Differential Mitochondrial Genome Expression of Four Hylid Frog Species under Low-Temperature Stress and Its Relationship with Amphibian Temperature Adaptation
Abstract
:1. Introduction
2. Results
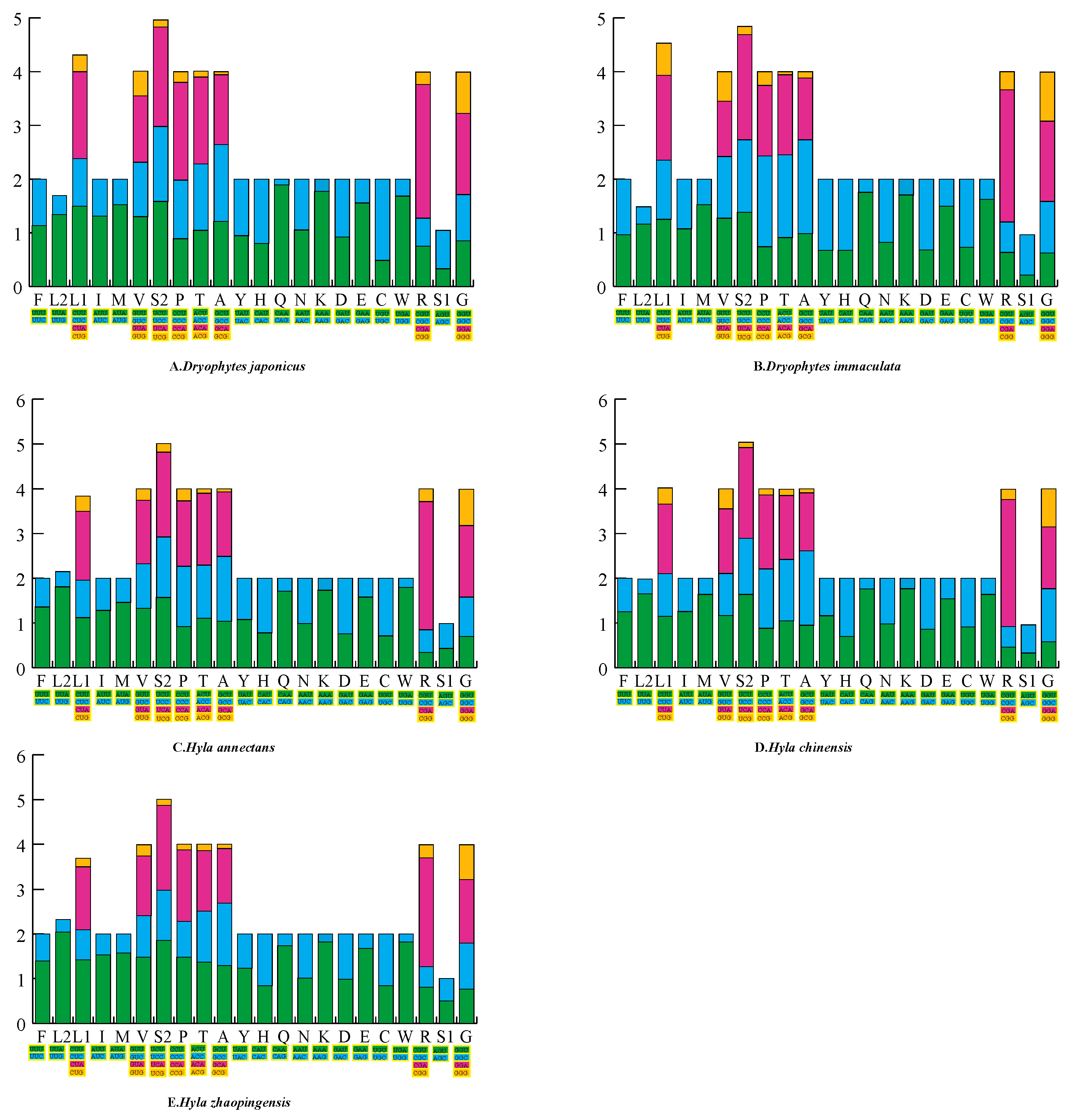
2.1. General Features of the Mitogenome
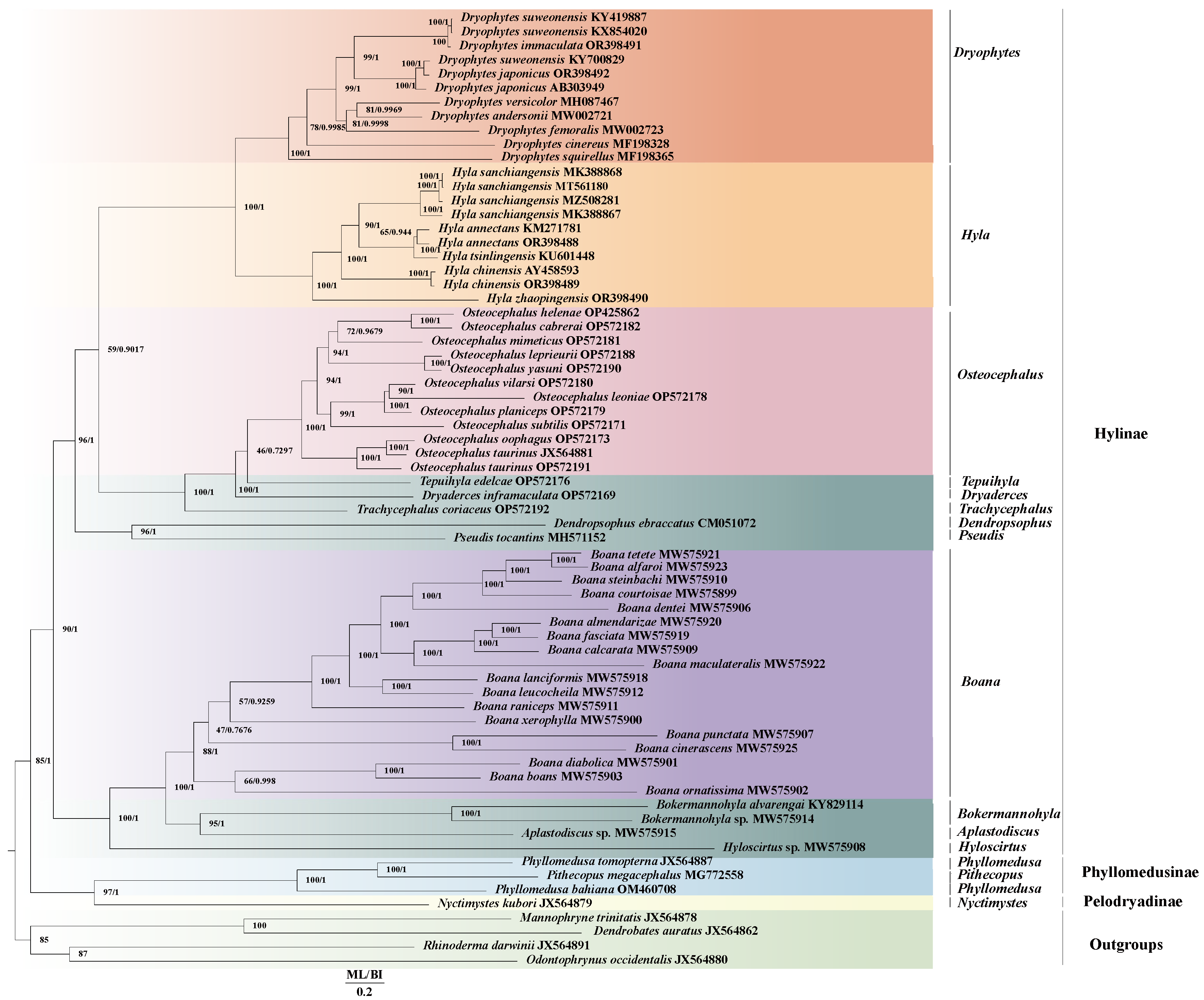
2.2. Genetic Distance and Phylogenetic Relationships
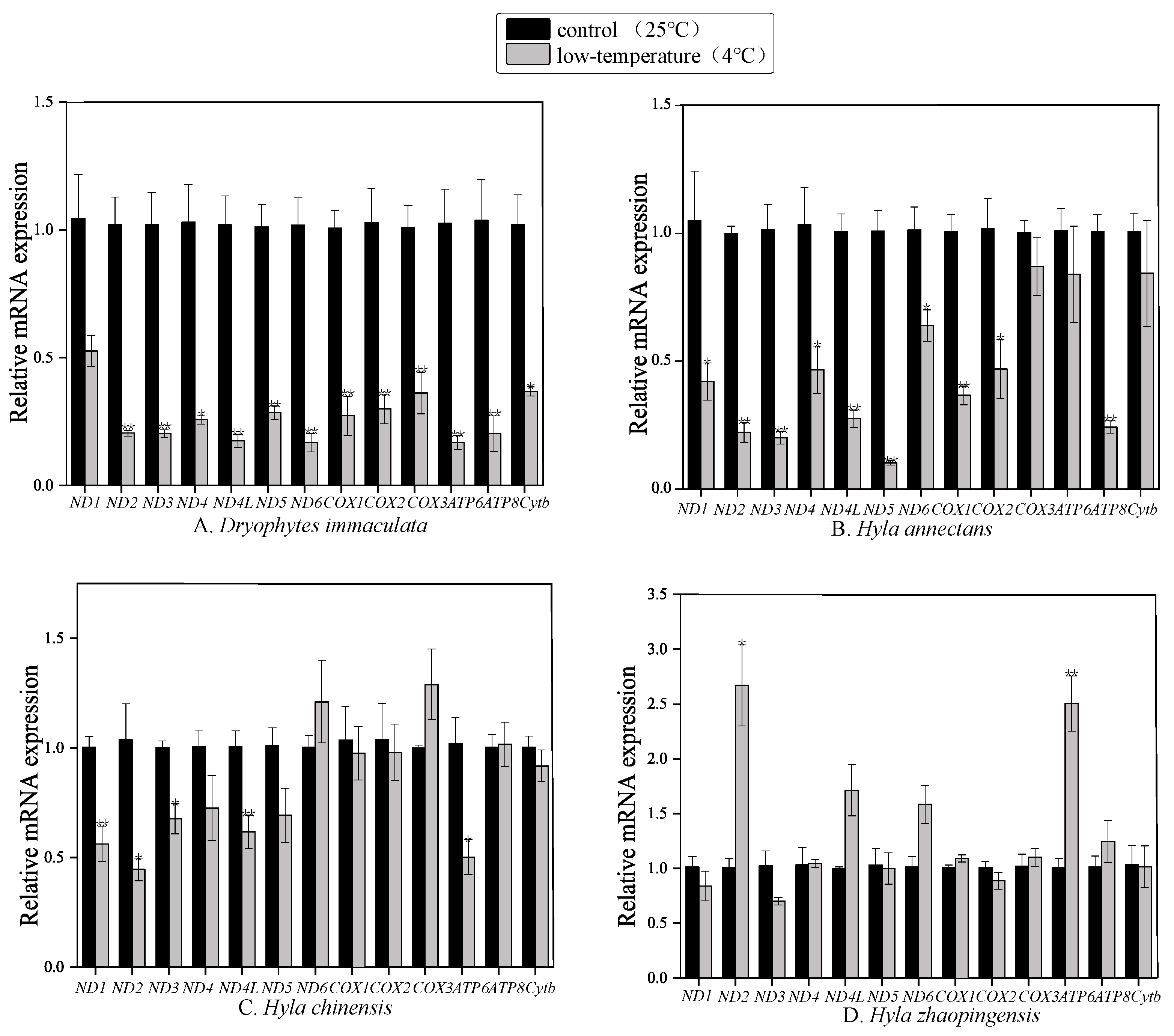
2.3. Effect of Cold Exposure on Transcript Levels of PCGs
3. Discussion
3.1. Mitogenome Structure, Genetic Distance and Phylogeny of Hylidae
3.2. Different mt Gene Expression between Different Species of Dryophytes and Hyla
3.3. The Relationship between Mitochondrial Gene Expression and Temperature Adaptation
4. Materials and Methods
4.1. Sample Collection and Cold-Stress Treatment
4.2. Total DNA Extraction, Primer Design, PCR Amplification and Sequencing
4.3. Mitogenome Annotation and Sequence Analyses
4.4. Genetic Distance and Phylogenetic Analyses
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantitative Primer Design and Relative mRNA Quantification
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faivovich, J. A new species of Scinax (Anura: Hylidae) from Misiones, Argentina. Herpetol. J. 2005, 61, 69–77. [Google Scholar] [CrossRef]
- Garda, A.A.; Cannatella, D.C. Phylogeny and biogeography of paradoxical frogs (Anura, Hylidae, Pseudae) inferred from 12S and 16S mitochondrial DNA. Mol. Phylogenetics Evol. 2007, 44, 104–114. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference, Version 6.2. Available online: https://amphibiansoftheworld.amnh.org (accessed on 20 June 2023).
- Amphibia China. The Database of Chinese Amphibians. Electronic Database. Available online: http://www.amphibiachina.org/ (accessed on 20 June 2023).
- Boettger, O. Aufzählung Einiger neu Erworbener Reptilien und Batrachier aus Ost-Asien; Bericht die Senckenbergische Naturforschende Gesellschaft in Frankfurt am Main: Franckfort, Germany, 1888; p. 189. [Google Scholar]
- Jerdon, T.C. Notes on Indian herpetology. Proc. Asiat. Soc. Bengal 1870, 1870, 66–85. [Google Scholar]
- Günther, A.C.L.G. Neue Batrachier in der Sammlung des britischen Museums. Arch. Für Naturgeschichte Berl. 1858, 24, 319–328. [Google Scholar] [CrossRef]
- Tang, Y.X.; Zhang, Z.J. A new species of amphibian from Guangxi. Acta Zootaxon. Sin. 1984, 9, 441–443. [Google Scholar]
- Suwon, C.; Eugene, C.; Inna, V.; Jong Ryol, C.; Hang, L.; Mi-Sook, M. Genetic diversity of Korean tree frog (Hyla suweonensis and Hyla japonica): Assessed by mitochondrial cytochrome b gene and cytochrome oxidase subunit I gene. Korean J. Herpetol. 2012, 4, 31–41. [Google Scholar]
- Park, S.; Jeong, G.; Jang, Y. No reproductive character displacement in male advertisement signals of Hyla japonica in relation to the sympatric H. suweonensis. Behav. Ecol. Sociobiol. 2013, 67, 1345–1355. [Google Scholar] [CrossRef]
