Prenatal Hypoxia Triggers a Glucocorticoid-Associated Depressive-like Phenotype in Adult Rats, Accompanied by Reduced Anxiety in Response to Stress
Abstract
1. Introduction
2. Results
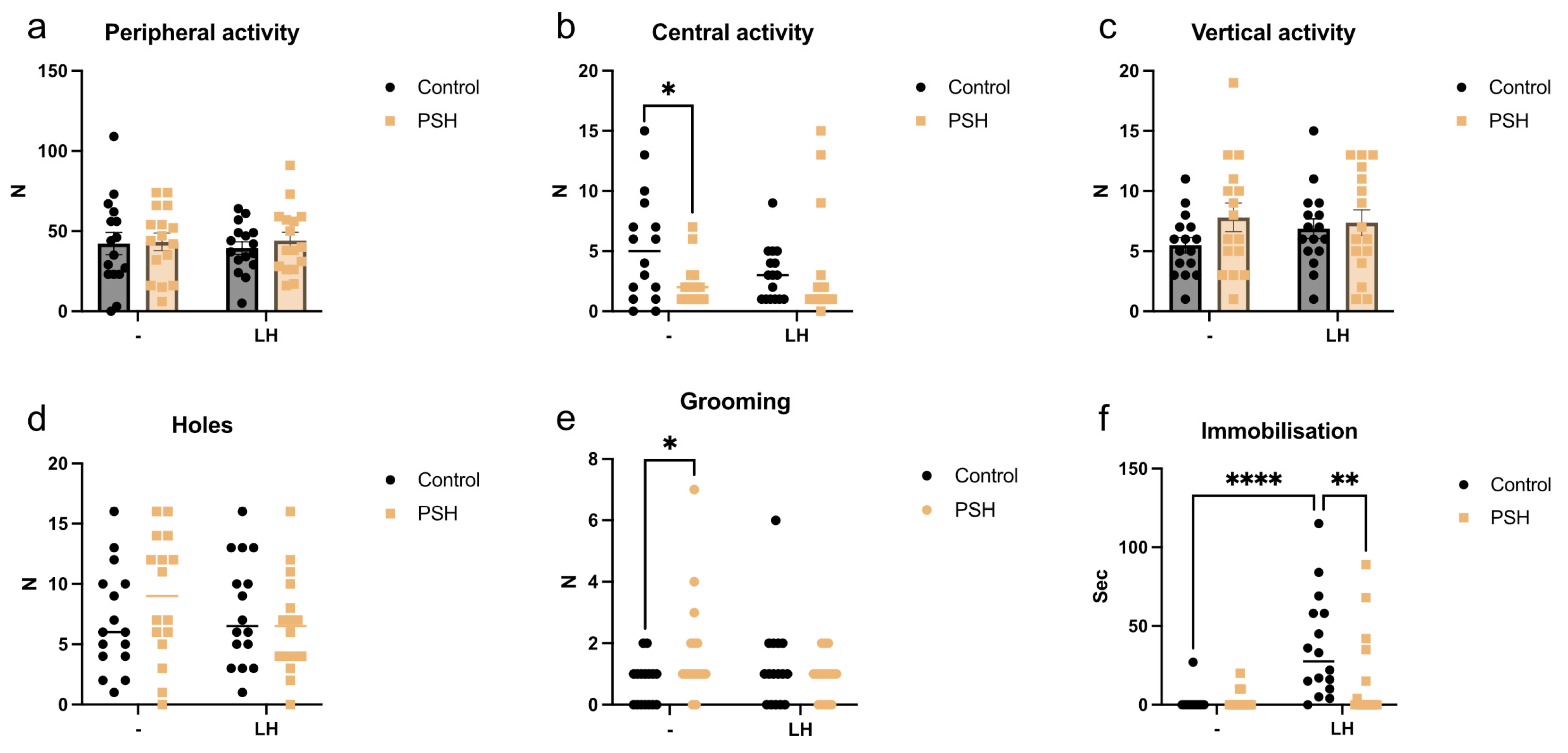
2.1. Impact of Prenatal Severe Hypoxia on Rat Behavior in Normal Adulthood and after Learned Helplessness
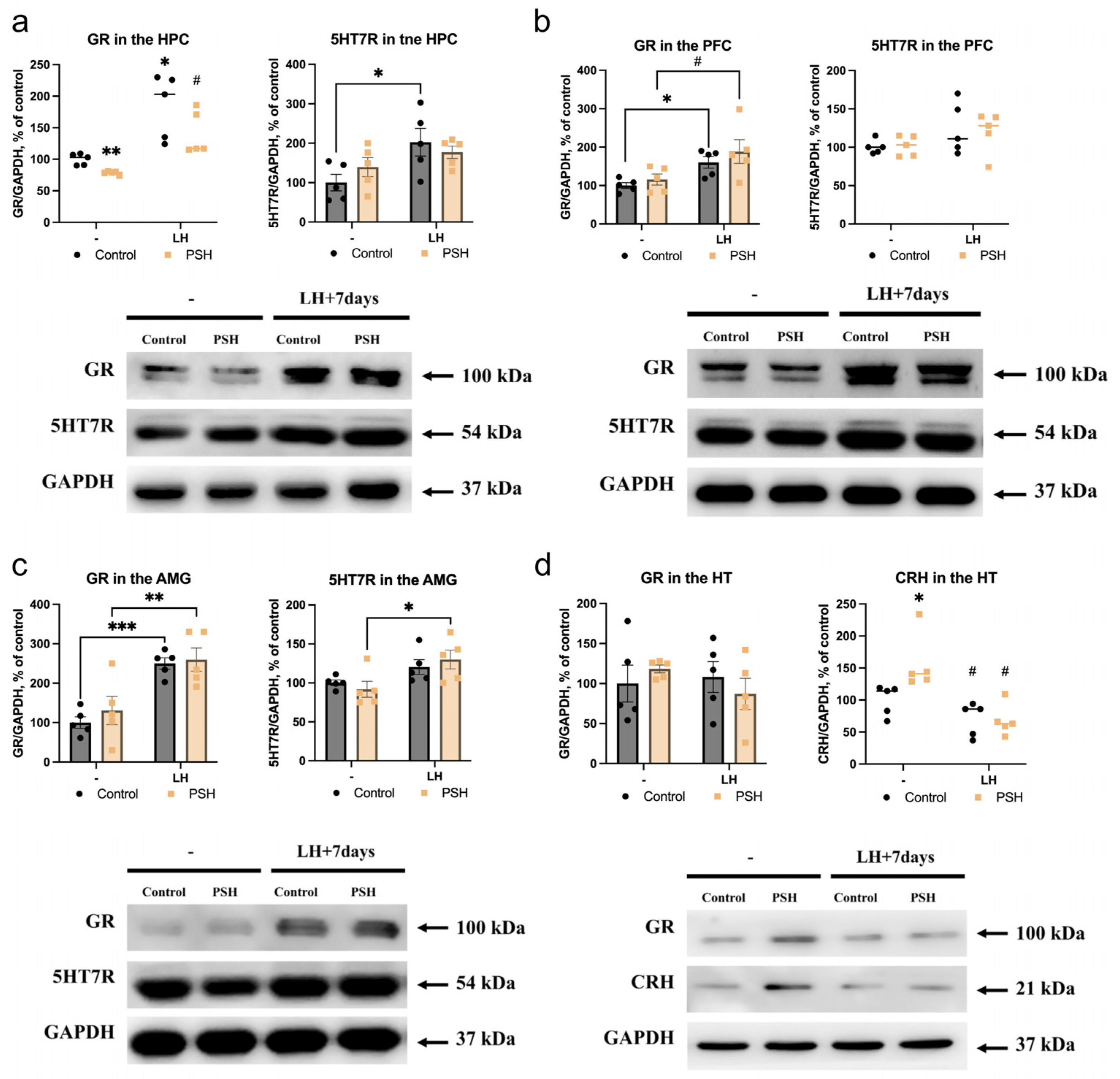
2.2. Impact of Prenatal Hypoxia on the Expression of 5HT7R and Glucocorticoid Receptors in the Extrahypothalamic Brain Structures in Normal Adulthood and after Learned Helplessness
2.3. Impact of Prenatal Hypoxia on the Hypothalamic Expression of Glucocorticoid Receptors and Corticotropin-Releasing Hormone in Normal Adulthood and after Learned Helplessness
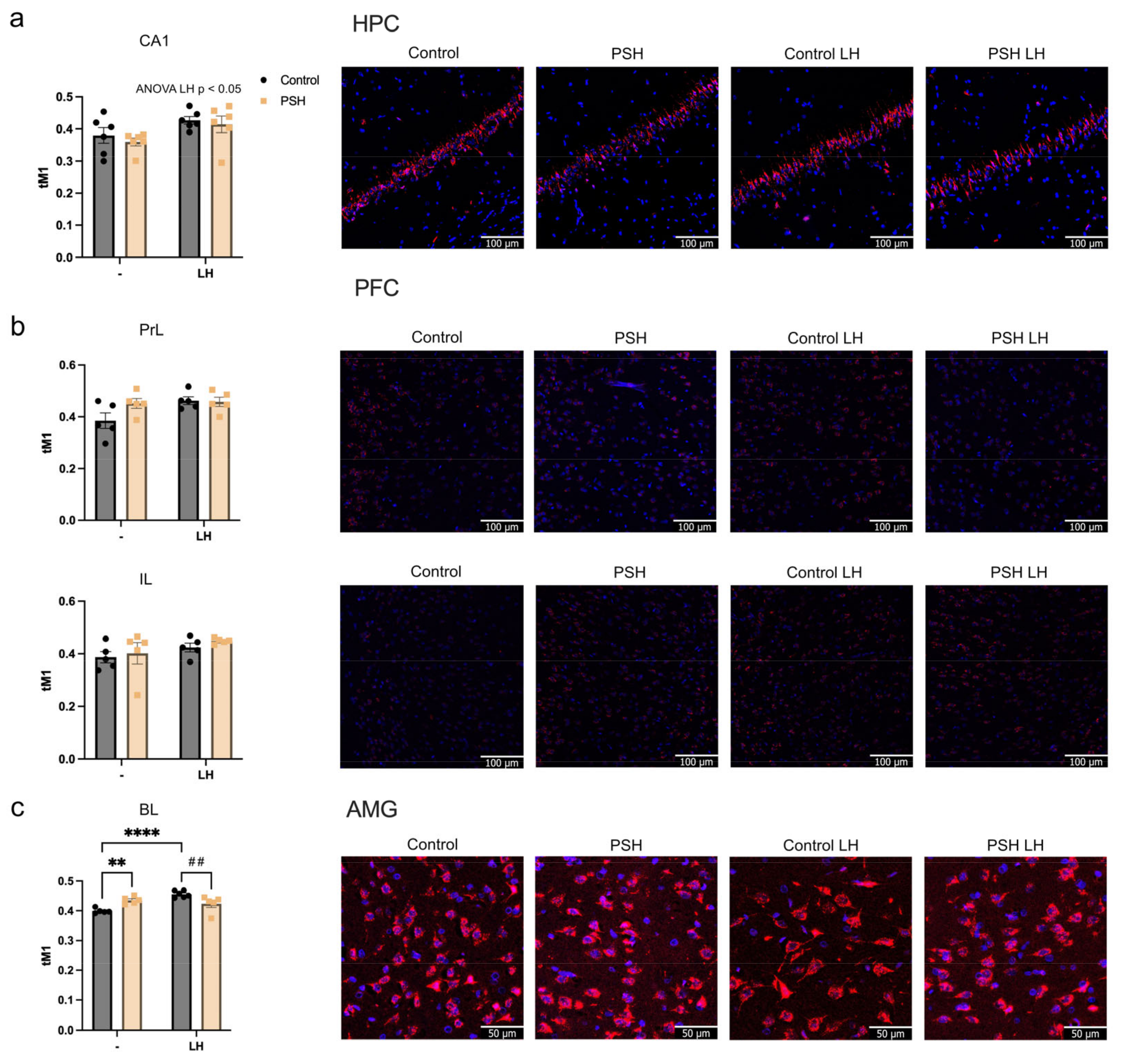
2.4. Impact of Prenatal Hypoxia on Glucocorticoid Receptor Nuclear Translocation in the Extrahypothalamic Brain Structures in Normal Adulthood and after Learned Helplessness
3. Discussion
4. Materials and Methods
4.1. Animals
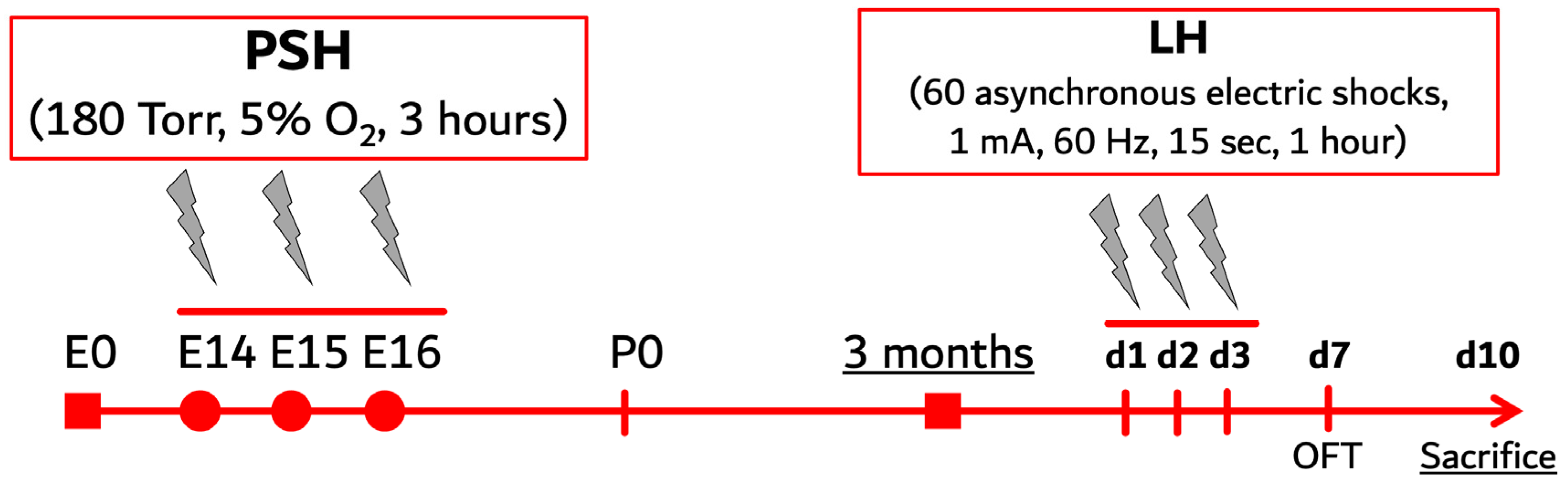
4.2. Prenatal Severe Hypoxia
4.3. Learned Helplessness Paradigm
4.4. Open Field Test
4.5. Sample Preparation
4.6. Western Blotting
4.7. Immunofluorescent Analysis
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haleem, D.J. Glucocorticoids in the physiological and transcriptional regulation of 5-HT1A receptor and the pathogenesis of depression. Neuroscientist 2022, 28, 59–68. [Google Scholar] [CrossRef]
- Porter, R.J.; Gallagher, P.; Watson, S.; Young, A.H. Corticosteroid-serotonin interactions in depression: A review of the human evidence. Psychopharmacology 2004, 173, 1–17. [Google Scholar] [CrossRef]
- Tafet, G.E.; Nemeroff, C.B. Pharmacological treatment of anxiety disorders: The role of the HPA axis. Front. Psychiatry 2020, 11, 529446. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.L.; Solanki, R.R.; Watt, M.J.; Renner, K.J.; Forster, G.L. Chronic administration of glucocorticoid receptor ligands increases anxiety-like behavior and selectively increase serotonin transporters in the ventral hippocampus. Brain Res. 2023, 1800, 148189. [Google Scholar] [CrossRef]
