Genome-Wide Identification and Expression Analysis of the YTH Domain-Containing RNA-Binding Protein Family in Cinnamomum camphora
Abstract
:1. Introduction
2. Results
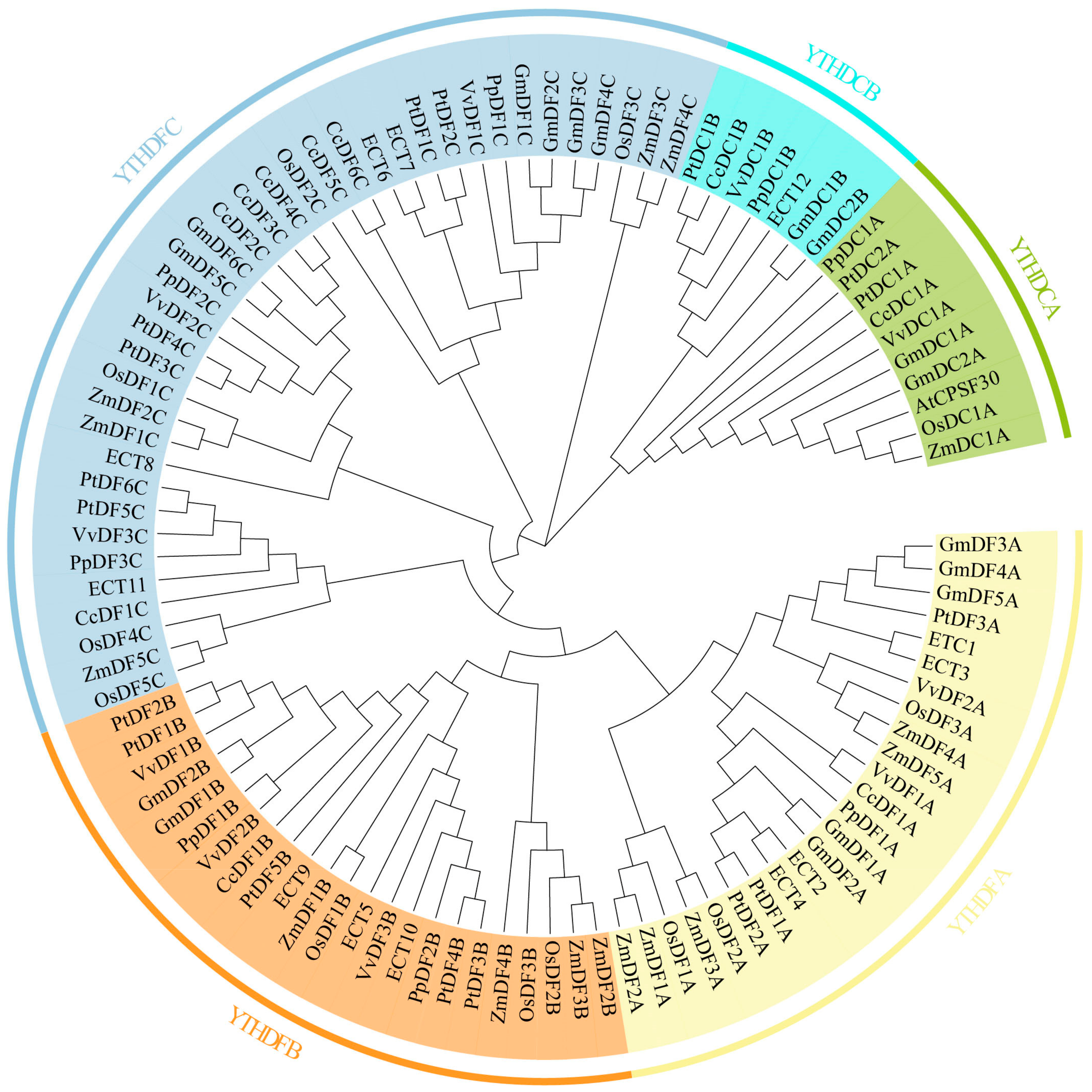
2.1. Identification and Phylogenetic Analysis of the C. camphora YTH Gene Family
2.2. Synteny Analysis of the CcYTH Genes
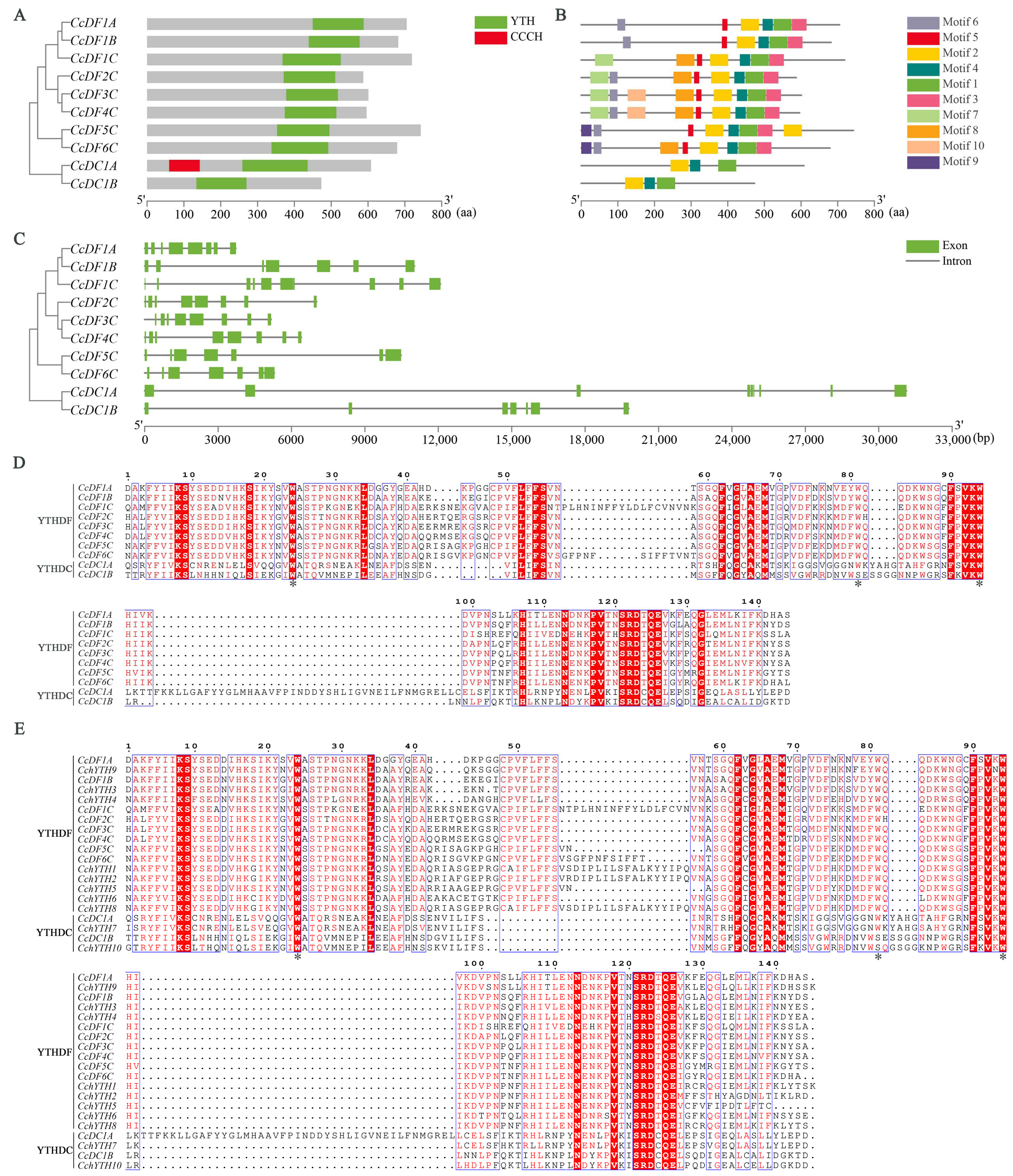
2.3. Protein Features and Gene Structure of the CcYTH Genes
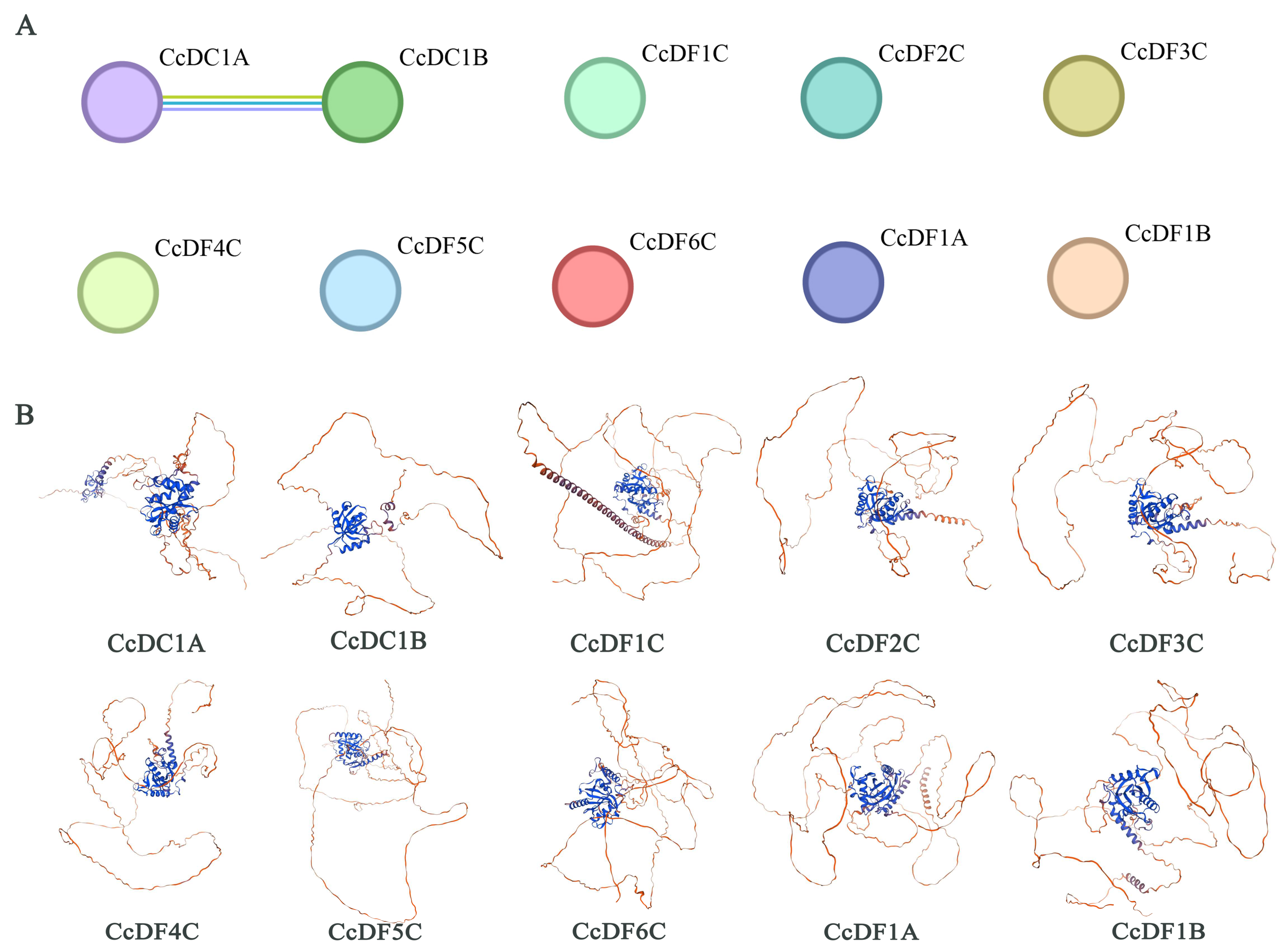
2.4. The Interaction and Tertiary Structure Prediction of CcYTH Proteins
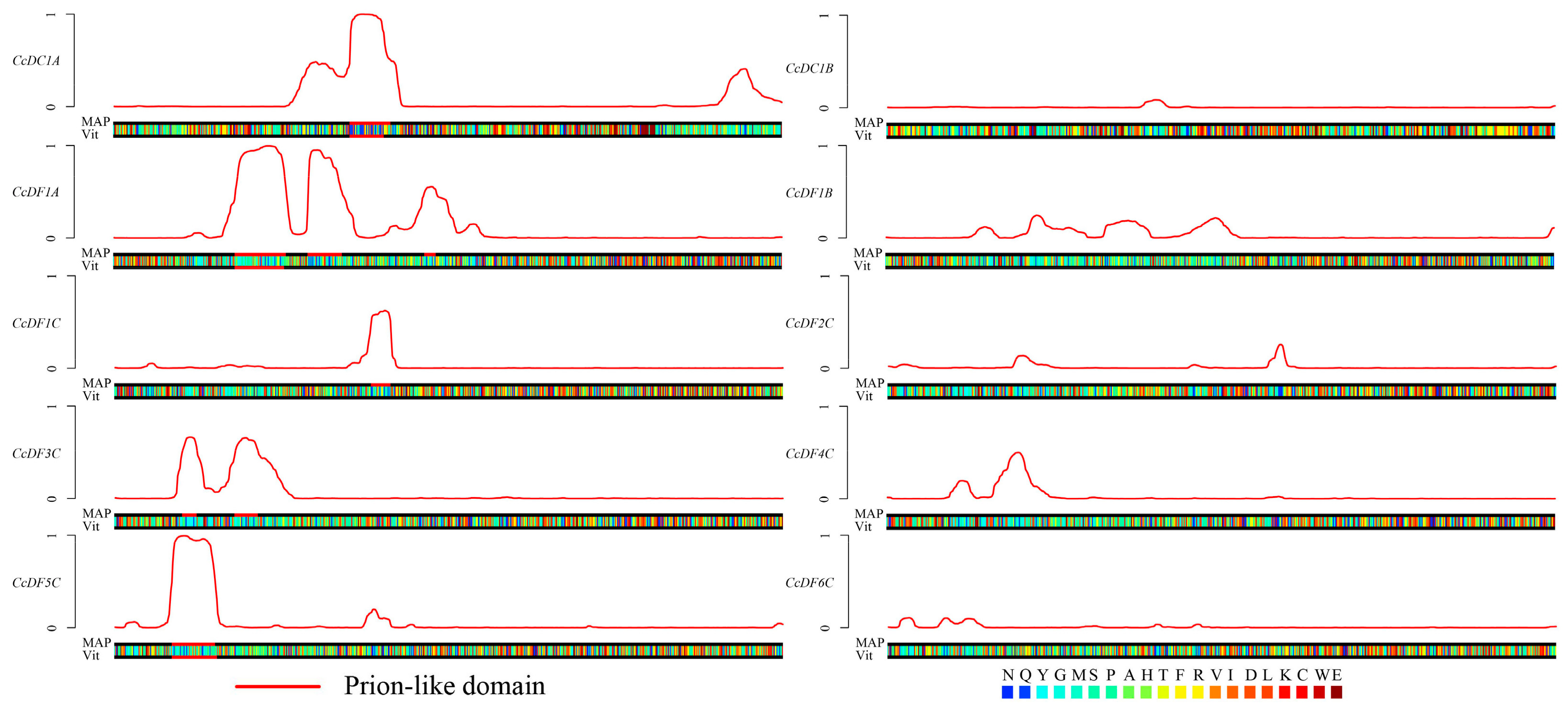
2.5. Prion Subsequences Analysis of the CcYTH Proteins Family
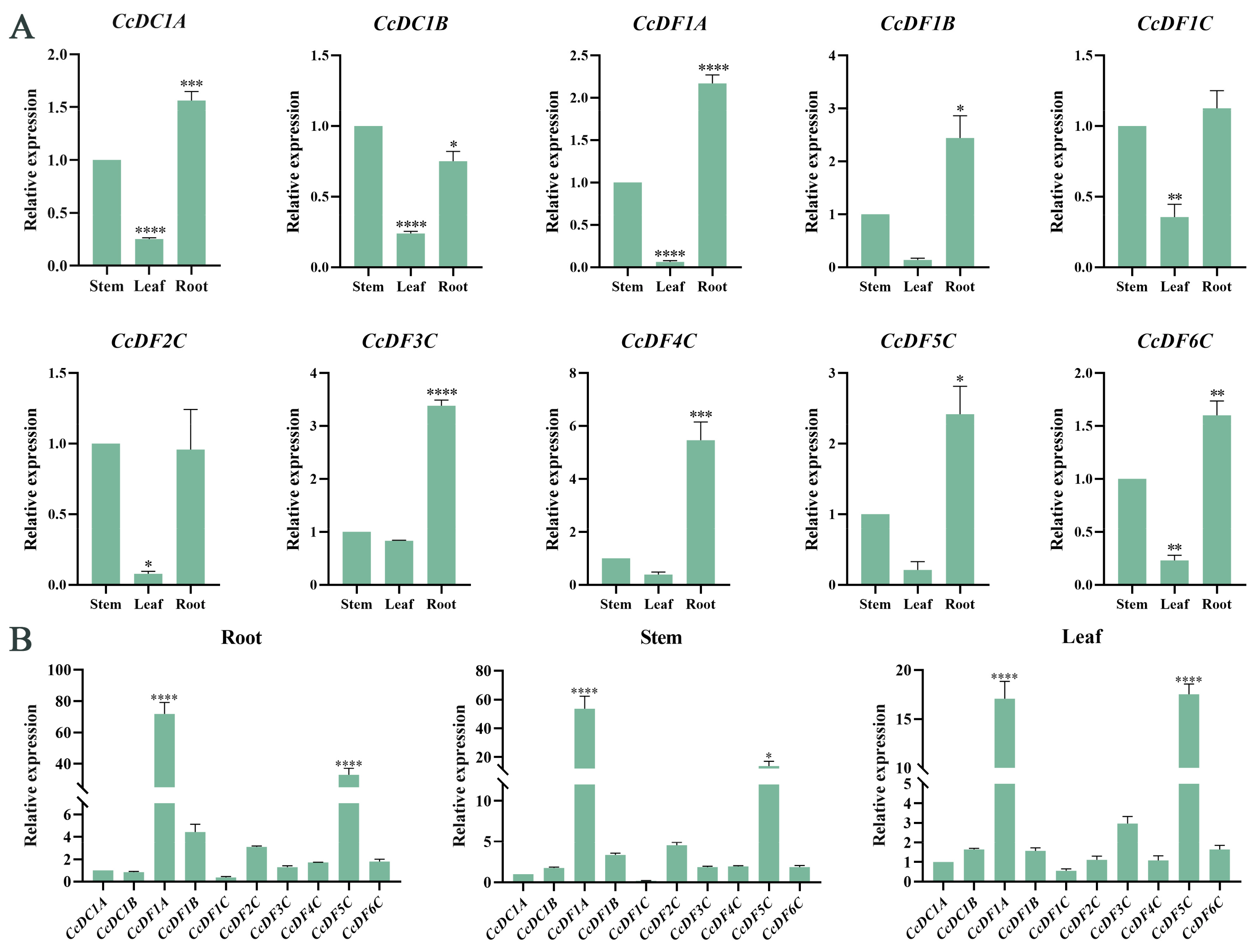
2.6. Tissue Expression Patterns of CcYTH Genes
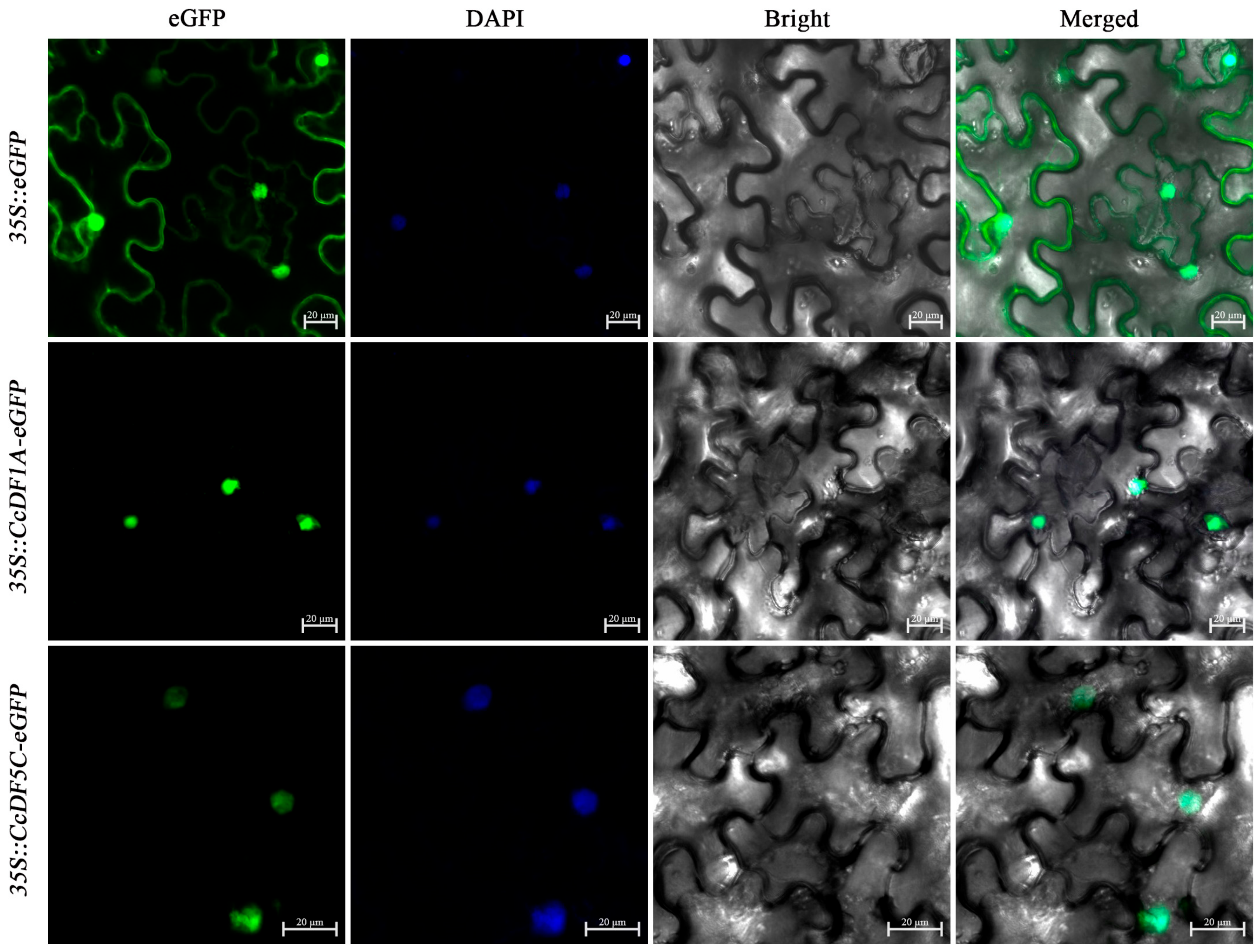
2.7. Subcellular Localization of CcDF1A and CcDF5C Proteins
2.8. Analysis of Cis-Regulatory Elements in the Promoter Region of the CcYTH Genes
2.9. Expression Patterns of CcYTH Genes under Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification of the YTH Genes in C. camphora
4.3. Phylogenetic Analysis, Chromasomal Location and Synteny Analysis
4.4. Gene Structure, Conserved Motifs and Consevered Domain Analyses
4.5. Identification of Protein-Protein Interactions, Tertiary Structures, and Prion-like Subsequences of the CcYTH Proteins Family
4.6. Cis-Regulatory Elements Analysis of the CcYTH Genes Promoter
4.7. Subcellular Localization of CcDF1A and CcDF5C Proteins
4.8. Total RNA Extraction, and Expression of the CcYTH Genes Analyzed by qRT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cappannini, A.; Ray, A.; Purta, E.; Mukherjee, S.; Boccaletto, P.; Moafinejad, S.N.; Lechner, A.; Barchet, C.; Klaholz, B.P.; Stefaniak, F.; et al. MODOMICS: A database of RNA modifications and related information. 2023 update. Nucleic Acids Res. 2024, 52, D239–D244. [Google Scholar] [CrossRef]
