Metabolomic Profiling Reveals the Anti-Herbivore Mechanisms of Rice (Oryza sativa)
Abstract
1. Introduction
2. Results
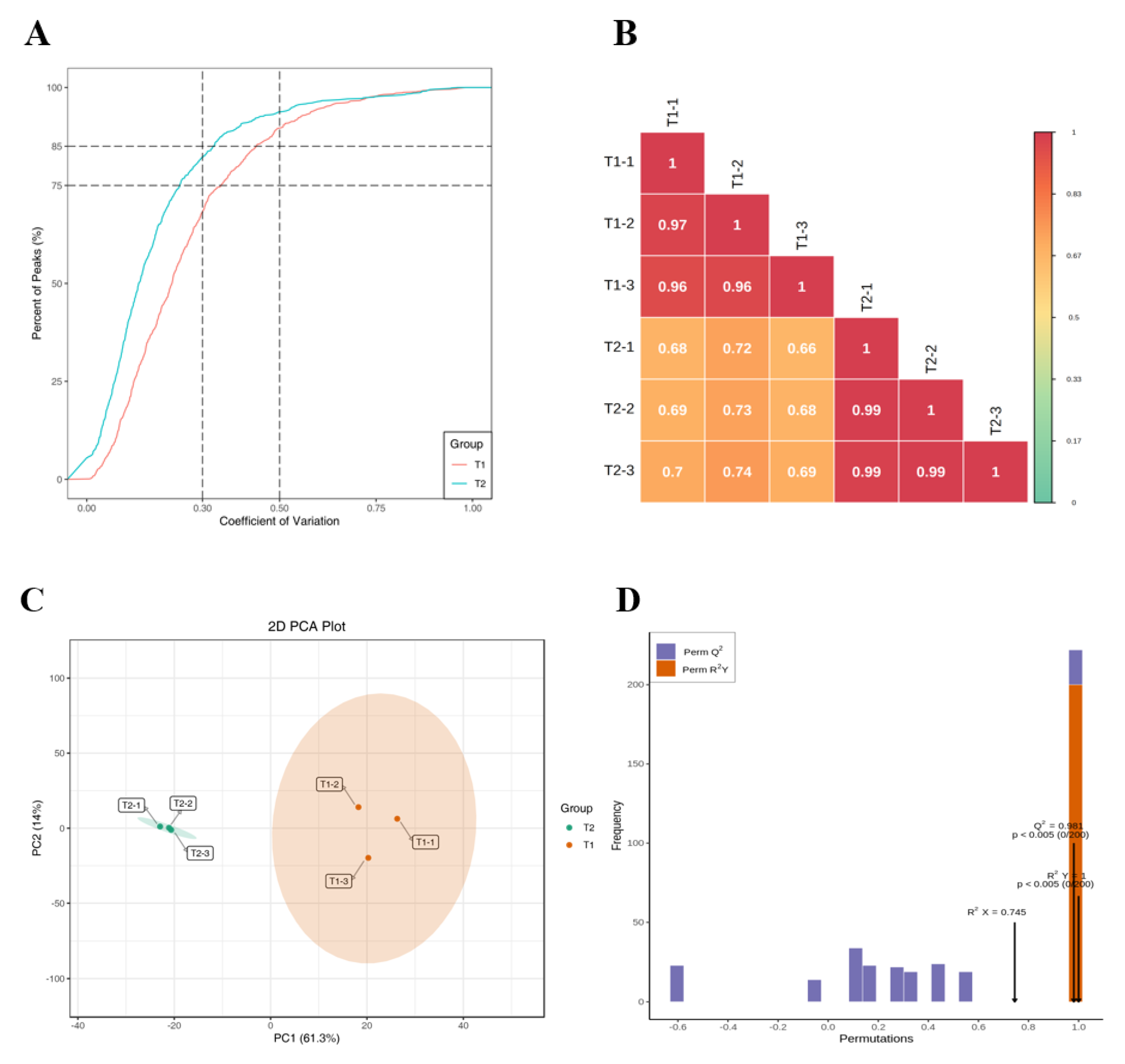
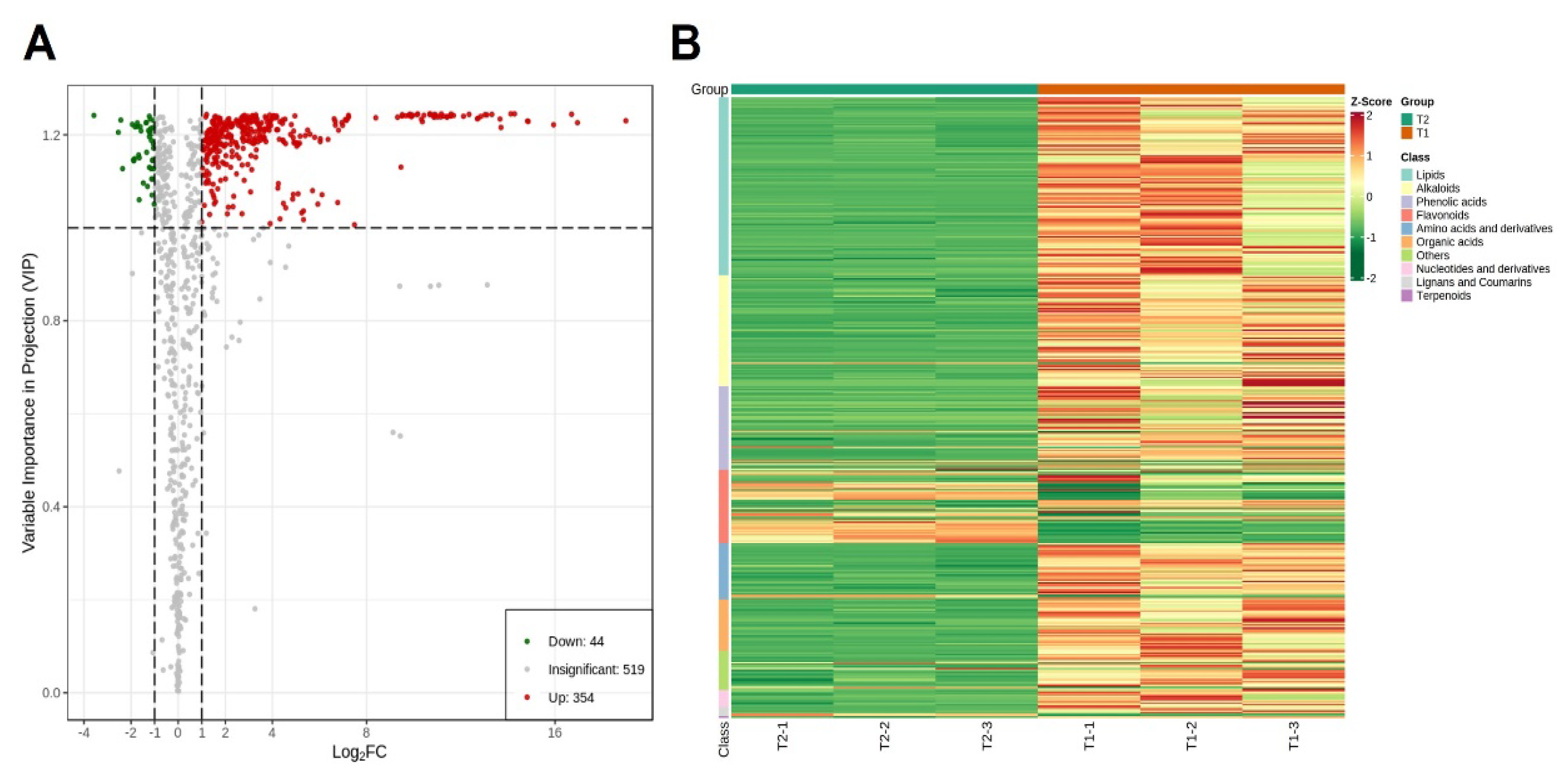
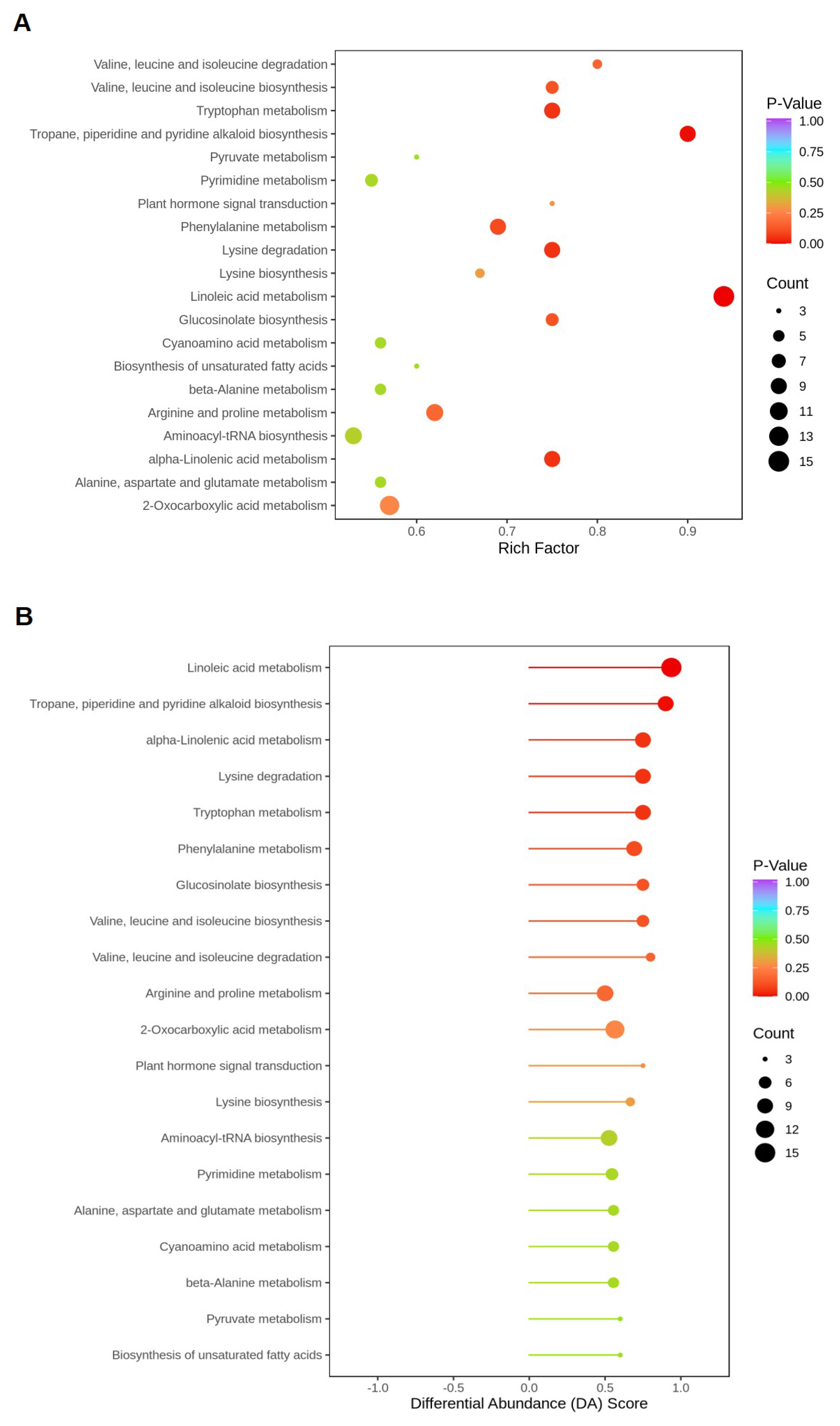
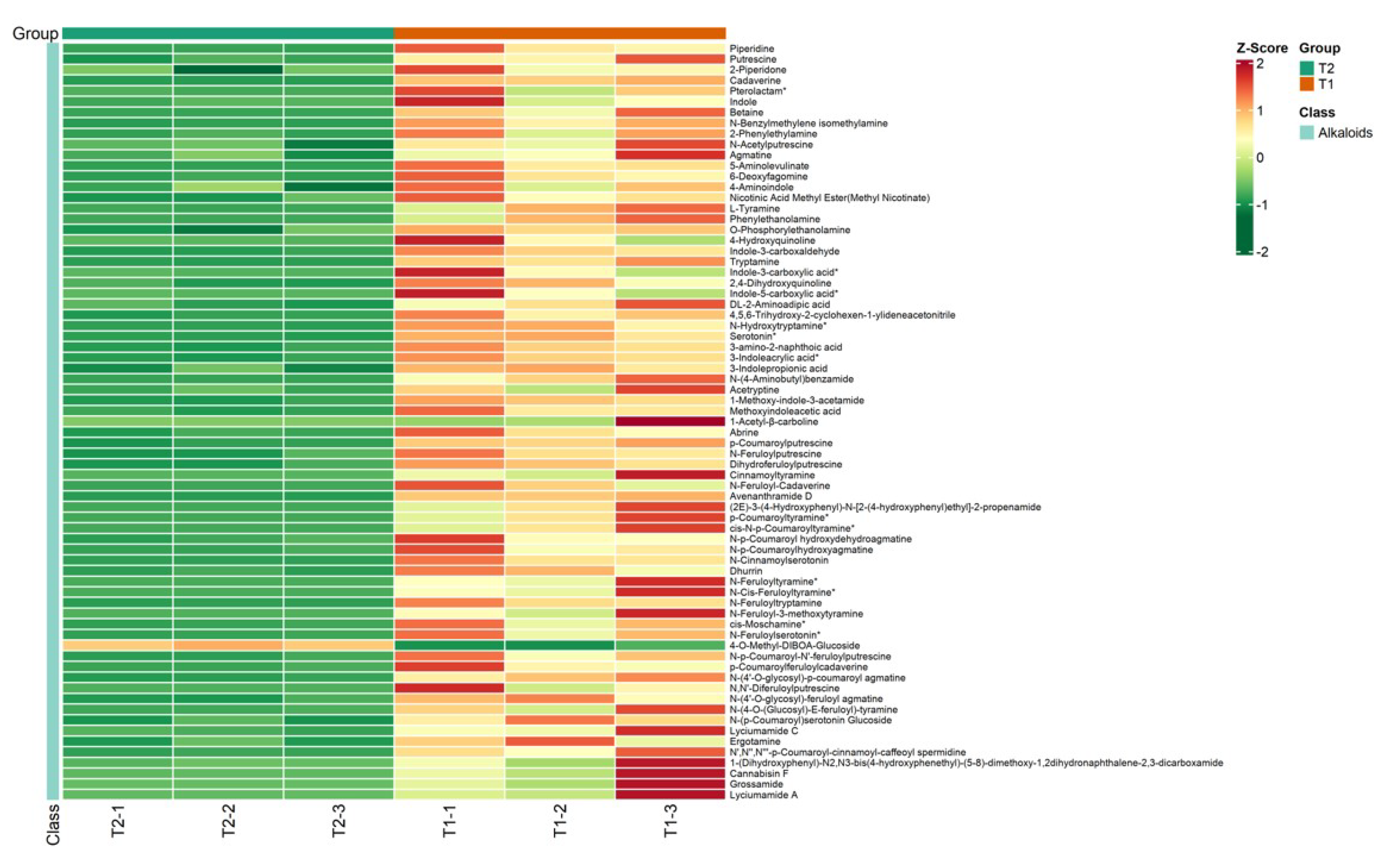
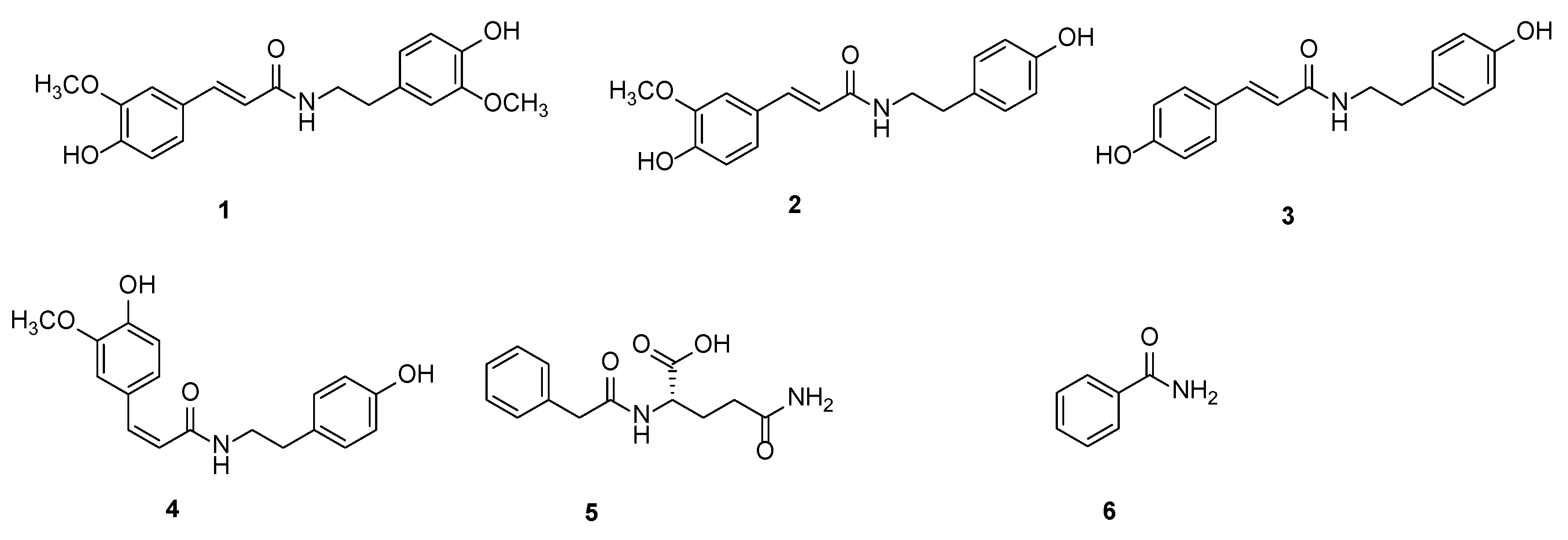
2.1. Results of Metabolites
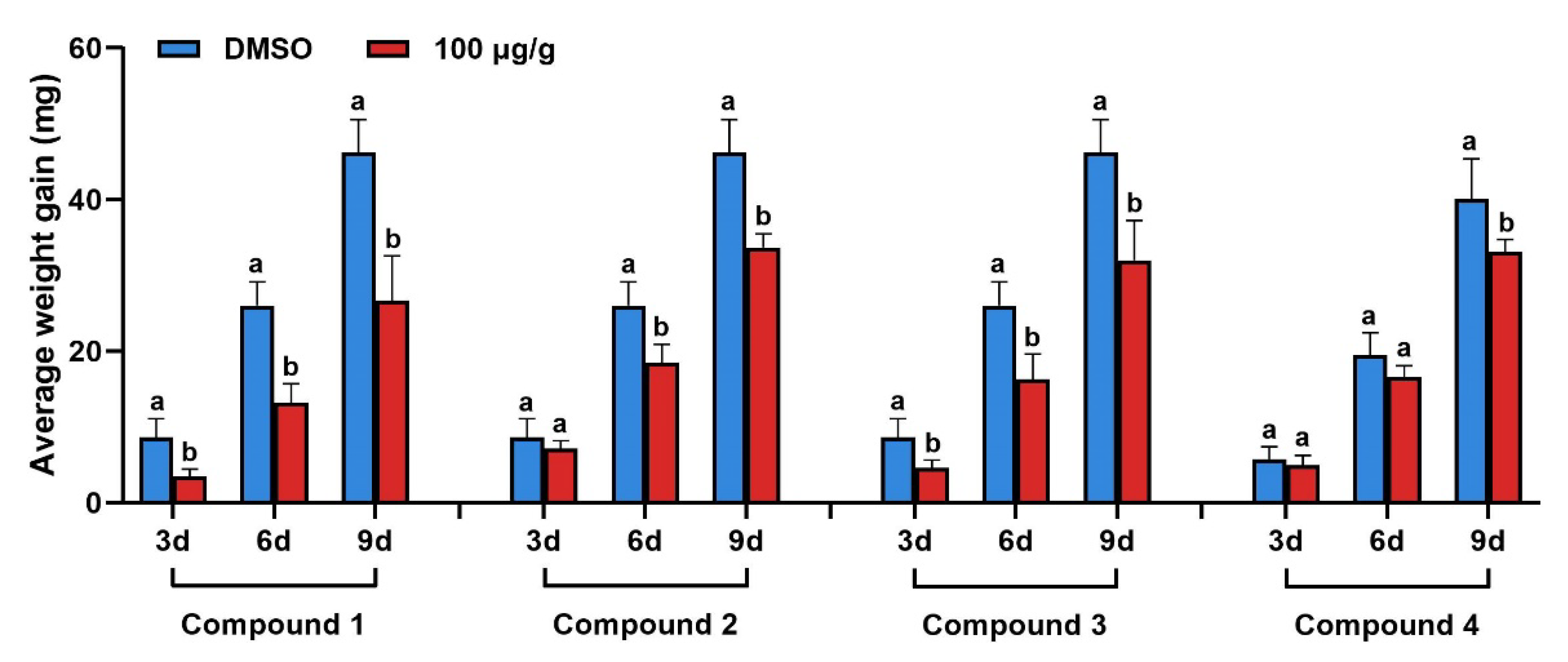
2.2. Results of Insecticidal Activity
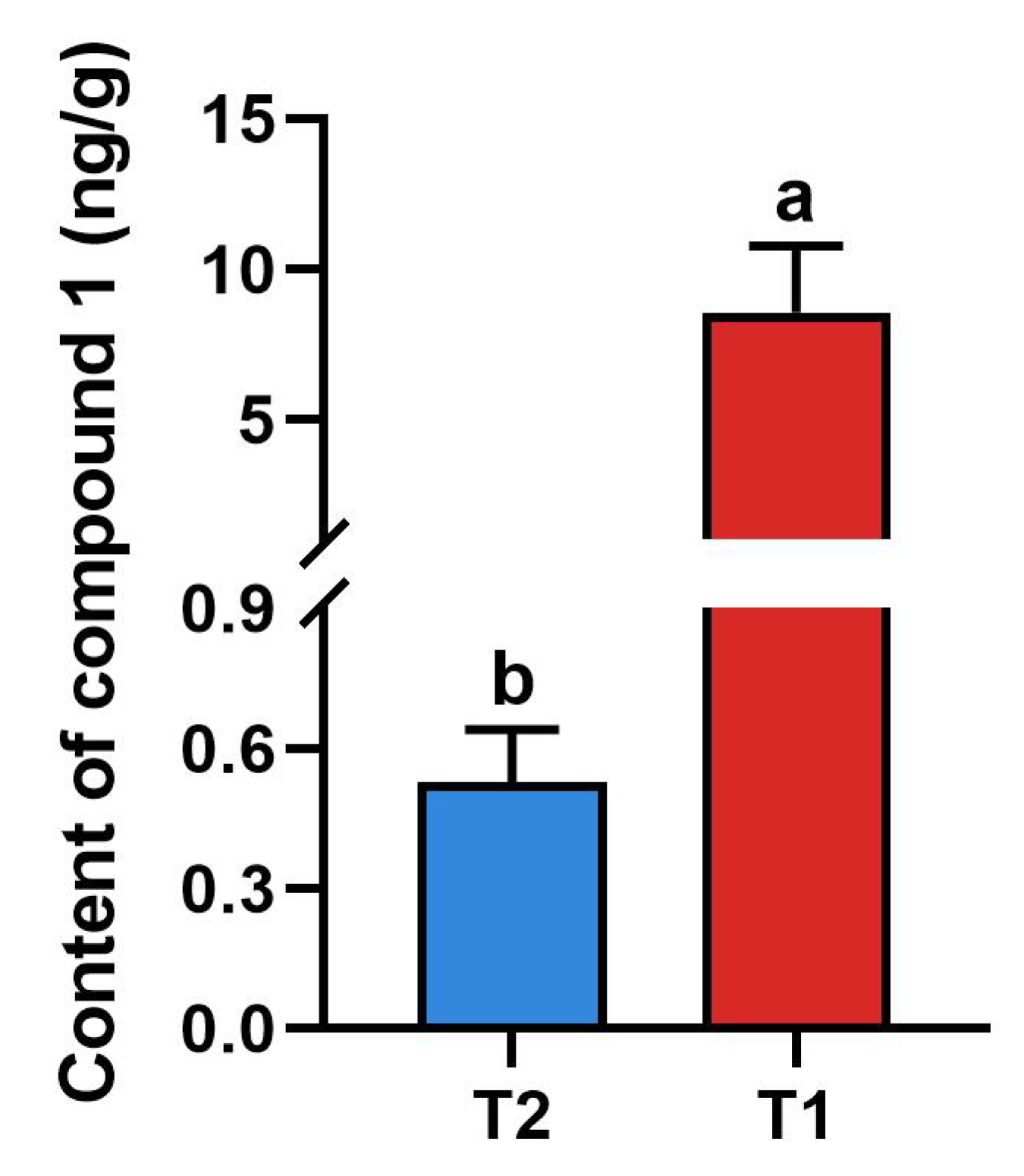
2.3. Contents of Compound 1 in Different Treatments of Rice
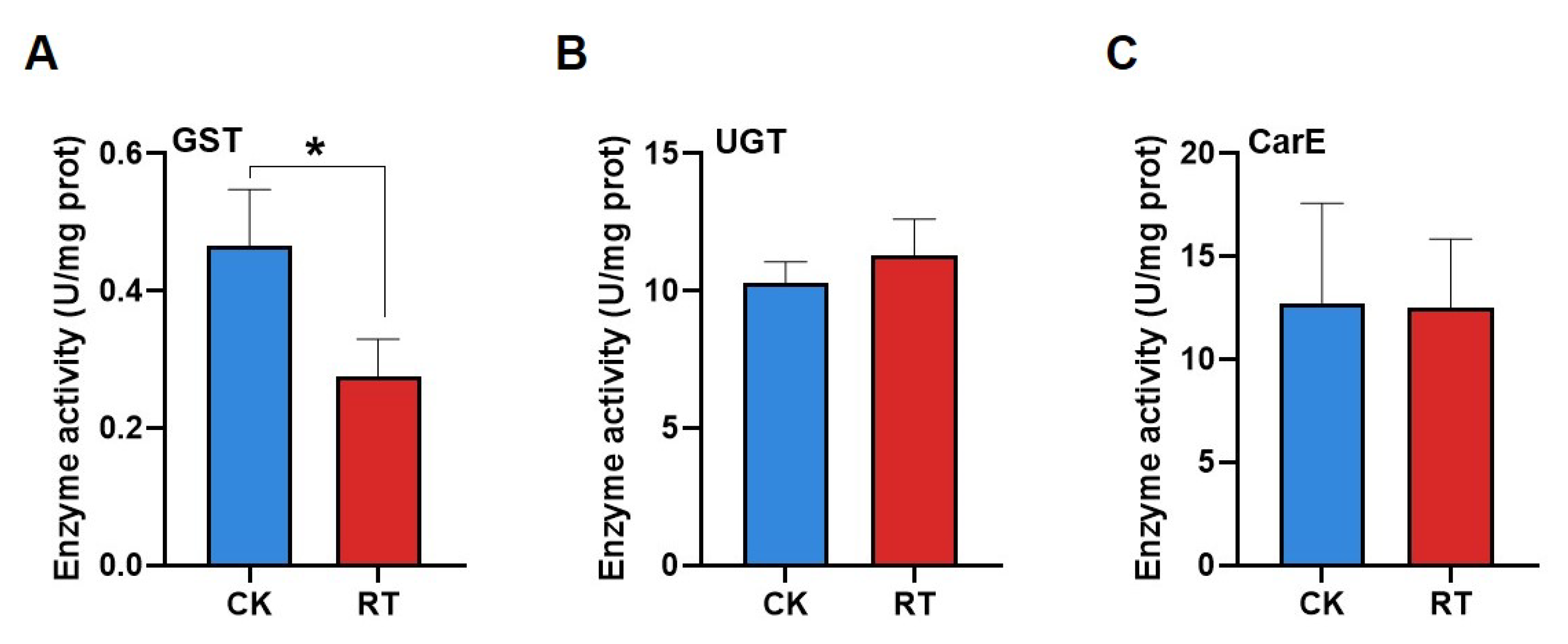
2.4. Effects of Compound 1 on Detoxification Enzyme Activities
2.5. Results of Transcriptome Sequencing
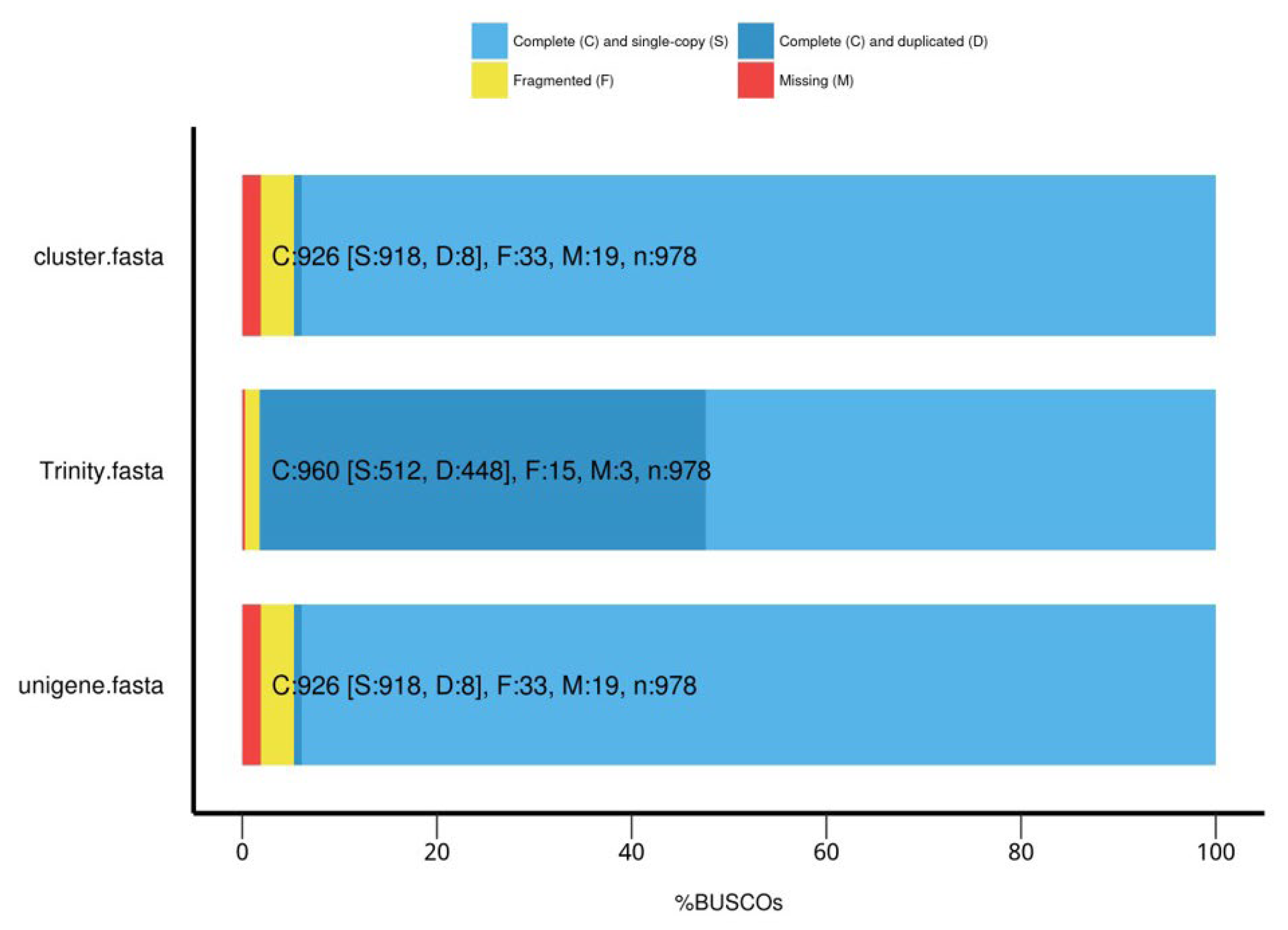
2.5.1. Quality of Transcriptome Sequencing
2.5.2. Quality of Transcripts
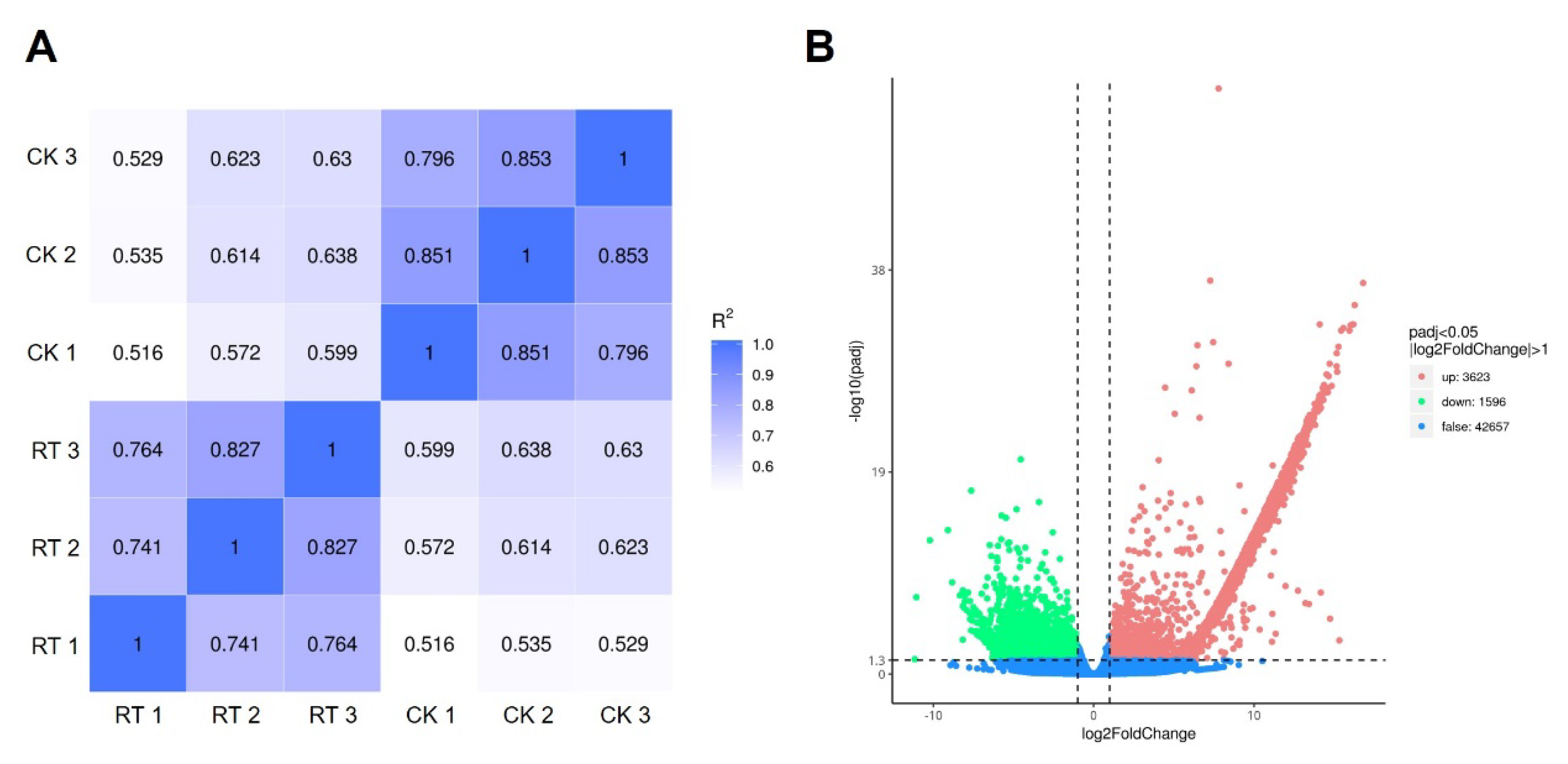
2.5.3. Analysis of Differentially Expressed Genes between CK Group and RT Group
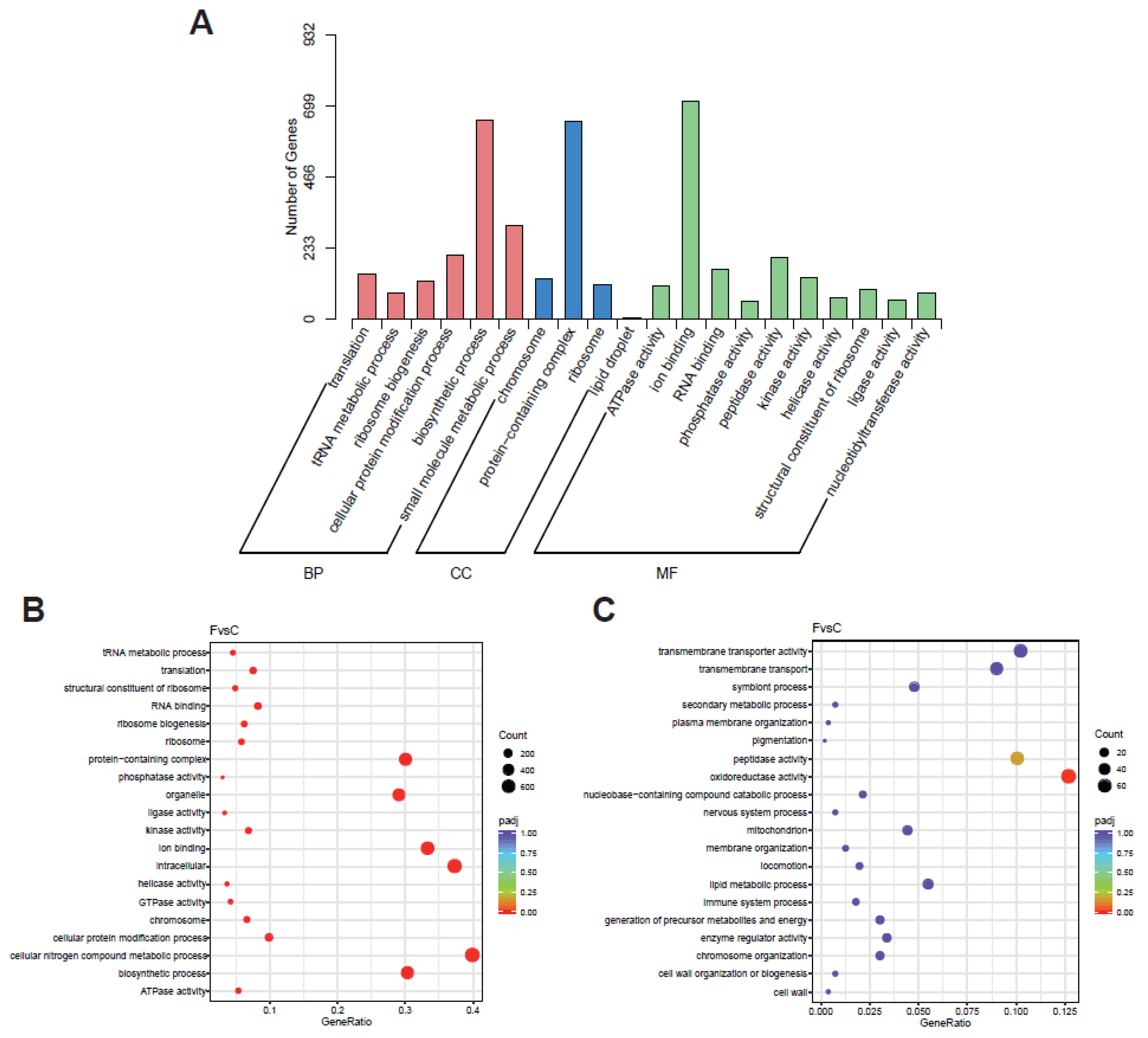
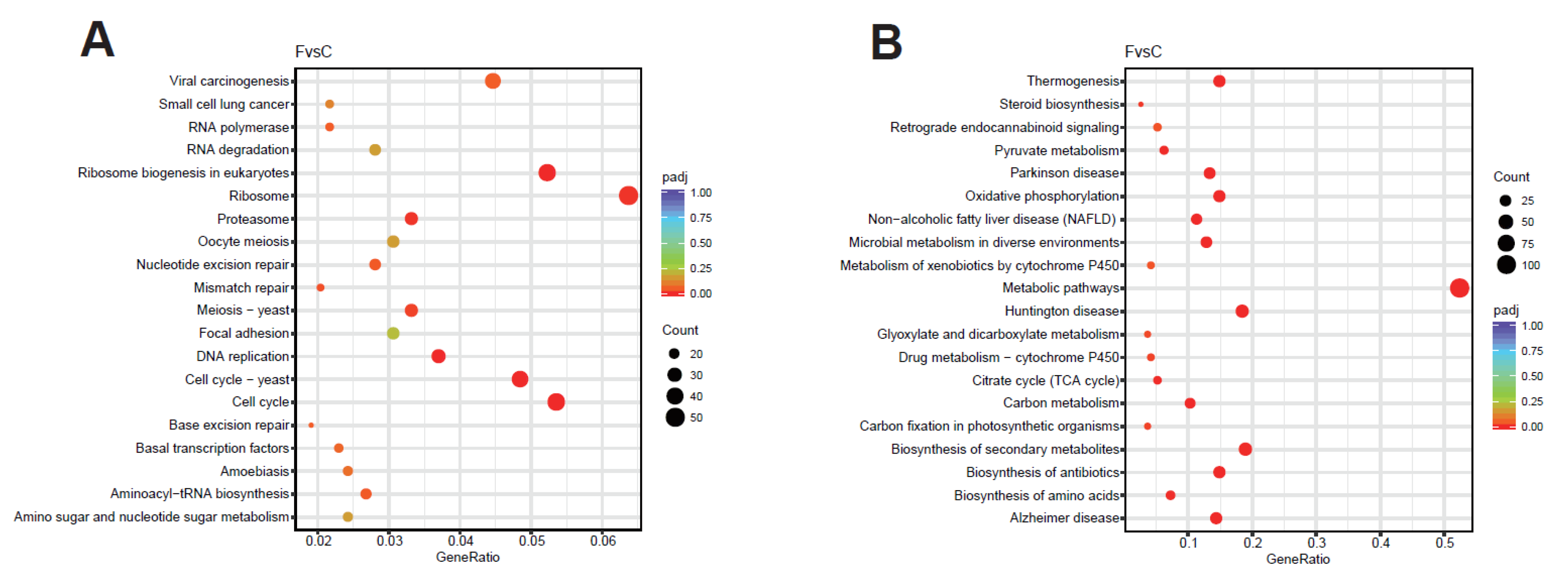
2.5.4. Functional Annotation and Enrichment Analysis of DEGs
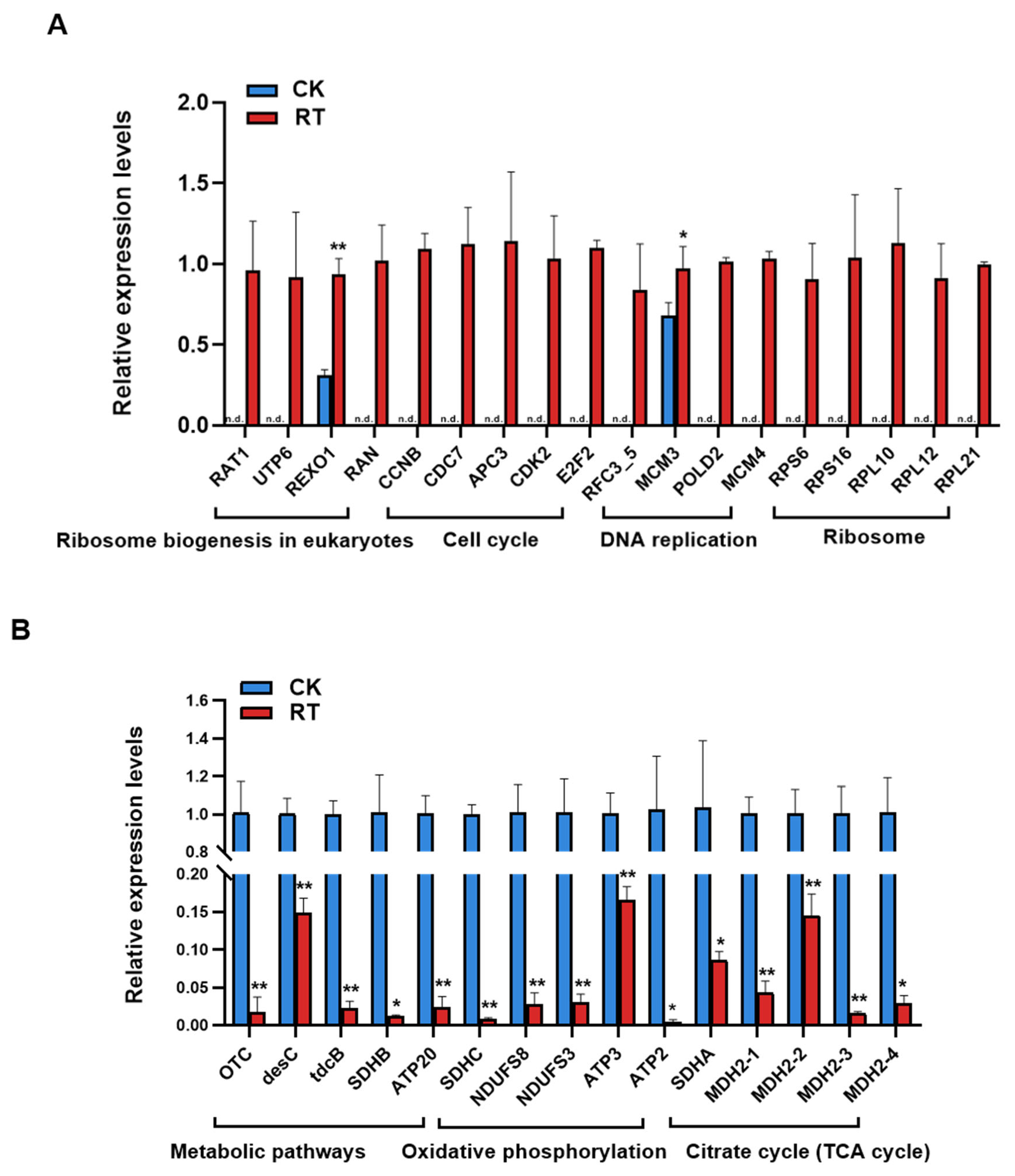
2.6. Verification by RT-qPCR
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Insects and Rice
5.2. Reagents and Instruments
5.3. Metabolites
5.3.1. Extraction and Preparation of Samples
