Intestinal Microbiota and Derived Metabolites in Myocardial Fibrosis and Postoperative Atrial Fibrillation
Abstract
1. Introduction
2. Results
2.1. Histological Evaluation of the Right Atrium
2.2. ELISA Results
2.3. Regression Analysis
2.4. Secondary Endpoints: Thoracentesis
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Inclusion Criteria
4.2. Endpoints
4.3. Sample Size Calculation
4.4. Sample Collection and Analysis
- Collagen type 1 [COL1], assay Hs00164004_m1
- Collagen type 3 [COL3], assay Hs00943809_m1
- Fibronectin, assay Hs00365052_m1
- TGFb, assay Hs00998133_m1
- SMAD-2, assay Hs00998187_m1
- sP-selectin, a marker of platelet activation, Thermofisher BMS219-4
- Lipopolysaccharide (LPS), a marker of bacterial presence, Cusabio CSB-E09945h
- Zonulin (ZNL), a marker of intestinal permeability, Cusabio CSB-EQ027649HU
- TGFb, a marker of fibrosis, Thermofisher BMS249-4
- TMAO, as the main metabolic product of the gut microbiota, MyBioSource MBS7269386
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.C.; Voskoboinik, A.; Gerche, A.; Marwick, T.H.; McMullen, J.R. Prevention of Pathological Atrial Remodeling and Atrial Fibrillation, JACC State-of-the-Art Review. J. Am. Coll Cardiol. 2021, 77, 2846–2864. [Google Scholar] [CrossRef] [PubMed]
- Xintarakou, A.; Tzeis, S.; Psarras, S.; Asvestas, D.; Vardas, P. Atrial fibrosis as a dominant factor for the development of atrial fibrillation, facts and gaps. Europace 2020, 22, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Arora, R.; Jalife, J. Atrial Myopathy. JACC Basic Transl. Sci. 2019, 4, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2020, 41, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [CrossRef]
- Li, X.; Geng, J.; Zhao, J.; Ni, Q.; Zhao, C.; Zheng, Y.; Chen, X.; Wang, L. Trimethylamine N-Oxide Exacerbates Cardiac Fibrosis via Activating the NLRP3 Inflammasome. Front. Physiol. 2019, 10, 866. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Z.; Yan, J.; Liu, H.; Liu, Q.; Deng, Y.; Ou, C.; Chen, M. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab. Investig. 2019, 99, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, S.; Zhu, J.; Jiang, H.; Jia, D.; Ou, T.; Qi, Z.; Zou, Y.; Qian, J.; Sun, A.; et al. Gut microbe-derived metabolite trimethylamine N-oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J. Mol. Cell. Cardiol. 2019, 134, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Backhed, F.; Landmesser, U.; Hazen, S.L. Intestinal Microbiota in Cardiovascular Health and Disease, JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2089–2105. [Google Scholar] [CrossRef]
- Linz, D.; Gawałko, M.; Sanders, P.; Penders, J.; Li, N.; Nattel, S.; Dobrev, D. Does gut microbiota affect atrial rhythm? Causalities and speculations. Eur. Heart J. 2021, 42, 3521–3525. [Google Scholar] [CrossRef]
- Liu, L.; Su, J.; Li, R.; Luo, F. Changes in Intestinal Flora Structure and Metabolites Are Associated with Myocardial Fibrosis in Patients with Persistent Atrial Fibrillation. Front. Nutr. 2021, 8, 702085. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Zou, X.; Zhang, H. The Relationship between Atrial Fibrillation and Intestinal Flora With Its Metabolites. Front. Cardiovasc. Med. 2022, 9, 948755. [Google Scholar] [CrossRef] [PubMed]
