Sex-Based Mechanisms of Cardiac Development and Function: Applications for Induced-Pluripotent Stem Cell Derived-Cardiomyocytes
Abstract
1. Introduction
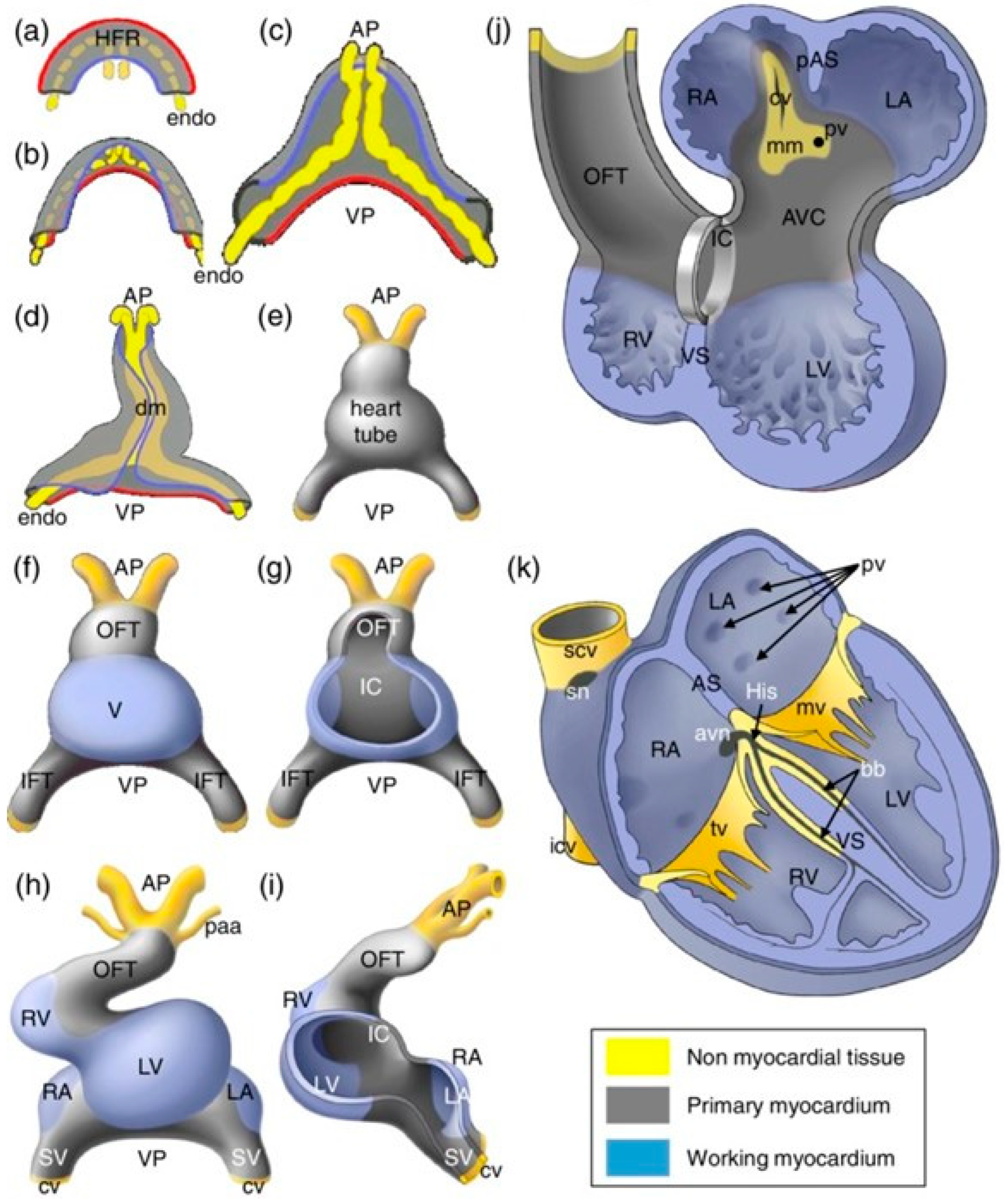
2. Cardiac Development and Sex-Specific Differences
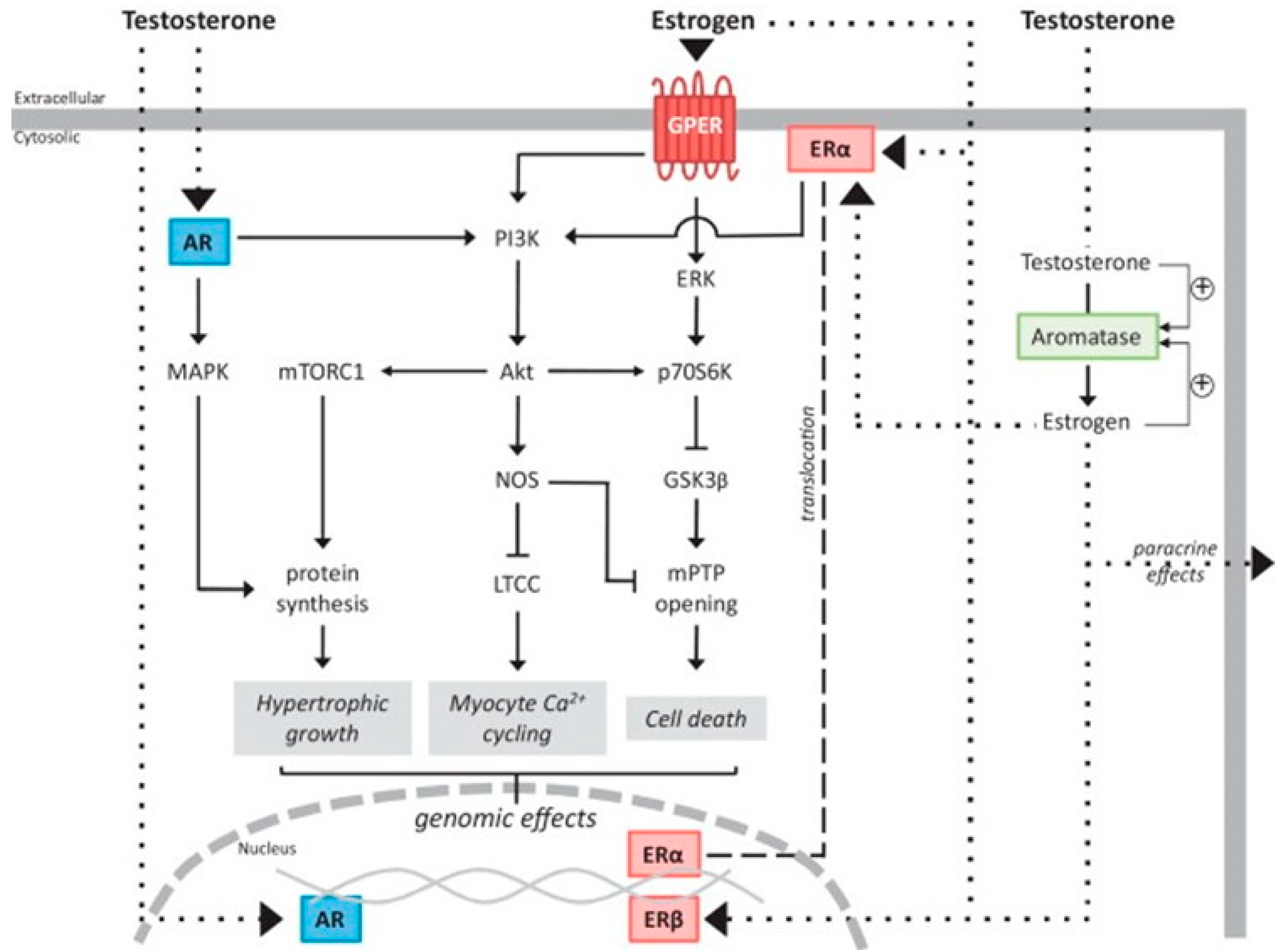
3. Sex Hormone Signaling Pathways and Mechanisms of Action
3.1. Testosterone
3.2. Estrogen
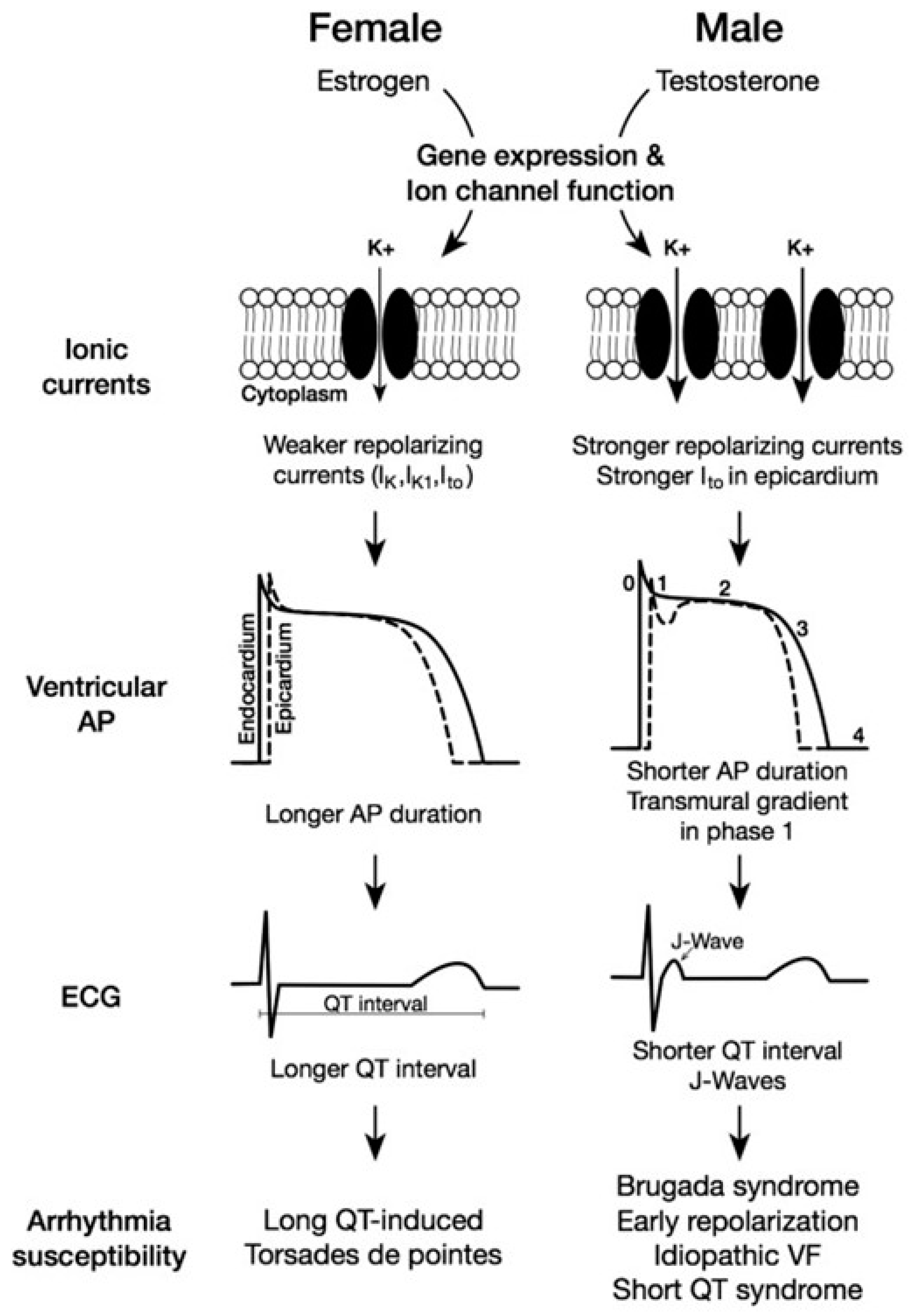
4. The Effects of Hormonal Variation on Cardiac Status
5. Sex-Based Differences in Mature Human Cardiomyocytes
6. Using Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs) to Better Understand Sex-Based Differences in Cardiac Function and Dysfunction
7. Future Directions in Understanding Sex-Specific Cardiac Diseases
8. Conclusions
Funding
Conflicts of Interest
References
- Deegan, D.F.; Engel, N. Sexual Dimorphism in the Age of Genomics: How, When, Where. Front. Cell Dev. Biol. 2019, 7, 186. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Sheng, X.; Dorr, K.M.; Hutton, J.E.; Emerson, J.I.; Davies, H.A.; Andrade, T.D.; Wasson, L.K.; Greco, T.M.; Hashimoto, Y.; et al. Cardiac proteomics reveals sex chromosome-dependent differences between males and females that arise prior to gonad formation. Dev. Cell 2021, 56, 3019–3034.e7. [Google Scholar] [CrossRef] [PubMed]
- Nussey, S.; Whitehead, S. The gonad. In Endocrinology: An Integrated Approach; BIOS Scientific Publishers: Milton Park, UK, 2001. [Google Scholar]
- Albalushi, H.; Sahlin, L.; Åkesson, E.; Kurek, M.; Kjartansdóttir, K.R.; Lindh, R.; Söder, O.; Rotstein, E.; Hovatta, O.; Stukenborg, J.-B. Hormone Production by Human First-Trimester Gonads in a Functional In Vitro System. Endocrinology 2019, 160, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Trexler, C.L.; Odell, A.T.; Jeong, M.Y.; Dowell, R.D.; Leinwand, L.A. Transcriptome and Functional Profile of Cardiac Myocytes Is Influenced by Biological Sex. Circ. Cardiovasc. Genet. 2017, 10, e001770. [Google Scholar] [CrossRef] [PubMed]
- Lock, R.; Al Asafen, H.; Fleischer, S.; Tamargo, M.; Zhao, Y.; Radisic, M.; Vunjak-Novakovic, G. A framework for developing sex-specific engineered heart models. Nat. Rev. Mater. 2022, 7, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.S.; Kelly, R.P.; Collins, P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc. Res. 2000, 46, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.A.; Ross, E.G.; Bolli, R.; Pepine, C.J.; Leeper, N.J.; Yang, P.C. The Promise and Challenge of Induced Pluripotent Stem Cells for Cardiovascular Applications. JACC Basic Transl. Sci. 2016, 1, 510–523. [Google Scholar] [CrossRef]
- Beauchamp, P.; Jackson, C.B.; Ozhathil, L.C.; Agarkova, I.; Galindo, C.L.; Sawyer, D.B.; Suter, T.M.; Zuppinger, C. 3D Co-culture of hiPSC-Derived Cardiomyocytes with Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids. Front. Mol. Biosci. 2020, 7, 14. [Google Scholar] [CrossRef]
- Tan, C.M.J.; Lewandowski, A.J. The Transitional Heart: From Early Embryonic and Fetal Development to Neonatal Life. Fetal Diagn. Ther. 2020, 47, 373–386. [Google Scholar] [CrossRef]
- Bulatovic, I.; Månsson-Broberg, A.; Sylvén, C.; Grinnemo, K.-H. Human fetal cardiac progenitors: The role of stem cells and progenitors in the fetal and adult heart. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 58–68. [Google Scholar] [CrossRef]
- Dhanantwari, P.; Lee, E.; Krishnan, A.; Samtani, R.; Yamada, S.; Anderson, S.; Lockett, E.; Donofrio, M.; Shiota, K.; Leatherbury, L.; et al. Human cardiac development in the first trimester: A high-resolution magnetic resonance imaging and episcopic fluorescence image capture atlas. Circulation 2009, 120, 343–351. [Google Scholar] [CrossRef]
- Sizarov, A.; Ya, J.; de Boer, B.A.; Lamers, W.H.; Christoffels, V.M.; Moorman, A.F.M. Formation of the building plan of the human heart: Morphogenesis, growth, and differentiation. Circulation 2011, 123, 1125–1135. [Google Scholar] [CrossRef]
- Buijtendijk, M.F.J.; Barnett, P.; van den Hoff, M.J.B. Development of the human heart. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 7–22. [Google Scholar] [CrossRef]
- Faber, J.W.; Hagoort, J.; Moorman, A.F.M.; Christoffels, V.M.; Jensen, B. Quantified growth of the human embryonic heart. Biol. Open 2021, 10, bio057059. [Google Scholar] [CrossRef]
