Advancing Diabetes Research: A Novel Islet Isolation Method from Living Donors
Abstract
:1. Introduction
2. Results
2.1. Patient Characterization and Surgical Procedure
Study Design and Experimental Procedures
- -
- Oral Glucose Tolerance Test:
- -
- Hyperinsulinemic–Euglycemic Clamp Procedure:
- -
- Hyperglycemic Clamp Procedure:
- -
- Mixed-Meal Test:
2.2. Surgical Procedures
2.3. Samples Collection and Tissue Digestion
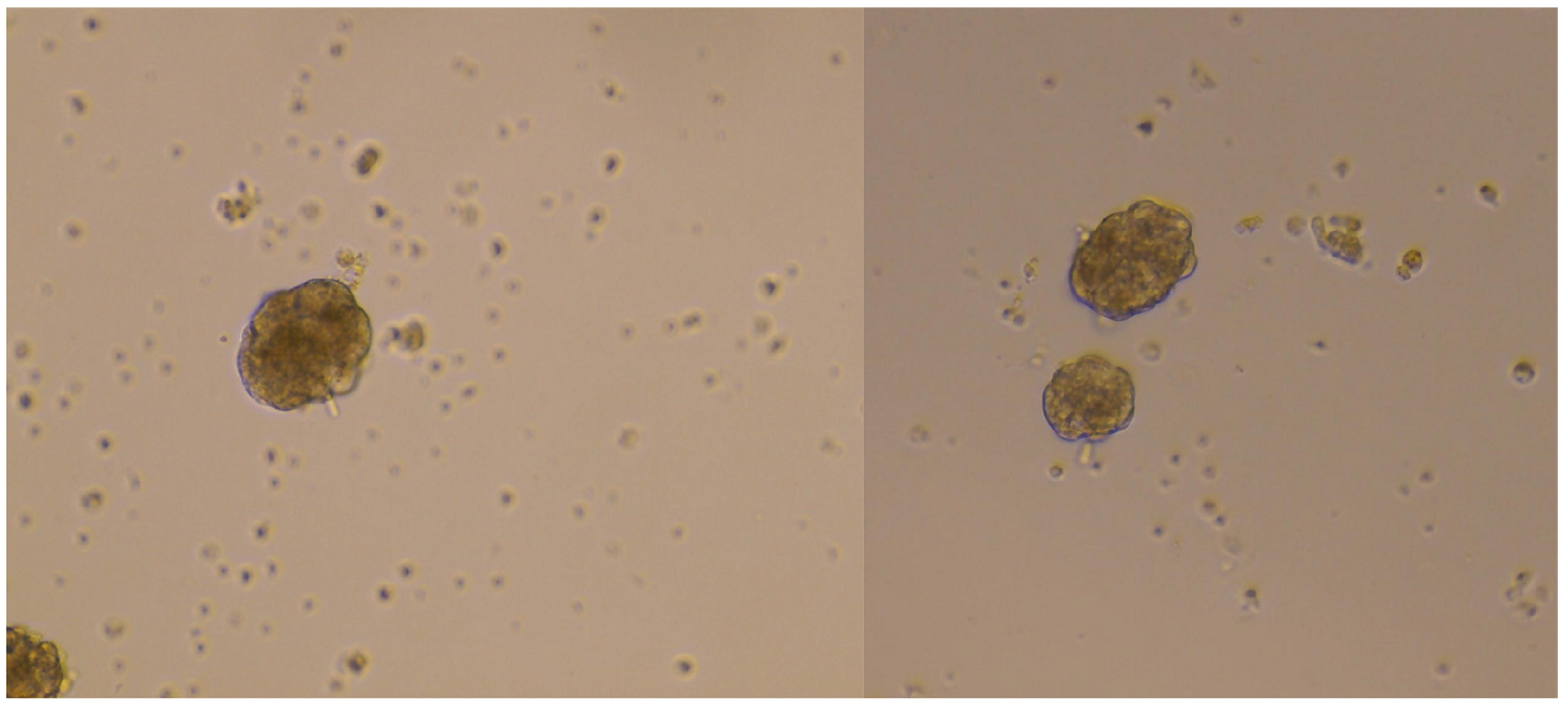
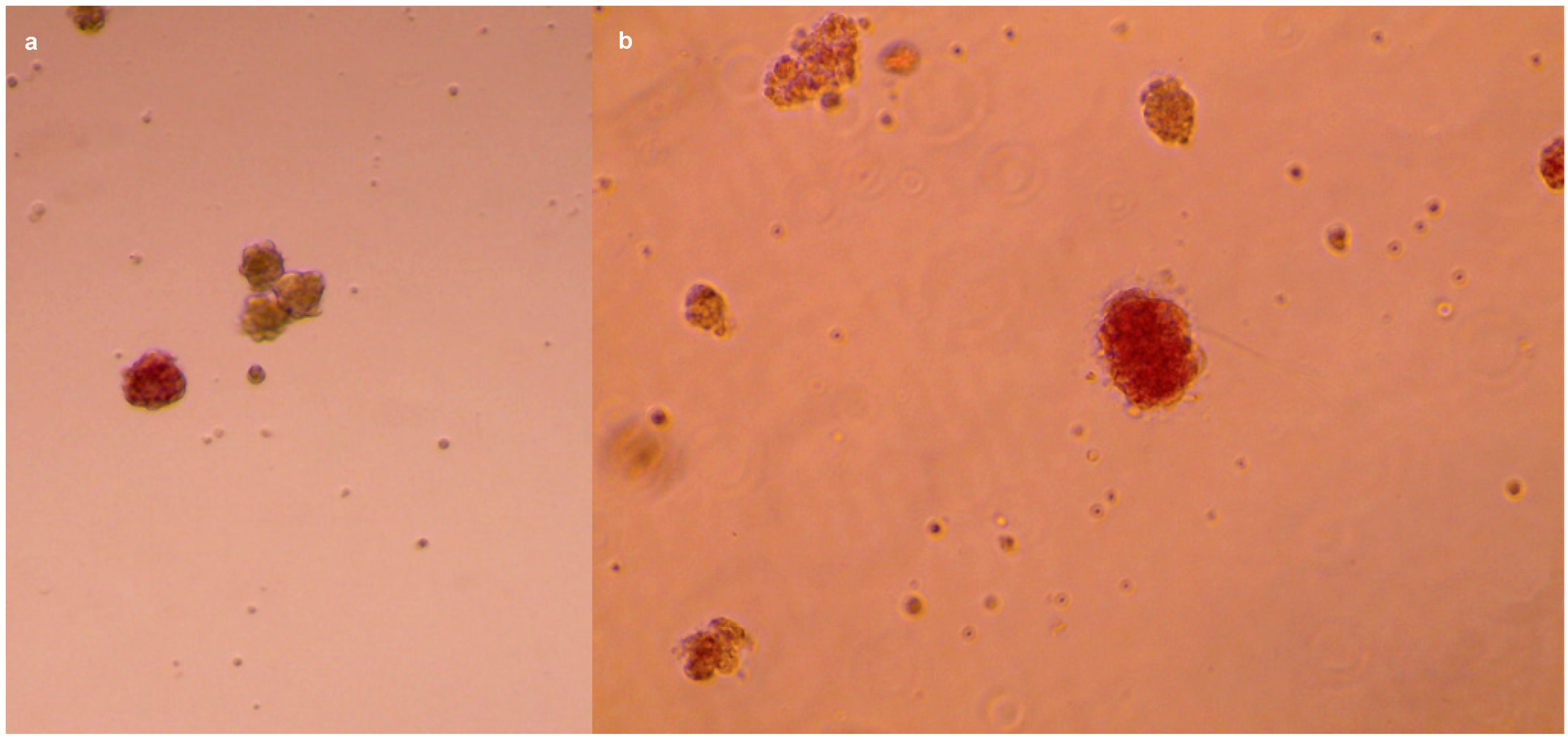
2.4. Islets Isolation: Filtration and Density Gradient
2.5. Glucose Stimulation and Insulin Secretion
2.5.1. Islets Stimulation
- -
- Basal glucose, 3.3 mM;
- -
- High glucose, 16.7 mM;
- -
- Basal glucose 3.3 mM+ arginine 20 mM (referred to as arginine 20 mM).
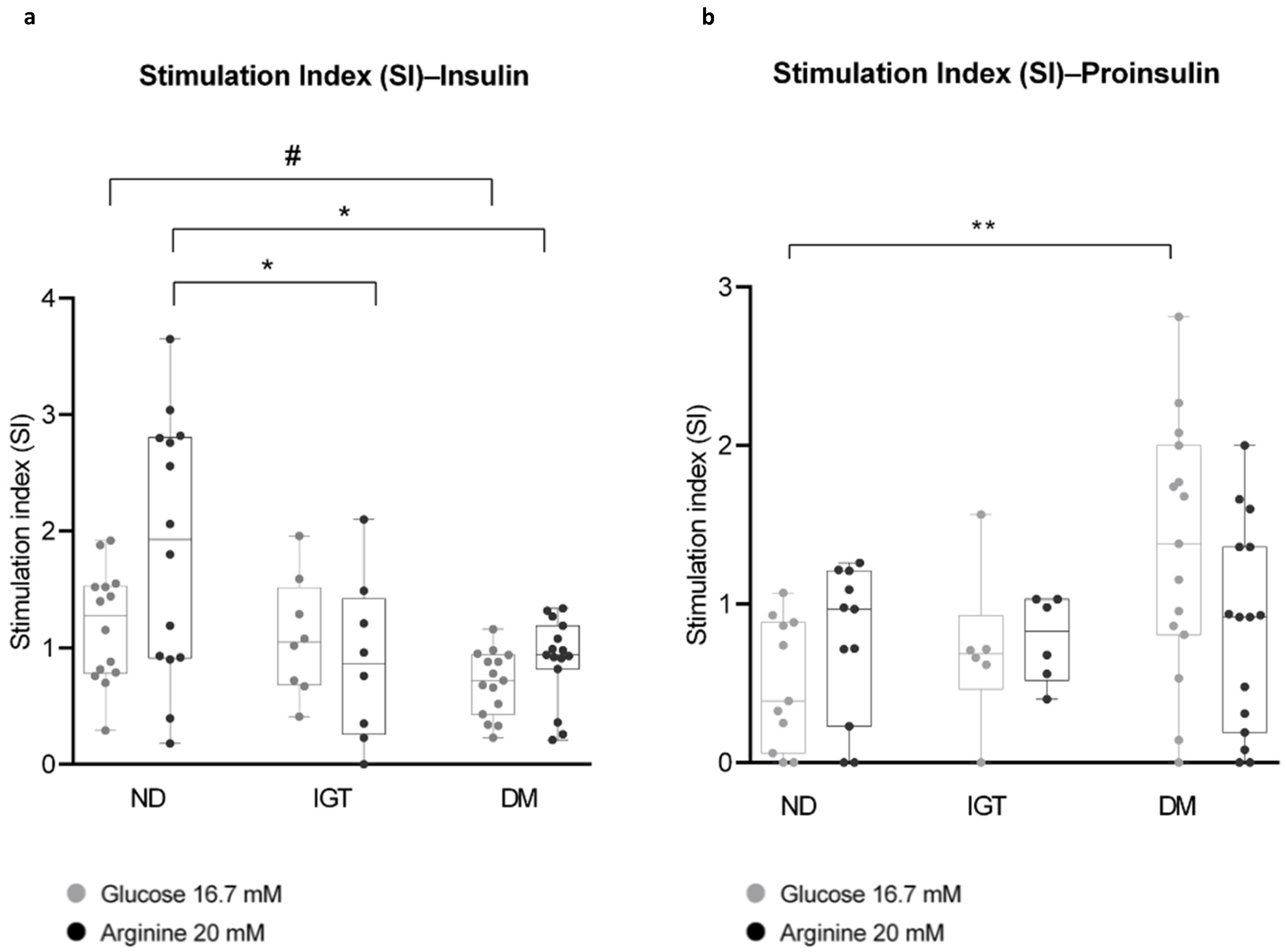
2.5.2. Assessment of Insulin and Proinsulin Secretion
- SI (Glucose 16.7 mM) = (OD insulin (or proinsulin) at glucose 16.7 mM)/(OD insulin (or proinsulin) at glucose 3.3 mM);
- SI (Arginine 20 mM) = (OD insulin (or proinsulin) at arginine 20 mM)/(OD insulin (or proinsulin) at glucose 3.3 mM);
- Mean differences among the three groups were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s HSD multiple comparison test.
3. Discussion
4. Materials (List of Reagents)
