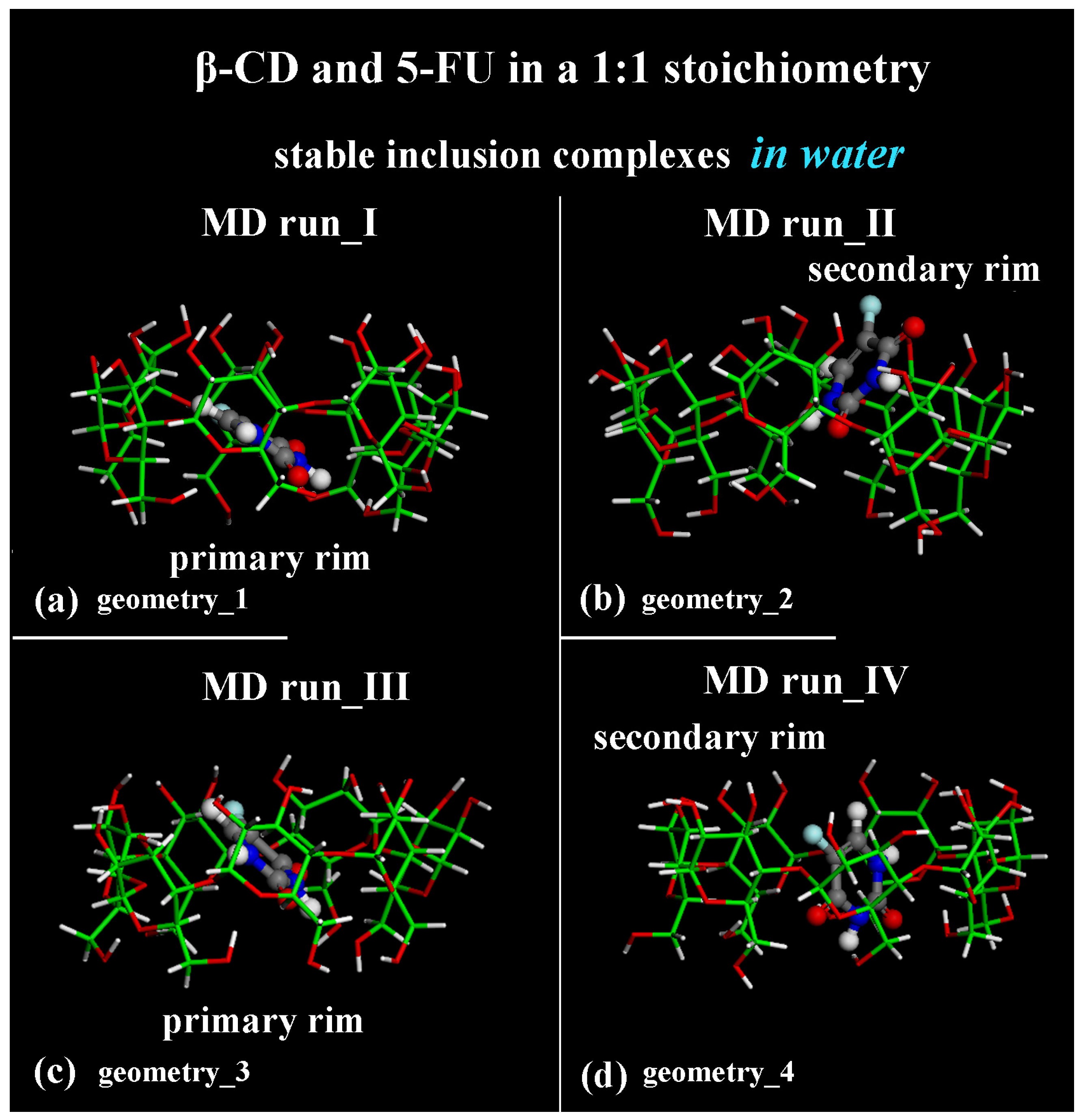
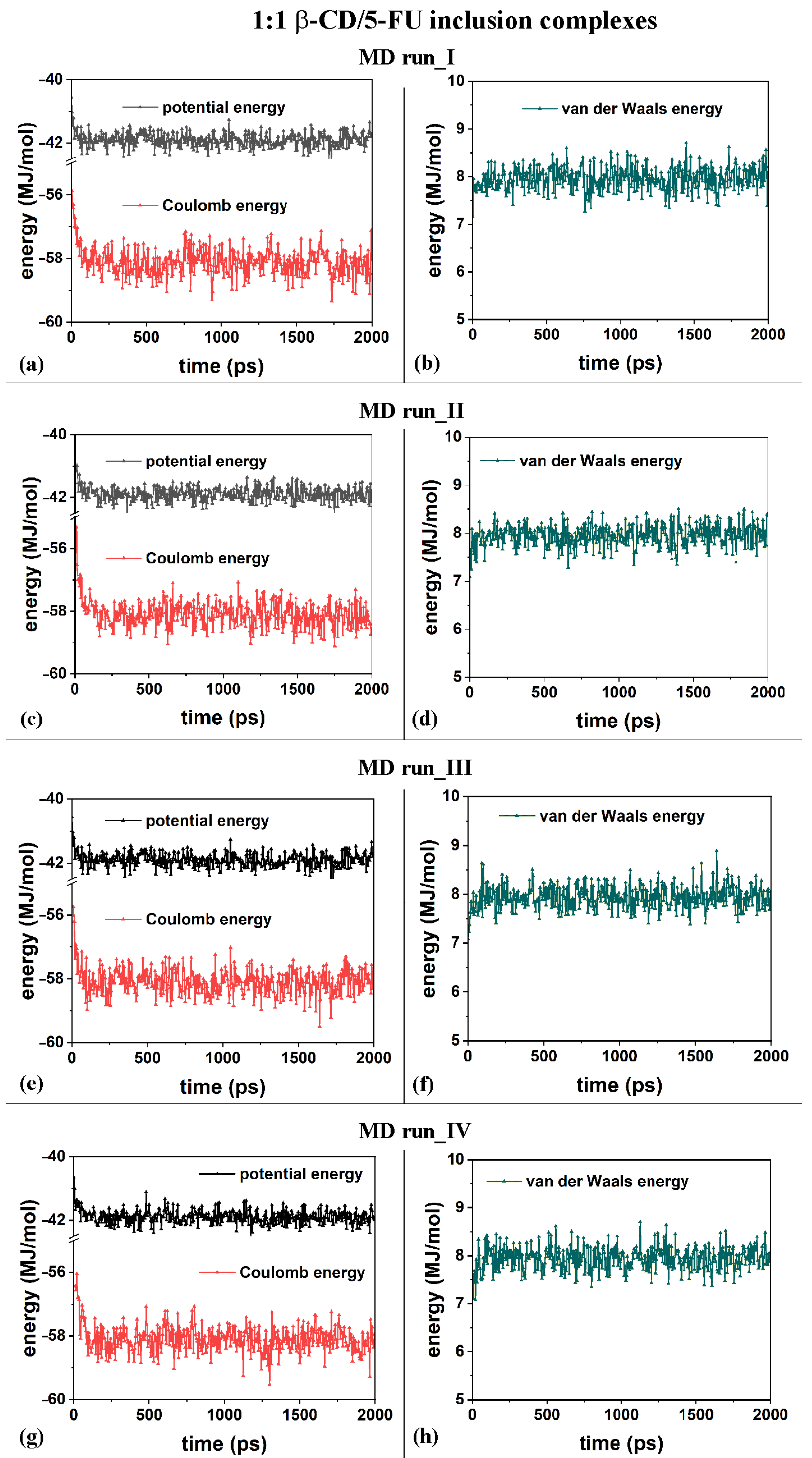
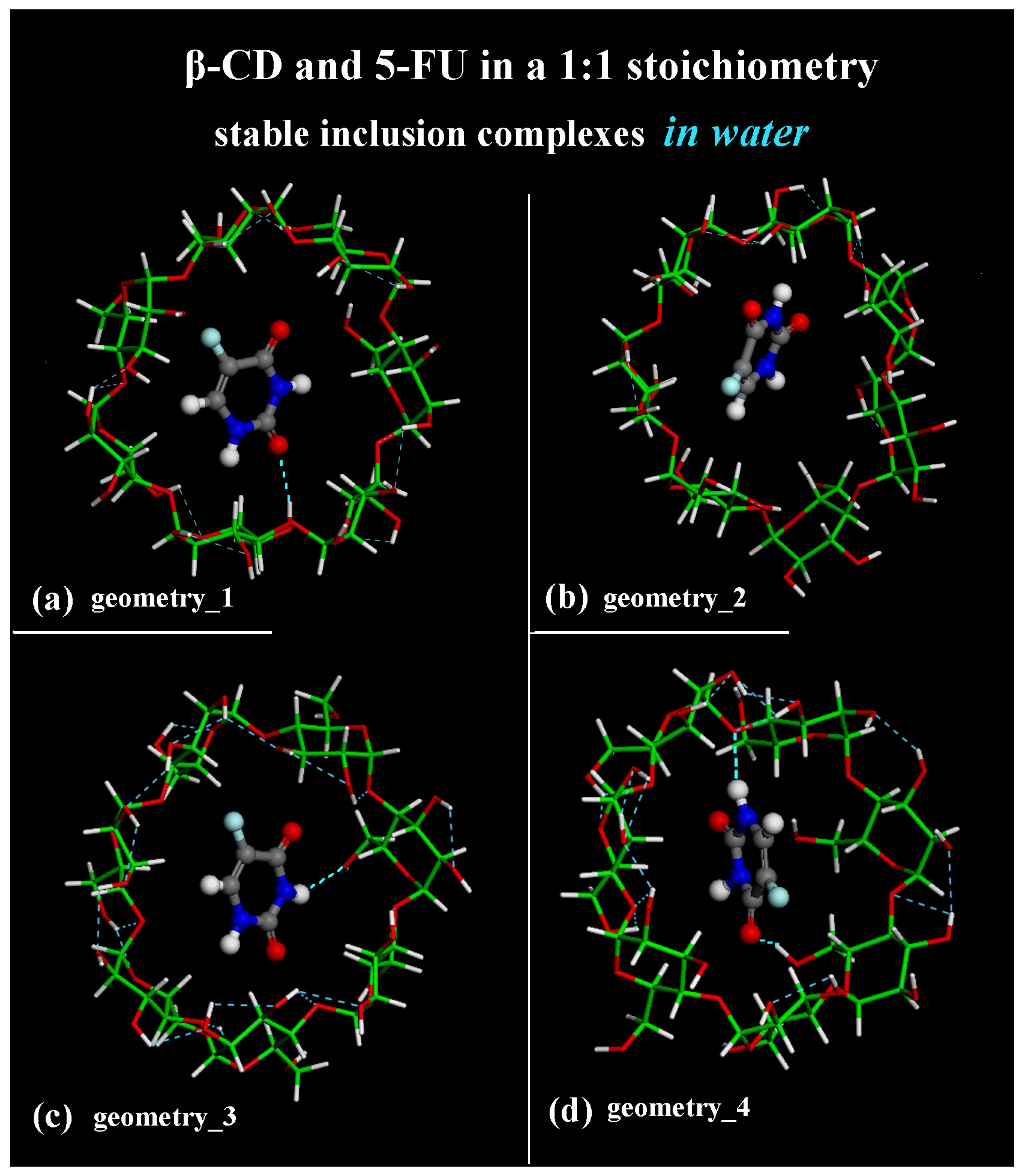
2.1. β-CD/5-FU Inclusion Complexes in a 1:1 Host–Guest Stoichiometry in Water
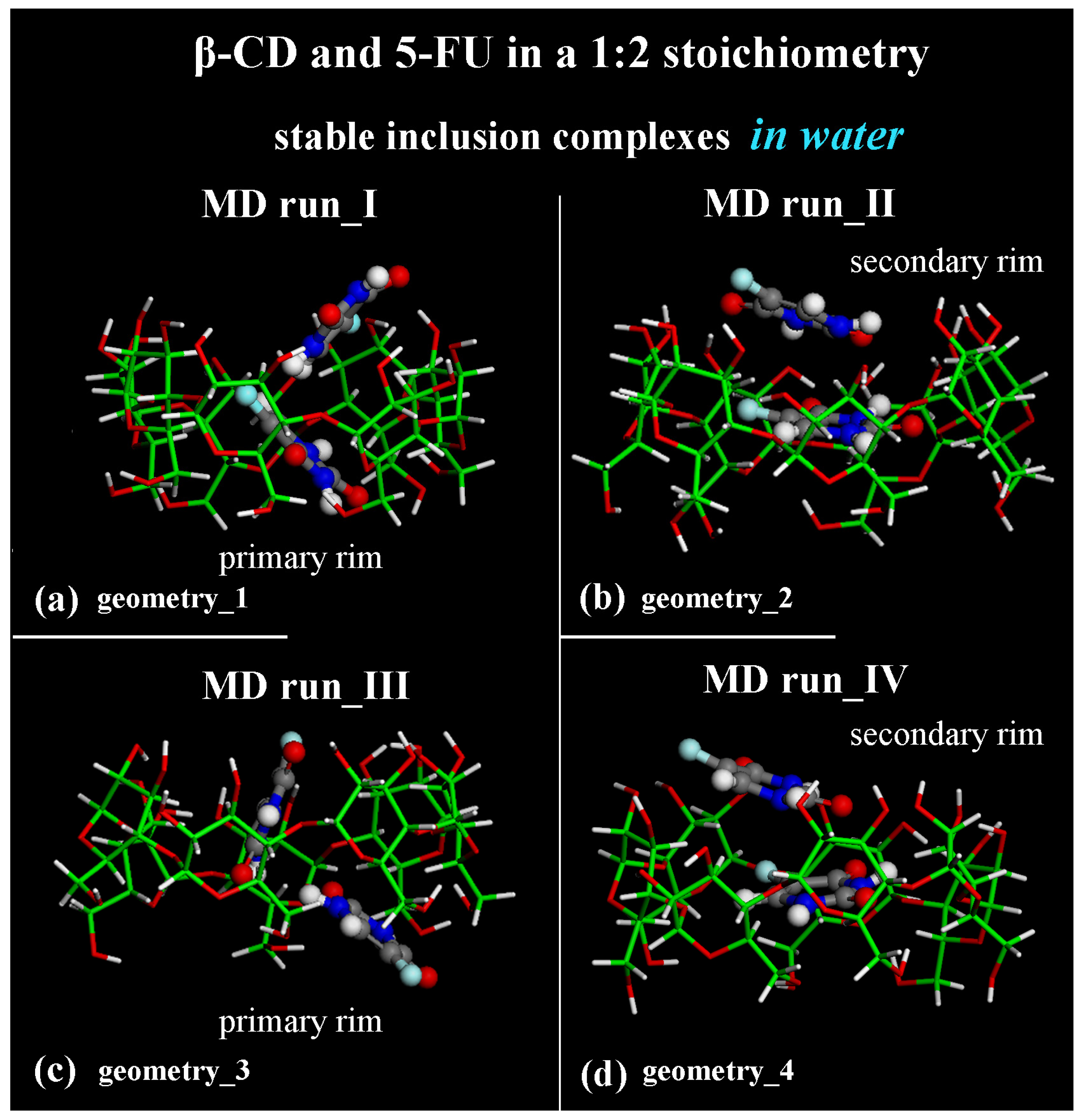
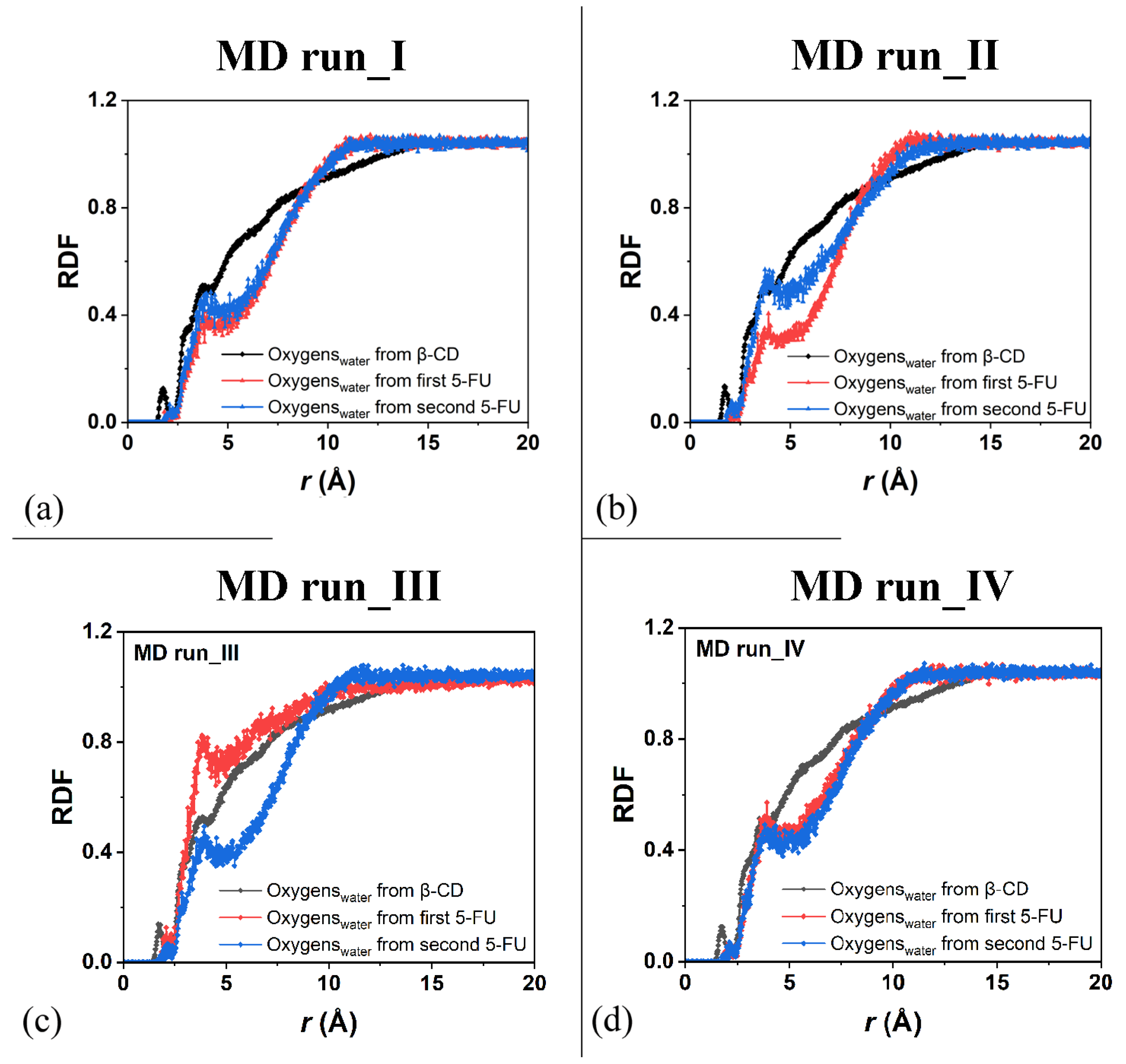
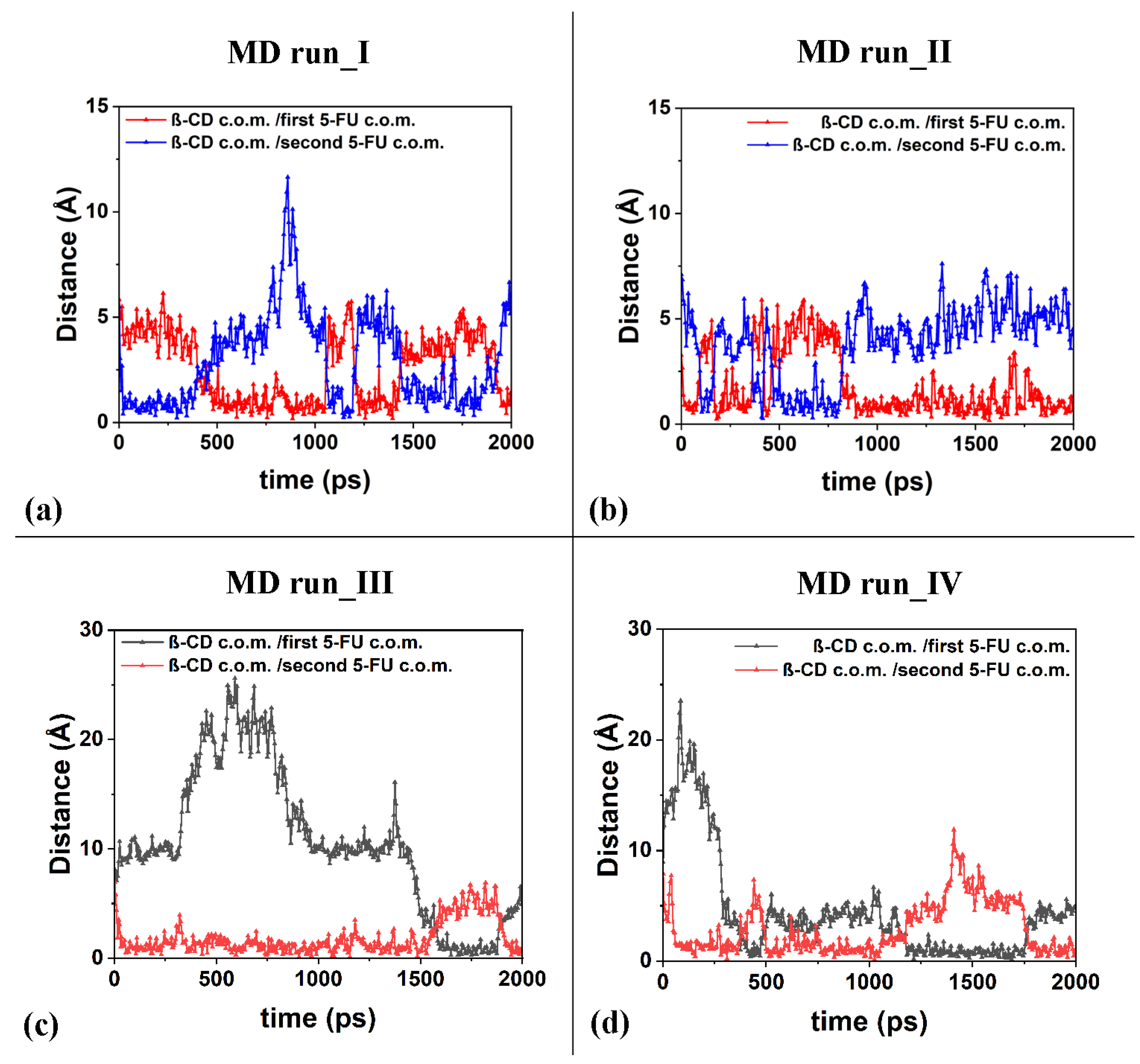
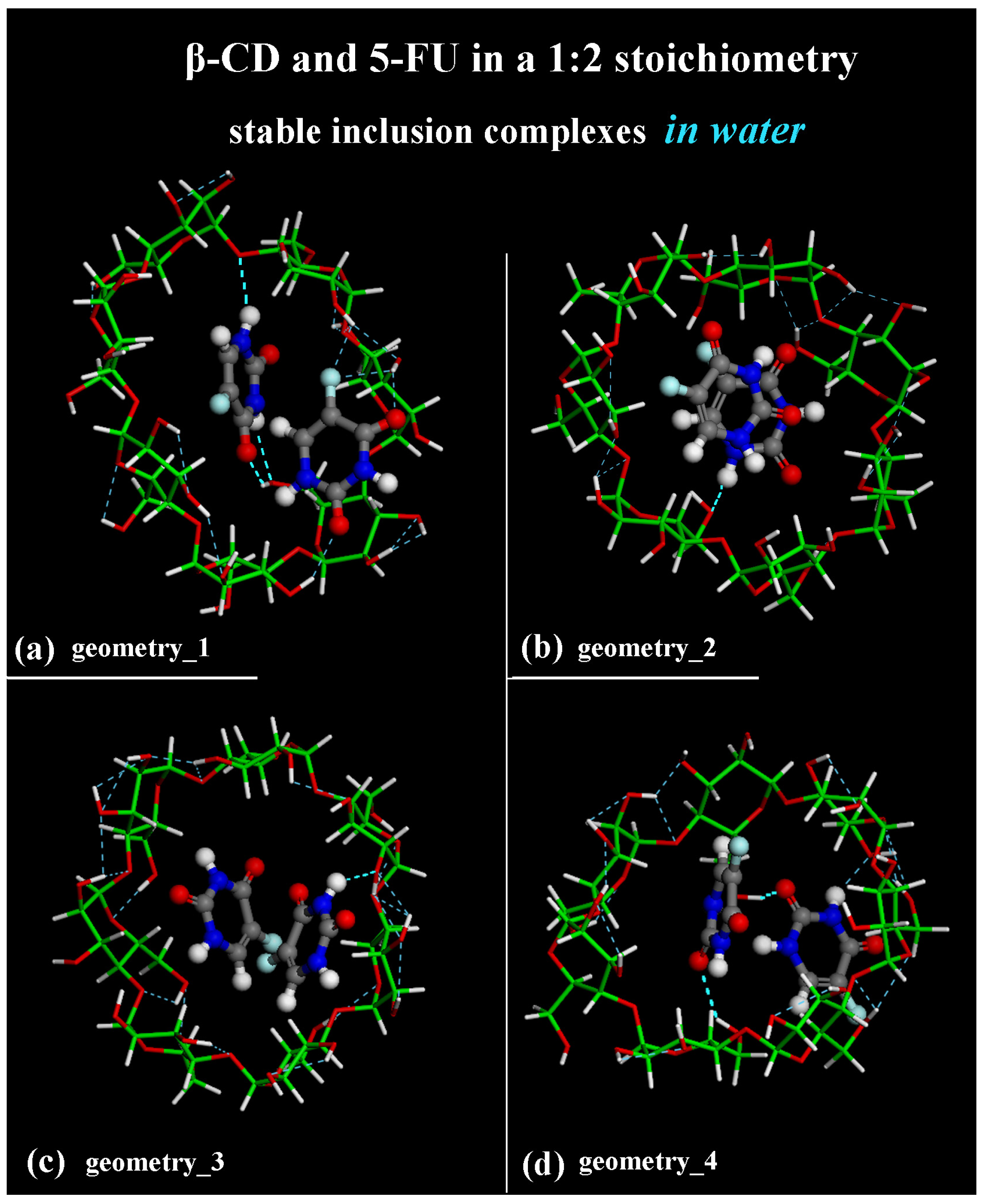
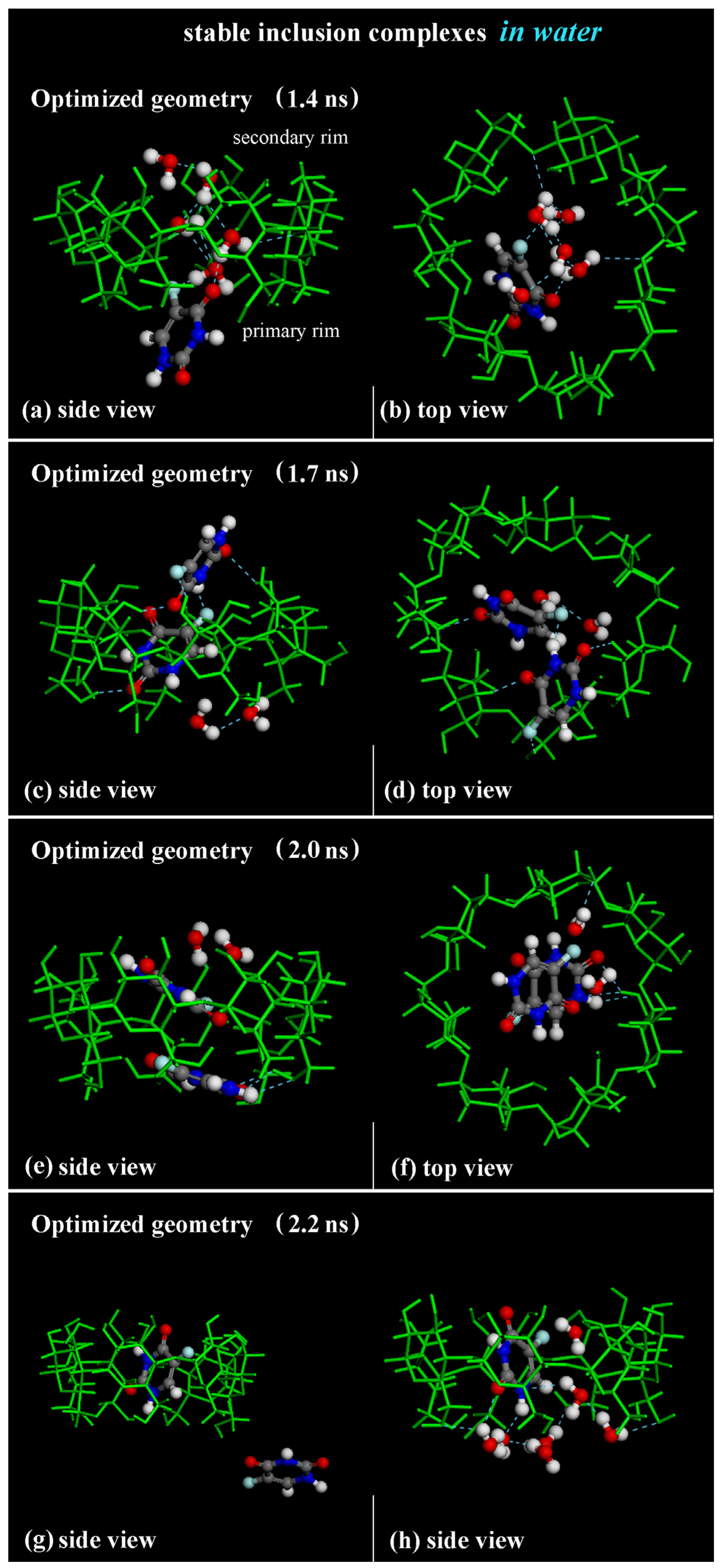
2.2. β-CD/5-FU Inclusion Complexes in a 1:2 Host–Guest Stoichiometry in Water
2.3. β-CD and Two 5-FU Molecules in a Random Arrangement in Water: From 1:1 to 1:2 Inclusion Complex Formation with Different Stabilities
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G. Review: A history of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular Chemistry Receptors, Catalysts, and Carriers. Science 1985, 227, 849–857. [Google Scholar] [CrossRef]
- Das, S.K.; Rajabalaya, R.; David, S.; Gani, N.; Khanam, J.; Nanda, A. Cyclodextrins-The Molecular Container. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1694–1720. [Google Scholar]
- Lee, T.-C.; Kalenius, E.; Lazar, A.I.; Assaf, K.I.; Kuhnert, N.; Grün, C.H.; Jänis, J.; Scherman, O.A.; Nau, W.M. Chemistry inside molecular containers in the gas phase. Nat. Chem. 2013, 5, 376–382. [Google Scholar] [CrossRef]
- Ceborska, M.; Szwed, K.; Suwinska, K. β-Cyclodextrin as the suitable molecular container for isopulegol enantiomers. Carbohydr. Polym. 2013, 97, 546–550. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Davis, M.; Brewster, M. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Stella, V.J.; Rao, V.M.; Zannou, E.A.; Zia, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Sakai, S.; Hirano, Y.; Kobayashi, Y.; Arai, N. Effect of temperature on the structure and drug-release behaviour of inclusion complex of β-cyclodextrin with cyclophosphamide: A molecular dynamics study. Soft Matter 2023, 19, 2902–2907. [Google Scholar] [CrossRef]
- Bencini, M.; Ranucci, E.; Ferruti, P.; Trotta, F.; Donalisio, M.; Cornaglia, M.; Lembo, D.; Cavalli, R. Preparation and in vitro evaluation of the antiviral activity of the Acyclovir complex of a β-cyclodextrin/poly(amidoamine) copolymer. J. Control. Release 2008, 126, 17–25. [Google Scholar] [CrossRef]
- Li, B.-L.; Wang, C.-L.; Wang, Q.; Yang, J.-M.; Chi, S.-M.; Chen, J.-C.; Zhang, J.; Zhao, Y. β-Cyclodextrin-based supramolecular nanoparticles: pH-sensitive nanocarriers for the sustained release of anti-tumor drugs. New J. Chem. 2022, 46, 21823–21833. [Google Scholar] [CrossRef]
- Treccani, S.; Alongi, J.; Manfredi, A.; Ferruti, P.; Cavalli, R.; Raffaini, G.; Ranucci, E. L-Arginine-Derived Polyamidoamine Oligomers Bearing at Both Ends β-Cyclodextrin Units as pH-Sensitive Curcumin Carriers. Polymers 2022, 14, 3193. [Google Scholar] [CrossRef]
