Biological Characterization and Genomic Analysis of Three Novel Serratia- and Enterobacter-Specific Virulent Phages
Abstract
:1. Introduction
2. Results and Discussion
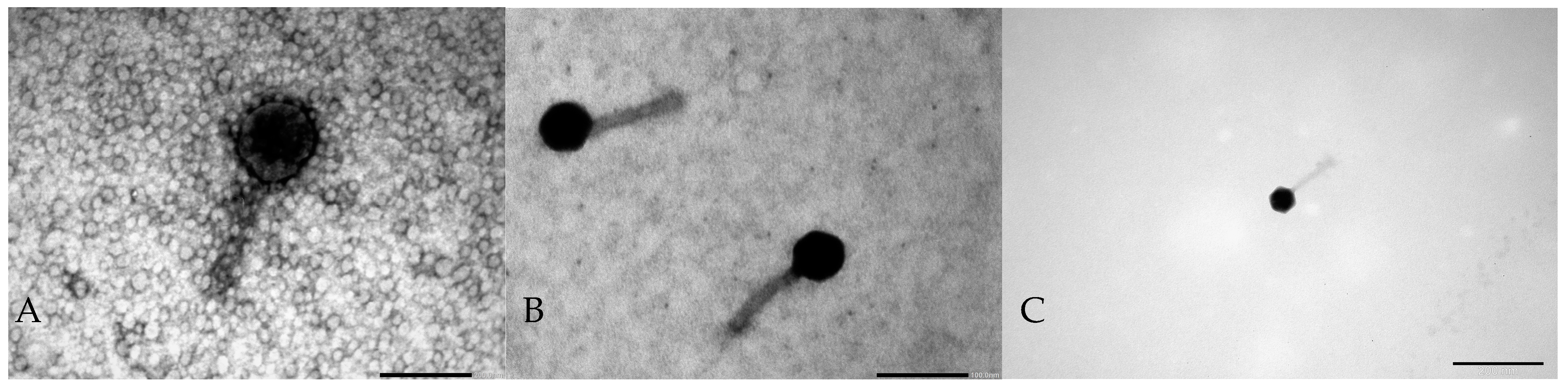
2.1. Phage Morphology
2.2. Bacterial Host Range of Bacteriophages
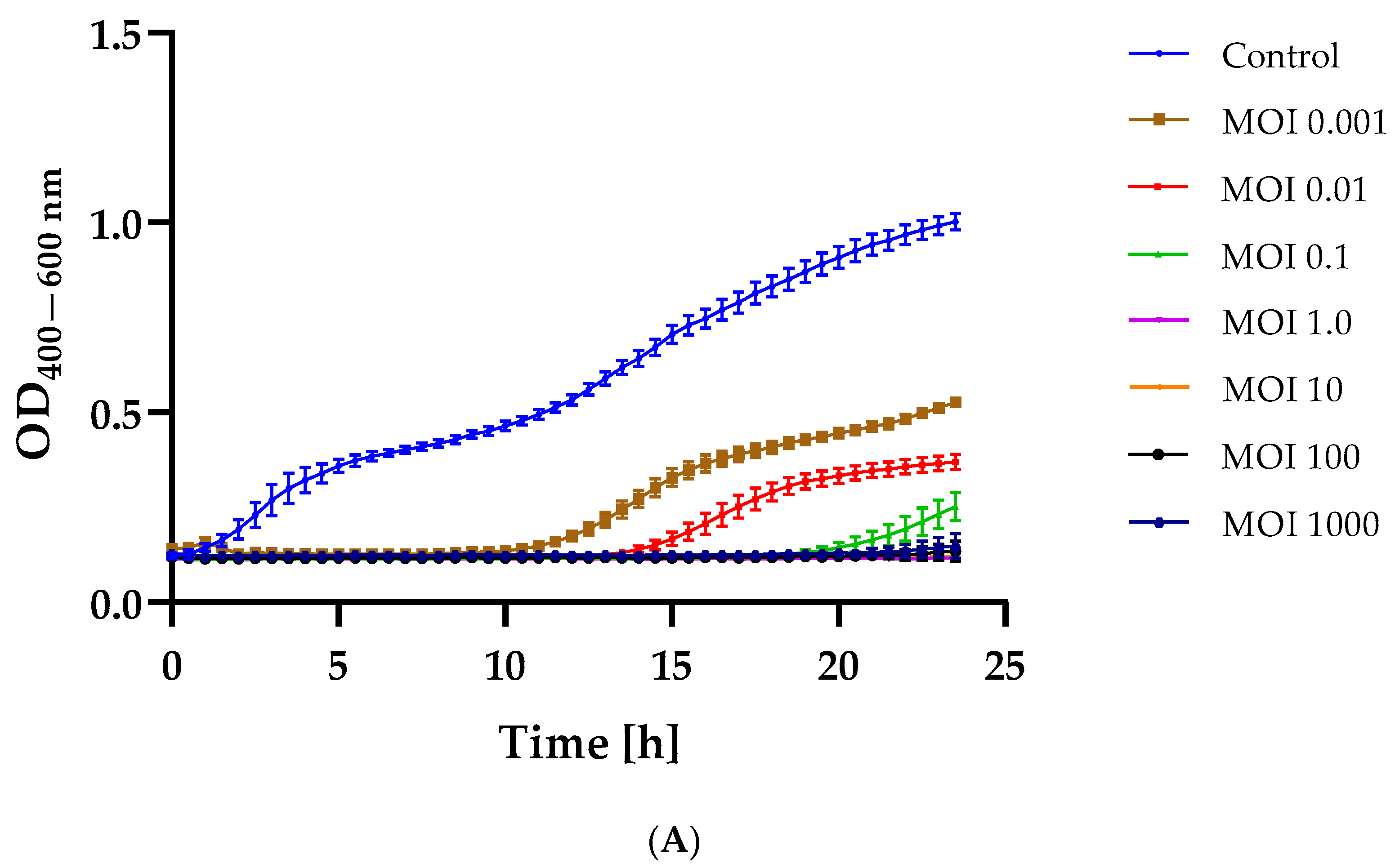
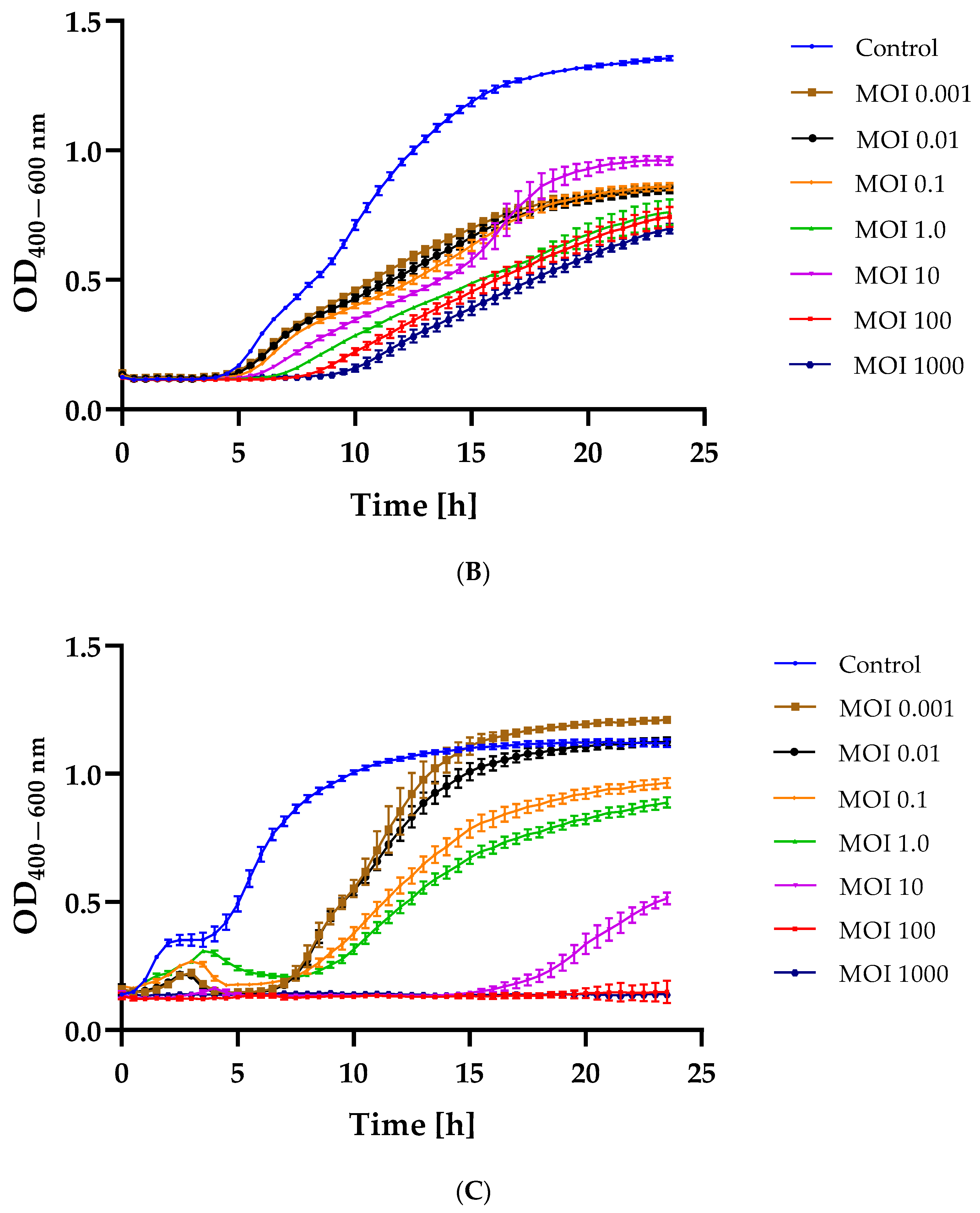
2.3. Bacterial Growth Inhibition after Phage Infection
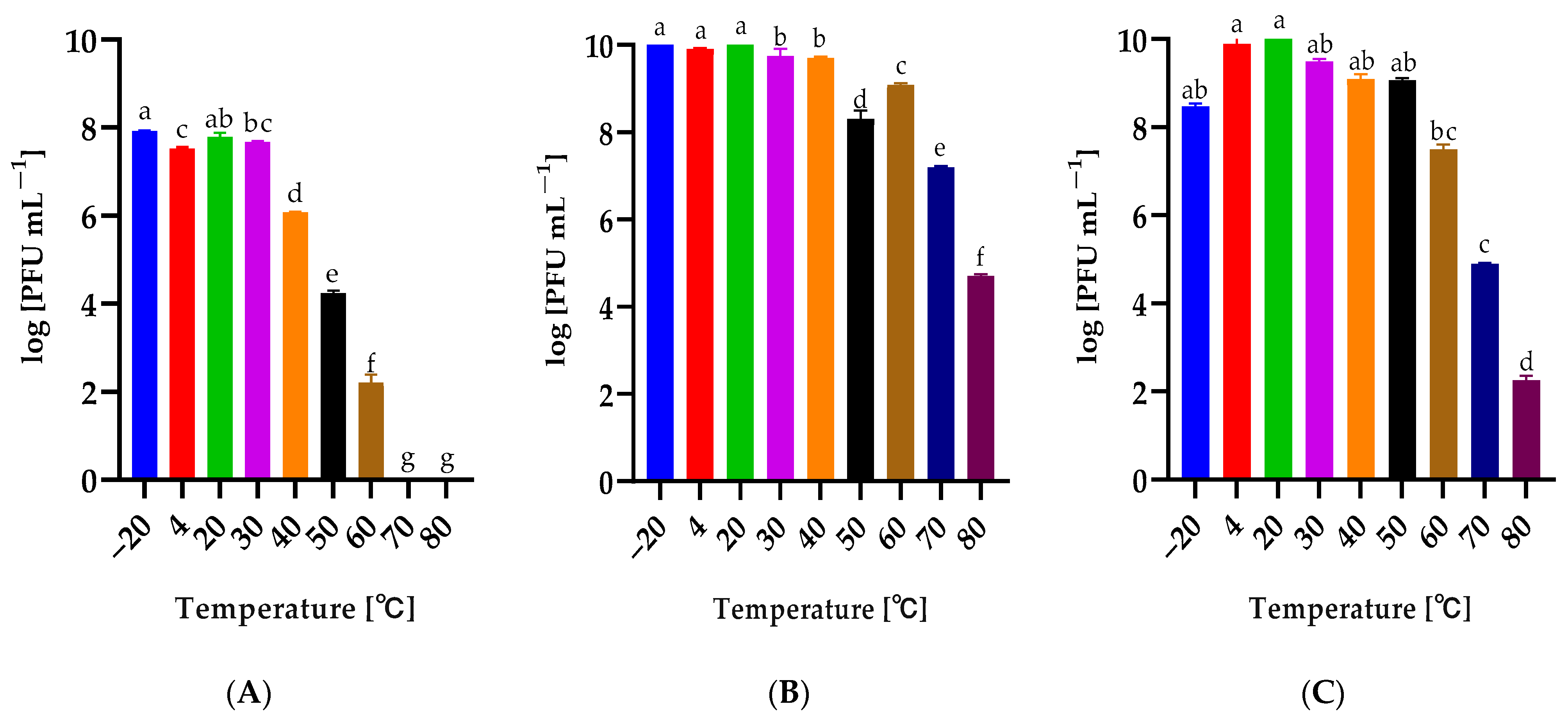
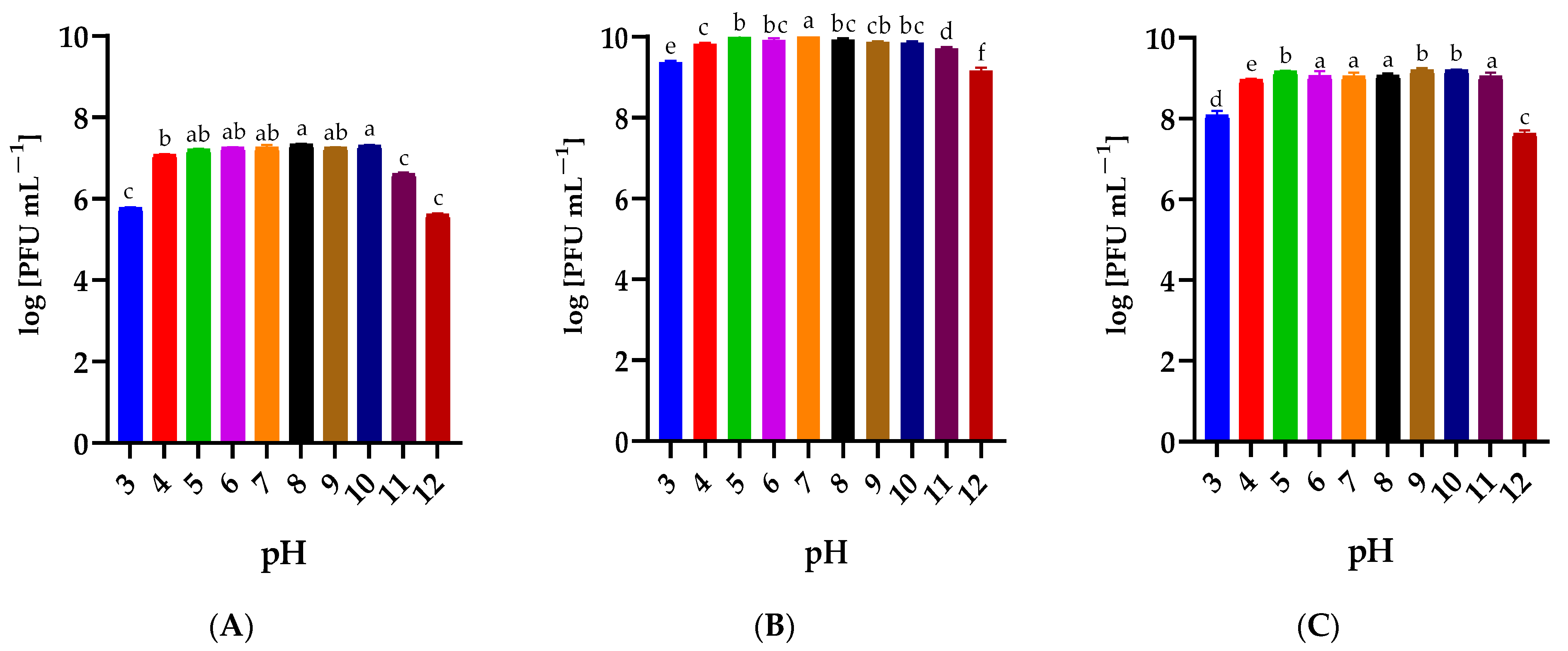
2.4. Influence of Temperature and pH on the Phage Stability
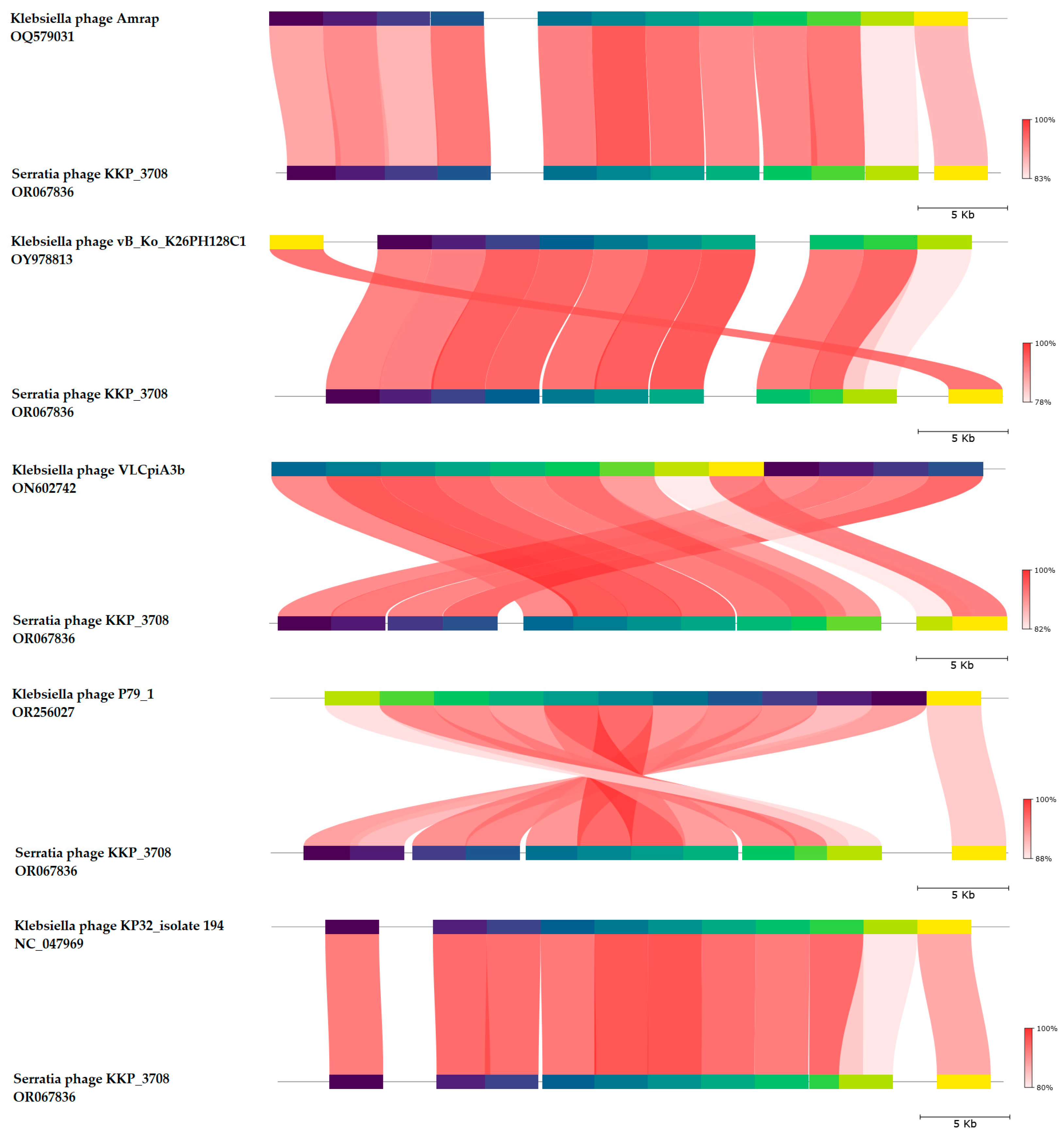
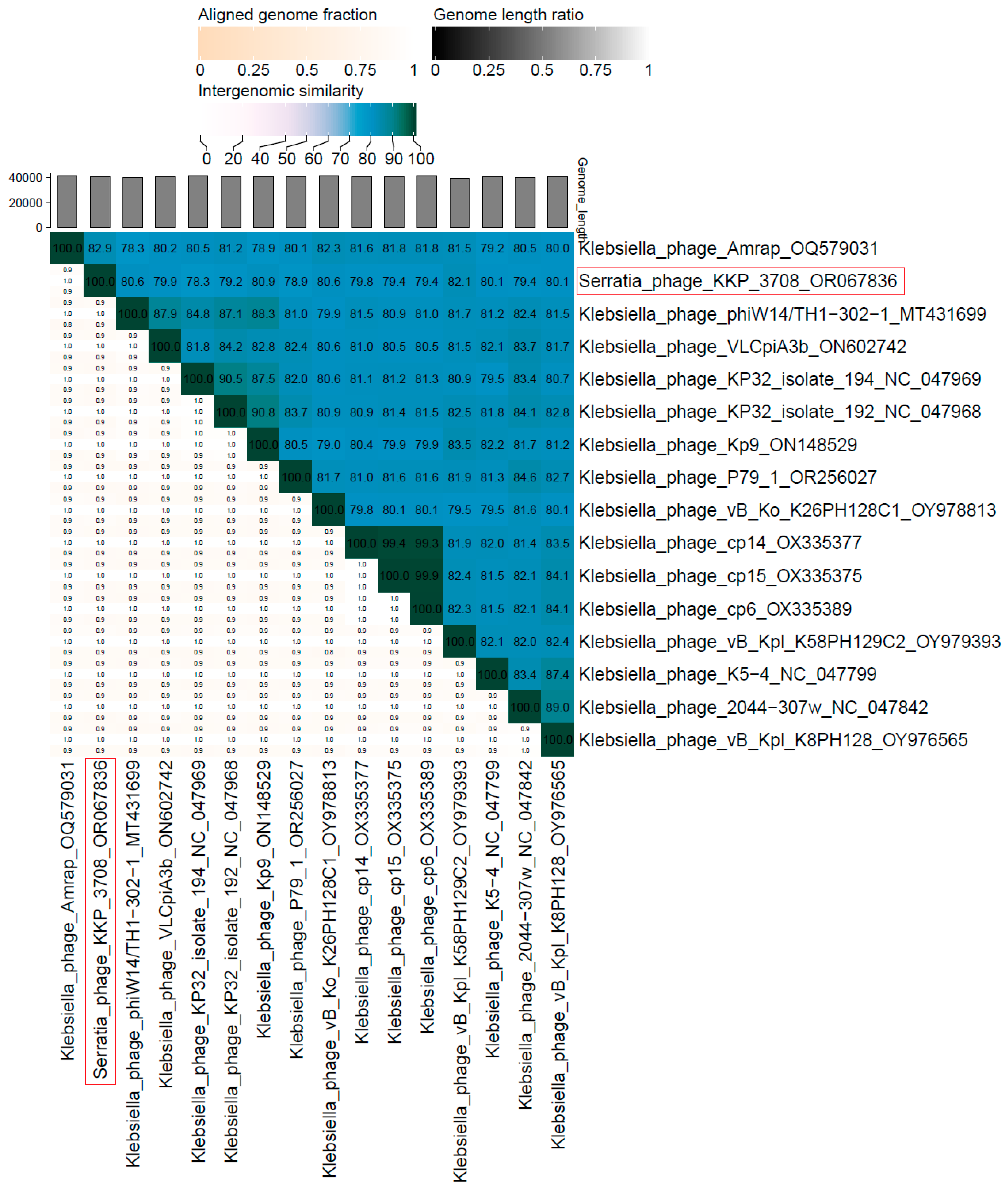
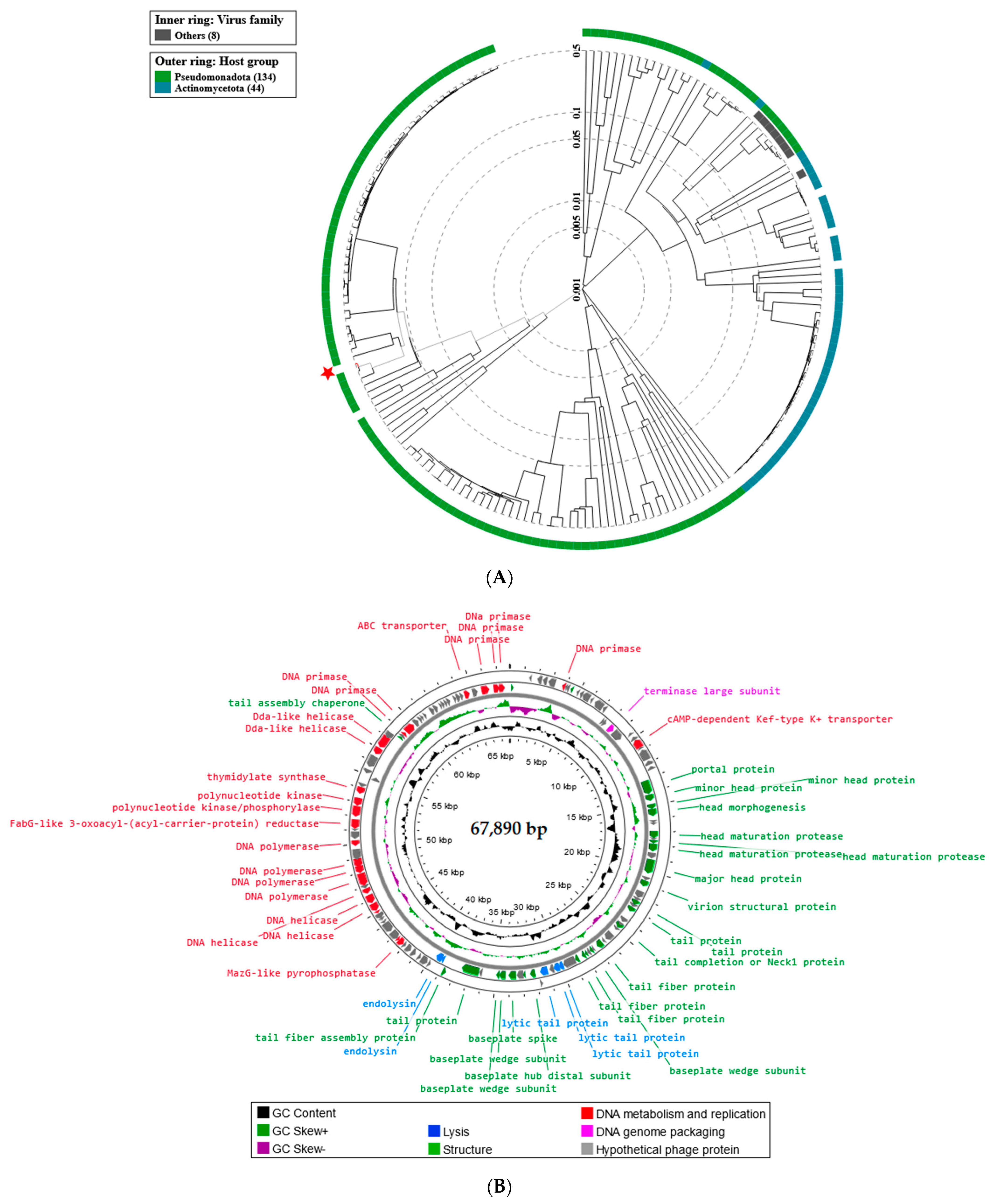
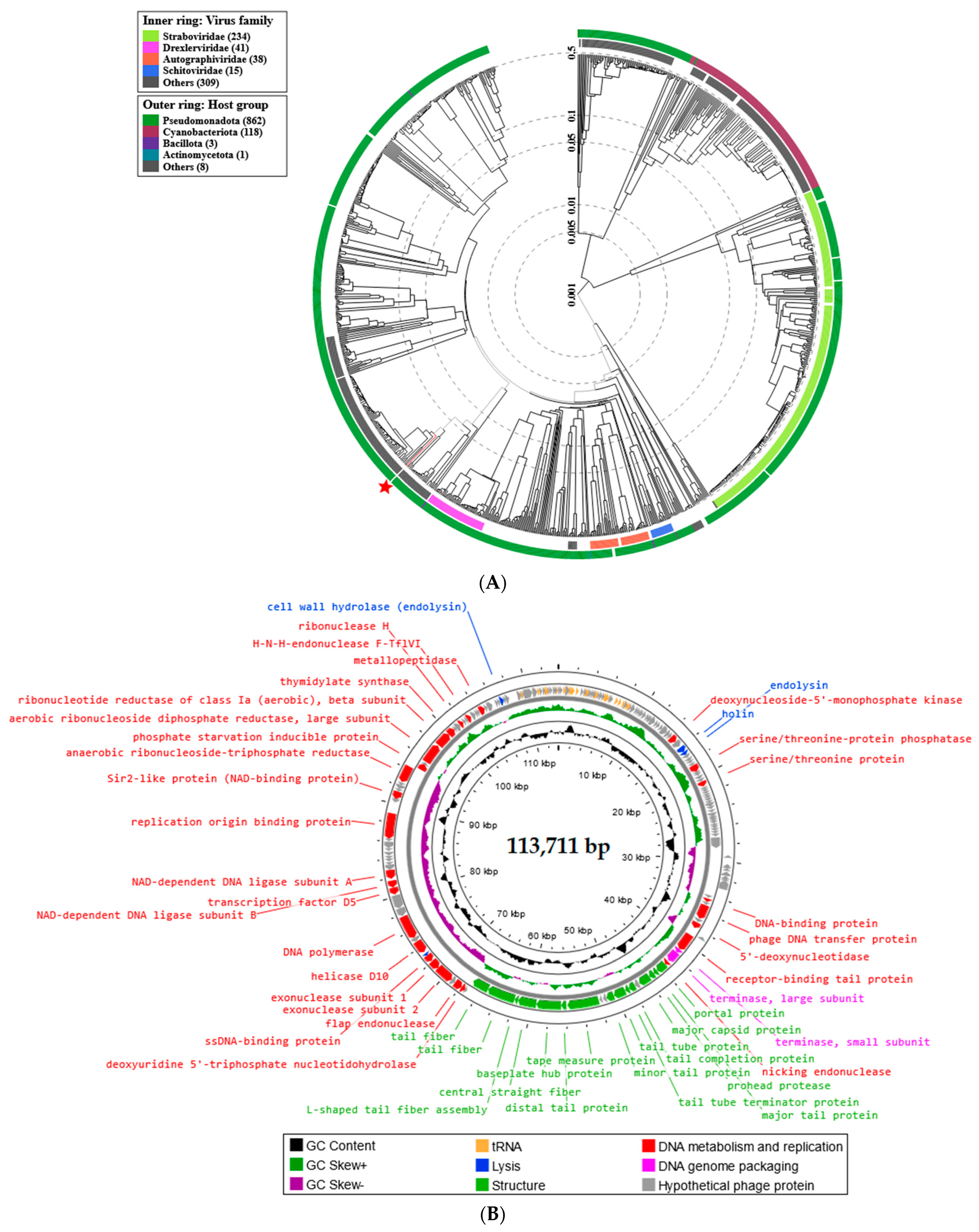
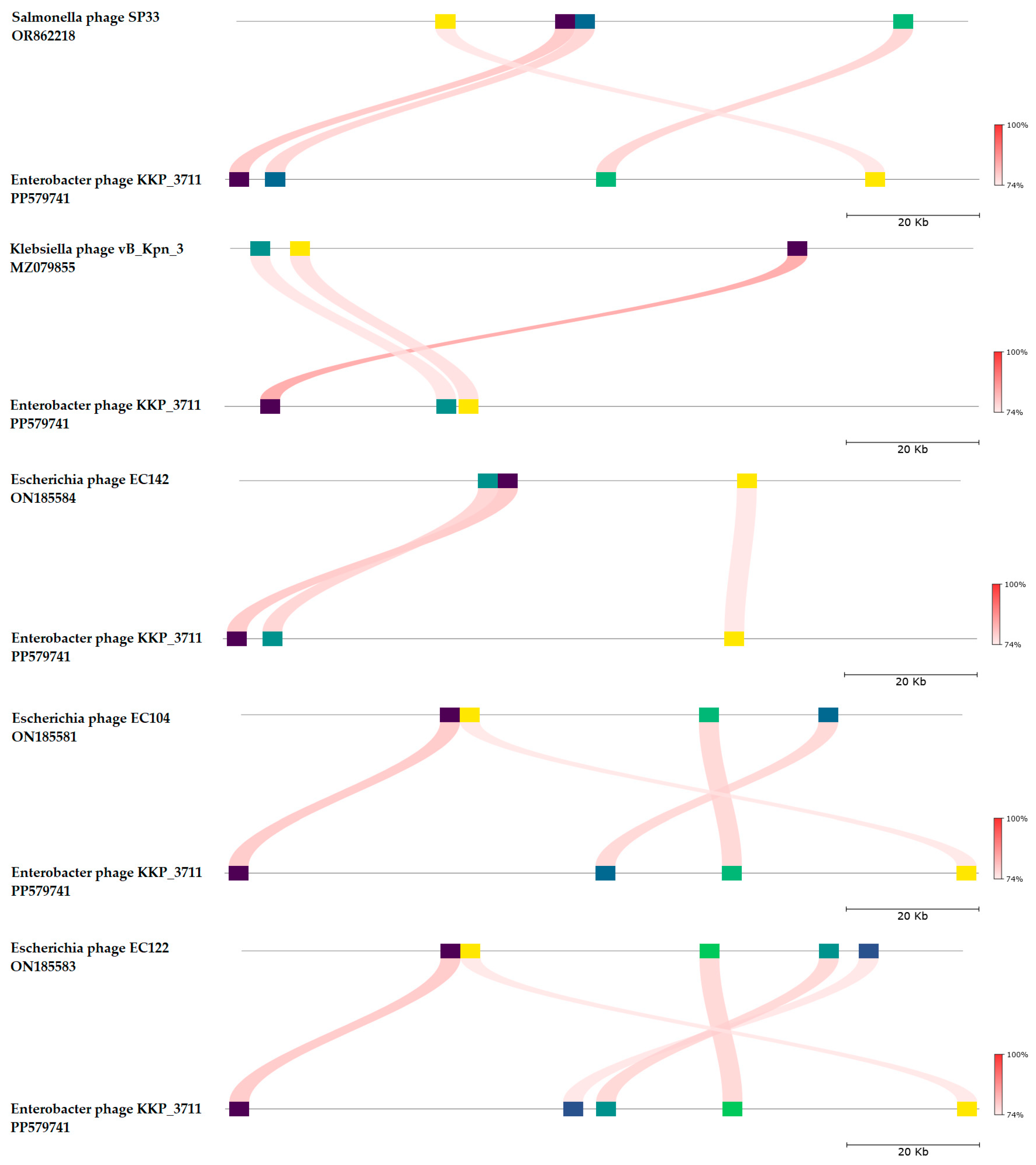
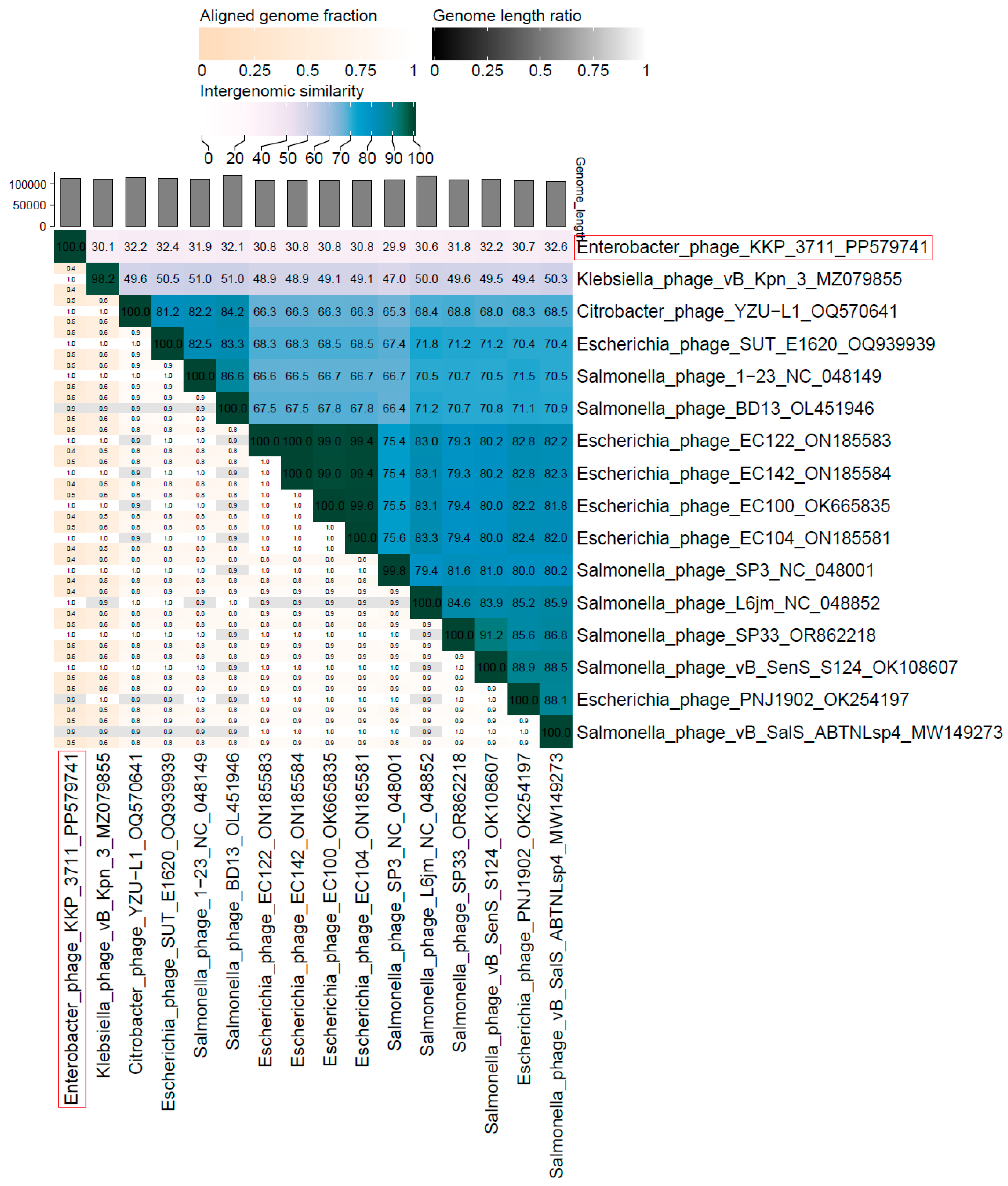
2.5. Phage Genome Sequencing and Analysis Using Bioinformatics Approaches
3. Materials and Methods
3.1. Bacterial Host Strains
3.2. Isolation of Bacteriophages, Propagation and Purification of Phage Particles
3.3. Electron Micrographs of Phage Particles
3.4. Determination of the Bacterial Host Range for the Tested Phages
3.5. Changes in the Growth Kinetics of Bacterial Hosts after Phage Infection at Different MOIs
3.6. Phage Stability after Exposure to Selected Factors
3.7. Extraction of Bacteriophage Genomic DNA
3.8. Genome Sequencing and Bioinformatic Analysis
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Głąbska, D.; Guzek, D.; Groele, B.; Gutkowska, K. Fruit and Vegetable Intake and Mental Health in Adults: A Systematic Review. Nutrients 2020, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Coppens, P. The Importance of Food Supplements for Public Health and Well-Being. World Rev. Nutr. Diet. 2020, 121, 66–72. [Google