- HBSS (Hank’s Balanced Salts Solution w/Calcium w/Magnesium w/Phenol Red; Euroclone, Pero (MI), Italy, Cat. #ECB4006L);
- 96-Well, Cell Culture-Treated (#353072, Falcon®, Corning, NY, USA);
- 24-Well, Cell Culture-Treated (#3524, Corning®, Corning, NY, USA);
- Fetal Bovine Serum (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, Cat. #F7524);
- Collagenase P, 1.5 U/mg (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, Cat. #11249002001);
- Lympholyte®-H, sterile liquid (Euroclone, Pero (MI), Italy, Cat. #DVCL5020);
- DMEM, no glucose, no glutamine, no phenol red (Gibco™, Thermo Fisher Scientific Waltham, MA, USA, Cat. #A1443001);
- Bovine Calf Serum (BCS), US Origin (Cytiva, Global Life Sciences Solutions Marlborough, MA, USA, Cat. #SH30073.03);
- L-Arginine Minimum 98% (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, Cat. #A-5006);
- D(+)-Glucose Anhydrous (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, Cat. #G-5767);
- Amphotericin B (Fungizone→) 250 μg/mL (100 mL) (Euroclone, Pero (MI), Italy, Cat. #ECM0009D);
- Penicillin–Streptomycin 10,000 U-10 mg (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, Cat. #P0781);
- L-Glutamine Solution 200 mM (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany);
- HEPES buffer 1 M (Eurobio Scientific, Les Ulis, France, Cat. #CSTHEP00-0U);
- Gentamicin solution 50 mg/mL (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, Cat. #G1397);
