Petanin Potentiated JNK Phosphorylation to Negatively Regulate the ERK/CREB/MITF Signaling Pathway for Anti-Melanogenesis in Zebrafish
Abstract
1. Introduction
2. Results and Discussion
2.1. Melanin Inhibition Rate
2.2. Tyrosinase Activity in Zebrafish
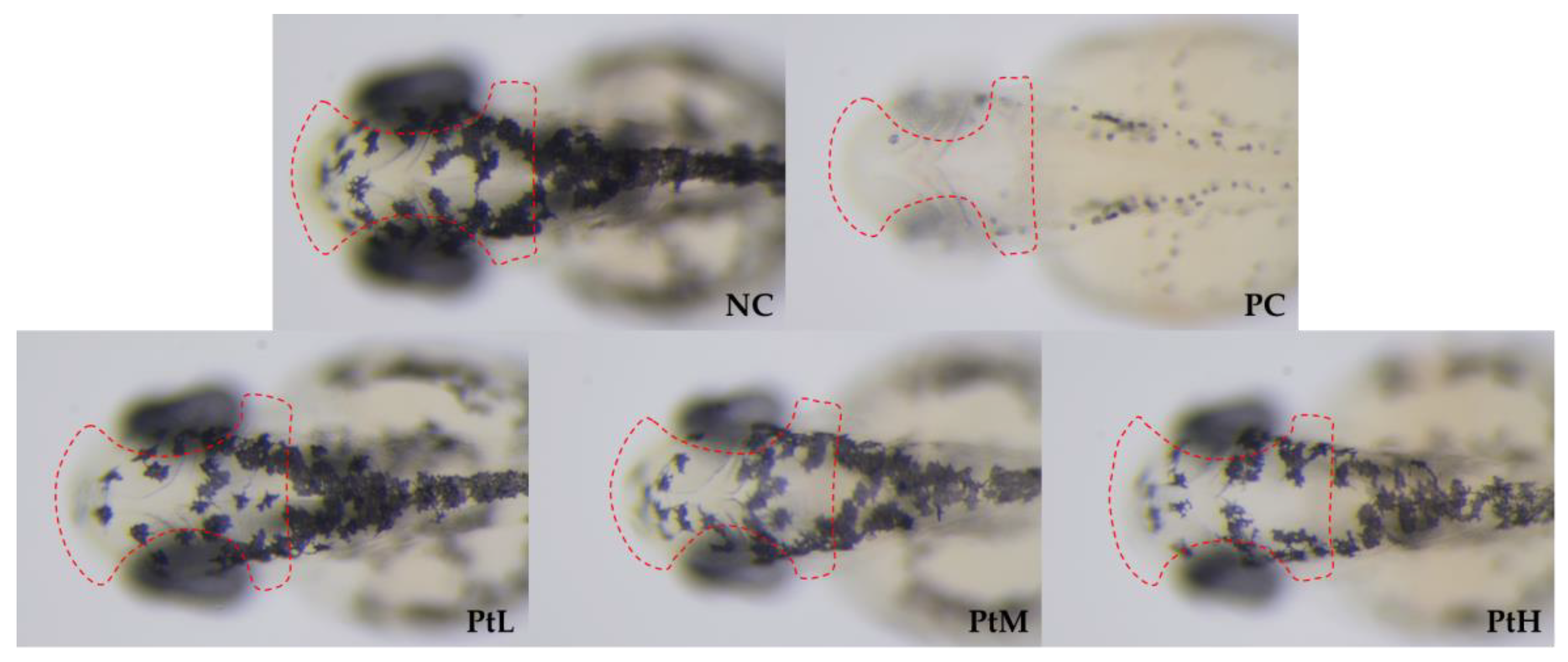
2.3. Structure and Distribution of Melanocytes and Melanosomes
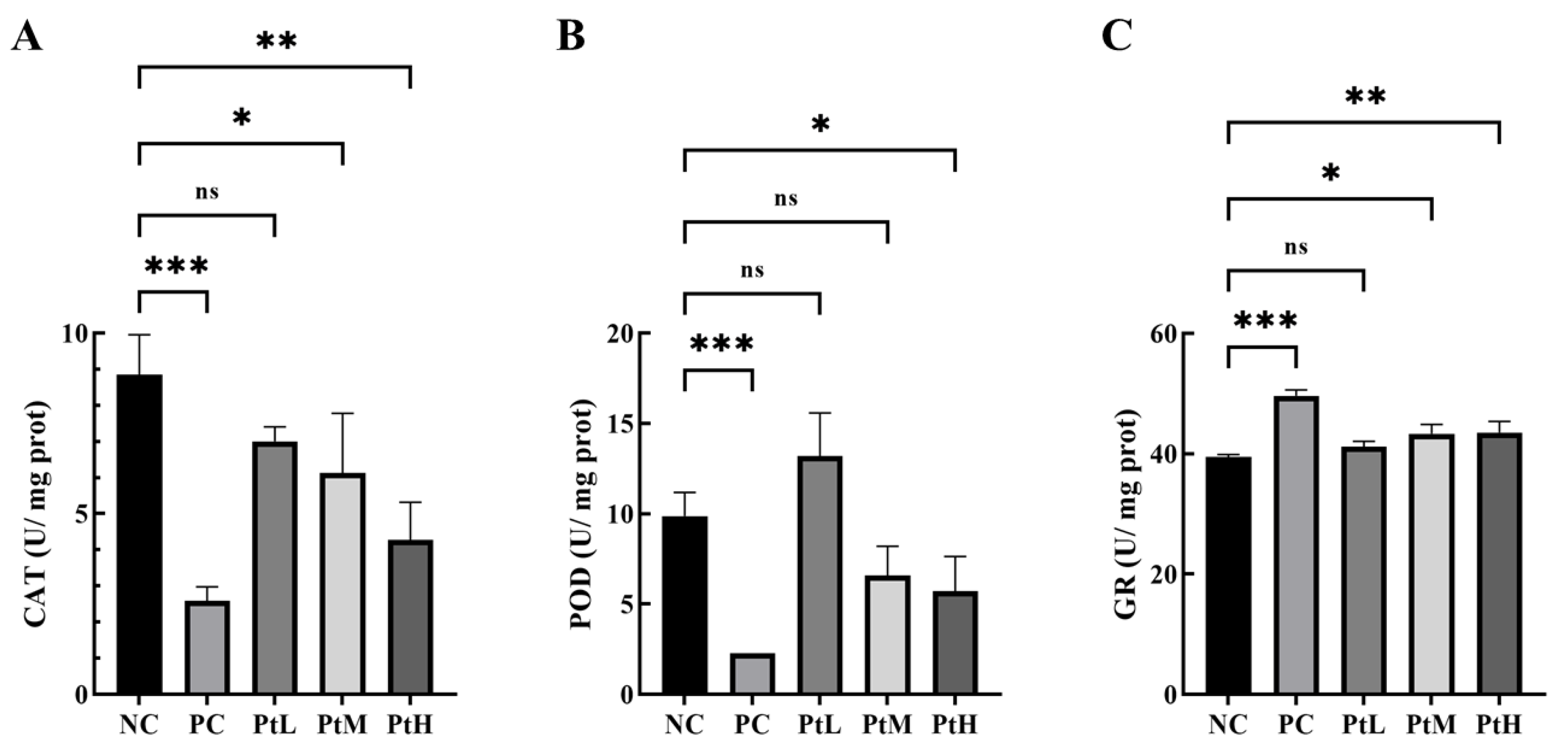
2.4. Effect of Petanin on Oxidoreductase Activity in Zebrafish
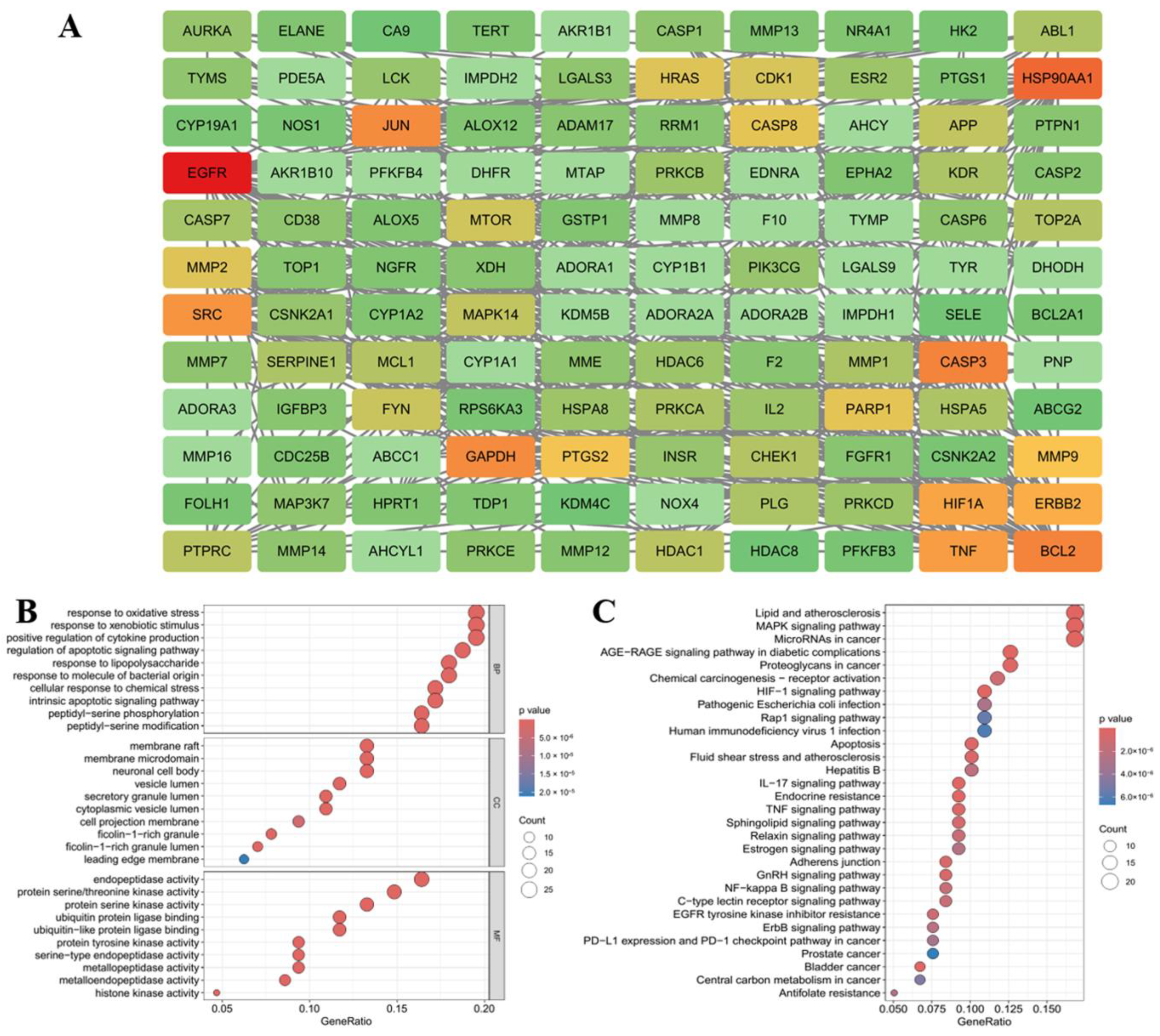
2.5. Network Pharmacological Analysis of the Anti-Melanogenic Effect of Petanin
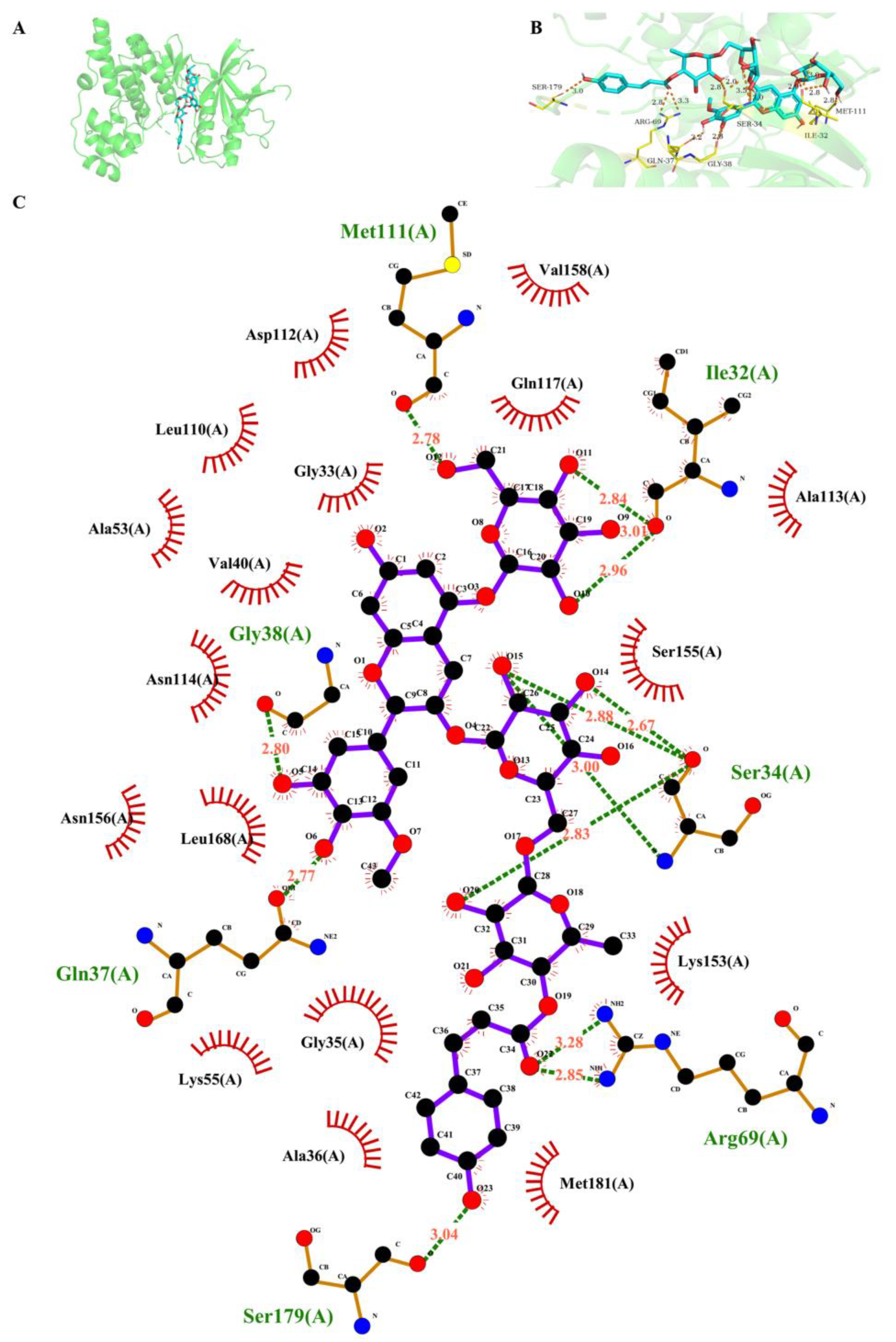
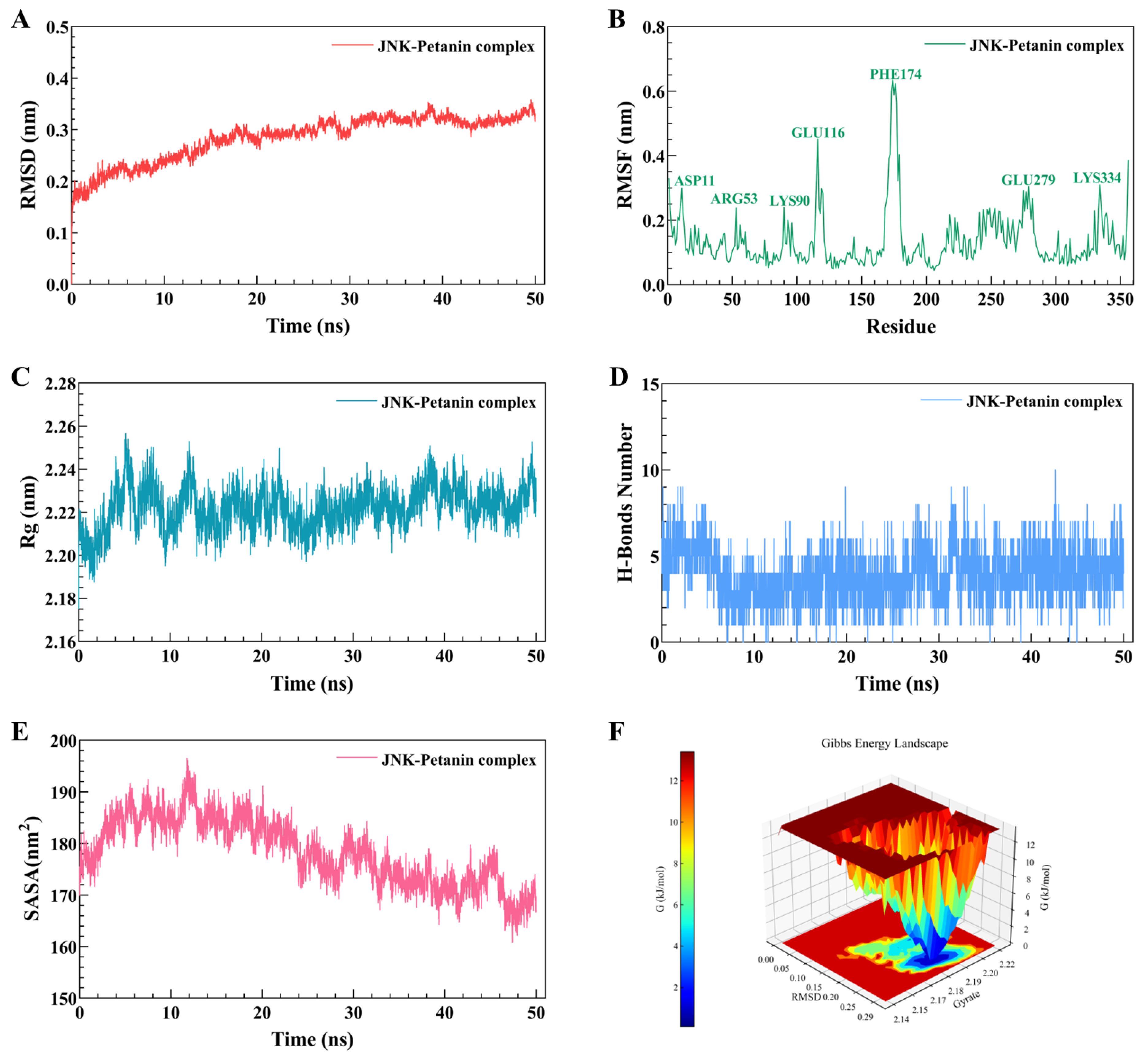
2.6. Molecular Docking and Dynamic Simulation of Petanin with Key Signaling Pathway Proteins
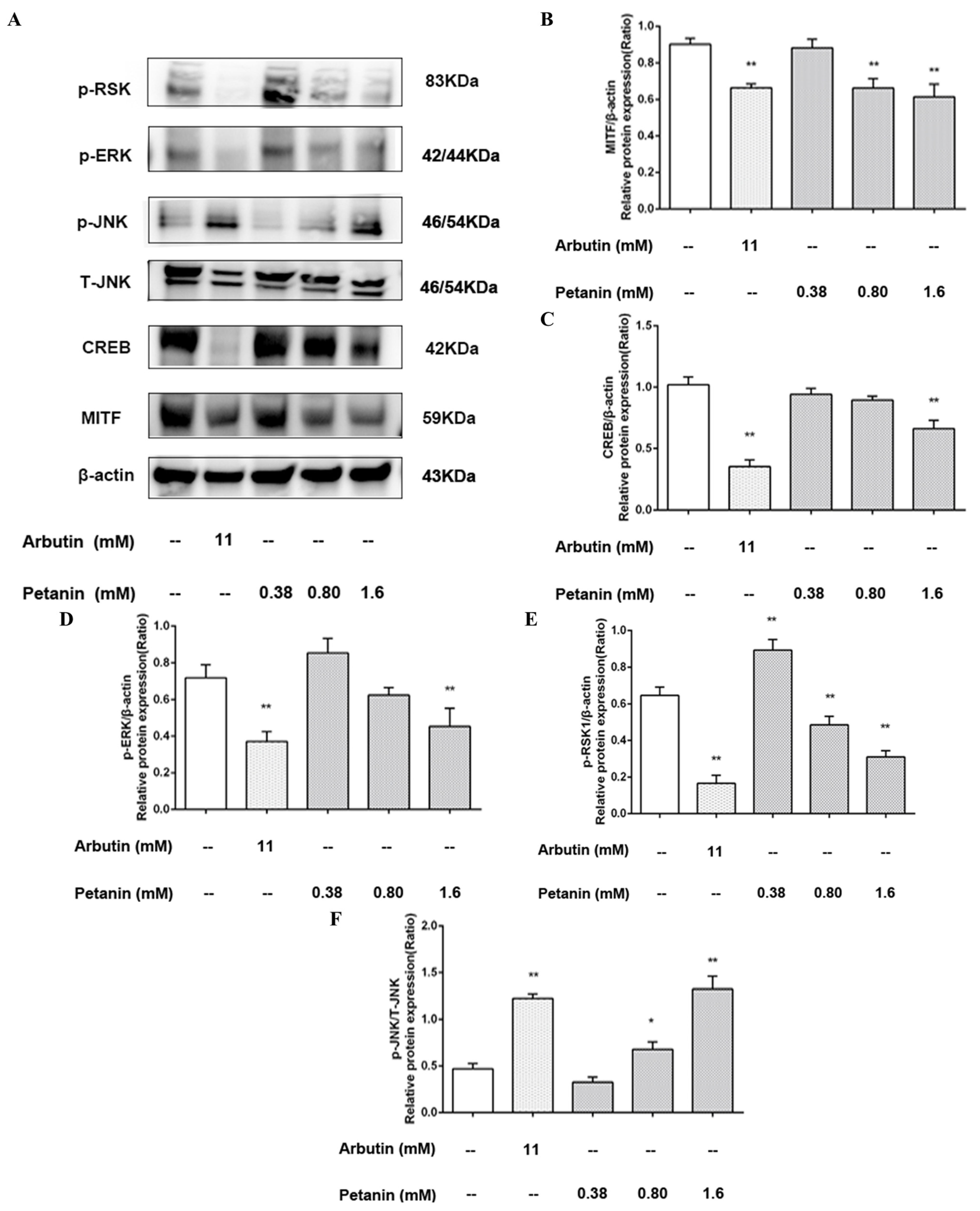
2.7. Effect of Petanin on the Expression of Proteins Related to Melanin Production in Zebrafish
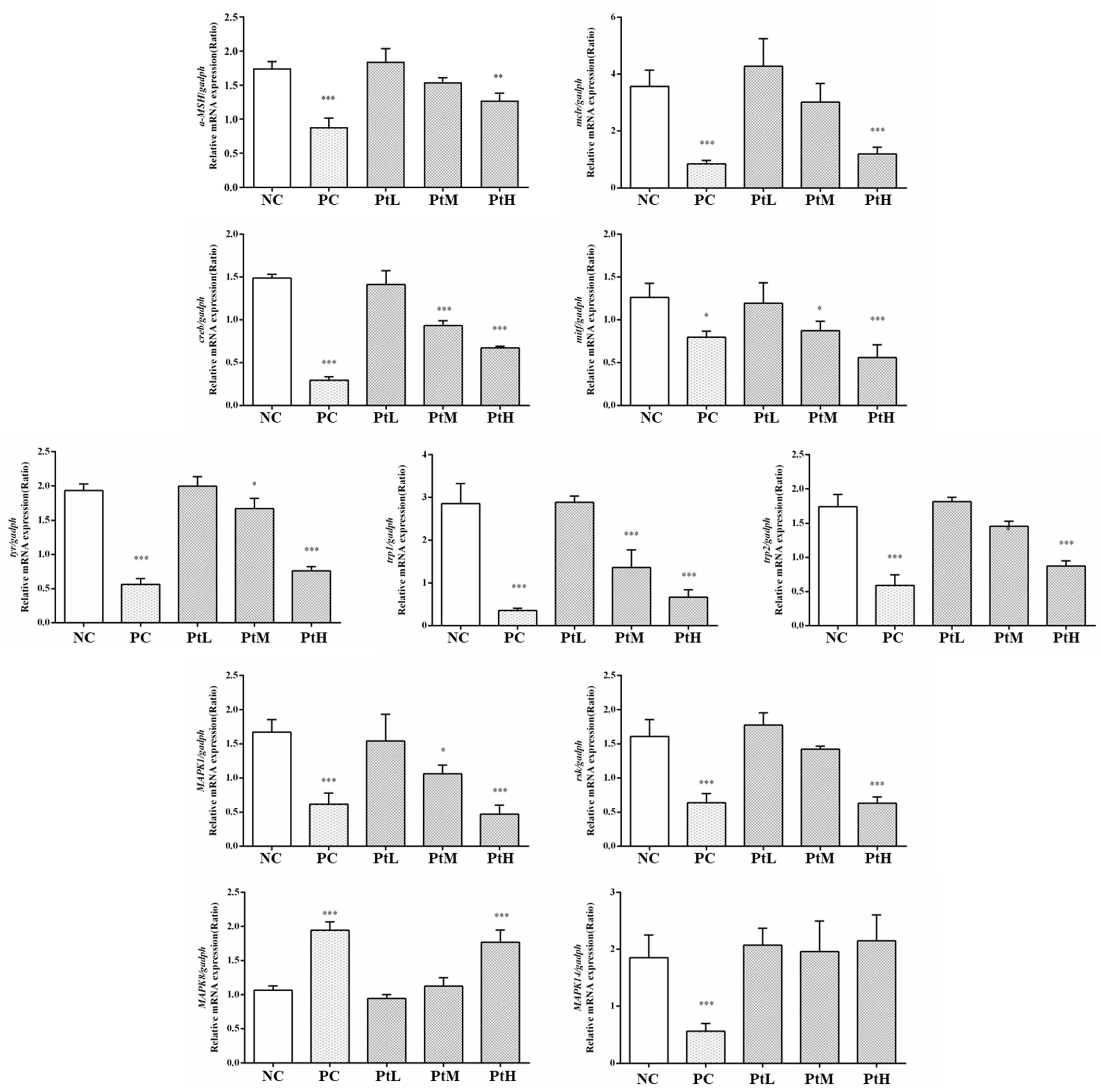
2.8. Effect of Petanin on Transcription of mRNAs Related to Melanogenesis in Zebrafish
3. Materials and Methods
3.1. Samples and Reagents
3.2. Melanin Inhibition Rate in Zebrafish
3.3. Tyrosinase Activity Assay in Zebrafish
3.4. Detection of CAT, POD and GR Contents in Zebrafish Larvae
3.5. HE Staining
3.6. TEM
3.7. Network Pharmacological Analysis
3.8. Molecular Docking and Molecular Dynamics’ Simulation
3.9. Western Blot
3.10. Gene Expression Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yotsumoto, H.; Tian, Y.; Sakamoto, K. α-Mangostin suppressed melanogenesis in B16F10 murine melanoma cells through GSK3β and ERK signaling pathway. Biochem. Biophys. Rep. 2021, 26, 100949. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Ko, D.; Friedman, B.J.; Lim, H.W.; Mohammad, T.F. Disorders of hyperpigmentation. Part I. Pathogenesis and clinical features of common pigmentary disorders. J. Am. Acad. Dermatol. 2023, 88, 271–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-Y.; You, Y.-J.; Liu, Y.-J.; Hou, C.-W.; Wu, C.-S.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Sesamol inhibited melanogenesis by regulating melanin-related signal transduction in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 1108. [Google Scholar] [CrossRef] [PubMed]