- Zagami, R.; Barattucci, A.; Scolaro, L.M.; Viale, M.; Raffaini, G.; Bonaccorsi, P.M.; Mazzaglia, A. Curcumin/amphiphilic cyclodextrin nanoassemblies: Theoretical and spectroscopic studies to address their debut in anticancer therapy. J. Mol. Liq. 2023, 389, 122841. [Google Scholar] [CrossRef]
- Xiong, T.; Zou, C.; Lin, S. β-Cyclodextrin magnetic graphene oxide (β-CD@MGO) for efficient removal of Ni(II) and phenol: Adsorption performance, reusability and adsorption mechanism studies. Diam. Relat. Mater. 2023, 139, 110269. [Google Scholar] [CrossRef]
- Liu, W.D. Dissolution behavior of 5-fluorouracil in fourteen neat solvents: Solubility, correlation, solvent effect, Hansen solubility parameter, molecular simulation and thermodynamic analysis. J. Mol. Liq. 2022, 353, 118733. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T.; Bodor, N. Applications of chemically-modified cyclodextrins: Use of hydroxypropyl-β-cyclodextrin as an enabling excipient for brain targeting, redox-based derivatives of estradiol: A review of preclinical and clinical findings. J. Drug Deliv. Sci. Technol. 2004, 14, 21–34. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef]
- Altoom, N.; Adlii, A.; Othman, S.I.; Allam, A.A.; Alqhtani, H.A.; Al-Otaibi, F.S.; Abukhadra, M.R. Synthesis and characterization of β-cyclodextrin functionalized zeolite-A as biocompatible carrier for Levofloxacin drug; loading, release, cytotoxicity, and anti-inflammatory studies. J. Solid State Chem. 2022, 312, 123280. [Google Scholar] [CrossRef]
- Ferruti, P.; Ranucci, E.; Trotta, F.; Gianasi, E.; Evagorou, E.G.; Wasil, M.; Wilson, G.; Duncan, R. Synthesis, characterisation and antitumour activity of platinum(II) complexes of novel functionalised poly(amido amine)s. Macromol. Chem. Phys. 1999, 200, 1644–1654. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host-Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Ryzhakov, A.; Thi, T.D.; Stappaerts, J.; Bertoletti, L.; Kimpe, K.; Couto, A.R.S.; Saokham, P.; Van den Mooter, G.; Augustijns, P.; Somsen, G.W.; et al. Self-Assembly of Cyclodextrins and Their Complexes in Aqueous Solutions. J. Pharm. Sci. 2016, 105, 2556–2569. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Cavalli, R.; Donalisio, M.; Civra, A.; Ferruti, P.; Ranucci, E.; Trotta, F.; Lembo, D. Enhanced antiviral activity of Acyclovir loaded into β-cyclodextrin-poly(4-acryloylmorpholine) conjugate nanoparticles. J. Control. Release 2009, 137, 116–122. [Google Scholar] [CrossRef]
- Iacovino, R.; Caso, J.V.; Rapuano, F.; Russo, A.; Isidori, M.; Lavorgna, M.; Malgieri, G.; Isernia, C. Physicochemical characterization and cytotoxic activity evaluation of hydroxymethylferrocene: β-Cyclodextrin inclusion complex. Molecules 2012, 17, 6056–6070. [Google Scholar] [CrossRef]
