Novel Insights into Psychosis and Antipsychotic Interventions: From Managing Symptoms to Improving Outcomes
Abstract
:1. Introduction
- To summarize what is known about the role of premature cellular/neuronal senescence in the pathogenesis of SCZ and SLDs.
- To discuss potential strategies for improving sustained recovery in SCZ and SLDs via natural senotherapeutics, microbial phenazines, aryl hydrocarbon receptor (AhR) antagonists, membrane lipid replacement (MLR), and mitochondrial transplantation.
2. Premature Cellular Senescence in Schizophrenia
2.1. Ferrosenescence vs. Ferroptosis
2.2. Senescent Gut Barrier
3. Aryl Hydrocarbon, the Master Regulator of Cellular Senescence
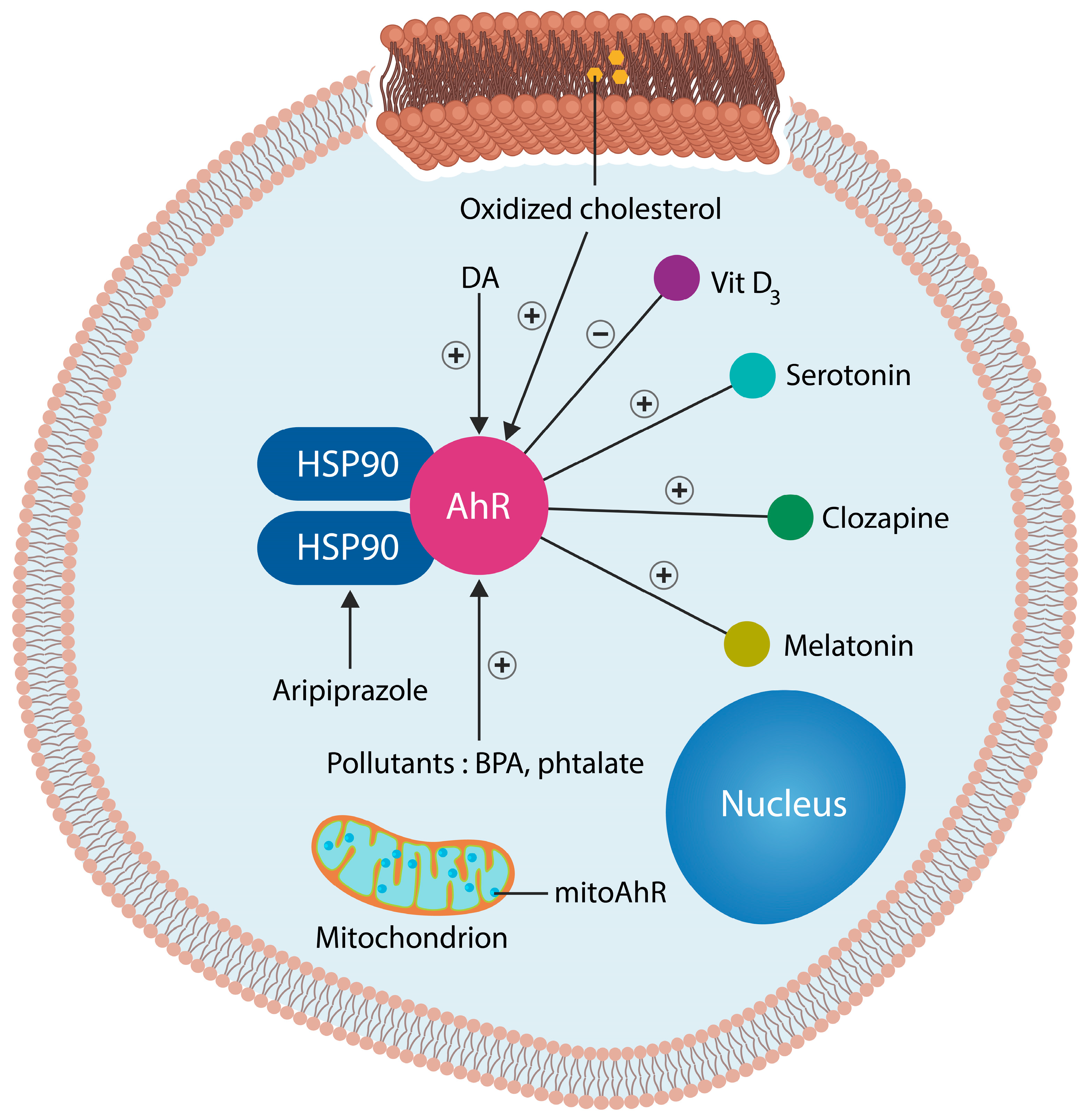
3.1. Gray and White Matter Loss
3.2. Dopamine-Sparing Antipsychotics
4. Mitochondrial Dysfunction and Loss of Gamma-Band Oscillations

Entrainment of Gamma-Band Oscillations in Schizophrenia
5. Senotherapeutics
6. Membrane Lipid Replacement (MLR)
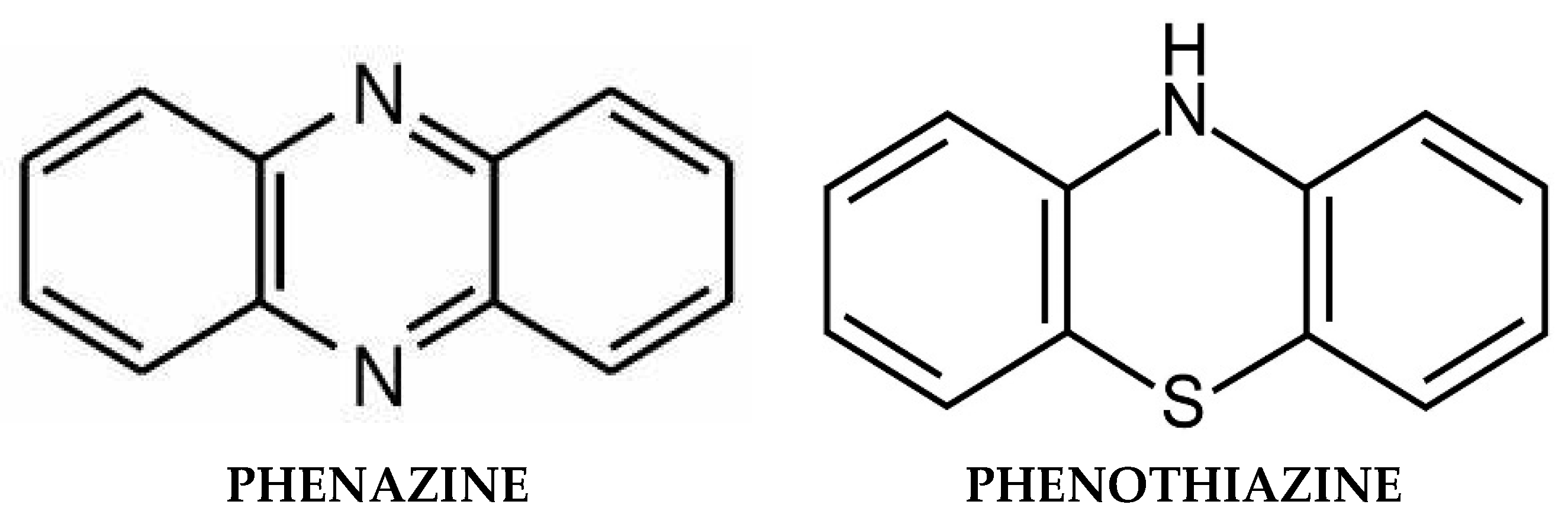
7. Phenazines and Antioxidant Phenothiazines
7.1. Natural Phenazines
7.2. Synthetic Phenazine Derivatives
7.3. Antioxidant Phenothiazines and Their Derivatives
8. Mitochondrial Transfer and Transplantation
9. Using AhR Antagonists as Antipsychotics
- Quercetin is a natural flavonoid and plant pigment that exerts antioxidant and anticancer properties. In the CNS, quercetin is a negative allosteric modulator of GABARs as well as an enhancer of glutamatergic neurotransmission, a signaling pathway deficient in SCZ [209]. In addition, quercetin inhibits the apoptosis of cortical neurons, likely preventing gray matter loss.
- Apigenin is a plant-based remedy extract from Elsholtzia rugulosa used by traditional practitioners from Africa for treating mental illness. Aside from antagonizing the AhR, apigenin exhibits vasorelaxant, antioxidant, and antipsychotic properties [209].
- Luteolin is a natural antipsychotic that exerts its beneficial actions by reducing microglial inflammation [202]. Luteolin is currently under clinical trials for SCZ treatment (NCT05204407).
10. Synthetic AhR Antagonists
11. Recombinant Interleukin-22
- SCZ is often comorbid with IBD, conditions associated with increased gut barrier permeability and microbial translocation from the GI tract into host tissues, including the brain.
- Translocation markers, including soluble CD14 (sCD14) and lipopolysaccharide-binding protein (LBP), are elevated in SCZ, suggesting bacterial translocation.
- Increased BBB permeability in SCZ enables translocated gut microbes to reach the brain.
- The Escherichia coli (E. coli) outbreak in 2011 in Germany has been associated with cases of new-onset psychosis.