- Tweezers;
- Single-use stainless surgical blades (Paragon Medical, Pierceton, IN, USA, Cat. #P301);
- Polypropylene 15 mL–50 mL Graduated Tubes (Sarstedt, Nümbrecht, Germany, Cat. #62 554502, #62 547254);
- Petri dish, 60 × 15 mm, transparent, with ventilation cams (Sarstedt, Nümbrecht, Germany, Cat. #82.1194.500);
- Corning® Cell strainer, pore size 100 μm and 40 μm (Cat. #431752, #431750);
- Pipettes and tips (2–100 μL);
- MACROMAN Pipette controller (Gilson, Middleton, WI, USA, Cat. #F110120), serological pipettes;
- Shaking water bath;
- SL 16 Centrifuge Series (Thermo Scientific™, Thermo Fisher Scientific, Waltham, MA, USA, Cat. #75004031);
- Series 8000 Direct-Heat CO2 Incubator (Thermo Scientific™, Thermo Fisher Scientific Waltham, MA, USA, Cat. #3540-MAR);
- Ethanol 70%;
- 3.5 mL Transferpipette (Sarstedt, Nümbrecht, Germany, Cat. # 86.1171.001);
- Human Insulin ELISA kit (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, #EZHI-14K);
- Human Total Proinsulin ELISA kit (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, #EZHPI-15K);
- Dithizone (Merck-Millipore–Sigma-Aldrich, Darmstadt, Germany, Cat. #D5130);
- CytoScan™ LDH Cytotoxicity Assay (G-Biosciences, St. Louis, MO, USA, #786-324);
- GraphPad Prism v8.0 (GraphPad Software, Boston, MA, USA).