- Li, J.T.; Wang, J.S.; Nian, H.H.; Litvinchuk, S.N.; Wang, J.; Li, Y.; Rao, D.Q.; Klaus, S. Amphibians crossing the Bering Land Bridge: Evidence from holarctic treefrogs (Hyla, Hylidae, Anura). Mol. Phylogenetics Evol. 2015, 87, 80–90. [Google Scholar] [CrossRef]
- Dufresnes, C.; Litvinchuk, S.N.; Borzee, A.; Jang, Y.; Li, J.T.; Miura, I.; Perrin, N.; Stock, M. Phylogeography reveals an ancient cryptic radiation in East-Asian tree frogs (Hyla japonica group) and complex relationships between continental and island lineages. BMC Evol. Biol. 2016, 16, 253. [Google Scholar] [CrossRef]
- Borzée, A.; Kong, S.; Didinger, C.; Nguyen, H.Q.; Jang, Y. A ring-species or a ring of species? Phylogenetic relationship between two treefrog species around the Yellow Sea: Dryophytes suweonensis and D. immaculatus. Herpetol. J. 2018, 28, 160–170. [Google Scholar]
- Borzee, A.; Messenger, K.R.; Chae, S.; Andersen, D.; Groffen, J.; Kim, Y.I.; An, J.; Othman, S.N.; Ri, K.; Nam, T.Y.; et al. Yellow sea mediated segregation between North East Asian Dryophytes species. PLoS ONE 2020, 15, e0234299. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Karoly, D.; Vicarelli, M.; Neofotis, P.; Wu, Q.; Casassa, G.; Menzel, A.; Root, T.L.; Estrella, N.; Seguin, B. Attributing physical and biological impacts to anthropogenic climate change. Nature 2008, 453, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J. Global warming. Rep. Prog. Phys. 2005, 68, 1343–1403. [Google Scholar] [CrossRef]
- Yousefkhani, S.S.H.; Yasser, A.; Naser, M.; Yousefabadi, F.; Gasimova, G. Response to global warming of Eichwald’s toad, Bufo eichwaldi Litvinchuk, Borkin, Skorinov and Rosanov, 2008 (Anura; Amphibia) in Iran and Azerbaijan. Folia Biol. 2022, 70, 119–125. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Raffel, T.R.; Romansic, J.M.; Halstead, N.T.; McMahon, T.A.; Venesky, M.D.; Rohr, J.R. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 2013, 3, 146–151. [Google Scholar] [CrossRef]
- Catenazzi, A. State of the world’s amphibians. Annu. Rev. Env. Resour. 2015, 40, 91–119. [Google Scholar] [CrossRef]
- Carey, C.; Alexander, M.A. Climate change and amphibian declines: Is there a link? Divers. Distrib. 2003, 9, 111–121. [Google Scholar] [CrossRef]
- Becker, C.G.; Fonseca, C.R.; Haddad, C.F.B.; Batista, R.F.; Prado, P.I. Habitat split and the global decline of amphibians. Science 2007, 318, 1775–1777. [Google Scholar] [CrossRef] [PubMed]
- Mosavi, J.; Vaissi, S.; Dastansara, N.; Sharifi, M. Effects of temperature on growth, development and survival in larvae of Pelophylax ridibundus (Pallas, 1771) (Amphibia: Anura): Linking global warming to amphibian development. Acta Zool. Bulg. 2017, 69, 541–546. [Google Scholar]
- Ron, S.R.; Duellman, W.E.; Coloma, L.A.; Bustamante, M.R. Population decline of the Jambato toad Atelopus ignescens (Anura: Bufonidae) in the Andes of Ecuador. J. Herpetol. 2003, 37, 116–126. [Google Scholar] [CrossRef]