- Gawałko, M.; A Agbaedeng, T.; Saljic, A.; Müller, D.N.; Wilck, N.; Schnabel, R.; Penders, J.; Rienstra, M.; van Gelder, I.; Jespersen, T.; et al. Gut microbiota, dysbiosis and atrial fibrillation. Arrhythmogenic mechanisms and potential clinical implications. Cardiovasc. Res. 2022, 118, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Wolino, K.S.; Cardozo, L.F.M.d.F.; Leal, V.d.O.; Mafra, D.; Stockler-Pinto, M.B. Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur. J. Nutr. 2021, 60, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Svingen, G.F.; Zuo, H.; Ueland, P.M.; Seifert, R.; Løland, K.H.; Pedersen, E.R.; Schuster, P.M.; Karlsson, T.; Tell, G.S.; Schartum-Hansen, H.; et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int. J. Cardiol. 2018, 267, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Büttner, P.; Okun, J.G.; Hauke, J.; Holzwirth, E.; Obradovic, D.; Hindricks, G.; Thiele, H.; Kornej, J. Trimethylamine N-oxide in atrial fibrillation progression. Int. J. Cardiol. Heart Vasc. 2020, 29, 100554. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gimenez, R.; Ahmed-Khodja, W.; Molina, Y.; Peiró, O.M.; Bonet, G.; Carrasquer, A.; Fragkiadakis, G.A.; Bulló, M.; Bardaji, A.; Papandreou, C. Gut Microbiota-Derived Metabolites and Cardiovascular Disease Risk, A Systematic Review of Prospective Cohort Studies. Nutrients 2022, 14, 2654. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Yang, R.; Zhao, Q.; Li, X.D.; Wang, Y.T. A systematic review and meta-analysis of the gut microbiota-dependent metabolite trimethylamine N-oxide with the incidence of atrial fibrillation. Ann. Palliat. Med. 2021, 10, 11512–11523. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, R.; Sanjanwala, R.; Le, M.L.; Yamashita, M.H.; Arora, R.C. Postoperative Atrial Fibrillation after Cardiac Surgery, A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 111, 544–554. [Google Scholar] [CrossRef]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.B.; Nattel, S. Postoperative atrial fibrillation, mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Drapkina, O.M.; Yafarova, A.A.; Kaburova, A.N.; Kiselev, A.R. Targeting Gut Microbiota as a Novel Strategy for Prevention and Treatment of Hypertension, Atrial Fibrillation and Heart Failure, Current Knowledge and Future Perspectives. Biomedicines 2022, 10, 2019. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.; Li, L.; Yang, M.-Z.; Wang, Q.-X.; Bai, X.-W.; Gao, F.; Yuan, Y.-Q.; Yu, Z.-J.; Ren, Z.-G. The correlation between gut microbiome and atrial fibrillation: Pathophysiology and therapeutic perspectives. Mil. Med. Res. 2023, 10, 51. [Google Scholar] [CrossRef]
- Huang, R.; Yan, L.; Lei, Y. The Gut Microbial-Derived Metabolite Trimethylamine N-Oxide and Atrial Fibrillation, Relationships, Mechanisms, and Therapeutic Strategies. Clin. Interv. Aging 2021, 16, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Zhang, L.; Zhang, Y.; Wang, F.; Zhao, Z.; Zhou, X. Gut Microbial Metabolite Trimethylamine N-Oxide Is Related to Thrombus Formation in Atrial Fibrillation Patients. Am. J. Med. Sci. 2019, 358, 422–428. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Piccini, J.; Mack, M. Postoperative atrial fibrillation: From mechanisms to treatment. Eur. Heart J. 2023, 44, 1020–1039. [Google Scholar] [CrossRef]
- Caldonazo, T.; Kirov, H.; Rahouma, M.; Robinson, N.B.; Demetres, M.; Gaudino, M.; Doenst, T.; POAF-MA Group. Atrial fibrillation after cardiac surgery: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2023, 165, 94–103.e24. [Google Scholar] [CrossRef]
- Seo, E.J.; Hong, J.; Lee, H.J.; Son, Y.J. Perioperative risk factors for new-onset postoperative atrial fibrillation after coronary artery bypass grafting: A systematic review. BMC Cardiovasc. Disord. 2021, 21, 418. [Google Scholar] [CrossRef]