Tips Section
- Minimize the sample processing time as much as possible, always using ice-cold buffers/media (except for Lympholyte). Prepare HBSS-FBS fresh aliquots before starting.
- If the tissue is very fatty or fibrotic, discard the unsuitable parts during cutting. This will improve the overall quality of digestion, especially in the case of fat, which tends to form a superficial oily layer that significantly lowers the final yield.
- If the starting specimen is particularly small in size, repeat the filtration step through the 40 μm cell strainer to minimize the loss of islets, and thoroughly wash the mesh of the strainers with higher volumes of ice-cold HBSS-FBS.
- Glucose stimulation experiments are performed by dividing the sample equally into three tubes. This requires a homogeneous cell suspension that can be achieved by pipetting 1/3 of the sample volume from the bottom to the top at least 3–4 times and then drawing the desired volume from the center of the suspension.
- It is uncommon to see the ‘cell ring’ at the lower interface (3 mL), but it is crucial to proceed nonetheless to improve the yield.
- It is necessary to handle the density gradient with extreme care, as the interfaces are delicate and prone to easy remixing, resulting in material loss. A 3.5 mL transfer pipette can be very helpful in recollecting almost the whole ‘cell ring’ at the interfaces; however, using a p1000 pipette can be more manageable for beginners.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wesolowska-Andersen, A.; Brorsson, C.A.; Bizzotto, R.; Mari, A.; Tura, A.; Koivula, R.; Mahajan, A.; Vinuela, A.; Tajes, J.F.; Sharma, S.; et al. Four groups of type 2 diabetes contribute to the etiological and clinical heterogeneity in newly diagnosed individuals: An IMI DIRECT study. Cell Rep. Med. 2022, 3, 100477. [Google Scholar] [CrossRef] [PubMed]
- Camunas-Soler, J.; Dai, X.Q.; Hang, Y.; Bautista, A.; Lyon, J.; Suzuki, K.; Kim, S.K.; Quake, S.R.; MacDonald, P.E. Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Cell Metab. 2020, 31, 1017–1031.e4. [Google Scholar] [CrossRef]
- Segerstolpe, Å.; Palasantza, A.; Eliasson, P.; Andersson, E.M.; Andréasson, A.C.; Sun, X.; Picelli, S.; Sabirsh, A.; Clausen, M.; Bjursell, M.K.; et al. Single-Cell Transcriptome Profiling of Human Pancreatic Islets in Health and Type 2 Diabetes. Cell Metab. 2016, 24, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Avrahami, D.; Wang, Y.J.; Schug, J.; Feleke, E.; Gao, L.; Liu, C.; HPAP Consortium; Naji, A.; Glaser, B.; Kaestner, K.H. Single-cell transcriptomics of human islet ontogeny defines the molecular basis of β-cell dedifferentiation in T2D. Mol. Metab. 2020, 42, 101057. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Ibberson, M.; Marchetti, P.; Powers, A.C.; Rorsman, P.; Sander, M.; Solimena, M. Every islet matters: Improving the impact of human islet research. Nat. Metab. 2022, 4, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Pokrywczynska, M.; Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, C.; Williams, D.; Gores, P. Isolation of human cadaveric pancreatic islets for clinical transplantation. Methods Mol. Biol. 2013, 1001, 227–259. [Google Scholar] [CrossRef]
- De Paep, D.L.; Van Hulle, F.; Ling, Z.; Vanhoeij, M.; Hilbrands, R.; Distelmans, W.; Gillard, P.; Keymeulen, B.; Pipeleers, D.; Jacobs-Tulleneers-Thevissen, D. Utility of Islet Cell Preparations from Donor Pancreases After Euthanasia. Cell Transplant. 2022, 31, 9636897221096160. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, C.; Lacy, P.E.; Finke, E.H.; Olack, B.J.; Scharp, D.W. Automated method for isolation of human pancreatic islets. Diabetes 1988, 37, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, C.; Lacy, P.E.; Scharp, D.W. Automated Islet Isolation from Human Pancreas. Diabetes 1989, 38 (Suppl. 1), 140–142. [Google Scholar] [CrossRef]
- Wei, L. Isolation and Purification of Human Pancreatic Islets. In Type-1 Diabetes; Moore, A., Wang, P., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2023; Volume 2592, pp. 219–232. [Google Scholar] [CrossRef]
- Bötticher, G.; Sturm, D.; Ehehalt, F.; Knoch, K.P.; Kersting, S.; Grützmann, R.; Baretton, G.B.; Solimena, M.; Saeger, H.D. Isolation of human islets from partially pancreatectomized patients. J. Vis. Exp. 2011, 53, e2962. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Barbaro, B.; Wang, S.; Wang, Y.; Hansen, M.; Oberholzer, J. Human pancreatic islet isolation: Part II: Purification and culture of human islets. J. Vis. Exp. 2009, 27, e1343. [Google Scholar] [CrossRef] [PubMed]
