DNA Methylation Mediates Sperm Quality via piwil1 and piwil2 Regulation in Japanese Flounder (Paralichthys olivaceus)
Abstract
1. Introduction
2. Results and Discussion
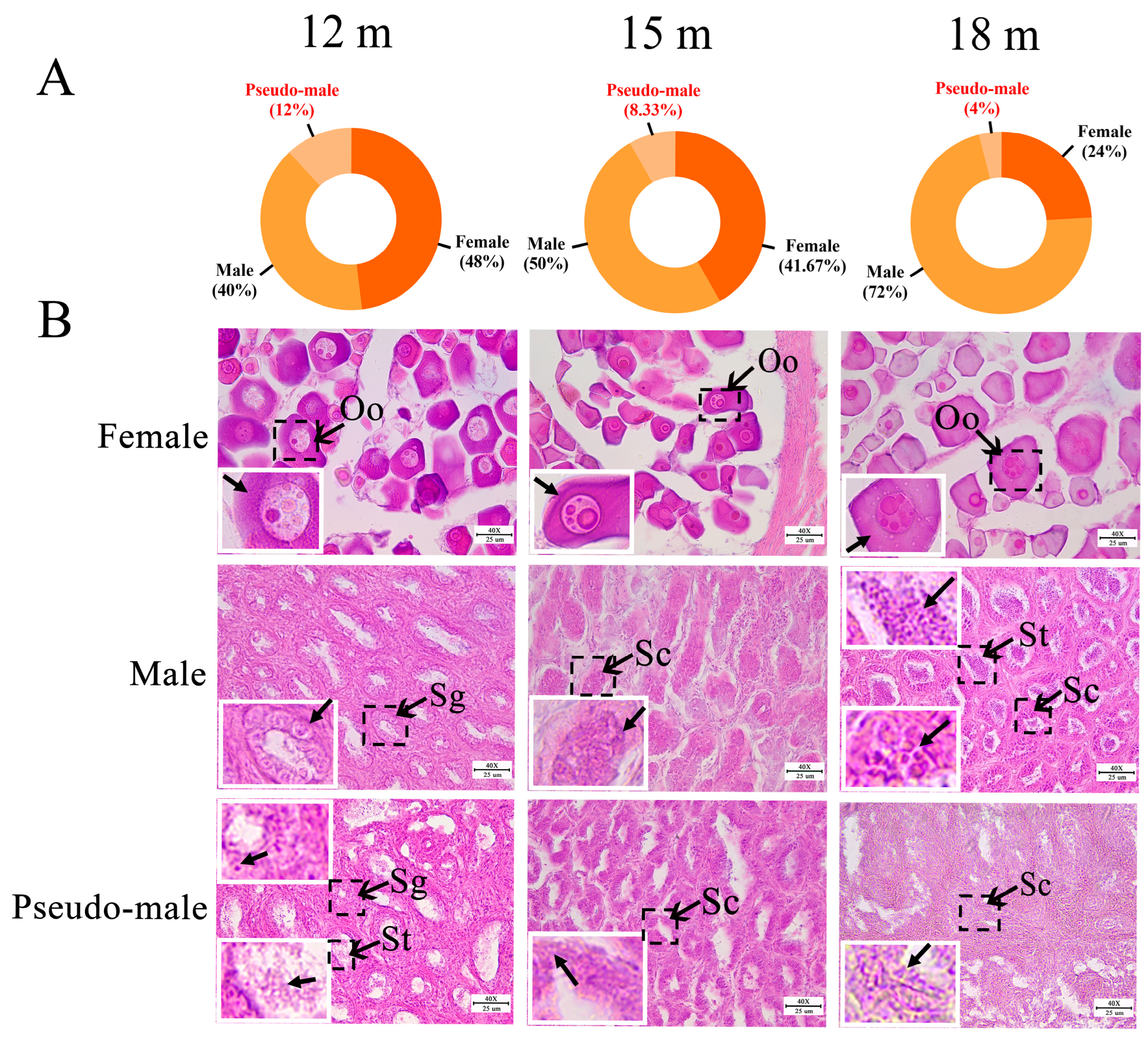
2.1. Identification of Genetic Sex in P. olivaceus
2.2. Gonadal Histology of P. olivaceus
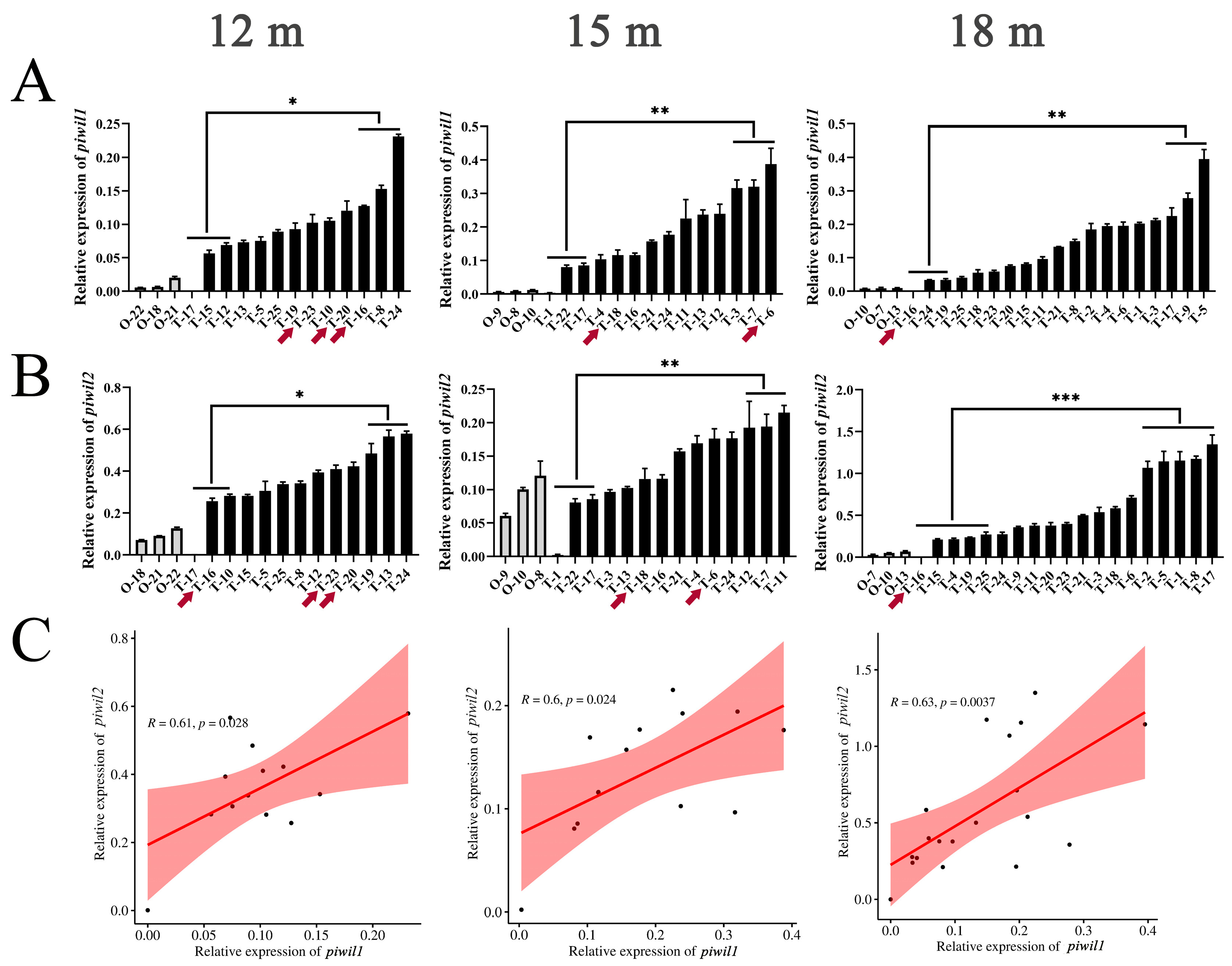
2.3. Expression Profile of piwil1 and piwil2 Genes in P. olivaceus Gonads
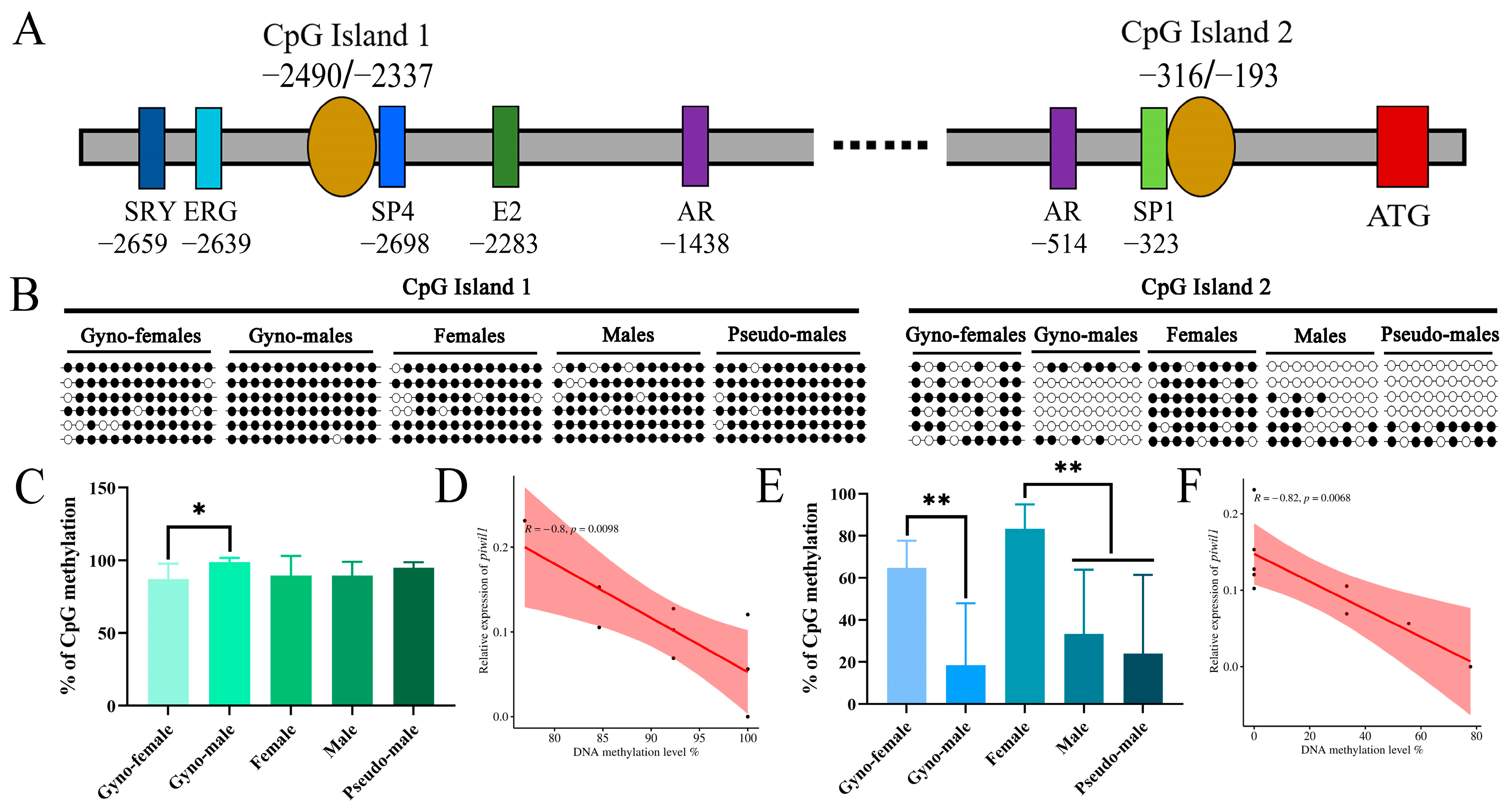
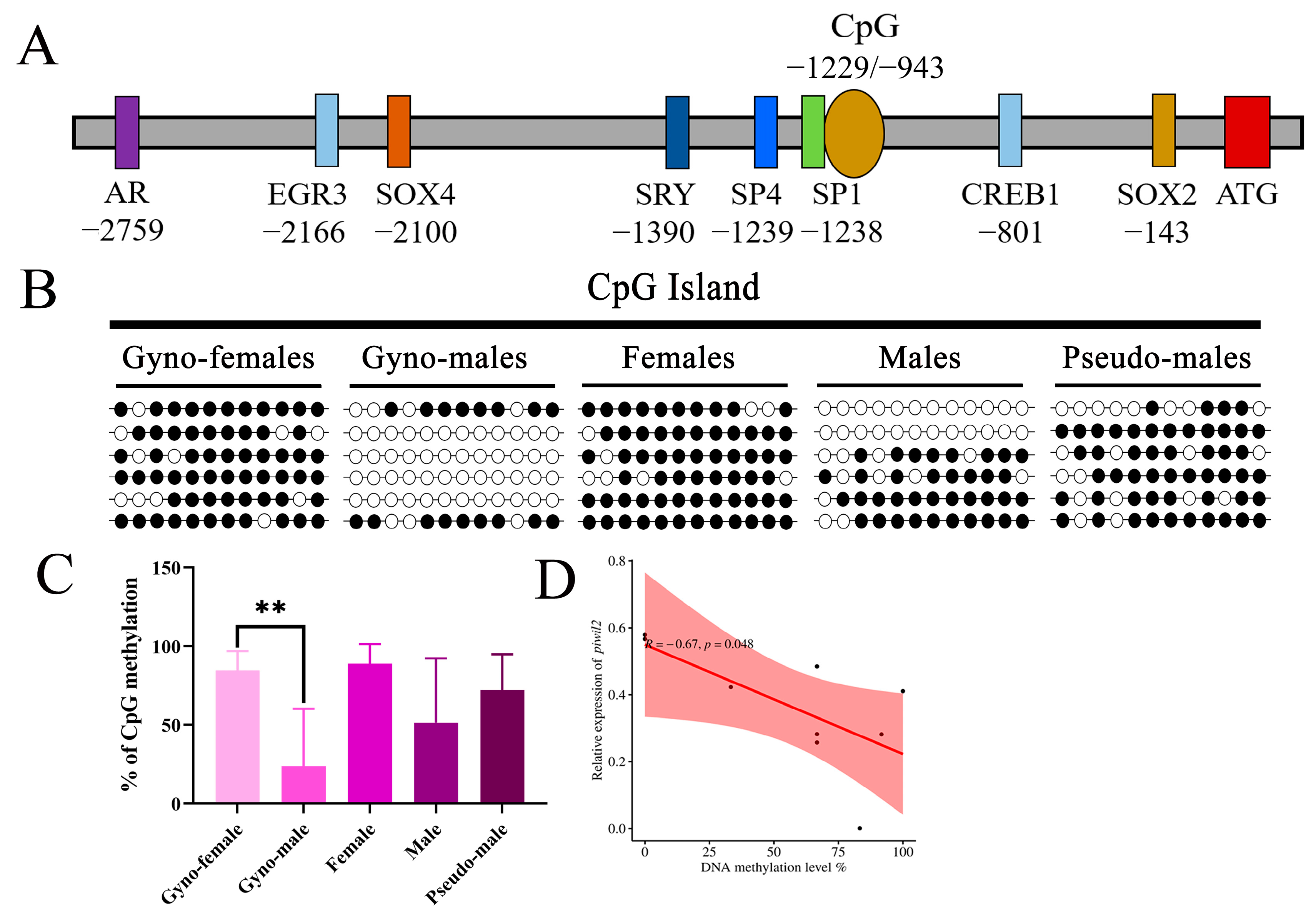
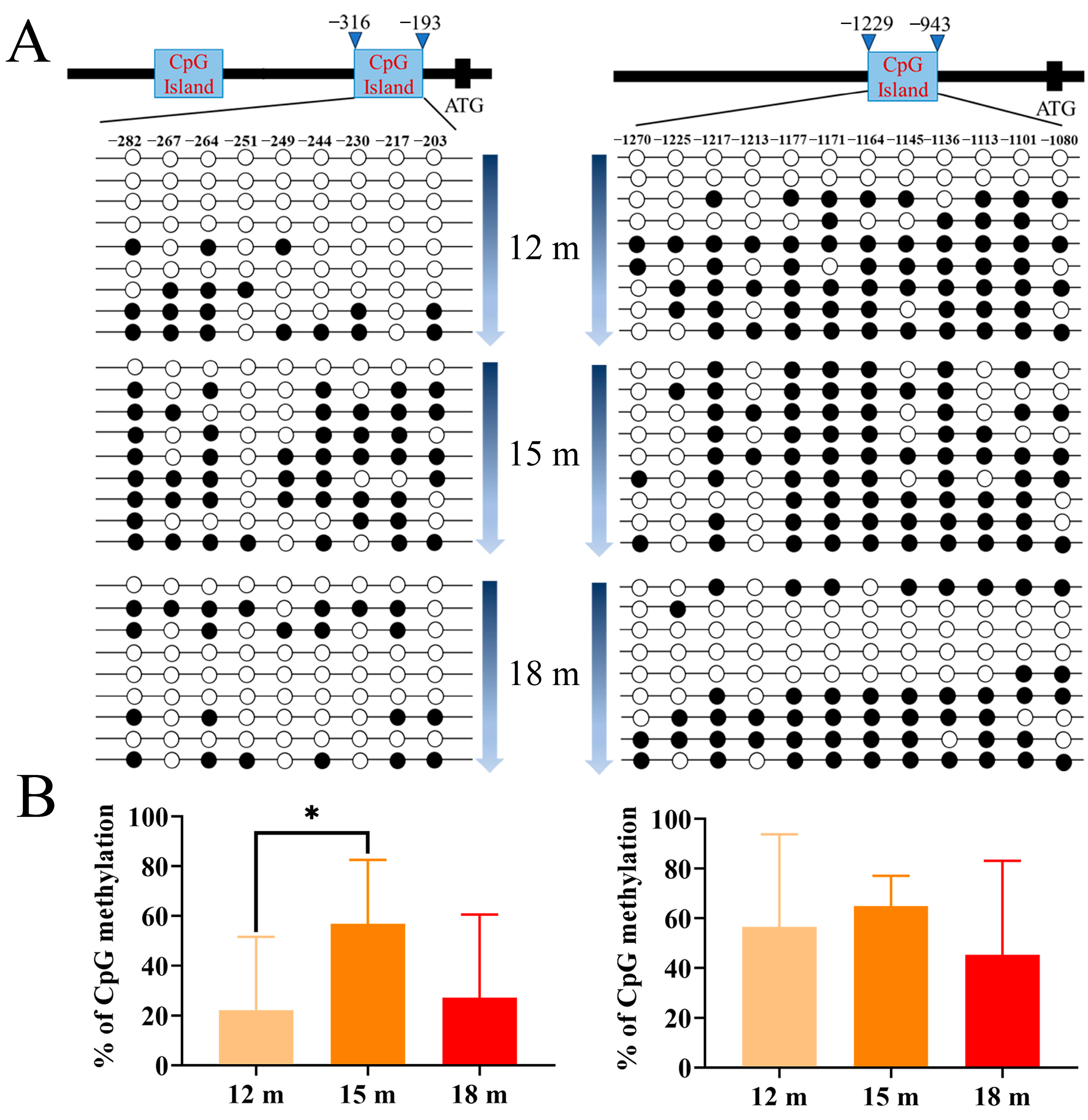
2.4. DNA Methylation Dynamics of piwi Genes in P. olivaceus Gonads
2.5. DNA Methylation Profile of piwi Genes at Different Stages
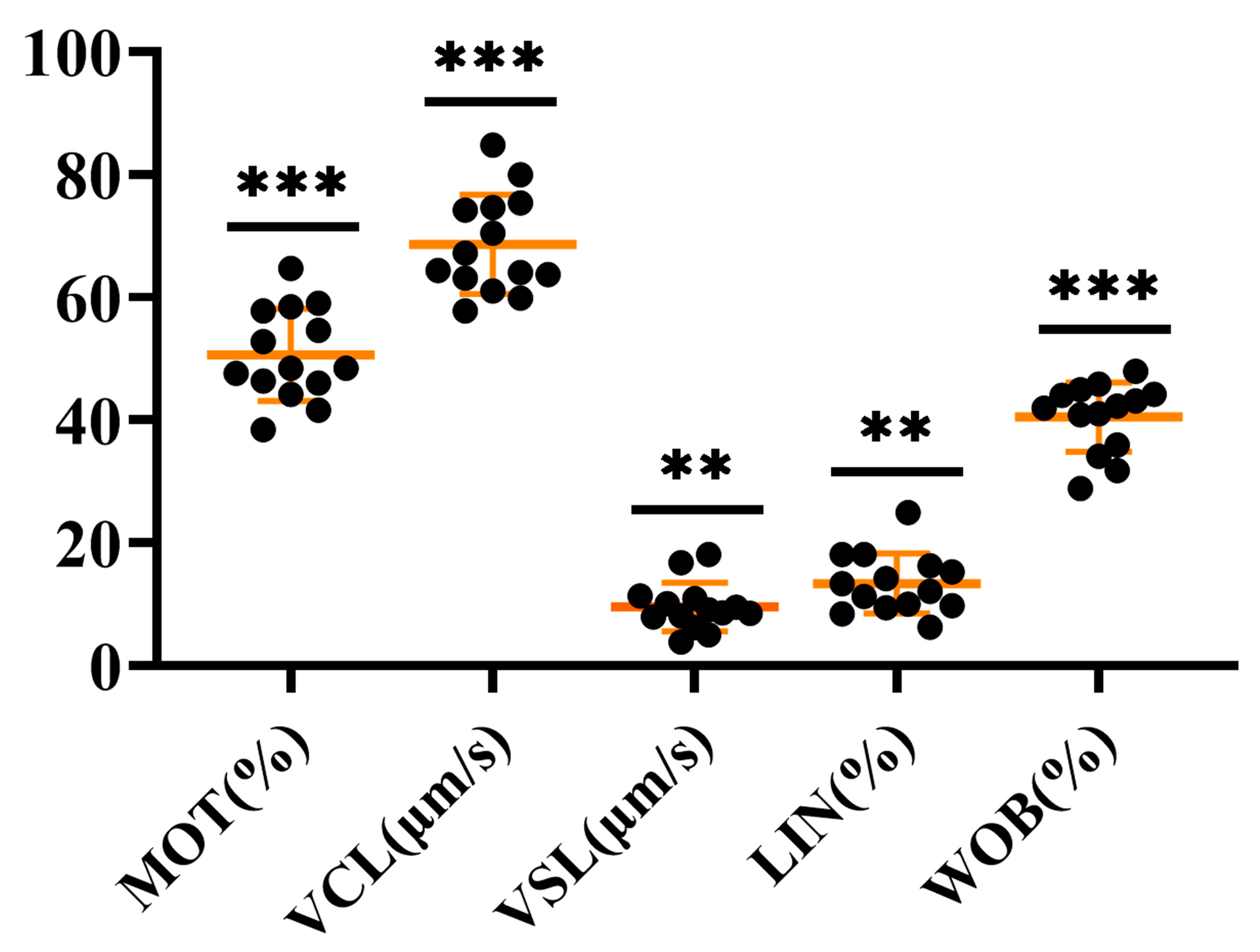
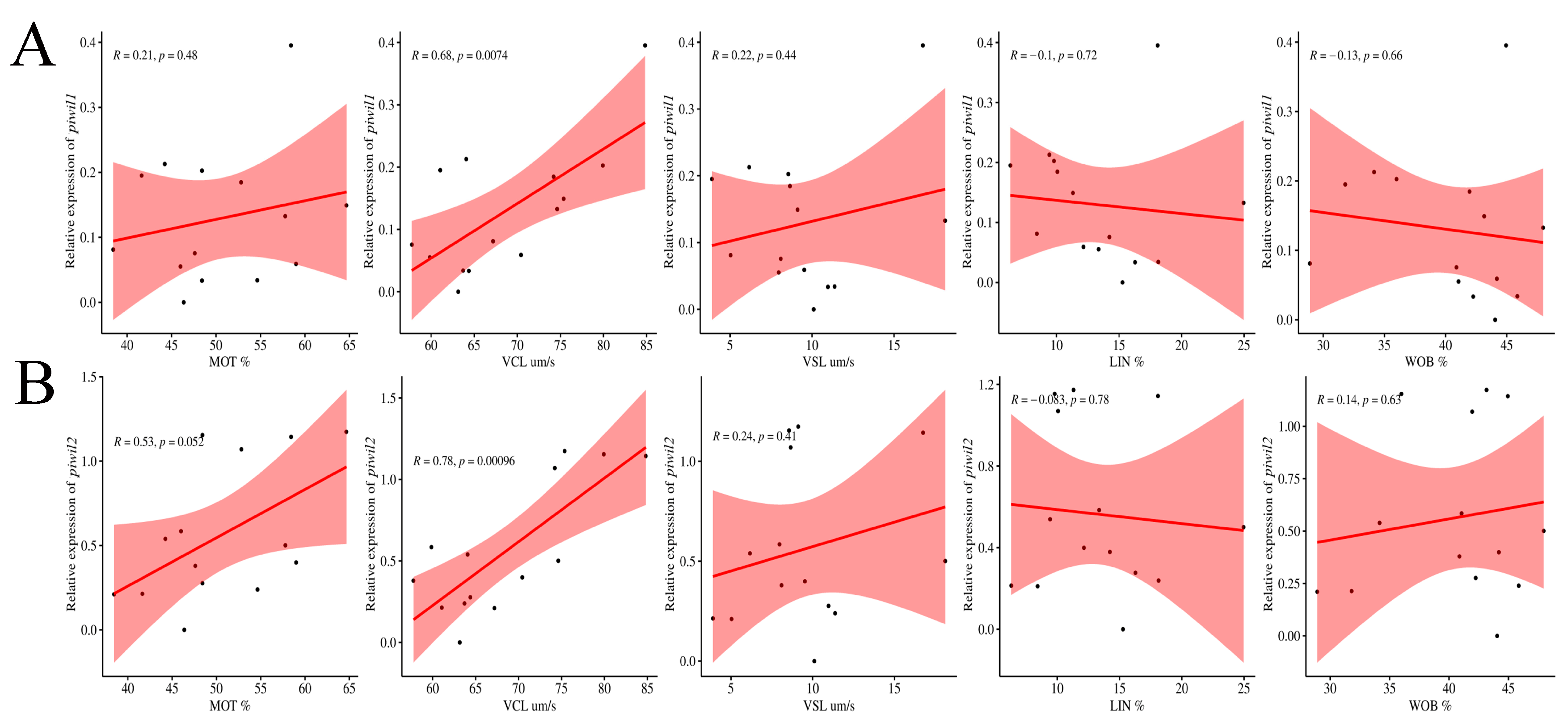
2.6. Characterization of P. olivaceus Sperm Quality with piwi Expression
3. Materials and Methods
3.1. Material Statement
3.2. DNA Isolation and PCR
3.3. Histological Examination of P. olivaceus Gonads
3.4. Total RNA Isolation and qRT-PCR
3.5. Prediction of CpG Islands
3.6. Bisulfite Modification Sequencing
3.7. Prediction of Transcription Factor Binding Sites in the Promoter Region
3.8. Sperm Quality Analysis of P. olivaceus
3.9. Pearson Coefficient Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomson, T.; Lin, H. The biogenesis and function of PIWI proteins and piRNAs: Progress and prospect. Annu. Rev. Cell Dev. Biol. 2009, 25, 355–376. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.; Seto, A.G.; Kim, J.; Kuramochi-Miyagawa, S.; Nakano, T.; Bartel, D.P.; Kingston, R.E. Characterization of the piRNA complex from rat testes. Science 2006, 313, 363–367. [Google Scholar] [CrossRef]
