Chondroitin Sulfate-Based Nanocapsules as Nanocarriers for Drugs and Nutraceutical Supplements
Abstract
:1. Introduction
2. Results and Discussion
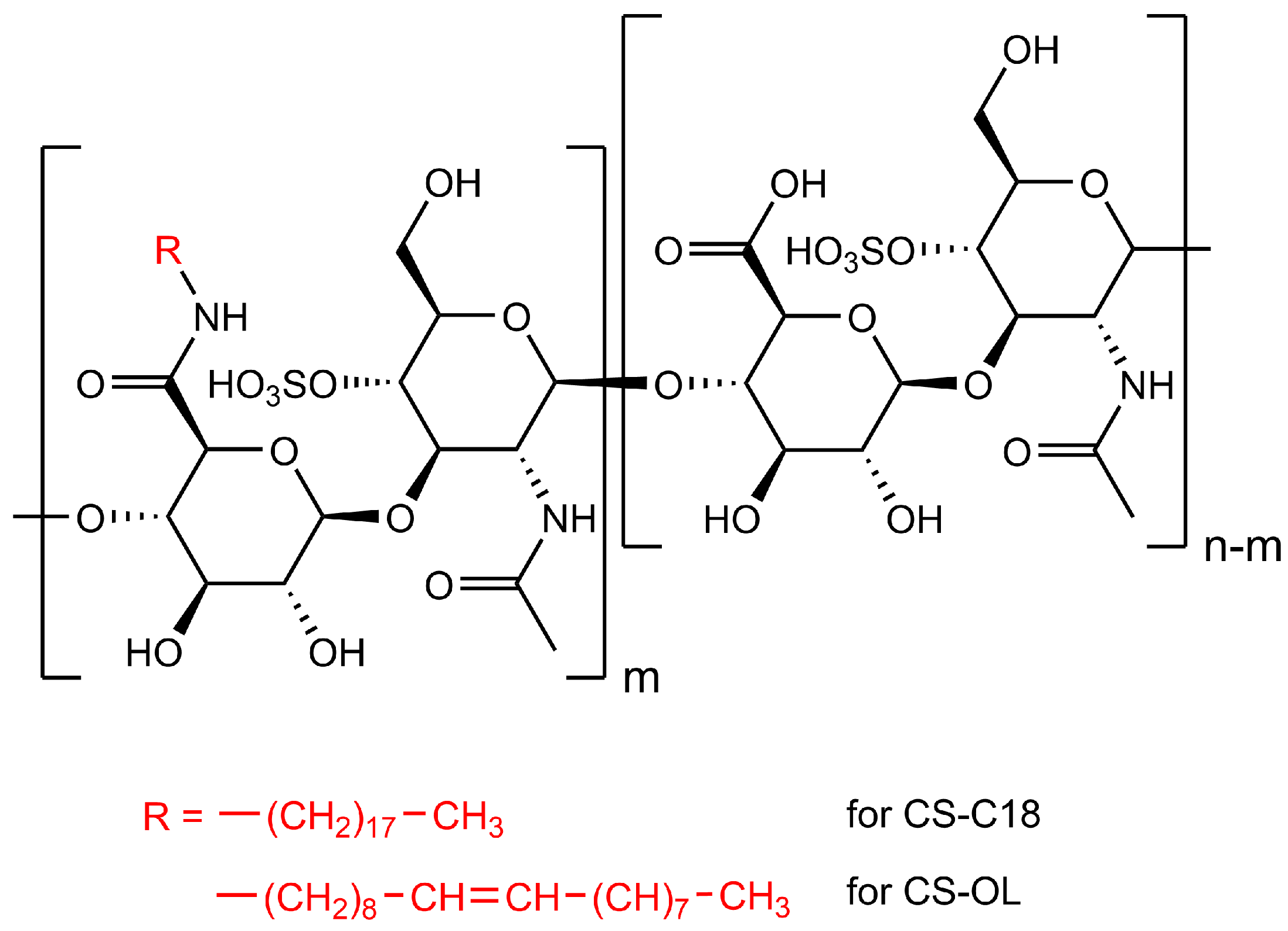
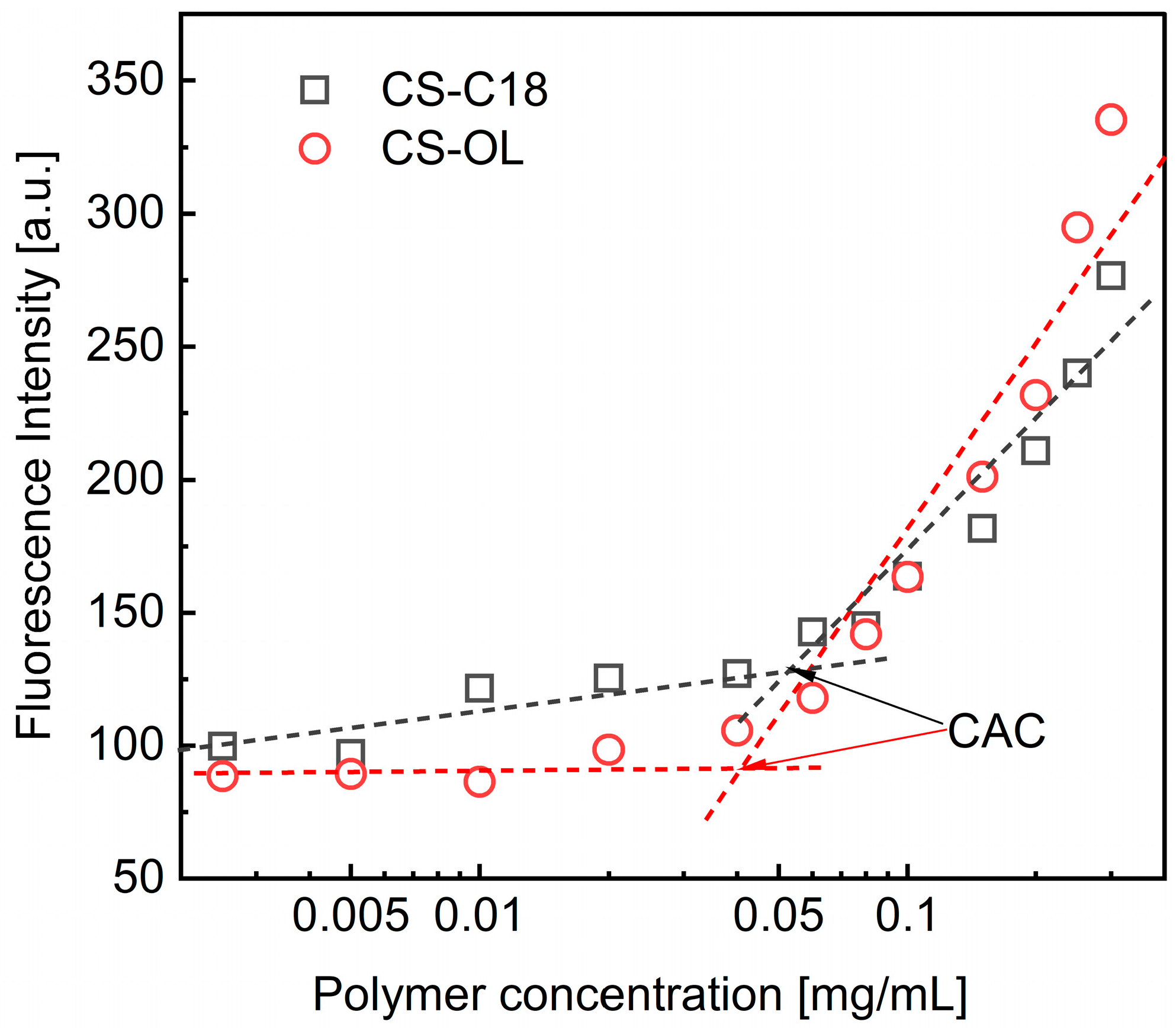
2.1. Synthesis of the Amphiphilic CS Derivatives and Their Properties in Water
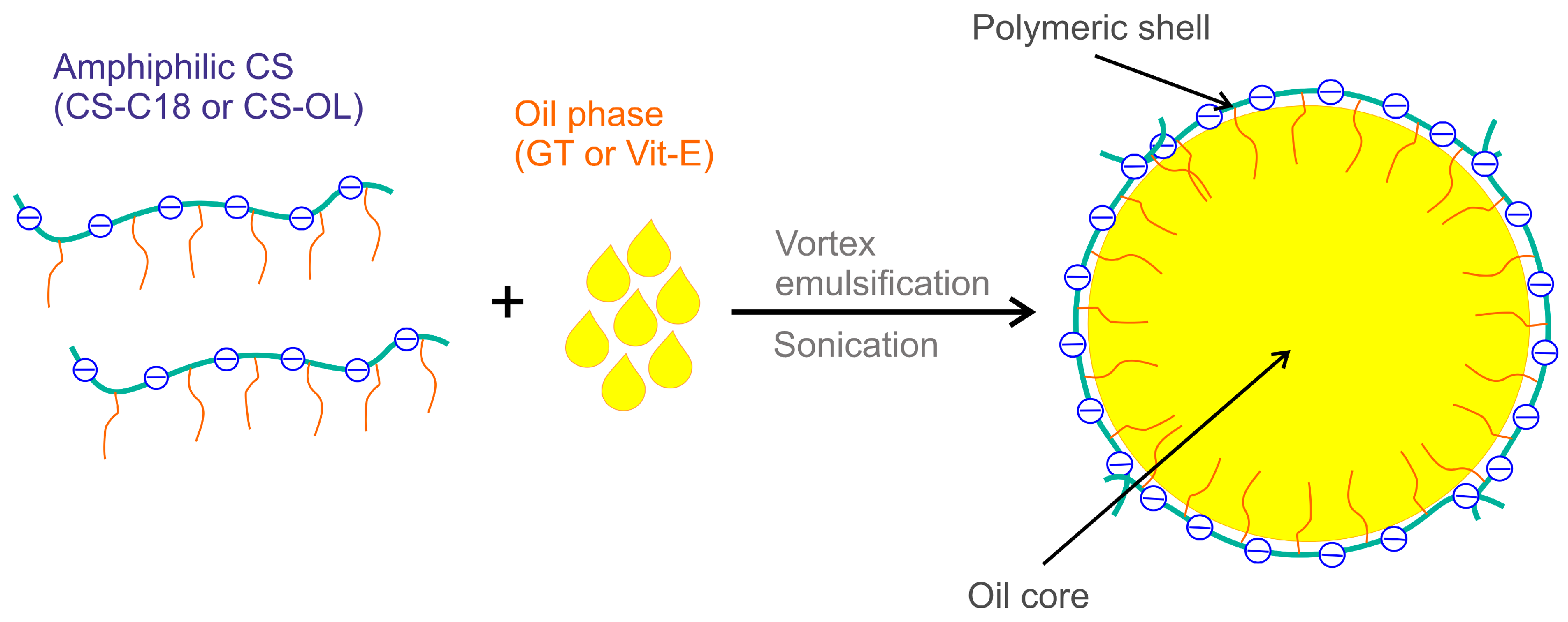
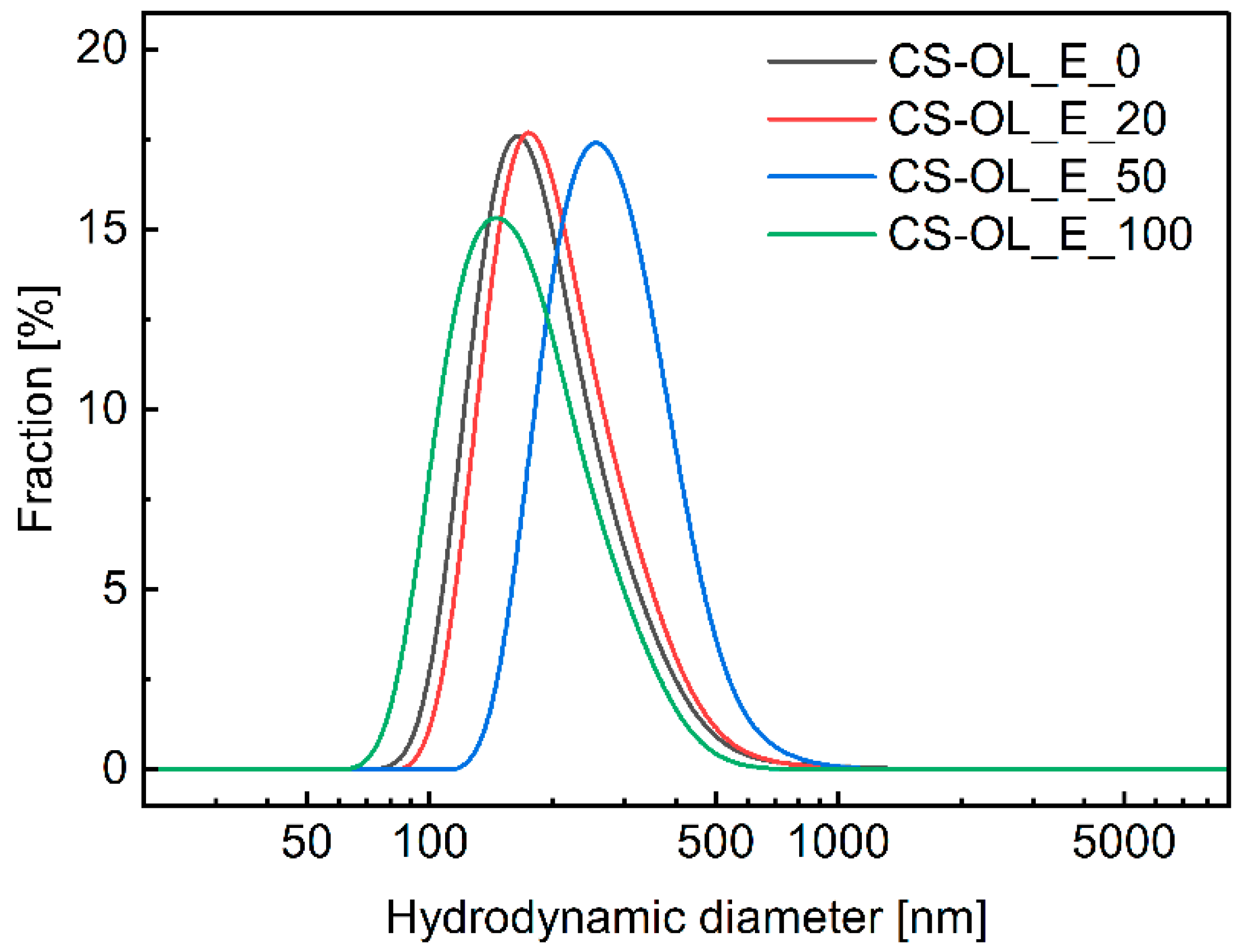
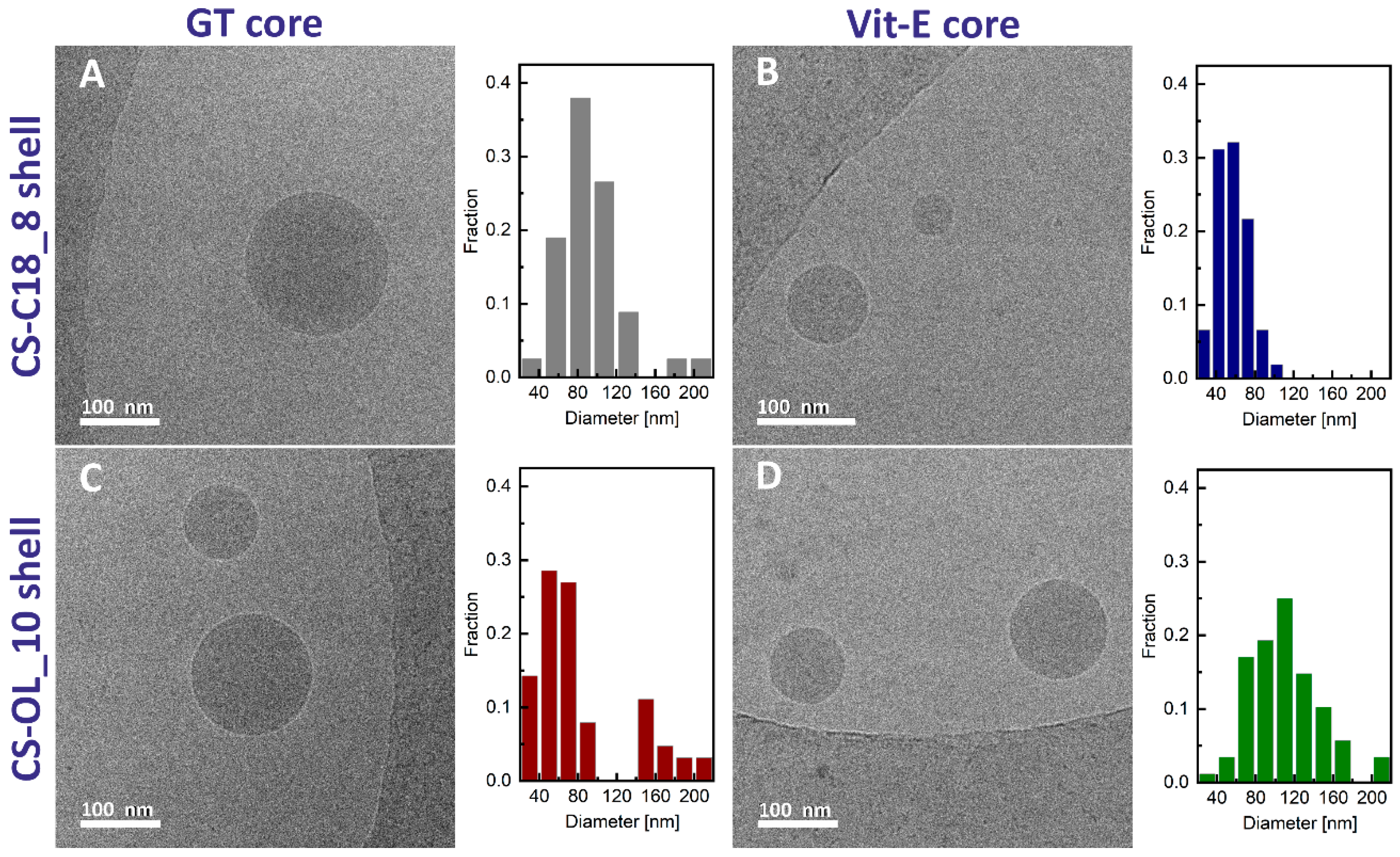
2.2. Formulation and Properties of Nanocapsules
2.3. Intracellular Uptake of the Nanocapsules
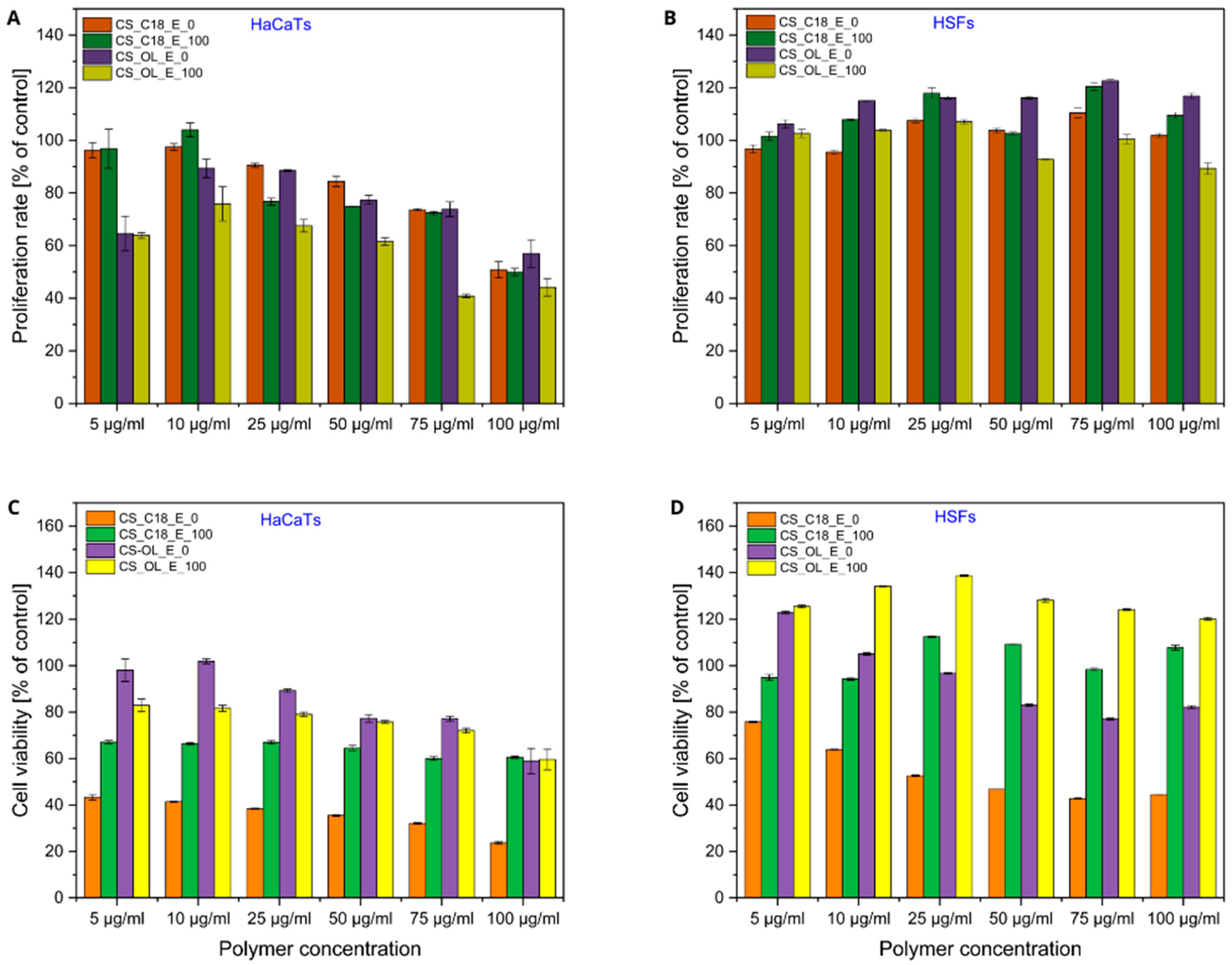
2.4. Effects of Polymer Dispersions and CS-Based Nanocapsules on Proliferation and Viability of Human Skin Cells
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Amphiphilic CS Derivatives
3.3. Preparation of Nanocapsules
3.4. Critical Aggregation Concentrations (CACs)
3.5. Dynamic Light Scattering (DLS) and Zeta Potential Measurements
3.6. Cryo-TEM
3.7. Cytotoxicity and Proliferation Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crecente-Campo, J.; Alonso, M.J. Engineering, on-demand manufacturing, and scaling-up of polymeric nanocapsules. Bioeng. Transl. Med. 2018, 26, 38–50. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Jasinski, K.; Węglarz, W.P.; Zapotoczny, S.; Chłopicki, S. Low Dose Curcumin Administered in Hyaluronic Acid-Based Nanocapsules Induces Hypotensive Effect in Hypertensive Rats. Int. J. Nanomed. 2021, 16, 1377–1390. [Google Scholar] [CrossRef]
