The Influence of Oxidized Imino-Allantoin in the Presence of OXOG on Double Helix Charge Transfer: A Theoretical Approach
Abstract
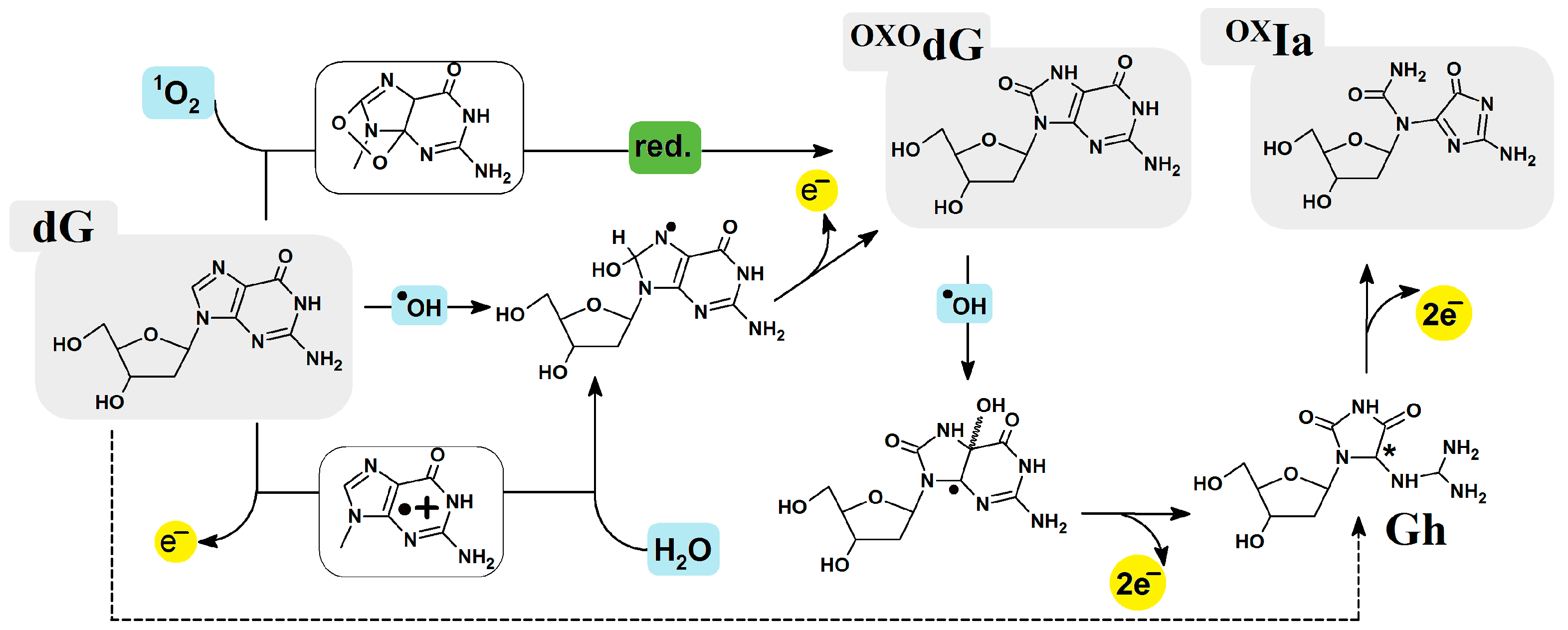
1. Introduction
2. Results
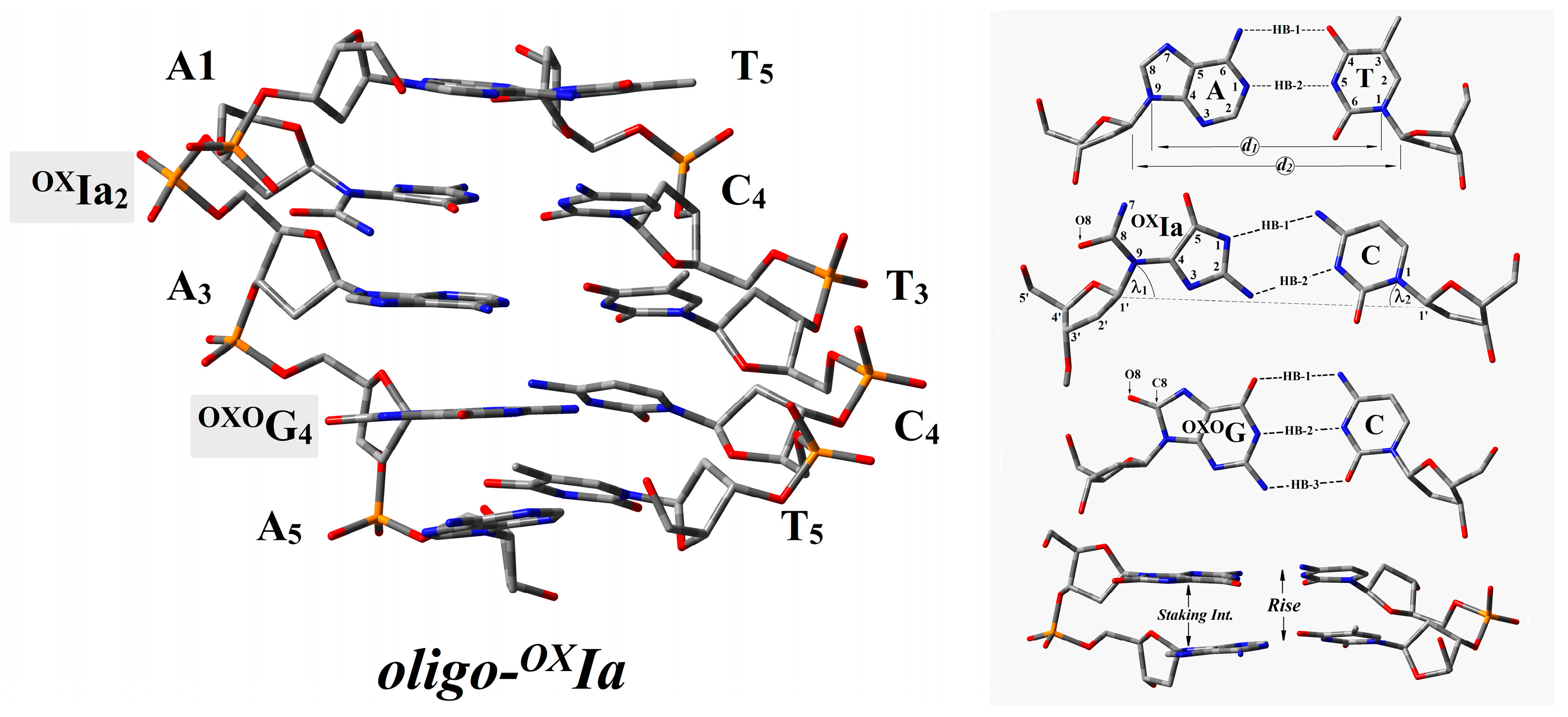
2.1. The Influence of OXIa as Part of a CDL on Double Helix Electronic Properties
| Base Pair | HB-1 | HB-2 | HB-3 | EHB | λ1 | λ2 | d1 | d2 | Base Pair Dimer | Rise | EST |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1T5 | 2.80 | 2.99 | 10.84 | 51.5 | 52.0 | 10.7 | 8.83 | A1T5|OXIa2C4 | 3.00 | 14.77 | |
| OXIa2C4 | 3.00 | 2.99 | 12.40 | 42.5 | 55.5 | 11.4 | 9.37 | OXIa2C4|A3T3 | 2.99 | 15.90 | |
| A3T3 | 2.84 | 2.89 | 10.74 | 48.7 | 50.7 | 10.8 | 8.95 | A3T3|OXOG2C4 | 2.97 | 16.70 | |
| OXOG2C4 | 2.86 | 2.88 | 2.86 | 17.54 | 52.1 | 55.5 | 10.8 | 9.00 | OXOG2C4|A5T1 | 2.95 | 15.19 |
| A5T1 | 2.82 | 2.99 | 10.51 | 53.7 | 56.1 | 10.5 | 8.85 | ||||
| Parameters calculated for ideal mode [35] | |||||||||||
| AT [16] | 2.96 | 3.05 | 10.81 | 54.5 | 54.5 | 10.7 | 8.58 | GC||AT [17] | 2.96 | 14.56 | |
| GC [16] | 3.00 | 3.00 | 2.87 | 17.23 | 54.2 | 54.5 | 10.8 | 9.01 | AT||GC [17] | 3.31 | 13.59 |
2.2. Influence of OXIa as Part of a CDL on Charge Distribution and Electronic Properties of Basepairs Present in oligo-OXIa
2.3. The Influence of OXIa as Part of a CDL on Charge Migration through oligo-OXIa
3. Discussion
4. Materials and Methods
The oligo-OXIageometry Optimization in Condensed Phase
5. Conclusions and Further Perspectives
- A structural analysis revealed that a clustered damage site consisting of OXIa and OXOG in the oligo-OXIa structure had negligible influence according to the standard DNA reference frame parameters.