- Vagin, V.V.; Sigova, A.; Li, C.; Seitz, H.; Gvozdev, V.; Zamore, P.D. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 2006, 313, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 2007, 128, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nishida, K.M.; Mori, T.; Kawamura, Y.; Miyoshi, K.; Nagami, T.; Siomi, H.; Siomi, M.C. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006, 20, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, S.; Matushansky, I. Piwis and piwi-interacting RNAs in the epigenetics of cancer. J. Cell. Biochem. 2012, 113, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.D.; Hannon, G.J. Small RNAs as guardians of the genome. Cell 2009, 136, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.H.; Zhao, Y.M. The regulatory functions of piRNA/PIWI in spermatogenesis. Yi Chuan Hered. 2017, 39, 683–691. [Google Scholar]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-cell RNA sequencing analysis reveals sequential cell fate transition during human spermatogenesis. Cell Stem Cell 2018, 23, 599–614.e4. [Google Scholar] [CrossRef]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Houwing, S.; Berezikov, E.; Ketting, R.F. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J. 2008, 27, 2702–2711. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Duan, J.; Cheng, N.; Nagahama, Y. Specific expression of Olpiwi1 and Olpiwi2 in medaka (Oryzias latipes) germ cells. Biochem. Biophys. Res. Commun. 2012, 418, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, D.; Li, X.; Zhao, C.; Wang, T.; Qian, X.; Yin, S. Differential expression of two Piwil orthologs during embryonic and gonadal development in pufferfish, Takifugu fasciatus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 219, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Sun, L.; Chen, J.; Shi, H.; Wang, D. Genomic identification, rapid evolution, and expression of Argonaute genes in the tilapia, Oreochromis niloticus. Dev. Genes Evol. 2016, 226, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Liu, J.; Li, A.; Zhu, H.; Wang, X.; Zhang, Q. Piwil1 gene is regulated by hypothalamic-pituitary-gonadal axis in turbot (Scophthalmus maximus): A different effect in ovaries and testes. Gene 2018, 658, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, W.; Shao, C.; Zhang, N.; Li, H.; Liu, K.; Dong, Z.; Qi, Q.; Zhao, W.; Chen, S. Cloning, expression and methylation analysis of piwil2 in half-smooth tongue sole (Cynoglossus semilaevis). Mar. Genom. 2014, 18, 45–54. [Google Scholar] [CrossRef]
- Nagase, H.; Ghosh, S. Epigenetics: Differential DNA methylation in mammalian somatic tissues. FEBS J. 2008, 275, 1617–1623. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Lee, L.; Asada, H.; Kizuka, F.; Tamura, I.; Maekawa, R.; Taketani, T.; Sato, S.; Yamagata, Y.; Tamura, H.; Sugino, N. Changes in histone modification and DNA methylation of the StAR and Cyp19a1 promoter regions in granulosa cells undergoing luteinization during ovulation in rats. Endocrinology 2013, 154, 458–470. [Google Scholar] [CrossRef]
- Ghai, S.; Monga, R.; Mohanty, T.K.; Chauhan, M.S.; Singh, D. Tissue-specific promoter methylation coincides with Cyp19 gene expression in buffalo (Bubalus bubalis) placenta of different stages of gestation. Gen. Comp. Endocrinol. 