- Bednorz, J.; Smela, K.; Zapotoczny, S. Tailoring Properties of Hyaluronate-Based Core–Shell Nanocapsules with Encapsulation of Mixtures of Edible Oils. Int. J. Mol. Sci. 2023, 24, 14995. [Google Scholar] [CrossRef]
- Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules 2020, 25, 3966. [Google Scholar] [CrossRef]
- Sarkar, A.; Mackie, A.R. Engineering oral delivery of hydrophobic bioactives in real-world scenarios. Curr. Opin. Colloid Interface Sci. 2020, 48, 40–52. [Google Scholar] [CrossRef]
- Borel, P.; Caillaud, D.; Cano, N.J. Vitamin D bioavailability: State of the art. Crit. Rev. Food Sci. Nutr. 2015, 55, 1193–1205. [Google Scholar] [CrossRef]
- Vieira, M.C.; Bakof, K.K.; Schuch, N.J.; Skupien, J.A.; Boeck, C.R. The benefits of omega-3 fatty acid nanocapsulation for the enrichment of food products: A review. Rev. Nutr. 2020, 33, e190165. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P. Overview of carotenoid bioavailability determinants: From dietary factors to host genetic variations. Trends. Food Sci. Technol. 2017, 69, 270–280. [Google Scholar] [CrossRef]