- Theoretical studies revealed that the radical cation prefers to settle on the OXOG moiety, irrespective of the presence of OXIa in a ds-oligo. The lowest vertical (5.94 [eV]) and adiabatic (5.52 [eV]) ionization potential values were found for the OXOG:::C base pair. Conversely, the highest vertical and adiabatic electron affinity was assigned for OXIaC as follows: 3.15 and 3.49 [eV], respectively.
- The Hirshfeld charge and spin analysis indicated that the radical cation is mainly located on the OXOG4C2 base pair, regardless of its state—vertical or adiabatic. Moreover, no differences were noted when non-equilibrated and equilibrated solvent-solute interactions were considered. Similar observations were noted for negatively charged oligo-OXIa, i.e., the OXIa2C4 moiety was noted as the spot of a radical anion in all the discussed cases.
- The calculated values of driving force (ΔG) and activation energy (Ea) of A1T5→OXIa2C4, OXIa2C4←A3T3, and OXIa2C4←OXOG4C2 settled this process in the inverted region, which is unfavorable from a thermodynamic perspective. The following values of kCT were found: 3.56 × 10−22, 6.32 × 10−20, and 3.73 × 10−21 [s−1], respectively. At the same point, the electron transfer towards OXOGC should be privileged, and the following kCT values were noted: 1.05 × 1014 and 3.12 × 1010 [s−1] for A3T3→OXOG4C2 and OXOG4C2←A5T1, respectively.
Further Perspectives
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minchin, S.; Lodge, J. Understanding biochemistry: Structure and function of nucleic acids. Essays Biochem. 2019, 63, 433–456. [Google Scholar] [CrossRef]
- Cohen, N.; Dagan, T.; Stone, L.; Graur, D. GC composition of the human genome: In search of isochores. Mol. Biol. Evol. 2005, 22, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P. DNA Damage Repair: Historical Perspectives, Mechanistic Pathways and Clinical Translation for Targeted Cancer Therapy; Springer: Greer, SA, USA, 2021; ISBN 4139202100648. [Google Scholar]
- Sudhir Ambekar, S. DNA: Damage and Repair Mechanisms in Humans. Glob. J. Pharm. Pharm. Sci. 2017, 3, 555613. [Google Scholar] [CrossRef][Green Version]
- Burrows, C.J.; Muller, J.G. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 1998, 98, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Rusling, J.F. Oxidation Chemistry of DNA and p53 Tumor Suppressor Gene. ChemistryOpen 2019, 8, 252–265. [Google Scholar] [CrossRef]
- Kanvah, S.; Joseph, J.; Schuster, G.B.; Barnett, R.N.; Cleveland, C.L.; Landman, U.Z.I. Oxidation of DNA: Damage to Nucleobases. Acc. Chem. Res. 2010, 43, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Muller, J.G.; Rachlin, E.M.; Burrows, C.J. Characterization of Hydantoin Products from One-Electron Oxidation of 8-Oxo-7,8-dihydroguanosine in a Nucleoside Model. Chem. Res. Toxicol. 2001, 14, 927–938. [Google Scholar] [CrossRef]
- Cadet, J.; Richard Wagner, J. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Giglia-mari, G.; Zotter, A.; Vermeulen, W. DNA Damage Response. Cold Spring Harb. Perspect. Biol. 2011, 3, a000745. [Google Scholar] [CrossRef]
- Hang, B. Base excision repair. In DNA Repair, Genetic Instability, and Cancer; World Scientific Publishing: Singapore, 2007; pp. 23–64. [Google Scholar] [CrossRef]
