Unveiling the Chemical Composition and Biofunctionality of Hericium spp. Fungi: A Comprehensive Overview †
Abstract
:1. Introduction
2. Toxicology Studies
3. Identification of Novel Bioactive Compounds in Hericium spp. and Their Biological Functions
3.1. Polysaccharides
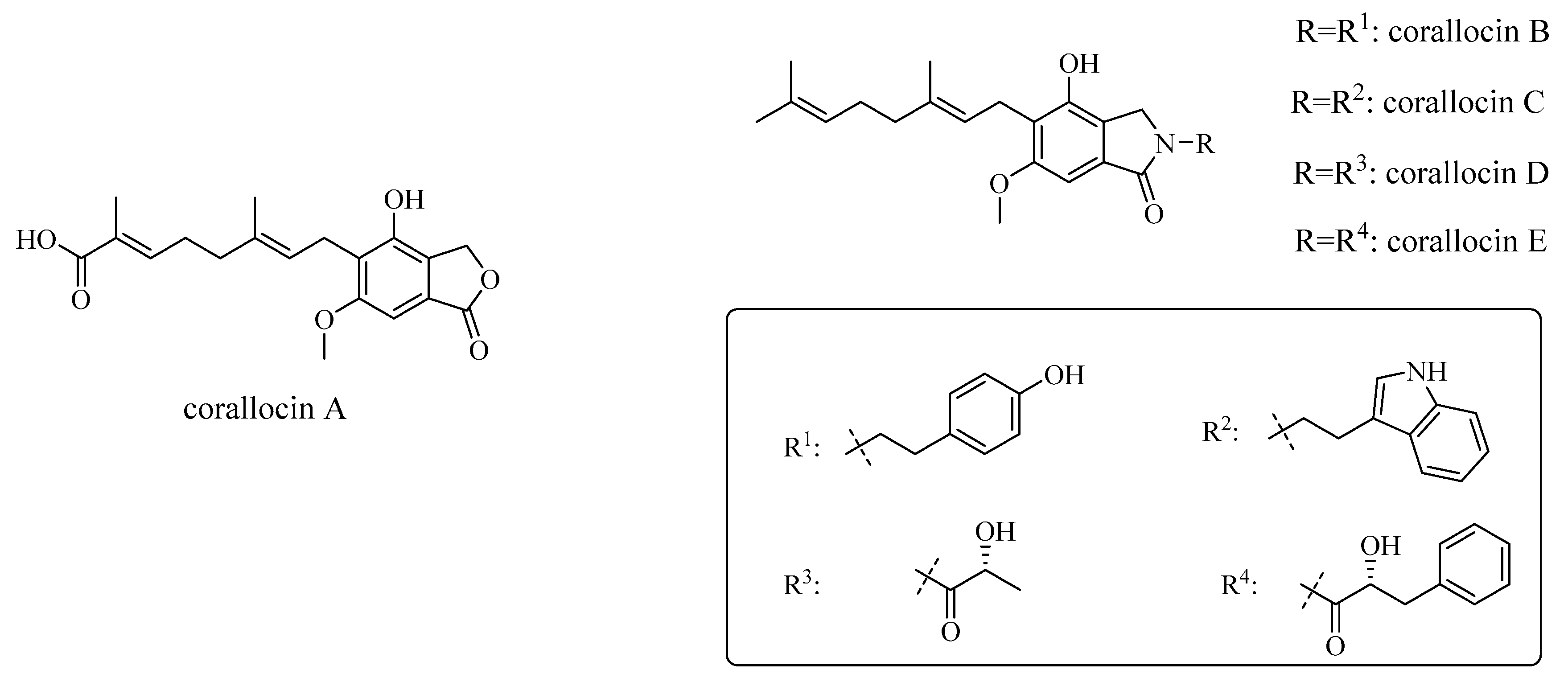
3.2. Corallocins
3.3. Erinacerins
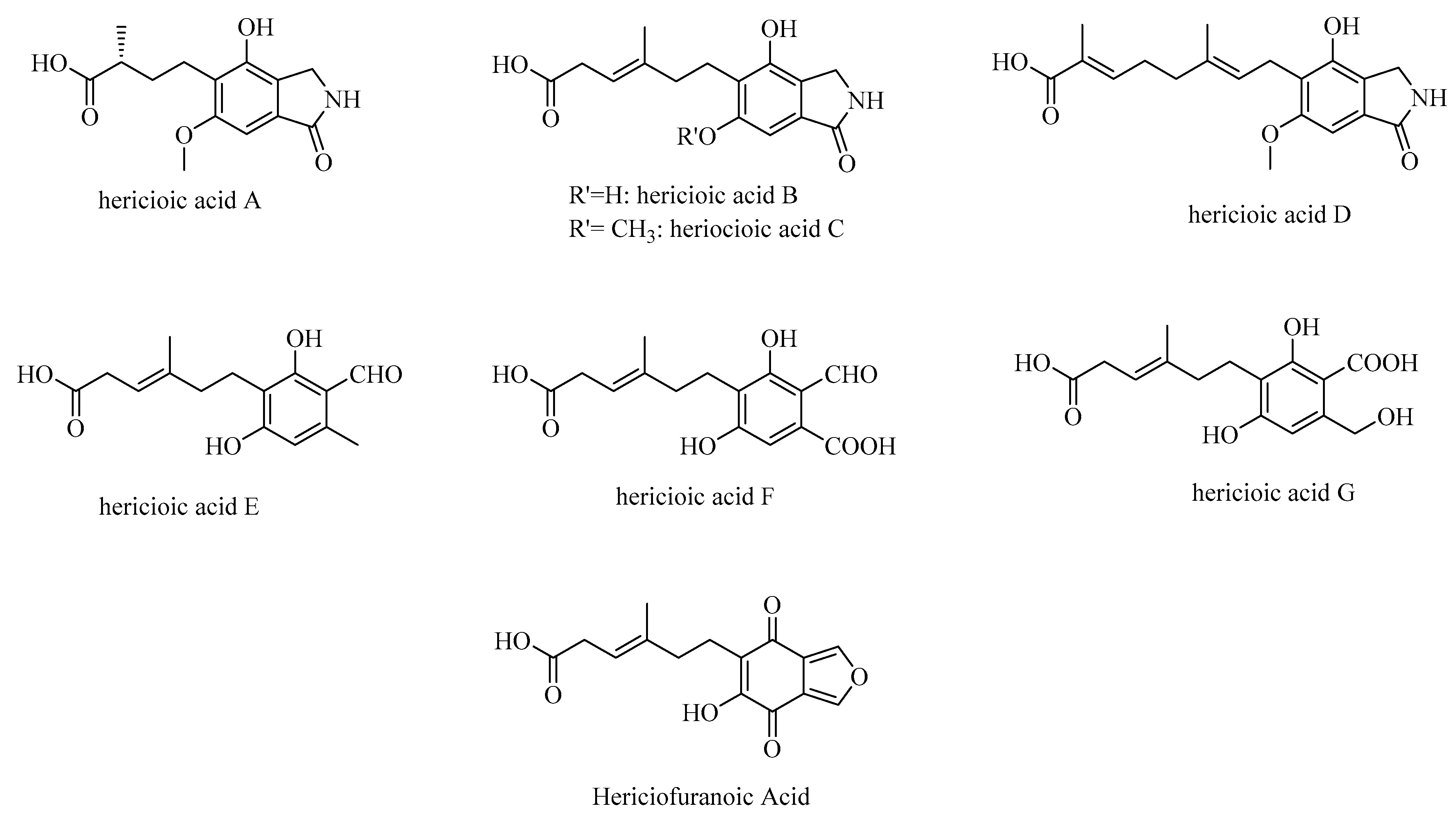
3.4. Hericioic Acids
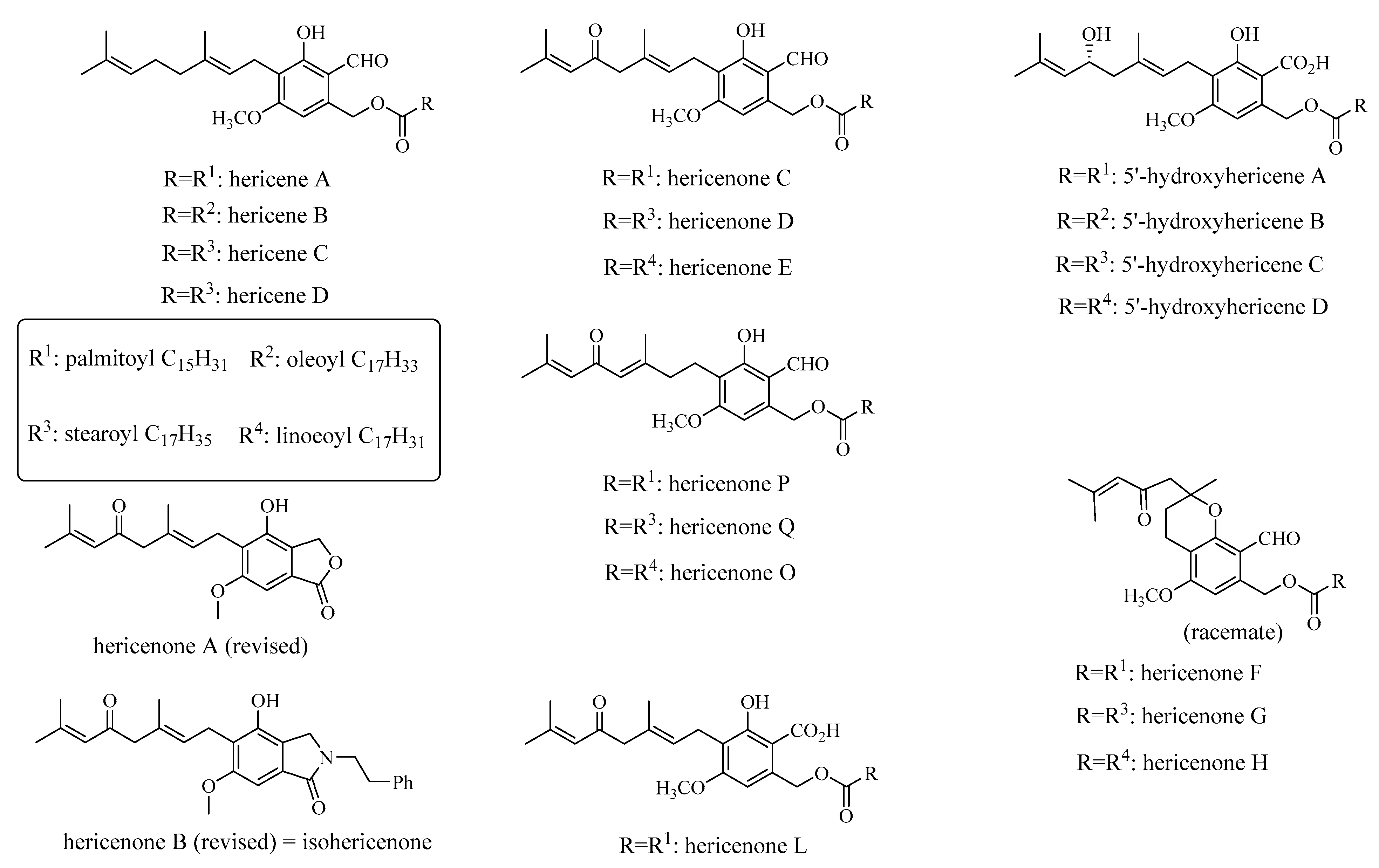
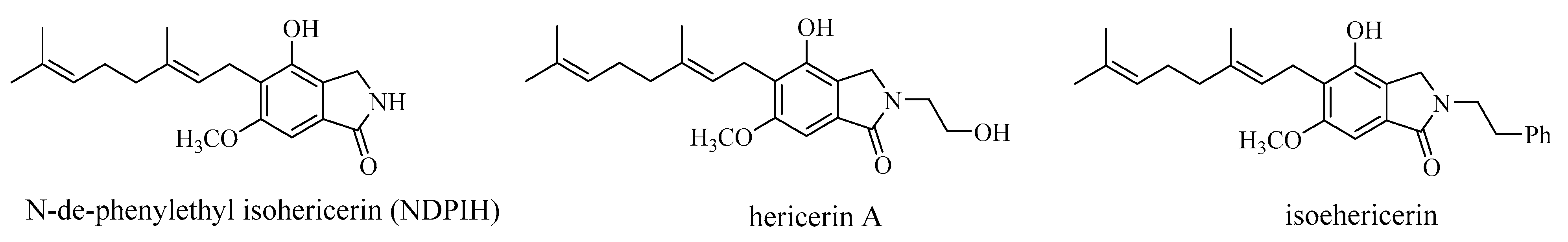
3.5. Hericenes, Hericenones and Hericerins
3.6. Erinacines
3.7. Other Specialized Metabolites
3.7.1. Lovastatin and Ergothioneine
3.7.2. Herialpin A, Herialpin B, and Avenacein
4. Speculated Biosynthesis
4.1. Biosynthesis of Geranyl-Resorcinol-Type Metabolites
4.2. Biosynthesis of Erinacines Metabolites
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Hericenones C, D and E, stimulators of nerve growth factor (NGF)-synthesis, from the mushroom Hericium erinaceum. Tetrahedron Lett. 1991, 32, 4561. [Google Scholar] [CrossRef]
- Mori, K.; Obara, Y.; Hirota, M.; Azumi, Y.; Kinugasa, S.; Inatomi, S.; Nakahata, N. Nerve Growth Factor-Inducing Activity of Hericium erinaceus in 1321N1 Human Astrocytoma Cells. Biol. Pharm. Bull. 2008, 31, 1727. [Google Scholar] [CrossRef] [PubMed]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: A double-blind placebo-controlled clinical trial. Phytother. Res. 2009, 23, 367. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-T.; Feng, T.; Zhang, L.; Dong, Z.-J.; Li, Z.-H.; Liu, J.-K. Cyathane diterpenoids from fruiting bodies of Phellodon niger. Nat. Prod. Bioprospecting 2011, 1, 37. [Google Scholar] [CrossRef]
- Bhandari, D.R.; Shen, T.; Römpp, A.; Zorn, H.; Spengler, B. Analysis of cyathane-type diterpenoids from Cyathus striatus and Hericium erinaceus by high-resolution MALDI MS imaging. Anal. Bioanal. Chem. 2014, 406, 695. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-W.; Liu, L.; Gao, J.-M.; Zhang, A.-L. Cyathane diterpenes from Chinese mushroom Sarcodon scabrosus and their neurite outgrowth-promoting activity. Eur. J. Med. Chem. 2011, 46, 3112. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Zhang, Y.; Guo, C.; Yang, Y.; Han, J.; Yu, B.; Yin, W.; Liu, H. Reconstitution of biosynthetic pathway for mushroom-derived cyathane diterpenes in yeast and generation of new “non-natural” analogues. Acta Pharm. Sin. B 2021, 11, 2945. [Google Scholar] [CrossRef]
- Tan, Y.-F.; Mo, J.-S.; Wang, Y.-K.; Zhang, W.; Jiang, Y.-P.; Xu, K.-P.; Tan, G.-S.; Liu, S.; Li, J.; Wang, W.-X. The ethnopharmacology, phytochemistry and pharmacology of the genus Hericium. J. Ethnopharmacol. 2024, 319, 117353. [Google Scholar] [CrossRef]
- Guan, Y.; Shi, D.; Wang, S.; Sun, Y.; Song, W.; Liu, S.; Wang, C. Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota. Nutrients 2023, 15, 3799. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Jain, S. 111Hericium erinaceus (Bull.) and Hericium coralloides. In Edible and Medicinal Mushrooms of the Himalayas; CRC Press: Boca Raton, FL, USA, 2023; Volume 111. [Google Scholar]
- Song, X.; Gaascht, F.; Schmidt-Dannert, C.; Salomon, C.E. Discovery of Antifungal and Biofilm Preventative Compounds from Mycelial Cultures of a Unique North American Hericium sp. Fungus. Molecules 2020, 25, 963. [Google Scholar] [CrossRef] [PubMed]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two New Cyathane Diterpenoids from Mycelial Cultures of the Medicinal Mushroom Hericium erinaceus and the Rare Species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed]
- Brandalise, F.; Roda, E.; Ratto, D.; Goppa, L.; Gargano, M.L.; Cirlincione, F.; Priori, E.C.; Venuti, M.T.; Pastorelli, E.; Savino, E.; et al. Hericium erinaceus in Neurodegenerative Diseases: From Bench to Bedside and Beyond, How Far from the Shoreline? J. Fungi 2023, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Fung, M.-L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 163. [Google Scholar] [CrossRef]