- Mendes, C.; Meirelles, G.C.; Barp, C.G.; Assreuy, J.; Silva, M.A.S.; Ponchel, G. Cyclodextrin based nanosponge of norfloxacin: Intestinal permeation enhancement and improved antibacterial activity. Carbohydr. Polym. 2018, 195, 586–592. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Understanding Surface Interaction and Inclusion Complexes between Piroxicam and Native or Crosslinked β-Cyclodextrins: The Role of Drug Concentration. Molecules 2020, 25, 2848. [Google Scholar] [CrossRef]
- Castiglione, F.; Ganazzoli, F.; Malpezzi, L.; Mele, A.; Panzeri, W.; Raffaini, G. Inclusion complexes of β-cyclodextrin with tricyclic drugs: An X-ray diffraction, NMR and molecular dynamics study. Beilstein J. Org. Chem. 2017, 13, 714–719. [Google Scholar] [CrossRef]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Swaminathan, S.; Cavalli, R.; Trotta, F. Cyclodextrin-based nanosponges: A versatile platform for cancer nanotherapeutics development. Wiley interdisciplinary reviews. Nanomed. Nanobiotechnol. 2016, 8, 579–601. [Google Scholar] [CrossRef]
- Hirayama, F.; Uekama, K. Cyclodextrin-based controlled drug release system. Adv. Drug Deliv. Rev. 1999, 36, 125–141. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Signorile, R.; Fini, P.; Cecone, C.; Matencio, A.; Trotta, F.; Cosma, P. Cyclodextrin nanosponges as adsorbent material to remove hazardous pollutants from water: The case of ciprofloxacin. Chem. Eng. J. 2021, 411, 128514. [Google Scholar] [CrossRef]
- Yang, G.; Fang, D.; Yang, L.; Wei, Z.; Tu, Y.; Shao, P.; Hua, Z.; Wang, Z.; Luo, X. Tailored construction of β-cyclodextrin covalently-supported tannic acid polymer nanosponge towards highly selective lead recovery. J. Clean. Prod. 2022, 330, 129882. [Google Scholar] [CrossRef]
- Nazerdeylami, S.; Ghasemi, J.B.; Ziarani, G.M.; Amiri, A.; Badiei, A. Direct monitoring of diclofenac using a supramolecular fluorescent approach based on β-cyclodextrin nanosponge. J. Mol. Liq. 2021, 336, 116104. [Google Scholar] [CrossRef]
- Mhlanga, S.D.; Marnba, B.B.; Krause, R.I.; Malefetse, T.J. Removal of organic contaminants from water using nanosponge cyclodextrin polyurethanes. J. Chem. Technol. Biotechnol. 2007, 82, 382–388. [Google Scholar] [CrossRef]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-β-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef]
- Fang, G.; Yang, X.; Chen, S.; Wang, Q.; Zhang, A.; Tang, B. Cyclodextrin-based host-guest supramolecular hydrogels for local drug delivery. Coord. Chem. Rev. 2022, 454, 214352. [Google Scholar] [CrossRef]
- Liu, K.L.; Zhang, Z.; Li, J. Supramolecular hydrogels based on cyclodextrin-polymer polypseudorotaxanes: Materials design and hydrogel properties. Soft Matter 2011, 7, 11290–11297. [Google Scholar] [CrossRef]