- New-onset psychosis, or its exacerbation, has been identified in E. coli-associated UTIs.
12. Vehicles: Lipid Nanoparticles
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamb, H.R. Deinstitutionalization and the homeless mentally ill. Hosp. Community Psychiatry 1984, 35, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Scott, J. Homelessness and mental illness. Br. J. Psychiatry 1993, 162, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Insel, T. Rethinking schizophrenia. Nature 2010, 468, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Delport, A.; Harvey, B.H.; Petzer, A.; Petzer, J.P. The monoamine oxidase inhibition properties of selected structural analogues of methylene blue. Toxicol. Appl. Pharmacol. 2017, 325, 1–8. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Juola, P.; Hirvonen, N.; McGrath, J.J.; Saha, S.; Isohanni, M.; Veijola, J.; Miettunen, J. A Systematic Review and Meta-Analysis of Recovery in Schizophrenia. Schizophr. Bull. 2012, 39, 1296–1306. [Google Scholar] [CrossRef]
- Warner, R. Recovery from Schizophrenia Psychiatry and Political Economy, 3rd ed.; Brunner-Routledge: Hove, UK; New York, NY, USA, 1997; p. 74. [Google Scholar]
- Üçok, A.; Polat, A.; Çakır, S.; Genç, A. One year outcome in first episode schizophrenia: Predictors of relapse. Eur. Arch. Psychiatry Clin. Neurosci. 2005, 256, 37–43. [Google Scholar] [CrossRef]
- Holm, M.; Taipale, H.; Tanskanen, A.; Tiihonen, J.; Mitterdorfer-Rutz, E. Employment among people with schizophrenia or bipolar disorder: A population-based study using nationwide registers. Acta Psychiatr. Scand. 2020, 143, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, I.S.; Abdel-Baki, A. Homeless youth with first-episode psychosis: A 2-year outcome study. Schizophr. Res. 2019, 216, 460–469. [Google Scholar] [CrossRef]
- Lai, C.H.; Wu, Y.T.; Chen, C.Y.; Hou, Y.C. Gray matter increases in fronto-parietal regions of depression patients with aripiprazole monotherapy: An exploratory study. Medicine 2016, 95, e4654. [Google Scholar] [CrossRef]
- Rollema, H.; Lu, Y.; Schmidt, A.W.; Zorn, S.H. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur. J. Pharmacol. 1997, 338, R3–R5. [Google Scholar] [CrossRef]
- Papanastasiou, E.; Gaughran, F.; Smith, S. Schizophrenia as segmental progeria. J. R. Soc. Med. 2011, 104, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Prinzi, G.; Proietti, S.; Lamonaca, P.; Frustaci, A.; Boccia, S.; Amore, R.; Lorenzi, M.; Onder, G.; Marzetti, E.; et al. Shorter telomere length in schizophrenia: Evidence from a real-world population and meta-analysis of Most recent literature. Schizophr. Res. 2018, 202, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Dada, O.; Adanty, C.; Dai, N.; Jeremian, R.; Alli, S.; Gerretsen, P.; Graff, A.; Strauss, J.; De Luca, V. Biological aging in schizophrenia and psychosis severity: DNA methylation analysis. Psychiatry Res. 2021, 296, 113646. [Google Scholar] [CrossRef] [PubMed]
- Schnack, H.G.; van Haren, N.E.; Nieuwenhuis, M.; Hulshoff Pol, H.E.; Cahn, W.; Kahn, R.S. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am. J. Psychiatry 2016, 173, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Langhi Prata, L.G.P.; Tchkonia, T.; Kirkland, J.L. Cell senescence, the senescence-associated secretory phenotype, and cancers. PLoS Biol. 2023, 21, e3002326. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Stec, A.; Maciejewska, M.; Zaremba, M.; Paralusz-Stec, K.; Michalska, M.; Rudnicka, L.; Sikora, M. The Clinical Significance of Serum Biomarkers of the Intestinal Barrier in Systemic Sclerosis: A Cross-Sectional Study. J. Pers. Med. 2023, 13, 678. [Google Scholar] [CrossRef]
- Sepich-Poore, G.D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J.A.; Knight, R. The microbiome and human cancer. Science 2021, 371, eabc4552. [Google Scholar] [CrossRef]
- White, M.G.; Wargo, J.A. Gut Microbes’ Impact on Oncogenic Drivers: Location Matters. Mol. Cell 2020, 79, 878–880. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Berni Canani, R.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, X.; Trakalo, J.; Valente, J.; Azevedo, M.H.; Pato, M.T.; Pato, C.N.; Kennedy, J.L. Human p53 tumor suppressor gene (TP53) and schizophrenia: Case-control and family studies. Neurosci. Lett. 2005, 388, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Nickles, J.; Rodriguez-Armendariz, A.G.; McFarland, B.C.; Ajami, N.J.; Ballester, L.Y.; Wargo, J.A.; Esquenazi, Y. Glioma and the gut-brain axis: Opportunities and future perspectives. Neurooncol. Adv. 2022, 4, vdac054. [Google Scholar] [CrossRef] [PubMed]
- Palacios, E.; Lobos-González, L.; Guerrero, S.; Kogan, M.J.; Shao, B.; Heinecke, J.W.; Quest, A.F.G.; Leyton, L.; Valenzuela-Valderrama, M. Helicobacter pylori outer membrane vesicles induce astrocyte reactivity through nuclear factor-κappa B activation and cause neuronal damage in vivo in a murine model. J. Neuroinflamm. 2023, 20, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Tian, H.; Song, X.; Jiang, D.; Chen, G.; Cai, Z.; Ping, J.; Cheng, L.; Zhou, C.; Chen, C.; et al. Microglia and cognitive impairment in schizophrenia: Translating scientific progress into novel therapeutic interventions. Schizophrenia 2023, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacol. Rev. 2017, 42, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Wiwanitkit, V. Hemolysis in E. coli O104, H4 Infection. Indian J. Hematol. Blood Transfus. 2012, 28, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karrer, T.M.; Josef, A.K.; Mata, R.; Morris, E.D.; Samanez-Larkin, G.R. Reduced dopamine receptors and transporters but not synthesis capacity in normal aging adults: A meta-analysis. Neurobiol. Aging 2017, 57, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kim, T.W.; Han, Y.; Nair, M.S.; Harschnitz, O.; Zhu, J.; Wang, P.; Koo, S.Y.; Lacko, L.A.; Chandar, V.; et al. SARS-CoV-2 infection causes dopaminergic neuron senescence. Cell Stem Cell 2024, 31, 196–211.e6. [Google Scholar] [CrossRef] [PubMed]
- Fettucciari, K.; Fruganti, A.; Stracci, F.; Spaterna, A.; Marconi, P.; Bassotti, G. Clostridioides difficile Toxin B Induced Senescence: A New Pathologic Player for Colorectal Cancer? Int. J. Mol. Sci. 2023, 24, 8155. [Google Scholar] [CrossRef]
- Vinithakumari, A.A.; Padhi, P.; Hernandez, B.; Lin, S.J.; Dunkerson-Kurzhumov, A.; Showman, L.; Breitzman, M.; Stokes, C.; Sulaiman, Y.; Tangudu, C.; et al. Clostridioides difficile Infection Dysregulates Brain Dopamine Metabolism. Microbiol. Spectr. 2022, 10, e0007322. [Google Scholar] [CrossRef]
- Feng, Y.; Shen, J.; He, J.; Lu, M. Schizophrenia and cell senescence candidate genes screening, machine learning, diagnostic models, and drug prediction. Front. Psychiatry 2023, 14, 1105987. [Google Scholar] [CrossRef] [PubMed]
- Ling, E.; Nemesh, J.; Goldman, M.; Kamitaki, N.; Reed, N.; Handsaker, R.E.; Genovese, G.; Vogelgsang, J.S.; Gerges, S.; Kashin, S.; et al. A concerted neuron–astrocyte program declines in ageing and schizophrenia. Nature 2024, 627, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Ishida, I.; Ogura, J.; Aizawa, E.; Ota, M.; Hidese, S.; Yomogida, Y.; Matsuo, J.; Yoshida, S.; Kunugi, H. Gut permeability and its clinical relevance in schizophrenia. Neuropsychopharmacol. Rep. 2022, 42, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Scheurink, T.A.W.; Borkent, J.; Gangadin, S.S.; El Aidy, S.; Mandl, R.; Sommer, I.E.C. Association between gut permeability, brain volume, and cognition in healthy participants and patients with schizophrenia spectrum disorder. Brain Behav. 2023, 13, e3011. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, J.; Gawlik-Kotelnicka, O. Intestinal permeability and its significance in psychiatric disorders—A narrative review and future perspectives. Behav. Brain Res. 2023, 448, 114459. [Google Scholar] [CrossRef] [PubMed]