- Qi, J.; Wu, J.; Kang, S.-J.; Gao, J.-M.; Kawagishi, H.; Liu, H.; Liu, C. The chemical structures, biosynthesis, and biological activities of secondary metabolites from the culinary-medicinal mushrooms of the genus Hericium: A review. Chin. J. Nat. Med. 2024, 22, 1–23. [Google Scholar] [CrossRef]
- Sutthibutpong, T.; Posansee, K.; Liangruksa, M.; Termsaithong, T.; Piyayotai, S.; Phitsuwan, P.; Saparpakorn, P.; Hannongbua, S.; Laomettachit, T. Combining Deep Learning and Structural Modeling to Identify Potential Acetylcholinesterase Inhibitors from Hericium erinaceus. ACS Omega 2024, 9, 16311. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Chen, Y.-L.; Lee, L.-Y.; Chen, W.-P.; Tsai, Y.-T.; Chen, C.-C.; Chen, C.-S. Evaluation of the toxicological safety of erinacine A-enriched Hericium erinaceus in a 28-day oral feeding study in Sprague–Dawley rats. Food Chem. Toxicol. 2014, 70, 61. [Google Scholar] [CrossRef] [PubMed]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Chen, S.N.; Chang, C.S.; Yang, M.F.; Chen, S.; Soni, M.; Mahadevan, B. Subchronic toxicity and genotoxicity studies of Hericium erinaceus β-glucan extract preparation. Curr. Res. Toxicol. 2022, 3, 100068. [Google Scholar] [CrossRef]
- Li, I.C.; Chen, Y.-L.; Chen, W.-P.; Lee, L.-Y.; Tsai, Y.-T.; Chen, C.-C.; Chen, C.-S. Genotoxicity profile of erinacine A-enriched Hericium erinaceus mycelium. Toxicol. Rep. 2014, 1, 1195. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, H.; Raman, J.; David, P.; Wong, K.-H.; Naidu, M.; Sabaratnam, V. Haematological, biochemical and histopathological aspects of Hericium erinaceus ingestion in a rodent model: A sub-chronic toxicological assessment. J. Ethnopharmacol. 2016, 194, 1051. [Google Scholar] [CrossRef] [PubMed]
- Li, I.-C.; Chang, H.-H.; Lin, C.-H.; Chen, W.-P.; Lu, T.-H.; Lee, L.-Y.; Chen, Y.-W.; Chen, Y.-P.; Chen, C.-C.; Lin, D.P.-C. Prevention of Early Alzheimer’s Disease by Erinacine A-Enriched Hericium erinaceus Mycelia Pilot Double-Blind Placebo-Controlled Study. Front. Aging Neurosci. 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Morales, P.; Fernández-Ruiz, V.; Ferreira, I.C.F.R. Chemical composition, antioxidant activity and bioaccessibility studies in phenolic extracts of two Hericium wild edible species. LWT—Food Sci. Technol. 2015, 63, 475. [Google Scholar] [CrossRef]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.-T.; Yang, Y.-C.; Li, Y.-H.; Mau, J.-L.; Wasser, S.P. Chemical Composition and Nutritional and Medicinal Value of Fruit Bodies and Submerged Cultured Mycelia of Culinary-Medicinal Higher Basidiomycetes Mushrooms. Int. J. Med. Mushrooms 2014, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, H.; Feng, G.; Zhang, S.; Wang, J.; Cui, L. Rapid Identification of Chemical Constituents in Hericium erinaceus Based on LC-MS/MS Metabolomics. J. Food Qual. 2021, 2021, 5560626. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q. Research Progress of Flavonoids Regulating Endothelial Function. Pharmaceuticals 2023, 16, 1201. [Google Scholar] [CrossRef]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, 27533. [Google Scholar] [CrossRef]
- Hao, B.; Yang, Z.; Liu, H.; Liu, Y.; Wang, S. Advances in Flavonoid Research: Sources, Biological Activities, and Developmental Prospectives. Curr. Issues Mol. Biol. 2024, 46, 2884. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fan, P.; Feng, S.; Shao, P.; Sun, P. Isolation and Purification of Two Isoflavones from Hericium erinaceum Mycelium by High-Speed Counter-Current Chromatography. Molecules 2018, 23, 560. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S. Total Synthesis of Geranyl-Resorcinols Isolated from Mushrooms of Genus Hericium. Synthesis 2022, 55, 417. [Google Scholar] [CrossRef]