5.3.2. UPLC and MS Conditions, Qualitative and Quantitative of Metabolites, and Data Processing and Analysis (See Supporting Information S1)
5.4. Insecticidal Activity of Metabolites
5.5. Determination of Compound 1 in Rice
5.5.1. Sample and Standard Preparation
5.5.2. UPLC-QqQ MS Conditions
5.6. Detoxifying Enzyme Activity Assay of Chilo Suppressalis
5.6.1. The Procedure for Aqueous Enzymatic Extraction in CarE and UGT Assays
5.6.2. The Procedure for Aqueous Enzymatic Extraction in P450 and GST Assays
5.7. Transcriptome Sequencing
5.7.1. RNA Extraction
5.7.2. Construction of RNA-Seq Library, Quality Control, and Sequencing
5.7.3. Differentially Expressed Genes’ Identification and Functional Enrichment Analysis
5.8. Real-Time Quantitative PCR (RT-qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karban, R.; Baldwin, I. Induced responses to herbivory. Ecol. Entomol. 2006, 241, 122–123. [Google Scholar]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Frost, C.J. New evidence for a multifunctional role of herbivore-induced plant volatiles in defense against herbivores. Plant Signal. Behav. 2010, 5, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Orre, G.U.S.; Wratten, S.D.; Jonsson, M. Effects of an herbivore-induced plant volatile on arthropods from three trophic levels in brassicas. Biol. Control 2010, 53, 62–67. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Malook, S.U.; Qi, J.F.; Hettenhausen, C.; Xu, Y.Y.; Zhang, C.P.; Zhang, J.X.; Lu, C.K.; Li, J.; Wang, L.; Wu, J.Q. The oriental armyworm (Mythimna separata) feeding induces systemic defence responses within and between maize leaves. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180307. [Google Scholar] [CrossRef] [PubMed]
- Kenari, H.M.; Kordafshari, G.; Moghimi, M.; Eghbalian, F.; Taherkhani, D. Review of Pharmacological Properties and Chemical Constituents of Pastinaca sativa. J. Pharmacopunct. 2021, 24, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Bai, J.B.; Liu, J.J.; Chen, X.; Zhou, Y.F.; Cheng, X.S. Effects of different insect mouthparts feeding on tobacco leaf’s signal molecules and secondary metabolite contents. Tobacco Sci. Technol. 2017, 50, 16–22. [Google Scholar]
- Kariyat, R.R.; Gaffoor, I.; Sattar, S.; Dixon, C.W.; Frock, N.; Moen, J.; De Moraes, C.M.; Mescher, M.C.; Thompson, G.A.; Chopra, S. Sorghum 3-Deoxyanthocyanidin Flavonoids Confer Resistance against Corn Leaf Aphid. J. Chem. Ecol. 2019, 45, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Alan, W.B.; Kennaway, B.M.; Barry, J. Gamma-aminobutyrate: Defense against invertebrate pests? Trends Plant Sci. 2006, 11, 424–427. [Google Scholar]