- Lotan, A.; Luza, S.; Opazo, C.M.; Ayton, S.; Lane, D.J.R.; Mancuso, S.; Pereira, A.; Sundram, S.; Weickert, C.S.; Bousman, C.; et al. Perturbed iron biology in the prefrontal cortex of people with schizophrenia. Mol. Psychiatry 2023, 28, 2058–2070. [Google Scholar] [CrossRef]
- Dichtl, S.; Demetz, E.; Haschka, D.; Tymoszuk, P.; Petzer, V.; Nairz, M.; Seifert, M.; Hoffmann, A.; Brigo, N.; Würzner, R.; et al. Dopamine Is a Siderophore-Like Iron Chelator That Promotes Salmonella enterica Serovar Typhimurium Virulence in Mice. mBio 2019, 10, e02624-18. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Lactate as a regulator of iron homeostasis. Life Metab. 2023, 2, load033. [Google Scholar] [CrossRef]
- Cai, Z.; Deng, L.; Fan, Y.; Ren, Y.; Ling, Y.; Tu, J.; Cai, Y.; Xu, X.; Chen, M. Dysregulation of Ceramide Metabolism Is Linked to Iron Deposition and Activation of Related Pathways in the Aorta of Atherosclerotic Miniature Pigs. Antioxidants 2024, 13, 4. [Google Scholar] [CrossRef]
- de la Monte, S.M. Triangulated mal-signaling in Alzheimer’s disease: Roles of neurotoxic ceramides, ER stress, and insulin resistance reviewed. J. Alzheimers Dis. 2012, 30 (Suppl. S2), S231–S249. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, P.; Syeda, W.T.; Jayaram, M.; Rushmore, R.J.; Moffat, B.; Lin, A.P.; Lyall, A.E.; Merritt, A.H.; Yaghmaie, N.; Laskaris, L.; et al. In Vivo 7-Tesla MRI Investigation of Brain Iron and Its Metabolic Correlates in Chronic Schizophrenia. Schizophrenia 2022, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, J.; Qu, C.; Yang, L.; Wu, X.; Wang, S.; Yang, T.; Liu, H.; Fang, Y.; Sun, P. Identification of Ferroptosis-Related Genes in Schizophrenia Based on Bioinformatic Analysis. Genes 2022, 13, 2168. [Google Scholar] [CrossRef]
- Lian, K.; Li, Y.; Yang, W.; Ye, J.; Liu, H.; Wang, T.; Yang, G.; Cheng, Y.; Xu, X. Hub genes, a diagnostic model, and immune infiltration based on ferroptosis-linked genes in schizophrenia. IBRO Neurosci. Rep. 2024, 16, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Go, S.; Kang, M.; Kwon, S.P.; Jung, M.; Jeon, O.H.; Kim, B.S. The Senolytic Drug JQ1 Removes Senescent Cells via Ferroptosis. Tissue Eng. Regen. Med. 2021, 18, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Clatworthy, S.A.S.; Gamell, C.; Meggyesy, P.M.; Rigopoulos, A.T.; Haupt, S.; Haupt, Y.; Denoyer, D.; Adlard, P.A.; Bush, A.I.; et al. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 2018, 14, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A.; Osorio, C.; Maguire, G.; Rahman, L.; Afzaal, J.; Cummings, M.; Maldonado, J.C. COVID-19, ferrosenescence and neurodegeneration, a mini-review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110230. [Google Scholar] [CrossRef]
- Sfera, A.; Bullock, K.; Price, A.; Inderias, L.; Osorio, C. Ferrosenescence: The iron age of neurodegeneration? Mech. Ageing Dev. 2018, 174, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Boshkovski, T.; Cohen-Adad, J.; Misic, B.; Arnulf, I.; Corvol, J.C.; Vidailhet, M.; Lehéricy, S.; Stikov, N.; Mancini, M. The Myelin-Weighted Connectome in Parkinson’s Disease. Mov. Disord. 2022, 37, 724–733. [Google Scholar] [CrossRef]
- Belaidi, A.A.; Masaldan, S.; Southon, A.; Kalinowski, P.; Acevedo, K.; Appukuttan, A.T.; Portbury, S.; Lei, P.; Agarwal, P.; Leurgans, S.E.; et al. Apolipoprotein E potently inhibits ferroptosis by blocking ferritinophagy. Mol. Psychiatry 2022, 29, 211–220. [Google Scholar] [CrossRef]
- Arnold, S.E.; Joo, E.; Martinoli, M.G.; Roy, N.; Trojanowski, J.Q.; Gur, R.E.; Cannon, T.; Price, R.A. Apolipoprotein E genotype in schizophrenia: Frequency, age of onset, and neuropathologic features. Neuroreport. 1997, 8, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Kampman, O.; Anttila, S.; Illi, A.; Mattila, K.M.; Rontu, R.; Leinonen, E.; Lehtimäki, T. Apolipoprotein E polymorphism is associated with age of onset in schizophrenia. J. Hum. Genet. 2004, 49, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Guo, Y.; Cheng, J.; Xue, K.; Yang, M.; Song, X.; Feng, Y.; Cheng, J. Brain iron assessment in patients with First-episode schizophrenia using quantitative susceptibility mapping. Neuroimage Clin. 2021, 31, 102736. [Google Scholar] [CrossRef] [PubMed]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Pruett, B.S.; Meador-Woodruff, J.H. Evidence for altered energy metabolism, increased lactate, and decreased pH in schizophrenia brain: A focused review and meta-analysis of human postmortem and magnetic resonance spectroscopy studies. Schizophr. Res. 2020, 223, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.M.; Yen, Y.H.; Yuan, F.; Zhang, S.C.; Chong, C.M. Neuronal Senescence in the Aged Brain. Aging Dis. 2023, 14, 1618–1632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Gamma oscillations weaken with age in healthy elderly in human EEG. Neuroimage 2020, 215, 116826. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Emerging Interrelationship between the Gut Microbiome and Cellular Senescence in the Context of Aging and Disease: Perspectives and Therapeutic Opportunities. Probiotics Antimicrob. Proteins 2022, 14, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Venturelli, S.; Zender, L.; Bitzer, M. Cellular senescence in gastrointestinal diseases: From pathogenesis to therapeutics. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 81–95. [Google Scholar] [CrossRef]
- Maes, M.; Kanchanatawan, B.; Sirivichayakul, S.; Carvalho, A.F. In Schizophrenia, Increased Plasma IgM/IgA Responses to Gut Commensal Bacteria Are Associated with Negative Symptoms, Neurocognitive Impairments, and the Deficit Phenotype. Neurotox. Res. 2019, 35, 684–698. [Google Scholar] [CrossRef]
- Hennigar, S.R.; McClung, J.P. Nutritional Immunity: Starving Pathogens of Trace Minerals. Am. J. Lifestyle Med. 2016, 10, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, L.; Kell, D.B.; Pretorius, E. Iron Dysregulation and Dormant Microbes as Causative Agents for Impaired Blood Rheology and Pathological Clotting in Alzheimer’s Type Dementia. Front. Neurosci. 2018, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Link, C.D. Is There a Brain Microbiome? Neurosci. Insights 2021, 16, 26331055211018709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dworkin, J.; Shah, I.M. Exit from dormancy in microbial organisms. Nat. Rev. Microbiol. 2010, 8, 890–896. [Google Scholar] [CrossRef]
- Peyrusson, F.; Nguyen, T.K.; Najdovski, T.; Van Bambeke, F. Host Cell Oxidative Stress Induces Dormant Staphylococcus aureus Persisters. Microbiol. Spectr. 2022, 10, e0231321. [Google Scholar] [CrossRef]
- Berthelot, J.M.; de la Cochetière, M.F.; Potel, G.; Le Goff, B.; Maugars, Y. Evidence supporting a role for dormant bacteria in the pathogenesis of spondylarthritis. Jt. Bone Spine 2013, 80, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Sroka, K.; Binienda, A.; Jurk, D.; Fichna, J. A new face of old cells: An overview about the role of senescence and telomeres in inflammatory bowel diseases. Ageing Res. Rev. 2023, 91, 102083. [Google Scholar] [CrossRef]
- Qian, L.; He, X.; Gao, F.; Fan, Y.; Zhao, B.; Ma, Q.; Yan, B.; Wang, W.; Ma, X.; Yang, J. Estimation of the bidirectional relationship between schizophrenia and inflammatory bowel disease using the mendelian randomization approach. Schizophrenia 2022, 8, 31. [Google Scholar] [CrossRef]
- Bartocci, B.; Dal Buono, A.; Gabbiadini, R.; Busacca, A.; Quadarella, A.; Repici, A.; Mencaglia, E.; Gasparini, L.; Armuzzi, A. Mental Illnesses in Inflammatory Bowel Diseases: Mens sana in corpore sano. Medicina 2023, 59, 682. [Google Scholar] [CrossRef]
- Zevin, A.S.; McKinnon, L.; Burgener, A.; Klatt, N.R. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr. Opin. HIV AIDS 2016, 11, 182–190. [Google Scholar] [CrossRef]
- Sfera, A.; Jafri, N.; Rahman, L. F-652 (Recombinant Human Interleukin-22) For Schizophrenia. Arch. Pharmacal Res. 2023, 3, 1–6. [Google Scholar]
