Anti-Inflammatory and Immunomodulatory Effects of 0.1 Sub-Terahertz Irradiation in Collagen-Induced Arthritis Mice
Abstract
1. Introduction
2. Results
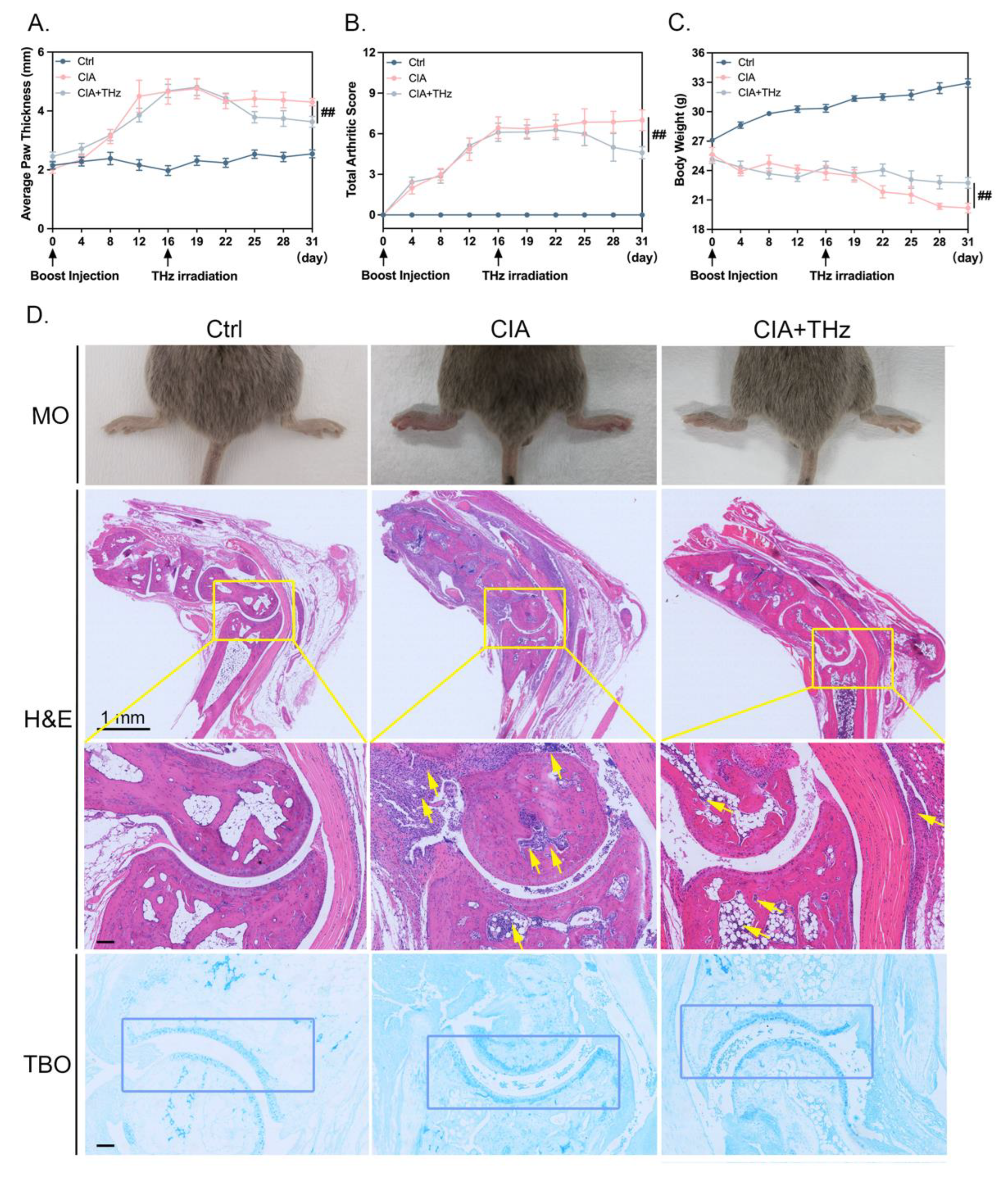
2.1. Irradiation of 0.1 Sub-THz Attenuated Joint Swelling and Cartilage Damage in CIA Mice
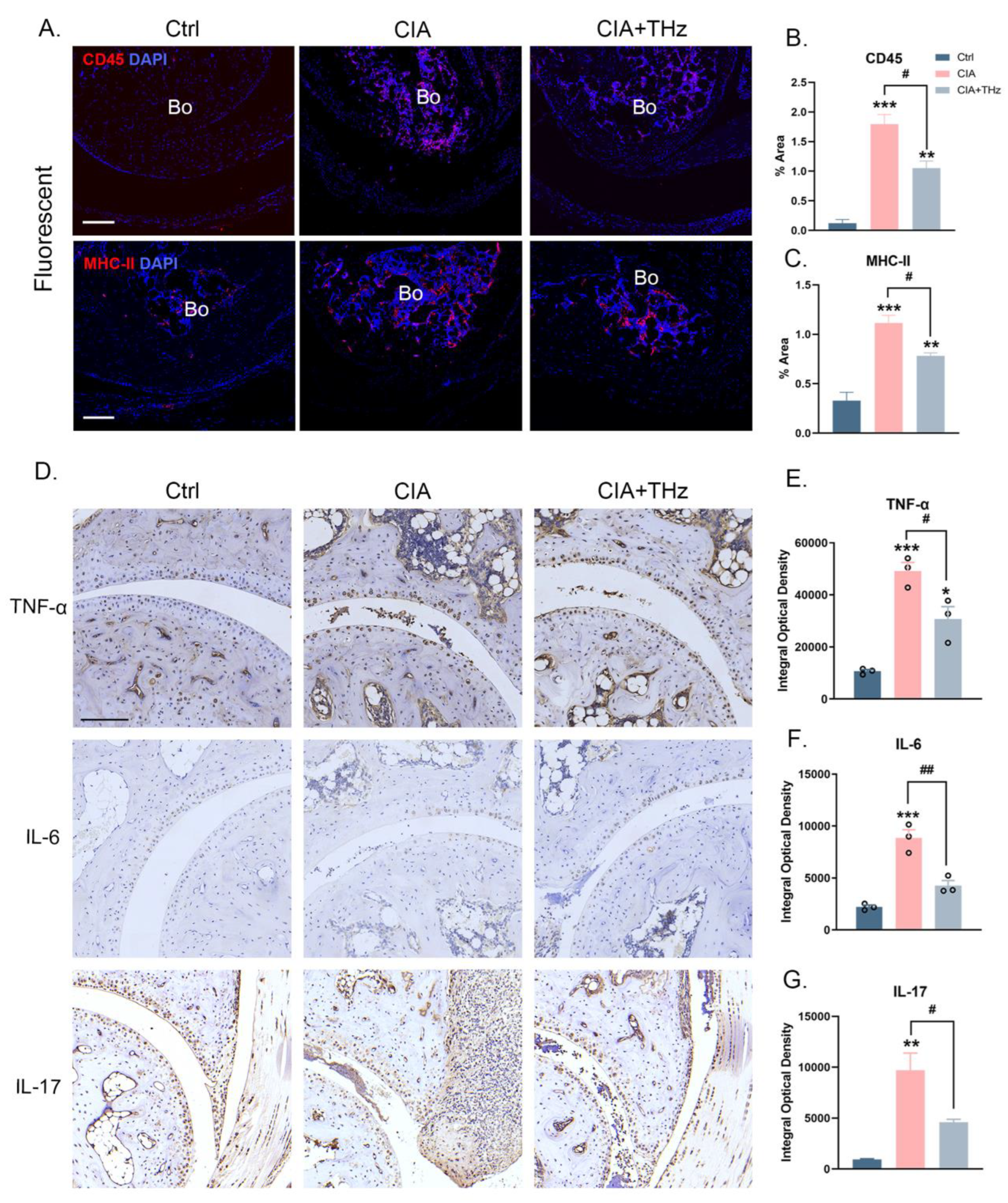
2.2. Irradiation of 0.1 Sub-THz Reduced Proinflammatory Cytokine Levels in the Ankle Joint
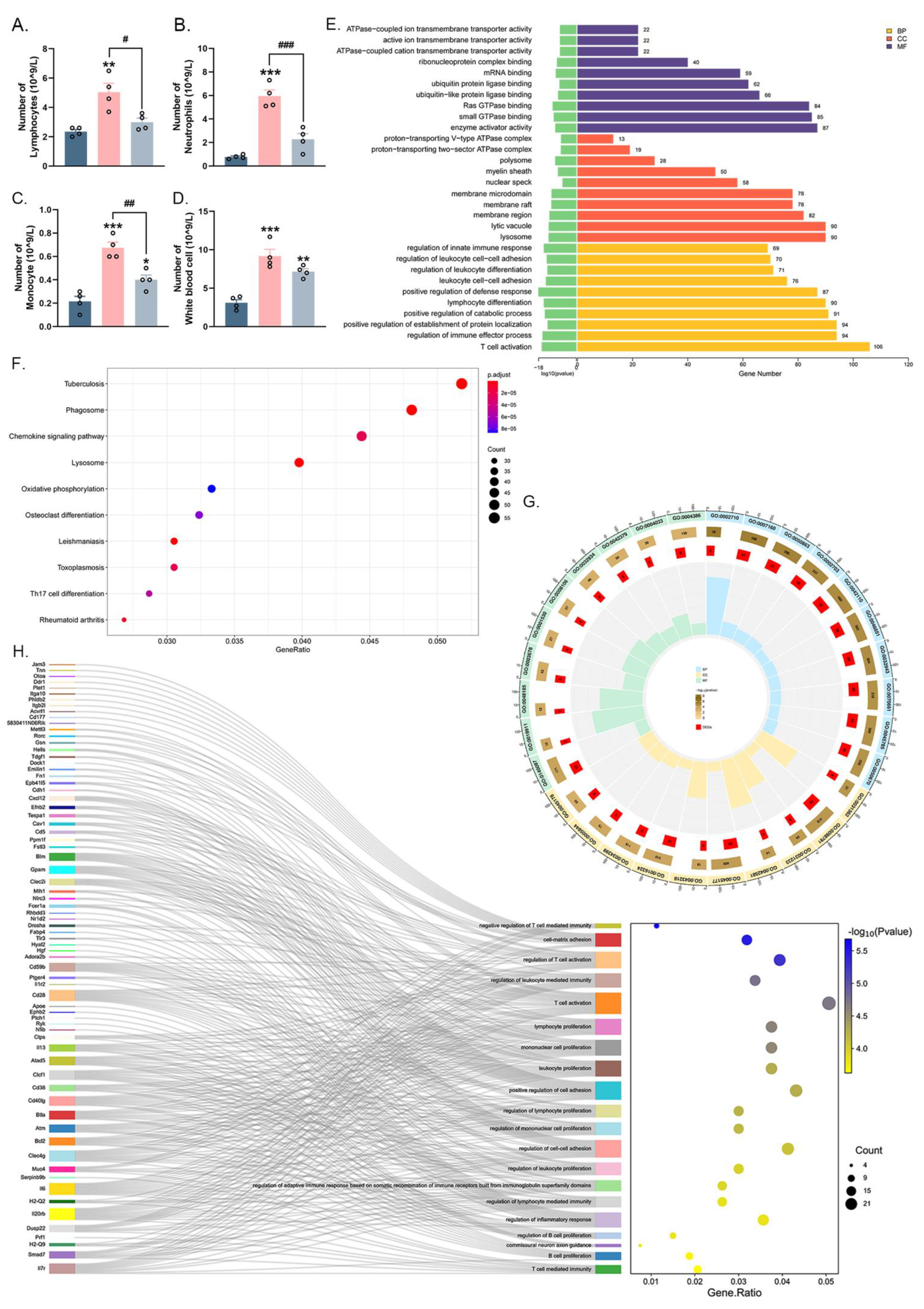
2.3. Irradiation of 0.1 Sub-THz Attenuated the Leukocytes Enhancement and Altered the Gene Expression of Blood Leukocytes in CIA Mice
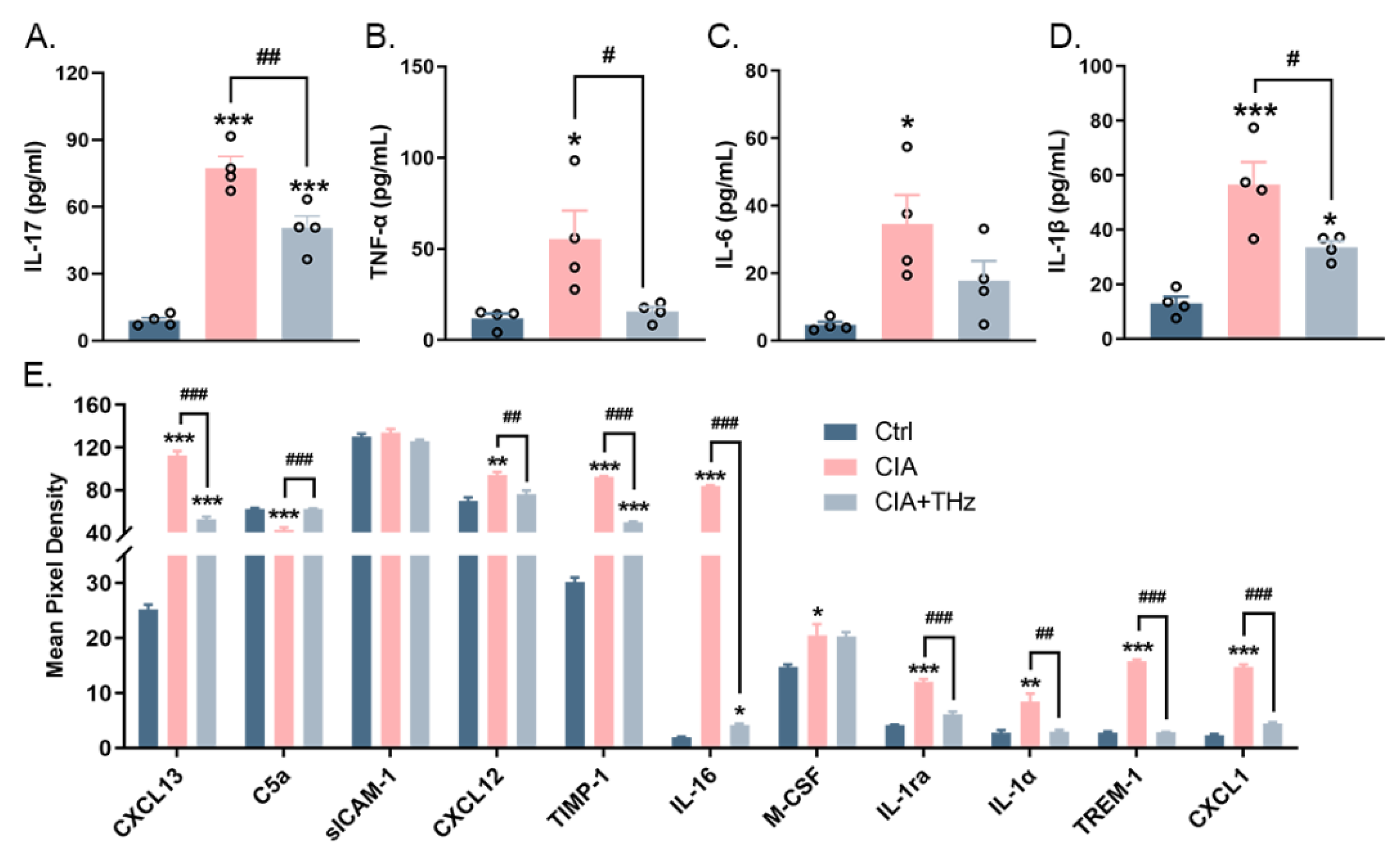
2.4. Irradiation of 0.1 Sub-THz Reduced the Levels of Proinflammatory Cytokines in the Serum
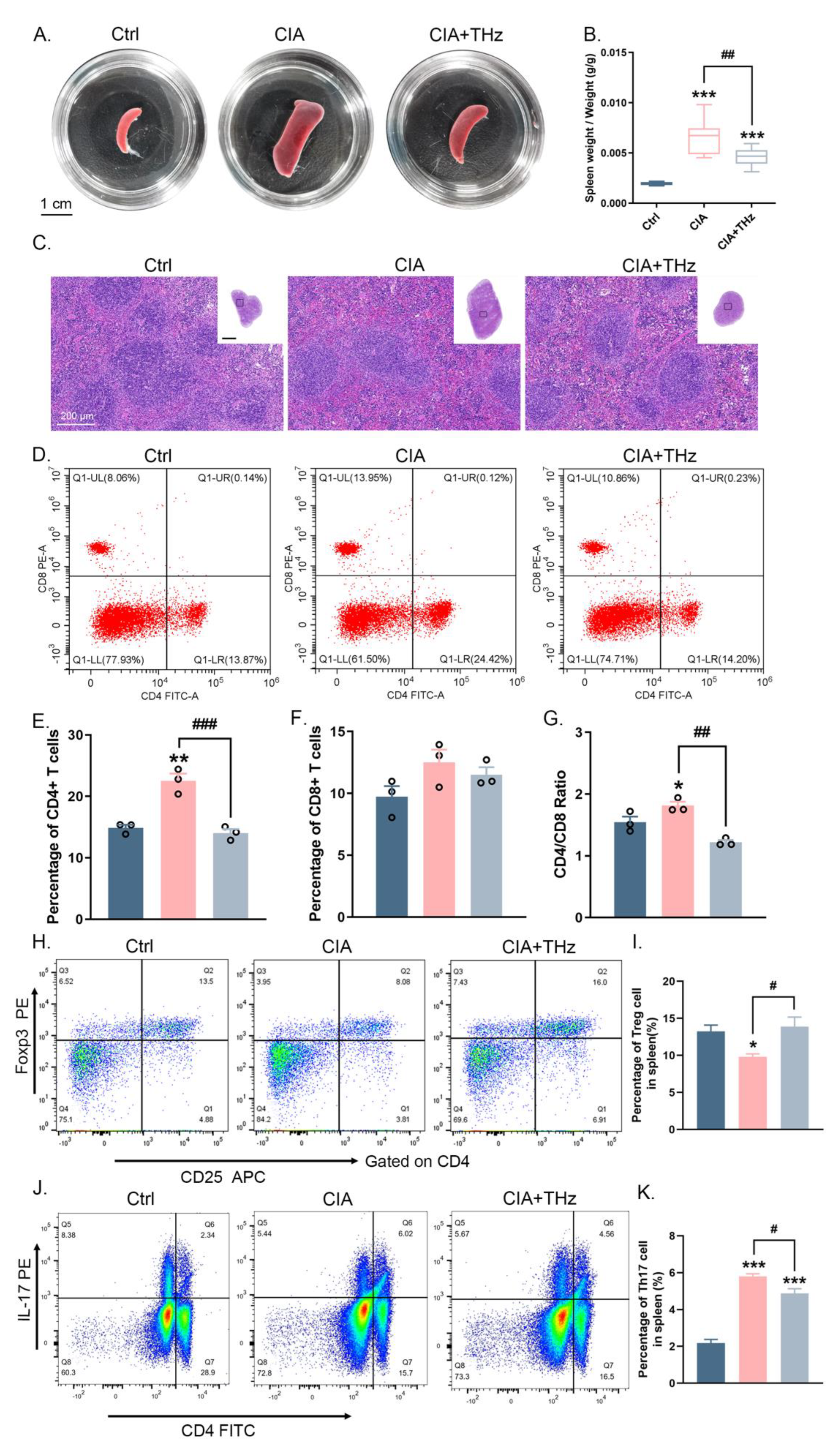
2.5. Radiation of 0.1 Sub-THz Reduced Immune Activation in the Spleen of CIA Mice
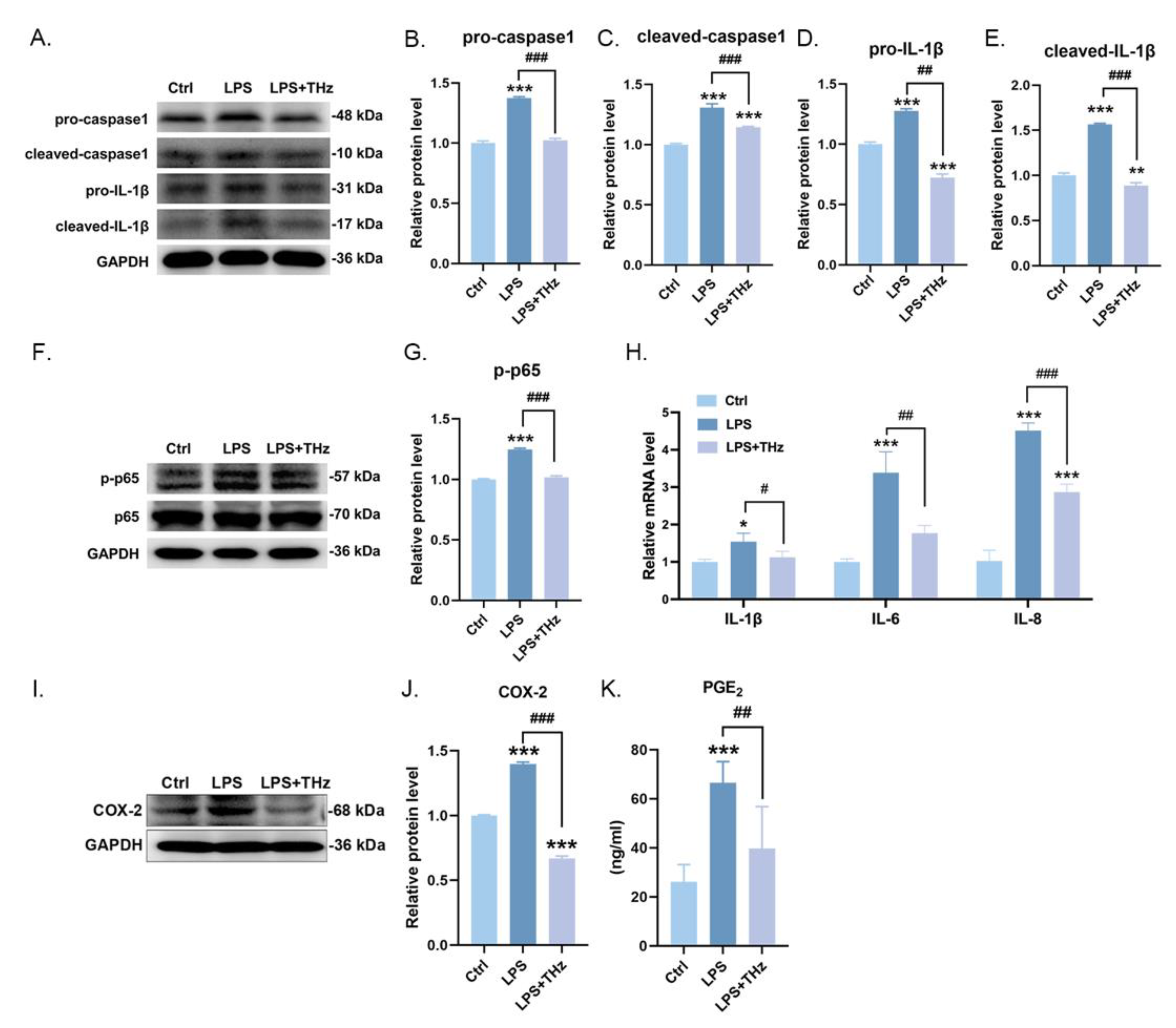
2.6. Irradiation of 0.1 Sub-THz Elevated the LPS-Stimulated Keratocytes Inflammatory Responses