- Kundu, D.; Mondal, S.K.; Banerjee, T. Development of β-Cyclodextrin-Cellulose/Hemicellulose-Based Hydrogels for the Removal of Cd(II) and Ni(II): Synthesis, Kinetics, and Adsorption Aspects. J. Chem. Eng. Data 2019, 64, 2601–2617. [Google Scholar] [CrossRef]
- Yue, Y.D.; Cui, Y.Y.; Yang, C.-X. Construction of cyclodextrin microporous organic network based drug delivery platform for controllable release and targeting delivery of doxorubicin. Chem. Eng. J. Adv. 2023, 14, 100487. [Google Scholar] [CrossRef]
- Raffaini, G.; Catauro, M. Surface Interactions between Ketoprofen and Silica-Based Biomaterials as Drug Delivery System Synthesized via Sol–Gel: A Molecular Dynamics Study. Materials 2022, 15, 2759. [Google Scholar] [CrossRef]
- Yi, S.; Zheng, J.; Lv, P.; Zhang, D.; Zheng, X.; Zhang, Y.; Liao, R. Controlled Drug Release from Cyclodextrin-Gated Mesoporous Silica Nanoparticles Based on Switchable Host-Guest Interactions. Bioconjug. Chem. 2018, 29, 2884–2891. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, P.; Cao, Z.; Liang, W.; Yan, J.; Xu, H.; Wu, L.; Sun, L.; Gong, L.; Peng, C.; et al. Simultaneous solubilization and extended release of insoluble drug as payload in highly soluble particles of γ-cyclodextrin metal-organic frameworks. Int. J. Pharm. 2022, 619, 121685. [Google Scholar] [CrossRef]
- Tian, B.R.; Liu, Y.M.; Liu, J. Cyclodextrin as a magic switch in covalent and non-covalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Perkins, G.; Hirota, K. Targeting cholesterol with β-cyclodextrin sensitizes cancer cells for apoptosis. FEBS Lett. 2015, 589, 4097–4105. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Lavorgna, M.; Iacovino, R.; Russo, C.; Di Donato, C.; Piscitelli, C.; Isidori, M. A New Approach for Improving the Antibacterial and Tumor Cytotoxic Activities of Pipemidic Acid by Including It in Trimethyl—Cyclodextrin. Int. J. Mol. Sci. 2019, 20, 416. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. A Molecular Dynamics Study of a Photodynamic Sensitizer for Cancer Cells: Inclusion Complexes of γ-Cyclodextrins with C70. Int. J. Mol. Sci. 2019, 20, 4831. [Google Scholar] [CrossRef]
- Raffaini, G.; Mazzaglia, A.; Catauro, M. Molecular Dynamics Study of Sorafenib Anti-Cancer Drug: Inclusion Complex in Amphiphilic Cyclodextrin. Macromol. Symp. 2021, 395, 2000201. [Google Scholar] [CrossRef]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Pereira-Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-based delivery systems for in vivo-tested anticancer therapies. Drug Deliv. Transl. Res. 2020, 11, 49–71. [Google Scholar] [CrossRef]
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef]
- Straub, J.O. Combined environmental risk assessment for 5-fluorouracil and capecitabine in Europe. Integr. Environ. Assess. Manag. 2010, 6, 540–566. [Google Scholar] [CrossRef]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef]