- Schubert, K.O.; Föcking, M.; Cotter, D.R. Proteomic pathway analysis of the hippocampus in schizophrenia and bipolar affective disorder implicates 14-3-3 signaling, aryl hydrocarbon receptor signaling, and glucose metabolism: Potential roles in GABAergic interneuron pathology. Schizophr. Res. 2015, 167, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Juricek, L.; Coumoul, X. The Aryl Hydrocarbon Receptor and the Nervous System. Int. J. Mol. Sci. 2018, 19, 2504. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.; Nichols, R.; Zhang, L.; Patterson, A.D.; Perdew, G.H. Expression of the aryl hydrocarbon receptor contributes to the establishment of intestinal microbial community structure in mice. Sci. Rep. 2016, 6, 33969. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Jin, U.H.; Karki, K.; Jayaraman, A.; Allred, C.; Michelhaugh, S.K.; Mittal, S.; Chapkin, R.S.; Safe, S. Dopamine is an aryl hydrocarbon receptor agonist. Biochem. J. 2020, 477, 3899–3910. [Google Scholar] [CrossRef] [PubMed]
- Fehsel, K.; Schwanke, K.; Kappel, B.A.; Fahimi, E.; Meisenzahl-Lechner, E.; Esser, C.; Hemmrich, K.; Haarmann-Stemmann, T.; Kojda, G.; Lange-Asschenfeldt, C. Activation of the aryl hydrocarbon receptor by clozapine induces preadipocyte differentiation and contributes to endothelial dysfunction. J. Psychopharmacol. 2022, 36, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Moura-Alves, P.; Faé, K.; Houthuys, E.; Dorhoi, A.; Kreuchwig, A.; Furkert, J.; Barison, N.; Diehl, A.; Munder, A.; Constant, P.; et al. AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512, 387–392. [Google Scholar] [CrossRef]
- Bai, Z.; Yang, P.; Yu, F.; Li, Z.; Yao, Z.; Martinez, J.; Li, M.; Xu, H. Combining adoptive NK cell infusion with a dopamine-releasing peptide reduces senescent cells in aged mice. Cell Death Dis. 2022, 13, 305. [Google Scholar] [CrossRef]
- Yurchenko, O.P.; Grigoriev, N.G.; Turpaev, T.M.; Konjević, D.; Rakić, L. Intracellular injection of dopamine enhances acetylcholine responses of neuron R2 in the Aplysia abdominal ganglion. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1987, 87, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Tanaka, H.; Koga, T. Cellular Senescence Variation by Metabolic and Epigenomic Remodeling. Trends Cell Biol. 2020, 30, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Meng, F.; Lyu, W.; He, J.; Wei, R.; Du, Z.; Zhang, C. Histone lactylation antagonizes senescence and skeletal muscle aging via facilitating gene expression reprogramming. bioRxiv 2023. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, H.; Liu, M.; Zhou, T.; Cheng, X.; Huang, W.; Cao, L. The role and mechanism of histone lactylation in health and diseases. Front. Genet. 2022, 13, 949252. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yang, X.; Wang, J.; Wang, Z.; Wang, Q.; Ding, Y.; Yu, A. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFκB signaling pathway. J. Neuroinflamm. 2023, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.Y.; McNeely, T.L.; Baker, D.J. Untangling senescent and damage-associated microglia in the aging and diseased brain. FEBS J. 2023, 290, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Béchade, C.; D‘Andrea, I.; St-Pierre, M.K.; Henry, M.S.; Roumier, A.; Tremblay, M.E. Microglia Gone Rogue: Impacts on Psychiatric Disorders across the Lifespan. Front. Mol. Neurosci. 2018, 10, 421. [Google Scholar] [CrossRef]
- Zhu, H.; Guan, A.; Liu, J.; Peng, L.; Zhang, Z.; Wang, S. Noteworthy perspectives on microglia in neuropsychiatric disorders. J. Neuroinflamm. 2023, 20, 223. [Google Scholar] [CrossRef]
- Galle, E.; Wong, C.W.; Ghosh, A.; Desgeorges, T.; Melrose, K.; Hinte, L.C.; Castellano-Castillo, D.; Engl, M.; de Sousa, J.A.; Ruiz-Ojeda, F.J.; et al. H3K18 lactylation marks tissue-specific active enhancers. Genome Biol. 2022, 23, 207. [Google Scholar] [CrossRef]
- Hagihara, H.; Shoji, H.; Otabi, H.; Toyoda, A.; Katoh, K.; Namihira, M.; Miyakawa, T. Protein lactylation induced by neural excitation. Cell Rep. 2021, 37, 109820. [Google Scholar] [CrossRef]
- Föcking, M.; Doyle, B.; Munawar, N.; Dillon, E.T.; Cotter, D.; Cagney, G. Epigenetic Factors in Schizophrenia: Mechanisms and Experimental Approaches. Mol. Neuropsychiatry 2019, 5, 6–12. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef]
- Chen, A.N.; Luo, Y.; Yang, Y.H.; Fu, J.T.; Geng, X.M.; Shi, J.P.; Yang, J. Lactylation, a Novel Metabolic Reprogramming Code: Current Status and Prospects. Front. Immunol. 2021, 12, 688910. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Tawara, K.; Nakagawa, H.; Honda, R.; Kido, T.; Nishijo, H.; Saito, S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin in maternal breast milk newborn head circumference. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kinney, D.K.; Teixeira, P.; Hsu, D.; Napoleon, S.C.; Crowley, D.J.; Miller, A.; Hyman, W.; Huang, E. Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: A role for prenatal vitamin d deficiency and infections? Schizophr. Bull. 2009, 35, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Cummings, C.; Chapman, G.E.; Shatalina, E. Neuroimaging in schizophrenia: An overview of findings and their implications for synaptic changes. Neuropsychopharmacology 2022, 48, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Cheung, C.; Yu, K.; Yip, B.; Sham, P.; Li, Q.; Chua, S.; McAlonan, G. Gray Matter in First-Episode Schizophrenia Before and After Antipsychotic Drug Treatment. Anatomical Likelihood Estimation Meta-analyses with Sample Size Weighting. Schizophr. Bull. 2009, 37, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Smieskova, R.; Kempton, M.; Ho, B.; Andreasen, N.; Borgwardt, S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013, 37, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Veijola, J.; Guo, J.Y.; Moilanen, J.S.; Jääskeläinen, E.; Miettunen, J.; Kyllönen, M.; Haapea, M.; Huhtaniska, S.; Alaräisänen, A.; Mäki, P.; et al. Longitudinal changes in total brain volume in schizophrenia: Relation to symptom severity, cognition and antipsychotic medication. PLoS ONE. 2014, 18, e101689. [Google Scholar] [CrossRef]
- Cahn, W.; Pol, H.E.H.; Lems, E.B.; van Haren, N.E.; Schnack, H.G.; van der Linden, J.A.; Schothorst, P.F.; van Engeland, H.; Kahn, R.S. Brain volume changes in first-episode schizophrenia: A 1-year follow-up study. Arch. Gen. Psychiatry 2002, 59, 1002–1010. [Google Scholar] [CrossRef]
- Banwinkler, M.; Dzialas, V. Parkinson’s Progression Markers Initiative; Hoenig MC, van Eimeren T. Gray Matter Volume Loss in Proposed Brain-First and Body-First Parkinson’s Disease Subtypes. Mov Disord. 2022, 37, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, Y.J.; Kim, Y.J.; Lee, H.S.; Jeong, S.H.; Hong, J.M.; Sohn, Y.H.; Yun, M.; Jeong, Y.; Lee, P.H. Association between White Matter Networks and the Pattern of Striatal Dopamine Depletion in Patients with Parkinson Disease. Neurology 2022, 99, e2672–e2682. [Google Scholar] [CrossRef]
- Dean, D.C., 3rd; Sojkova, J.; Hurley, S.; Kecskemeti, S.; Okonkwo, O.; Bendlin, B.B.; Theisen, F.; Johnson, S.C.; Alexander, A.L.; Gallagher, C.L. Alterations of Myelin Content in Parkinson’s Disease: A Cross-Sectional Neuroimaging Study. PLoS ONE 2016, 11, e0163774. [Google Scholar] [CrossRef]
- Brown, J.S., Jr. Effects of bisphenol-A and other endocrine disruptors compared with abnormalities of schizophrenia: An endocrine-disruption theory of schizophrenia. Schizophr. Bull. 2009, 35, 256–278. [Google Scholar] [CrossRef] [PubMed]
- Newbury, J.B.; Stewart, R.; Fisher, H.L.; Beevers, S.; Dajnak, D.; Broadbent, M.; Pritchard, M.; Shiode, N.; Heslin, M.; Hammoud, R.; et al. Association between air pollution exposure and mental health service use among individuals with first presentations of psychotic and mood disorders: Retrospective cohort study. Br. J. Psychiatry 2021, 219, 678–685. [Google Scholar] [CrossRef]
