A Comprehensive Analytical Review of Polyphenols: Evaluating Neuroprotection in Alzheimer’s Disease
Abstract
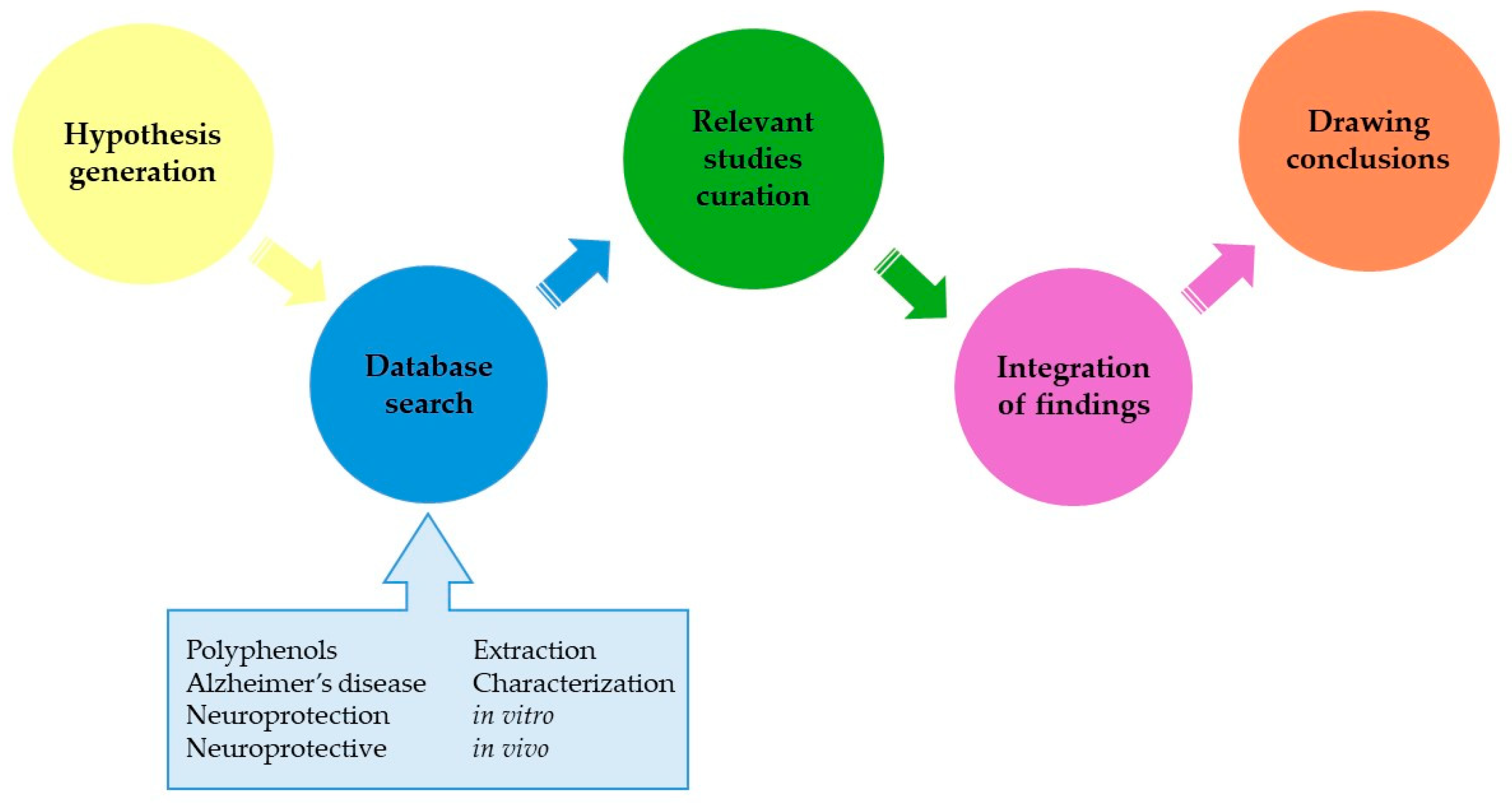
:1. Introduction
2. Polyphenols and Their Obtention
2.1. Polyphenols: Characteristics, Sources, and Applications
2.2. Extraction Methods for Recovering Polyphenols
2.2.1. Conventional Extraction Techniques
2.2.2. Non-Conventional Extraction Techniques
2.2.3. Purification of Phenolic Extracts
2.3. Analytical Methods for the Characterization of Phenolic Extracts
2.3.1. Spectrophotometric Methods for Quantification of Total Phenolics
2.3.2. Chromatographic Methods Used in Separation, Qualitative and Quantitative Polyphenol Analysis
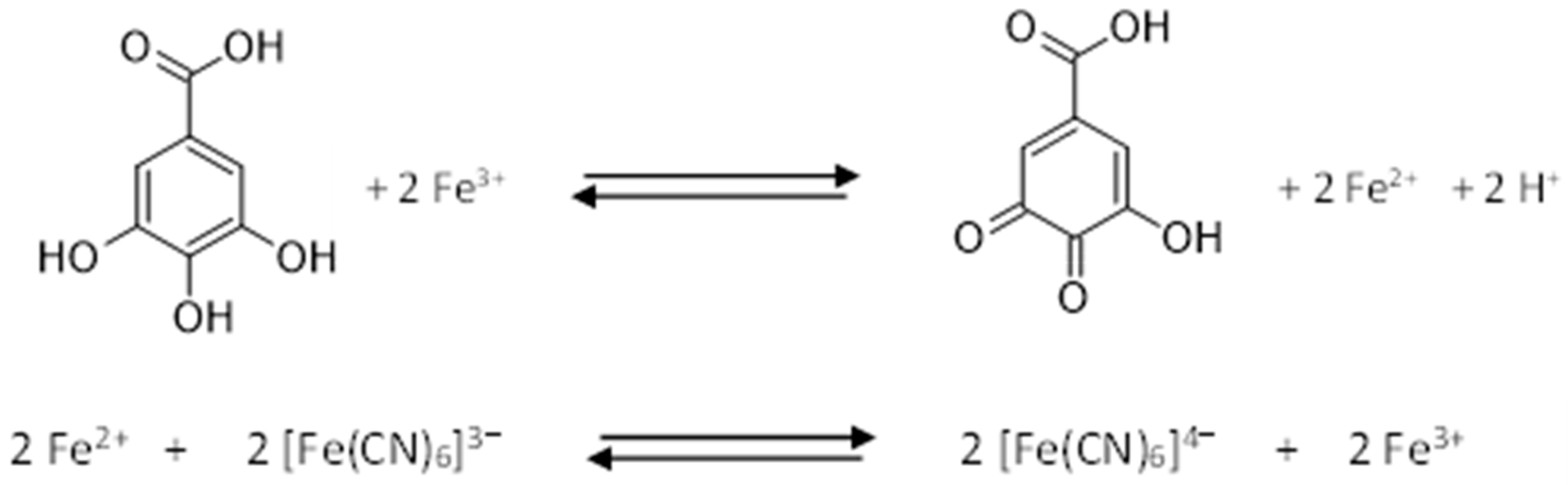
2.3.3. Other Analytical Characterization Methods
2.4. Application of Chemometrics in Polyphenol Characterization
2.4.1. ANOVA and Post-Hoc Methods
2.4.2. Correlation Analysis, Multivariate Linear Regression and Principal Component Analysis
2.4.3. Experimental Design
3. Polyphenols and Their In Vitro Impact on AD Treatment
3.1. Exploring Interactions between Polyphenols and Proteins Involved in AD
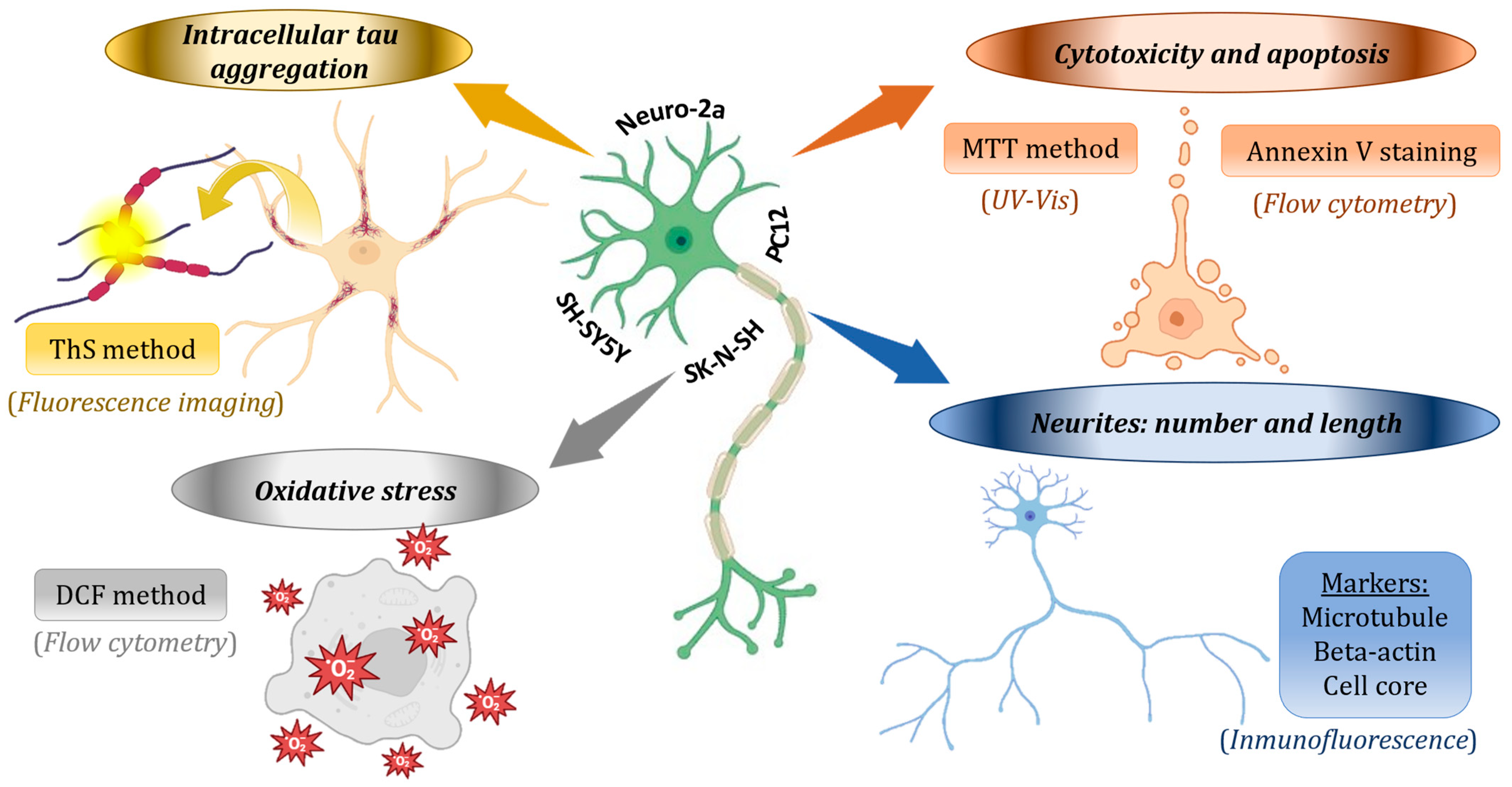
3.2. Neuroprotective Effects in Cell Lines: Mechanisms and Effectiveness
4. Polyphenols and Their Role in AD Treatment: In Vivo Studies
4.1. Overview of Key In Vivo Models in AD Research
4.2. Behavioral Assessments with Models of Alzheimer’s Disease
4.3. Histopathological Examinations: Polyphenols’ Effects in the Brain
4.4. Bioanalytical Assays: Polyphenols’ Influence in AD Models
5. Emerging Trends and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H. Dementia Epidemiology Fact Sheet 2022. Ann. Rehabil. Med. 2022, 46, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to Be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C.; Bifani, V. Natural Additives in Bioactive Edible Films and Coatings: Functionality and Applications in Foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Guuaadaoui, A.; Benaicha, S.; Elmajdoui, N.; Bellaoui, M.; Hamal, A. What Is a Bioactive Compound? A Combined Definition for a Preliminary Consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Coelho, M.S.; Salas-Mellado, M.D.L.M. Bioactive Compounds as Ingredients of Functional Foods: Polyphenols, Carotenoids, Peptides from Animal and Plant Sources New; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128147757. [Google Scholar]
- Scarano, A.; Laddomada, B.; Blando, F.; De Santis, S.; Verna, G.; Chieppa, M.; Santino, A. The Chelating Ability of Plant Polyphenols Can Affect Iron Homeostasis and Gut Microbiota. Antioxidants 2023, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.B.; de Aguiar, A.C.; Rostagno, M.A. Extraction of Natural Products Using Supercritical Fluids and Pressurized Liquids Assisted by Ultrasound: Current Status and Trends. Ultrason. Sonochem. 2021, 74, 105584. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Gomez-Zavaglia, A. Sustainable Food Systems in Fruits and Vegetables Food Supply Chains. Front. Nutr. 2022, 9, 829061. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and Characterization of Phenolic Compounds and Their Potential Antioxidant Activities. Environ. Sci. Pollut. Res. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.-F.; Jiang, C.-L.; Kong, Y.-S.; Luo, J.-L.; Yin, P.; Guo, G.-Y. Recent Advances in Analytical Methods for Determination of Polyphenols in Tea: A Comprehensive Review. Foods 2022, 11, 1425. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cui, K.; Li, X.; Zhao, J.; Zeng, Z.; Song, R.; Qi, X.; Xu, W. Effect of Polyphenols on Cognitive Function: Evidence from Population-Based Studies and Clinical Trials. J. Nutr. Health Aging 2021, 25, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, P.; Upadhyay, A. Flavonoid-Based Nanomedicines in Alzheimer’s Disease Therapeutics: Promises Made, a Long Way To Go. ACS Pharmacol. Transl. Sci. 2021, 4, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E. Unravelling the In Vitro and In Vivo Potential of Selenium Nanoparticles in Alzheimer’s Disease: A Bioanalytical Review. Talanta 2024, 269, 125519. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Moradi, S.Z.; Cao, H.; Khan, H.; Xiao, J. Effects of Polyphenols on Oxidative Stress, Inflammation, and Interconnected Pathways during Spinal Cord Injury. Oxid. Med. Cell. Longev. 2022, 2022, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, T.; Shi, M.; Wei, Y.; Huang, X.; Shen, J.; Zhang, X.; Xie, Z.; Huang, P.; Yuan, K.; et al. Polyphenols: Natural Food Grade Biomolecules for Treating Neurodegenerative Diseases from a Multi-Target Perspective. Front. Nutr. 2023, 10, 1139558. [Google Scholar] [CrossRef] [PubMed]
- Gentile, M.T.; Camerino, I.; Ciarmiello, L.; Woodrow, P.; Muscariello, L.; De Chiara, I.; Pacifico, S. Neuro-Nutraceutical Polyphenols: How Far Are We? Antioxidants 2023, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef] [PubMed]
- Kalogiouri, N.P.; Aalizadeh, R.; Dasenaki, M.E.; Thomaidis, N.S. Application of High Resolution Mass Spectrometric Methods Coupled with Chemometric Techniques in Olive Oil Authenticity Studies—A Review. Anal. Chim. Acta 2020, 1134, 150–173. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) for Multivariate Association between Bioactive Compounds and Functional Properties in Foods: A Critical Perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Chiriac, E.; Chiţescu, C.; Geană, E.-I.; Gird, C.; Socoteanu, R.; Boscencu, R. Advanced Analytical Approaches for the Analysis of Polyphenols in Plants Matrices—A Review. Separations 2021, 8, 65. [Google Scholar] [CrossRef]
- Feizi, N.; Hashemi-Nasab, F.S.; Golpelichi, F.; Saburouh, N.; Parastar, H. Recent Trends in Application of Chemometric Methods for GC-MS and GC×GC-MS-Based Metabolomic Studies. TrAC Trends Anal. Chem. 2021, 138, 116239. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of Polyphenols and Their Properties. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–44. [Google Scholar] [CrossRef]
- di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple Functions of Polyphenols in Plants Inhabiting Unfavorable Mediterranean Areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A Critical Review of Methods for Characterisation of Polyphenolic Compounds in Fruits and Vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and Vegetable Waste Management: Conventional and Emerging Approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, B.R.; Corrêa, R.C.G.; de Lima Sampaio, S.; Barros, L. Bioactive Compounds from Food and Its By-Products: Current Applications and Future Perspectives; Springer: Berlin/Heidelberg, Germany, 2023; pp. 3–41. [Google Scholar]