2010, 169, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Liu, Z.; Zhang, L.; Zhang, W. Epigenetic modifications during sex change repress gonadotropin stimulation of cyp19a1a in a teleost ricefield eel (Monopterus albus). Endocrinology 2013, 154, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Martín, L.; Vinas, J.; Ribas, L.; Diaz, N.; Gutierrez, A.; Di Croce, L.; Piferrer, F. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 2011, 7, e1002447. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yi, S.; Gao, Z.; Abbas, K.; Zhou, X. DNA methylation mediates gonadal development via regulating the expression levels of cyp19a1a in loach Misgurnus anguillicaudatus. Int. J. Biol. Macromol. 2023, 235, 123794. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, X.; Jin, C.; Du, X.; He, Y.; Zhang, Q. Transcriptome profiling insights the feature of sex reversal induced by high temperature in tongue sole Cynoglossus semilaevis. Front. Genet. 2019, 10, 437067. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, J.; Gu, Z.; Chen, L.; Luo, C.; Chi, M.; Liu, S.; Jiang, W.; Cheng, S. Cloning of Sox9 gene and the relationship between CpG island methylation and gene expression in Culter alburnus. Acta Hydrobiol. Sin. 2019, 43, 473–478. [Google Scholar]
- Ferreira, H.J.; Heyn, H.; Garcia del Muro, X.; Vidal, A.; Larriba, S.; Munoz, C.; Villanueva, A.; Esteller, M. Epigenetic loss of the PIWI/piRNA machinery in human testicular tumorigenesis. Epigenetics 2014, 9, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Heyn, H.; Ferreira, H.J.; Bassas, L.; Bonache, S.; Sayols, S.; Sandoval, J.; Esteller, M.; Larriba, S. Epigenetic disruption of the PIWI pathway in human spermatogenic disorders. PLoS ONE 2012, 7, e47892. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.W.; Jung, J.; Park, K.; Lee, S.; Park, I.; Kim, W.J.; Kim, D.S. Characterization of sexual size dimorphism and sex-biased genes expression profile in the olive flounder. Mol. Biol. Rep. 2020, 47, 8317–8324. [Google Scholar] [CrossRef]