- Yang, Y.; Hsu, P.J.; Chen, Y.S.; Yang, Y.G. Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 2018, 28, 616–624. [Google Scholar] [CrossRef]
- Ambrosone, A.; Costa, A.; Leone, A.; Grillo, S. Beyond transcription: RNA-binding proteins as emerging regulators of plant response to environmental constraints. Plant Sci. 2012, 182, 12–18. [Google Scholar] [CrossRef]
- Harvey, R.; Dezi, V.; Pizzinga, M.; Willis, A.E. Post-transcriptional control of gene expression following stress: The role of RNA-binding proteins. Biochem. Soc. Trans. 2017, 45, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Scherrer, T.; Gerber, A.P.; Janga, S.C. Interplay between Posttranscriptional and Posttranslational Interactions of RNA-Binding Proteins. J. Mol. Biol. 2011, 409, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.L.; Li, H.Y.; Bodi, Z.; Button, J.; Vespa, L.; Herzog, M.; Fray, R.G. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 2008, 20, 1278–1288. [Google Scholar] [CrossRef]
- Shen, L.S.; Liang, Z.; Yu, H. N6-Methyladenosine RNA Modification Regulates Shoot Stem Cell Fate in Arabidopsis. Mech. Dev. 2017, 145, S171. [Google Scholar] [CrossRef]
- Ruzicka, K.; Zhang, M.; Campilho, A.; Bodi, Z.; Kashif, M.; Saleh, M.; Eeckhout, D.; El-Showk, S.; Li, H.Y.; Zhong, S.L.; et al. Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017, 215, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bodi, Z.; Mackinnon, K.; Zhong, S.L.; Archer, N.; Mongan, N.P.; Simpson, G.G.; Fray, R.G. Two zinc finger proteins with functions in m6A writing interact with HAKAI. Nat. Commun. 2022, 13, 15. [Google Scholar] [CrossRef]