- Drevelegka, I.; Goula, A.M. Recovery of Grape Pomace Phenolic Compounds through Optimized Extraction and Adsorption Processes. Chem. Eng. Process.-Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic Compounds in Fruits—An Overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, J.; Xiao, A.; Liu, L. Antibacterial Activity of Polyphenols: Structure-Activity Relationship and Influence of Hyperglycemic Condition. Molecules 2017, 22, 1913. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Functional Foods: Concepts and Application to Inulin and Oligofructose. Br. J. Nutr. 2002, 87 (Suppl. S2), S139–S143. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Gu, X.; Wink, M.; Tencomnao, T. Vitis Vinifera Leaf Extract Protects Against Glutamate-Induced Oxidative Toxicity in HT22 Hippocampal Neuronal Cells and Increases Stress Resistance Properties in Caenorhabditis Elegans. Front. Nutr. 2021, 8, 634100. [Google Scholar] [CrossRef] [PubMed]
- Khokar, R.; Hachani, K.; Hasan, M.; Othmani, F.; Essam, M.; Al Mamari, A.; UM, D.; Khan, S.A. Anti-Alzheimer Potential of a Waste by-Product (Peel) of Omani Pomegranate Fruits: Quantification of Phenolic Compounds, in-Vitro Antioxidant, Anti-Cholinesterase and in-Silico Studies. Biocatal. Agric. Biotechnol. 2021, 38, 102223. [Google Scholar] [CrossRef]
- Arcone, R.; D’Errico, A.; Nasso, R.; Rullo, R.; Poli, A.; Di Donato, P.; Masullo, M. Inhibition of Enzymes Involved in Neurodegenerative Disorders and Aβ1–40 Aggregation by Citrus Limon Peel Polyphenol Extract. Molecules 2023, 28, 6332. [Google Scholar] [CrossRef] [PubMed]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and Leaves from Wild Blueberry Plants Contain Diverse Polyphenols and Decrease Neuroinflammatory Responses in Microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Dos Santos, L.C.; Mendiola, J.A.; Sánchez-camargo, A.D.P.; Álvarez-rivera, G.; Viganó, J.; Cifuentes, A.; Ibáñez, E.; Martínez, J. Selective Extraction of Piceatannol from Passiflora Edulis By-products: Application of Hsps Strategy and Inhibition of Neurodegenerative Enzymes. Int. J. Mol. Sci. 2021, 22, 6248. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Issa, M.Y.; Ebrahim, H.S.; Alaaeldin, R.; Elrehany, M.A.; Abd El-Kadder, E.M.; Abdelmohsen, U.R. Anti-Alzheimer Chemical Constituents of Morus Macroura Miq.: Chemical Profiling, In Silico and In Vitro Investigations. Food Funct. 2021, 12, 8078–8089. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Beigi, H.; Aliakbari, F.; Sahin, C.; Lomax, C.; Tawfike, A.; Schafer, N.P.; Amiri-Nowdijeh, A.; Eskandari, H.; Møller, I.M.; Hosseini-Mazinani, M.; et al. Oleuropein Derivatives from Olive Fruit Extracts Reduce α-Synuclein Fibrillation and Oligomer Toxicity. J. Biol. Chem. 2019, 294, 4215–4232. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Odubanjo, V.O.; Bello, F.; Ademosun, A.O.; Oyeleye, S.I.; Nwanna, E.E.; Ademiluyi, A.O. Aqueous Extracts of Avocado Pear (Persea americana Mill.) Leaves and Seeds Exhibit Anti-Cholinesterases and Antioxidant Activities In Vitro. J. Basic. Clin. Physiol. Pharmacol. 2016, 27, 131–140. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.G.; Pimenta, L.P.S.; Melo, J.O.F.; Mendonça, H.d.O.P.; Augusti, R.; Takahashi, J.A. Phytochemicals of Avocado Residues as Potential Acetylcholinesterase Inhibitors, Antioxidants, and Neuroprotective Agents. Molecules 2022, 27, 1892. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, N.M.; Shehata, M.G.; Alsulami, T.; Badr, A.N.; Elbakatoshy, M.R.; Ali, H.S.; El-Sohaimy, S.A. Characterization of Orange Peel Extract and Its Potential Protective Effect against Aluminum Chloride-Induced Alzheimer’s Disease. Pharmaceuticals 2023, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Balawejder, M.; Piechowiak, T.; Kapusta, I.; Chęciek, A.; Matłok, N. In Vitro Analysis of Selected Antioxidant and Biological Properties of the Extract from Large-Fruited Cranberry Fruits. Molecules 2023, 28, 7895. [Google Scholar] [CrossRef] [PubMed]
- Azib, L.; Debbache-Benaida, N.; Da Costa, G.; Atmani-Kilani, D.; Saidene, N.; Ayouni, K.; Richard, T.; Atmani, D. Pistacia lentiscus L. Leaves Extract and Its Major Phenolic Compounds Reverse Aluminium-Induced Neurotoxicity in Mice. Ind. Crops Prod. 2019, 137, 576–584. [Google Scholar] [CrossRef]
- Ciaramelli, C.; Palmioli, A.; Angotti, I.; Colombo, L.; De Luigi, A.; Sala, G.; Salmona, M.; Airoldi, C. NMR-Driven Identification of Cinnamon Bud and Bark Components with Anti-Aβ Activity. Front. Chem. 2022, 10, 896253. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.; Freschi, M.; Marrazzo, P.; Hrelia, S.; Beghelli, D.; Juan-García, A.; Juan, C.; Caprioli, G.; Sagratini, G.; Angeloni, C. Antioxidant and Anti-Inflammatory Profiles of Spent Coffee Ground Extracts for the Treatment of Neurodegeneration. Oxid. Med. Cell. Longev. 2021, 2021, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Valu, M.V.; Ducu, C.; Moga, S.; Negrea, D.; Hritcu, L.; Boiangiu, R.S.; Vamanu, E.; Balseanu, T.A.; Carradori, S.; Soare, L.C. Effects of the Hydroethanolic Extract of Lycopodium selago L. On Scopolamine-Induced Memory Deficits in Zebrafish. Pharmaceuticals 2021, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Aliaño-González, M.J.; Barea-Sepúlveda, M.; Espada-Bellido, E.; Ferreiro-González, M.; López-Castillo, J.G.; Palma, M.; Barbero, G.F.; Carrera, C. Ultrasound-Assisted Extraction of Total Phenolic Compounds and Antioxidant Activity in Mushrooms. Agronomy 2022, 12, 1812. [Google Scholar] [CrossRef]
- Guo, C.; Valdés, A.; Sánchez-Martínez, J.D.; Ibáñez, E.; Bi, J.; Cifuentes, A. Neuroprotective Potential of Thinned Peaches Extracts Obtained by Pressurized Liquid Extraction after Different Drying Processes. Foods 2022, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Montenegro, Z.J.; Ballesteros-Vivas, D.; Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. Neuroprotective Potential of Tamarillo (Cyphomandra betacea) Epicarp Extracts Obtained by Sustainable Extraction Process. Front. Nutr. 2021, 8, 769617. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Rodríguez, G.; Ramón Vidal, D.; Martorell, P.; Plaza, M.; Marina, M.L. Composition of Nonextractable Polyphenols from Sweet Cherry Pomace Determined by DART-Orbitrap-HRMS and Their In Vitro and In Vivo Potential Antioxidant, Antiaging, and Neuroprotective Activities. J. Agric. Food Chem. 2022, 70, 7993–8009. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mejía, E.; Vicente-Zurdo, D.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Screening the Extraction Process of Phenolic Compounds from Pressed Grape Seed Residue: Towards an Integrated and Sustainable Management of Viticultural Waste. LWT 2022, 169, 113988. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.Y.; Gan, R.Y.; Zheng, J.; Li, Y.; Zhang, J.J.; Xu, D.P.; Li, H. bin Optimization of Ultrasound-Assisted Extraction of Antioxidant Polyphenols from the Seed Coats of Red Sword Bean (Canavalia gladiate (Jacq.) DC.). Antioxidants 2019, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M. Olive Mill and Winery Wastes as Viable Sources of Bioactive Compounds: A Study on Polyphenols Recovery. Antioxidants 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Recovery of Added-Value Compounds from Orange and Spinach Processing Residues: Green Extraction of Phenolic Compounds and Evaluation of Antioxidant Activity. Antioxidants 2021, 10, 1800. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging Opportunities for the Effective Valorization of Wastes and By-Products Generated during Olive Oil Production Process: Non-Conventional Methods for the Recovery of High-Added Value Compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Žlabur, J.; Voća, S.; Brnčić, M.; Rimac-Brnčić, S. New Trends in Food Technology for Green Recovery of Bioactive Compounds From Plant Materials. In Role of Materials Science in Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 1–36. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. New Insights into the Application of MSPD in Various Fields of Analytical Chemistry. TrAC Trends Anal. Chem. 2019, 112, 29–51. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Mikkelsen, L.H.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. A Combined Approach Based on Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles and Liquid Chromatography to Determine Polyphenols from Grape Residues. J. Chromatogr. A 2021, 1644, 462128–462139. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Laganà, A.; Piovesana, S.; Samperi, R. Recent Trends in Matrix Solid-Phase Dispersion. TrAC Trends Anal. Chem. 2013, 43, 53–66. [Google Scholar] [CrossRef]
- Tomaz, I.; Huzanić, N.; Preiner, D.; Stupić, D.; Andabaka, Ž.; Maletić, E.; Kontić, J.K.; Ašperger, D. Extraction Methods of Polyphenol from Grapes: Extractions of Grape Polyphenols. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 151–167. [Google Scholar] [CrossRef]
- Di Donato, P.; Taurisano, V.; Tommonaro, G.; Pasquale, V.; Jiménez, J.M.S.; de Pascual-Teresa, S.; Poli, A.; Nicolaus, B. Biological Properties of Polyphenols Extracts from Agro Industry’s Wastes. Waste Biomass Valorization 2018, 9, 1567–1578. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Determination of Phenolic Compounds in Residual Brewing Yeast Using Matrix Solid-Phase Dispersion Extraction Assisted by Titanium Dioxide Nanoparticles. J. Chromatogr. A 2019, 1601, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Celeiro, M.; Rubio, L.; Bañobre, A.; Otero-Otero, M.; Garcia-Jares, C.; Lores, M. Optimization of Bioactives Extraction from Grape Marc via a Medium Scale Ambient Temperature System and Stability Study. Front. Nutr. 2022, 9, 1008457. [Google Scholar] [CrossRef] [PubMed]
- Lucci, P.; Saurina, J.; Núñez, O. Trends in LC-MS and LC-HRMS Analysis and Characterization of Polyphenols in Food. TrAC Trends Anal. Chem. 2017, 88, 1–24. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric Analysis of Phenolic Compounds in Grapes and Wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- Razem, M.; Ding, Y.; Morozova, K.; Mazzetto, F.; Scampicchio, M. Analysis of Phenolic Compounds in Food by Coulometric Array Detector: A Review. Sensors 2022, 22, 7498. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu Assay Revisited: Improvement of Its Specificity for Total Phenolic Content Determination. Anal. Methods 2013, 5, 5990. [Google Scholar] [CrossRef]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Gallina-Toschi, T.; Fernández-Gutiérrez, A. Analytical Determination of Polyphenols in Olive Oils. J. Sep. Sci. 2005, 28, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Kim, C.; Neilson, A.P.; Griffin, L.E.; Peck, G.M.; O’Keefe, S.F.; Stewart, A.C. Comparison of Common Analytical Methods for the Quantification of Total Polyphenols and Flavanols in Fruit Juices and Ciders. J. Food Sci. 2019, 84, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Székelyhidi, R.; Lakatos, E.; Kapcsándi, V.; Ajtony, Z. Analytical Procedures for Determination of Phenolics Active Herbal Ingredients in Fortified Functional Foods: An Overview. Eur. Food Res. Technol. 2022, 248, 329–344. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue Assays to Estimate The Total Phenolic Content of Juices and Teas Using 96-Well Microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef] [PubMed]
- Mammen, D.; Daniel, M. A Critical Evaluation on the Reliability of Two Aluminum Chloride Chelation Methods for Quantification of Flavonoids. Food Chem. 2012, 135, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Vijayalaxmi, S.; Jayalakshmi, S.K.; Sreeramulu, K. Polyphenols from Different Agricultural Residues: Extraction, Identification and Their Antioxidant Properties. J. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Extraction and Analysis of Phenolics in Food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021, 11, 8276. [Google Scholar] [CrossRef]
- Osman, M.; Mohd Hassan, N.; Khatib, A.; Tolos, S. Antioxidant Activities of Dialium indum L. Fruit and Gas Chromatography-Mass Spectrometry (GC-MS) of the Active Fractions. Antioxidants 2018, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Ifeanacho, M.O.; Ikewuchi, C.C.; Ikewuchi, J.C. Investigation of the Profile of Phenolic Compounds in the Leaves and Stems of Pandiaka Heudelotii Using Gas Chromatography Coupled with Flame Ionization Detector. Food Sci. Nutr. 2017, 5, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Motilva, M.-J.; Serra, A.; Macià, A. Analysis of Food Polyphenols by Ultra High-Performance Liquid Chromatography Coupled to Mass Spectrometry: An Overview. J. Chromatogr. A 2013, 1292, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Žuvela, P.; Skoczylas, M.; Jay Liu, J.; Ba̧czek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column Characterization and Selection Systems in Reversed-Phase High-Performance Liquid Chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Sentkowska, A. Chromatographic Analysis of Polyphenols. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 353–364. [Google Scholar]