- Töns, H.A.M.; Baranski, A.G.; Terpstra, O.T.; Bouwman, E. Isolation of the islets of Langerhans from the human pancreas with magnetic retraction. Transplant. Proc. 2008, 40, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Miki, A.; Ricordi, C.; Messinger, S.; Yamamoto, T.; Mita, A.; Barker, S.; Haetter, R.; Khan, A.; Alejandro, R.; Ichii, H. Toward improving human islet isolation from younger donors: Rescue purification is efficient for trapped islets. Cell Transplant. 2009, 18, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Saliba, Y.; Farès, N. Isolation, Purification, and Culture of Mouse Pancreatic Islets of Langerhans. Methods Mol. Biol. 2019, 1940, 255–265. [Google Scholar] [CrossRef]
- O’Dowd, J.F.; Stocker, C.J. Isolation and Purification of Rodent Pancreatic Islets of Langerhans. Methods Mol. Biol. 2020, 2076, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Li, D.S.; Yuan, Y.H.; Tu, H.J.; Liang, Q.L.; Dai, L.J. A protocol for islet isolation from mouse pancreas. Nat. Protoc. 2009, 4, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Corbin, K.L.; West, H.L.; Brodsky, S.; Whitticar, N.B.; Koch, W.J.; Nunemaker, C.S. A Practical Guide to Rodent Islet Isolation and Assessment Revisited. Biol. Proced. Online 2021, 23, 7. [Google Scholar] [CrossRef]
- Heiser, A.; Ulrichs, K.; Müller-Ruchholtz, W. Isolation of porcine pancreatic islets: Low trypsin activity during the isolation procedure guarantees reproducible high islet yields. J. Clin. Lab. Anal. 1994, 8, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Krickhahn, M.; Bühler, C.; Meyer, T.; Thiede, A.; Ulrichs, K. The morphology of islets within the porcine donor pancreas determines the isolation result: Successful isolation of pancreatic islets can now be achieved from young market pigs. Cell Transplant. 2002, 11, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, H.; Johnson, P.R.V.; Brandhorst, D. Pancreatic Islets: Methods for Isolation and Purification of Juvenile and Adult Pig Islets. Adv. Exp. Med. Biol. 2016, 938, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pu, Z.; Chen, J.; Deng, J.; Deng, Y.; Zhu, S.; Xu, C.; Yao, F.; Wu, Z.; Ni, Y.; et al. Adult Pig Islet Isolation. J. Vis. Exp. 2021, 176, 63017. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.J.; Longacre, M.J.; Stoker, S.W.; Kendrick, M.; Thonpho, A.; Brown, L.J.; Hasan, N.M.; Jitrapakdee, S.; Fukao, T.; Hanson, M.S.; et al. Differences between human and rodent pancreatic islets: Low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J. Biol. Chem. 2011, 286, 18383–18396. [Google Scholar] [CrossRef] [PubMed]
- Levetan, C.S.; Pierce, S.M. Distinctions between the islets of mice and men: Implications for new therapies for type 1 and 2 diabetes. Endocr. Pract. 2013, 19, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Dunn, T.B. Transplant strategies for type 1 diabetes: Whole pancreas, islet and porcine beta cell therapies. Diabetologia 2020, 63, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, M.; George, J.J.; Loganathan, G.; Narayanan, S.; Hughes, M.G.; Williams, S.K.; Balamurugan, A.N. Pig islet xenotransplantation. Curr. Opin. Organ. Transplant. 2017, 22, 452–462. [Google Scholar] [CrossRef]
- Muraro, M.J.; Dharmadhikari, G.; Grün, D.; Groen, N.; Dielen, T.; Jansen, E.; van Gurp, L.; Engelse, M.A.; Carlotti, F.; de Koning, E.J.; et al. A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst. 2016, 3, 385–394.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Klughammer, J.; Farlik, M.; Penz, T.; Spittler, A.; Barbieux, C.; Berishvili, E.; Bock, C.; Kubicek, S. Single-cell transcriptomes reveal characteristic features of human pancreatic islet cell types. EMBO Rep. 2016, 17, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Ciregia, F.; Bugliani, M.; Ronci, M.; Giusti, L.; Boldrini, C.; Mazzoni, M.R.; Mossuto, S.; Grano, F.; Cnop, M.; Marselli, L.; et al. Palmitate-induced lipotoxicity alters acetylation of multiple proteins in clonal β cells and human pancreatic islets. Sci. Rep. 2017, 7, 13445. [Google Scholar] [CrossRef] [PubMed]