- D’Amen, M.; Bombi, P. Global warming and biodiversity: Evidence of climate-linked amphibian declines in Italy. Biol. Conserv. 2009, 142, 3060–3067. [Google Scholar] [CrossRef]
- Zeisset, I.; Beebee, T. Amphibian phylogeography: A model for understanding historical aspects of species distributions. Heredity 2008, 101, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Lannoo, M. Amphibian Declines: The Conservation Status of United States Species; University California Press: Berkeley, CA, USA, 2005; pp. 915–925. [Google Scholar]
- Adlassnig, W.; Schmidt, B.; Jirsa, F.; Gradwohl, A.; Ivesic, C.; Koller-Peroutka, M. The Arsenic–Antimony Creek at Sauerbrunn/Burgenland, Austria: A toxic habitat for amphibians. Int. J. Environ. Res. Public Health 2022, 19, 6010. [Google Scholar] [CrossRef] [PubMed]
- Aragón, P.; Rodríguez, M.; Olalla-Tárraga, M.; Lobo, J. Predicted impact of climate change on threatened terrestrial vertebrates in central Spain highlights differences between endotherms and ectotherms. Anim. Conserv. 2010, 13, 363–373. [Google Scholar] [CrossRef]
- Piha, H.; Luoto, M.; Piha, M.; Merilä, J. Anuran abundance and persistence in agricultural landscapes during a climatic extreme. Glob. Change Biol. 2007, 13, 300–311. [Google Scholar] [CrossRef]
- Girardello, M.; Griggio, M.; Whittingham, M.J.; Rushton, S.P. Models of climate associations and distributions of amphibians in Italy. Ecol. Res. 2010, 25, 103–111. [Google Scholar] [CrossRef]
- Dastansara, N.; Vaissi, S.; Mosavi, J.; Sharifi, M. Impacts of temperature on growth, development and survival of larval Bufo (Pseudepidalea) viridis (Amphibia: Anura): Implications of climate change. Zool. Ecol. 2017, 27, 228–234. [Google Scholar] [CrossRef]
- Costanzo, J.P.; Lee Jr, R.E. Cryoprotection by urea in a terrestrially hibernating frog. J. Exp. Biol. 2005, 208, 4079–4089. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.T.; Lee, J.R.E. Geographic variation in energy storage and physiological responses to freezing in the gray treefrogs Hyla versicolor and H. chrysoscelis. J. Exp. Biol. 2003, 206, 2859–2867. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Molecular physiology of freeze tolerance in vertebrates. Physiol. Rev. 2017, 97, 623–665. [Google Scholar] [CrossRef] [PubMed]
- Biggar, K.K.; Dubuc, A.; Storey, K.B. MicroRNA regulation below zero: Differential expression of miRNA-21 and miRNA-16 during freezing in wood frogs. Cryobiology 2009, 59, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.J.; Biggar, K.K.; Storey, K.B. Transcript expression of the freeze responsive gene fr10 in Rana sylvatica during freezing, anoxia, dehydration, and development. Mol. Cell. Biochem. 2015, 399, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.M.; Wu, S.; Storey, K.B. Mitochondria and the frozen frog. Antioxidants 2021, 10, 543. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.I.; Bulakhova, N.A.; Meshcheryakova, E.N. The Siberian wood frog survives for months underwater without oxygen. Sci. Rep. 2019, 9, 13594. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Storey, K.B. Freeze-induced suppression of pyruvate kinase in liver of the wood frog (Rana sylvatica). Adv. Biol. Regul. 2022, 88, 100944. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Storey, K.B. Hepatic citrate synthase suppression in the freeze-tolerant wood frog (Rana sylvatica). Int. J. Biol. Macromol. 2023, 242, 124718. [Google Scholar] [CrossRef]
- Biggar, K.K.; Storey, K.B. The emerging roles of microRNAs in the molecular responses of metabolic rate depression. J. Mol. Cell Biol. 2010, 3, 167–175. [Google Scholar] [CrossRef]
- Trzcionka, M.; Withers, K.W.; Klingenspor, M.; Jastroch, M. The effects of fasting and cold exposure on metabolic rate and mitochondrial proton leak in liver and skeletal muscle of an amphibian, the cane toad Bufo marinus. J. Exp. Biol. 2008, 211, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sainz, A.G.; Shadel, G.S. Mitochondrial DNA: Cellular genotoxic stress sentinel. Trends Biochem. Sci. 2021, 46, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Roe, B.A.; Ma, D.; Wilson, R.; Wong, J. The complete nucleotide sequence of the Xenopus laevis mitochondrial genome. J. Biol. Chem. 1985, 260, 9759–9774. [Google Scholar] [CrossRef] [PubMed]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.B.; Wang, Y.Q.; Zhou, K.Y.; Zhu, W.Q.; Nie, J.S.; Wang, C.L. Complete mitochondrial DNA sequence of Chinese alligator, Alligator sinensis, and phylogeny of crocodiles. Chin. Sci. Bull. 2003, 48, 2050–2054. [Google Scholar] [CrossRef]
- Sano, N.; Kurabayashi, A.; Fujii, T.; Yonekawa, H.; Sumida, M. Complete nucleotide sequence and gene rearrangement of the mitochondrial genome of the bell-ring frog, Buergeria buergeri (Family Rhacophoridae). Genes Genet. Syst. 2004, 79, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Zhang, L.P.; Yu, D.N.; Storey, K.B.; Zheng, R.Q. Complete mitochondrial genomes of Nanorana taihangnica and N. yunnanensis (Anura: Dicroglossidae) with novel gene arrangements and phylogenetic relationship of Dicroglossidae. BMC Evol. Biol. 2018, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Kurabayashi, A.; Usuki, C.; Mikami, N.; Fujii, T.; Yonekawa, H.; Sumida, M.; Hasegawa, M. Complete nucleotide sequence of the mitochondrial genome of a Malagasy poison frog Mantella madagascariensis: Evolutionary implications on mitochondrial genomes of higher anuran groups. Mol. Phylogenetics Evol. 2006, 39, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Kurabayashi, A.; Yoshikawa, N.; Sato, N.; Hayashi, Y.; Oumi, S.; Fujii, T.; Sumida, M. Complete mitochondrial DNA sequence of the endangered frog Odorrana ishikawae (family Ranidae) and unexpected diversity of mt gene arrangements in ranids. Mol. Phylogenetics Evol. 2010, 56, 543–553. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Luu, B.E.; Yu, D.N.; Zhang, L.P.; Al-Attar, R.; Storey, K.B. The complete mitochondrial genome of Dryophytes versicolor: Phylogenetic relationship among Hylidae and mitochondrial protein-coding gene expression in response to freezing and anoxia. Int. J. Biol. Macromol. 2019, 132, 461–469. [Google Scholar] [CrossRef]