- Azaroual, L.; Liazid, A.; Mansouri, F.E.; Brigui, J.; Ruíz-Rodriguez, A.; Barbero, G.F.; Palma, M. Optimization of the Microwave-Assisted Extraction of Simple Phenolic Compounds from Grape Skins and Seeds. Agronomy 2021, 11, 1527. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus Peels Waste as a Source of Value-Added Compounds: Extraction and Quantification of Bioactive Polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of Polyphenols Using Liquid Chromatography–Tandem Mass Spectrometry Technique (LC–MS/MS): A Review. Antioxidants 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass. Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Bhumireddy, S.R.; Giuberti, G.; Mandal, R.; Lucini, L.; Wishart, D.S. Edible Nuts Deliver Polyphenols and Their Transformation Products to the Large Intestine: An In Vitro Fermentation Model Combining Targeted/Untargeted Metabolomics. Food Res. Int. 2019, 116, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Arribas, A.S.; Martínez-Fernández, M.; Chicharro, M. The Role of Electroanalytical Techniques in Analysis of Polyphenols in Wine. TrAC Trends Anal. Chem. 2012, 34, 78–96. [Google Scholar] [CrossRef]
- Arráez-Román, D.; Fu, S.; Sawalha, S.M.S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC/CE-ESI-TOF-MS Methods for the Characterization of Polyphenols in Almond-Skin Extracts. Electrophoresis 2010, 31, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Mazumder, P.M. Cellular Investigations to Uncover Curative Potentials of Polyphenols—An In Vitro Study of Apple Cider Vinegar (ACV) and Chrysin against Alzheimer’s like Pathology via down-Regulation of AChE Activity. Indian J. Tradit. Knowl. 2021, 20, 320–328. [Google Scholar]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.E.; Cho, D.Y.; Kim, I.S.; Cho, E.A.; Ganesan, P.; Choi, D.K. Ameliorative Potential of Desalted Salicornia europaea L. Extract in Multifaceted Alzheimer’s-like Scopolamine-Induced Amnesic Mice Model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.Q.; Pan, R.; Tang, Y.; Zhou, X.G.; Wu, J.M.; Yu, L.; Law, B.Y.K.; Ai, W.; Yu, C.L.; Qin, D.L.; et al. Lychee Seed Polyphenol Inhibits Aβ-Induced Activation of NLRP3 Inflammasome via the LRP1/AMPK Mediated Autophagy Induction. Biomed. Pharmacother. 2020, 130, 110575. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Valero, M.S.; Moliner, C.; Weinkove, D.; López, V.; Gómez-Rincón, C. Jasonia glutinosa (L.) Dc., a Traditional Herbal Tea, Exerts Antioxidant and Neuroprotective Properties in Different In Vitro and In Vivo Systems. Biology 2021, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Moliner, C.; Barros, L.; Dias, M.I.; Reigada, I.; Ferreira, I.C.F.R.; López, V.; Langa, E.; Rincón, C.G. Viola Cornuta and Viola x Wittrockiana: Phenolic Compounds, Antioxidant and Neuroprotective Activities on Caenorhabditis Elegans. J. Food Drug Anal. 2019, 27, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.H.P.; Najimudin, N.; Watanabe, N.; Shamsuddin, S.; Azzam, G. P-Coumaric Acid Attenuates the Effects of Aβ42 In Vitro and in a Drosophila Alzheimer’s Disease Model. Behav. Brain Res. 2023, 452, 114568. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Bertolini, A.; Stefani, M.; Bucciantini, M. Evoo Polyphenols Relieve Synergistically Autophagy Dysregulation in a Cellular Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7225. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Vasarri, M.; Carnemolla, F.; Oriente, F.; Cabaro, S.; Stio, M.; Degl’Innocenti, D.; Stefani, M.; Bucciantini, M. EVOO Polyphenols Exert Anti-Inflammatory Effects on the Microglia Cell through TREM2 Signaling Pathway. Pharmaceuticals 2023, 16, 933. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Costanzo, P.; Masullo, M.; D’Errico, A.; Nasso, R.; Bonacci, S.; Mollace, V.; Oliverio, M.; Arcone, R. Hydroxytyrosol–Donepezil Hybrids Play a Protective Role in an In Vitro Induced Alzheimer’s Disease Model and in Neuronal Differentiated Human SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 13461. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bosco, F.; Guarnieri, L.; Nucera, S.; Ruga, S.; Oppedisano, F.; Tucci, L.; Muscoli, C.; Palma, E.; Giuffrè, A.M.; et al. Protective Role of an Extract Waste Product from Citrus Bergamia in an In Vitro Model of Neurodegeneration. Plants 2023, 12, 2126. [Google Scholar] [CrossRef] [PubMed]
- Taram, F.; Ignowski, E.; Duval, N.; Linseman, D.A. Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons. Molecules 2018, 23, 2956. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Lu, Y.; Wu, Q.; Yang, J.; Chen, J.; Zhong, S.; Eliezer, D.; Tan, Q.; Wu, C. Fisetin Inhibits Tau Aggregation by Interacting with the Protein and Preventing the Formation of β-Strands. Int. J. Biol. Macromol. 2021, 178, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Hole, K.L.; Staniaszek, L.E.; Menon Balan, G.; Mason, J.M.; Brown, J.T.; Williams, R.J. Oral (−)-Epicatechin Inhibits Progressive Tau Pathology in RTg4510 Mice Independent of Direct Actions at GSK3β. Front. Neurosci. 2021, 15, 697319. [Google Scholar] [CrossRef] [PubMed]
- Mahnashi, M.H.; Ashraf, M.; Alhasaniah, A.H.; Ullah, H.; Zeb, A.; Ghufran, M.; Fahad, S.; Ayaz, M.; Daglia, M. Polyphenol-Enriched Desmodium Elegans DC. Ameliorate Scopolamine-Induced Amnesia in Animal Model of Alzheimer’s Disease: In Vitro, In Vivo and In Silico Approaches. Biomed. Pharmacother. 2023, 165, 115144. [Google Scholar] [CrossRef]
- Reutzel, M.; Grewal, R.; Silaidos, C.; Zotzel, J.; Marx, S.; Tretzel, J.; Eckert, G.P. Effects of Long-Term Treatment with a Blend of Highly Purified Olive Secoiridoids on Cognition and Brain ATP Levels in Aged NMRI Mice. Oxid. Med. Cell Longev. 2018, 2018, 4070935. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.-H.; Lin, Y.-Y.; Wang, K.-C.; Chang, C.-L.; Chen, R.-Y.; Wu, C.-C.; Cheng, I.H. Curcuminoid Submicron Particle Ameliorates Cognitive Deficits and Decreases Amyloid Pathology in Alzheimer’s Disease Mouse Model. Oncotarget 2018, 9, 10681–10697. [Google Scholar] [CrossRef] [PubMed]
- Kenchappa, P.G.; Karthik, Y.; Vijendra, P.D.; Hallur, R.L.S.; Khandagale, A.S.; Pandurangan, A.K.; Jayanna, S.G.; Alshehri, M.A.; Alasmari, A.; Sayed, S.; et al. In Vitro Evaluation of the Neuroprotective Potential of Olea Dioica against Aβ Peptide-Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells. Front. Pharmacol. 2023, 14, 1139606. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS Profiling of Secondary Metabolites from Opuntia Ficus-Indica Cladode, Peel and Fruit Pulp Extracts and Their Antioxidant, Neuroprotective Effect in Rats with Aluminum Chloride Induced Neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Dostal, V.; Roberts, C.M.; Link, C.D. Genetic Mechanisms of Coffee Extract Protection in a Caenorhabditis Elegans Model of β-Amyloid Peptide Toxicity. Genetics 2010, 186, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Palmioli, A.; Mazzoni, V.; De Luigi, A.; Bruzzone, C.; Sala, G.; Colombo, L.; Bazzini, C.; Zoia, C.P.; Inserra, M.; Salmona, M.; et al. Alzheimer’s Disease Prevention through Natural Compounds: Cell-Free, In Vitro, and In Vivo Dissection of Hop (Humulus lupulus L.) Multitarget Activity. ACS Chem. Neurosci. 2022, 13, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