- Deegan, D.F.; Karbalaei, R.; Madzo, J.; Kulathinal, R.J.; Engel, N. The developmental origins of sex-biased expression in cardiac development. Biol. Sex Differ. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Chang, S.; Wang, Y.; Xin, Y.; Wang, S.; Luo, Y.; Wang, L.; Zhang, H.; Li, J. DNA methylation abnormalities of imprinted genes in congenital heart disease: A pilot study. BMC Med. Genomics 2021, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Cassis, L.A.; Eghbali, M.; Reue, K.; Sandberg, K. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 746–756. [Google Scholar] [CrossRef] [PubMed]
- St Pierre, S.R.; Peirlinck, M.; Kuhl, E. Sex Matters: A Comprehensive Comparison of Female and Male Hearts. Front. Physiol. 2022, 13, 831179. [Google Scholar] [CrossRef]
- McClain, A.K.; Monteleone, P.P.; Zoldan, J. Sex in cardiovascular disease: Why this biological variable should be considered in in vitro models. Sci. Adv. 2024, 10, eadn3510. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, P.S.; Arnold, A.P. A primer on the use of mouse models for identifying direct sex chromosome effects that cause sex differences in non-gonadal tissues. Biol. Sex Differ. 2016, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Snell, D.M.; Turner, J.M.A. Sex Chromosome Effects on Male-Female Differences in Mammals. Curr. Biol. 2018, 28, R1313–R1324. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Chen, M.; Xue, Q.; Xiao, D.; Zhang, L. Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts. Circ. Res. 2010, 107, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Ghnenis, A.; Padmanabhan, V.; Vyas, A. Sexual dimorphism in testosterone programming of cardiomyocyte development in sheep. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H607–H621. [Google Scholar] [CrossRef] [PubMed]
- Pedernera, E.; Gómora, M.J.; Meneses, I.; De Ita, M.; Méndez, C. Androgen receptor is expressed in mouse cardiomyocytes at prenatal and early postnatal developmental stages. BMC Physiol. 2017, 17, 7. [Google Scholar] [CrossRef]
- Prajapati, C.; Koivumäki, J.; Pekkanen-Mattila, M.; Aalto-Setälä, K. Sex differences in heart: From basics to clinics. Eur. J. Med. Res. 2022, 27, 241. [Google Scholar] [CrossRef] [PubMed]
- Rautaharju, P.M.; Zhou, S.H.; Wong, S.; Calhoun, H.P.; Berenson, G.S.; Prineas, R.; Davignon, A. Sex differences in the evolution of the electrocardiographic QT interval with age. Can. J. Cardiol. 1992, 8, 690–695. [Google Scholar] [PubMed]
- Wong, G.R.; Nalliah, C.J.; Lee, G.; Voskoboinik, A.; Chieng, D.; Prabhu, S.; Parameswaran, R.; Sugumar, H.; Al-Kaisey, A.; McLellan, A.; et al. Sex-Related Differences in Atrial Remodeling in Patients with Atrial Fibrillation: Relationship to Ablation Outcomes. Circ. Arrhythm. Electrophysiol. 2022, 15, e009925. [Google Scholar] [CrossRef]
- Villareal, R.P.; Woodruff, A.L.; Massumi, A. Gender and cardiac arrhythmias. Tex. Heart Inst. J. 2001, 28, 265–275. [Google Scholar]
- Reue, K.; Wiese, C.B. Illuminating the Mechanisms Underlying Sex Differences in Cardiovascular Disease. Circ. Res. 2022, 130, 1747–1762. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.R.; Bernasochi, G.B.; Varma, U.; Raaijmakers, A.J.A.; Delbridge, L.M.D. Sex and sex hormones in cardiac stress—Mechanistic insights. J. Steroid Biochem. Mol. Biol. 2013, 137, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Kurokawa, J. Involvement of sex hormonal regulation of K+ channels in electrophysiological and contractile functions of muscle tissues. J. Pharmacol. Sci. 2019, 139, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Goodale, T.; Sadhu, A.; Petak, S.; Robbins, R. Testosterone and the Heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, R.; Donoiu, I.; Mirea, O.; Bălşeanu, T.A. Testosterone, cardiomyopathies, and heart failure: A narrative review. Asian J. Androl. 2021, 23, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.-Y.; Nheu, L.; Komesaroff, P.; Ling, S. Testosterone protects cardiac myocytes from superoxide injury via NF-κB signalling pathways. Life Sci. 2015, 133, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.; Wu, S.; Liu, J.; Wong, T.M. Testosterone protects rat hearts against ischaemic insults by enhancing the effects of alpha(1)-adrenoceptor stimulation. Br. J. Pharmacol. 2008, 153, 693–709. [Google Scholar] [CrossRef]