- Galbally, M.; Watson, S.J.; van IJzendoorn, M.; Saffery, R.; Ryan, J.; de Kloet, E.R.; Oberlander, T.F.; Lappas, M.; Lewis, A.J. The role of glucocorticoid and mineralocorticoid receptor DNA methylation in antenatal depression and infant stress regulation. Psychoneuroendocrinology 2020, 115, 104611. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 28, 3243–3256. [Google Scholar] [CrossRef] [PubMed]
- Vahid-Ansari, F.; Albert, P.R. Rewiring of the serotonin system in major depression. Front. Psychiatry 2021, 12, 802581. [Google Scholar] [CrossRef]
- Roshan-Milani, S.; Seyyedabadi, B.; Saboory, E.; Parsamanesh, N.; Mehranfard, N. Prenatal stress and increased susceptibility to anxiety-like behaviors: Role of neuroinflammation and balance between GABAergic and glutamatergic transmission. Stress 2021, 24, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; D’Arcy, C.; Meng, X. Research review: Developmental origins of depression—A systematic review and meta-analysis. J. Child Psychol. Psychiatry 2021, 62, 1050–1066. [Google Scholar] [CrossRef]
- Lautarescu, A.; Craig, M.C.; Glover, V. Prenatal stress: Effects on fetal and child brain development. Int. Rev. Neurobiol. 2020, 150, 17–40. [Google Scholar] [CrossRef]
- French, S.J.; Totterdell, S. Individual nucleus accumbens-projection neurons receive Both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 2003, 119, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Laplante, D.P.; Brunet, A.; King, S. The effects of maternal stress and illness during pregnancy on infant temperament: Project Ice Storm. Pediatr. Res. 2016, 79, 107–113. [Google Scholar] [CrossRef]
- Lucassen, P.J.; Pruessner, J.; Sousa, N.; Almeida, O.F.X.; Van Dam, A.M.; Rajkowska, G.; Swaab, D.F.; Czéh, B. Neuropathology of stress. Acta Neuropathol. 2014, 127, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, R. Placental insufficiency and its consequences. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, S99–S107. [Google Scholar] [CrossRef] [PubMed]