- Luckenbach, J.A.; Borski, R.J.; Daniels, H.V.; Godwin, J. Sex determination in flatfishes: Mechanisms and environmental influences. Semin. Cell Dev. Biol. 2009, 20, 256–263. [Google Scholar] [CrossRef]
- Song, H.; Xing, C.; Lu, W.; Liu, Z.; Wang, X.; Cheng, J.; Zhang, Q. Rapid evolution of piRNA pathway and its transposon targets in Japanese flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. D Genom. Proteom. 2019, 31, 100609. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, H.; Song, H.; Jin, C.; Peng, M.; Gao, C.; Yang, F.; Du, X.; Qi, J.; Zhang, Q.; et al. Evolutionary significance and regulated expression of Tdrd family genes in gynogenetic Japanese flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. D Genom. Proteom. 2019, 31, 100593. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, S.; Guo, S.; Lu, W.; Zhang, Q.; Cheng, J. Evolutionary dynamics and conserved function of the Tudor domain-containing (TDRD) proteins in teleost fish. Mar. Life Sci. Technol. 2022, 4, 18–30. [Google Scholar] [CrossRef]
- Ni, F.; Yu, H.; Liu, Y.; Meng, L.; Yan, W.; Zhang, Q.; Wang, X. Roles of piwil1 gene in gonad development and gametogenesis in Japanese flounder, Paralichthys olivaceus. Gene 2019, 701, 104–112. [Google Scholar] [CrossRef]