- Kino, K.; Sugiyama, H. UVR-induced G–C to C–G transversions from oxidative DNA damage. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 571, 33–42. [Google Scholar] [CrossRef]
- Elias, B.; Shao, F.; Barton, J.K. Charge Migration along the DNA Duplex: Hole versus Electron Transport. J. Am. Chem. Soc. 2008, 130, 1152–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fleming, A.M.; Orendt, A.M.; Burrows, C.J. Burrowsa pH-Dependent equilibrium between 5-guanidinohydantoin and iminoallantoin affects nucleotide insertion opposite the DNA lesion. J. Org. Chem. 2016, 176, 351–359. [Google Scholar] [CrossRef]
- Fleming, A.M.; Muller, J.G.; Ji, I.; Burrows, C.J. Characterization of 2′-deoxyguanosine oxidation products observed in the Fenton-like system Cu(ii)/H2O2/reductant in nucleoside and oligodeoxynucleotide contexts. Org. Biomol. Chem. 2011, 9, 3338–3348. [Google Scholar] [CrossRef]
- Alshykhly, O.R.; Fleming, A.M.; Burrows, C.J. 5-Carboxamido-5-formamido-2-iminohydantoin, in Addition to 8-oxo-7,8-Dihydroguanine, Is the Major Product of the Iron-Fenton or X-ray Radiation-Induced Oxidation of Guanine under Aerobic Reducing Conditions in Nucleoside and DNA Contexts. J. Org. Chem. 2015, 80, 6996–7007. [Google Scholar] [CrossRef] [PubMed]
- White, B.; Smyth, M.R.; Stuart, J.D.; Rusling, J.F. Oscillating formation of 8-oxoguanine during DNA oxidation. J. Am. Chem. Soc. 2003, 125, 6604–6605. [Google Scholar] [CrossRef]
- Kornyushyna, O.; Berges, A.M.; Muller, J.G.; Burrows, C.J. In vitro nucleotide misinsertion opposite the oxidized guanosine lesions spiroiminodihydantoin and guanidinohydantoin and DNA synthesis past the lesions using Escherichia coli DNA polymerase I (Klenow fragment). Biochemistry 2002, 41, 15304–15314. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.M.; Burrows, C.J. Formation and processing of DNA damage substrates for the hNEIL enzymes. Free. Radic. Biol. Med. 2017, 107, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Henderson, P.T.; Delaney, J.C.; Muller, J.G.; Neeley, W.L.; Tannenbaum, S.R.; Burrows, C.J.; Essigmann, J.M. The hydantoin lesions formed from oxidation of 7,8-dihydro-8-oxoguanine are potent sources of replication errors in vivo. Biochemistry 2003, 42, 9257–9262. [Google Scholar] [CrossRef]
- Delaney, S.; Delaney, J.C.; Essigmann, J.M. Chemical-biological fingerprinting: Probing the properties of DNA lesions formed by peroxynitrite. Chem. Res. Toxicol. 2007, 20, 1718–1729. [Google Scholar] [CrossRef]
- Neeley, W.L.; Delaney, S.; Alekseyev, Y.O.; Jarosz, D.F.; Delaney, J.C.; Walker, G.C.; Essigmann, J.M. DNA polymerase V allows bypass of toxic guanine oxidation products in vivo. J. Biol. Chem. 2007, 282, 12741–12748. [Google Scholar] [CrossRef]
- Morland, I.; Rolseth, V.; Luna, L.; Rognes, T.; Bjørås, M.; Seeberg, E. Human DNA glycosylases of the bacterial Fpg/MutM superfamily: An alternative pathway for the repair of 8-oxoguanine and other oxidation products in DNA. Nucleic Acids Res. 2002, 30, 4926–4936. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, V.; Sunkara, S.; Wallace, S.S.; Bond, J.P. A novel human DNA glycosylase that removes oxidative DNA damage and is homologous to Escherichia coli endonuclease VIII. DNA Repair 2002, 1, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Syed, A.; Tainer, J.A. Charge Transport Communication through DNA by Protein Fe−S Clusters: How Far Is Not Too Far? ACS Cent. Sci. 2019, 5, 9–11. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.H.S.; da Silva, A.E.; de Oliveira, I.M.; Henriques, J.A.P.; Agnez-Lima, L.F. MutY-glycosylase: An overview on mutagenesis and activities beyond the GO system. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2014, 769, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kairupan, C.; Scott, R.J. Base excision repair and the role of MUTYH. Hered. Cancer Clin. Pract. 2007, 5, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Eccles, L.J.; Neill, P.O.; Lomax, M.E. Mutation Research / Fundamental and Molecular Mechanisms of Mutagenesis Delayed repair of radiation induced clustered DNA damage: Friend or foe? Mutat. Res./Fundam. Mol. Mech. Mutagen. 2011, 711, 134–141. [Google Scholar] [CrossRef]
- Singleton, B.K.; Griffin, C.S.; Thacker, J. Clustered DNA damage leads to complex genetic changes in irradiated human cells. Cancer Res. 2002, 62, 6263–6269. [Google Scholar]
- France, C. Clustered DNA damages as dosemeters for ionising radiation exposure and biological responses. Radiat. Prot. Dosim. 2001, 97, 33–38. [Google Scholar]
- Olson, W.K.; Bansal, M.; Burley, S.K.; Dickerson, R.E.; Gerstein, M.; Harvey, S.C.; Heinemann, U.; Lu, X.J.; Neidle, S.; Shakked, Z.; et al. A standard reference frame for the description of nucleic acid base-pair geometry. J. Mol. Biol. 2001, 313, 229–237. [Google Scholar] [CrossRef]
- Karwowsk, B.T. The influence of single, tandem, and clustered DNA damage on the electronic properties of the double helix: A theoretical study. Molecules 2020, 25, 3126. [Google Scholar] [CrossRef]
- Karwowski, B.T. The Influence of (5′R)- and (5′S)-5′,8-Cyclo-2′-Deoxyadenosine on UDG and hAPE1 Activity. Tandem Lesions are the Base Excision Repair System’s Nightmare. Cells 2019, 8, 1303. [Google Scholar] [CrossRef]
- Nazari, Z.E.; Herrero, J.G.; Fojan, P.; Gurevich, L. Formation of Conductive DNA-Based Nanowires via Conjugation of dsDNA with Cationic Peptide. Nanomaterials 2017, 7, 128. [Google Scholar] [CrossRef]
- Karwowski, B. How Clustered DNA Damage Can Change the Electronic Properties of ds-DNA, Differences between GAG, GAOXOG, OXOGAOXOG. Biomolecules 2023, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Adhikary, A.; Sevilla, M.D.; Close, D.M. One-electron oxidation of ds(5′-GGG-3′) and ds(5′-G(8OG)G-3′) and the nature of hole distribution: A density functional theory (DFT) study. Phys. Chem. Chem. Phys. 2020, 22, 5078–5089. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.B.; Vorpagel, E.R.; Wang, L.S. Direct experimental observation of the low ionization potentials of guanine in free oligonucleotides by using photoelectron spectroscopy. Proc. Natl. Acad. Sci. USA 2004, 101, 17588–17592. [Google Scholar] [CrossRef]
- Diamantis, P.; Tavernelli, I.; Rothlisberger, U. Redox Properties of Native and Damaged DNA from Mixed Quantum Mechanical/Molecular Mechanics Molecular Dynamics Simulations. J. Chem. Theory Comput. 2020, 16, 6690–6701. [Google Scholar] [CrossRef]