- Del Marmol, V.; Beermann, F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996, 381, 165–168. [Google Scholar] [CrossRef]
- Oh, G.-W.; Ko, S.-C.; Heo, S.-Y.; Nguyen, V.-T.; Kim, G.; Jang, C.H.; Park, W.S.; Choi, I.-W.; Qian, Z.-J.; Jung, W.-K. A novel peptide purified from the fermented microalga Pavlova lutheri attenuates oxidative stress and melanogenesis in B16F10 melanoma cells. Process Biochem. 2015, 50, 1318–1326. [Google Scholar] [CrossRef]
- Karkoszka, M.; Rok, J.; Wrześniok, D. Melanin Biopolymers in Pharmacology and Medicine-Skin Pigmentation Disorders, Implications for Drug Action, Adverse Effects and Therapy. Pharmaceuticals 2024, 17, 521. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ding, Y.; Wang, D.; Deng, Y.; Zhao, Y. Radio frequency-assisted enzymatic extraction of anthocyanins from Akebia trifoliata (Thunb.) Koidz. flowers: Process optimization, structure, and bioactivity determination. Ind. Crops Prod. 2020, 149, 112327. [Google Scholar]
- Karunarathne, W.A.H.M.; Molagoda, I.M.N.; Park, S.R.; Kim, J.W.; Lee, O.-K.; Kwon, H.Y.; Oren, M.; Choi, Y.H.; Ryu, H.W.; Oh, S.-R. Anthocyanins from Hibiscus syriacus L. inhibit melanogenesis by activating the ERK signaling pathway. Biomolecules 2019, 9, 645. [Google Scholar] [CrossRef]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Germanò, M.P. The hull of ripe pistachio nuts (Pistacia vera L.) as a source of new promising melanogenesis inhibitors. Plant Foods Hum. Nutr. 2021, 76, 111–117. [Google Scholar] [CrossRef]
- Hanamura, T.; Uchida, E.; Aoki, H. Skin-lightening effect of a polyphenol extract from Acerola (Malpighia emarginata DC.) fruit on UV-induced pigmentation. Biosci. Biotechnol. Biochem. 2008, 72, 3211–3218. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Kim, J.H.; Kim, M.O.; Jang, S.; Kang, M.; Oh, S.W.; Nho, Y.H.; Kang, S.H.; Kim, M.H.; Park, S.-H. Afzelin positively regulates melanogenesis through the p38 MAPK pathway. Chem.-Biol. Interact. 2016, 254, 167–172. [Google Scholar] [CrossRef]