- Iwata, K.; Wu, Q.; Ferdousi, F.; Sasaki, K.; Tominaga, K.; Uchida, H.; Arai, Y.; Szele, F.G.; Isoda, H. Sugarcane (Saccharum officinarum L.) Top Extract Ameliorates Cognitive Decline in Senescence Model SAMP8 Mice: Modulation of Neural Development and Energy Metabolism. Front. Cell Dev. Biol. 2020, 8, 573487. [Google Scholar] [CrossRef] [PubMed]
- Eggers, C.; Fujitani, M.; Kato, R.; Smid, S. Novel Cannabis Flavonoid, Cannflavin A Displays Both a Hormetic and Neuroprotective Profile against Amyloid β-Mediated Neurotoxicity in PC12 Cells: Comparison with Geranylated Flavonoids, Mimulone and Diplacone. Biochem. Pharmacol. 2019, 169, 113609. [Google Scholar] [CrossRef] [PubMed]
- Chethana, K.; Sasidhar, B.; Naika, M.; Keri, R. Phytochemical Composition of Caesalpinia Crista Extract as Potential Source for Inhibiting Cholinesterase and β-Amyloid Aggregation: Significance to Alzheimer’s Disease. Asian Pac. J. Trop. Biomed. 2018, 8, 500–512. [Google Scholar] [CrossRef]
- Kundo, N.K.; Manik, M.I.N.; Biswas, K.; Khatun, R.; Al-Amin, M.Y.; Alam, A.H.M.K.; Tanaka, T.; Sadik, G. Identification of Polyphenolics from Loranthus Globosus as Potential Inhibitors of Cholinesterase and Oxidative Stress for Alzheimer’s Disease Treatment. Biomed. Res. Int. 2021, 2021, 9154406. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and Characterization of Anthocyanins and Non-Anthocyanin Phenolics from Australian Native Fruits and Their Antioxidant, Antidiabetic, and Anti-Alzheimer Potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Arribas, L.V.; León-González, M.E.; Rosales-Conrado, N. Learning Principal Component Analysis by Using Data from Air Quality Networks. J. Chem. Educ. 2017, 94, 458–464. [Google Scholar] [CrossRef]
- Sereia, A.L.; de Oliveira, M.T.; Baranoski, A.; Medeiros Marques, L.L.; Ribeiro, F.M.; Isolani, R.G.; de Medeiros, D.C.; Chierrito, D.; Lazarin-Bidóia, D.; Ferreira Zielinski, A.A.; et al. In Vitro Evaluation of the Protective Effects of Plant Extracts against Amyloid-Beta Peptide-Induced Toxicity in Human Neuroblastoma SH-SY5Y Cells. PLoS ONE 2019, 14, e0212089. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Tyagi, A.; Ham, H.J.; Elahi, F.; Oh, D.H. Effect of Fermentation on the Bioactive Compounds of the Black Soybean and Their Anti-Alzheimer’s Activity. Front. Nutr. 2022, 9, 880361. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Tyagi, A.; Ham, H.J.; Oh, D.H. Comprehensive Profiling of Bioactive Compounds in Germinated Black Soybeans via UHPLC-ESI-QTOF-MS/MS and Their Anti-Alzheimer’s Activity. PLoS ONE 2022, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Zhang, H.; Zhang, Z.; Zhang, H.; Zhou, L.; Chen, T.; Feng, S.; Ding, C.; Yuan, M. The Extraction, Antioxidant and against β-Amyloid Induced Toxicity of Polyphenols from Alsophila Spinulosa Leaves. Arab. J. Chem. 2022, 15, 103707. [Google Scholar] [CrossRef]
- Francenia Santos-Sánchez, N.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Mueed, A.; Shibli, S.; Al-Quwaie, D.A.; Ashkan, M.F.; Alharbi, M.; Alanazi, H.; Binothman, N.; Aljadani, M.; Majrashi, K.A.; Huwaikem, M.; et al. Extraction, Characterization of Polyphenols from Certain Medicinal Plants and Evaluation of Their Antioxidant, Antitumor, Antidiabetic, Antimicrobial Properties, and Potential Use in Human Nutrition. Front. Nutr. 2023, 10, 1125106. [Google Scholar] [CrossRef] [PubMed]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Towards Transparent Valorization of Food Surplus, Waste and Loss: Clarifying Definitions, Food Waste Hierarchy, and Role in the Circular Economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef] [PubMed]
- Crespo, L.; Sede Lucena, B.; Martínez, F.G.; Mozzi, F.; Pescuma, M. Selenium Bioactive Compounds Produced by Beneficial Microbes. Adv. Appl. Microbiol. 2024, 126, 63–92. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.R.; Chowdhury, N.M.; Yasmin, H.; Kabir, M.T.; Akter, R.; Perveen, A.; Ashraf, G.M.; Akter, S.; Rahman, M.H.; Sweilam, S.H. Unveiling the Potential of Polyphenols as Anti-Amyloid Molecules in Alzheimer’s Disease. Curr. Neuropharmacol. 2023, 21, 787–807. [Google Scholar] [CrossRef] [PubMed]
- El Gaamouch, F.; Chen, F.; Ho, L.; Lin, H.-Y.; Yuan, C.; Wong, J.; Wang, J. Benefits of Dietary Polyphenols in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 1019942. [Google Scholar] [CrossRef] [PubMed]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase Targeting by Polyphenols: A Therapeutic Approach for the Treatment of Alzheimer’s Disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Nasso, R.; Pagliara, V.; D’Angelo, S.; Rullo, R.; Masullo, M.; Arcone, R. Annurca Apple Polyphenol Extract Affects Acetyl- Cholinesterase and Mono-Amine Oxidase In Vitro Enzyme Activity. Pharmaceuticals 2021, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Zendehcheshm, S.; Khademi, F. Green Synthesis, In Vitro Cytotoxicity, Antioxidant Activity and Interaction Studies of CuO Nanoparticles with DNA, Serum Albumin, Hemoglobin and Lysozyme. ChemistrySelect 2022, 7, e202202916. [Google Scholar] [CrossRef]
- Al-Radadi, N.S. Single-Step Green Synthesis of Gold Conjugated Polyphenol Nanoparticle Using Extracts of Saudi’s Myrrh: Their Characterization, Molecular Docking and Essential Biological Applications. Saudi Pharm. J. 2022, 30, 1215–1242. [Google Scholar] [CrossRef] [PubMed]
- Thatyana, M.; Dube, N.P.; Kemboi, D.; Manicum, A.-L.E.; Mokgalaka-Fleischmann, N.S.; Tembu, J.V. Advances in Phytonanotechnology: A Plant-Mediated Green Synthesis of Metal Nanoparticles Using Phyllanthus Plant Extracts and Their Antimicrobial and Anticancer Applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In Vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Sheeler, C.; Rosa, J.-G.; Ferro, A.; McAdams, B.; Borgenheimer, E.; Cvetanovic, M. Glia in Neurodegeneration: The Housekeeper, the Defender and the Perpetrator. Int. J. Mol. Sci. 2020, 21, 9188. [Google Scholar] [CrossRef] [PubMed]
- Jávega, B.; Herrera, G.; Martínez-Romero, A.; O’Connor, J.-E. Flow Cytometry of Oxygen and Oxygen-Related Cellular Stress. Oxygen 2023, 3, 222–255. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef] [PubMed]