- Hutter, D.; Kingdom, J.; Jaeggi, E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: A review. Int. J. Pediatr. 2010, 2010, 401323. [Google Scholar] [CrossRef]
- Tong, W.; Giussani, D.A. Preeclampsia link to gestational hypoxia. J. Dev. Orig. Health Dis. 2019, 10, 322–333. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Role of prenatal hypoxia in brain development, cognitive functions, and neurodegeneration. Front. Neurosci. 2018, 12, 825. [Google Scholar] [CrossRef]
- Graham, E.M.; Ruis, K.A.; Hartman, A.L.; Northington, F.J.; Fox, H.E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 2008, 199, 587–595. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Piešová, M.; Mach, M. Impact of perinatal hypoxia on the developing brain. Physiol. Res. 2020, 69, 199–213. [Google Scholar] [CrossRef]
- Stratilov, V.; Vetrovoy, O.; Potapova, S.; Tyulkova, E. The prenatal hypoxic pathology associated with maternal stress predisposes to dysregulated expression of the Chrna7 gene and the subsequent development of nicotine addiction in adult offspring. Neuroendocrinology 2024, 114, 423–438. [Google Scholar] [CrossRef]
- Vetrovoy, O.; Tyulkova, E.; Stratilov, V.; Baranova, K.; Nimiritsky, P.; Makarevich, P.; Rybnikova, E. Long-term effects of prenatal severe hypoxia on central and peripheral components of the glucocorticoid system in rats. Dev. Neurosci. 2021, 42, 145–158. [Google Scholar] [CrossRef]
- Miyagawa, K.; Tsuji, M.; Ishii, D.; Takeda, K.; Takeda, H. Prenatal stress induces vulnerability to stress together with the disruption of central serotonin neurons in mice. Behav. Brain Res. 2015, 277, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Hecht, P.M.; Hudson, M.; Connors, S.L.; Tilley, M.R.; Liu, X.; Beversdorf, D.Q. Maternal serotonin transporter genotype affects risk for ASD with exposure to prenatal stress. Autism Res. 2016, 9, 1151–1160. [Google Scholar] [CrossRef]
- Sandman, C.A. Prenatal CRH: An integrating signal of fetal distress. Dev. Psychopathol. 2018, 30, 941–952. [Google Scholar] [CrossRef]