- Mosleh, M.K.; Hassan, Q.K.; Chowdhury, E.H. Application of remote sensors in mapping rice area and forecasting its production: A review. Sensors 2015, 15, 769–791. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, D.Y.; Liu, K.; Chu, G.; Huang, L.Y.; Tian, X.H.; Zhang, Y.B. Inbred varieties outperformed hybrid rice varieties under dense planting with reducing nitrogen. Sci. Rep. 2020, 10, 8769. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.R.; Xiao, M.L. The insect-resistance physiology of plants: A review. Chin. Agr. Sci. Bull. 2021, 37, 130–136. [Google Scholar]
- Heidel-Fischer, H.M.; Vogel, H. Molecular mechanisms of insect adaptation to plant secondary compounds. Curr. Opin. Insect Sci. 2015, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.J.; Du, B.; He, G.C.; Li, J.R. Research progress of chemical interactions between rice and brown planthopper. Plant Physiol. J. 2018, 54, 528–538. [Google Scholar]
- Gill, R.I.; Ellis, B.E.; Isman, M.B. Tryptamine-induced resistance in tryptophan decarboxylase transgenic poplar and tobacco plants against their specific herbivores. J. Chem. Ecol. 2003, 29, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Hashimoto, Y.; Miyagawa, H. Induction of serotonin accumulation by feeding of rice striped stem borer in rice leaves. Plant Signal. Behav. 2008, 3, 714–716. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.H. Comparision of Defense Chemicals of Rice Plants Infested by Different Herbivores and Setup of Rice Lines with Antisense Inhibition of Defense-Related Gene OPR1 or PLD3. Master’s Thesis, Zhejiang University, Hangzhou, China, 2007. [Google Scholar]