- Lewis, F.D.; Liu, J.; Weigel, W.; Rettig, W.; Kurnikov, I.V.; Beratan, D.N. Donor-bridge-acceptor energetics determine the distance dependence of electron tunneling in DNA. Proc. Natl. Acad. Sci. USA 2002, 99, 12536–12541. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.A. Electron Transfer Reactions in Chemistry: Theory and Experiment (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Karwowski, B.T. The AT Interstrand Cross-Link: Structure, Electronic Properties, and Influence on Charge Transfer in dsDNA. Mol. Ther.-Nucleic Acids 2018, 13, 665–685. [Google Scholar] [CrossRef]
- Parada, G.A.; Goldsmith, Z.K.; Kolmar, S.; Rimgard, B.P.; Mercado, B.Q.; Hammarström, L.; Hammes-schiffer, S.; James, M. Concerted proton-electron transfer reactions in the Marcus inverted region. Science 2020, 364, 471–475. [Google Scholar] [CrossRef]
- Mignon, P.; Loverix, S.; Steyaert, J.; Geerlings, P. Influence of the π-π interaction on the hydrogen bonding capacity of stacked DNA/RNA bases. Nucleic Acids Res. 2005, 33, 1779–1789. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.R.; Grodick, M.A.; Barton, J.K. Review DNA Charge Transport: From Chemical Principles to the Cell. Cell Chem. Biol. 2016, 23, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Boal, A.K.; Yavin, E.; Lukianova, O.A.; O’Shea, V.L.; David, S.S.; Barton, J.K. DNA-bound redox activity of DNA repair glycosylases containing [4Fe-4S] clusters. Biochemistry 2005, 44, 8397–8407. [Google Scholar] [CrossRef] [PubMed]
- Cammack, R. Iron-Sulfur Proteins, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, ISBN 9780123786319. [Google Scholar]
- Lomax, M.E.; Folkes, L.K.; Neill, P.O. Biological Consequences of Radiation-induced DNA Damage: Relevance to Radiotherapy Statement of Search Strategies Used and Sources of Information Why Radiation Damage is More Effective than Endogenous Damage at Killing Cells Ionising Radiation-induced Do. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Sage, E.; Harrison, L. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis Clustered DNA lesion repair in eukaryotes: Relevance to mutagenesis and cell survival. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2011, 711, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. Fapy dG in the Shadow of OXO dG—A Theoretical Study of Clustered DNA Lesions. Int. J. Mol. Sci. 2023, 24, 5361. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. The influence of oxoG on the electronic properties of ds-DNA. Damageversus mismatch: A theoretical approach. Comput. Biol. Chem. 2021, 92, 107485. [Google Scholar] [CrossRef] [PubMed]
- Voityuk, A.A.; Jortner, J.; Bixon, M.; Rösch, N. Energetics of hole transfer in DNA. Chem. Phys. Lett. 2000, 324, 430–434. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Grozema, F.C.; Guerra, C.F.; Bickelhaupt, F.M.; Siebbeles, L.D.A. Mapping the Sites for Selective Oxidation of Guanines in DNA. J. Am. Chem. Soc. 2003, 125, 13658–13659. [Google Scholar] [CrossRef]
- Kawai, K.; Majima, T. Hole transfer kinetics of DNA. Acc. Chem. Res. 2013, 46, 2616–2625. [Google Scholar] [CrossRef]
- Kumar, A.; Sevilla, M.D. DFT Studies of the Extent of Hole Delocalization in One-electron Oxidized Adenine and Guanine base Stacks. J. Phys. Chem. B 2011, 115, 4990–5000. [Google Scholar] [CrossRef] [PubMed]
- Suppan, P. The marcus inverted region. In Photoinduced Electron Transfer IV; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1992; Volume 163, pp. 95–130. [Google Scholar] [CrossRef]