- Watt, M.; Mohammadzadeh, P.; Pinsinski, E.; Hollinshead, F.K.; Bouma, G.J. Corticotropin releasing hormone is present in the feline placenta and maternal serum. Front. Endocrinol. 2023, 14, 1132743. [Google Scholar] [CrossRef]
- St-Pierre, J.; Laurent, L.; King, S.; Vaillancourt, C. Effects of prenatal maternal stress on serotonin and fetal development. Placenta 2016, 48, S66–S71. [Google Scholar] [CrossRef] [PubMed]
- Kassotaki, I.; Valsamakis, G.; Mastorakos, G.; Grammatopoulos, D.K. Placental CRH as a signal of pregnancy adversity and impact on fetal neurodevelopment. Front. Endocrinol. 2021, 12, 714214. [Google Scholar] [CrossRef]
- Pastor, V.; Antonelli, M.C.; Pallarés, M.E. Unravelling the link between prenatal stress, dopamine and substance use disorder. Neurotox. Res. 2016, 31, 169–186. [Google Scholar] [CrossRef]
- Pastor, V.; Pallarés, M.E.; Antonelli, M.C. Prenatal stress increases adult vulnerability to cocaine reward without affecting pubertal anxiety or novelty response. Behav. Brain Res. 2018, 339, 186–194. [Google Scholar] [CrossRef]
- Reynaert, M.L.; Marrocco, J.; Gatta, E.; Mairesse, J.; Van Camp, G.; Fagioli, F.; Maccari, S.; Nicoletti, F.; Morley-Fletcher, S. A self-medication hypothesis for increased vulnerability to drug abuse in prenatally restraint stressed rats. Adv. Neurobiol. 2015, 10, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Said, N.; Lakehayli, S.; El Khachibi, M.; El Ouahli, M.; Nadifi, S.; Hakkou, F.; Tazi, A. Prenatal stress induces vulnerability to nicotine addiction and alters D2 receptors’ expression in the nucleus accumbens in adult Rats. Neuroscience 2015, 304, 279–285. [Google Scholar] [CrossRef]
- Stratilov, V.A.; Vetrovoy, O.V.; Tyulkova, E.I. Prenatal hypoxia affects nicotine consumption and withdrawal in adult rats via impairment of the glutamate system in the brain. Mol. Neurobiol. 2022, 59, 4550–4561. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008, 32, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Vetrovoy, O.; Stratilov, V.; Lomert, E.; Tyulkova, E. Prenatal hypoxia-induced adverse reaction to mild stress is associated with depressive-like changes in the glucocorticoid system of rats. Neurochem. Res. 2023, 48, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Laugesen, K.; Sørensen, H.T.; Jørgensen, J.O.L.; Petersen, I. In utero exposure to glucocorticoids and risk of anxiety and depression in childhood or adolescence. Psychoneuroendocrinology 2022, 141, 105766. [Google Scholar] [CrossRef]
- Vallée, M.; Mayo, W.; Dellu, F.; Le Moal, M.; Simon, H.; Maccari, S. Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: Correlation with stress-induced corticosterone secretion. J. Neurosci. 1997, 17, 2626–2636. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, B.M.; Bhagya, V.; Harish, G.; Srinivas Bharath, M.M.; Shankaranarayana Rao, B.S. Environmental enrichment ameliorates chronic immobilisation stress-induced spatial learning deficits and restores the expression of BDNF, VEGF, GFAP and glucocorticoid receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 76, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Golan, H.; Huleihel, M. The effect of prenatal hypoxia on brain development: Short- and long-term consequences demonstrated in rodent models. Dev. Sci. 2006, 9, 338–349. [Google Scholar] [CrossRef]
- Crudo, A.; Petropoulos, S.; Moisiadis, V.G.; Iqbal, M.; Kostaki, A.; Machnes, Z.; Szyf, M.; Matthews, S.G. Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: Multigenerational effects. Endocrinology 2012, 153, 3269–3283. [Google Scholar] [CrossRef]
- Jafari, Z.; Mehla, J.; Kolb, B.E.; Mohajerani, M.H. Gestational stress augments postpartum β-amyloid pathology and cognitive decline in a mouse model of alzheimer’s disease. Cereb. Cortex 2019, 29, 3712–3724. [Google Scholar] [CrossRef]
- Vetrovoy, O.; Stratilov, V.; Nimiritsky, P.; Makarevich, P.; Tyulkova, E. Prenatal hypoxia induces premature aging accompanied by impaired function of the glutamatergic system in rat hippocampus. Neurochem. Res. 2021, 46, 550–563. [Google Scholar] [CrossRef]