- Martinez-Perez, M.; Aparicio, F.; Lopez-Gresa, M.P.; Belles, J.M.; Sanchez-Navarro, J.A.; Pallas, V. Arabidopsis m6A demethylase activity modulates viral infection of a plant virus and the m(6)A abundance in its genomic RNAs. Proc. Natl. Acad. Sci. USA 2017, 114, 10755–10760. [Google Scholar] [CrossRef]
- Duan, H.C.; Wei, L.H.; Zhang, C.; Wang, Y.; Chen, L.; Lu, Z.K.; Chen, P.R.; He, C.; Jia, G.F. ALKBH10B Is an RNA N6-Methyladenosine Demethylase Affecting Arabidopsis Floral Transition. Plant Cell 2017, 29, 2995–3011. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.P.; Pickering, B.F.; Jaffrey, S.R. Reading m6A in the Transcriptome: m6A-Binding Proteins. Trends Cell Biol. 2018, 28, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Theler, D.; Kaminska, K.H.; Hiller, M.; de la Grange, P.; Pudimat, R.; Rafalska, I.; Heinrich, B.; Bujnicki, J.M.; Allain, F.H.T.; et al. The YTH Domain Is a Novel RNA Binding Domain. J. Biol. Chem. 2010, 285, 14701–14710. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Matsuo, N.; Ogawa, S.; Tohyama, M.; Takagi, T. Cloning of a gene, YT521, for a novel RNA splicing-related protein induced by hypoxia/reoxygenation. Brain Res. Mol. Brain Res. 1998, 53, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, P.; Rafalska, I.; Stamm, S. YTH: A new domain in nuclear proteins. Trends Biochem. Sci. 2002, 27, 495–497. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Liu, K.; Roundtree, I.A.; Tempel, W.; Li, Y.J.; Lu, Z.K.; He, C.; Min, J.R. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat. Chem. Biol. 2014, 10, 927–929. [Google Scholar] [CrossRef]
- Liao, S.H.; Sun, H.B.; Xu, C. YTH Domain: A Family of N6-methyladenosine (m6A) Readers. Genom. Proteom. Bioinform. 2018, 16, 99–107. [Google Scholar] [CrossRef]