- Shi, J.H.; Sun, Z.; Hu, X.J.; Jin, H.N.; Foba, C.N.; Liu, H.; Wang, C.; Liu, L.; Li, F.F.; Wang, M.Q. Rice defense responses are induced upon leaf rolling by an insect herbivore. BMC Plant Biol. 2019, 19, 514. [Google Scholar] [CrossRef]
- Lin, Y.B.; Lin, X.H.; Ding, C.H.; Xia, M.; Xue, R.R.; Sun, Z.X.; Chen, D.Q.; Zhu, S.K.; Zeng, R.S.; Song, Y.Y. Priming of rice defense against a sap-sucking insect pest brown planthopper by silicon. J. Pest Sci. 2022, 95, 1371–1385. [Google Scholar] [CrossRef]
- Qi, J.F.; Saif, U.M.; Shen, G.J.; Gao, L.; Zhang, C.P.; Li, J.; Zhang, J.X.; Wang, L.; Wu, J.Q. Current understanding of maize and rice defense against insect herbivores. Plant Divers. 2018, 40, 189–195. [Google Scholar] [CrossRef]
- Divya, D.; Madhavi, K.R.; Dass, M.A. Expression Profile of Defense Genes in Rice Lines Pyramided with Resistance Genes Against Bacterial Blight, Fungal Blast and Insect Gall Midge. Rice 2018, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Stout, M.J.; Qian, Q.; Chen, F. Genetic, Molecular and Genomic Basis of Rice Defense against Insects. Crit. Rev. Plant Sci. 2012, 31, 74–91. [Google Scholar] [CrossRef]