- Cadete, A.A.; Olivera, M.; Besev, P.K.; Dhal, L.; Gonçalves, A.J.; Almeida, G.; Bastiat, J.; Benoit, M.; Fuente, M.; Garcia-Fuentes, M.; et al. Torres, Self-assembled hyaluronan nanocapsules for the intracellular delivery of anticancer drugs. Sci. Rep. 2019, 9, 11565. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Jain, A.; Hurkat, P.; Jain, S.K. Chondroitin sulphate: A focus on osteoarthritis. Glycoconj. J. 2016, 33, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Kitagawa, H. Biosynthesis and function of chondroitin sulfate. Biochim. Biophys. Acta 2013, 1830, 4719–4733. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, P.; Cheng, Y.; Zhang, X.; Sheng, J.; Wang, D.; Li, J.; Zhang, Q.; Zhong, C.; Cao, R.; et al. Enhancing the Intestinal Absorption of Low Molecular Weight Chondroitin Sulfate by Conjugation with α-Linolenic Acid and the Transport Mechanism of the Conjugates. Int. J. Pharm. 2014, 465, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Chung, S.J.; Cho, H.J.; Kim, D.D. Phenylboronic Acid-Decorated Chondroitin Sulfate A-Based Theranostic Nanoparticles for Enhanced Tumor Targeting and Penetration. Adv. Funct. Mater. 2015, 25, 3705–3717. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 Interactions as Potential Targets for Cancer Therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, M.; Pawar, V.K.; Jaiswal, A.K.; Dube, A.; Paliwal, S.K.; Chourasia, M.K. Chondroitin nanocapsules enhanced doxorubicin induced apoptosis against leishmaniasis via Th1 immune response. Int. J. Biol. Macromol. 