Scholar] [PubMed]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for the Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A.; Carlucci, E.; Leneveu-Jenvrin, C.; Remize, F.; Scarfato, P. Active Coated Films to Decrease Browning of Minimally Processed Banana Fruit. Macromol. Symp. 2022, 405, 2100256. [Google Scholar] [CrossRef]
- Collado-López, S.; Betanzos-Robledo, L.; Téllez-Rojo, M.M.; Lamadrid-Figueroa, H.; Reyes, M.; Ríos, C.; Cantoral, A. Heavy Metals in Unprocessed or Minimally Processed Foods Consumed by Humans Worldwide: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 8651. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.; Odeyemi, O.A.; Strateva, M.; Stratev, D. Microbial Spoilage of Vegetables, Fruits and Cereals. Appl. Food Res. 2022, 2, 100122. [Google Scholar] [CrossRef]
- Morya, S.; Amoah, A.E.D.D.; Snaebjornsson, S.O. Food Poisoning Hazards and Their Consequences over Food Safety. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 383–400. [Google Scholar]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- Dorado-García, A.; Smid, J.H.; Van Pelt, W.; Bonten, M.J.M.; Fluit, A.C.; Van Den Bunt, G.; Wagenaar, J.A.; Hordijk, J.; Dierikx, C.M.; Veldman, K.T.; et al. Molecular Relatedness of ESBL/AmpC-Producing Escherichia coli from Humans, Animals, Food and the Environment: A Pooled Analysis. J. Antimicrob. Chemother. 2018, 73, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Chelaghma, W.; Loucif, L.; Bendahou, M.; Rolain, J.M. Vegetables and Fruit as a Reservoir of β-Lactam and Colistin-Resistant Gram-Negative Bacteria: A Review. Microorganisms 2021, 9, 2534. [Google Scholar] [CrossRef]