- Zamudio Díaz, D.F.; Busch, L.; Kröger, M.; Klein, A.L.; Lohan, S.B.; Mewes, K.R.; Vierkotten, L.; Witzel, C.; Rohn, S.; Meinke, M.C. Significance of melanin distribution in the epidermis for the protective effect against UV light. Sci. Rep. 2024, 14, 3488. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.L.; Praetorius, C.; Bergsteinsdóttir, K.; Hallsson, J.H.; Gísladóttir, B.K.; Schepsky, A.; Swing, D.A.; O’Sullivan, T.N.; Arnheiter, H.; Bismuth, K. The role of MITF phosphorylation sites during coat color and eye development in mice analyzed by bacterial artificial chromosome transgene rescue. Genetics 2009, 183, 581–594. [Google Scholar] [CrossRef]
- Yardman-Frank, J.M.; Fisher, D.E. Skin pigmentation and its control: From ultraviolet radiation to stem cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Fisher, D.E. MITF and UV responses in skin: From pigmentation to addiction. Pigment Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef]
- Goding, C.R.; Arnheiter, H. MITF—The first 25 years. Genes Dev. 2019, 33, 983–1007. [Google Scholar] [CrossRef]
- Primot, A.; Mogha, A.; Corre, S.; Roberts, K.; Debbache, J.; Adamski, H.; Dreno, B.; Khammari, A.; Lesimple, T.; Mereau, A. ERK-regulated differential expression of the Mitf 6a/b splicing isoforms in melanoma. Pigment Cell Melanoma Res. 2010, 23, 93–102. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hong, A.-r.; Kim, Y.-H.; Yoo, H.; Kang, S.-W.; Chang, S.E.; Song, Y. JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression. Theranostics 2020, 10, 4017. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.-A.; Cho, S.K. Phytol suppresses melanogenesis through proteasomal degradation of MITF via the ROS-ERK signaling pathway. Chem.-Biol. Interact. 2018, 286, 132–140. [Google Scholar] [CrossRef] [PubMed]
- In, K.-R.; Kang, M.A.; Kim, S.D.; Shin, J.; Kang, S.U.; Park, T.J.; Kim, S.-J.; Lee, J.-S. Anhydrous Alum Inhibits α-MSH-Induced Melanogenesis by Down-Regulating MITF via Dual Modulation of CREB and ERK. Int. J. Mol. Sci. 2023, 24, 14662. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hu, J.; Ni, H.; Jiang, Z.; Wang, L. Research progress in melanogenesis signaling pathway. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2019, 35, 1633–1642. [Google Scholar]