- Van Kuilenburg, A.B. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur. J. Cancer 2004, 40, 939–950. [Google Scholar] [CrossRef]
- Buur, A.; Bundgaard, H.; Falch, E. Prodrugs of 5-fluorouracil. IV. Hydrolysis kinetics, bioactivation and physicochemical properties of various N-acyloxymethyl derivatives of 5-fluorouracil. Int. J. Pharmaceut. 1985, 24, 43–60. [Google Scholar] [CrossRef]
- Dongsar, T.T.; Dongsar, T.S.; Gupta, N.; Almalki, W.H.; Sahebkar, A.; Kesharwani, P. Emerging potential of 5-Fluorouracil-loaded chitosan nanoparticles in cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104371. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2019, 209, 107447. [Google Scholar] [CrossRef]
- Caballero, G.A.; Ausman, R.K.; Quebbeman, E.J. Long-term, ambulatory, continuous IV infusions of 5-FU for the treatment of advanced adenocarcinomas. Cancer Treat. Rep. 1985, 69, 13–15. [Google Scholar] [PubMed]
- Rajaei, M.; Rashedi, H.; Yazdian, F.; Navaei-Nigjeh, M.; Rahdar, A.; Díez-Pascual, A.M. Chitosan/agarose/graphene oxide nanohydrogel as drug delivery system of 5-fluorouracil in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 82, 104307. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, A.; Guo, Y.; Ma, T.; Feng, S. The relationship between tumor metabolism and 5-fluorouracil resistance. Biochem. Pharmacol. 2023, 218, 115902. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B.; Pushparaj, P.N. 5-Fluorouracil and capecitabine therapies for the treatment of colorectal cancer (Review). Oncol. Rep. 2023, 50, 175. [Google Scholar] [CrossRef] [PubMed]
- Mafi, A.; Rezaee, M.; Hedayati, N.; Hogan, S.D.; Reiter, R.J.; Aarabi, M.-H.; Asemi, Z. Melatonin and 5-fluorouracil combination chemotherapy: Opportunities and efficacy in cancer therapy. J. Cell Commun. Signal. 2023, 21, 33. [Google Scholar] [CrossRef]
- Mohammadian, S.; Khazaei, M.; Maghami, P.; Avan, A.; Rezaei, M. Polycaprolactone-based Nanocarriers Containing 5-fluorouracil as a Therapeutic Guided Drug Delivery Approach for Enhancing Anticancer Activity. Curr. Cancer Drug Targets 2023, 23, 524–533. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Okasha, A.T.; Al Othman, S.I.; Alfassam, H.E.; Alenazi, N.A.; AlHammadi, A.A.; Allam, A.A. Synthesis and Characterization of Mg-Hydroxyapatite and Its β-Cyclodextrin Composite as Enhanced Bio-Carrier of 5-Fluorouracil Drug; Equilibrium and Release Kinetics. ACS Omega 2023, 8, 30247–30261. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R. A comparative study of the interactions of 5-fluorouracil and Amlodipine Besylate in aqueous β-cyclodextrin solution and drug release studies. Polym. Bull. 2023, 81, 2719–2740. [Google Scholar] [CrossRef]