2015, 79, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Valverde-Fraga, L.; Haddad, R.; Alrabadi, N.; Sánchez, S.; Remuñán-López, C.; Csaba, N. Design and in vitro assessment of chitosan nanocapsules for the pulmonary delivery of rifabutin. Eur. J. Pharm. Sci. 2023, 1, 106484. [Google Scholar] [CrossRef]
- Zak, A.; Łazarski, G.; Wytrwal-Sarna, M.; Jamroz, D.; Górniewicz, M.; Forys, A.; Trzebicka, B.; Kepczynski, M. Molecular insights into the self-assembly of hydrophobically modified chondroitin sulfate in aqueous media. Carbohydr. Polym. 2022, 297, 119999. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, J.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yuan, Z.; Yuan, Q.; Huang, Y.; Yu, Q.; Ren, J.; Liang, L.; Jin, H.; Yu, J. Preparation and characterization of amphiphilic nanoparticles based on chondroitin sulfate a conjugated with hydrophobic drug for enhanced doxorubicin delivery. Colloid Polym. Sci. 2021, 299, 129–136. [Google Scholar] [CrossRef]
- Lewandowska, J.; Kępczyński, M.; Bednar, J.; Rząd, E.; Moravcikova, V.; Jachimska, B.; Nowakowska, M. Silicone-stabilized liposomes. Colloid Polym. Sci. 2010, 288, 37–45. [Google Scholar] [CrossRef]
- Wytrwal, M.; Koczurkiewicz, P.; Wojcik, K.; Michalik, M.; Kozik, B.; Zylewski, M.; Nowakowska, M.; Kepczynski, M. Synthesis of strong polycations with improved biological properties. J. Biomed. Mater Res. Part A 2014, 102A, 721–731. [Google Scholar] [CrossRef]