- Lam, Y.T.; Lecce, L.; Tan, J.T.M.; Bursill, C.A.; Handelsman, D.J.; Ng, M.K.C. Androgen Receptor-Mediated Genomic Androgen Action Augments Ischemia-Induced Neovascularization. Endocrinology 2016, 157, 4853–4864. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Katus, H.A.; Olson, E.N.; Hill, J.A. Hypertrophy of the heart: A new therapeutic target? Circulation 2004, 109, 1580–1589. [Google Scholar] [CrossRef]
- Marsh, J.D.; Lehmann, M.H.; Ritchie, R.H.; Gwathmey, J.K.; Green, G.E.; Schiebinger, R.J. Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 1998, 98, 256–261. [Google Scholar] [CrossRef]
- Altamirano, F.; Oyarce, C.; Silva, P.; Toyos, M.; Wilson, C.; Lavandero, S.; Uhlén, P.; Estrada, M. Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J. Endocrinol. 2009, 202, 299–307. [Google Scholar] [CrossRef]
- Duran, J.; Oyarce, C.; Pavez, M.; Valladares, D.; Basualto-Alarcon, C.; Lagos, D.; Barrientos, G.; Troncoso, M.F.; Ibarra, C.; Estrada, M. GSK-3β/NFAT Signaling Is Involved in Testosterone-Induced Cardiac Myocyte Hypertrophy. PLoS ONE 2016, 11, e0168255. [Google Scholar] [CrossRef]
- Lopes, R.A.M.; Neves, K.B.; Pestana, C.R.; Queiroz, A.L.; Zanotto, C.Z.; Chignalia, A.Z.; Valim, Y.M.; Silveira, L.R.; Curti, C.; Tostes, R.C. Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1485–H1494. [Google Scholar] [CrossRef]
- Ribeiro Júnior, R.F.; Ronconi, K.S.; Jesus, I.C.G.; Almeida, P.W.M.; Forechi, L.; Vassallo, D.V.; Guatimosim, S.; Stefanon, I.; Fernandes, A.A. Testosterone deficiency prevents left ventricular contractility dysfunction after myocardial infarction. Mol. Cell. Endocrinol. 2018, 460, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Corrêa, R.d.A.; Ribeiro Júnior, R.F.; Mendes, S.B.O.; Dos Santos, P.M.; da Silva, M.V.A.; Silva, D.F.; Biral, I.P.; de Batista, P.R.; Vassallo, D.V.; Bittencourt, A.S.; et al. Testosterone deficiency reduces the effects of late cardiac remodeling after acute myocardial infarction in rats. PLoS ONE 2019, 14, e0213351. [Google Scholar] [CrossRef] [PubMed]
- Cavasin, M.A.; Tao, Z.-Y.; Yu, A.-L.; Yang, X.-P. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2043–H2050. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Ropero, A.B.; Eghbali, M.; Minosyan, T.Y.; Tang, G.; Toro, L.; Stefani, E. Heart estrogen receptor alpha: Distinct membrane and nuclear distribution patterns and regulation by estrogen. J. Mol. Cell. Cardiol. 2006, 41, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Gourdy, P.; Guillaume, M.; Fontaine, C.; Adlanmerini, M.; Montagner, A.; Laurell, H.; Lenfant, F.; Arnal, J.-F. Estrogen receptor subcellular localization and cardiometabolism. Mol. Metab. 2018, 15, 56–69. [Google Scholar] [CrossRef]
- Pugach, E.K.; Blenck, C.L.; Dragavon, J.M.; Langer, S.J.; Leinwand, L.A. Estrogen receptor profiling and activity in cardiac myocytes. Mol. Cell. Endocrinol. 2016, 431, 62–70. [Google Scholar] [CrossRef]
- Brouillette, J.; Lupien, M.-A.; St-Michel, C.; Fiset, C. Characterization of ventricular repolarization in male and female guinea pigs. J. Mol. Cell. Cardiol. 2007, 42, 357–366. [Google Scholar] [CrossRef]
- Lizotte, E.; Grandy, S.A.; Tremblay, A.; Allen, B.G.; Fiset, C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2009, 23, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.A.; Budish, R.A.; Kashyap, S.; Lindsey, S.H. GPER-novel membrane oestrogen receptor. Clin. Sci. Lond. Engl. 1979 2016, 130, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Korzick, D.H. Estrogen and the female heart. Mol. Cell. Endocrinol. 2014, 389, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, S.; Fritschka, S.; Dworatzek, E.; Pham, T.H.; Becher, E.; Kuehne, A.; Davidson, M.M.; Regitz-Zagrosek, V. Nuclear factor-kappaB regulates estrogen receptor-alpha transcription in the human heart. J. Biol. Chem. 2009, 284, 24705–24714. [Google Scholar] [CrossRef] [PubMed]