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethical Approval
5.2. Animals and Reagents
5.3. Collagen-Induced Arthritis (CIA) Model Establishment and Experimental Design
5.4. Irradiation Treatment of 0.1 Sub-THz and Real-Time Temperature Monitoring
5.5. Arthritic Score, Paw Thickness, Body Weight, and Spleen Organ Index Assessment
5.6. Histopathology, Immunohistochemistry, and Immunofluorescence Analysis
5.7. Mouse Hemocyte Composition Analysis and Serum Cytokines Assay
5.8. Mice Splenic Lymphocytes Separation and Flow Cytometry Analysis
5.9. Murine Hemocytes mRNA Sequencing and Bioinformatics Analysis
5.10. Cell Culture and LPS/THz Treatment
5.11. RNA Extraction and RT-qPCR Program
5.12. Western Blotting Analysis
5.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Grundt, J.E.; Cerna, C.; Roth, C.C.; Ibey, B.L.; Lipscomb, D.; Echchgadda, I.; Wilmink, G.J. Terahertz radiation triggers a signature gene expression profile in human cells. In Proceedings of the 2011 International Conference on Infrared, Millimeter, and Terahertz Waves, Houston, TX, USA, 2–7 October 2011; p. 12442108. [Google Scholar]
- Echchgadda, I.; Cerna, C.Z.; Sloan, M.A.; Elam, D.P.; Ibey, B.L. Effects of different terahertz frequencies on gene expression in human keratinocytes. In Optical Interactions with Tissue and Cells XXVI; SPIE: San Francisco, CA, USA, 2015. [Google Scholar]
- Titova, L.V.; Ayesheshim, A.K.; Golubov, A.; Rodriguez-Juarez, R.; Woycicki, R.; Hegmann, F.A.; Kovalchuk, O. Intense THz pulses down-regulate genes associated with skin cancer and psoriasis: A new therapeutic avenue? Sci. Rep. 2013, 3, 2363. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Ahn, J.; Mun, J.; Bae, S.; Jeong, Y.U.; Vinokurov, N.A.; Kim, P. In vivo analysis of THz wave irradiation induced acute inflammatory response in skin by laser-scanning confocal microscopy. Opt. Express 2014, 22, 11465–11475. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Park, J.; Jo, S.J.; Jung, S.; Kwon, O.S.; Gallerano, G.P.; Park, W.-Y.; Park, G.-S. High-power femtosecond-terahertz pulse induces a wound response in mouse skin. Sci. Rep. 2013, 3, 2296. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Frommer, K.; Diller, M.; Müller-Ladner, U. Rheumatoide Arthritis. Z. fur Rheumatol. 2018, 77, 769–775. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Pandey, A. Inflammation and rheumatoid arthritis. J. Physiol. Biochem. 2013, 69, 335–347. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Kirichuk, V.F.; Antipova, O.N.; Krylova, Y.A. Effect of continuous irradiation with terahertz electromagnetic waves of the NO frequency range on behavioral reactions of male albino rats under stress conditions. Bull. Exp. Biol. Med. 2014, 157, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Hur, J.K.; Hwang, W.; Yang, H.-J.; Son, J.-H. Epigenetic modification of gene expression in cancer cells by terahertz demethylation. Sci. Rep. 2023, 13, 4930. [Google Scholar] [CrossRef]
- Murakami, M.; Nishimoto, N. IL-6 inhibitors prevent bone loss and cartilage degeneration in rheumatoid arthritis. Clin. Calcium 2015, 25, 1851–1857. [Google Scholar]
- Sander, W.J.; O’Neill, H.G.; Pohl, C.H. Prostaglandin E2 as a Modulator of Viral Infections. Front. Physiol. 2017, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.P.; Schulze-Koops, H.; Aringer, M. The central role of T cells in rheumatoid arthritis. Clin. Exp. Rheumatol. 2007, 25, S4–S11. [Google Scholar] [PubMed]
- Cooles, F.A.H.; Isaacs, J.D.; Anderson, A.E. Treg cells in rheumatoid arthritis: An update. Curr. Rheumatol. Rep. 2013, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Zheng, Y.; Li, R.; Guo, T.B.; He, D.; Fang, L.; Liu, X.; Xiao, L.; Chen, X.; Wan, B.; et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat. Med. 2013, 19, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tachizaki, T.; Sakaguchi, R.; Terada, S.; Kamei, K.