- Ouyang, J.; Hu, N.; Wang, H. Isolation, Purification and Tyrosinase Inhibitory Activity of Anthocyanins and Their Novel Degradation Compounds from Solanum tuberosum L. Molecules 2024, 29, 1492. [Google Scholar] [CrossRef] [PubMed]
- Kulkeaw, K.; Ishitani, T.; Kanemaru, T.; Ivanovski, O.; Nakagawa, M.; Mizuochi, C.; Horio, Y.; Sugiyama, D. Cold exposure down-regulates zebrafish pigmentation. Genes Cells 2011, 16, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Nakamura, K.I.; Kondo, S. Pigment cell distributions in different tissues of the zebrafish, with special reference to the striped pigment pattern. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 234, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Nakamura, K.i.; Kanemaru, T.; Shibata, Y.; Kondo, S. Pigment cell organization in the hypodermis of zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2003, 227, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yang, Y.; Lv, M.; Song, W.; Song, Z. Oxidative stress and DNA damage in zebrafish liver due to hydroxyapatite nanoparticles-loaded cadmium. Chemosphere 2018, 202, 498–505. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, Y.; Yu, R.; Chen, L.; Zhao, X. Biological response of zebrafish after short-term exposure to azoxystrobin. Chemosphere 2018, 202, 56–64. [Google Scholar] [CrossRef]
- Mao, L.; Jia, W.; Zhang, L.; Zhang, Y.; Zhu, L.; Sial, M.U.; Jiang, H. Embryonic development and oxidative stress effects in the larvae and adult fish livers of zebrafish (Danio rerio) exposed to the strobilurin fungicides, kresoxim-methyl and pyraclostrobin. Sci. Total Environ. 2020, 729, 139031. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Lu, J.; Xue, H.; Lou, Y.; Li, S.; Liu, T.; Ding, Z.; Chen, X. Anthocyanins from Lycium ruthenicum Murray Mitigate Cadmium-Induced Oxidative Stress and Testicular Toxicity by Activating the Keap1/Nrf2 Signaling Pathway. Pharmaceuticals 2024, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wu, C.-Y.; Wang, C.C.; Lee, C.-H. Exposure to phenols reduces melanogenesis in B16F10 cells and zebrafish. Aquat. Toxicol. 