- Formenti, N.; Calò, S.; Parisio, G.; Guarneri, F.; Birbes, L.; Pitozzi, A.; Scali, F.; Tonni, M.; Guadagno, F.; Giovannini, S.; et al. Esbl/Ampc-Producing Escherichia coli in Wild Boar: Epidemiology and Risk Factors. Animals 2021, 11, 1855. [Google Scholar] [CrossRef]
- Begrem, S.; Jérôme, M.; Leroi, F.; Delbarre-Ladrat, C.; Grovel, O.; Passerini, D. Genomic Diversity of Serratia proteamaculans and Serratia liquefaciens Predominant in Seafood Products and Spoilage Potential Analyses. Int. J. Food Microbiol. 2021, 354, 109326. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.L.; Doidge, N.P.; Bushell, R.N.; Browning, G.F.; Marenda, M.S. Healthcare-Associated Infections Caused by Chlorhexidine-Tolerant Serratia marcescens Carrying a Promiscuous IncHI2 Multi-Drug Resistance Plasmid in a Veterinary Hospital. PLoS ONE 2022, 17, e0264848. [Google Scholar] [CrossRef] [PubMed]
- Labbate, M.; Queck, S.Y.; Koh, K.S.; Rice, S.A.; Givskov, M.; Kjelleberg, S. Quorum Sensing-Controlled Biofilm Development in Serratia liquefaciens MG1. J. Bacteriol. 2004, 186, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.A.; Baglinière, F.; Vanetti, M.C.D. Spoilage Potential of a Heat-Stable Lipase Produced by Serratia liquefaciens Isolated from Cold Raw Milk. LWT 2020, 126, 109289. [Google Scholar] [CrossRef]
- Lavizzari, T.; Breccia, M.; Bover-Cid, S.; Vidal-Carou, M.C.; Veciana-Nogués, M.T. Histamine, Cadaverine, and Putrescine Produced in Vitro by Enterobacteriaceae and Pseudomonadaceae Isolated from Spinach. J. Food Prot. 2010, 73, 385–389. [Google Scholar] [CrossRef]
- Özkütük, A.S. Antimicrobial Effects of Carnosic Acid, Kaempferol and Luteolin on Biogenic Amine Production by Spoilage and Food-Borne Pathogenic Bacteria. Food Biosci. 2022, 46, 101588. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, G.; Liu, X.; Wang, J. Genomic Analysis and Biological Properties of the Novel Serratia liquefaciens Phage VB_SlqM_MQ-4. Arch. Virol. 2023, 168, 38. [Google Scholar] [CrossRef] [PubMed]