- McIntyre, W.F. Post-operative atrial fibrillation after cardiac surgery: Challenges throughout the patient journey. Front. Cardiovasc. Med. 2023, 10, 1156626. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.; Ahmed, A.; Massie, V.; Marshall, E.; Harky, A. Determinants of atrial fibrillation after cardiac surgery. Rev. Cardiovasc. Med. 2021, 22, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Higgs, M.; Sim, J.; Traynor, V. Incidence and risk factors for new-onset atrial fibrillation following coronary artery bypass grafting: A systematic review and meta-analysis. Intensive Crit. Care Nurs. 2020, 60, 102897. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.; Li, R.; Jiang, H.; Tao, D.; Zhao, K.; Yin, Z.; Zhang, J.; Wang, H. Gut Microbiota in Patients with Postoperative Atrial Fibrillation Undergoing Off-Pump Coronary Bypass Graft Surgery. J. Clin. Med. 2023, 12, 1493. [Google Scholar] [CrossRef]
- Malagon, I.; Onkenhout, W.; Klok, G.; van der Poel, P.F.; Bovill, J.G.; Hazekamp, M.G. Gut permeability in paediatric cardiac surgery. Br. J. Anaesth. 2005, 94, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zwischenberger, J.B.; Nguyen, T.T.; Vertrees, R.A.; McDaniel, L.B.; Nutt, L.K.; Herndon, D.N.; Kramer, G.C. Gut mucosal ischemia during normothermic cardiopulmonary bypass results from blood flow redistribution and increased oxygen demand. J. Thorac. Cardiovasc. Surg. 1995, 110, 819–828. [Google Scholar] [CrossRef]
- Liblik, K.; Zucker, J.; Baranchuk, A.; Fernandez, A.L.; Zhang, S.; Diasty, M.E. The role of pericardial fluid biomarkers in predicting post-operative atrial fibrillation, a comprehensive review of current literature. Trends Cardiovasc. Med. 2024, 34, 244–247. [Google Scholar] [CrossRef]
- Khan, M.S.; Yamashita, K.; Sharma, V.; Ranjan, R.; Dosdall, D.J. RNAs and Gene Expression Predicting Postoperative Atrial Fibrillation in Cardiac Surgery Patients Undergoing Coronary Artery Bypass Grafting. J. Clin. Med. 2020, 9, 1139. [Google Scholar] [CrossRef]
- Olek, K.; Kuczaj, A.A.; Warwas, S.; Hrapkowicz, T.; Przybyłowski, P.; Tanasiewicz, M. Gut Microbiome in Patients after Heart Transplantation-Current State of Knowledge. Biomedicines 2023, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Yuzefpolskaya, M.; Bohn, B.; Javaid, A.; Mondellini, G.M.; Braghieri, L.; Pinsino, A.; Onat, D.; Cagliostro, B.; Kim, A.; Takeda, K.; et al. Levels of Trimethylamine N-Oxide Remain Elevated Long Term after Left Ventricular Assist Device and Heart Transplantation and Are Independent from Measures of Inflammation and Gut Dysbiosis. Circ. Heart Fail. 2021, 14, e007909. [Google Scholar] [CrossRef]
- Yuzefpolskaya, M.; Bohn, B.; Nasiri, M.; Zuver, A.M.; Onat, D.D.; Royzman, E.A.; Nwokocha, J.; Mabasa, M.; Pinsino, A.; Brunjes, D.; et al. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant. J. Heart Lung. Transplant. 2020, 39, 880–890. [Google Scholar] [CrossRef]
- Sen, T.; Thummer, R.P. The Impact of Human Microbiotas in Hematopoietic Stem Cell and Organ Transplantation. Front. Immunol. 2022, 13, 932228. [Google Scholar] [CrossRef]
- Jennings, D.L.; Bohn, B.; Zuver, A.; Onat, D.; Gaine, M.; Royzman, E.; Hupf, J.; Brunjes, D.; Latif, F.; Restaino, S.; et al. Gut microbial diversity, inflammation, and oxidative stress are associated with tacrolimus dosing requirements early after heart transplantation. PLoS ONE 2020, 15, e0233646. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Mayerhofer, C.C.; Broch, K.; Arora, S.; Svardal, A.; Hov, J.R.; Andreassen, A.K.; Gude, E.; Karason, K.; Dellgren, G.; et al. The carnitine-butyrobetaine-TMAO pathway after cardiac transplant, Impact on cardiac allograft vasculopathy and acute rejection. J. Heart Lung. Transplant. 2019, 38, 1097–1103. [Google Scholar] [CrossRef]
- Svinarich, J.T. The functional medicine approach to atrial fibrillation, can a cure for atrial fibrillation be found in the gut? Curr. Opin. Cardiol. 2021, 36, 44–50. [Google Scholar] [CrossRef]