- Aust, A.E.; Eveleigh, J.F. Mechanisms of DNA oxidation. Proc. Soc. Exp. Biol. Med. 1999, 222, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Sanii, L.; Schuster, G.B. Long-Distance Charge Transport in DNA: Sequence-Dependent Radical Cation Injection Efficiency. J. Am. Chem. Soc. 2000, 122, 11545–11546. [Google Scholar] [CrossRef]
- Schuster, G.B.; Landman, U. The Mechanism of Long-Distance Radical Cation Transport in Duplex DNA: Ion-Gated Hopping of Polaron-Like Distortions. In Long-Range Charge Transfer in DNA I; Springer: Berlin/Heidelberg, Germany, 2012; pp. 139–161. [Google Scholar] [CrossRef]
- Wang, J.; Ding, T.; Gao, K.; Wang, L.; Zhou, P.; Wu, K. Marcus inverted region of charge transfer from low-dimensional semiconductor materials. Nat. Commun. 2021, 12, 6333. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. Charge Transfer Depends on Its Diastereomeric Form: A Theoretical Study. Antioxidants 2023, 12, 881. [Google Scholar] [CrossRef] [PubMed]
- Karwowski, B.T. The 2Ih and OXOG Proximity Consequences on Charge Transfer through ds -DNA: Theoretical Studies of Clustered DNA Damage. Molecules 2023, 28, 2180. [Google Scholar] [CrossRef] [PubMed]
- Dapprich, S.; Komáromi, I.; Byun, K.S.; Morokuma, K.; Frisch, M.J. A new ONIOM implementation in Gaussian98. Part I. The calculation of energies, gradients, vibrational frequencies and electric field derivatives. J. Mol. Struct. THEOCHEM 1999, 461–462, 1–21. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, J.; Lynch, B.J.; Truhlar, D.G. Tests of second-generation and third-generation density functionals for thermochemical kineticsElectronic supplementary information (ESI) available: Mean errors for pure and hybrid DFT methods. Phys. Chem. Chem. Phys. 2004, 6, 673–676. [Google Scholar] [CrossRef]
- Gu, J.; Xie, Y.; Schaefer, H.F. Electron attachment to nucleotides in aqueous solution. Chemphyschem 2006, 7, 1885–1887. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Leszczynski, J. Electron attachment-induced DNA single-strand breaks at the pyrimidine sites. Nucleic Acids Res. 2010, 38, 5280–5290. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Leszczynski, J. Electron Attachment-Induced DNA Single Strand Breaks: C3′–O3′ σ-Bond Breaking of Pyrimidine Nucleotides Predominates. J. Am. Chem. Soc. 2006, 9322–9323. [Google Scholar] [CrossRef] [PubMed]
- Hehre, W.J.; Radom, L.; Schleyer, P.R.; Pople, J.; Wiley, J.; Wiberg, K.B.; Clark, T.; York, N. Ab Initio Molecular Orbital Theory by A Handbook of Computational Chem-istry: A Practical Guide to Chemical Structure and Energy Calculations. J. Comput. Chem. 1986, 7, 379–383. [Google Scholar]
- Lange, A.W.; Herbert, J.M. Both intra- and interstrand charge-transfer excited states in aqueous B-DNA are present at energies comparable to, or just above, the 1ππ* excitonic bright states. J. Am. Chem. Soc. 2009, 131, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Tong, P.Q. Time-dependent density-functional theory for multicomponent systems. Phys. Rev. A 1986, 34, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Miertus, S.; Tomasi, J. Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem. Phys. 1982, 65, 239–245. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Cave, R.J.; Newton, M.D. Generalization of the Mulliken-Hush treatment for the calculation of electron transfer matrix elements. Chem. Phys. Lett. 1996, 249, 15–19. [Google Scholar] [CrossRef]