- Menazza, S.; Murphy, E. The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ. Res. 2016, 118, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Kim, J.K. The Role of Estrogen and Estrogen Receptors on Cardiomyocytes: An Overview. Can. J. Cardiol. 2016, 32, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.E.; Singh, H.; Lu, R.; Olde, B.; Leeb-Lundberg, L.M.F.; Bopassa, J.C. G Protein-Coupled Estrogen Receptor 1 Mediates Acute Estrogen-Induced Cardioprotection via MEK/ERK/GSK-3β Pathway after Ischemia/Reperfusion. PLoS ONE 2015, 10, e0135988. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodzadeh, S.; Dworatzek, E. The Role of 17β-Estradiol and Estrogen Receptors in Regulation of Ca2+ Channels and Mitochondrial Function in Cardiomyocytes. Front. Endocrinol. 2019, 10, 310. [Google Scholar] [CrossRef]
- Iorga, A.; Umar, S.; Ruffenach, G.; Aryan, L.; Li, J.; Sharma, S.; Motayagheni, N.; Nadadur, R.D.; Bopassa, J.C.; Eghbali, M. Estrogen rescues heart failure through estrogen receptor Beta activation. Biol. Sex Differ. 2018, 9, 48. [Google Scholar] [CrossRef]
- Firth, J.M.; Yang, H.-Y.; Francis, A.J.; Islam, N.; MacLeod, K.T. The Effect of Estrogen on Intracellular Ca2+ and Na+ Regulation in Heart Failure. JACC Basic Transl. Sci. 2020, 5, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.A.; Longcope, C.; Derby, C.A.; Johannes, C.B.; Araujo, A.B.; Coviello, A.D.; Bremner, W.J.; McKinlay, J.B. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J. Clin. Endocrinol. Metab. 2002, 87, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Shabsigh, R.; Katz, M.; Yan, G.; Makhsida, N. Cardiovascular issues in hypogonadism and testosterone therapy. Am. J. Cardiol. 2005, 96, 67M–72M. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rastrelli, G.; Monami, M.; Guay, A.; Buvat, J.; Sforza, A.; Forti, G.; Mannucci, E.; Maggi, M. Hypogonadism as a risk factor for cardiovascular mortality in men: A meta-analytic study. Eur. J. Endocrinol. 2011, 165, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, J.; Malhotra, A.; Schaible, T.F.; Capasso, J. Effects of gonadectomy and hormonal replacement on rat hearts. Circ. Res. 1987, 61, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sebag, I.A.; Gillis, M.-A.; Calderone, A.; Kasneci, A.; Meilleur, M.; Haddad, R.; Noiles, W.; Patel, B.; Chalifour, L.E. Sex hormone control of left ventricular structure/function: Mechanistic insights using echocardiography, expression, and DNA methylation analyses in adult mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1706–H1715. [Google Scholar] [CrossRef] [PubMed]
- Curl, C.L.; Delbridge, L.M.D.; Canny, B.J.; Wendt, I.R. Testosterone modulates cardiomyocyte Ca2+ handling and contractile function. Physiol. Res. 2009, 58, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, O.; Banga, S.; Heinze-Milne, S.; Rose, R.A.; Pyle, W.G.; Howlett, S.E. Long-term testosterone deficiency modifies myofilament and calcium-handling proteins and promotes diastolic dysfunction in the aging mouse heart. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H768–H780. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Kranias, E.G. Phospholamban: A crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell Biol. 2003, 4, 566–577. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Banga, S.; Mishra, M.; Heinze-Milne, S.D.; Jansen, H.J.; Rose, R.A.; Howlett, S.E. Chronic testosterone deficiency increases late inward sodium current and promotes triggered activity in ventricular myocytes from aging male mice. Am. J. Physiol. Heart Circ. Physiol. 2023, 325, H264–H277. [Google Scholar] [CrossRef] [PubMed]
- Thibault, S.; Ton, A.-T.; Huynh, F.; Fiset, C. Connexin Lateralization Contributes to Male Susceptibility to Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 10696. [Google Scholar] [CrossRef] [PubMed]
- Thibault, S.; Long, V.; Fiset, C. Higher Na+-Ca2+ Exchanger Function and Triggered Activity Contribute to Male Predisposition to Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 10724. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, F.; Palacios, S. Hormonal changes during menopause. Maturitas 2009, 63, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Fares, E.; Pyle, W.G.; Ray, G.; Rose, R.A.; Denovan-Wright, E.M.; Chen, R.P.; Howlett, S.E. The impact of ovariectomy on calcium homeostasis and myofilament calcium sensitivity in the aging mouse heart. PLoS ONE 2013, 8, e74719. [Google Scholar] [CrossRef]