- Mohsina, K.; Ratkowsky, D.A.; Bowman, J.P.; Powell, S.; Kaur, M.; Tamplin, M.L. Effect of Glucose, PH and Lactic Acid on Carnobacterium maltaromaticum, Brochothrix thermosphacta and Serratia liquefaciens within a Commercial Heat-Shrunk Vacuum-Package Film. Food Microbiol. 2020, 91, 103515. [Google Scholar] [CrossRef]
- Khayat, M.T.; Elbaramawi, S.S.; Nazeih, S.I.; Safo, M.K.; Khafagy, E.S.; Ali, M.A.M.; Abbas, H.A.; Hegazy, W.A.H.; Seleem, N.M. Diminishing the Pathogenesis of the Food-Borne Pathogen Serratia marcescens by Low Doses of Sodium Citrate. Biology 2023, 12, 504. [Google Scholar] [CrossRef]
- Liu, J.; Yu, S.; Han, B.; Chen, J. Effects of Benzalkonium Chloride and Ethanol on Dual-Species Biofilms of Serratia liquefaciens S1 and Shewanella putrefaciens S4. Food Control 2017, 78, 196–202. [Google Scholar] [CrossRef]
- Iguchi, A.; Nagaya, Y.; Pradel, E.; Ooka, T.; Ogura, Y.; Katsura, K.; Kurokawa, K.; Oshima, K.; Hattori, M.; Parkhill, J.; et al. Genome Evolution and Plasticity of Serratia marcescens, an Important Multidrug-Resistant Nosocomial Pathogen. Genome Biol. Evol. 2014, 6, 2096–2110. [Google Scholar] [CrossRef]
- Åttman, E.; Korhonen, P.; Tammela, O.; Vuento, R.; Aittoniemi, J.; Syrjänen, J.; Mattila, E.; Österblad, M.; Huttunen, R.A. Serratia marcescens Outbreak in a Neonatal Intensive Care Unit Was Successfully Managed by Rapid Hospital Hygiene Interventions and Screening. Acta Paediatr. 2018, 107, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Fang, Z.; Chen, Z.; Wu, J.; Fang, X. Antibacterial Mechanism of Metabolites of Leuconostoc mesenteroides against Serratia liquefaciens. LWT 2023, 187, 115335. [Google Scholar] [CrossRef]