- Wang, L.L.; Zheng, W.S.; Chen, S.H.; Han, Y.X.; Jiang, J.D. Development of rectal delivered thermo-reversible gelling film encapsulating a 5-fluorouracil hydroxypropyl-β-cyclodextrin complex. Carbohydr. Polym. 2016, 137, 9–18. [Google Scholar] [CrossRef]
- Di Donato, C.; Lavorgna, M.; Fattorusso, R.; Isernia, C.; Isidori, M.; Malgieri, G.; Piscitelli, C.; Russo, C.; Russo, L.; Iacovino, R. Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation. Molecules 2016, 21, 1644. [Google Scholar] [CrossRef] [PubMed]
- Safdari, F.; Raissi, H.; Shahabi, M.; Zaboli, M. DFT Calculations and Molecular Dynamics Simulation Study on the Adsorption of 5-Fluorouracil Anticancer Drug on Graphene Oxide Nanosheet as a Drug Delivery Vehicle. J. Inorg. Organomet. Polym. Mater. 2017, 27, 805–817. [Google Scholar] [CrossRef]
- Harris, R.A. Molecular dynamics simulations of hydrophilic pores in phospholipid bilayers and Fe3O4 nanoparticles loaded with the 5-fluorouracil anticancer drug. J. Nanopart. Res. 2023, 25, 244. [Google Scholar] [CrossRef]
- Ghazali, F.; Hosseini, S.; Ketabi, S. DFT and Molecular Simulation Study of Gold Clusters as Effective Drug Delivery Systems for 5-Fluorouracil Anticancer Drug. J. Clust. Sci. 2023, 34, 1499–1509. [Google Scholar] [CrossRef]
- Mahdi, W.A.; Hussain, A.; Ramzan, M. 5-Fluorouracil Loaded Biogenic and Albumin Capped Gold Nanoparticles Using Bacterial Enzyme-In Vitro-In Silico Gastroplus® Simulation and Prediction. Processes 2020, 8, 1579. [Google Scholar] [CrossRef]
- Kavitha, K.; Srinivasa Rao, A.; Nalini, C.N. An Investigation on Enhancement of Solubility of 5 Fluorouracil by Applying Complexation Technique-Characterization, Dissolution and Molecular-Modeling Studies. J. Appl. Pharm. Sci. 2013, 3, 162–166. [Google Scholar]
- Buczek, A.; Stas, M.; Hebenstreit, C.; Maller, C.; Broda, M.A.; Kupka, T.; Kelterer, A.M. Interaction of 5-fluorouracil with β-cyclodextrin: A density functional theory study with dispersion correction. Int. J. Quantum Chem. 2021, 121, e26487. [Google Scholar] [CrossRef]
- Raffaini, G.; Elli, S.; Ganazzoli, F.; Catauro, M. Inclusion Complexes Between β-cyclodextrin and the Anticancer Drug 5-Fluorouracil for its Solubilization: A Molecular Dynamics Study at Different Stoichiometries. Macromol. Symp. 2022, 404, 2100305. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F.; Malpezzi, L.; Fuganti, C.; Fronza, G.; Panzeri, W.; Mele, A. Validating a Strategy for Molecular Dynamics Simulations of Cyclodextrin Inclusion Complexes through Single-Crystal X-ray and NMR Experimental Data: A Case Study. J. Phys. Chem. B 2009, 113, 9110–9122. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Hydration and flexibility of α-, β-, γ- and δ-cyclodextrin: A molecular dynamics study. Chem. Phys. 2007, 333, 128–134. [Google Scholar] [CrossRef]