2024, 266, 106806. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Lin, C.-P.; Gowrisankar, Y.V.; Huang, P.-J.; Chang, W.-L.; Shrestha, S.; Hseu, Y.-C. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated α-MSH pathways via Nrf2 activation in keratinocytes. Biochem. Pharmacol. 2021, 185, 114454. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Ho, Y.-G.; Mathew, D.C.; Yen, H.-R.; Chen, X.-Z.; Yang, H.-L. The in vitro and in vivo depigmenting activity of Coenzyme Q10 through the down-regulation of α-MSH signaling pathways and induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated skin keratinocytes. Biochem. Pharmacol. 2019, 164, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg. Chem. 2018, 81, 168–174. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.-H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yoon, J.-H.; Youn, K.; Jun, M. Decursin prevents melanogenesis by suppressing MITF expression through the regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef]
- Singh, S.K.; Sarkar, C.; Mallick, S.; Saha, B.; Bera, R.; Bhadra, R. Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigment Cell Res. 2005, 18, 113–121. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H. Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell. Physiol. 2019, 234, 7330–7340. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-H.; Chiang, Y.-C.; Tsai, M.-H.; Liang, C.-J.; Hsu, L.-F.; Li, S.-Y.; Wang, M.-C.; Yen, F.-L.; Lee, C.-W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Liu, X.; Zheng, Y.; Zhang, Z.; Li, Y.; Che, B.; Liu, G.; Zhang, L.; Dong, C.; Aisa, H.A. The mechanisms of melanogenesis inhibition by glabridin: Molecular docking, PKA/MITF and MAPK/MITF pathways. Food Sci. Hum. Wellness 2023, 12, 212–222. [Google Scholar] [CrossRef]
- Wu, K.-C.; Hseu, Y.-C.; Shih, Y.-C.; Sivakumar, G.; Syu, J.-T.; Chen, G.-L.; Lu, M.-T.; Chu, P.-C. Calycosin, a common dietary isoflavonoid, suppresses melanogenesis through the downregulation of PKA/CREB and p38 MAPK signaling pathways. Int. J. Mol. Sci. 2022, 23, 1358. [Google Scholar] [CrossRef]