- Mladenović, K.G.; Grujović, M.; Kiš, M.; Furmeg, S.; Tkalec, V.J.; Stefanović, O.D.; Kocić-Tanackov, S.D. Enterobacteriaceae in Food Safety with an Emphasis on Raw Milk and Meat. Appl. Microbiol. Biotechnol. 2021, 105, 8615–8627. [Google Scholar] [CrossRef]
- Liu, Y.M.; Chen, Y.S.; Toh, H.S.; Huang, C.C.; Lee, Y.L.; Ho, C.M.; Lu, P.L.; Ko, W.C.; Chen, Y.H.; Wang, J.H.; et al. In Vitro Susceptibilities of Non-Enterobacteriaceae Isolates from Patients with Intra-Abdominal Infections in the Asia-Pacific Region from 2003 to 2010: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 2012, 40, S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Rossi, F.; Baquero, F.; Hsueh, P.R.; Woods, G.L.; Satishchandran, V.; Snyder, T.A.; Harvey, C.M.; Teppler, H.; DiNubile, M.J.; et al. In Vitro Susceptibilities of Aerobic and Facultative Gram-Negative Bacilli Isolated from Patients with Intra-Abdominal Infections Worldwide: The 2003 Study for Monitoring Antimicrobial Resistance Trends (SMART). J. Antimicrob. Chemother. 2005, 55, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Budiarso, T.Y.; Amarantini, C.; Restiani, R.; Sitanggang, P.K.; Samodra, E.M.A.; Mamoto, A.V.; Puteri, Y.; Kindangen, V.; Wattimury, A.; Yokebeth, I.P. Isolation and Biochemical Characterization of Enterobacter cloacae Isolates from Ready-to-Eat Foods Using API 20E. AIP Conf. Proc. 2023, 2556, 030006. [Google Scholar]
- Nyenje, M.E.; Odjadjare, C.E.; Tanih, N.F.; Green, E.; Ndip, R.N. Foodborne Pathogens Recovered from Ready-to-Eat Foods from Roadside Cafeterias and Retail Outlets in Alice, Eastern Cape Province, South Africa: Public Health Implications. Int. J. Environ. Res. Public Health 2012, 9, 2608–2619. [Google Scholar] [CrossRef]