- Mai Huong, L.; Huu Nghi, D.; Dinh Luyen, N.; Thu Quynh, D.; Huong, P.T.T.; Tai, B.H.; Kiem, P.V. Hericium VN, an undescribed compound isolated from Hericium erinaceus and its cytotoxic activity on human brain astrocytoma. J. Asian Nat. Prod. Res. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, Y.; Xu, D.; Konishi, T.; Gao, Q. Hericium erinaceus (Yamabushitake): A unique resource for developing functional foods and medicines. Food Funct. 2014, 5, 3055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lv, H.; Zhang, X. Erinacerins, Novel Glioma Inhibitors from Hericium erinaceus, Induce Apoptosis of U87 Cells through Bax/Capase-2 Pathway. Anti-Cancer Agents Med. Chem. 2020, 20, 2082. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-F.; Chen, J.-H.; Teng, C.-C.; Shen, C.-H.; Hsieh, M.-C.; Lu, C.-C.; Lee, K.-C.; Lee, L.-Y.; Chen, W.-P.; Chen, C.-C.; et al. Protective Effects of Hericium erinaceus Mycelium and Its Isolated Erinacine A against Ischemia-Injury-Induced Neuronal Cell Death via the Inhibition of iNOS/p38 MAPK and Nitrotyrosine. Int. J. Mol. Sci. 2014, 15, 15073. [Google Scholar] [CrossRef] [PubMed]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.-J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef]
- Lee, K.-F.; Tung, S.-Y.; Teng, C.-C.; Shen, C.-H.; Hsieh, M.C.; Huang, C.-Y.; Lee, K.-C.; Lee, L.-Y.; Chen, W.-P.; Chen, C.-C.; et al. Post-Treatment with Erinacine A, a Derived Diterpenoid of H. erinaceus, Attenuates Neurotoxicity in MPTP Model of Parkinson’s Disease. Antioxidants 2020, 9, 137. [Google Scholar] [CrossRef]
- Boddy, L.; Crockatt, M.E.; Ainsworth, A.M. Ecology of Hericium cirrhatum, H. coralloides and H. erinaceus in the UK. Fungal Ecol. 2011, 4, 163. [Google Scholar] [CrossRef]
- Gonkhom, D.; Luangharn, T.; Hyde, K.D.; Stadler, M.; Thongklang, N. Optimal conditions for mycelial growth of medicinal mushrooms belonging to the genus Hericium. Mycol. Prog. 2022, 21, 82. [Google Scholar] [CrossRef]
- Turk, A.; Yeon, S.W.; Ryu, S.H.; Ko, S.M.; Kim, B.S.; Hwang, B.Y.; Lee, M.K. Effect of culture conditions on the content of hericene A, an α-glucosidase inhibitory constituent of Hericium erinaceus. Sci. Hortic. 2021, 288, 110407. [Google Scholar] [CrossRef]
- Atila, F.; Tuzel, Y.; Fernández, J.A.; Cano, A.F.; Sen, F. The effect of some agro– industrial wastes on yield, nutritional characteristics and antioxidant activities of Hericium erinaceus isolates. Sci. Hortic. 2018, 238, 246. [Google Scholar] [CrossRef]
- Khatib, S.; Pereman, I.; Kostanda, E.; Zdouc, M.M.; Ezov, N.; Schweitzer, R.; van der Hooft, J.J.J. Olive mill solid waste induces beneficial mushroom-specialized metabolite diversity: A computational metabolomics study. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhang, D.-d.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Recent developments in Hericium erinaceus polysaccharides: Extraction, purification, structural characteristics and biological activities. Crit. Rev. Food Sci. Nutr. 2019, 59, S96–S115. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-K.; Ding, Z.-C.; Gao, X.; Wang, Y.-Y.; Yang, Y.; Wu, D.; Zhang, H.-N. Comparative study of physicochemical properties and bioactivity of Hericium erinaceus polysaccharides at different solvent extractions. Carbohydr. Polym. 2018, 193, 373. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Song, M.; Wang, C.; Guo, Z.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Hericium erinaceus fermented mycelium and its pharmacological basis for application in Alzheimer’s disease: Oxidative stress related calcium homeostasis. Int. J. Biol. Macromol. 2021, 193, 358. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Huang, H. Structural characterization of a novel polysaccharide fraction from Hericium erinaceus and its signaling pathways involved in macrophage immunomodulatory activity. J. Funct. Foods 2017, 37, 574. [Google Scholar] [CrossRef]