- Pretorius, C.J.; Zeiss, D.R.; Dubery, I.A. The presence of oxygenated lipids in plant defense in response to biotic stress: A metabolomics appraisal. Plant Signal. Behav. 2021, 16, 1989215. [Google Scholar] [CrossRef]
- Saini, R.; Kumar, S. Genome-wide identification, characterization and in-silico profiling of genes encoding FAD (fatty acid desaturase) proteins in chickpea (Cicer arietinum L.). Plant Gene 2019, 18, 100180. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef]
- Luo, T.; Lu, H.P.; Shu, Q.Y. The pathway of tryptophan metabolism influences the defense response of rice to brown planthopper. In Proceedings of the 7th Symposium on Plant Allelopathy in China, Kunming, China, 27–30 October 2015; p. 32. [Google Scholar]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in Chemical Structures and Biological Properties of Plant Alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.Y.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F.Y. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Rui, H.Y.; Yu, L.; Jin, N.; Liu, W.; Guo, C.; Cheng, Y.M.; Lou, Y.G. Four-Chlorophenoxyacetic Acid Treatment Induces the Defense Resistance of Rice to White-Backed Planthopper Sogatella furcifera. Int. J. Mol. Sci. 2023, 24, 15722. [Google Scholar] [CrossRef] [PubMed]