- Achsel, T.; Bagni, C. Cooperativity in RNA-protein interactions: The complex is more than the sum of its partners. Curr. Opin. Neurobiol. 2016, 39, 146–151. [Google Scholar] [CrossRef]
- Hou, Y.F.; Sun, J.; Wu, B.X.; Gao, Y.Y.; Nie, H.B.; Nie, Z.T.; Quan, S.X.; Wang, Y.; Cao, X.F.; Li, S.S. CPSF30-L-mediated recognition of mRNA m6A modification controls alternative polyadenylation of nitrate signaling-related gene transcripts in Arabidopsis. Mol. Plant. 2021, 14, 688–699. [Google Scholar] [CrossRef]
- Wei, L.H.; Song, P.Z.; Wang, Y.; Lu, Z.K.; Tang, Q.; Yu, Q.; Xiao, Y.; Zhang, X.; Duan, H.C.; Jia, G.F. The m6A Reader ECT2 Controls Trichome Morphology by Affecting mRNA Stability in Arabidopsis. Plant Cell 2018, 30, 968–985. [Google Scholar] [CrossRef]
- Arribas-Hernandez, L.; Bressendorff, S.; Hansen, M.H.; Poulsen, C.; Erdmann, S.; Brodersen, P. An m6A-YTH Module Controls Developmental Timing and Morphogenesis in Arabidopsis. Plant Cell 2018, 30, 952–967. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Hernandez, L.; Simonini, S.; Hansen, M.H.; Botterweg Paredes, E.; Bressendorff, S.; Dong, Y.; Ostergaard, L.; Brodersen, P. Recurrent requirement for the m6A-ECT2/ECT3/ECT4 axis in the control of cell proliferation during plant organogenesis. Development 2020, 147, 189134. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Zhang, H.J.; Hong, Y.B.; Huang, L.; Li, X.H.; Zhang, Y.F.; Ouyang, Z.G.; Song, F.M. Genome-Wide Identification, Biochemical Characterization, and Expression Analyses of the YTH Domain-Containing RNA-Binding Protein Family in Arabidopsis and Rice. Plant Mol. Biol. Rep. 2014, 32, 1169–1186. [Google Scholar] [CrossRef]