- Vega-Avila, E.; Pugsley, M.K. An Overview of Colorimetric Assay Methods Used to Assess Survival or Proliferation of Mammalian Cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar] [PubMed]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, 087379. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Naitoh, M.; Kubota, H.; Yamawaki, S.; Aya, R.; Ishiko, T.; Morimoto, N. Chondroitin Sulfate Promotes the Proliferation of Keloid Fibroblasts Through Activation of the Integrin and Protein Kinase B Pathways. Int. J. Mol. Sci. 2020, 21, 1955. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Park, S.; Kim, H.; Lee, S.H.; Ahn, Y.; Jung, W.; Kim, H.-J.; Cho, Y.W. Potential anti-ageing effect of chondroitin sulphate through skin regeneration. Int. J. Cosmet. Sci. 2020, 42, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Rother, S.; Zimmermann, H.; Lee, P.S.; Moeller, S.; Schnabelrauch, M.; Koul, V.; Jordan, R.; Hintze, V.; Scharnweber, D. Biomimetic electrospun scaffolds from main extracellular matrix components for skin tissue engineering application—The role of chondroitin sulfate and sulfated hyaluronan. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.C.; Ke, Y.L.; Lai, Y.H.; Hsieh, W.C.; Lin, C.H.; Huang, S.S.; Peng, J.Y.; Chen, C.H. Chondroitin Sulfate Enhances Proliferation and Migration via Inducing β-Catenin and Intracellular ROS as Well as Suppressing Metalloproteinases through Akt/NF-κB Pathway Inhibition in Human Chondrocytes. J. Nutr. Health Aging 2022, 26, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.H.; Foong, W.C.; Cao, T.; Bay, B.H.; Ouyang, H.W.; Yip, G.W. Chondroitin Sulfate in Palatal Wound Healing. J. Dent. Res. 2004, 83, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Wytrwal, M.; Szmajnta, K.; Kucharski, M.; Nowak, J.; Oclon, E.; Kepczynski, M. Kartogenin-loaded liposomes coated with alkylated chondroitin sulfate for cartilage repair. Int. J. Pharm. 2023, 646, 123436. [Google Scholar] [CrossRef]
- Maalouf, S.; El-Sabban, M.; Darwiche, N.; Gali-Muhtasib, H. Protective Effect of Vitamin E on Ultraviolet B Light–Induced Damage in Keratinocytes. Mol. Carcinog. 2002, 34, 121–130. [Google Scholar] [CrossRef]
- Makpol, S.; Jam, F.A.; Yusof, Y.A.M.; Ngah, W.Z.W. Modulation of collagen synthesis and its gene expression in human skin fibroblasts by tocotrienol-rich fraction. Arch. Med. Sci. 2011, 7, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.; Mehmood, A.; Ali, M.; Tasneem, S.; Anjum, M.S.; Tarar, M.N.; Khan, S.N.; Riazuddin, S. Protective role of vitamin E preconditioning of human dermal fibroblasts against thermal stress in vitro. Life Sci. 2017, 184, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.; Mehmood, A.; Ali, M.; Tasneem, S.; Tarar, M.N.; Khan, S.N.; Riazuddin, S. Vitamin E preconditioning alleviates in vitro thermal stress in cultured human epidermal keratinocytes. Life Sci. 2019, 239, 116972. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, N.; Kopec, W.; Wilkosz, N.; Jamróz, D.; Hub, J.S.; Zatorska, M.; Petka, R.; Nowakowska, M.; Kepczynski, M. Molecular Mechanism of Polycation-Induced Pore Formation in Biomembranes. ACS Biomater. Sci. Eng. 2019, 5, 780–794. [Google Scholar] [CrossRef]
- Wnuk, D.; Paw, M.; Ryczek, K.; Bochenek, G.; Sladek, K.; Madeja, Z.; Michalik, M. Enhanced asthma-related fibroblast to myofibroblast transition is the result of profibrotic TGF-β/Smad2/3 pathway intensification and antifibrotic TGF-β/Smad1/5/(8)9 pathway impairment. Sci. Rep. 2020, 10, 16492. [Google Scholar] [CrossRef]



| Polymer | DS [%] | CAC [µg/mL] | dz [nm] | PI | ζ [mV] |
|---|---|---|---|---|---|
| CS-C18 | 8 | 53.1 | 202 ± 71 | 0.36 ± 0.01 | −33.3 ± 0.3 |
| CS-OL | 10.5 | 40.0 | 95 ±21 | 0.50 ± 0.05 | −36.8 ± 1.7 |
| Sample | Weight of GT [mg] | Weight of Vit-E [mg] | dz [nm] | PI | RSD [%] | ζ [mV] |
|---|---|---|---|---|---|---|
| CS-C18_E_0 | 10 | 0 | 315.7 ± 5.7 | 0.34 ± 0.02 | 1.8 | −24.6 ± 2.1 |
| CS-C18_E_20 | 8 | 2 (20%) | 304.7 ± 6.0 | 0.22 ± 0.02 | 2.0 | −24.5 ± 0.7 |