- Zhao, N.; Su, X.; Li, H.; Li, Z.; Wang, Y.; Chen, J.; Zhuang, W. Schisandrin B inhibits α-melanocyte-stimulating hormone-induced melanogenesis in B16F10 cells via downregulation of MAPK and CREB signaling pathways. Biosci. Biotechnol. Biochem. 2021, 85, 834–841. [Google Scholar] [CrossRef]
- Fu, T.; Chai, B.; Shi, Y.; Dang, Y.; Ye, X. Fargesin inhibits melanin synthesis in murine malignant and immortalized melanocytes by regulating PKA/CREB and P38/MAPK signaling pathways. J. Dermatol. Sci. 2019, 94, 213–219. [Google Scholar] [CrossRef]
- Kim, T.; Hyun, C.-G. Imperatorin positively regulates melanogenesis through signaling pathways involving Pka/Creb, Erk, Akt, and Gsk3β/Β-catenin. Molecules 2022, 27, 6512. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.H.; Lee, E.S.; Yoo, J.W.; Lee, S.H.; Ko, J.Y.; Kim, Y.J.; Lee, T.R.; Kim, D.Y.; Lee, C.S. Mannosylerythritol lipids inhibit melanogenesis via suppressing ERK-CREB-MiTF-tyrosinase signalling in normal human melanocytes and a three-dimensional human skin equivalent. Exp. Dermatol. 2019, 28, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Niwano, T.; Terazawa, S.; Nakajima, H.; Imokawa, G. The stem cell factor-stimulated melanogenesis in human melanocytes can be abrogated by interrupting the phosphorylation of MSK1: Evidence for involvement of the p38/MSK1/CREB/MITF axis. Arch. Dermatol. Res. 2018, 310, 187–196. [Google Scholar] [CrossRef]
- Tagashira, H.; Miyamoto, A.; Kitamura, S.-i.; Tsubata, M.; Yamaguchi, K.; Takagaki, K.; Imokawa, G. UVB stimulates the expression of endothelin B receptor in human melanocytes via a sequential activation of the p38/MSK1/CREB/MITF pathway which can be interrupted by a French maritime pine bark extract through a direct inactivation of MSK1. PLoS ONE 2015, 10, e0128678. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, M.; Han, J.; Lee, J.-h.; Bae, I.-H.; Choi, H.; Son, E.D.; Park, Y.-H.; Lim, K.-M. Liver X receptor activation inhibits melanogenesis through the acceleration of ERK-mediated MITF degradation. J. Investig. Dermatol. 2013, 133, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Su, T.-R.; Lin, J.-J.; Tsai, C.-C.; Huang, T.-K.; Yang, Z.-Y.; Wu, M.-O.; Zheng, Y.-Q.; Su, C.-C.; Wu, Y.-J. Inhibition of melanogenesis by gallic acid: Possible involvement of the PI3K/Akt, MEK/ERK and Wnt/β-catenin signaling pathways in B16F10 cells. Int. J. Mol. Sci. 2013, 14, 20443–20458. [Google Scholar] [CrossRef]