- Bouige, A.; Fourcade, C.; Bicart-See, A.; Félicé, M.P.; Gautie, L.; Krin, G.; Lourtet-Hascoet, J.; Marlin, P.; Giordano, G.; Bonnet, E. Characteristics of Enterobacter cloacae Prosthetic Joint Infections. Med. Mal. Infect. 2019, 49, 511–518. [Google Scholar] [CrossRef]
- Yang, H.F.; Pan, A.J.; Hu, L.F.; Liu, Y.Y.; Cheng, J.; Ye, Y.; Li, J.B. Galleria Mellonella as an in vivo Model for Assessing the Efficacy of Antimicrobial Agents against Enterobacter cloacae Infection. J. Microbiol. Immunol. Infect. 2017, 50, 55–61. [Google Scholar] [CrossRef]
- Ganbold, M.; Seo, J.; Wi, Y.M.; Kwon, K.T.; Ko, K.S. Species Identification, Antibiotic Resistance, and Virulence in Enterobacter cloacae Complex Clinical Isolates from South Korea. Front. Microbiol. 2023, 14, 1122691. [Google Scholar] [CrossRef] [PubMed]
- Mezzatesta, M.L.; Gona, F.; Stefani, S. Enterobacter cloacae Complex: Clinical Impact and Emerging Antibiotic Resistance. Future Microbiol. 2012, 7, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, W.; Żabicka, D. Stanowisko Zespołu Roboczego ds. Oznaczania Lekowrażliwości Zgodnie z Zaleceniami EUCAST w Sprawie Najczęściej Zgłaszanych pytań Dotyczących Stosowania Rekomendacji EUCAST–wersja 6.0 z dnia 30 maja 2023 [Position of the Working Group on Antimicrobial Susceptibility Testing in Accordance with EUCAST Recommendations on the Most Frequently Reported Questions Regarding the Application of EUCAST Recommendations-version 6.0 of May 30, 2023]. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://korld.nil.gov.pl/wp-content/uploads/2023/07/Zalecenia-Zespolu-wersja-6_30-05-2023.pdf&ved=2ahUKEwijkNDh5uaFAxUrFBAIHV5XDygQFnoECA8QAQ&usg=AOvVaw0Ho3V3wKxxD25Fxh3DibJM (accessed on 23 April 2024).