- Hattori, R.S.; Kumazawa, K.; Nakamoto, M.; Nakano, Y.; Yamaguchi, T.; Kitano, T.; Yamamoto, E.; Fuji, K.; Sakamoto, T. Y-specific amh allele, amhy, is the master sex-determining gene in Japanese flounder Paralichthys olivaceus. Front. Genet. 2022, 13, 1007548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B. Identification and Application of Sex Markers and Sex-Related Gene in Pseudomale Cynoglossus semilaevis. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Zhang, B.; Zhao, N.; Jia, L.; Che, J.; He, X.; Liu, K.; Bao, B. Identification and application of piwi-interacting RNAs from seminal plasma exosomes in Cynoglossus semilaevis. BMC Genom. 2020, 21, 302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Jia, L.; Deng, Q.; Zhu, C.; Zhang, B. Comparative piRNAs profiles give a clue to transgenerational inheritance of sex-biased piRNAs in Cynoglossus semilaevis. Mar. Biotechnol. 2022, 24, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Skaar, K.S.; Nobrega, R.H.; Magaraki, A.; Olsen, L.C.; Schulz, R.W.; Male, R. Proteolytically activated, recombinant anti-Müllerian hormone inhibits androgen secretion, proliferation, and differentiation of spermatogonia in adult zebrafish testis organ cultures. Endocrinology 2011, 152, 3527–3540. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Wang, R.; Chen, S. Transcriptomics analysis revealing candidate networks and genes for the body size sexual dimorphism of Chinese tongue sole (Cynoglossus semilaevis). Funct. Integr. Genom. 2018, 18, 327–339. [Google Scholar] [CrossRef]
- Li, W. Studies on the Artificial Induction of Polyploidy and Its Effect on Growth and Development of Triploid in Half-Smooth Tongue Sole (Cynoglossus semilaevis). Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2012. [Google Scholar]