- Loftsson, T.; Sigurdsson, H.H.; Jansook, P. Anomalous Properties of Cyclodextrins and Their Complexes in Aqueous Solutions. Materials 2023, 16, 2223. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Coates, J.H.; Lincoln, S.F. Kinetic and equilibrium studies of cyclomalto-octaose (γ-cyclodextrin) -methyl orange inclusion complexes. Carbohydr. Res. 1984, 127, 181–191. [Google Scholar] [CrossRef]
- Clarke, R.J.; Coates, J.H.; Lincoln, S.F. Inclusion Complexes of the Cyclomalto-Oligosaccharides (Cyclodextrins). Adv. Carbohydr. Chem. Biochem. 1988, 46, 205–249. [Google Scholar]
- Melnikova, D.L.; Badrieva, Z.F.; Kostin, M.A.; Maller, C.; Stas, M.; Buczek, A.; Broda, M.A.; Kupka, T.; Kelterer, A.-M.; Tolstoy, P.M.; et al. On Complex Formation between 5-Fluorouracil and β-Cyclodextrin in Solution and in the Solid State: IR Markers and Detection of Short-Lived Complexes by Diffusion NMR. Molecules 2020, 25, 5706. [Google Scholar] [CrossRef] [PubMed]
- Rzepiela, K.; Buczek, A.; Kupka, T.; Broda, M.A. Factors governing the chemical stability and NMRparameters of uracil tautomers and Its 5-halogen derivatives. Molecules 2020, 25, 3931. [Google Scholar] [CrossRef]
- Scanlan, M.J.; Hillier, I.H. An ab initio study of tautomerism of uracil, thymine, 5-fluorouracil, and cytosine. J. Am. Chem. Soc. 1984, 106, 3737–3745. [Google Scholar] [CrossRef]
- Rastogi, V.; Palafox, M. Vibrational spectra, tautomerism and thermodynamics of anticarcinogenic drug: 5-Fluorouracil. Spectrochim. Acta A 2011, 79, 970–977. [Google Scholar] [CrossRef]
- Markova, N.; Enchev, V.; Timtcheva, I. Oxo-hydroxy tautomerism of 5-fluorouracil: Water-assisted proton transfer. J. Phys. Chem. A 2005, 109, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Crampton, K.T.; Rathur, A.I.; Nei, Y.W.; Berden, G.; Oomens, J.; Rodgers, M.T. Protonation preferentially stabilizes minor tautomers of the halouracils: IRMPD action spectroscopy and theoretical studies. J. Am. Soc. Mass Spectr. 2012, 23, 1469–1478. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F. Surface hydration of polymeric (bio)materials: A molecular dynamics simulation study. J. Biomed. Mat. Res. A. 2010, 92A, 1382–1391. [Google Scholar] [CrossRef]
- Baba, H.; Urano, R.; Nagai, T.; Okazaki, S. Prediction of self-diffusion coefficients of chemically diverse pure liquids by all-atom molecular dynamics simulations. J. Comput. Chem. 2022, 43, 1892–1900. [Google Scholar] [CrossRef] [PubMed]
- Raffaini, G.; Ganazzoli, F. Different Conformational Properties and Frequencies of Periodic Motion in the β- and γ-Cyclodextrin. Macromol. Symp. 2023, 411, 2200160. [Google Scholar] [CrossRef]
- Materials Studio BIOVIA version 7.0. Accelrys Inc. InsightII 2000. Accelrys Inc.: San Diego, CA, USA, 2000. Available online: http://www.accelrys.com (accessed on 4 April 2021).