- Domínguez-Acosta, O.; Vega, L.; Estrada-Muñiz, E.; Rodríguez, M.S.; Gonzalez, F.J.; Elizondo, G. Activation of aryl hydrocarbon receptor regulates the LPS/IFNγ-induced inflammatory response by inducing ubiquitin-proteosomal and lysosomal degradation of RelA/p65. Biochem. Pharmacol. 2018, 155, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Youngren, K.; Inglis, F.; Pivirotto, P.; Jedema, H.P.; Bradberry, C.W.; Goldman-Rakic, P.S.; Roth, R.H.; Moghaddam, B. Clozapine Preferentially Increases Dopamine Release in the Rhesus Monkey Prefrontal Cortex Compared with the Caudate Nucleus. Neuropsychopharmacology 1999, 20, 403–412. [Google Scholar] [CrossRef]
- Tronchin, G.; Akudjedu, T.N.; Ahmed, M.; Holleran, L.; Hallahan, B.; Cannon, D.M.; McDonald, C. Progressive subcortical volume loss in treatment-resistant schizophrenia patients after commencing clozapine treatment. Neuropsychopharmacology 2020, 45, 1353–1361, Erratum in Neuropsychopharmacology 2021, 46, 1857–1858. [Google Scholar] [CrossRef]
- van Erp, T.G.M.; Walton, E.; Hibar, D.P.; Schmaal, L.; Jiang, W.; Glahn, D.C.; Pearlson, G.D.; Yao, N.; Fukunaga, M.; Hashimoto, R.; et al. Cortical brain abnormalities in 4474 individuals with schizophrenia and 5098 control subjects via the enhancing neuro imaging genetics through meta analysis (ENIGMA) consortium. Biol. Psychiatry 2018, 84, 644–654. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Liao, J.; Jiang, S.; Yan, J.; Yue, W.; Zhang, D.; Yan, H. Progressive Grey Matter Volume Changes in Patients with Schizophrenia over 6 Weeks of Antipsychotic Treatment and Their Relationship to Clinical Improvement. Neurosci. Bull. 2018, 34, 816–826. [Google Scholar] [CrossRef]
- Liu, N.; Xiao, Y.; Zhang, W.; Tang, B.; Zeng, J.; Hu, N.; Chandan, S.; Gong, Q.; Lui, S. Characteristics of gray matter alterations in never-treated and treated chronic schizophrenia patients. Transl. Psychiatry 2020, 10, 136. [Google Scholar] [CrossRef]
- Van Haren, N.E.; Hulshoff Pol, H.E.; Schnack, H.G.; Cahn, W.; Mandl, R.C.; Collins, D.L.; Evans, A.C.; Kahn, R.S. Focal gray matter changes in schizophrenia across the course of the illness: A 5-year follow-up study. Neuropsychopharmacology 2007, 32, 2057–2066. [Google Scholar] [CrossRef]
- Alamri, A.S.; Alhomrani, M.; Alsanie, W.F.; Alyami, H.; Shakya, S.; Habeeballah, H.; Abdulaziz, O.; Alamri, A.; Alkhatabi, H.A.; Felimban, R.I.; et al. Spectroscopic and Molecular Docking Analysis of π-Acceptor Complexes with the Drug Barbital. Appl. Sci. 2022, 12, 10130. [Google Scholar] [CrossRef]
- Refat, M.S.; Gaber, A.; Althobaiti, Y.S.; Alyami, H.; Alsanie, W.F.; Shakya, S.; Adam, A.M.A.; Kobeasy, M.I.; Asla, K.A. Spectroscopic and Molecular Docking Studies of Cu(II), Ni(II), Co(II), and Mn(II) Complexes with Anticonvulsant Therapeutic Agent Gabapentin. Molecules 2022, 27, 4311. [Google Scholar] [CrossRef]
- Hulshoff Pol, H.E.; Schnack, H.G.; Mandl, R.C.; van Haren, N.E.; Koning, H.; Collins, D.L.; Evans, A.C.; Kahn, R.S. Focal gray matter density changes in schizophrenia. Arch. Gen. Psychiatry 2001, 58, 1118–1125. [Google Scholar] [CrossRef]
- Winkler, T.E.; Lederer, S.L.; Kim, E.; Ben-Yoav, H.; Kelly, D.L.; Payne, G.F.; Ghodssi, R. Molecular processes in an electrochemical clozapine sensor. Biointerphases 2017, 12, 02B401. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.M.A.; Saad, H.A.; Refat, M.S.; Hegab, M.S. Charge-transfer complexes of antipsychotic drug sulpiride with inorganic and organic acceptors generated through two different approaches: Spectral characterization. J. Mol. Liq. 2022, 357, 119092. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Zaravinos, A.; Apidianakis, Y.; Lagoumintzis, G. Editorial: Microbiota and mitochondria: Impact on cell signaling, physiology, and disease. Front. Microbiol. 2022, 13, 1056499. [Google Scholar] [CrossRef]
- Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The Similarities between Human Mitochondria and Bacteria in the Context of Structure, Genome, and Base Excision Repair System. Molecules 2020, 25, 2857. [Google Scholar] [CrossRef]
- Liesa, M.; Shirihai, O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013, 17, 491–506. [Google Scholar] [CrossRef]
- Johnson, E.L.; Heaver, S.L.; Waters, J.L.; Kim, B.I.; Bretin, A.; Goodman, A.L.; Gewirtz, A.T.; Worgall, T.S.; Ley, R.E. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020, 11, 2471. [Google Scholar] [CrossRef]
- Venable, M.E.; Lee, J.Y.; Smyth, M.J.; Bielawska, A.; Obeid, L.M. Role of ceramide in cellular senescence. J. Biol. Chem. 1995, 270, 30701–30708. [Google Scholar] [CrossRef]
- Dadsena, S.; Bockelmann, S.; Mina, J.G.M.; Hassan, D.G.; Korneev, S.; Razzera, G.; Jahn, H.; Niekamp, P.; Müller, D.; Schneider, M.; et al. Ceramides bind VDAC2 to trigger mitochondrial apoptosis. Nat. Commun. 2019, 10, 1832. [Google Scholar] [CrossRef]
- Colombini, M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim. Biophys. Acta 2010, 1797, 1239–1244. [Google Scholar] [CrossRef]
- Dutta, D.; Kanca, O.; Byeon, S.K.; Marcogliese, P.C.; Zuo, Z.; Shridharan, R.V.; Park, J.H.; Undiagnosed Diseases Network; Lin, G.; Ge, M.; et al. A defect in mitochondrial fatty acid synthesis impairs iron metabolism and causes elevated ceramide levels. Nat. Metab. 2023, 5, 1595–1614. [Google Scholar] [CrossRef]
- Zietzer, A.; Düsing, P.; Reese, L.; Nickenig, G.; Jansen, F. Ceramide Metabolism in Cardiovascular Disease: A Network with High Therapeutic Potential. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1220–1228. [Google Scholar] [CrossRef]
- Smesny, S.; Schmelzer, C.E.; Hinder, A.; Köhler, A.; Schneider, C.; Rudzok, M.; Schmidt, U.; Milleit, B.; Milleit, C.; Nenadic, I.; et al. Skin ceramide alterations in first-episode schizophrenia indicate abnormal sphingolipid metabolism. Schizophr. Bull. 2013, 39, 933–941. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhao, F.; Tian, H.; Chen, J.; Li, Q.; Yang, L.; Ping, J.; Li, R.; Wang, L.; Xu, Y.; et al. Acid sphingomyelinase/ceramide system in schizophrenia: Implications for therapeutic intervention as a potential novel target. Transl. Psychiatry 2022, 12, 260. [Google Scholar] [CrossRef]
- Yuan, X.; Bhat, O.M.; Zou, Y.; Li, X.; Zhang, Y.; Li, P.L. Endothelial Acid Sphingomyelinase Promotes NLRP3 Inflammasome and Neointima Formation During Hypercholesterolemia. J. Lipid Res. 2022, 63, 100298. [Google Scholar] [CrossRef]
- Xia, Q.S.; Wu, F.; Wu, W.B.; Dong, H.; Huang, Z.Y.; Xu, L.; Lu, F.E.; Gong, J. Berberine reduces hepatic ceramide levels to improve insulin resistance in HFD-fed mice by inhibiting HIF-2α. Biomed. Pharmacother. 2022, 150, 112955. [Google Scholar] [CrossRef]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria know no boundaries: Mechanisms and functions of intercellular mitochondrial transfer. Front. Cell. Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Fairley, L.H.; Grimm, A.; Eckert, A. Mitochondria transfer in brain injury and disease. Cells 2022, 11, 3603. [Google Scholar] [CrossRef]
- Jackson, J.G.; Robinson, M.B. Regulation of mitochondrial dynamics in astrocytes: Mechanisms, consequences, and unknowns. Glia 2018, 66, 1213–1234. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.B.; Jetté, N.; Fiest, K.M.; Roberts, J.I.; Pearson, D.; Smith, E.E.; Roach, P.; Kirk, A.; Pringsheim, T.; Maxwell, C.J. The Prevalence and Incidence of Frontotemporal Dementia: A Systematic Review. Can. J. Neurol. Sci. 2016, 43 (Suppl. S1), S96–S109. [Google Scholar] [CrossRef] [PubMed]
- Course, M.M.; Wang, X. Transporting mitochondria in neurons. F1000Research 2016, 5, 1735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nuñez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Thayyullathil, F.; Cheratta, A.R.; Alakkal, A.; Subburayan, K.; Pallichankandy, S.; Hannun, Y.A.; Galadari, S. Acid sphingomyelinase-dependent autophagic degradation of GPX4 is critical for the execution of ferroptosis. Cell Death Dis. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Morsy, S.M.Y.; Sleem, A.A. The effect of different antidepressant drugs on oxidative stress after lipopolysaccharide administration in mice. EXCLI J. 2011, 10, 290–302. [Google Scholar] [PubMed]