- Arribas-Hernandez, L.; Rennie, S.; Koster, T.; Porcelli, C.; Lewinski, M.; Staiger, D.; Andersson, R.; Brodersen, P. Principles of mRNA targeting via the Arabidopsis m6A-binding protein ECT2. eLife 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Arribas-Hernandez, L.; Rennie, S.; Schon, M.; Porcelli, C.; Enugutti, B.; Andersson, R.; Nodine, M.D.; Brodersen, P. The YTHDF proteins ECT2 and ECT3 bind largely overlapping target sets and influence target mRNA abundance, not alternative polyadenylation. eLife 2021, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Song, P.Z.; Yang, J.B.; Wang, C.L.; Lu, Q.; Shi, L.Q.; Tayier, S.; Jia, G.F. Arabidopsis N6-methyladenosine reader CPSF30-L recognizes FUE signals to control polyadenylation site choice in liquid-like nuclear bodies. Mol. Plant. 2021, 14, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Boeynaems, S.; Alberti, S.; Fawzi, N.L.; Mittag, T.; Polymenidou, M.; Rousseau, F.; Schymkowitz, J.; Shorter, J.; Wolozin, B.; Van den Bosch, L.; et al. Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol. 2018, 28, 420–435. [Google Scholar] [CrossRef]
- Lancaster, A.K.; Nutter-Upham, A.; Lindquist, S.; King, O.D. PLAAC: A web and command-line application to identify proteins with prion-like amino acid composition. Bioinformatics 2014, 30, 2501–2502. [Google Scholar] [CrossRef] [PubMed]
- Ries, R.J.; Zaccara, S.; Klein, P.; Olarerin-George, A.; Namkoong, S.; Pickering, B.F.; Patil, D.P.; Kwak, H.; Lee, J.H.; Jaffrey, S.R. m6A enhances the phase separation potential of mRNA. Nature 2019, 571, 424–428. [Google Scholar] [CrossRef]
- Yin, S.Q.; Ao, Q.J.; Tan, C.Y.; Yang, Y.W. Genome-wide identification and characterization of YTH domain-containing genes, encoding the m6A readers, and their expression in tomato. Plant Cell Rep. 2021, 40, 1229–1245. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Q.; Zheng, G.Q.; He, C.; Parisien, M.; Pan, T. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 2015, 518, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Adhikari, S.; Dahal, U.; Chen, Y.S.; Hao, Y.J.; Sun, B.F.; Sun, H.Y.; Li, A.; Ping, X.L.; Lai, W.Y.; et al. Nuclear m6A Reader YTHDC1 Regulates mRNA Splicing. Mol. Cell 2016, 61, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.J.; Zhu, Y.F.; Ma, H.H.; Guo, Y.H.; Shi, X.D.; Liu, Y.Y.; Qi, M.J.; Lu, Z.K.; Shi, H.L.; Wang, J.Y.; et al. YTHDC2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017, 27, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, B.S.; Roundtree, I.A.; Lu, Z.K.; Han, D.L.; Ma, H.H.; Weng, X.C.; Chen, K.; Shi, H.L.; He, C. N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 2015, 161, 1388–1399. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhao, Y.; He, J.Q.; Zhang, Y.; Xi, H.R.; Liu, M.F.; Ma, J.B.; Wu, L.G. YTHDF2 destabilizes m6A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat. Commun. 2016, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.L.; Wang, X.; Lu, Z.K.; Zhao, B.X.S.; Ma, H.H.; Hsu, P.J.; Liu, C.; He, C. YTHDF3 facilitates translation and decay of N6-methyladenosine-modified RNA. Cell Res. 2017, 27, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Scutenaire, J.; Deragon, J.M.; Jean, V.; Benhamed, M.; Raynaud, C.; Favory, J.J.; Merret, R.; Bousquet-Antonelli, C. The YTH Domain Protein ECT2 Is an m6A Reader Required for Normal Trichome Branching in Arabidopsis. Plant Cell 2018, 30, 986–1005. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bie, X.M.; Wang, N.; Zhang, X.S.; Gao, X.Q. Genome-wide identification and expression analysis of YTH domain-containing RNA-binding protein family in common wheat. BMC Plant Biol. 2020, 20, 14. [Google Scholar] [CrossRef]