- Terazawa, S.; Imokawa, G. Signaling cascades activated by UVB in human melanocytes lead to the increased expression of melanocyte receptors, endothelin B receptor and c-KIT. Photochem. Photobiol. 2018, 94, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.B.; Ahmed, A.; Motin, M.A.; Kim, S.; Lee, S.-H. Attenuation of melanogenesis by Nymphaea nouchali (Burm. f) flower extract through the regulation of cAMP/CREB/MAPKs/MITF and proteasomal degradation of tyrosinase. Sci. Rep. 2018, 8, 13928. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.h.; Lee, S.H. Sesamol decreases melanin biosynthesis in melanocyte cells and zebrafish: Possible involvement of MITF via the intracellular cAMP and p38/JNK signalling pathways. Exp. Dermatol. 2015, 24, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Ma, P.-C.; Chen, Z.-Q.; Zhou, W.-Q.; Fu, Y.-J.; Li, L.-J.; Li, C.-R. Inhibition of MITF and tyrosinase by paeonol-stimulated JNK/SAPK to reduction of phosphorylated CREB. Am. J. Chin. Med. 2008, 36, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, T.; Moresco, J.J.; Vaughan, J.M.; Matsumura, S.; Yates, J.R., III; Montminy, M. Analysis of a cAMP regulated coactivator family reveals an alternative phosphorylation motif for AMPK family members. PLoS ONE 2017, 12, e0173013. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Oh, S.W.; Park, S.-H.; Lee, J.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Yu, E.; Cho, J.Y. Melanogenic effects of maclurin are mediated through the activation of cAMP/PKA/CREB and p38 MAPK/CREB signaling pathways. Oxidative Med. Cell. Longev. 2019, 2019, 9827519. [Google Scholar] [CrossRef]
- Jin, M.L.; Park, S.Y.; Kim, Y.H.; Park, G.; Son, H.-J.; Lee, S.-J. Suppression of α-MSH and IBMX-induced melanogenesis by cordycepin via inhibition of CREB and MITF, and activation of PI3K/Akt and ERK-dependent mechanisms. Int. J. Mol. Med. 2012, 29, 119–124. [Google Scholar]