- Tripathi, S.J.; Chakraborty, S.; Srikumar, B.N.; Raju, T.R.; Shankaranarayana Rao, B.S. Prevention of chronic immobilization stress-induced enhanced expression of glucocorticoid receptors in the prefrontal cortex by inactivation of basolateral amygdala. J. Chem. Neuroanat. 2019, 95, 134–145. [Google Scholar] [CrossRef]
- Weidenfeld, J.; Ovadia, H. The role of the amygdala in regulating the hypothalamic-pituitary-adrenal axis. In The Amygdala—Where Emotions Shape Perception, Learning and Memories; InTech: Cambridge, ON, Canada, 2017. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Mcklveen, J.M.; Solomon, M.B.; Carvalho-Netto, E.; Myers, B. Neural regulation of the stress response: Glucocorticoid feedback mechanisms. Braz. J. Med. Biol. Res. 2012, 45, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kolber, B.J.; Roberts, M.S.; Howell, M.P.; Wozniak, D.F.; Sands, M.S.; Muglia, L.J. Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc. Natl. Acad. Sci. USA 2008, 105, 12004. [Google Scholar] [CrossRef] [PubMed]
- Berumen, L.C.; Rodríguez, A.; Miledi, R.; García-Alcocer, G. Serotonin receptors in hippocampus. Sci. World J. 2012, 2012, 823493. [Google Scholar] [CrossRef]
- Laplante, P.; Diorio, J.; Meaney, M.J. Serotonin regulates hippocampal glucocorticoid receptor expression via a 5-HT7 receptor. Dev. Brain Res. 2002, 139, 199–203. [Google Scholar] [CrossRef]
- Bąk, J.; Bobula, B.; Hess, G. Restraint stress and repeated corticosterone administration differentially affect neuronal excitability, synaptic transmission and 5-HT7 receptor reactivity in the dorsal raphe nucleus of young adult male rats. Int. J. Mol. 2022, 23, 14303. [Google Scholar] [CrossRef]
- Pehrson, A.L.; Sanchez, C. Serotonergic modulation of glutamate neurotransmission as a strategy for treating depression and cognitive dysfunction. CNS Spectr. 2014, 19, 121–133. [Google Scholar] [CrossRef]