- Wang, N.; Yue, Z.Y.; Liang, D.; Ma, F.W. Genome-wide identification of members in the YTH domain-containing RNA-binding protein family in apple and expression analysis of their responsiveness to senescence and abiotic stresses. Gene 2014, 538, 292–305. [Google Scholar] [CrossRef]
- Sun, X.C.; Wu, W.L.; Yang, Y.F.; Wilson, I.; Shao, F.J.; Qiu, D.Y. Genome-Wide Identification of m6A Writers, Erasers and Readers in Poplar 84K. Genes 2022, 13, 16. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, L.F.; Jiang, L.W.; Liu, S.Q. Genome-wide identification and expression analysis of YTH domain-containing RNA-binding protein family in cucumber (Cucumis sativus). Genes Genom. 2018, 40, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhang, J.J.; Cheng, X.; Wang, D.B.; Yu, W.Y.; Ji, K.S.; Yu, Q. Genome-Wide Identification and Characterization of the YTH Domain-Containing RNA-Binding Protein Family in Liriodendron chinense. Int. J. Mol. Sci. 2023, 24, 17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yao, S.; Zhang, J.J.; Wang, D.B.; Xu, S.J.; Yu, Q.; Ji, K.S. Genome-Wide Identification and Expression Analysis of YTH Gene Family for Abiotic Stress Regulation in Camellia chekiangoleosa. Int. J. Mol. Sci. 2024, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Xiang, S.; Zhang, Q.G.; Xiao, L.; Zhang, D.Y.; Zhang, P.L.; Chen, D.Q.; Hao, Y.; Liu, D.K.; Ding, L.; et al. The camphor tree genome enhances the understanding of magnoliid evolution. J. Genet. Genom. 2022, 49, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Rio, D.C. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Locus | CDS (bp) | Protein Length (aa) | MW (kDa) | Aliphatic Index | GRAVY | pI | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|
| CcDC1A | GWHGBGXC018157 | Chr05 | 1827 | 608 | 66.7 | 61.64 | −0.539 | 5.91 | Nucleus |