- Li, X.; Xu, L.; Cui, Y.; Pang, M.; Wang, F.; Qi, J. Anti-Bacteria Effect of Active Ingredients of Cacumen Platycladi on the Spoilage Bacteria of Sauced Pork Head Meat. IOP Conf. Ser. Mater. Sci. Eng. 2018, 275, 012013. [Google Scholar] [CrossRef]
- Liu, F.; Jin, P.; Sun, Z.; Du, L.; Wang, D.; Zhao, T.; Doyle, M.P. Carvacrol Oil Inhibits Biofilm Formation and Exopolysaccharide Production of Enterobacter cloacae. Food Control 2021, 119, 107473. [Google Scholar] [CrossRef]
- Shymialevich, D.; Wójcicki, M.; Świder, O.; Średnicka, P.; Sokołowska, B. Characterization and Genome Study of a Newly Isolated Temperate Phage Belonging to a New Genus Targeting Alicyclobacillus acidoterrestris. Genes 2023, 14, 1303. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, M.; Świder, O.; Średnicka, P.; Shymialevich, D.; Ilczuk, T.; Koperski, Ł.; Cieślak, H.; Sokołowska, B.; Juszczuk-Kubiak, E. Newly Isolated Virulent Salmophages for Biocontrol of Multidrug-Resistant Salmonella in Ready-to-Eat Plant-Based Food. Int. J. Mol. Sci. 2023, 24, 10134. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, M.; Świder, O.; Gientka, I.; Błażejak, S.; Średnicka, P.; Shymialevich, D.; Cieślak, H.; Wardaszka, A.; Emanowicz, P.; Sokołowska, B.; et al. Effectiveness of a Phage Cocktail as a Potential Biocontrol Agent against Saprophytic Bacteria in Ready-To-Eat Plant-Based Food. Viruses 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Dakheel, K.H.; Rahim, R.A.; Neela, V.K.; Al-Obaidi, J.R.; Hun, T.G.; Isa, M.N.M.; Yusoff, K. Genomic Analyses of Two Novel Biofilm-Degrading Methicillin-Resistant Staphylococcus aureus Phages. BMC Microbiol. 2019, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Shymialevich, D.; Wójcicki, M.; Wardaszka, A.; Świder, O.; Sokołowska, B.; Błażejak, S. Application of Lytic Bacteriophages and Their Enzymes to Reduce Saprophytic Bacteria Isolated from Minimally Processed Plant-Based Food Products-In Vitro Studies. Viruses 2022, 15, 9. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage Diversity, Genomics and Phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Ribeiro, J.M.; Pereira, G.N.; Durli, I.; Teixeira, G.M.; Bertozzi, M.M.; Verri, W.A.; Kobayashi, R.K.T.; Nakazato, G. Comparative Analysis of Effectiveness for Phage Cocktail Development against Multiple Salmonella Serovars and Its Biofilm Control Activity. Sci. Rep. 2023, 13, 13054. [Google Scholar] [CrossRef]
- Green, S.I.; Kaelber, J.T.; Ma, L.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Bacteriophages from ExPEC Reservoirs Kill Pandemic Multidrug-Resistant Strains of Clonal Group ST131 in Animal Models of Bacteremia. Sci. Rep. 2017, 7, 46151. [Google Scholar] [CrossRef]
- Gibson, S.B.; Green, S.L.; Liu, C.G.; Salazar, K.C.; Clark, J.R.; Terwiliger, A.L.; Kaplan, H.B.; Maresso, A.W.; Trautner, B.W.; Ramig, R.F. Constructing and characterizing bacteriophages libraries for phage therapy of human infections. Front. Microbiol. 2019, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Lukman, C.; Yonathan, C.; Magdalena, S.; Waturangi, D.E. Isolation and characterization of pathogenic Escherichia coli bacteriophages from chicken and beef offal. BMC Res. Notes 2020, 13, 8. [Google Scholar] [CrossRef]
- Łobocka, M.; Hejnowicz, M.S.; Gągała, U.; Weber-Dąbrowska, B.; Węgrzyn, G.; Dadlez, M. The First Step to Bacteriophage Therapy: How to Choose the Correct Phage. In Phage Therapy: Current Research and Applications; Borysowski, J., Międzybrodzki, R., Górski, A., Eds.; Caister Academic Press: Norfalk, UK, 2014; pp. 23–67. [Google Scholar]
- Guerrero-Bustamante, C.A.; Dedrick, R.M.; Garlena, R.A.; Russell, D.A.; Hatfull, G.F. Toward a Phage Cocktail for Tuberculosis: Susceptibility and Tuberculocidal Action of Mycobacteriophages against Diverse Mycobacterium tuberculosis Strains. MBio 2021, 12, e00973-21. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Casjens, S.R.; Millard, A.D.; Harrison, C.; Gannon, L.; Chattaway, M.A. Genomic analysis of Anderson typing phages of Salmonella Typhimrium: Towards understanding the basis of bacteria-phage interaction. Sci. Rep. 2023, 13, 10484. [Google Scholar] [CrossRef] [PubMed]
- Vipra, A.; Desay, S.N.; Junjappa, R.P.; Roy, P.; Poonacha, N.; Ravinder, P.; Sriram, B.; Padmanabhan, S. Determining the minimum inhibitory concentration of bacteriophages: Potential advantages. Adv. Microbiol. 2013, 3, 32359. [Google Scholar] [CrossRef]
- Cieślik, M.; Harhala, M.; Orwat, F.; Dąbrowska, K.; Górski, A.; Jończyk-Matysiak, E. Two Newly Isolated Enterobacter-Specific Bacteriophages: Biological Properties and Stability Studies. Viruses 2022, 14, 1518. [Google Scholar] [CrossRef] [PubMed]
- Wójcicki, M.; Średnicka, P.; Błażejak, S.; Gientka, I.; Kowalczyk, M.; Emanowicz, P.; Świder, O.; Sokołowska, B.; Juszczuk-Kubiak, E. Characterization and Genome Study of Novel Lytic Bacteriophages against Prevailing Saprophytic Bacterial Microflora of Minimally Processed Plant-Based Food Products. Int. J. Mol. Sci. 2021, 22, 12460. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Sun, Q.; Chen, H.; Wu, Q.; Chen, M.; Yang, S.; Du, M.; Zha, F.; Ye, Q.; Zhang, J. Isolation and Characterization of a Novel Salmonella Phage VB_SalP_TR2. Front. Microbiol. 2021, 12, 664810. [Google Scholar] [CrossRef]
- Egido, J.E.; Costa, A.R.; Aparicio-Maldonado, C.; Haas, P.J.; Brouns, S.J.J. Mechanisms and Clinical Importance of Bacteriophage Resistance. FEMS Microbiol. Rev. 2022, 46, fuab048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Dutta, V.; Elhanafi, D.; Lee, S.; Osborne, J.A.; Kathariou, S. A Novel Restriction-Modification System Is Responsible for Temperature-Dependent Phage Resistance in Listeria monocytogenes ECII. Appl. Environ. Microbiol. 2012, 78, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.L.; Josephsen, J. The Methyltransferase from the LlaDII Restriction-Modification System Influences the Level of Expression of Its Own Gene. J. Bacteriol. 2004, 186, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, M.È.; Villion, M.; Magadán, A.H.; Moineau, S. CRISPR-Cas and Restriction-Modification Systems Are Compatible and Increase Phage Resistance. Nat. Commun. 2013, 4, 2087. [Google Scholar] [CrossRef] [PubMed]
- Morozova, V.; Bokovaya, O.; Kozlova, Y.; Kurilshikov, A.; Babkin, I.; Tupikin, A.; Bondar, A.; Ryabchikova, E.; Brayanskaya, A.; Peltek, S.; et al. A Novel Thermophilic Aeribacillus Bacteriophage AP45 Isolated from the Valley of Geysers, Kamchatka: Genome Analysis Suggests the Existence of a New Genus within the Siphoviridae Family. Extremophiles 2019, 23, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Estilo, E.E.C.; Nakano, H. Effects of Diluents, Temperature and PH on the Enumeration and Growth Kinetics of Alicyclobacillus acidoterrestris in Standard Growth Media. LWT 2019, 104, 148–158. [Google Scholar] [CrossRef]
- Shang, C.; Zhang, T.; Xu, J.; Zhao, N.; Zhang, W.; Fan, M. Exploring the Growth Characteristics of Alicyclobacillus acidoterrestris for Controlling Juice Spoilage with Zero Additives. Food Chem. X 2023, 19, 100790. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Shkoporov, A.N.; Lood, C.; Millard, A.D.; Dutilh, B.E.; Alfenas-Zerbini, P.; van Zyl, L.J.; Aziz, R.K.; Oksanen, H.M.; Poranen, M.M.; et al. Abolishment of morphology-based taxa and change to binomial species names: 2022 taxonomy update of the ICTV bacterial viruses subcommittee. Arch. Virol. 2023, 168, 74. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Nishimura, Y.; Shimizu, Y.; Nishiyama, H.; Yoshikawa, G.; Uehara, H.; Hingamp, P.; Goto, S.; Ogata, H. Linking Virus Genomes with Host Taxonomy. Viruses 2016, 8, 66. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Wei, F.; Yu, F.; Zhao, Z. Bactericidal Activity of a Holin-Endolysin System Derived from Vibrio alginolyticus Phage HH109. Microb. Pathog. 2021, 159, 105135. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, Ł.; Łepek, K.; Stasiłojć, M.; Kosznik-Kwaśnicka, K.; Zdrojewska, K.; Maciąg-Dorszyńska, M.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Encoded Enzymes Destroying Bacterial Cell Membranes and Walls, and Their Potential Use as Antimicrobial Agents. Microbiol. Res. 2021, 248, 126746. [Google Scholar] [CrossRef] [PubMed]