- Li, J.; Mo, J.-R.; Hu, S.-Y.; Dong, X.; Li, J.-W.; Yang, L.-Y.; Wu, Y.-J. Effects of Hericium erinaceus polysaccharide in porcine IPEC-J2 intestinal epithelial cells against apoptosis induced by oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 280, 109902. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Lv, G.-Y.; Song, T.-T.; Xu, Z.-W.; Wang, M.-Y. Effects of different extraction methods on the structural and biological properties of Hericium coralloides polysaccharides. Food Chem. 2024, 445, 138752. [Google Scholar] [CrossRef]
- Li, H.; Feng, J.; Liu, C.; Hou, S.; Meng, J.; Liu, J.-Y.; Zilong, S.; Chang, M.-C. Polysaccharides from an edible mushroom, Hericium erinaceus, alleviate ulcerative colitis in mice by inhibiting the NLRP3 inflammasomes and reestablish intestinal homeostasis. Int. J. Biol. Macromol. 2024, 267, 131251. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A–C, Nerve Growth and Brain-Derived Neurotrophic Factor Inducing Metabolites from the Mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Hong, S.M.; Khan, Z.; Lee, S.K.; Vishwanath, M.; Turk, A.; Yeon, S.W.; Jo, Y.H.; Lee, D.H.; Lee, J.K.; et al. Neurotrophic isoindolinones from the fruiting bodies of Hericium erinaceus. Bioorganic Med. Chem. Lett. 2021, 31, 127714. [Google Scholar] [CrossRef] [PubMed]
- Sum, W.C.; Gonkhom, D.; Ibrahim, M.A.A.; Stadler, M.; Ebada, S.S. New isoindolinone derivatives isolated from the fruiting bodies of the basidiomycete Hericium coralloides. Mycol. Prog. 2023, 23, 4. [Google Scholar] [CrossRef]
- Martínez-Mármol, R.; Chai, Y.; Conroy, J.N.; Khan, Z.; Hong, S.-M.; Kim, S.B.; Gormal, R.S.; Lee, D.H.; Lee, J.K.; Coulson, E.J.; et al. Hericerin derivatives activates a pan-neurotrophic pathway in central hippocampal neurons converging to ERK1/2 signaling enhancing spatial memory. J. Neurochem. 2023, 165, 791. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Bao, L.; Qi, Q.; Zhao, F.; Ma, K.; Pei, Y.; Liu, H. Erinacerins C–L, Isoindolin-1-ones with α-Glucosidase Inhibitory Activity from Cultures of the Medicinal Mushroom Hericium erinaceus. J. Nat. Prod. 2015, 78, 146. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Chen, Y.-P.; Lin, T.-W.; Li, T.-J.; Chen, Y.-W.; Li, I.-C.; Chen, C.-C. Discovery of a New Compound, Erinacerin W, from the Mycelia of Hericium erinaceus, with Immunomodulatory and Neuroprotective Effects. Molecules 2024, 29, 812. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.; Amen, Y.; Allam, A.E.; Kudo, T.; Nagata, M.; Ohnuki, K.; Shimizu, K. New isoindolinones from the fruiting bodies of the fungus Hericium erinaceus. Phytochem. Lett. 2019, 32, 10. [Google Scholar] [CrossRef]
- Wang, K.; Bao, L.; Ma, K.; Liu, N.; Huang, Y.; Ren, J.; Wang, W.; Liu, H. Eight new alkaloids with PTP1B and α-glucosidase inhibitory activities from the medicinal mushroom Hericium erinaceus. Tetrahedron 2015, 71, 9557. [Google Scholar] [CrossRef]
- Bailly, C. Discovery and current developments of isoindolinone-based fungal natural products. Eur. J. Med. Chem. Rep. 2023, 9, 100112. [Google Scholar] [CrossRef]
- Sum, W.C.; Ebada, S.S.; Kirchenwitz, M.; Kellner, H.; Ibrahim, M.A.A.; Stradal, T.E.B.; Matasyoh, J.C.; Stadler, M. Hericioic Acids A–G and Hericiofuranoic Acid; Neurotrophic Agents from Cultures of the European Mushroom Hericium flagellum. J. Agric. Food Chem. 2023, 71, 11094. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, W.; Kim, E.-J.; Shim, S.H.; Kang, H.K.; Kim, Y.H. Isolation and identification of aromatic compounds in Lion’s Mane Mushroom and their anticancer activities. Food Chem. 2015, 170, 336. [Google Scholar] [CrossRef] [PubMed]
- Arnone, A.; Cardillo, R.; Nasini, G.; de Pava, O.V. Secondary Mold Metabolites: Part 46. Hericenes A-C and Erinapyrone C, New Metabolites Produced by the Fungus Hericium erinaceus. J. Nat. Prod. 1994, 57, 602. [Google Scholar] [CrossRef]