- Fares, E.; Parks, R.J.; Macdonald, J.K.; Egar, J.M.S.; Howlett, S.E. Ovariectomy enhances SR Ca2+ release and increases Ca2+ spark amplitudes in isolated ventricular myocytes. J. Mol. Cell. Cardiol. 2012, 52, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Paigel, A.S.; Ribeiro, R.F.; Fernandes, A.A.; Targueta, G.P.; Vassallo, D.V.; Stefanon, I. Myocardial contractility is preserved early but reduced late after ovariectomy in young female rats. Reprod. Biol. Endocrinol. 2011, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, G.M.; Kam, K.W.L.; Liu, J.; Wu, S.; Wong, T.M. Altered Ca2+ handling by ryanodine receptor and Na+-Ca2+ exchange in the heart from ovariectomized rats: Role of protein kinase A. Am. J. Physiol. Cell Physiol. 2007, 292, C1625–C1635. [Google Scholar] [CrossRef]
- Mayne, B.T.; Bianco-Miotto, T.; Buckberry, S.; Breen, J.; Clifton, V.; Shoubridge, C.; Roberts, C.T. Large Scale Gene Expression Meta-Analysis Reveals Tissue-Specific, Sex-Biased Gene Expression in Humans. Front. Genet. 2016, 7, 183. [Google Scholar] [CrossRef]
- Raznahan, A.; Parikshak, N.N.; Chandran, V.; Blumenthal, J.D.; Clasen, L.S.; Alexander-Bloch, A.F.; Zinn, A.R.; Wangsa, D.; Wise, J.; Murphy, D.G.M.; et al. Sex-chromosome dosage effects on gene expression in humans. Proc. Natl. Acad. Sci. USA 2018, 115, 7398–7403. [Google Scholar] [CrossRef]
- Tukiainen, T.; Villani, A.-C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X chromosome inactivation across human tissues. Nature 2017, 550, 244–248. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef]
- Godfrey, A.K.; Naqvi, S.; Chmátal, L.; Chick, J.M.; Mitchell, R.N.; Gygi, S.P.; Skaletsky, H.; Page, D.C. Quantitative analysis of Y-Chromosome gene expression across 36 human tissues. Genome Res. 2020, 30, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz-Weiss, P.; Tomek, R.J.; Mathew, J.; Eghbali, M. Gender-specific differences in expression of mRNAs for functional and structural proteins in rat ventricular myocardium. J. Mol. Cell. Cardiol. 1994, 26, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Schwertz, D.W.; Beck, J.M.; Kowalski, J.M.; Ross, J.D. Sex differences in the response of rat heart ventricle to calcium. Biol. Res. Nurs. 2004, 5, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.; Fontes, S.K.; Bautista, E.N.; Cheng, Z. Physiological and pathological roles of protein kinase A in the heart. Cardiovasc. Res. 2022, 118, 386–398. [Google Scholar] [CrossRef]
- Chung, A.K.; Das, S.R.; Leonard, D.; Peshock, R.M.; Kazi, F.; Abdullah, S.M.; Canham, R.M.; Levine, B.D.; Drazner, M.H. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: The Dallas Heart Study. Circulation 2006, 113, 1597–1604. [Google Scholar] [CrossRef]
- Petersen, S.E.; Aung, N.; Sanghvi, M.M.; Zemrak, F.; Fung, K.; Paiva, J.M.; Francis, J.M.; Khanji, M.Y.; Lukaschuk, E.; Lee, A.M.; et al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2017, 19, 18. [Google Scholar] [CrossRef]
- Sotomi, Y.; Hikoso, S.; Nakatani, D.; Mizuno, H.; Okada, K.; Dohi, T.; Kitamura, T.; Sunaga, A.; Kida, H.; Oeun, B.; et al. Sex Differences in Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e018574. [Google Scholar] [CrossRef]
- Jiao, L.; Machuki, J.O.; Wu, Q.; Shi, M.; Fu, L.; Adekunle, A.O.; Tao, X.; Xu, C.; Hu, X.; Yin, Z.; et al. Estrogen and calcium handling proteins: New discoveries and mechanisms in cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H820–H829. [Google Scholar] [CrossRef] [PubMed]
- Machuki, J.O.; Zhang, H.-Y.; Geng, J.; Fu, L.; Adzika, G.K.; Wu, L.; Shang, W.; Wu, J.; Kexue, L.; Zhao, Z.; et al. Estrogen regulation of cardiac cAMP-L-type Ca2+ channel pathway modulates sex differences in basal contraction and responses to β2AR-mediated stress in left ventricular apical myocytes. Cell Commun. Signal. CCS 2019, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Trépanier-Boulay, V.; St-Michel, C.; Tremblay, A.; Fiset, C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ. Res. 2001, 89, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; El Khoury, N.; Rivard, K.; Paradis, P.; Nemer, M.; Fiset, C. Angiotensin II Overstimulation Leads to an Increased Susceptibility to Dilated Cardiomyopathy and Higher Mortality in Female Mice. Sci. Rep. 2018, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Papp, R.; Bett, G.C.L.; Lis, A.; Rasmusson, R.L.; Baczkó, I.; Varró, A.; Salama, G. Genomic upregulation of cardiac Cav1.2α and NCX1 by estrogen in women. Biol. Sex Differ. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef]