- Al Mamun, A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Polyphenols Targeting NF-ΚB Pathway in Neurological Disorders: What We Know So Far? Int. J. Biol. Sci. 2024, 20, 1332–1355. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Berbel, I.; Espargaró, A.; Viayna, A.; Caballero, A.B.; Busquets, M.A.; Gámez, P.; Luque, F.J.; Sabaté, R. Three to Tango: Inhibitory Effect of Quercetin and Apigenin on Acetylcholinesterase, Amyloid-β Aggregation and Acetylcholinesterase-Amyloid Interaction. Pharmaceutics 2022, 14, 2342. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.; Cortés, N.; Pastor, G.; Gonzalez, A.; Ramos-Escobar, N.; Pastene, E.; Rojo, L.E.; MacCioni, R.B. N-Acetyl Cysteine and Catechin-Derived Polyphenols: A Path Toward Multi-Target Compounds against Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 75, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Kang, S.S.; Lee, S.K.; Han, B.H. Polyphenolic Biflavonoids Inhibit Amyloid-Beta Fibrillation and Disaggregate Preformed Amyloid-Beta Fibrils. Biomol. Ther. 2020, 28, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ayyalasomayajula, N.; Ajumeera, R.; Chellu, C.S.; Challa, S. Mitigative Effects of Epigallocatechin Gallate in Terms of Diminishing Apoptosis and Oxidative Stress Generated by the Combination of Lead and Amyloid Peptides in Human Neuronal Cells. J. Biochem. Mol. Toxicol. 2019, 33, e22393. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Abreu, G.E.; Martínez-Díaz, J.A.; Hernández-Aguilar, M.E.; Rojas-Durán, F.; Herrera-Covarrubias, D.; García-Hernández, L.I.; Mestizo-Gutiérrez, S.L. Expression of Proteins Linked to Alzheimer’s Disease in C6 Rat Glioma Cells under the Action of Lipopolysaccharide (LPS), Nimesulide, Resveratrol and Citalopram. Turk. J. Biochem. 2020, 45, 793–801. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Pomegranate Polyphenol Punicalagin Improves Learning Memory Deficits, Redox Homeostasis, and Neuroinflammation in Aging Mice. Phytother. Res. 2023, 37, 3655–3674. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Hu, X.; Lu, Y.; Shi, S.; Yang, D.; Yao, T. New Strategy for Reducing Tau Aggregation Cytologically by A Hairpinlike Molecular Inhibitor, Tannic Acid Encapsulated in Liposome. ACS Chem. Neurosci. 2020, 11, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; D’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.M.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of a Polyphenolic Extract from Arabidopsis Thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K.; et al. Rosmarinic Acid Suppresses Alzheimer’s Disease Development by Reducing Amyloid β Aggregation by Increasing Monoamine Secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cui, Y.; Liang, H.; Li, Z.; Wang, N.; Wang, Y.; Zheng, G. Multifunctional Selenium Nanoparticles with Different Surface Modifications Ameliorate Neuroinflammation through the Gut Microbiota-NLRP3 Inflammasome-Brain Axis in APP/PS1 Mice. ACS Appl. Mater. Interfaces 2022, 14, 30557–30570. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, S.; La Vitola, P.; Pagano, K.; Brandi, E.; Santamaria, G.; Galante, D.; D’Arrigo, C.; Moni, L.; Lambruschini, C.; Banfi, L.; et al. Biophysical and In Vivo Studies Identify a New Natural-Based Polyphenol, Counteracting Aβ Oligomerization In Vitro and Aβ Oligomer-Mediated Memory Impairment and Neuroinflammation in an Acute Mouse Model of Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 4462–4475. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, C.M.; Li, H.J.; Zhang, Z.P.; Cui, W.B.; An, F.L.; Zhang, Z.X.; Wang, D.S.; Fei, D.Q. Ethyl Caffeate Attenuates Aβ-Induced Toxicity in Caenorhabditis Elegans AD Models via the Insulin/Insulin-like Growth Factor-1 Signaling Pathway. Bioorg Chem. 2023, 139, 106714. [Google Scholar] [CrossRef] [PubMed]
- Prüßing, K.; Voigt, A.; Schulz, J.B. Drosophila Melanogaster as a Model Organism for Alzheimer’s Disease. Mol. Neurodegener. 2013, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Uversky, V.N.; Chinnathambi, S. Baicalein Inhibits Heparin-Induced Tau Aggregation by Initializing Non-Toxic Tau Oligomer Formation. Cell Commun. Signal. 2021, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, D.; Lee, L.; Palmeri, A.; Calabrese, G.; Arancio, O. Behavioral Assays with Mouse Models of Alzheimer’s Disease: Practical Considerations and Guidelines. Biochem. Pharmacol. 2014, 88, 450–467. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.; Rammes, G.; Blobner, M.; Kellermann, K.; Bratke, S.; Fendl, D.; Kaichuan, Z.; Schneider, G.; Jungwirth, B. Cognitive Decline in Tg2576 Mice Shows Sex-Specific Differences and Correlates with Cerebral Amyloid-Beta. Behav. Brain Res. 2019, 359, 408–417. [Google Scholar] [CrossRef] [PubMed]
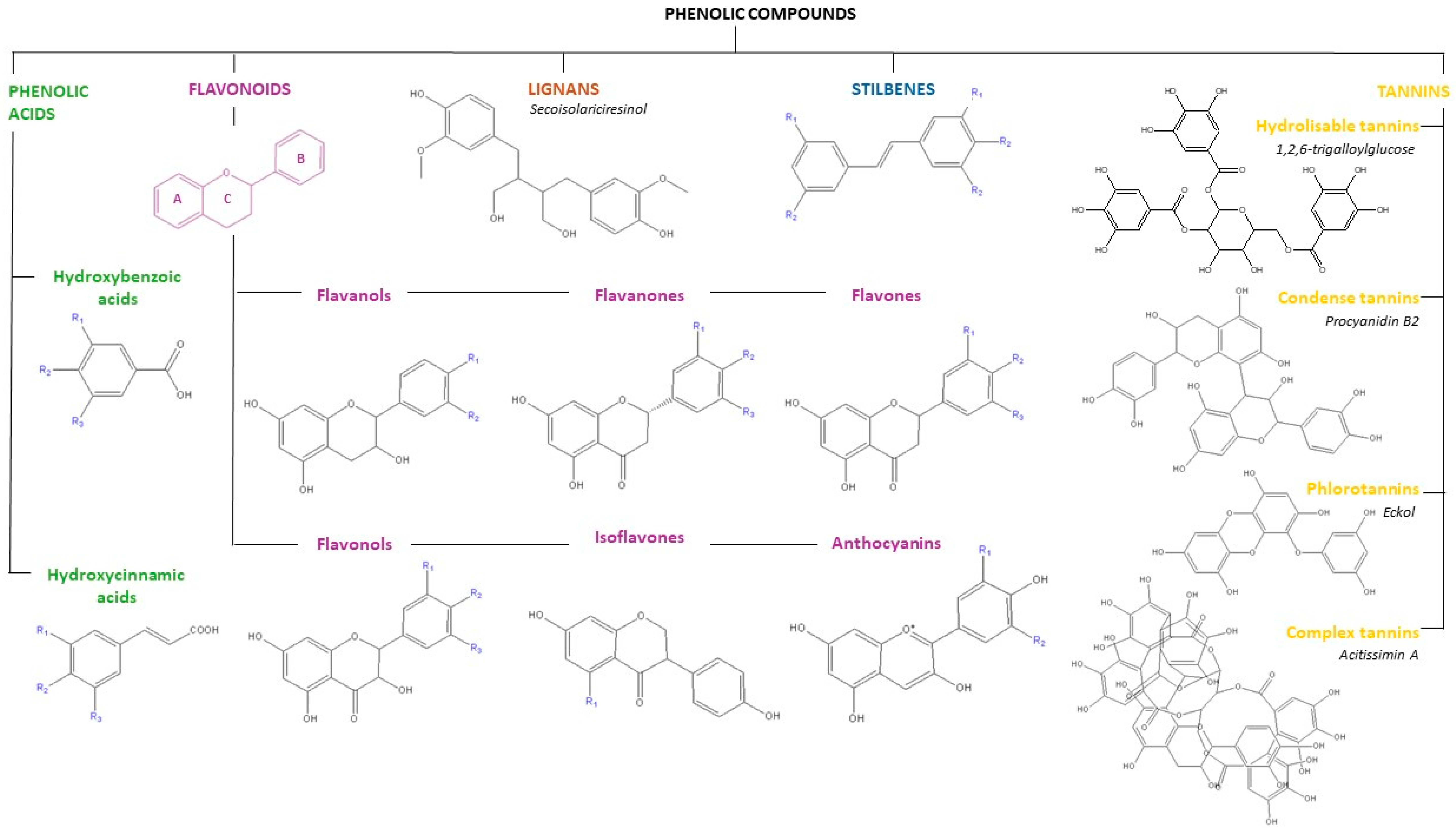


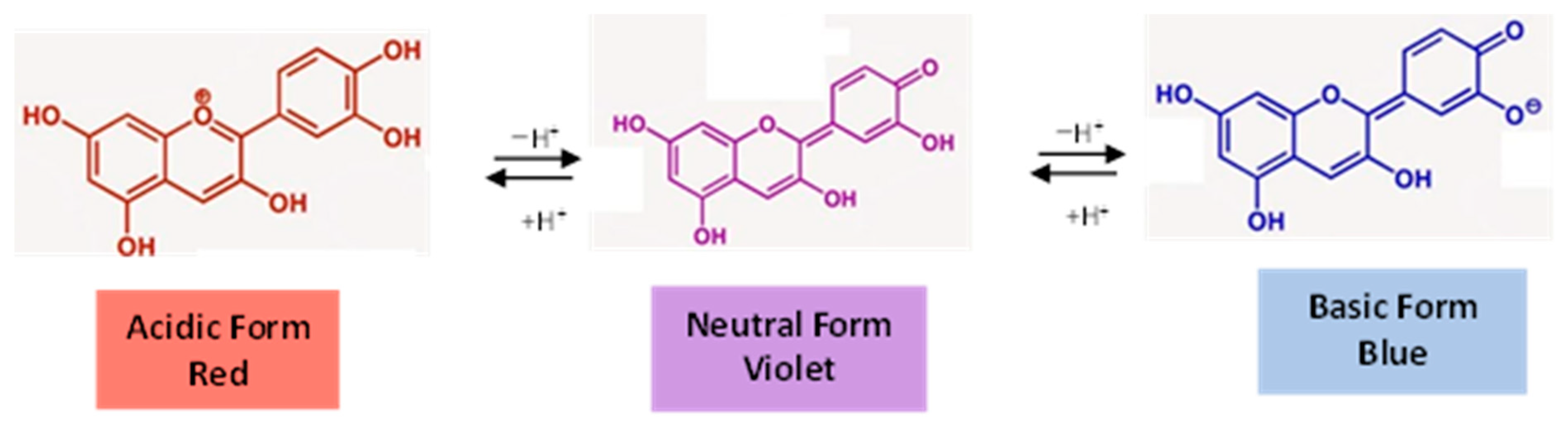

| Phenolic Family | Dietary Sources | Agri-Food Residue or Non-Edible Sources |
|---|---|---|
| Phenolic acid | ||
| Hydroxycinnamic acid | Almonds, cereals, cherries, citrus juices and fruits, coffee, corn flour, peaches, plums, potato, rice flour, spinach, tomatoes, and wheat flour. | Apple pomace, artichoke wastewaters, banana peel, citrus peels, olive mill wastewaters, and spent coffee grounds. |
| Hydroxybenzoic acid | Black currant, blackberry, cereals, coffee, cowpea, oilseeds, raspberry, and wheat flour. | Citrus peels, grape pomace, residual brewing yeast, squash shells and seeds. |
| Flavonoids | ||
| Flavonols | Apples, apricot, arugula, beans, capers, cloves, leeks, lettuce, onions, saffron, and tomatoes. | Apples peels, banana peels, grape pomace and seeds, guava peels and seeds, onion peels, olive leaves and pomace. |
| Flavones | Artichoke, black olive, celery, citrus fruits, oregano, peanut, parsley, pepper, spinach. | Artichokes steams, camu-camu peels and peanut skin and shell |
| Isoflavones | Red clover, soybeans, soymilk and soy flour. | Soy processing waste and peanut skins and shells. |
| Flavanols | Grapes, apples, tomatoes, leeks, lettuces, curly kale, berries, onions, red grapes, beans, green and black, cider, tea, red wine. | Appel peel, grape seeds, peels and pomace and tea by-products. |
| Anthocyanins | Eggplant, grape, plums, pomegranate, raspberries, red and back-currants, red cabbage, red wine, strawberries, and radish. | Grape skins and seeds, grape pomace and floral tepals (saffron). |
| Flavanones | Citrus juices, citrus fruits, peppermint, fennel and rosemary. | Banana peels, citrus seeds, peels and pomace and residual brewing yeast. |
| Stilbenes | Almonds, grapes, red wine and berries. | Avocado peels, grape skins, seeds, pomace and stems. |
| Lignans | Broccoli, flaxseed, kale, lentil, sesame seeds, tea, wine and wheat. | Coffee, soybeans and wine residues. |