- Li, S.A.; Qi, L.X.; Tan, X.Y.; Li, S.F.; Fang, J.C.; Ji, R. Small Brown Planthopper Nymph Infestation Regulates Plant Defenses by Affecting Secondary Metabolite Biosynthesis in Rice. Int. J. Mol. Sci. 2023, 24, 4764. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef]
- Meng, X.K.; Wu, Z.L.; Jiang, C.Y.; Guan, D.J.; Zhang, N.; Jiang, H.; Shen, Q.W.; Qain, K.; Wang, J.J. Identification and characterization of glutathione S-transferases and their potential roles in detoxification of abamectin in the rice stem borer, Chilo suppressalis. Pestic. Biochem. Phys. 2022, 182, 105050. [Google Scholar] [CrossRef]
- Cheng, Y.B. Mechanisms of ROS-CncC Pathway Regulating Insecticide Tolerance in Response to Phytochemicals in Spodoptera litura. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2021. [Google Scholar]
- Cheng, Y.B.; Li, Y.M.; Li, W.R.; Song, Y.Y.; Zeng, R.S.; Lu, K. Effect of hepatocyte nuclear factor 4 on the fecundity of Nilaparvata lugens: Insights from RNA interference combined with transcriptomic analysis. Genomics 2020, 112, 4585–4594. [Google Scholar] [CrossRef]

| Sample | Raw Reads | Clean Reads | Clean Bases | Error Rate | Q20 | Q30 | GC_pct |
|---|---|---|---|---|---|---|---|
| CK1 | 22633197 | 21769138 | 6.5 | 0.03 | 97.47 | 92.59 | 41.64 |
| CK2 | 23337632 | 22542267 | 6.8 | 0.03 | 97.34 | 92.3 | 41.98 |
| CK3 | 22768375 | 21925915 | 6.6 | 0.03 | 97.56 | 92.71 | 41.00 |