- Hwang, M.-J.; Ni, X.; Waldman, M.; Ewig, C.S.; Hagler, A.T. Derivation of class II force fields. VI. Carbohydrate compounds and anomeric effects. Biopolymers 1998, 45, 435–468. [Google Scholar] [CrossRef]
- Petaccia, M.; Gentili, P.; Besker, N.; D’Abramo, M.; Giansanti, L.; Leonelli, F.; La Bella, A.; Villalva, D.G.; Mancini, G. Kinetics and mechanistic study of competitive inhibition of thymidine phosphorylase by 5-fluoruracil derivatives. Colloids Surf. B Biointerfaces 2016, 140, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Petaccia, M.; Condello, M.; Giansanti, L.; La Bella, A.; Leonelli, F.; Meschini, S.; Villalva, D.G.; Pellegrini, E.; Ceccacci, F.; Galantini, L.; et al. Inclusion of new 5-fluorouracil amphiphilic derivatives in liposome formulation for cancer treatment. Medchemcomm 2015, 6, 1639–1642. [Google Scholar] [CrossRef]
- Raffaini, G.; Ganazzoli, F.; Mele, A.; Castiglione, F. A molecular dynamics study of cyclodextrin nanosponge models. J. Incl. Phenom. Macrocycl. Chem. 2013, 75, 263–268. [Google Scholar] [CrossRef]
- Pyrak, B.; Rogacka-Pyrak, K.; Gubica, T.; Szeleszczuk, L. Exploring Cyclodextrin-Based Nanosponges as Drug Delivery Systems: Understanding the Physicochemical Factors Influencing Drug Loading and Release Kinetics. Int. J. Mol. Sci. 2024, 25, 3527. [Google Scholar] [CrossRef]
- Suvarna, V.; Singh, V.; Sharma, D.; Murahari, M. Experimental and computational insight of the supramolecular complexes of Irbesartan with β-cyclodextrin based nanosponges. J. Drug Deliv. Sci. Technol. 2021, 63, 102494. [Google Scholar] [CrossRef]
- Sadr, M.K.; Cheraghi, M.; Lorestani, B.; Sobhanardakani, S.; Golkarian, H. Removal of fluorouracil from aqueous environment using magnetite graphene oxide modified with γ-cyclodextrin. Environ. Monit. Assess. 2024, 196, 116. [Google Scholar] [CrossRef]


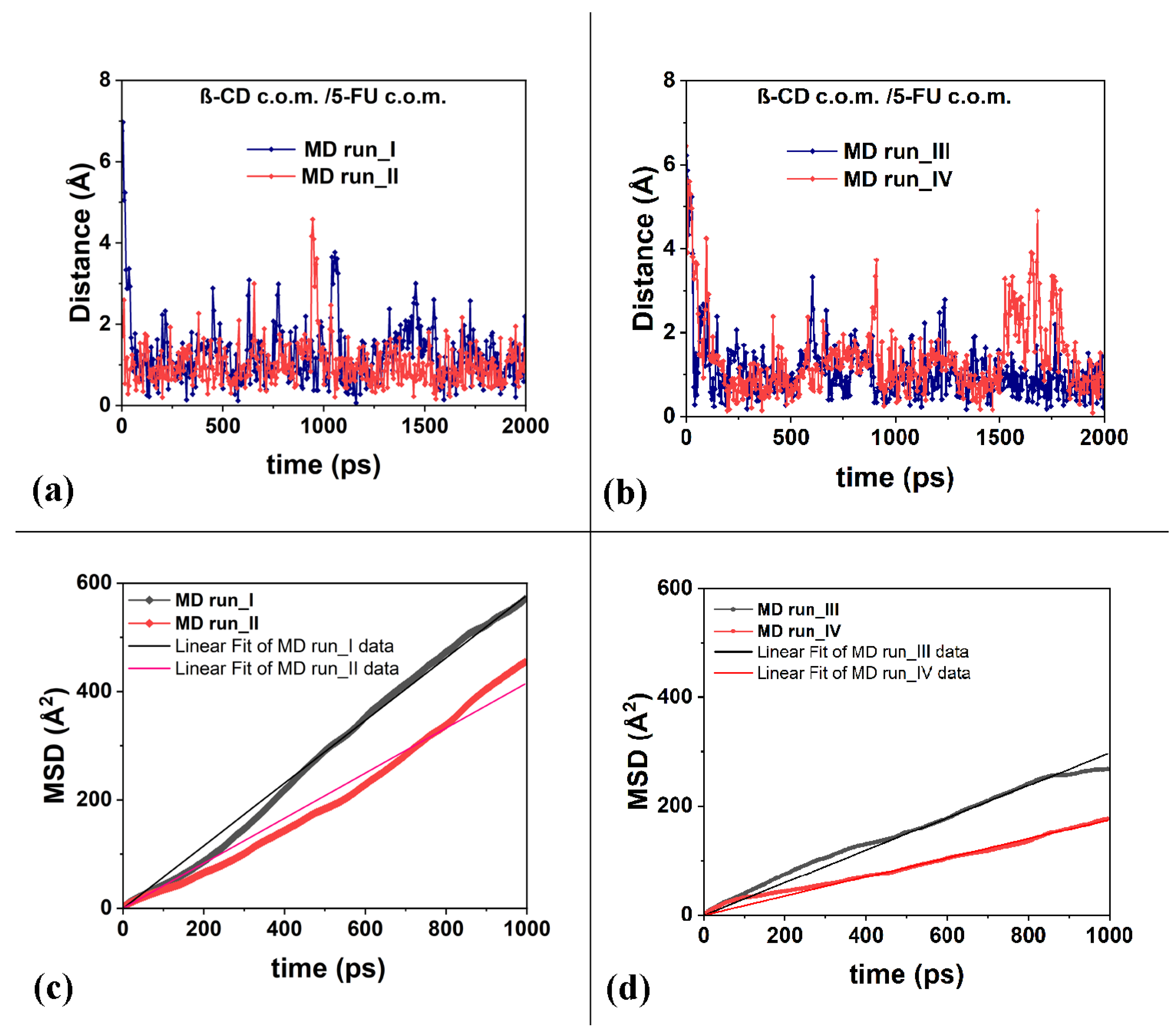


| Slope (Å2/ps) | D (m2/s) | R2 | |
| MD run_I | 0.57774 ± 0.00184 | 9.6290 × 10−10 | 0.99798 |
| MD run_II | 0.41579 ± 0.00256 | 6.9628 × 10−10 | 0.99254 |
| MD run_III | 0.29751 ± 0.00130 | 4.9585 × 10−10 | 0.99620 |
| MD run_IV | 0.17505 ± 0.00072 | 2.9175 × 10−10 | 0.99656 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffaini, G.; Elli, S.; Catauro, M.; D’Angelo, A. Different Drug Mobilities in Hydrophobic Cavities of Host–Guest Complexes between β-Cyclodextrin and 5-Fluorouracil at Different Stoichiometries: A Molecular Dynamics Study in Water. Int. J. Mol. Sci. 2024, 25, 5888. https://doi.org/10.3390/ijms25115888
Raffaini G, Elli S, Catauro M, D’Angelo A. Different Drug Mobilities in Hydrophobic Cavities of Host–Guest Complexes between β-Cyclodextrin and 5-Fluorouracil at Different Stoichiometries: A Molecular Dynamics Study in Water. International Journal of Molecular Sciences. 2024; 25(11):5888. https://doi.org/10.3390/ijms25115888
Chicago/Turabian StyleRaffaini, Giuseppina, Stefano Elli, Michelina Catauro, and Antonio D’Angelo. 2024. "Different Drug Mobilities in Hydrophobic Cavities of Host–Guest Complexes between β-Cyclodextrin and 5-Fluorouracil at Different Stoichiometries: A Molecular Dynamics Study in Water" International Journal of Molecular Sciences 25, no. 11: 5888. https://doi.org/10.3390/ijms25115888