| Tannins | ||
| Hydrolizable tannins | Pomegranates, raspberries and tea | Pomegranate peels and seeds and tea by-products. |
| Condense tannins | Apples, chestnut, grapes, pears, peaches, and hazelnuts. | Grape seeds |
| Phlorotannins | - | Brown seaweed |
| Complex tannins | - | Cork by-products (e.g., tree bark) |
| Extraction Technique | Source (Amount) | Sample Treatment | Extraction Solvent (Volume) | Extraction Conditions | Major Polyphenols (Amount) | Ref. |
|---|---|---|---|---|---|---|
| Soxhlet | Grape leaves (Vitis vinifera) (40 g) | Air-dried and ground | Ethanol (not specified) | Extraction at 78–80 °C for 36 h, followed by filtration and solvent evaporation | Catechin and quercetin (55–197, mg·100 g−1) | [50] |
| Pomegranate peels (Punica granatum L.) (not specified) | Air-dried and ground | Chloroform Ethyl acetate Butanol (not specified) | Extraction for 6 h at temperature above the boiling point, followed solvent evaporation | Total polyphenol (6–9 mg CAE·g−1) | [51] | |
| Solid-liquid extraction | Lemon peel (Citrus limon) (1.0 g) | Freeze-dried and ground | Ethanol-Water 80:20 (v/v) (25 mL) | Stirring for 2 h min. Then the extract was centrifuged, filtered and the solvent was evaporated | Not specified | [52] |
| Wild blueberry leaves (Vaccinium carymbosum) (1.0 g) | Dried with liquid nitrogen and ground | Acetone-Water 80:20 (v/v) acidified with 0.2% (v/v) formic acid (5 mL) | Vortex stirring and shaken on ice for 30 min, followed by centrifugation | Delphinidin and cyanidin glucosides (22–117 mg·100 g−1) | [53] | |
| Passion fruit seeds (Passiflora edulis) (5.0 g) | Oven-dried at 45 °C, ground and defatted | Ethanol-Water 79:21 (v/v) (30 mL) | Mechanical stirring for 80 min at 85 °C and 250 rpm, followed by centrifugation | Piceatannol (23.4 mg·g−1) | [54] | |
| Mulberry leaves (Morus macrour) (1.0 kg) | Dried | Ethanol-Water 80:20 (v/v) (500 mL) | Extraction was performed three consecutive times, then the solvent was evaporated | Resveratrol, chrysin, moracin D and ferulic acid (not specified) | [55] | |
| Olive mesocarp (Olea europaea) (3.0 g) | Freeze-dried and ground | Methanol (12 mL) | Vortex stirring for 1 min at 20 °C. Then the extract was centrifuged and lyophilized | Oleuropein and derivatives (not specified) | [56] | |
| Maceration | Avocado leaves and seeds (Persea americana Mill.) (1.0 g) | Cut and ground | Water (100 mL) | Soaking the sample for 24 h, final filtration and centrifugation | Total polyphenol (92 mg GAE·g−1) | [57] |
| Avocado peels and seeds (Persea americana Mill.) (50.0 and 100 g) | Dried, cut and ground | 1st Hexane 2nd Ethanol (100 mL) | Fractionated extraction. The extractions were performed three times for 24 h. The solvent was removed in a rotary evaporator (60 °C) | Caffeic acid, (epi)catechin, rutin, B- type procyanidins (not specified) | [58] | |
| Orange peel (Citrus sinensis) (20 mg) | Oven-dried at 50 °C and ground | Water (200 mL) | Soaking the sample for 72 h at 37 °C, further filtration, solvent evaporation and freeze-dried of the extract | Gallic acid, quercetin, naringenin, propyl gallate and rutin (3.0–1.2, mg·100 g−1) | [59] | |
| Cranberry (Vaccinium oxycoccos) (500 g) | Air-dried and ground | Ethanol (2.5 L) | Soaking the sample for 48 h, followed by filtration and solvent evaporation | Cyanidin-, peonidin-, myricetin- and quercetin 3-O-glucoside (96–227, mg·100 g−1) | [60] | |
| Mastic leaves (Pistacia lentiscus L.) (150 g) | Air-dried and ground | Ethanol (600 mL) | Agitation for 24 h, followed by decantation, centrifugation, and solvent evaporation | Total polyphenol (95 g GAE·kg−1) | [61] | |
| UAE | Cinnamon barks and buds (Cinnamomum zeylanicum and Cinnamomum cassia) (2.0 g) | Ground | Ethanol (20 mL) | Sonication in ultrasonic bath at 37 kHz, 45 °C for 60 min, followed by centrifugation, filtration, evaporation of the solvent and lyophilization | B-type procyanidins (not quantified) | [62] |
| Spent coffee grounds (Coffea arabica) (10 g) | Oven-dried at 50 °C and ground | Methanol Water Methanol-Water 50:50 (v/v) Ethanol-Water 70:30 (v/v) (50 mL) | Sonication in ultrasonic bath at 40 kHz, 20 °C for 120 min, followed by filtration and lyophilization | 5-caffeoylquinic acid and 3,5-dicaffeoylquinic acid (52–0.9 mg·g−1) | [63] | |
| Moss (Lycopodium selago) (1.0 g) | Dried and crushed | Ethanol-Water 70:30 (v/v) (30 mL) | Ultrasonic probe sonication at 20 kHz and 70% amplitude, followed by centrifugation and lyophilization | Total polyphenol (9.21 mg GAE·g−1) | [64] | |
| Mushrooms (0.2 g) | Freeze-dried and crushed | Methanol-Water 93.6:6.4 (v/v) (15.3 mL) | Ultrasonic probe sonication at 20 Hz, 70 W, 16.86% amplitude, 0.71 s−1 cycles and 60 °C for 5 min, followed by centrifugation | Total polyphenol (10–13, mg GAE·g−1) | [65] | |
| PLE | Passion fruit seeds (Passiflora edulis) (1.0 g) | Oven-dried at 45 °C, ground and defatted | Ethanol (25 mL) | Sample mixing with 1.0 g of sea sand and extraction at 110 °C and 1500 psi for 20 min, with 3 cycles | Piceatannol (56.5 mg·g−1) | [54] |
| Peach (Prunus persica) (1.0 g) | Freeze-dried at 45 °C and ground | Ethanol-Water 50:50 (v/v) (no specified) | Sample mixing with 1.0 g of sea sand and extraction at 180 °C and 10 MPa for 5 min. The solvent was evaporated, and the extract was then freeze-dried | Total polyphenol (100 mg GAE·g−1) | [66] | |
| Tamarillo peels (Cyphomandra betacea (Cav.) Sendt) (1.0 g) | Freeze-dried at 45 °C and ground | Ethanol (no specified) | Extraction at 180 °C and 1500 psi for 20 min, followed by centrifugation | Hydroxycinnamic acid derivatives (not quantified) | [67] | |
| EAE | Sweet cherry pomace (Prunus avium) (0.38 g) | Ground | Phosphate buffer 100 mM (1 mL) | Extraction was performed at 70 °C, pH = 10 for 40 min with 137.4 µL·g−1 Promod enzyme | (Epi)catechin (not quantified) | [68] |
| MSPD | Grape seeds (0.1 g) | Defatted by cold pressing | EtOH-Water 80:20 (v/v) (15 mL) | The sample was blended for 2 min with 0.5 g of diatomaceous earth and stirred (US bath) with 2 mL of the solvent for 5 min. Ultimately the extract was centrifuged | Catechin, gallic acid and dihydroxibenzoic acid (140–295, mg·kg−1) | [69] |
| Phenolic Compounds | Family | Source | Mechanisms | References |
|---|---|---|---|---|
| Quercetin and apigenin | Flavonoid | Pure standards | Reduced Aβ40 aggregation, elongation rate and fibrils lenght. Generation of amorphous aggregates. AChE inhibition. | [155] |
| Catechin and epicatechin- derived adducts | Flavonoid | Pure standards | Reduced tau aggregation Increased neurites number and length in Neuro-2a cells. | [156] |