- Yang, X.; Mao, X.; Xu, G.; Xing, S.; Chattopadhyay, A.; Jin, S.; Salama, G. Estradiol up-regulates L-type Ca2+ channels via membrane-bound estrogen receptor/phosphoinositide-3-kinase/Akt/cAMP response element-binding protein signaling pathway. Heart Rhythm 2018, 15, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Parks, R.J.; Howlett, S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflug. Arch. 2013, 465, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.H.; Izadnegahdar, M.; Sedlak, T.; Saw, J.; Johnston, N.; Schenck-Gustafsson, K.; Shah, R.U.; Regitz-Zagrosek, V.; Grewal, J.; Vaccarino, V.; et al. Sex differences in cardiovascular disease—Impact on care and outcomes. Front. Neuroendocrinol. 2017, 46, 46–70. [Google Scholar] [CrossRef]
- Tadros, R.; Ton, A.-T.; Fiset, C.; Nattel, S. Sex differences in cardiac electrophysiology and clinical arrhythmias: Epidemiology, therapeutics, and mechanisms. Can. J. Cardiol. 2014, 30, 783–792. [Google Scholar] [CrossRef]
- Vicente, J.; Johannesen, L.; Galeotti, L.; Strauss, D.G. Mechanisms of sex and age differences in ventricular repolarization in humans. Am. Heart J. 2014, 168, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, J.; Trépanier-Boulay, V.; Fiset, C. Effect of androgen deficiency on mouse ventricular repolarization. J. Physiol. 2003, 546, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Brouillette, J.; Rivard, K.; Lizotte, E.; Fiset, C. Sex and strain differences in adult mouse cardiac repolarization: Importance of androgens. Cardiovasc. Res. 2005, 65, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Ciobotaru, A.; Bopassa, J.C.; Toro, L.; Stefani, E.; Eghbali, M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circ. Res. 2009, 105, 343–352. [Google Scholar] [CrossRef] [PubMed]
- El Gebeily, G.; El Khoury, N.; Mathieu, S.; Brouillette, J.; Fiset, C. Estrogen regulation of the transient outward K+ current involves estrogen receptor α in mouse heart. J. Mol. Cell. Cardiol. 2015, 86, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Arathimos, R.; Sharp, G.C.; Granell, R.; Tilling, K.; Relton, C.L. Associations of sex hormone-binding globulin and testosterone with genome-wide DNA methylation. BMC Genet. 2018, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Kovács, T.; Szabó-Meleg, E.; Ábrahám, I.M. Estradiol-Induced Epigenetically Mediated Mechanisms and Regulation of Gene Expression. Int. J. Mol. Sci. 2020, 21, 3177. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.; Bretherton, I.; Pang, K.; Mansell, T.; Czajko, A.; Kim, B.; Vlahos, A.; Zajac, J.D.; Saffery, R.; Cheung, A.; et al. Gender-affirming hormone therapy induces specific DNA methylation changes in blood. Clin. Epigenet. 2022, 14, 24. [Google Scholar] [CrossRef]
- Shin, H.S.; Shin, H.H.; Shudo, Y. Current Status and Limitations of Myocardial Infarction Large Animal Models in Cardiovascular Translational Research. Front. Bioeng. Biotechnol. 2021, 9, 673683. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.W.; Janssen, P.M.L. The Case for, and Challenges of, Human Cardiac Tissue in Advancing Phosphoprotein Research. Front. Physiol. 2022, 13, 853511. [Google Scholar] [CrossRef]
- Matsa, E.; Burridge, P.W.; Yu, K.-H.; Ahrens, J.H.; Termglinchan, V.; Wu, H.; Liu, C.; Shukla, P.; Sayed, N.; Churko, J.M.; et al. Transcriptome Profiling of Patient-Specific Human iPSC-Cardiomyocytes Predicts Individual Drug Safety and Efficacy Responses In Vitro. Cell Stem Cell 2016, 19, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Hnatiuk, A.P.; Briganti, F.; Staudt, D.W.; Mercola, M. Human iPSC modeling of heart disease for drug development. Cell Chem. Biol. 2021, 28, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Buikema, J.W.; Lee, S.; Goodyer, W.R.; Maas, R.G.; Chirikian, O.; Li, G.; Miao, Y.; Paige, S.L.; Lee, D.; Wu, H.; et al. Wnt Activation and Reduced Cell-Cell Contact Synergistically Induce Massive Expansion of Functional Human iPSC-Derived Cardiomyocytes. Cell Stem Cell 2020, 27, 50–63.e5. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hua, Y.; Miyagawa, S.; Zhang, J.; Li, L.; Liu, L.; Sawa, Y. hiPSC-Derived Cardiac Tissue for Disease Modeling and Drug Discovery. Int. J. Mol. Sci. 2020, 21, 8893. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Bao, X.; Wang, Y.; Lu, T.; Zhang, L. Human induced pluripotent stem cell (hiPSC)-derived cardiomyocyte modelling of cardiovascular diseases for natural compound discovery. Biomed. Pharmacother. 2023, 157, 113970. [Google Scholar] [CrossRef] [PubMed]