- Jin, W.T.; Guan, J.Y.; Dai, X.Y.; Wu, G.J.; Zhang, L.P.; Storey, K.B.; Zhang, J.Y.; Zheng, R.Q.; Yu, D.N. Mitochondrial gene expression in different organs of Hoplobatrachus rugulosus from China and Thailand under low-temperature stress. BMC Zool. 2022, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Graham, C.H.; Moen, D.S.; Smith, S.A.; Reeder, T.W. Evolutionary and ecological causes of the latitudinal diversity gradient in hylid frogs: Treefrog trees unearth the roots of high tropical diversity. Am. Nat. 2006, 168, 579–596. [Google Scholar] [CrossRef]
- Yan, P.; Pan, T.; Wu, G.Y.; Kang, X.; Ali, I.; Zhou, W.L.; Li, J.T.; Wu, X.B.; Zhang, B.W. Species Delimitation and Evolutionary History of Tree Frogs in the Hyla chinensis Group (Hylidae, Amphibian). Front. Ecol. Evol. 2020, 8, 234. [Google Scholar] [CrossRef]
- Duellman, W.E.; Marion, A.B.; Hedges, S.B. Phylogenetics, classification, and biogeography of the treefrogs (Amphibia: Anura: Arboranae). Zootaxa 2016, 4104, 1–109. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.Y.; Guo, J.L.; Geng, X.F.; Li, P.F.; Sun, J.Y.; Wang, Q.W.; Xu, C.S. Proteome analysis of the liver in the Chinese fire-bellied newt Cynops orientalis. Genet. Mol. Res. 2016, 15, 15037993. [Google Scholar] [CrossRef] [PubMed]
- Borzee, A.; Didinger, C.; Jang, Y. Complete mitochondrial genome of Dryophytes suweonensis (Anura Hylidae). Mitochondrial DNA B Resour. 2017, 2, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Jeon, H.S.; Min, M.; An, J. Sequencing and analysis of the complete mitochondrial genome of Hyla suweonensis (Anura: Hylidae). Mitochondrial DNA B 2017, 2, 126–127. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Kurabayashi, A.; Usuki, C.; Fujii, T.; Sumida, M. Complete mitochondrial genomes of three neobatrachian anurans: A case study of divergence time estimation using different data and calibration settings. Gene 2008, 407, 116–129. [Google Scholar] [CrossRef]
- Ye, L.T.; Zhu, C.C.; Yu, D.N.; Zhang, Y.P.; Zhang, J.Y. The complete mitochondrial genome of Hyla annectans (Anura: Hylidae). Mitochondrial DNA A 2016, 27, 1593–1594. [Google Scholar] [CrossRef]
- Kang, X.; Sun, Z.L.; Guo, W.B.; Wu, J.; Qian, L.F.; Pan, T.; Wang, H.; Li, K.; Zhang, B.W. Sequencing of complete mitochondrial genome for Tsinling Tree Toad (Hyla tsinlingensis). Mitochondrial DNA B 2016, 1, 466–467. [Google Scholar] [CrossRef]