- Ha, J.H.; Jeong, Y.J.; Xuan, S.H.; Lee, J.-Y.; Park, J.; Park, S.N. Methyl-2-acetylamino-3-(4-hydroxyl-3, 5-dimethoxybenzoylthio) propanoate suppresses melanogenesis through ERK signaling pathway mediated MITF proteasomal degradation. J. Dermatol. Sci. 2018, 91, 142–152. [Google Scholar] [CrossRef]
- Cooper, C.D. Insights from zebrafish on human pigment cell disease and treatment. Dev. Dyn. 2017, 246, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, X.; Liang, F.; Li, S.; Zhong, H.; Lin, S. Characterization of two novel small molecules targeting melanocyte development in zebrafish embryogenesis. Pigment Cell Melanoma Res. 2012, 25, 446–453. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef] [PubMed]




| Sample | Melanin Inhibition Rate | Inhibition Rate of Tyrosinase | p-Value |
|---|---|---|---|
| NC | - | - | - |
| PC | 93.52% | 70.59% | ˂0.001 |
| PtL | 13.26% | 14.12% | ˂0.05 |
| PtM | 18.64% | 17.65% | ˂0.01 |
| PtH | 24.86% | 25.88% | ˂0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Hu, N.; Wang, H. Petanin Potentiated JNK Phosphorylation to Negatively Regulate the ERK/CREB/MITF Signaling Pathway for Anti-Melanogenesis in Zebrafish. Int. J. Mol. Sci. 2024, 25, 5939. https://doi.org/10.3390/ijms25115939
Ouyang J, Hu N, Wang H. Petanin Potentiated JNK Phosphorylation to Negatively Regulate the ERK/CREB/MITF Signaling Pathway for Anti-Melanogenesis in Zebrafish. International Journal of Molecular Sciences. 2024; 25(11):5939. https://doi.org/10.3390/ijms25115939
Chicago/Turabian StyleOuyang, Jian, Na Hu, and Honglun Wang. 2024. "Petanin Potentiated JNK Phosphorylation to Negatively Regulate the ERK/CREB/MITF Signaling Pathway for Anti-Melanogenesis in Zebrafish" International Journal of Molecular Sciences 25, no. 11: 5939. https://doi.org/10.3390/ijms25115939
APA StyleOuyang, J., Hu, N., & Wang, H. (2024). Petanin Potentiated JNK Phosphorylation to Negatively Regulate the ERK/CREB/MITF Signaling Pathway for Anti-Melanogenesis in Zebrafish. International Journal of Molecular Sciences, 25(11), 5939. https://doi.org/10.3390/ijms25115939