- Deicher, A.; Seeger, T. Human Induced Pluripotent Stem Cells as a Disease Model System for Heart Failure. Curr. Heart Fail. Rep. 2021, 18, 1–11. [Google Scholar] [CrossRef]
- Reilly, L.; Munawar, S.; Zhang, J.; Crone, W.C.; Eckhardt, L.L. Challenges and innovation: Disease modeling using human-induced pluripotent stem cell-derived cardiomyocytes. Front. Cardiovasc. Med. 2022, 9, 966094. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, J.; Clouse, H.; Lagrutta, A.; Sannajust, F. HiPSC-CMs from different sex and ethnic origin donors exhibit qualitatively different responses to several classes of pharmacological challenges. J. Pharmacol. Toxicol. Methods 2019, 99, 106598. [Google Scholar] [CrossRef]
- Costa, S.; Saguner, A.M.; Gasperetti, A.; Akdis, D.; Brunckhorst, C.; Duru, F. The Link Between Sex Hormones and Susceptibility to Cardiac Arrhythmias: From Molecular Basis to Clinical Implications. Front. Cardiovasc. Med. 2021, 8, 644279. [Google Scholar] [CrossRef]
- Huo, J.; Wei, F.; Cai, C.; Lyn-Cook, B.; Pang, L. Sex-Related Differences in Drug-Induced QT Prolongation and Torsades de Pointes: A New Model System with Human iPSC-CMs. Toxicol. Sci. Off. J. Soc. Toxicol. 2019, 167, 360–374. [Google Scholar] [CrossRef]
- Salem, J.-E.; Yang, T.; Moslehi, J.J.; Waintraub, X.; Gandjbakhch, E.; Bachelot, A.; Hidden-Lucet, F.; Hulot, J.-S.; Knollmann, B.C.; Lebrun-Vignes, B.; et al. Androgenic Effects on Ventricular Repolarization: A Translational Study from the International Pharmacovigilance Database to iPSC-Cardiomyocytes. Circulation 2019, 140, 1070–1080. [Google Scholar] [CrossRef]
- El Khoury, N.; Ross, J.L.; Long, V.; Thibault, S.; Ethier, N.; Fiset, C. Pregnancy and oestrogen regulate sinoatrial node calcium homeostasis and accelerate pacemaking. Cardiovasc. Res. 2018, 114, 1605–1616. [Google Scholar] [CrossRef]
- Akdis, D.; Saguner, A.M.; Shah, K.; Wei, C.; Medeiros-Domingo, A.; von Eckardstein, A.; Lüscher, T.F.; Brunckhorst, C.; Chen, H.S.V.; Duru, F. Sex hormones affect outcome in arrhythmogenic right ventricular cardiomyopathy/dysplasia: From a stem cell derived cardiomyocyte-based model to clinical biomarkers of disease outcome. Eur. Heart J. 2017, 38, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhang, H.; Ong’achwa Machuki, J.; Zhang, T.; Han, L.; Sang, L.; Wu, L.; Zhao, Z.; James Turley, M.; Hu, X.; et al. GPER mediates estrogen cardioprotection against epinephrine-induced stress. J. Endocrinol. 2021, 249, 209–222. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Zhao, Z.; Lan, H.; Schünemann, J.-D.; Sattler, K.; Buljubasic, F.; Patocskai, B.; Li, X.; Yücel, G.; Lang, S.; et al. Estradiol protection against toxic effects of catecholamine on electrical properties in human-induced pluripotent stem cell derived cardiomyocytes. Int. J. Cardiol. 2018, 254, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Discher, D.E.; Leong, K.W.; Vunjak-Novakovic, G.; Wu, J.C. Challenges and opportunities for the next generation of cardiovascular tissue engineering. Nat. Methods 2022, 19, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Gehmlich, K.; Denning, C.; Pavlovic, D. Complex Relationship between Cardiac Fibroblasts and Cardiomyocytes in Health and Disease. J. Am. Heart Assoc. 2021, 10, e019338. [Google Scholar] [CrossRef]
- da Rocha, A.M.; Campbell, K.; Mironov, S.; Jiang, J.; Mundada, L.; Guerrero-Serna, G.; Jalife, J.; Herron, T.J. hiPSC-CM Monolayer Maturation State Determines Drug Responsiveness in High Throughput Pro-Arrhythmia Screen. Sci. Rep. 2017, 7, 13834. [Google Scholar] [CrossRef] [PubMed]