- Cooper, T.G.; Noonan, E.; Von Eckardstein, S.; Auger, J.; Baker, H.G.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Chen, S.L.; Ji, X.S.; Shao, C.W.; Li, W.L.; Yang, J.F.; Liang, Z.; Liao, X.L.; Xu, G.B.; Xu, Y.; Song, W.T. Induction of mitogynogenetic diploids and identification of WW super-female using sex-specific SSR markers in half-smooth tongue sole (Cynoglossus semilaevis). Mar. Biotechnol. 2012, 14, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, W.; Ma, L.; Jie, J.; Zhang, Y.; Wang, H.; Li, H. New locus reveals the genetic architecture of sex reversal in the Chinese tongue sole (Cynoglossus semilaevis). Heredity 2018, 121, 319–326. [Google Scholar] [CrossRef]
- Gallego, V.; Herranz-Jusdado, J.G.; Rozenfeld, C.; Perez, L.; Asturiano, J.F. Subjective and objective assessment of fish sperm motility: When the technique and technicians matter. Fish Physiol. Biochem. 2018, 44, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Ma, W.; Jing, J.; Zhang, J.; Dan, C.; Gui, J.F.; Mei, J. An miR-200 cluster on chromosome 23 regulates sperm motility in zebrafish. Endocrinology 2018, 159, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Riesco, M.F.; Valcarce, D.G.; Martínez-Vazquez, J.M.; Martin, I.; Calderon-Garcia, A.A.; Gonzalez-Nunez, V.; Robles, V. Male reproductive dysfunction in Solea senegalensis: New insights into an unsolved question. Reprod. Fertil. Dev. 2019, 31, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Gunes, S.; Arslan, M.A.; Hekim, G.N.T.; Asci, R. The role of epigenetics in idiopathic male infertility. J. Assist. Reprod. Genet. 2016, 33, 553–569. [Google Scholar] [CrossRef]
- Woods, L.C., III; Li, Y.; Ding, Y.; Liu, J.; Reading, B.J.; Fuller, S.A.; Song, J. DNA methylation profiles correlated to striped bass sperm fertility. BMC Genom. 2018, 19, 244. [Google Scholar] [CrossRef]