- Ma, B.-J.; Yu, H.-Y.; Shen, J.-W.; Ruan, Y.; Zhao, X.; Zhou, H.; Wu, T.-T. Cytotoxic aromatic compounds from Hericium erinaceum. J. Antibiot. 2010, 63, 713. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Kang, H.-W.; Park, C.-G.; Ahn, Y.-S.; Shin, Y. Isolation and identification of phytochemicals and biological activities of Hericium ernaceus and their contents in Hericium strains using HPLC/UV analysis. J. Ethnopharmacol. 2016, 184, 219. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Ando, M.; Shinba, K.; Sakamoto, H.; Yoshida, S.; Ojima, F.; Ishiguro, Y.; Ukai, N.; Furukawa, S. Chromans, hericenones F, G and H from the mushroom Hericium erinaceum. Phytochemistry 1992, 32, 175. [Google Scholar] [CrossRef]
- Kawagishi, H.; Ando, M.; Mizuno, T. Hericenone A and B as cytotoxic principles from the mushroom hericium erinaceum. Tetrahedron Lett. 1990, 31, 373. [Google Scholar] [CrossRef]
- Rama Rao, A.V.; Reddy, R.G. First unambiguous total synthesis of hericenone A: Proposed structure revised. Tetrahedron Lett. 1992, 33, 4061. [Google Scholar] [CrossRef]
- Ma, B.-J.; Ma, J.-C.; Ruan, Y. Hericenone L, a new aromatic compound from the fruiting bodies of Hericium erinaceums. Chin. J. Nat. Med. 2012, 10, 363. [Google Scholar] [CrossRef]
- Thongkongkaew, T.; Jariyasopit, N.; Khoomrung, S.; Siritutsoontorn, S.; Jitrapakdee, S.; Kittakoop, P.; Ruchirawat, S. Anti-Xanthine Oxidase 5′-Hydroxyhericenes A–D from the Edible Mushroom Hericium erinaceus and Structure Revision of 3-[2,3-Dihydroxy-4-(hydroxymethyl)tetrahydrofuran-1-yl]-pyridine-4,5-diol. ACS Omega 2023, 8, 46284. [Google Scholar] [CrossRef]
- Kimura, Y.; Nishibe, M.; Nakajima, H.; Hamasaki, T.; Shimada, A.; Tsuneda, A.; Shigematsu, N. Hericerin, a New Pollen Growth Inhibitor from the Mushroom Hericium erinaceum. Agric. Biol. Chem. 1991, 55, 2673. [Google Scholar] [CrossRef]
- Kobayashi, S.; Inoue, T.; Ando, A.; Tamanoi, H.; Ryu, I.; Masuyama, A. Total Synthesis and Structural Revision of Hericerin. J. Org. Chem. 2012, 77, 5819. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Han, C.; Wang, D.; Inoue, Y.; Amen, Y.; Othman, A.; Mittraphab, Y.; Nagata, M.; Shimizu, K. New benzaldehyde derivatives from the fruiting bodies of Hericium erinaceus with cytotoxic activity. Nat. Prod. Res. 2023, 37, 4089. [Google Scholar] [CrossRef] [PubMed]
- Tamrakar, S.; Wang, D.; Hiraki, E.; Han, C.; Ruan, Y.; Allam, A.E.; Amen, Y.; Katakura, Y.; Shimizu, K. Deacylated Derivative of Hericenone C Treated by Lipase Shows Enhanced Neuroprotective Properties Compared to Its Parent Compound. Molecules 2023, 28, 4549. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Yin, X.; Zhang, C.C.; Jia, Q.; Gao, J.M. Structure Diversity, Synthesis, and Biological Activity of Cyathane Diterpenoids in Higher Fungi. Curr. Med. Chem. 2015, 22, 2375. [Google Scholar] [CrossRef] [PubMed]
- Kenmoku, H.; Shimai, T.; Toyomasu, T.; Kato, N.; Sassa, T. Erinacine Q, a New Erinacine from Hericium erinaceum, and its Biosynthetic Route to Erinacine C in the Basidiomycete. Biosci. Biotechnol. Biochem. 2002, 66, 571. [Google Scholar] [CrossRef]
- Lee, E.W.; Shizuki, K.; Hosokawa, S.; Suzuki, M.; Suganuma, H.; Inakuma, T.; Li, J.; Ohnishi-Kameyama, M.; Nagata, T.; Furukawa, S.; et al. Two novel diterpenoids, erinacines H and I from the mycelia of Hericium erinaceum. Biosci. Biotechnol. Biochem. 2000, 64, 2402. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.L.; Whitehead, C.R. Recent progress on the synthesis of cyathane type diterpenes. A review. Org. Prep. Proced. Int. 2000, 32, 307. [Google Scholar] [CrossRef]
- Chen, C.-C.; Tzeng, T.-T.; Chen, C.-C.; Ni, C.-L.; Lee, L.-Y.; Chen, W.-P.; Shiao, Y.-J.; Shen, C.-C. Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438. [Google Scholar] [CrossRef]