- Marselli, L.; Piron, A.; Suleiman, M.; Colli, M.L.; Yi, X.; Khamis, A.; Carrat, G.R.; Rutter, G.A.; Bugliani, M.; Giusti, L.; et al. Persistent or Transient Human β Cell Dysfunction Induced by Metabolic Stress: Specific Signatures and Shared Gene Expression with Type 2 Diabetes. Cell Rep. 2020, 33, 108466. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Eich, T.M.; Felldin, M.; Foss, A.; Källen, R.; Salmela, K.; Tibell, A.; Tufveson, G.; Fujimori, K.; Engkvist, M.; et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 2004, 78, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Skog, O.; Korsgren, S.; Wiberg, A.; Danielsson, A.; Edwin, B.; Buanes, T.; Krogvold, L.; Korsgren, O.; Dahl-Jørgensen, K. Expression of human leukocyte antigen class I in endocrine and exocrine pancreatic tissue at onset of type 1 diabetes. Am. J. Pathol. 2015, 185, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Suleiman, M.; Marselli, L. Organ donor pancreases for the study of human islet cell histology and pathophysiology: A precious and valuable resource. Diabetologia 2018, 61, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Cefalo, C.M.A.; Cinti, F.; Quero, G.; Pontecorvi, A.; Alfieri, S.; Holst, J.J.; Giaccari, A. Endocrine and Metabolic Insights from Pancreatic Surgery. Trends Endocrinol. Metab. 2020, 31, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Clemente, G.; Sorice, G.P.; Conte, C.; De Rose, A.M.; Sun, V.A.; Cefalo, C.M.; Pontecorvi, A.; Nuzzo, G.; Giaccari, A. Metabolic consequences of the occlusion of the main pancreatic duct with acrylic glue after pancreaticoduodenectomy. Am. J. Surg. 2015, 210, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Ferraro, P.M.; Di Giuseppe, G.; Moffa, S.; Cefalo, C.M.; Cinti, F.; Impronta, F.; Capece, U.; Quero, G.; Pontecorvi, A.; et al. Pancreaticoduodenectomy model demonstrates a fundamental role of dysfunctional β cells in predicting diabetes. J. Clin. Investig. 2021, 131, e146788. [Google Scholar] [CrossRef]
- Mezza, T.; Sorice, G.P.; Conte, C.; Sun, V.A.; Cefalo, C.M.; Moffa, S.; Pontecorvi, A.; Mari, A.; Kulkarni, R.N.; Giaccari, A. β-Cell Glucose Sensitivity Is Linked to Insulin/Glucagon Bihormonal Cells in Nondiabetic Humans. J. Clin. Endocrinol. Metab. 2016, 101, 470–475. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef] [PubMed]
- Berkova, Z.; Saudek, F.; Girman, P.; Zacharovova, K.; Kriz, J.; Fabryova, E.; Leontovyc, I.; Koblas, T.; Kosinova, L.; Neskudla, T.; et al. Combining Donor Characteristics with Immunohistological Data Improves the Prediction of Islet Isolation Success. J. Diabetes Res. 2016, 2016, 4214328. [Google Scholar] [CrossRef]
- Komatsu, H.; Cook, C.; Wang, C.H.; Medrano, L.; Lin, H.; Kandeel, F.; Tai, Y.C.; Mullen, Y. Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS ONE 2017, 12, e0183780. [Google Scholar] [CrossRef] [PubMed]
- Dybala, M.P.; Hara, M. Heterogeneity of the Human Pancreatic Islet. Diabetes 2019, 68, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Harrington, S.; Stehno-Bittel, L. The Flaws and Future of Islet Volume Measurements. Cell Transplant. 2018, 27, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, E.; Caporale, R.; Buchi, F.; Masala, E.; Gozzini, A.; Sanna, A.; Sassolini, F.; Valencia, A.; Bosi, A.; Santini, V. Distinct signal transduction abnormalities and erythropoietin response in bone marrow hematopoietic cell subpopulations of myelodysplastic syndrome patients. Clin. Cancer Res. 2012, 18, 3079–3089. [Google Scholar] [CrossRef]
- Colasanti, T.; Alessandri, C.; Capozzi, A.; Sorice, M.; Delunardo, F.; Longo, A.; Pierdominici, M.; Conti, F.; Truglia, S.; Siracusano, A.; et al. Autoantibodies specific to a peptide of β2-glycoprotein I cross-react with TLR4, inducing a proinflammatory phenotype in endothelial cells and monocytes. Blood 2012, 120, 3360–3370. [Google Scholar] [CrossRef] [PubMed]
- Pievani, A.; Belussi, C.; Klein, C.; Rambaldi, A.; Golay, J.; Introna, M. Enhanced killing of human B-cell lymphoma targets by combined use of cytokine-induced killer cell (CIK) cultures and anti-CD20 antibodies. Blood 2011, 117, 510–518. [Google Scholar] [CrossRef] [PubMed]
- NIH CIT Consortium Chemistry Manufacturing Controls Monitoring Committee; NIH CIT Consortium. Purified Human Pancreatic Islet: Qualitative and Quantitative Assessment of Islets Using Dithizone (DTZ): Standard Operating Procedure of the NIH Clinical Islet Transplantation Consortium. CellR4 Cell. Repair Replace. Regen. Reprogramming 2015, 3, e1369. [Google Scholar]