| CS-C18_E_50 | 5 | 5 (50%) | 391.5 ± 2.5 | 0.38 ± 0.03 | 0.6 | −18.1 ± 1.8 |
| CS-C18_E_100 | 0 | 10 (100%) | 406 ± 13 | 0.37 ± 0.02 | 3.2 | −31.8 ± 0.9 |
| CS-OL_E_0 | 10 | 0 | 390.4 ± 0.5 | 0.33 ± 0.04 | 0.1 | −30.4 ± 1.7 |
| CS-OL_ E_20 | 8 | 2 (20%) | 366.1 ± 3.5 | 0.27 ± 0.02 | 0.9 | −21.6 ± 2.1 |
| CS-OL_E_50 | 5 | 5 (50%) | 571 ± 29 | 0.42 ± 0.05 | 5.0 | −15.0 ± 2.5 |
| CS-OL_E_100 | 0 | 10 (100%) | 371 ± 15 | 0.47 ± 0.07 | 4.0 | −31.6 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górniewicz, M.; Wnuk, D.; Foryś, A.; Trzebicka, B.; Michalik, M.; Kepczynski, M. Chondroitin Sulfate-Based Nanocapsules as Nanocarriers for Drugs and Nutraceutical Supplements. Int. J. Mol. Sci. 2024, 25, 5897. https://doi.org/10.3390/ijms25115897
Górniewicz M, Wnuk D, Foryś A, Trzebicka B, Michalik M, Kepczynski M. Chondroitin Sulfate-Based Nanocapsules as Nanocarriers for Drugs and Nutraceutical Supplements. International Journal of Molecular Sciences. 2024; 25(11):5897. https://doi.org/10.3390/ijms25115897
Chicago/Turabian StyleGórniewicz, Magdalena, Dawid Wnuk, Aleksander Foryś, Barbara Trzebicka, Marta Michalik, and Mariusz Kepczynski. 2024. "Chondroitin Sulfate-Based Nanocapsules as Nanocarriers for Drugs and Nutraceutical Supplements" International Journal of Molecular Sciences 25, no. 11: 5897. https://doi.org/10.3390/ijms25115897