-I.; Hirori, H. Terahertz pulse-altered gene networks in human induced pluripotent stem cells. Opt. Lett. 2020, 45, 6078–6081. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, M.; Liu, S. Collagen-Induced Arthritis Models. In Rheumatoid Arthritis: Methods and Protocols; Methods in Molecular Biology 1868; Springer: New York, NY, USA, 2024; pp. 3–7. [Google Scholar]
- Grötsch, B.; Bozec, A.; Schett, G. In Vivo Models of Rheumatoid Arthritis. In Bone Research Protocols; Methods in Molecular Biology 1914; Idris, A.I., Ed.; Springer: New York, NY, USA, 2019; pp. 269–280. [Google Scholar]
- Bergholt, N.L.; Lysdahl, H.; Lind, M.; Foldager, C.B. A Standardized Method of Applying Toluidine Blue Metachromatic Staining for Assessment of Chondrogenesis. Cartilage 2019, 10, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Cardiff, R.D. Methods of Immunohistochemistry and Immunofluorescence: Converting Invisible to Visible. In The Tumor Microenvironment: Methods and Protocols; Methods in Molecular Biology 1458; Springer: New York, NY, USA, 2016; pp. 1–12. [Google Scholar]
- Liu, C.; He, L.; Wang, J.; Wang, Q.; Sun, C.; Li, Y.; Jia, K.; Wang, J.; Xu, T.; Ming, R.; et al. Anti-angiogenic effect of Shikonin in rheumatoid arthritis by downregulating PI3K/AKT and MAPKs signaling pathways. J. Ethnopharmacol. 2020, 260, 113039. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Zhang, Y.; Fang, K.; Shi, Y.; Zhang, T.; Chen, R.; Wu, D.; Yang, R.; Huang, X. Application of genetic screening processor in screening neonatal glucose-6-phosphate dehydrogenase deficiency. Zhejiang Da Xue Xue Bao Yi Xue Ban 2021, 50, 487–493. [Google Scholar] [CrossRef]
- DeLuca, D.S.; Levin, J.Z.; Sivachenko, A.; Fennell, T.; Nazaire, M.D.; Williams, C.; Reich, M.; Winckler, W.; Getz, G. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 2012, 28, 1530–1532. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Shang, S.; Li, X.; Lu, X. Anti-Inflammatory and Immunomodulatory Effects of 0.1 Sub-Terahertz Irradiation in Collagen-Induced Arthritis Mice. Int. J. Mol. Sci. 2024, 25, 5963. https://doi.org/10.3390/ijms25115963
Zhang Q, Shang S, Li X, Lu X. Anti-Inflammatory and Immunomodulatory Effects of 0.1 Sub-Terahertz Irradiation in Collagen-Induced Arthritis Mice. International Journal of Molecular Sciences. 2024; 25(11):5963. https://doi.org/10.3390/ijms25115963
Chicago/Turabian StyleZhang, Qi, Sen Shang, Xu Li, and Xiaoyun Lu. 2024. "Anti-Inflammatory and Immunomodulatory Effects of 0.1 Sub-Terahertz Irradiation in Collagen-Induced Arthritis Mice" International Journal of Molecular Sciences 25, no. 11: 5963. https://doi.org/10.3390/ijms25115963
APA StyleZhang, Q., Shang, S., Li, X., & Lu, X. (2024). Anti-Inflammatory and Immunomodulatory Effects of 0.1 Sub-Terahertz Irradiation in Collagen-Induced Arthritis Mice. International Journal of Molecular Sciences, 25(11), 5963. https://doi.org/10.3390/ijms25115963