- Hong, Y.H.; Huang, H.M.; Wu, L.; Storey, K.B.; Zhang, J.Y.; Zhang, Y.P.; Yu, D.N. Characterization of two mitogenomes of Hyla sanchiangensis (Anura: Hylidae), with phylogenetic relationships and selection pressure analyses of Hylidae. Animals 2023, 13, 1593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.E.; Lin, Y.F.; Ma, L.; Ding, G.H.; Lin, Z.H. Partial mitochondrial genome of the Sanchiang Tree Toad Hyla sanchiangensis (Anura: Hylidae). Mitochondrial DNA B 2020, 5, 2682–2683. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, H.; Chen, Y.Q.; Liu, Y.F.; Qu, L.H. Mitogenomic perspectives on the origin and phylogeny of living amphibians. Syst. Biol. 2005, 54, 391–400. [Google Scholar] [CrossRef]
- Ortiz, D.A.; Hoskin, C.J.; Werneck, F.P.; Réjaud, A.; Manzi, S.; Ron, S.R.; Fouquet, A. Historical biogeography highlights the role of Miocene landscape changes on the diversification of a clade of Amazonian tree frogs. Org. Divers. Evol. 2022, 23, 395–414. [Google Scholar] [CrossRef]
- Alexander, R.P.; Fang, G.; Rozowsky, J.; Snyder, M.; Gerstein, M.B. Annotating non-coding regions of the genome. Nat. Rev. Genet. 2010, 11, 559–571. [Google Scholar] [CrossRef]
- Somero, G.N. Linking biogeography to physiology: Evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2005, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Tribute to P. L. Lutz: Putting life on ‘pause’–molecular regulation of hypometabolism. J. Exp. Biol. 2007, 210, 1700–1714. [Google Scholar] [CrossRef] [PubMed]
- Saraste, M. Oxidative phosphorylation at the fin de siecle. Science 1999, 283, 1488–1493. [Google Scholar] [CrossRef]
- Lenaz, G.; Fato, R.; Genova, M.L.; Bergamini, C.; Bianchi, C.; Biondi, A. Mitochondrial complex I: Structural and functional aspects. Biochim. Biophys. Acta 2006, 1757, 1406–1420. [Google Scholar] [CrossRef]
- Kadenbach, B. Complex IV-the regulatory center of mitochondrial oxidative phosphorylation. Mitochondrion 2021, 58, 296–302. [Google Scholar] [CrossRef]
- Junge, W.; Nelson, N. ATP synthase. Annu. Rev. Biochem. 2015, 84, 631–657. [Google Scholar] [CrossRef]
- Barel, O.; Shorer, Z.; Flusser, H.; Ofir, R.; Narkis, G.; Finer, G.; Shalev, H.; Nasasra, A.; Saada, A.; Birk, O.S. Mitochondrial complex III deficiency associated with a homozygous mutation in UQCRQ. Am. J. Hum. Genet. 2008, 82, 1211–1216. [Google Scholar] [CrossRef]
- Okajima, Y.; Kumazawa, Y. Mitochondrial genomes of acrodont lizards: Timing of gene rearrangements and phylogenetic and biogeographic implications. BMC Evol. Biol. 2010, 10, 141. [Google Scholar] [CrossRef]
- Nitzan, T.; Slosman, T.; Gutkovich, D.; Weller, J.I.; Hulata, G.; Zak, T.; Benet, A.; Cnaani, A. Maternal effects in the inheritance of cold tolerance in blue tilapia (Oreochromis aureus). Environ. Biol. Fish. 2016, 99, 975–981. [Google Scholar] [CrossRef]
- Bozinovic, F.; Calosi, P.; Spicer, J.I. Physiological correlates of geographic range in animals. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 155–179. [Google Scholar] [CrossRef]