| Cannflavin A | Flavonoid | Pure standard | Reduction in Aβ42 fibrillation, density of aggregates. Binding with Aβ42 oligomes. Reduced Aβ42 induced cytotoxicity in PC12 cells | [130] |
| Mimulone | Flavonoid | Pure standard | Reduction in Aβ42 aggregates density. Reduced Aβ42 induced cytotoxicity in PC12 cells | [130] |
| Diplacone | Flavonoid | Pure standard | Reduction in Aβ42 fibrillation, density of aggregates. Reduced Aβ42 induced cytotoxicity in PC12 cells. | [130] |
| Amentoflavone | Biflavonoid | Pure standard | Inhibition of Aβ42 aggregation and disaggregation of preformed fibrils. Generation of amorphous Aβ42 aggregates | [157] |
| Caffeic acid Quercetin | Hydroxycinamic acid Flavonoid | Jasonia glutinosa (L.) | AChE, MAO-A and TYR inhibition | [112] |
| Gallic acid catechin | Hydroxybenzoic acid Flavonoid | Desmodium elegans DC. | AChE and BChE inhibition | [122] |
| Epigallocatechin gallate | Flavonoid | Pure standard | Protected against Aβ induced apoptosis, ROS and cytotoxicity in SH-SY5Y cells. | [158] |
| Resveratrol | Stilbene | Pure standard | Reduced tau expression and hyperphosphorilation in C6 cells. Reduced APP amyloidogenic pathway. | [159] |
| Punicalagin | Tannin | Pure standard | Reduced LPS induced cytotoxicity and neuroinflammation in BV2 cells. Reduced LPS-induced neuroinflammation and increased synaptic density in Neuro-2a cells | [160] |
| Tannic acid-loaded liposomes | Tannin | Pure standard | Reduced intracellular tau aggregation in SK-N-SH cells | [161] |
| Synapic acid | Hydroxycinamic acid | Arabidopsis thaliana | Reduced Aβ25–35 induced cytotoxicity and pro-inflamatory effect on BV2 cells | [162] |
| Animal Model | Description | Polyphenol or Extract | Studies in Animals | Behavioral Improvements | Effects on Brain | Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|
| APP/PS1 | Transgenic | SeNPs coated with dihydromyricetin | Spatial learning ability: Morris Water Maze | Number of times they pass through the platform area | Overexpression and release of cytokines | [164] | |
| Female Tg2576 mice | Transgenic | Rosmarinic acid | DNA microarray analysis in the brain, monoamine concentration in the brain, mRNA measurement in cerebral cortex | Increases monoamine neurotransmitters level | [163] | ||
| Male C57BL/6J mice | AD model induced D-gal 150 mg kg−1 day for 8 weeks | PU | Behavioral (Morris water maze, Y maze, open field test), brain Immunohistochemistry | Number of times they pass through the platform/rodents prefer to investigate a new arm | Increased survival neurons/improvement inflammatory process | [160] | |
| Male Sprague Dawley rats | AD model induced Aluminum chloride 70 mg kg−1, for six weeks | Opuntia ficus-indica cladode, peel and fruit pulp extracts | Learning and memory functions (passive avoidance). Total antioxidant capacity in blood. Monoamine neurotransmitters in brain | number of times they avoid the chamber in which they will suffer shocks | Increases monoamines neurotransmitters level | >2000 mg kg−1 body weight | [126] |
| Male albinos mice | AD model induced Aluminum chloride 100 ppm day for two months | P. lentiscus L. leaves extract | Behavioral (anxiety: head-dipping, black and white and elevated plus maze and memory: Morris water maze) histological (brain pathological changes) | Number of times they pass through the platform area | Protective effects in oxidative stress and lipid peroxidation | [61] | |
| Mice | AD model induced scopolamine at a dose of 1 mg kg−1 for up to 9 days | Crude extracts of Desmodium elegans | Behavioral (Water maze, Y maze, elevated plus maze) | Number of times they pass through the platform area/rodents prefer to investigate a new arm | [122] | ||
| C57BL/6 naive mice | Single intracerebroventricular (ICV) injection of Aβ Oligomers | Natural-based complex polyphenols | NOR. Memory task | Improvements in recognition of new objects | Reduction on microglial activation | [165] | |
| Male SAMP8 mice | Model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in AD | Sugarcane (Saccharum officinarum L.) Top Extract | Behavioral (Morris water maze), brain Immunohistochemistry, monoamine concentration in the brain, cRNA measurement in cerebral cortex | Number of times they pass through the platform area | Increases monoamines neurotransmitters level | [129] | |
| Female NMRI mice | Aging model | Olive secoiridoid polyphenols | Behavioral (Passive Avoidance, Y maze). ATP levels | latency to cross through the gate between compartments/rodents prefer to investigate a new arm | Increases ATP levels | [123] | |
| Caernorhabditis elegans | CL4176 transgenic sensible to temperature (25 °C), 5-hydroxytryptamine hypersensitivity | Ethyl caffeate | Paralysis symptoms | delayed the paralysis symptoms | Increases monoamines neurotransmitters level | >600 µM | [166] |
| Caenorhabditis elegans | Transgenic strain (CL4176 (smg-1 ts 131 (myo-3/Aβ42 long 30-UTR)) temperature-sensitive transgene | Jasonia glutinosa extract | Paralysis symptoms | delayed the paralysis symptoms | Increases monoamines neurotransmitters level | [112] | |
| Caenorhabditis elegans | CL2006 transgenic. expressing the Aβ3-42 peptide | Hop Extracts (Humulus lupulus L.) | Mobility Assay | Paralysis reduction | [128] | ||
| Drosophila flies | Drosophila melanogaster model | Extract of Arabidopsis thaliana | Locomotor dysfunction induced by Aβ42 | Improvement climbed ability | Anti-inflammatory | [162] | |
| Drosophila flies | Drosophila melanogaster model | p-Coumaric acid | Drosophila lines (GMR-Aβ42 for the eye assay and Actin5C-Aβ42 for the lifespan and locomotive assays) | [114] | |||
| Zebrafish | Scopolamine-Induced Memory Deficits | Extract of Lycopodium selago L. | Spatial Memory in Y-Maze, novel tank-diving, NOR | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Zurdo, D.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E. A Comprehensive Analytical Review of Polyphenols: Evaluating Neuroprotection in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5906. https://doi.org/10.3390/ijms25115906
Vicente-Zurdo D, Gómez-Mejía E, Rosales-Conrado N, León-González ME. A Comprehensive Analytical Review of Polyphenols: Evaluating Neuroprotection in Alzheimer’s Disease. International Journal of Molecular Sciences. 2024; 25(11):5906. https://doi.org/10.3390/ijms25115906
Chicago/Turabian StyleVicente-Zurdo, David, Esther Gómez-Mejía, Noelia Rosales-Conrado, and María Eugenia León-González. 2024. "A Comprehensive Analytical Review of Polyphenols: Evaluating Neuroprotection in Alzheimer’s Disease" International Journal of Molecular Sciences 25, no. 11: 5906. https://doi.org/10.3390/ijms25115906