- Kann, O. The energy demand of fast neuronal network oscillations: Insights from brain slice preparations. Front. Pharmacol. 2012, 2, 90. [Google Scholar] [CrossRef]
- Nakao, K.; Singh, M.; Sapkota, K.; Hagler, B.C.; Hunter, R.N.; Raman, C.; Hablitz, J.J.; Nakazawa, K. GSK3β inhibition restores cortical gamma oscillation and cognitive behavior in a mouse model of NMDA receptor hypofunction relevant to schizophrenia. Neuropsychopharmacology 2020, 45, 2207–2218. [Google Scholar] [CrossRef]
- Linseman, D.A.; Butts, B.D.; Precht, T.A.; Phelps, R.A.; Le, S.S.; Laessig, T.A.; Bouchard, R.J.; Florez-McClure, M.L.; Heidenreich, K.A. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J. Neurosci. 2004, 24, 9993–10002. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.M.; McCarley, R.W. Gamma band oscillations: A key to understanding schizophrenia symptoms and neural circuit abnormalities. Curr. Opin. Psychiatry 2016, 29, 202–210. [Google Scholar] [CrossRef]
- Tada, M.; Kirihara, K.; Koshiyama, D.; Nagai, T.; Fujiouka, M.; Usui, K.; Satomura, Y.; Koike, S.; Sawada, K.; Matsuoka, J.; et al. Alterations of auditory-evoked gamma oscillations are more pronounced than alterations of spontaneous power of gamma oscillation in early stages of schizophrenia. Transl. Psychiatry 2023, 13, 218. [Google Scholar] [CrossRef]
- Williams, S.; Boksa, P. Gamma oscillations and schizophrenia. J. Psychiatry Neurosci. 2010, 35, 75–77. [Google Scholar] [CrossRef]
- Veit, J.; Handy, G.; Mossing, D.P.; Doiron, B.; Adesnik, H. Cortical VIP neurons locally control the gain but globally control the coherence of gamma band rhythms. Neuron 2023, 111, 405–417.e5. [Google Scholar] [CrossRef] [PubMed]
- Antonoudiou, P.; Tan, Y.L.; Kontou, G.; Upton, A.L.; Mann, E.O. Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations. J. Neurosci. 2020, 40, 7668–7687. [Google Scholar] [CrossRef]
- Betterton, R.; Mellor, J.; Tsaneva-Atanasova, K. Modulation of hippocampal gamma oscillations by acetylcholine: Insights from mathematical and in vitro optogenetic models. BMC Neurosci. 2015, 16 (Suppl. S1), P267. [Google Scholar] [CrossRef]
- Çalışkan, G.; French, T.; Enrile Lacalle, S.; Del Angel, M.; Steffen, J.; Heimesaat, M.M.; Rita Dunay, I.; Stork, O. Antibiotic-induced gut dysbiosis leads to activation of microglia and impairment of cholinergic gamma oscillations in the hippocampus. Brain Behav. Immun. 2022, 99, 203–217. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Klaver, R.; De Vries, H.E.; Schenk, G.J.; Geurts, J.J. Grey matter damage in multiple sclerosis: A pathology perspective. Prion 2013, 7, 66–75. [Google Scholar] [CrossRef]
- Keo, A.; Dzyubachyk, O.; van der Grond, J.; Hafkemeijer, A.; van de Berg, W.D.J.; van Hilten, J.J.; Reinders, M.J.T.; Mahfouz, A. Cingulate networks associated with gray matter loss in Parkinson’s disease show high expression of cholinergic genes in the healthy brain. Eur. J. Neurosci. 2021, 53, 3727–3739. [Google Scholar] [CrossRef]
- Yohn, S.E.; Weiden, P.J.; Felder, C.C.; Stahl, S.M. Muscarinic acetylcholine receptors for psychotic disorders: Bench-side to clinic. Trends Pharmacol. Sci. 2022, 43, 1098–1112. [Google Scholar] [CrossRef]
- Sahu, P.P.; Tseng, P. Gamma sensory entrainment for cognitive improvement in neurodegenerative diseases: Opportunities and challenges ahead. Front. Integr. Neurosci. 2023, 17, 1146687. [Google Scholar] [CrossRef]
- Yan, L.; Li, H.; Qian, Y.; Zhang, J.; Cong, S.; Zhang, X.; Wu, L.; Wang, Y.; Wang, M.; Yu, T. Transcutaneous vagus nerve stimulation: A new strategy for Alzheimer’s disease intervention through the brain-gut-microbiota axis? Front. Aging Neurosci. 2024, 16, 1334887. [Google Scholar] [CrossRef]
- Karpiński, P.; Żebrowska-Różańska, P.; Kujawa, D.; Łaczmański, Ł.; Samochowiec, J.; Jabłoński, M.; Plichta, P.; Piotrowski, P.; Bielawski, T.; Misiak, B. Gut microbiota alterations in schizophrenia might be related to stress exposure: Findings from the machine learning analysis. Psychoneuroendocrinology 2023, 155, 106335. [Google Scholar] [CrossRef]
- Attademo, L.; Bernardini, F.; Garinella, R.; Compton, M.T. Environmental pollution and risk of psychotic disorders: A review of the science to date. Schizophr. Res. 2017, 181, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Breno SDiniz, B.S.; Seitz-Holland, J.; Sehgal, R.; Kasamoto, J.; Higgins-Chen, A.T.; Lenze, E. Geroscience-Centric Perspective for Geriatric Psychiatry: Integrating Aging Biology with Geriatric Mental Health Research. Geriatr. Psychiatry 2024, 32, 1–16. [Google Scholar]
- Seeman, M.V. Subjective Overview of Accelerated Aging in Schizophrenia. Int. J. Environ. Res. Public Health 2023, 20, 737. [Google Scholar] [CrossRef] [PubMed]
- Marin, I.; Serrano, M.; Pietrocola, F. Recent insights into the crosstalk between senescent cells and CD8 T lymphocytes. NPJ Aging 2023, 9, 8. [Google Scholar] [CrossRef]
- Harris, M.J.; Jeste, D.V.; Gleghorn, A.; Sewell, D.D. New-onset psychosis in HIV-infected patients. J. Clin. Psychiatry 1991, 52, 369–376. [Google Scholar]
- Kozato, N.; Mishra, M.; Firdosi, M. New-onset psychosis due to COVID-19. BMJ Case Rep. 2021, 14, e242538. [Google Scholar] [CrossRef] [PubMed]
- Luís, C.; Maduro, A.T.; Pereira, P.; Mendes, J.J.; Soares, R.; Ramalho, R. Nutritional senolytics and senomorphics: Implications to immune cells metabolism and aging—From theory to practice. Front. Nutr. 2022, 9, 958563. [Google Scholar] [CrossRef]
- An, S.; Cho, S.Y.; Kang, J.; Lee, S.; Kim, H.S.; Min, D.J.; Son, E.; Cho, K.H. Inhibition of 3-phosphoinositide-dependent protein kinase 1 (PDK1) can revert cellular senescence in human dermal fibroblasts. Proc. Natl. Acad. Sci. USA 2020, 117, 31535–31546. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.M.; Kardakaris, R.; Valentine, E.R.; Bar-Peled, L.; Chen, A.L.; Blewett, M.M.; McCormick, M.A.; Williamson, J.R.; Kennedy, B.; Cravatt, B.F.; et al. Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. eLife 2018, 7, e40314. [Google Scholar] [CrossRef]
- Deakin, B.; Suckling, J.; Dazzan, P.; Joyce, E.; Lawrie, S.M.; Upthegrove, R.; Husain, N.; Chaudhry, I.B.; Dunn, G.; Jones, P.B.; et al. Minocycline for Negative Symptoms of Schizophrenia and Possible Mechanistic Actions: The BeneMin RCT; NIHR Journals Library: Southampton, UK, 2019. [Google Scholar]
- Abir, M.H.; Mahamud, A.G.M.S.U.; Tonny, S.H.; Anu, M.S.; Hossain, K.H.S.; Protic, I.A.; Khan, M.S.U.; Baroi, A.; Moni, A.; Uddin, M.J. Pharmacological potentials of lycopene against aging and aging-related disorders: A review. Food Sci. Nutr. 2023, 11, 5701–5735. [Google Scholar] [CrossRef] [PubMed]
- Perrott, K.M.; Wiley, C.D.; Desprez, P.Y.; Campisi, J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience 2017, 39, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Elsallabi, O.; Patruno, A.; Pesce, M.; Cataldi, A.; Carradori, S.; Gallorini, M. Fisetin as a Senotherapeutic Agent: Biopharmaceutical Properties and Crosstalk between Cell Senescence and Neuroprotection. Molecules 2022, 27, 738. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Kumari, A.; Gulati, A.; Padwad, Y.; Sharma, R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 2019, 20, 171–189. [Google Scholar] [CrossRef]
- Li, W.; He, Y.; Zhang, R.; Zheng, G.; Zhou, D. The curcumin analog EF24 is a novel senolytic agent. Aging 2019, 11, 771–782. [Google Scholar] [CrossRef]
- Dang, Y.; An, Y.; He, J.; Huang, B.; Zhu, J.; Gao, M.; Zhang, S.; Wang, X.; Yang, B.; Xie, Z. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression. Aging Cell 2020, 19, e13060. [Google Scholar] [CrossRef]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef] [PubMed]