- Sarikhani, M.; Garbern, J.C.; Ma, S.; Sereda, R.; Conde, J.; Krähenbühl, G.; Escalante, G.O.; Ahmed, A.; Buenrostro, J.D.; Lee, R.T. Sustained Activation of AMPK Enhances Differentiation of Human iPSC-Derived Cardiomyocytes via Sirtuin Activation. Stem Cell Rep. 2020, 15, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Garbern, J.C.; Helman, A.; Sereda, R.; Sarikhani, M.; Ahmed, A.; Escalante, G.O.; Ogurlu, R.; Kim, S.L.; Zimmerman, J.F.; Cho, A.; et al. Inhibition of mTOR Signaling Enhances Maturation of Cardiomyocytes Derived from Human-Induced Pluripotent Stem Cells via p53-Induced Quiescence. Circulation 2020, 141, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Garbern, J.C.; Liu, R.; Li, Q.; Mancheño Juncosa, E.; Elwell, H.L.T.; Sokol, M.; Aoyama, J.; Deumer, U.-S.; Hsiao, E.; et al. Tissue-embedded stretchable nanoelectronics reveal endothelial cell-mediated electrical maturation of human 3D cardiac microtissues. Sci. Adv. 2023, 9, eade8513. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.A.; Janbandhu, V.; Maatz, H.; Kanemaru, K.; Cranley, J.; Teichmann, S.A.; Hübner, N.; Schneider, M.D.; Harvey, R.P.; Noseda, M. Single-cell transcriptomics for the assessment of cardiac disease. Nat. Rev. Cardiol. 2023, 20, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.B.; Phipson, B.; Ziemann, M.; Rafehi, H.; Mills, R.J.; Watt, K.I.; Abu-Bonsrah, K.D.; Kalathur, R.K.R.; Voges, H.K.; Dinh, D.T.; et al. Sex-Specific Control of Human Heart Maturation by the Progesterone Receptor. Circulation 2021, 143, 1614–1628. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.A.; Squiers, G.T.; McLellan, M.A.; Bolisetty, M.T.; Robson, P.; Rosenthal, N.A.; Pinto, A.R. Single-Cell Transcriptional Profiling Reveals Cellular Diversity and Intercommunication in the Mouse Heart. Cell Rep. 2018, 22, 600–610. [Google Scholar] [CrossRef] [PubMed]
- McLellan, M.A.; Skelly, D.A.; Dona, M.S.I.; Squiers, G.T.; Farrugia, G.E.; Gaynor, T.L.; Cohen, C.D.; Pandey, R.; Diep, H.; Vinh, A.; et al. High-Resolution Transcriptomic Profiling of the Heart during Chronic Stress Reveals Cellular Drivers of Cardiac Fibrosis and Hypertrophy. Circulation 2020, 142, 1448–1463. [Google Scholar] [CrossRef]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Vaura, F.; Palmu, J.; Aittokallio, J.; Kauko, A.; Niiranen, T. Genetic, Molecular, and Cellular Determinants of Sex-Specific Cardiovascular Traits. Circ. Res. 2022, 130, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Meder, B.; Haas, J.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Frese, K.; Lai, A.; Nietsch, R.; Scheiner, C.; Mester, S.; Bordalo, D.M.; et al. Epigenome-Wide Association Study Identifies Cardiac Gene Patterning and a Novel Class of Biomarkers for Heart Failure. Circulation 2017, 136, 1528–1544. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Binek, A.; Parker, S.J.; Shah, S.H.; Zanni, M.V.; Van Eyk, J.E.; Ho, J.E. Sexual Dimorphism in Cardiovascular Biomarkers: Clinical and Research Implications. Circ. Res. 2022, 130, 578–592. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Safabakhsh, S.; Palumbo, A.; Fiset, C.; Shen, C.; Parker, J.; Foster, L.J.; Laksman, Z. Sex-Based Mechanisms of Cardiac Development and Function: Applications for Induced-Pluripotent Stem Cell Derived-Cardiomyocytes. Int. J. Mol. Sci. 2024, 25, 5964. https://doi.org/10.3390/ijms25115964
Luo Y, Safabakhsh S, Palumbo A, Fiset C, Shen C, Parker J, Foster LJ, Laksman Z. Sex-Based Mechanisms of Cardiac Development and Function: Applications for Induced-Pluripotent Stem Cell Derived-Cardiomyocytes. International Journal of Molecular Sciences. 2024; 25(11):5964. https://doi.org/10.3390/ijms25115964
Chicago/Turabian StyleLuo, Yinhan, Sina Safabakhsh, Alessia Palumbo, Céline Fiset, Carol Shen, Jeremy Parker, Leonard J. Foster, and Zachary Laksman. 2024. "Sex-Based Mechanisms of Cardiac Development and Function: Applications for Induced-Pluripotent Stem Cell Derived-Cardiomyocytes" International Journal of Molecular Sciences 25, no. 11: 5964. https://doi.org/10.3390/ijms25115964
APA StyleLuo, Y., Safabakhsh, S., Palumbo, A., Fiset, C., Shen, C., Parker, J., Foster, L. J., & Laksman, Z. (2024). Sex-Based Mechanisms of Cardiac Development and Function: Applications for Induced-Pluripotent Stem Cell Derived-Cardiomyocytes. International Journal of Molecular Sciences, 25(11), 5964. https://doi.org/10.3390/ijms25115964