- Fu, J.-T.; Yang, C.-J.; Lee, L.-Y.; Chen, W.-P.; Chen, Y.-W.; Chen, C.-C.; Sun, Y.-T.; Yang, C.-S.; Tzeng, S.-F. Erinacine S, a small active component derived from Hericium erinaceus, protects oligodendrocytes and alleviates mood abnormalities in cuprizone-exposed rodents. Biomed. Pharmacother. 2024, 173, 116297. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Bao, L.; Yang, Y.; Ma, K.; Liu, H. Three new cyathane diterpenes with neurotrophic activity from the liquid cultures of Hericium erinaceus. J. Antibiot. 2018, 71, 818. [Google Scholar] [CrossRef] [PubMed]
- Kenmoku, H.; Sassa, T.; Kato, N. Isolation of erinacine P, a new parental metabolite of cyathane-xylosides, from Hericium erinaceum and its biomimetic conversion into erinacines A and B. Tetrahedron Lett. 2000, 41, 4389. [Google Scholar] [CrossRef]
- Černelič Bizjak, M.; Jenko Pražnikar, Z.; Kenig, S.; Hladnik, M.; Bandelj, D.; Gregori, A.; Kranjc, K. Effect of erinacine A-enriched Hericium erinaceus supplementation on cognition: A randomized, double-blind, placebo-controlled pilot study. J. Funct. Foods 2024, 115, 106120. [Google Scholar] [CrossRef]
- Priori, E.C.; Ratto, D.; De Luca, F.; Sandionigi, A.; Savino, E.; Giammello, F.; Romeo, M.; Brandalise, F.; Roda, E.; Rossi, P. Hericium erinaceus Extract Exerts Beneficial Effects on Gut–Neuroinflammaging–Cognitive Axis in Elderly Mice. Biology 2024, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Shimbo, M.; Kawagishi, H.; Yokogoshi, H. Erinacine A increases catecholamine and nerve growth factor content in the central nervous system of rats. Nutr. Res. 2005, 25, 617. [Google Scholar] [CrossRef]
- Xu, J.; Lacoske, M.H.; Theodorakis, E.A. Neurotrophic natural products: Chemistry and biology. Angew. Chem. (Int. Ed. Engl.) 2014, 53, 956. [Google Scholar] [CrossRef] [PubMed]
- Valu, M.-V.; Soare, L.C.; Ducu, C.; Moga, S.; Negrea, D.; Vamanu, E.; Balseanu, T.-A.; Carradori, S.; Hritcu, L.; Boiangiu, R.S. Hericium erinaceus (Bull.) Pers. Ethanolic Extract with Antioxidant Properties on Scopolamine-Induced Memory Deficits in a Zebrafish Model of Cognitive Impairment. J. Fungi 2021, 7, 477. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, J.-y.; Feng, X.-l.; Zhang, Y.; Hu, X.; Hui, H.; Xue, X.; Qi, J. Unprecedented Neoverrucosane and Cyathane Diterpenoids with Anti-Neuroinflammatory Activity from Cultures of the Culinary-Medicinal Mushroom Hericium erinaceus. Molecules 2023, 28, 6380. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Liao, E.-C.; Chiang, W.-C.; Wang, K.-L. Antioxidative Activities of Micronized Solid-State Cultivated Hericium erinaceus Rich in Erinacine A against MPTP-Induced Damages. Molecules 2023, 28, 3386. [Google Scholar] [CrossRef]
- Yaovapa, A.; Teerachart, L.; Danita, P.; Wachiryah, T.-A. Benefits of Erinacines from Different Cultivate Formulas on Cognitive Deficits and Anxiety-like Behaviour in Mice with Trimethyltin-Induced Toxicity. Trop. Life Sci. Res. 2023, 34, 165. [Google Scholar] [CrossRef]
- Lazur, J.; Kała, K.; Krakowska, A.; Sułkowska-Ziaja, K.; Szewczyk, A.; Piotrowska, J.; Rospond, B.; Fidurski, M.; Marzec, K.; Muszyńska, B. Analysis of bioactive substances and essential elements of mycelia and fruiting bodies of Hericium spp. J. Food Compos. Anal. 2024, 127, 105981. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell. Signal. 2021, 87, 110122. [Google Scholar] [CrossRef]
- Fu, T.-T.; Shen, L. Ergothioneine as a Natural Antioxidant against Oxidative Stress-Related Diseases. Front. Pharmacol. 2022, 13, 850813. [Google Scholar] [CrossRef]
- Li, L.-N.; Wang, L.; Guo, X.-L. Chemical constituents from the culture of the fungus Hericium alpestre. J. Asian Nat. Prod. Res. 2019, 21, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yu, C.; Qi, J.; Wang, P.; Zhao, P.; Gong, W.; Xie, C.; Xia, X.; Liu, C. High-efficient production of mushroom polyketide compounds in a platform host Aspergillus oryzae. Microb. Cell Factories 2023, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.-H.; Yao, J.-N.; Peng, Y.-L.; Huang, R.; Feng, T.; Liu, J.-K. Isoindolinone-containing meroterpenoids with α-glucosidase inhibitory activity from mushroom Hericium caput-medusae. Fitoterapia 2017, 122, 107. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Cheng, M.; Zhu, J.-F.; Zhang, Y.; Cui, K.; Wang, X.; Qi, J. Comparative Genomic Analysis and Metabolic Potential Profiling of a Novel Culinary-Medicinal Mushroom, Hericium rajendrae (Basidiomycota). J. Fungi 2023, 9, 1018. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, L.; Li, J.; Chen, J.; Yang, M. Genome Sequencing of Hericium coralloides by a Combination of PacBio RS II and Next-Generation Sequencing Platforms. Int. J. Genom. 2022, 2022, 4017654. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, X.; Yang, Y.L.; Xing, Y.M.; Zhang, Q.; Li, J.M.; Ma, K.; Liu, H.W.; Guo, S.X. Genomic and transcriptomic analyses reveal differential regulation of diverse terpenoid and polyketides secondary metabolites in Hericium erinaceus. Sci. Rep. 2017, 7, 10151. [Google Scholar] [CrossRef]
- Luo, P.; Huang, J.-H.; Lv, J.-M.; Wang, G.-Q.; Hu, D.; Gao, H. Biosynthesis of fungal terpenoids. Nat. Prod. Rep. 2024, 41, 748–783. [Google Scholar] [CrossRef]


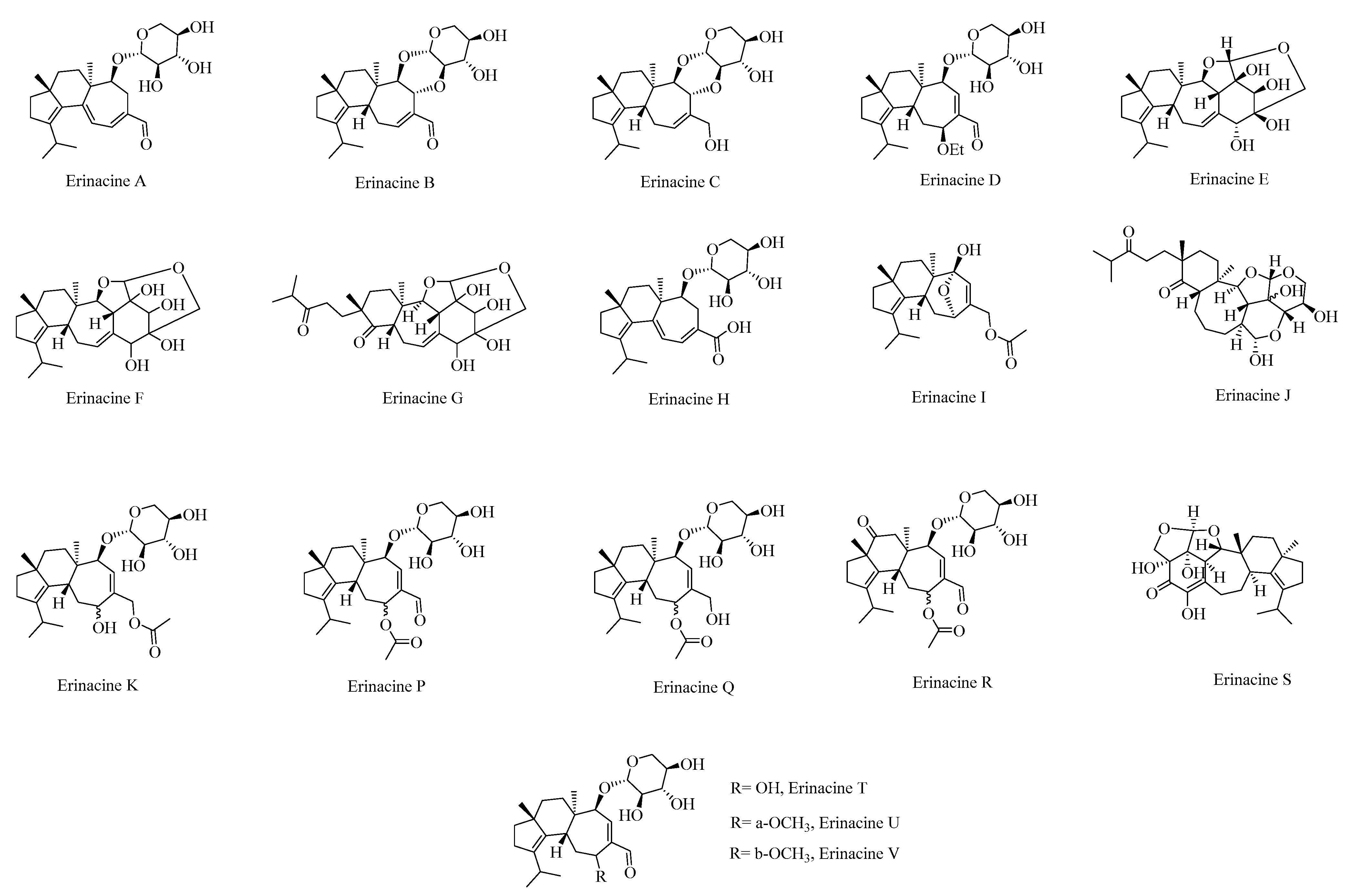
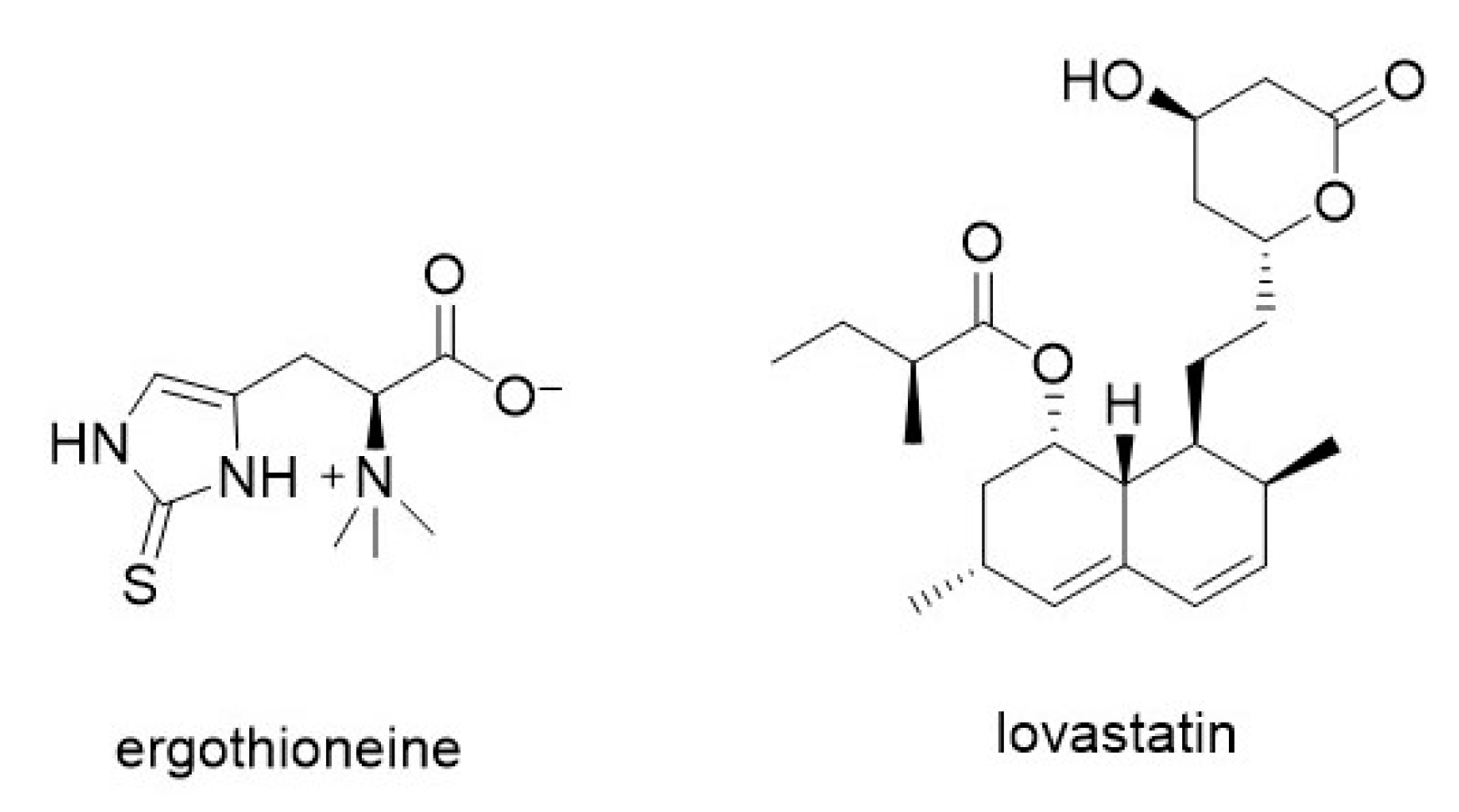

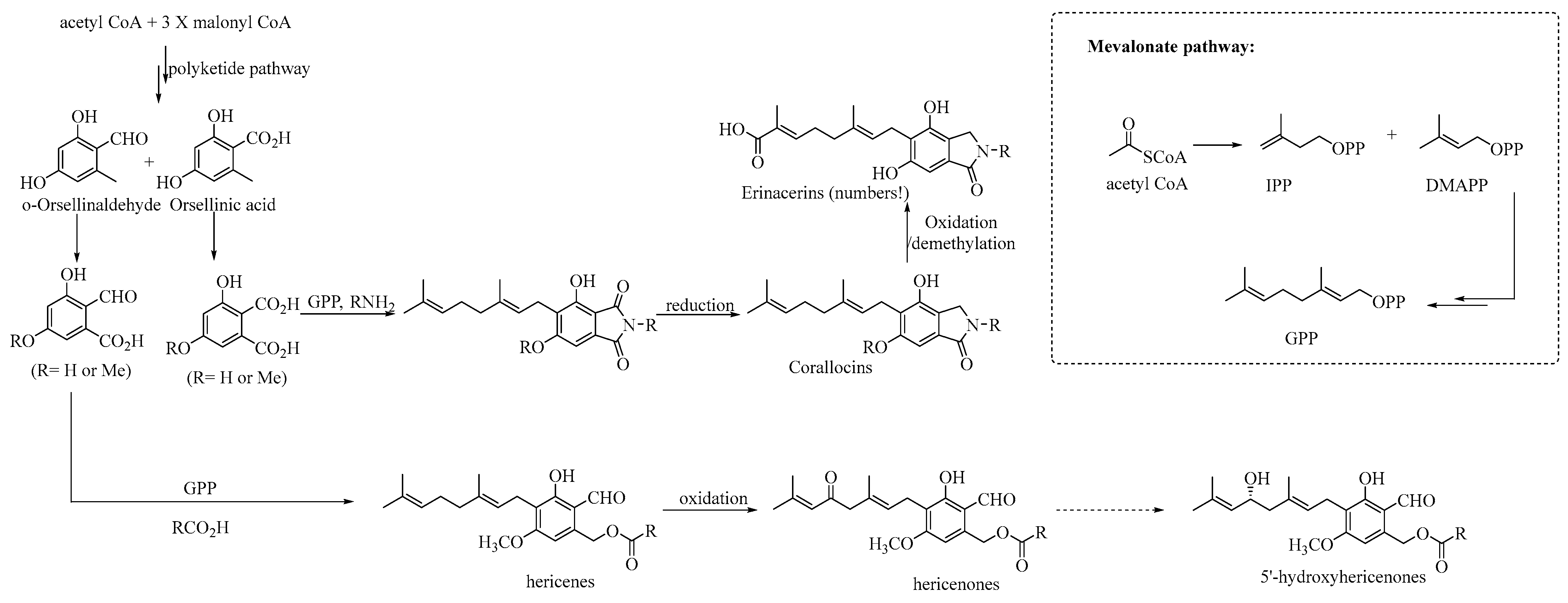
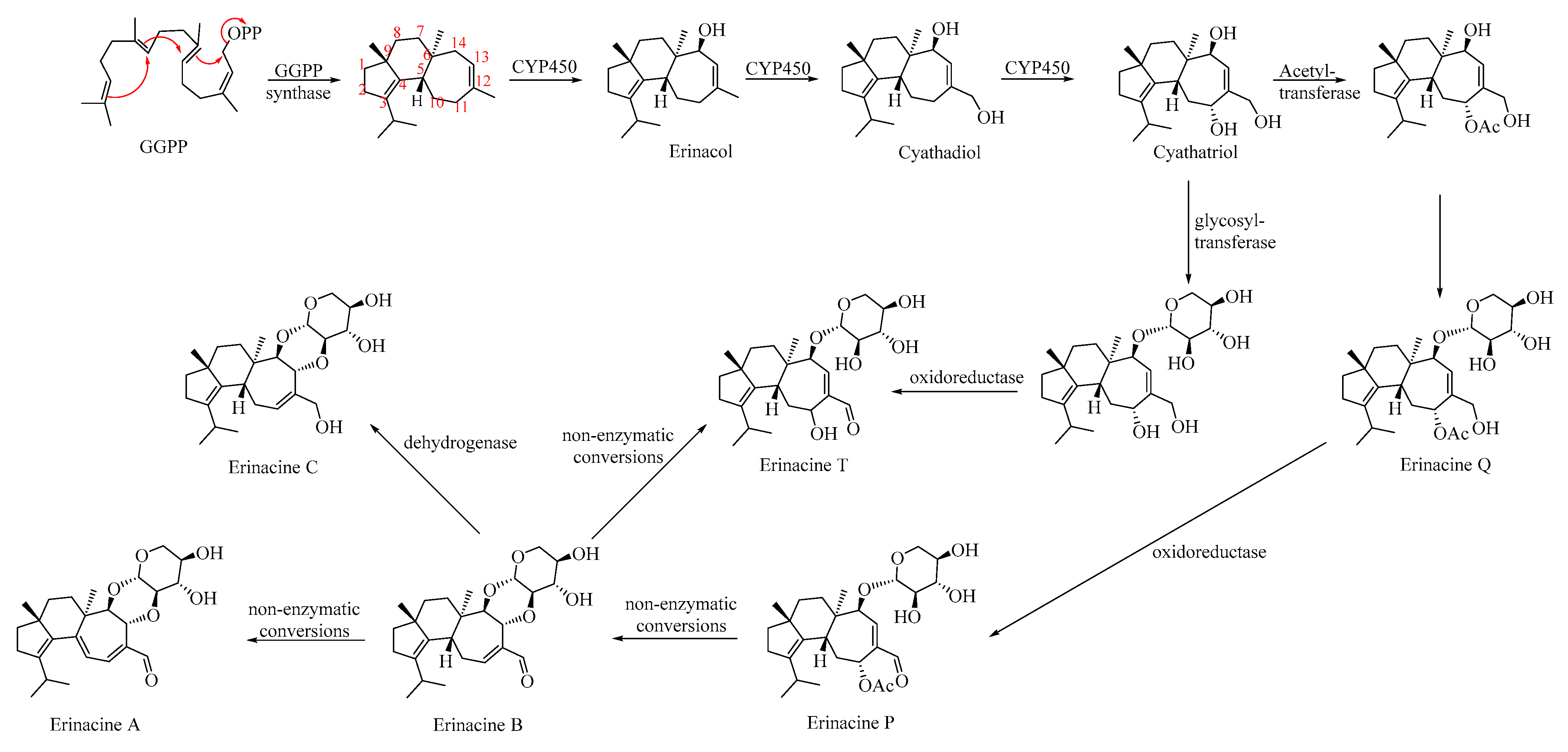
| Analyzed Compound | Sample Type | Biological Activity | Study Model | Reference |
|---|---|---|---|---|
| polysaccharides fraction HEP-C | H. erinaceus fruiting bodies | Antioxidant potential—scavenging activity Hypoglycemic potential—inhibitory effect on α-glycosidase and α-amylase | in vitro | [47] |
| polysaccharides fraction HEP-W | H. erinaceus fruiting bodies | Immunomodulatory potential—enhancing pinocytic and phagocytic capacity, increasing NO, IL-6, and TNF-α secretion | in vitro—RAW264.7 cells | [49] |
| polysaccharides fraction HEP | H. erinaceus fruiting bodies | Antioxidant potential—scavenging activity, reducing LDH and MDA production anti-apoptotic effect | in vitro—IPEC-J2 cells | [50] |
| polysaccharides fractions | H. coralloides fruiting bodies | Antioxidant potential—scavenging activities (ABTS, DPPH, hydroxyl, superoxide), protection against H2O2-induced cell death | in vitro—L929 cells | [51] |
| polysaccharides extract | H. erinaceus fruiting bodies | Antioxidant potential—decreased malondialdehyde levels, increased levels of the antioxidant enzymes (superoxide dismutase and catalase) in mice colon Anti-inflammatory—reducing pro-inflammatory factors (IL-6, IL-1β, and TNF-α), promoting the anti-inflammatory factor IL-10 | in vivo—male C57BL/6 J mice | [52] |
| polysaccharides fraction PHEB | H. erinaceus fermented mycelia | Neuroprotective and antioxidant activities—improving cell viability of L-glutamate-exposed cells, improving AD-model mice cognitivity, alleviating amyloid-beta deposition and tau hyperphosphorylation, and antioxidant activity in the brain by the regulation of calcium homeostasis | in vitro—HT22 cells in vivo—APP/PS1 mice | [48] |
| hericioic acids A–G, hericiofuranoic acid | H. flagellum fruiting bodies | Neurotrophic activity—increased neurite outgrowths in PC12 cells | in vitro—PC12 cells | [62] |
| corallocin A | H. coralloides fruiting bodies | Neurotrophic activity—upregulation of NGF expression in astrocytoma cells followed by neurite outgrowth in PC12 cells | in vitro—PC12 cells, 1321N1 cells | [53] |
| H. erinaceus fruiting bodies | Upregulation of NGF, BDNF, and SYP in glioma cells | in vitro—C6 cells, N2a cells | [54] | |
| H. erinaceus fruiting bodies | Elicitation of neurotrophic responses on hippocampal neuron culture | ex vivo—primary hippocampal neurons from E18 rat embryos | [56] | |
| corallocin B | H. coralloides fruiting bodies | Neurotrophic activity—upregulation of BDNF expression in astrocytoma cells | in vitro—1321N1 cells | [53] |
| corallocin C | H. coralloides fruiting bodies | Neurotrophic activity—upregulation of NGF expression in astrocytoma cells followed by neurite outgrowth in PC12 cells, upregulation of BDNF expression | in vitro—1321N1 cells and PC12 cells | [53] |