- von Kobbe, C. Targeting senescent cells: Approaches, opportunities, challenges. Aging 2019, 11, 12844–12861. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Shimizu, I.; Katsuumi, G.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Matsumoto, N.; Yoshida, Y.; Mikawa, R.; Katayama, A.; et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 2021, 1, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Poblocka, M.; Bassey, A.L.; Smith, V.M.; Falcicchio, M.; Manso, A.S.; Althubiti, M.; Sheng, X.; Kyle, A.; Barber, R.; Frigerio, M.; et al. Targeted clearance of senescent cells using an antibody-drug conjugate against a specific membrane marker. Sci. Rep. 2021, 11, 20358. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Ferreira de Mattos, G.; Ash, M.; Settineri, R.; Escribá, P.V. Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging. Membranes 2021, 11, 944. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3β. Oxid. Med. Cell. Longev. 2015, 2015, 481405. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Roh, M.S. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr. Drug Targets 2006, 7, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Zhang, L.; Wang, L. Kaempferol, a potential neuroprotective agent in neurodegenerative diseases: From chemistry to medicine. Biomed. Pharmacother. 2023, 165, 115215. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ash, M.E. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim. Biophys. Acta Biomembr. 2017, 1859 Pt B, 1704–1724. [Google Scholar] [CrossRef]
- Nadeem, A.; Meijler, M.M. Unraveling the Antibacterial and Iron Chelating Activity of N-Oxide Hydroxy-Phenazine natural Products and Synthetic Analogs against Staphylococcus aureus. Isr. J. Chem. 2023, 63, 5–6. [Google Scholar] [CrossRef]
- Heitmann, A.S.B.; Zanjani, A.A.H.; Klenow, M.B.; Mularski, A.; Sønder, S.L.; Lund, F.W.; Boye, T.L.; Dias, C.; Bendix, P.M.; Simonsen, A.C.; et al. Phenothiazines alter plasma membrane properties and sensitize cancer cells to injury by inhibiting annexin-mediated repair. J. Biol. Chem. 2021, 297, 101012. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.W.; Mishra, A.; Lai, G.H.; Davis, M.; Sanders, L.K.; Dat, T.; Garcia, A.; Tai, K.P.; McCray, J.; Paul, B.; et al. Criterion for amino acid composition of defensins and antimicrobial peptides based on geometry of membrane destabilization. J. Am. Chem. Soc. 2011, 133, 6720–6727. [Google Scholar] [CrossRef] [PubMed]
- Blankenfeldt, W.; Parsons, J.F. The structural biology of phenazine biosynthesis. Curr. Opin. Struct. Biol. 2014, 29, 26–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pierson, L.S., 3rd; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.A.; Kamer, A.M.A.; Al-Monofy, K.B.; Al-Madboly, L.A. Pseudomonas aeruginosa’s greenish-blue pigment pyocyanin: Its production and biological activities. Microb. Cell Fact. 2023, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Yohn, S.E.; Popiolek, M.; Miller, A.C.; Felder, C.C. Muscarinic Acetylcholine Receptor Agonists as Novel Treatments for Schizophrenia. Am. J. Psychiatry 2022, 179, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Abbas, M.; Zhang, Y.; Elshahawi, S.I.; Ponomareva, L.V.; Cui, Z.; Van Lanen, S.G.; Sajid, I.; Voss, S.R.; Shaaban, K.A.; et al. Divergent Fused Phenazine-Based Metabolites from a Himalayan Streptomyces. J. Nat. Prod. 2019, 82, 1686–1693. [Google Scholar] [CrossRef]
- Cha, J.W.; Lee, S.; Kim, M.C.; Thida, M.; Lee, J.W.; Park, J.S.; Kwon, H.C. Pontemazines a and B, phenazine derivatives containing a methylamine linkage from Streptomyces sp. UT1123 and their protective effect to HT-22 neuronal cells. Bioorganic Med. Chem. Lett. 2015, 25, 5083–5086. [Google Scholar] [CrossRef]
- Kim, W.G.; Ryoo, I.J.; Yun, B.S.; Shin-ya, K.; Seto, H.; Yoo, I.D. Phenazostatin C, a new diphenazine with neuronal cell protecting activity from Streptomyces sp. J. Antibiot. 1999, 52, 758–761. [Google Scholar] [CrossRef]
- Kato, S.; Shindo, K.; Yamagishi, Y.; Matsuoka, M.; Kawai, H.; Mochizuki, J. Phenazoviridin, a novel free radical scavenger from Streptomyces sp. taxonomy, fermentation, isolation, structure elucidation and biological properties. J. Antibiot. 1993, 46, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Boonnoy, P.; Jarerattanachat, V.; Karttunen, M.; Wong-ekkabut, J. Bilayer Deformation, Pores, and Micellation Induced by Oxidized Lipids. J. Phys. Chem. Lett. 2015, 6, 4884–4888. [Google Scholar] [CrossRef] [PubMed]
- Voronova, O.; Zhuravkov, S.; Korotkova, E.; Artamonov, A.; Plotnikov, E. Antioxidant Properties of New Phenothiazine Derivatives. Antioxidants 2022, 11, 1371. [Google Scholar] [CrossRef]
- Keynes, R.G.; Karchevskaya, A.; Riddall, D.; Griffiths, C.H.; Bellamy, T.C.; Chan, A.W.E.; Selwood, D.L.; Garthwaite, J. N10-carbonyl-substituted phenothiazines inhibiting lipid peroxidation and associated nitric oxide consumption powerfully protect brain tissue against oxidative stress. Chem. Biol. Drug Des. 2019, 94, 1680–1693. [Google Scholar] [CrossRef] [PubMed]
- Philot, E.A.; da Mata Lopes, D.; de Souza, A.T.; Braz, A.S.; Nantes, I.L.; Rodrigues, T.; Perahia, D.; Miteva, M.A.; Scott, L.P. Binding of phenothiazines into allosteric hydrophobic pocket of human thioredoxin 1. Eur. Biophys. J. 2016, 45, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.C.; Andreasen, N.C.; Ziebell, S.; Pierson, S.; Magnotta, V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 2011, 68, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Ayuk, E.L.; Igbojekwe, B.U.; Unaegbu, M. Potential Antioxidant Activity of New Tetracyclic and Pentacyclic Nonlinear Phenothiazine Derivatives. Biochem. Res. Int. 2016, 2016, 9896575. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Shay, J.W. Mitochondrial transformation of mammalian cells. Nature 1982, 295, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Katrangi, E.; D’Souza, G.; Boddapati, S.V.; Kulawiec, M.; Singh, K.K.; Bigger, B.; Weissig, V. Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function. Rejuvenation Res. 2007, 10, 561–570. [Google Scholar] [CrossRef]
- Pacak, A.C.; Preble, J.M.; Kondo, H.; Seibel, P.; Levitsky, S.; del Nido, P.J.; Cowan, D.B.; McCully, J.D. Actin-dependent mitochondrial internalization in cardiomyocytes: Evidence for rescue of mitochondrial function. Biol. Open 2015, 4, 622–626. [Google Scholar] [CrossRef]
- Sheng, Z.H. Mitochondrial trafficking and anchoring in neurons: New insight and implications. J. Cell Biol. 2014, 204, 1087–1098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali Pour, P.; Hosseinian, S.; Kheradvar, A. Mitochondrial transplantation in cardiomyocytes: Foundation, methods, and outcomes. Am. J. Physiol. Cell Physiol. 2021, 321, C489–C503. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, D.; Abe, J.; Takeda, A.; Harashima, H.; Yamada, Y. Transplantation of MITO cells, mitochondria activated cardiac progenitor cells, to the ischemic myocardium of mouse enhances the therapeutic effect. Sci. Rep. 2022, 12, 4344. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Majerníková, N.; Marmolejo-Garza, A.; Trombetta-Lima, M.; Sabogal-Guáqueta, A.M.; Zhang, Y.; Ten Kate, R.; Zuidema, M.; Mulder, P.P.M.F.A.; den Dunnen, W.; et al. Mitochondrial transplantation rescues neuronal cells from ferroptosis. Free Radic. Biol. Med. 2023, 208, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Um, J.-H.; Lee, K.-M.; Kim, Y.-Y.; Lee, D.-Y.; Kim, E.; Kim, D.-H.; Yun, J. Berberine Induces Mitophagy through Adenosine Monophosphate-Activated Protein Kinase and Ameliorates Mitochondrial Dysfunction in PINK1 Knockout Mouse Embryonic Fibroblasts. Int. J. Mol. Sci. 2024, 25, 219. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555, Erratum in Nature 2016, 539, 123. [Google Scholar] [CrossRef] [PubMed]