| CcDC1B | GWHGBGXC008948 | Chr02 | 1422 | 473 | 53.5 | 68.20 | −0.617 | 8.62 | Nucleus |
| CcDF1A | GWHGBGXC015419 | Chr04 | 2118 | 705 | 76.9 | 60.26 | −0.676 | 7.95 | Nucleus |
| CcDF1B | GWHGBGXC020092 | Chr05 | 2049 | 682 | 74.5 | 56.06 | −0.618 | 5.63 | Nucleus |
| CcDF1C | GWHGBGXC025982 | Chr08 | 2160 | 719 | 80.7 | 59.64 | −0.811 | 8.8 | Cell membrane or Nucleus |
| CcDF2C | GWHGBGXC023812 | Chr07 | 1764 | 587 | 64.9 | 60.29 | −0.580 | 5.9 | Nucleus |
| CcDF3C | GWHGBGXC017636 | Chr04 | 1806 | 601 | 66.5 | 56.76 | −0.676 | 5.42 | Nucleus |
| CcDF4C | GWHGBGXC008047 | Chr12 | 1791 | 596 | 65.8 | 61.16 | −0.644 | 5.21 | Nucleus |
| CcDF5C | GWHGBGXC005246 | Chr11 | 2232 | 743 | 81.2 | 59.84 | −0.655 | 6.64 | Nucleus |
| CcDF6C | GWHGBGXC005110 | Chr10 | 2040 | 679 | 74.3 | 73.03 | −0.513 | 8.17 | Chloroplast or Nucleus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Yao, S.; Cheng, X.; Zhao, Y.; Yu, W.; Ren, X.; Ji, K.; Yu, Q. Genome-Wide Identification and Expression Analysis of the YTH Domain-Containing RNA-Binding Protein Family in Cinnamomum camphora. Int. J. Mol. Sci. 2024, 25, 5960. https://doi.org/10.3390/ijms25115960
Zhang J, Yao S, Cheng X, Zhao Y, Yu W, Ren X, Ji K, Yu Q. Genome-Wide Identification and Expression Analysis of the YTH Domain-Containing RNA-Binding Protein Family in Cinnamomum camphora. International Journal of Molecular Sciences. 2024; 25(11):5960. https://doi.org/10.3390/ijms25115960
Chicago/Turabian StyleZhang, Jingjing, Sheng Yao, Xiang Cheng, Yulu Zhao, Wenya Yu, Xingyue Ren, Kongshu Ji, and Qiong Yu. 2024. "Genome-Wide Identification and Expression Analysis of the YTH Domain-Containing RNA-Binding Protein Family in Cinnamomum camphora" International Journal of Molecular Sciences 25, no. 11: 5960. https://doi.org/10.3390/ijms25115960