- Kaddis, J.S.; Danobeitia, J.S.; Niland, J.C.; Stiller, T.; Fernandez, L.A. Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes. Am. J. Transplant. 2010, 10, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Misawa, R.; Zielinski, M.C.; Cowen, P.; Jo, J.; Periwal, V.; Ricordi, C.; Khan, A.; Szust, J.; Shen, J.; et al. Regional differences in islet distribution in the human pancreas—Preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS ONE 2013, 8, e67454. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.J.; Zhai, X.; LeGatt, D.F.; Cheng, S.B.; Shapiro, A.M.J.; Lakey, J.R.T. Quantitative assessment of collagenase blends for human islet isolation. Transplantation 2005, 80, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Ko, S.H.; Cho, J.H.; Lee, J.M.; Ahn, Y.B.; Song, K.H.; Yoo, S.J.; Kang, M.I.; Cha, B.Y.; Lee, K.W.; et al. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J. Clin. Endocrinol. Metab. 2003, 88, 2300–2308. [Google Scholar] [CrossRef]
- Meier, J.J.; Breuer, T.G.K.; Bonadonna, R.C.; Tannapfel, A.; Uhl, W.; Schmidt, W.E.; Schrader, H.; Menge, B.A. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia 2012, 55, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Cohrs, C.M.; Chen, C.; Jahn, S.R.; Stertmann, J.; Chmelova, H.; Weitz, J.; Bähr, A.; Klymiuk, N.; Steffen, A.; Ludwig, B.; et al. Vessel Network Architecture of Adult Human Islets Promotes Distinct Cell-Cell Interactions In Situ and Is Altered after Transplantation. Endocrinology 2017, 158, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Uno, S.; Iwahashi, H.; Fujita, Y.; Yoshikawa, A.; Kozawa, J.; Okita, K.; Takiuchi, D.; Eguchi, H.; Nagano, H.; et al. Predominance of β-Cell Neogenesis Rather Than Replication in Humans with an Impaired Glucose Tolerance and Newly Diagnosed Diabetes. J. Clin. Endocrinol. Metab. 2013, 98, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Okitsu, T.; Iwanaga, Y.; Noguchi, H.; Nagata, H.; Yonekawa, Y.; Yamada, Y.; Nakai, Y.; Ueda, M.; Ishii, A.; et al. Insulin independence of unstable diabetic patient after single living donor islet transplantation. Transplant. Proc. 2005, 37, 3427–3429. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, Y.H.; Lee, S.; Lee, Y.N.; Go, H.S.; Hwang, D.W.; Song, K.B.; Lee, J.H.; Lee, W.; So, S.; et al. The Outcomes and Quality of Pancreatic Islet Cells Isolated from Surgical Specimens for Research on Diabetes Mellitus. Cells 2022, 11, 2335. [Google Scholar] [CrossRef] [PubMed]
- Krogvold, L.; Wiberg, A.; Edwin, B.; Buanes, T.; Jahnsen, F.L.; Hanssen, K.F.; Larsson, E.; Korsgren, O.; Skog, O.; Dahl-Jørgensen, K. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia 2016, 59, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Ehehalt, F.; Knoch, K.; Erdmann, K.; Krautz, C.; Jäger, M.; Steffen, A.; Wegbrod, C.; Meisterfeld, R.; Kersting, S.; Bergert, H.; et al. Impaired insulin turnover in islets from type 2 diabetic patients. Islets 2010, 2, 30–36. [Google Scholar] [CrossRef]
- Braganza, J.M.; Lee, S.H.; McCloy, R.F.; McMahon, M.J. Chronic pancreatitis. Lancet 2011, 377, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Eskaros, A.; Windon, A.L.; Saunders, D.C.; Bottino, R.; Human Pancreas Analysis Program; Brissova, M.; Powers, A.C. 1374-P: In Type 2 Diabetes, the Exocrine Pancreas Has Greater Fibrosis, Fat, Metaplastic Changes, and Microangiopathy. Diabetes 2022, 71 (Suppl. 1), 1374-P. [Google Scholar] [CrossRef]
- Collier, J.J.; Sparer, T.E.; Karlstad, M.D.; Burke, S.J. Pancreatic islet inflammation: An emerging role for chemokines. J. Mol. Endocrinol. 2017, 59, R33–R46. [Google Scholar] [CrossRef]
- Ferdek, P.E.; Krzysztofik, D.; Stopa, K.B.; Kusiak, A.A.; Paw, M.; Wnuk, D.; Jakubowska, M.A. When healing turns into killing—The pathophysiology of pancreatic and hepatic fibrosis. J. Physiol. 2022, 600, 2579–2612. [Google Scholar] [CrossRef] [PubMed]
- Rickels, M.R.; Robertson, R.P. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr. Rev. 2019, 40, 631–668. [Google Scholar] [CrossRef] [PubMed]
- Cayabyab, F.; Nih, L.R.; Yoshihara, E. Advances in Pancreatic Islet Transplantation Sites for the Treatment of Diabetes. Front. Endocrinol. 2021, 12, 732431. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.C. Glucose-induced insulin secretion in isolated human islets: Does it truly reflect β-cell function in vivo? Mol. Metab. 2021, 48, 101212. [Google Scholar] [CrossRef]
- Porte, D. Banting lecture 1990. Beta-cells in type II diabetes mellitus. Diabetes 1991, 40, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.K.; Bolgiano, D.C.; McKnight, B.; Halter, J.B.; Porte, D. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J. Clin. Investig. 1984, 74, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Gorden, P.; Hendricks, C.M.; Roth, J. Circulating proinsulin-like component in man: Increased proportion in hypoinsulinemic states. Diabetologia 1974, 10, 469–474. [Google Scholar] [CrossRef]