- Gaston, K.J.; Chown, S.L.; Calosi, P.; Bernardo, J.; Bilton, D.T.; Clarke, A.; Clusella-Trullas, S.; Ghalambor, C.K.; Konarzewski, M.; Peck, L.S.; et al. Macrophysiology: A conceptual reunification. Am. Nat. 2009, 174, 595–612. [Google Scholar] [CrossRef] [PubMed]
- Ghalambor, C.K.; Huey, R.B.; Martin, P.R.; Tewksbury, J.J.; Wang, G. Are mountain passes higher in the tropics? Janzen’s hypothesis revisited. Integr. Comp. Biol. 2006, 46, 5–17. [Google Scholar] [CrossRef]
- Janzen, D.H. Why mountain passes are high in the tropics. Am. Nat. 1967, 101, 233–249. [Google Scholar] [CrossRef]
- Sun, B.J.; Williams, C.M.; Li, T.; Speakman, J.R.; Jin, Z.G.; Lu, H.L.; Luo, L.G.; Du, W.G. Higher metabolic plasticity in temperate compared to tropical lizards suggests increased resilience to climate change. Ecol. Monogr. 2022, 92, e1512. [Google Scholar] [CrossRef]
- Somero, G.N. The physiology of global change: Linking patterns to mechanisms. Ann. Rev. Mar. Sci. 2012, 4, 39–61. [Google Scholar] [CrossRef]
- Hadj-Moussa, H.; Storey, K.B. Micromanaging freeze tolerance: The biogenesis and regulation of neuroprotective microRNAs in frozen brains. Cell. Mol. Life Sci. 2018, 75, 3635–3647. [Google Scholar] [CrossRef] [PubMed]
- Consuegra, S.; John, E.; Verspoor, E.; de Leaniz, C.G. Patterns of natural selection acting on the mitochondrial genome of a locally adapted fish species. Genet. Sel. Evol. 2015, 47, 58. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Y.; Shen, S.Q.; Lu, L.X.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. The complete mitochondrial genome of Pyxicephalus adspersus: High gene rearrangement and phylogenetics of one of the world’s largest frogs. PeerJ 2019, 7, e7532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liang, D.; Mao, R.L.; Hillis, D.M.; Wake, D.B.; Cannatella, D.C. Efficient sequencing of anuran mtDNAs and a mitogenomic exploration of the phylogeny and evolution of frogs. Mol. Biol. Evol. 2013, 30, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Genetic distance between populations. Am. Nat. 1972, 106, 283–292. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, G.H.; Tan, M.Y.; Zeng, D.L.; Wang, K.Z.; Yuan, Z.G.; Lin, R.Q.; Zhu, X.Q.; Liu, Y. Survey of Spirometra erinaceieuropaei spargana infection in the frog Rana nigromaculata of the Hunan Province of China. Vet. Parasitol. 2010, 173, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Bioinf. Methods Protoc. 2000, 132, 71–91. [Google Scholar]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenetics Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neuböck, R.; Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Rescour. 2020, 20, 348–355. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Xie, Z. DAMBE: Software package for data analysis in molecular biology and evolution. J. Hered. 2001, 92, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.D.A. Figtree Version 1.4.4. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 June 2023).