- Hauser, S.R.; Hedlund, P.B.; Roberts, A.J.; Sari, Y.; Bell, R.L.; Engleman, E.A. The 5-HT7 receptor as a potential target for treating drug and alcohol abuse. Front. Neurosci. 2015, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Seckl, J.R.; Holmes, M.C. Mechanisms of disease: Glucocorticoids, their placental metabolism and fetal “programming” of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 1, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Baranova, K.A.; Mironova, V.I.; Rybnikova, E.A.; Samoilov, M.O. Characteristics of the transcription factor HIF-1α expression in the rat brain during the development of depressive state and the antidepressive effects of hypoxic preconditioning. Neurochem. J. 2010, 4, 35–40. [Google Scholar] [CrossRef]
- Henkel, V.; Bussfeld, P.; Möller, H.J.; Hegerl, U. Cognitive-behavioural theories of helplessness/hopelessness: Valid models of depression? Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 240–249. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stratilov, V.; Potapova, S.; Safarova, D.; Tyulkova, E.; Vetrovoy, O. Prenatal Hypoxia Triggers a Glucocorticoid-Associated Depressive-like Phenotype in Adult Rats, Accompanied by Reduced Anxiety in Response to Stress. Int. J. Mol. Sci. 2024, 25, 5902. https://doi.org/10.3390/ijms25115902
Stratilov V, Potapova S, Safarova D, Tyulkova E, Vetrovoy O. Prenatal Hypoxia Triggers a Glucocorticoid-Associated Depressive-like Phenotype in Adult Rats, Accompanied by Reduced Anxiety in Response to Stress. International Journal of Molecular Sciences. 2024; 25(11):5902. https://doi.org/10.3390/ijms25115902
Chicago/Turabian StyleStratilov, Viktor, Sofiya Potapova, Diana Safarova, Ekaterina Tyulkova, and Oleg Vetrovoy. 2024. "Prenatal Hypoxia Triggers a Glucocorticoid-Associated Depressive-like Phenotype in Adult Rats, Accompanied by Reduced Anxiety in Response to Stress" International Journal of Molecular Sciences 25, no. 11: 5902. https://doi.org/10.3390/ijms25115902
APA StyleStratilov, V., Potapova, S., Safarova, D., Tyulkova, E., & Vetrovoy, O. (2024). Prenatal Hypoxia Triggers a Glucocorticoid-Associated Depressive-like Phenotype in Adult Rats, Accompanied by Reduced Anxiety in Response to Stress. International Journal of Molecular Sciences, 25(11), 5902. https://doi.org/10.3390/ijms25115902