- Mezza, T.; Ferraro, P.M.; Sun, V.A.; Moffa, S.; Cefalo, C.M.A.; Quero, G.; Cinti, F.; Sorice, G.P.; Pontecorvi, A.; Folli, F.; et al. Increased β-Cell Workload Modulates Proinsulin-to-Insulin Ratio in Humans. Diabetes 2018, 67, 2389–2396. [Google Scholar] [CrossRef]
- Wigger, L.; Barovic, M.; Brunner, A.D.; Marzetta, F.; Schöniger, E.; Mehl, F.; Kipke, N.; Friedland, D.; Burdet, F.; Kessler, C.; et al. Multi-omics profiling of living human pancreatic islet donors reveals heterogeneous beta cell trajectories towards type 2 diabetes. Nat. Metab. 2021, 3, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Veres, A.; Wolock, S.L.; Faust, A.L.; Gaujoux, R.; Vetere, A.; Ryu, J.H.; Wagner, B.K.; Shen-Orr, S.S.; Klein, A.M.; et al. A Single-Cell Transcriptomic Map of the Human and Mouse Pancreas Reveals Inter- and Intra-cell Population Structure. Cell Syst. 2016, 3, 346–360.e4. [Google Scholar] [CrossRef] [PubMed]
- Johnston, N.R.; Mitchell, R.K.; Haythorne, E.; Pessoa, M.P.; Semplici, F.; Ferrer, J.; Piemonti, L.; Marchetti, P.; Bugliani, M.; Bosco, D.; et al. Beta Cell Hubs Dictate Pancreatic Islet Responses to Glucose. Cell Metab. 2016, 24, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Takita, M.; Shimoda, M.; Itoh, T.; Sugimoto, K.; Noguchi, H.; Naziruddin, B.; Levy, M.F.; Matsumoto, S. Large human islets secrete less insulin per islet equivalent than smaller islets in vitro. Islets 2011, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Brusco, N.; Sebastiani, G.; Di Giuseppe, G.; Licata, G.; Grieco, G.E.; Fignani, D.; Nigi, L.; Formichi, C.; Aiello, E.; Auddino, S.; et al. Intra-islet insulin synthesis defects are associated with endoplasmic reticulum stress and loss of beta cell identity in human diabetes. Diabetologia 2023, 66, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Shirakawa, J.; Martinez, R.; Hu, J.; Giaccari, A.; Kulkarni, R.N. Nuclear Export of FoxO1 Is Associated with ERK Signaling in β-Cells Lacking Insulin Receptors. J. Biol. Chem. 2016, 291, 21485–21495. [Google Scholar] [CrossRef] [PubMed]

| Subject Characteristics | ND (n = 14) | IGT (n = 8) | DM (n = 15) | p Value |
|---|---|---|---|---|
| Mean age (y) | 58.1 ± 4.02 | 66 ± 4.38 | 73.4 ± 2.04 | |
| Gender (F/M) | 7/7 | 3/5 | 9/6 | |
| BMI (kg/m2) | 24.67 ± 1.56 | 25.04 ± 1.55 | 23.70 ± 0.94 | 0.77 |
| Insulin sensitivity (mg·kg−1·min−1) | 4.60 ± 1.36 | 3.20 ± 0.48 | 4.90 ± 1.37 | 0.64 |
| Fasting glucose (mg/dL) | 86.5 ± 2.29 | 95.6 ± 5.27 | 129.4 ± 12.63 | 0.003 * |
| Fasting insulin (µUI/mL) | 4 ± 0.40 | 4.9 ± 0.53 | 4.92 ± 1.11 | 0.358 |
| Fasting C-peptide (ng/mL) | 1.54 ± 0.29 | 1.13 ± 0.10 | 2.05 ± 0.29 | 0.87 |
| AUC insulin (µUI/mL) | 11,351.1 ± 1513.6 | 12,921.5 ± 1730.4 | 5433 ± 1643 | 0.0129 * |
| AUC C-peptide (ng/mL) | 1159.9 ± 107.8 | 1343.1 ± 112.5 | 703,7 ± 114.7 | 0.0036 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Piazza, E.; Todi, L.; Di Giuseppe, G.; Soldovieri, L.; Ciccarelli, G.; Brunetti, M.; Quero, G.; Alfieri, S.; Tondolo, V.; Pontecorvi, A.; et al. Advancing Diabetes Research: A Novel Islet Isolation Method from Living Donors. Int. J. Mol. Sci. 2024, 25, 5936. https://doi.org/10.3390/ijms25115936
Di Piazza E, Todi L, Di Giuseppe G, Soldovieri L, Ciccarelli G, Brunetti M, Quero G, Alfieri S, Tondolo V, Pontecorvi A, et al. Advancing Diabetes Research: A Novel Islet Isolation Method from Living Donors. International Journal of Molecular Sciences. 2024; 25(11):5936. https://doi.org/10.3390/ijms25115936
Chicago/Turabian StyleDi Piazza, Eleonora, Laura Todi, Gianfranco Di Giuseppe, Laura Soldovieri, Gea Ciccarelli, Michela Brunetti, Giuseppe Quero, Sergio Alfieri, Vincenzo Tondolo, Alfredo Pontecorvi, and et al. 2024. "Advancing Diabetes Research: A Novel Islet Isolation Method from Living Donors" International Journal of Molecular Sciences 25, no. 11: 5936. https://doi.org/10.3390/ijms25115936
APA StyleDi Piazza, E., Todi, L., Di Giuseppe, G., Soldovieri, L., Ciccarelli, G., Brunetti, M., Quero, G., Alfieri, S., Tondolo, V., Pontecorvi, A., Gasbarrini, A., Nista, E. C., Giaccari, A., Pani, G., & Mezza, T. (2024). Advancing Diabetes Research: A Novel Islet Isolation Method from Living Donors. International Journal of Molecular Sciences, 25(11), 5936. https://doi.org/10.3390/ijms25115936