- Broendum, S.S.; Buckle, A.M.; McGowan, S. Catalytic Diversity and Cell Wall Binding Repeats in the Phage-Encoded Endolysins. Mol. Microbiol. 2018, 110, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Jado, I.; López, R.; García, E.; Fenoll, A.; Casal, J.; García, P.; Pallares, R.; de la Campa, A.G.; Bouza, E.; Baquero, F.; et al. Phage Lytic Enzymes as Therapy for Antibiotic-Resistant Streptococcus Pneumoniae Infection in a Murine Sepsis Model. J. Antimicrob. Chemother. 2003, 52, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Chamakura, K.; Young, R. Phage Single-Gene Lysis: Finding the Weak Spot in the Bacterial Cell Wall. J. Biol. Chem. 2019, 294, 3350–3358. [Google Scholar] [CrossRef] [PubMed]
- Leon-Velarde, C.G.; Kropinski, A.M.; Chen, S.; Abbasifar, A.; Griffiths, M.W.; Odumeru, J.A. Complete Genome Sequence of Bacteriophage VB-YenP-AP5 Which Infects Yersinia enterocolitica of Serotype O:3. Virol. J. 2014, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.B.; Feiss, M. The Bacteriophage DNA Packaging Motor. Annu. Rev. Genet. 2008, 42, 647–681. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, M.; Zeng, Y.; Hu, X.; Tan, Y.; Rao, X.; Jin, X.; Li, S.; Zhu, J.; Zhang, K.; et al. Functional Identification of the DNA Packaging Terminase from Pseudomonas aeruginosa Phage PaP3. Arch. Virol. 2012, 157, 2133–2141. [Google Scholar] [CrossRef]
- Huiting, E.; Bondy-Denomy, J. Defining the expanding mechanisms of phage-mediated activation of bacterial immunity. Curr. Opin. Microbiol. 2023, 74, 102325. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, D.; Zhu, J.; Feng, H.; Luo, X.; Liu, S.; Yan, X.X.; Zhang, X.; Gao, P. Structural Insights into Assembly, Operation and Inhibition of a Type I Restriction–Modification System. Nat. Microbiol. 2020, 5, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Atanasiu, C.; Su, T.J.; Sturrock, S.S.; Dryden, D.T.F. Interaction of the Ocr Gene 0.3 Protein of Bacteriophage T7 with EcoKl Restriction/Modification Enzyme. Nucleic Acids Res. 2002, 30, 3936–3944. [Google Scholar] [CrossRef] [PubMed]
- Klemm, B.P.; Singh, D.; Smith, C.E.; Hsu, A.L.; Dillard, L.B.; Krahn, J.M.; London, R.E.; Mueller, G.A.; Borgnia, M.J.; Schaaper, R.M. Mechanism by Which T7 Bacteriophage Protein Gp1.2 Inhibits Escherichia coli DGTPase. Proc. Natl. Acad. Sci. USA 2022, 119, e2123092119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, D.; Huang, Y.; Zhu, X.; Rui, M.; Wan, T.; Zheng, X.; Shen, Y.; Chen, X.; Ma, K.; et al. Structural and Functional Characterization of Deep-Sea Thermophilic Bacteriophage GVE2 HNH Endonuclease. Sci. Rep. 2017, 7, 42542. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Rodriguez, R.L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC-a novel tool to calculate the intergenomic similarities of prokaryote-infecting viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A roadmap for genome-based phage taxonomy. Viruses 2021, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Bailly-Bechet, M.; Vergassola, M.; Rocha, E. Causes for the intriguing presence of tRNAs in phages. Genome Res. 2007, 17, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Kemp, P.; Garcia, L.R.; Molineux, I.J. Changes in Bacteriophage T7 Virion Structure at the Initiation of Infection. Virology 2005, 340, 307–317. [Google Scholar] [CrossRef]
- Jamal, M.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Das, C.R. Isolation and Characterization of a Bacteriophage and Its Utilization against Multi-Drug Resistant Pseudomonas aeruginosa-2995. Life Sci. 2017, 190, 21–28. [Google Scholar] [CrossRef]
- Mirzaei, K.; Nilsson, A.S. Isolation of phage for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef] [PubMed]
- Sváb, D.; Falgenhauer, L.; Rohde, M.; Szabó, J.; Chakraborty, T.; Tóth, I. Identification and characterization of T5-like bacteriophages representing two novel subgroups from food products. Front. Microbiol. 2018, 9, 222. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of Long, Error-Prone Reads Using Repeat Graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Mcnair, K.; Zhou, C.; Dinsdale, E.A.; Souza, B.; Edwards, R.A. PHANOTATE: A Novel Approach to Gene Identification in Phage Genomes. Bioinformatics 2019, 35, 4537–4542. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Q.; Li, M.; Xu, J.; Wang, C.; Zhang, J.; Xiao, M.; Bin, Y.; Xia, J. PhaGAA: An Integrated Web Server Platform for Phage Genome Annotation and Analysis. Bioinformatics 2023, 39, btad120. [Google Scholar] [CrossRef]
- Tynecki, P.; Guziński, A.; Kazimierczak, J.; Jadczuk, M.; Dastych, J.; Onisko, A. PhageAI-Bacteriophage Life Cycle Recognition with Machine Learning and Natural Language Processing. bioRXiv 2020, 198606. [Google Scholar]



| Bacterial Host Strain (n = 25) | GenBank Accession Number | Year of Isolation | Source of Isolation | Phage Strain (EOP) | ||
|---|---|---|---|---|---|---|
| KKP_3708 | KKP_3709 | KKP_3711 | ||||
| Enterobacter cloacae KKP 3082 | MZ827006 | 2018 | Salad mix with carrot | − | − | + (0.215) |
| Enterobacter ludwigii KKP 3083 | MZ827002 | 2018 | Salad mix with carrot | − | + (0.033) | − |
| Serratia fonticola KKP 3084 | MZ827668 | 2018 | Salad mix with beetroot | − | − | − |
| Escherichia coli KKP 3650 | OM287487 | 2018 | Salad mix with beetroot | − | − | − |
| Pantoea agglomerans KKP 3651 | OP978292 | 2018 | Washed spinach | − | − | + (0.091) |
| Serratia fonticola KKP 3652 | OM287486 | 2018 | Washed spinach | − | − | − |
| Serratia liquefaciens KKP 3654 | OP978313 | 2018 | Salad mix with beetroot | ++ (1) | − | − |
| Citrobacter freundii KKP 3655 | MZ827001 | 2018 | Rucola | − | − | − |
| Enterobacter cloacae KKP 3656 | OM304355 | 2018 | Unwashed spinach | + (0.274) | − | − |
| Enterobacter cloacae KKP 3684 | OM281790 | 2018 | Salad mix with beetroot | − | − | ++ (1) |
| Serratia fonticola KKP 3685 | OM281802 | 2018 | Unwashed spinach | − | − | − |
| Enterobacter cloacae KKP 3686 | OM281778 | 2018 | Unwashed spinach | − | − | + (0.305) |
| Serratia marcescens KKP 3687 | OK103977 | 2018 | Salad mix with beetroot | − | ++ (1) | − |
| Escherichia coli KKP 3688 | OM281784 | 2018 | Salad mix with beetroot | − | − | − |
| Raoultella terrigena KKP 3689 | OK085529 | 2018 | Salad mix with beetroot | − | − | − |
| Escherichia coli KKP 3691 | OM281773 | 2018 | Washed spinach | − | − | − |
| Enterobacter cloacae KKP 3692 | OM281803 | 2018 | Unwashed spinach | ++ (0.553) | − | + (0.00003) |
| Escherichia coli KKP 3705 | OM212647 | 2018 | Salad mix with beetroot | − | − | + (0.614) |
| Enterobacter cloacae KKP 3706 | OM278533 | 2018 | Salad mix with carrot | − | − | + (0.024) |
| Escherichia coli KKP 3707 | OM281777 | 2018 | Washed spinach | − | − | − |
| Escherichia coli KKP 3800 | OM250392 | 2022 | Rucola | − | − | − |
| Escherichia coli KKP 3801 | OM250391 | 2022 | Rucola | − | − | − |
| Escherichia coli KKP 3802 | OM250393 | 2022 | Washed spinach | − | − | − |
| Escherichia coli KKP 3824 | ON303636 | 2020 | Salad mix with carrot | − | − | − |
| Escherichia coli KKP 3825 | ON303626 | 2020 | Rucola | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shymialevich, D.; Błażejak, S.; Średnicka, P.; Cieślak, H.; Ostrowska, A.; Sokołowska, B.; Wójcicki, M. Biological Characterization and Genomic Analysis of Three Novel Serratia- and Enterobacter-Specific Virulent Phages. Int. J. Mol. Sci. 2024, 25, 5944. https://doi.org/10.3390/ijms25115944
Shymialevich D, Błażejak S, Średnicka P, Cieślak H, Ostrowska A, Sokołowska B, Wójcicki M. Biological Characterization and Genomic Analysis of Three Novel Serratia- and Enterobacter-Specific Virulent Phages. International Journal of Molecular Sciences. 2024; 25(11):5944. https://doi.org/10.3390/ijms25115944
Chicago/Turabian StyleShymialevich, Dziyana, Stanisław Błażejak, Paulina Średnicka, Hanna Cieślak, Agnieszka Ostrowska, Barbara Sokołowska, and Michał Wójcicki. 2024. "Biological Characterization and Genomic Analysis of Three Novel Serratia- and Enterobacter-Specific Virulent Phages" International Journal of Molecular Sciences 25, no. 11: 5944. https://doi.org/10.3390/ijms25115944