- Sun, Z. Histological Studies on Gonadal Ontogenesis, Differentiation and Development in Flounder (Paralichthys olivaceus). Master’s Thesis, Northeast Agricultural University, Harbin, China, 2008. [Google Scholar]
- Cheng, J.; Yang, F.; Liu, S.; Zhao, H.; Lu, W.; Zhang, Q. Transcriptomic analysis reveals functional interaction of mRNA-lncRAN-miRNA in steroidogenesis and spermatogenesis of gynogenetic Japanese flounder (Paralichthys olivceus). Biology 2022, 11, 213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, W.; Wang, Y.; Zhang, L.; Lu, W.; Li, W.; Wang, F.; Cheng, J. DNA Methylation Mediates Sperm Quality via piwil1 and piwil2 Regulation in Japanese Flounder (Paralichthys olivaceus). Int. J. Mol. Sci. 2024, 25, 5935. https://doi.org/10.3390/ijms25115935
Zong W, Wang Y, Zhang L, Lu W, Li W, Wang F, Cheng J. DNA Methylation Mediates Sperm Quality via piwil1 and piwil2 Regulation in Japanese Flounder (Paralichthys olivaceus). International Journal of Molecular Sciences. 2024; 25(11):5935. https://doi.org/10.3390/ijms25115935
Chicago/Turabian StyleZong, Wenyu, Yapeng Wang, Lingqun Zhang, Wei Lu, Weigang Li, Fengchi Wang, and Jie Cheng. 2024. "DNA Methylation Mediates Sperm Quality via piwil1 and piwil2 Regulation in Japanese Flounder (Paralichthys olivaceus)" International Journal of Molecular Sciences 25, no. 11: 5935. https://doi.org/10.3390/ijms25115935
APA StyleZong, W., Wang, Y., Zhang, L., Lu, W., Li, W., Wang, F., & Cheng, J. (2024). DNA Methylation Mediates Sperm Quality via piwil1 and piwil2 Regulation in Japanese Flounder (Paralichthys olivaceus). International Journal of Molecular Sciences, 25(11), 5935. https://doi.org/10.3390/ijms25115935