| RT1 | 24100758 | 23138018 | 6.9 | 0.03 | 97.37 | 92.48 | 43.40 |
| RT2 | 23487995 | 22675237 | 6.8 | 0.03 | 97.41 | 92.48 | 43.41 |
| RT3 | 21570901 | 20795171 | 6.2 | 0.03 | 97.63 | 92.91 | 42.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, C.; Zhang, Y.; Wang, M.; Lin, Y.; Zeng, B.; Zheng, X.; Song, Y.; Zeng, R. Metabolomic Profiling Reveals the Anti-Herbivore Mechanisms of Rice (Oryza sativa). Int. J. Mol. Sci. 2024, 25, 5946. https://doi.org/10.3390/ijms25115946
Gu C, Zhang Y, Wang M, Lin Y, Zeng B, Zheng X, Song Y, Zeng R. Metabolomic Profiling Reveals the Anti-Herbivore Mechanisms of Rice (Oryza sativa). International Journal of Molecular Sciences. 2024; 25(11):5946. https://doi.org/10.3390/ijms25115946
Chicago/Turabian StyleGu, Chengzhen, Yujia Zhang, Mengmeng Wang, Yangzheng Lin, Bixue Zeng, Xinyu Zheng, Yuanyuan Song, and Rensen Zeng. 2024. "Metabolomic Profiling Reveals the Anti-Herbivore Mechanisms of Rice (Oryza sativa)" International Journal of Molecular Sciences 25, no. 11: 5946. https://doi.org/10.3390/ijms25115946
APA StyleGu, C., Zhang, Y., Wang, M., Lin, Y., Zeng, B., Zheng, X., Song, Y., & Zeng, R. (2024). Metabolomic Profiling Reveals the Anti-Herbivore Mechanisms of Rice (Oryza sativa). International Journal of Molecular Sciences, 25(11), 5946. https://doi.org/10.3390/ijms25115946