- Karwowski, B.T. The influence of phosphorothioate on charge migration in single and double stranded DNA: A theoretical approach. Phys. Chem. Chem. Phys. 2015, 17, 21507–21516. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- BIOVIA. Discovery Studio Visualizer; v16.1.0.15350; BIOVIA: San Diego, CA, USA, 2015. [Google Scholar]
- Li, S.; Olson, W.K.; Lu, X.J. Web 3DNA 2.0 for the analysis, visualization, and modeling of 3D nucleic acid structures. Nucleic Acids Res. 2019, 47, W26–W34. [Google Scholar] [CrossRef]


| VIPNE | VIPEQ | AIP | VEANE | VEAEQ | AEA | |
|---|---|---|---|---|---|---|
| oligo-OXIa | (a) 6.50 | 5.88 | 5.49 | 2.42 | 3.08 | 3.59 |
| (b) 6.30 | 5.80 | 5.39 | 1.86 | 2.87 | 3.24 | |
| oligo-N* | (a) 6.72 | 6.08 | 5.65 | 0.84 | 1.58 | 2.09 |
| (b) 6.48 | 5.98 | 5.58 | 0.60 | 1.34 | 1.90 | |
| RMSD: Anion versus Neutral | RMSD: Cation versus Neutral | |||||
| ds-DNA | BP | PS-Frame | ds-DNA | BP | PS-Frame | |
| oligo-OXIa | 0.49 | 0.36 | 0.59 | 0.18 | 0.13 | 0.22 |
| oligo-N [35] | 0.17 | 0.16 | 0.17 | 0.36 | 0.29 | 0.42 |
| Electron-Hole Transfer | Excess-Electron Transfer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| System | λ | ΔG | Ea | V12 | KCT | System | λ | ΔG | Ea | V12 | kCT |
| A1OXIa2A3OXOG4A5 | A1OXIa2A3OXOG4A5 | ||||||||||
| A1T5←OXIa2C4 | −0.01 | −0.39 | −5.51 | 0.38 | 0.00 | A1T5→OXIa2C4 | 0.35 | −2.10 | 2.19 | 0.38 | 3.56 × 10−22 |
| OXIa2C4→A3T3 | −0.01 | −0.44 | −6.27 | 0.09 | 0.00 | OXIa2C4←A3T3 | 0.36 | −2.07 | 2.05 | 0.32 | 6.32 × 10−20 |
| A3T3→OXOG4C2 | 0.42 | −1.10 | 0.27 | 0.43 | 1.13 × 1011 | A3T3→OXOG4C2 | 0.003 | −0.08 | 0.58 | 0.02 | 1.05 × 1014 |
| OXOG4C2←A5T1 | 0.35 | −1.16 | 0.46 | 0.36 | 5.59 × 107 | OXOG4C2←A5T1 | 0.008 | −0.08 | 0.16 | 0.01 | 3.12 × 1010 |
| A1T5→A3T3 | 0.00 | −0.05 | −0.16 | 0.03 | 0.00 | A1T5→A3T3 | 0.01 | −0.03 | 0.01 | 0.07 | 5.61 × 1014 |
| OXIa2C4→OXOG4C2 | 0.41 | −1.54 | 0.76 | 0.05 | 7.04 | OXIa2C4←OXOG4C2 | 0.33 | −1.98 | 2.04 | 0.06 | 3.73 × 10−21 |
| A3T3←A5T1 | 0.00 | −0.06 | −0.24 | 0.04 | 0.00 | A3T3→A5T1 | 0.00 | −0.003 | 0.01 | 0.05 | 5.16 × 1015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowski, B.T. The Influence of Oxidized Imino-Allantoin in the Presence of OXOG on Double Helix Charge Transfer: A Theoretical Approach. Int. J. Mol. Sci. 2024, 25, 5962. https://doi.org/10.3390/ijms25115962
Karwowski BT. The Influence of Oxidized Imino-Allantoin in the Presence of OXOG on Double Helix Charge Transfer: A Theoretical Approach. International Journal of Molecular Sciences. 2024; 25(11):5962. https://doi.org/10.3390/ijms25115962
Chicago/Turabian StyleKarwowski, Boleslaw T. 2024. "The Influence of Oxidized Imino-Allantoin in the Presence of OXOG on Double Helix Charge Transfer: A Theoretical Approach" International Journal of Molecular Sciences 25, no. 11: 5962. https://doi.org/10.3390/ijms25115962
APA StyleKarwowski, B. T. (2024). The Influence of Oxidized Imino-Allantoin in the Presence of OXOG on Double Helix Charge Transfer: A Theoretical Approach. International Journal of Molecular Sciences, 25(11), 5962. https://doi.org/10.3390/ijms25115962