- Goya-Jorge, E.; Jorge Rodríguez, M.E.; Veitía, M.S.; Giner, R.M. Plant Occurring Flavonoids as Modulators of the Aryl Hydrocarbon Receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Linck, V.M.; Ganzella, M.; Herrmann, A.P.; Okunji, C.O.; Souza, D.O.; Antonelli, M.C.; Elisabetsky, E. Original mechanisms of antipsychotic action by the indole alkaloid alstonine (Picralima nitida). Phytomedicine 2015, 22, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Cuzzocrea, S.; Crupi, R. An Update of Palmitoylethanolamide and Luteolin Effects in Preclinical and Clinical Studies of Neuroinflammatory Events. Antioxidants 2020, 9, 216. [Google Scholar] [CrossRef]
- McGovern, K.; Castro, A.C.; Cavanaugh, J.; Coma, S.; Walsh, M.; Tchaicha, J.; Syed, S.; Natarajan, P.; Manfredi, M.; Zhang, X.M.; et al. Discovery and Characterization of a Novel Aryl Hydrocarbon Receptor Inhibitor, IK-175, and Its Inhibitory Activity on Tumor Immune Suppression. Mol. Cancer Ther. 2022, 21, 1261–1272. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, C.; Safe, S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003, 111, 1877–1882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avancini, D.; Testori, A.; Fresolone, L.; Andolfi, G.; Vuono, M.; Martinelli, V.; Santoni de Sio, F.R.; Gregori, S. Aryl hydrocarbon receptor activity downstream of IL-10 signaling is required to promote regulatory functions in human dendritic cells. Cell Rep. 2023, 42, 112193. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; MacDonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248.e1. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G. Clozapine and the aryl hydrocarbon receptor. J. Psychopharmacol. 2022, 36, 516. [Google Scholar] [CrossRef] [PubMed]
- Korac, K.; Rajasekaran, D.; Sniegowski, T.; Schniers, B.K.; Ibrahim, A.F.; Bhutia, Y.D. Carbidopa, an activator of aryl hydrocarbon receptor, suppresses IDO1 expression in pancreatic cancer and decreases tumor growth. Biochem. J. 2022, 479, 1807–1824. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.L.; Ganem, L.G.; Fernandez-Salguero, P.; Gonzalez, F.; Jefcoate, C.R. Aryl-hydrocarbon receptor is an inhibitory regulator of lipid synthesis and of commitment to adipogenesis. J. Cell Sci. 1998, 111 Pt 22, 3311–3322. [Google Scholar] [CrossRef]
- Matsunawa, M.; Amano, Y.; Endo, K.; Uno, S.; Sakaki, T.; Yamada, S.; Makishima, M. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol. Sci. 2009, 109, 50–58. [Google Scholar] [CrossRef]
- Manzella, C.R.; Ackerman, M.; Singhal, M.; Ticho, A.L.; Ceh, J.; Alrefai, W.A.; Saksena, S.; Dudeja, P.K.; Gill, R.K. Serotonin Modulates AhR Activation by Interfering with CYP1A1-Mediated Clearance of AhR Ligands. Cell. Physiol. Biochem. 2020, 54, 126–141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guillon, C.; Meziani, M.; Abdelli, S.; Sigaudo Roussel, D.; Bonod, C.; Nosbaum, A. The aryl hydrocarbon receptor pathway plays a central role in the cutaneous response to pollutants. Eur. J. Dermatol. 2022, 32, 305–311. (In English) [Google Scholar] [CrossRef] [PubMed]
- Grycová, A.; Joo, H.; Maier, V.; Illés, P.; Vyhlídalová, B.; Poulíková, K.; Sládeková, L.; Nádvorník, P.; Vrzal, R.; Zemánková, L.; et al. Targeting the Aryl Hydrocarbon Receptor with Microbial Metabolite Mimics Alleviates Experimental Colitis in Mice. J. Med. Chem. 2022, 65, 6859–6868. [Google Scholar] [CrossRef]
- Kang, S.; Lee, A.G.; Im, S.; Oh, S.J.; Yoon, H.J.; Park, J.H.; Pak, Y.K. A Novel Aryl Hydrocarbon Receptor Antagonist HBU651 Ameliorates Peripheral and Hypothalamic Inflammation in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2022, 23, 14871. [Google Scholar] [CrossRef] [PubMed]
- Zai, W.; Chen, W.; Liu, H.; Ju, D. Therapeutic Opportunities of IL-22 in Non-Alcoholic Fatty Liver Disease: From Molecular Mechanisms to Clinical Applications. Biomedicines 2021, 9, 1912. [Google Scholar] [CrossRef] [PubMed]
- Sfera, A. Six Decades of Dopamine Hypothesis: Is Aryl Hydrocarbon Receptor the New D2? Reports 2023, 6, 36. [Google Scholar] [CrossRef]
- Shi, C.; Su, C.; Cen, L.; Han, L.; Tang, J.; Wang, Z.; Shi, X.; Ju, D.; Cao, Y.; Zhu, H. Vunakizumab-IL22, a Novel Fusion Protein, Promotes Intestinal Epithelial Repair and Protects against Gut Injury Induced by the Influenza Virus. Biomedicines 2023, 11, 1160. [Google Scholar] [CrossRef]
- Kim, C.J.; Nazli, A.; Rojas, O.L.; Chege, D.; Alidina, Z.; Huibner, S.; Mujib, S.; Benko, E.; Kovacs, C.; Shin, L.Y.; et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012, 5, 670–680. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Odeh, F.; Al-Adaileh, F.; Al-Taher, S.; Jaber, A.M.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Lipid nanostructures for targeting brain cancer. Heliyon 2021, 7, e07994. [Google Scholar] [CrossRef]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lu, Z.R. Structure and Function of Cationic and Ionizable Lipids for Nucleic Acid Delivery. Pharm. Res. 2023, 40, 27–46. [Google Scholar] [CrossRef]
- Brannagan, T.H., 3rd; Berk, J.L.; Gillmore, J.D.; Maurer, M.S.; Waddington-Cruz, M.; Fontana, M.; Masri, A.; Obici, L.; Brambatti, M.; Baker, B.F.; et al. Liver-directed drugs for transthyretin-mediated amyloidosis. J. Peripher. Nerv. Syst. 2022, 27, 228–237. [Google Scholar] [CrossRef]




| Senolytics | Sources | Reference |
|---|---|---|
| Lycopene | Grape skin, guava, grapefruit, blueberries, and tomatoes | [166] |
| Apigenin | Cabbage, blueberries, and acai berries | [167] |
| Fisetin | Strawberries, onions, apples, mangoes, persimmons, and kiwis | [168] |
| Curcumin and EF24 analog | Chicken, beef, tofu, and vegetables | [169] |
| Epigallocatechin gallate | Apples, blackberries, broad beans, cherries, black grapes, pears, raspberries, and chocolate | [170] |
| Berberine | Oregon grape, phellodendron, and tree turmeric | [171] |
| Quercetin | Fruits, apples, onions, parsley, sage, tea, and red wine | [172] |
| Kaempferol | Fruits and vegetables | [173] |
| Compound | Naturally Occurring | Synthetic |
|---|---|---|
| Phenazines | Geranyl-phenazine and bara-phenazines A–G | Pontemazines A and B and halogenated phenazines |
| Phenothiazines | Propenyl-phenothiazine and N10-carbonyl-substituted phenothiazines | |
| GSK-3β inhibitors | Kaempferol and curcumin | Lithium, valproic acid, clozapine, and olanzapine |
| AhR inhibitors | Quercetin, apigenin, alstonine, and luteolin | IK-175 and HBU651 |
| Acid sphingomyelinase (ASM) inhibitors | Fluvoxamine, rosuvastatin, and tricyclic antidepressants | |
| Dopamine D1R agonists | A68930, A77636, and dihydrexidine | |
| Mitochondrial export | SSRIs | |
| ACh agonists | Catharanthus roseus and Salvia spp. (Lamiaceae) | Cholinesterase inhibitors: donepezil, galantamine, and rivastigmine |
| Senotherapeutics | Please see Table 1 | Senotherapeutic antibiotics |
| Ferroptosis inhibitors | Natural flavonoids and berberine | Fluvoxamine, SSRIs, and N acetyl cysteine (NAC) |
| Recombinant IL-22 | ||
| Membrane lipid replacement | ||
| Mitochondrial transplantation | ||
| 40 HZ entrainment with sensory stimuli | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfera, A.; Imran, H.; Sfera, D.O.; Anton, J.J.; Kozlakidis, Z.; Hazan, S. Novel Insights into Psychosis and Antipsychotic Interventions: From Managing Symptoms to Improving Outcomes. Int. J. Mol. Sci. 2024, 25, 5904. https://doi.org/10.3390/ijms25115904
Sfera A, Imran H, Sfera DO, Anton JJ, Kozlakidis Z, Hazan S. Novel Insights into Psychosis and Antipsychotic Interventions: From Managing Symptoms to Improving Outcomes. International Journal of Molecular Sciences. 2024; 25(11):5904. https://doi.org/10.3390/ijms25115904
Chicago/Turabian StyleSfera, Adonis, Hassan Imran, Dan O. Sfera, Jacob J. Anton, Zisis Kozlakidis, and Sabine Hazan. 2024. "Novel Insights into Psychosis and Antipsychotic Interventions: From Managing Symptoms to Improving Outcomes" International Journal of Molecular Sciences 25, no. 11: 5904. https://doi.org/10.3390/ijms25115904