- Aguilar, O.A.; Hadj-Moussa, H.; Storey, K.B. Freeze-responsive regulation of MEF2 proteins and downstream gene networks in muscles of the wood frog, Rana sylvatica. J. Therm. Biol. 2017, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guide, R. Applied Biosystems StepOne™ and StepOnePlus™. Appl. Biosyst. 2010, 6, 4379704. [Google Scholar]
- Brosius, F. SPSS 21 [M]; MITP-Verlags GmbH & Co. KG: Frechen, Germany, 2013. [Google Scholar]
- Du, C.C.; Li, X.Y.; Wang, H.X.; Liang, K.; Wang, H.Y.; Zhang, Y.H. Identification of thyroid hormone receptors αand β genes and their expression profiles during metamorphosis in Rana chensinensis. Turk. J. Zool. 2017, 41, 454–463. [Google Scholar] [CrossRef]
- Barrow, L.N.; Soto-Centeno, J.A.; Warwick, A.R.; Lemmon, A.R.; Moriarty Lemmon, E. Evaluating hypotheses of expansion from refugia through comparative phylogeography of south-eastern Coastal Plain amphibians. J. Biogeogr. 2017, 44, 2692–2705. [Google Scholar] [CrossRef]
- Warwick, A.R.; Barrow, L.N.; Smith, M.L.; Means, D.B.; Lemmon, A.R.; Lemmon, E.M. Signatures of north-eastern expansion and multiple refugia: Genomic phylogeography of the Pine Barrens tree frog, Hyla andersonii (Anura: Hylidae). Biol. J. Linn. Soc. 2021, 133, 120–134. [Google Scholar] [CrossRef]
- Fouquet, A.; Marinho, P.; Réjaud, A.; Carvalho, T.R.; Caminer, M.A.; Jansen, M.; Rainha, R.N.; Rodrigues, M.T.; Werneck, F.P.; Lima, A.P. Systematics and biogeography of the Boana albopunctata species group (Anura, Hylidae), with the description of two new species from Amazonia. Syst. Biodivers. Sci. 2021, 19, 375–399. [Google Scholar] [CrossRef]
- Gatto, K.P.; Smith, J.J.; Lourenco, L.B. The mitochondrial genome of the endemic Brazilian paradoxical frog Pseudis tocantins (Hylidae). Mitochondrial DNA B 2018, 3, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Lima, N.G.d.S.; Carmo, A.O.d.; Martins, A.P.V.; Souza, R.C.C.d.; Kalapothakis, E.; Eterovick, P.C. Complete mitochondrial genome sequence of the high-altitude Brazilian tree frog Bokermannohyla alvarengai (Anura, Hylidae). Mitochondrial DNA B 2017, 2, 281–282. [Google Scholar] [CrossRef]
- Lima, N.G.d.S.; Carmo, A.O.d.; Souza, R.C.C.d.; Kalapothakis, E.; Eterovick, P.C. Complete mitochondrial genome sequence of the high altitude Brazilian treefrog Pithecopus megacephalus (Anura, Phyllomedusidae). Mitochondrial DNA B 2020, 5, 388–389. [Google Scholar] [CrossRef]

| Full Length * (bp) | A (%) | T (%) | C (%) | G (%) | A + T (%) | G + C (%) | AT Skew | GC Skew | |
|---|---|---|---|---|---|---|---|---|---|
| Dryophytes japonicus | 17,221 | 30.2 | 30.2 | 25.2 | 14.4 | 60.4 | 39.6 | −0.001 | −0.273 |
| Dryophytes immaculata | 18,186 | 29.4 | 28.5 | 27.3 | 14.9 | 57.9 | 42.2 | 0.015 | −0.295 |
| Hyla annectans | 17,060 | 30.3 | 30.5 | 24.8 | 14.5 | 60.8 | 39.3 | −0.004 | −0.263 |
| Hyla chinensis | 17,087 | 30.2 | 30 | 25.4 | 14.4 | 60.2 | 39.8 | 0.004 | −0.276 |
| Hyla zhaopingensis | 15,812 | 29.8 | 32.2 | 23.9 | 14.1 | 62 | 38 | −0.038 | −0.257 |
| KY700829 Dryophytes suweonensis | KY419887 Dryophytes suweonensis | KX854020 Dryophytes suweonensis | Dryophytes japonicus | Dryophytes immaculata | AB303949.1 Dryophytes japonicus | |
|---|---|---|---|---|---|---|
| KY700829 Dryophytes suweonensis | ||||||
| KY419887 Dryophytes suweonensis | 0.12158 | |||||
| KX854020 Dryophytes suweonensis | 0.12183 | 0.00176 | ||||
| Dryophytes japonicus | 0.01276 | 0.12164 | 0.12189 | |||
| Dryophytes immaculata | 0.12175 | 0.00831 | 0.00812 | 0.12189 | ||
| AB303949.1 Dryophytes japonicus | 0.02526 | 0.12061 | 0.12086 | 0.02479 | 0.12046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.-H.; Yuan, Y.-N.; Li, K.; Storey, K.B.; Zhang, J.-Y.; Zhang, S.-S.; Yu, D.-N. Differential Mitochondrial Genome Expression of Four Hylid Frog Species under Low-Temperature Stress and Its Relationship with Amphibian Temperature Adaptation. Int. J. Mol. Sci. 2024, 25, 5967. https://doi.org/10.3390/ijms25115967
Hong Y-H, Yuan Y-N, Li K, Storey KB, Zhang J-Y, Zhang S-S, Yu D-N. Differential Mitochondrial Genome Expression of Four Hylid Frog Species under Low-Temperature Stress and Its Relationship with Amphibian Temperature Adaptation. International Journal of Molecular Sciences. 2024; 25(11):5967. https://doi.org/10.3390/ijms25115967
Chicago/Turabian StyleHong, Yue-Huan, Ya-Ni Yuan, Ke Li, Kenneth B. Storey, Jia-Yong Zhang, Shu-Sheng Zhang, and Dan-Na Yu. 2024. "Differential Mitochondrial Genome Expression of Four Hylid Frog Species under Low-Temperature Stress and Its Relationship with Amphibian Temperature Adaptation" International Journal of Molecular Sciences 25, no. 11: 5967. https://doi.org/10.3390/ijms25115967