| erinacerins Q, S, T | H. erinaceus fruiting bodies | Antidiabetic potential—inhibitory activity against PTP1B phosphatase and α-glucosidase | in vitro | [60] |
| erinacerin W | H. erinaceus mycelia | Anti-inflammatory and neuroprotective activities—alleviation of the LPS-activated BV-2 microglia-induced overexpression of IL-6, IL-1β, and TNF-α on neuroblastoma cells | in vitro—BV2 microglia- SH-SY5Y neuroblastoma co-culture | [58] |
| 5′-hydroxyhericenes A–D | H. erinaceus fruiting bodies | Antioxidant potential—inhibition of xanthine oxidase activity | in vitro | [71] |
| hericenone L | H. erinaceus fruiting bodies | Anti-cancerous potential—against six human cancer cell lines | in vitro—SH-SY5Y, 1321N1, HCT-116, Caco-2, OVK18 and HeLa cells | [59] |
| hericenone Q | H. erinaceus fruiting bodies | Anti-cancerous potential—against human colorectal carcinoma and liver cancer cell lines | in vitro—Hep-G2 and HCT-116 cells | [74] |
| hericenone C and its deacylated derivative | H. erinaceus fruiting bodies | Neurotropic and neuroprotective activities—inducing BDNF expression and protection against H2O2-induced oxidative stress in astrocytoma cells | in vitro—1321N1 cells | [75] |
| N-dephenylethyl isohericerin, hericene A | H. erinaceus fruiting bodies | Neurotrophic activity—promoting extensive axon outgrowth and neurite branching in cultured hippocampal neurons | ex vivo—primary hippocampal neurons from E18 rat embryos | [56] |
| erinacine A | H. erinaceus mycelia | Antioxidant and neuroprotective potential—erinacine A-enriched extract impaired memory in an AD model of zebrafish | in vivo—wild type, short-fin strain zebrafish | [88] |
| H. erinaceus mycelia | Antioxidant and neuroprotective potential—erinacine A-enriched extract recovered dopamine levels in MPTP-treated mice and lowered ROS levels in the brain, liver, and blood | in vivo—C57BL/6Narl male mice | [90] | |
| H. erinaceus mycelia | Neuroprotective activities—preventing cytotoxicity of neuronal cells and the production of ROS in vitro and in MPTP-treated mice | ex vivo—mouse N2a cells, mouse neuron substantia nigra cells in vivo—C57BL/6 mice | [40] | |
| erinacine A, C, F | H. erinaceus mycelia | Neurotrophic activity—increased neurite outgrowths in PC12 cells Anti-inflammatory and neuroprotective activity—LPS-induced NO production inhibition in BV2 microglia cells | in vitro—PC12 cells, BV2 microglia cells | [89] |
| erinacine L | H. erinaceus mycelia | Neurotrophic activity—increased neurite outgrowths in PC12 cells Anti-inflammatory and neuroprotective activity—LPS-induced NO production inhibition in BV2 microglia cells | in vitro—PC12 cells, BV2 microglia cells | [89] |
| Nitric oxide synthesis inhibition | in silico—molecular docking simulation | |||
| erinacine S | H. erinaceus mycelia | Neuroprotective activity—preventing loss of oligodendrocytes and myelin during acute demyelination, and preserving myelin during chronic demyelination | ex vivo—oligodendrocyte cells from SD rat embryos in vivo—male SD rats | [81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostanda, E.; Musa, S.; Pereman, I. Unveiling the Chemical Composition and Biofunctionality of Hericium spp. Fungi: A Comprehensive Overview. Int. J. Mol. Sci. 2024, 25, 5949. https://doi.org/10.3390/ijms25115949
Kostanda E, Musa S, Pereman I. Unveiling the Chemical Composition and Biofunctionality of Hericium spp. Fungi: A Comprehensive Overview. International Journal of Molecular Sciences. 2024; 25(11):5949. https://doi.org/10.3390/ijms25115949
Chicago/Turabian StyleKostanda, Elizabeth, Sanaa Musa, and Idan Pereman. 2024. "Unveiling the Chemical Composition and Biofunctionality of Hericium spp. Fungi: A Comprehensive Overview" International Journal of Molecular Sciences 25, no. 11: 5949. https://doi.org/10.3390/ijms25115949