- Wang, G.; Kong, B.; Shuai, W.; Fu, H.; Jiang, X.; Huang, H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J. Nutr. Biochem. 2020, 78, 108341. [Google Scholar] [CrossRef]
- Chen, K.; Zheng, X.; Feng, M.; Li, D.; Zhang, H. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide Contributes to Cardiac Dysfunction in Western Diet-Induced Obese Mice. Front. Physiol. 2017, 8, 139. [Google Scholar] [CrossRef]
- Talari, K.; Goyal, M. Retrospective studies—Utility and caveats. J. R. Coll. Physicians Edinb. 2020, 50, 398–402. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Bloch, D.A.; Larsen, M.D. A simple method of sample size calculation for linear and logistic regression. Stat. Med. 1998, 17, 1623–1634. [Google Scholar] [CrossRef]
- Blackstone, E.H. Sufficient data. J. Thorac. Cardiovasc. Surg. 2016, 152, 1235–1236. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Dinsmore, R.E.; Fuster, V.; Glagov, S.; Insull, W., Jr.; Rosenfeld, M.E.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995, 92, 1355–1374. [Google Scholar] [CrossRef]
| All Patients N = 100 | POAF N = 38 | No POAF N = 62 | p Value | |
|---|---|---|---|---|
| Age | 69 (64–78) | 68.5 (64–78) | 69.5 (64–78) | 0.646 |
| Male sex | 58 (58%) | 24 (63.2%) | 34 (54.8%) | 0.413 |
| Hypertension | 98 (98%) | 37 (97.4%) | 60 (96.8%) | 0.866 |
| Dyslipidemia | 75 (75%) | 28 (73.7%) | 47 (75.8%) | 0.812 |
| Diabetes | 54 (54%) | 24 (63.2%) | 30 (48.4%) | 0.150 |
| Smoking habit | 61 (61%) | 29 (76.3%) | 52 (83.9%) | 0.350 |
| Alcohol use (units/week) | 4 (2–5) | 3 (1–5) | 4 (2–6) | 0.102 |
| Hemoglobin (g/dL) | 12.8 (11.7–13.9) | 12.2 (11.7–13.6) | 13.1 (11.8–14.0) | 0.062 |
| Creatinine (mg/dL) | 1.1 (0.9–1.3) | 1.1 (0.8–1.3) | 1.1 (0.9–1.3) | 0.421 |
| BMI (kg/m2) | 31.9 (29.1–34.5) | 31.9 (29.6–34.6) | 31.3 (28.4–34.3) | 0.432 |
| LVEDD (mm) | 51 (48–53) | 51.5 (48–53) | 49 (47–52) | 0.098 |
| LVEF (%) | 55 (50–60) | 55 (50–60) | 50 (50–55) | 0.081 |
| LAVI | 33 (31–35) | 33 (31–35) | 33 (30–35) | 0.731 |
| LAVI ≥ 34 | 44 (44%) | 17 (44.7%) | 27 (43.5%) | 0.907 |
| TAPSE (mm) | 21 (17–22) | 21 (17–23) | 19.5 (17–22) | 0.086 |
| Surgery type: | 0.857 | |||
| Revascularization | 82 (82%) | 32 (84.2%) | 50 (80.6%) | |
| Aortic valve | 8 (8%) | 3 (7.9%) | 5 (8.1%) | |
| Ascending aorta | 10 (10%) | 3 (7.9%) | 7 (11.3%) | |
| Duration CPB (min) | 55 (41–69) | 56 (45–71) | 54 (40–69) | 0.594 |
| Duration XCL (min) | 46 (30–58) | 47 (32–59) | 46 (30–58) | 0.604 |
| EuroSCORE II | 2.21 (1.70–3.03) | 2.27 (1.7–3.4) | 2.20 (1.6–2.8) | 0.454 |
| N = 100 | |
|---|---|
| Postoperative atrial fibrillation (POAF) | 38 (38%) |
| Thoracentesis | 26 (26%) |
| Postoperative length of stay (days) | 7 (5.5–8) |
| Number of red packed blood cells (units) | 1 (0–2) |
| In-hospital mortality | 0 (0%) |
| Postoperative bleeding requiring mediastinal re-exploration | 2 (2%) |
| Clinically relevant pericardial effusion (>1 cm or requiring drainage) | 3 (3%) |
| All Patients N = 100 | POAF N = 38 | No POAF N = 62 | p Value | |
|---|---|---|---|---|
| Fibrosis | 0.001 | |||
| Grade 0 | 18 (18%) | 0 (0%) | 18 (29.0%) | |
| Grade 1 | 34 (34%) | 6 (15.8%) | 28 (45.2%) | |
| Grade 2 | 23 (23%) | 12 (31.6%) | 11 (17.7%) | |
| Grade 3 | 25 (25%) | 20 (52.6%) | 5 (8.1%) | |
| Fibrosis grade ≥ 2 | 48 (48%) | 32 (84.2%) | 16 (25.8%) | 0.001 |
| Angiogenesis | 0.018 | |||
| Grade 0–1 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Grade 2 | 62 (62%) | 18 (47.4%) | 44 (71.0%) | |
| Grade 3 | 38 (38%) | 20 (52.6%) | 18 (29.0%) | |
| Inflammation | 0.033 | |||
| Grade 0 | 44 (44%) | 14 (36.8%) | 30 (48.4%) | |
| Grade 1 | 47 (47%) | 17 (44.7%) | 30 (48.4%) | |
| Grade 2 | 9 (9%) | 7 (18.4%) | 2 (3.2%) |
| All Patients N = 100 | POAF N = 38 | No POAF N = 62 | p Value | |
|---|---|---|---|---|
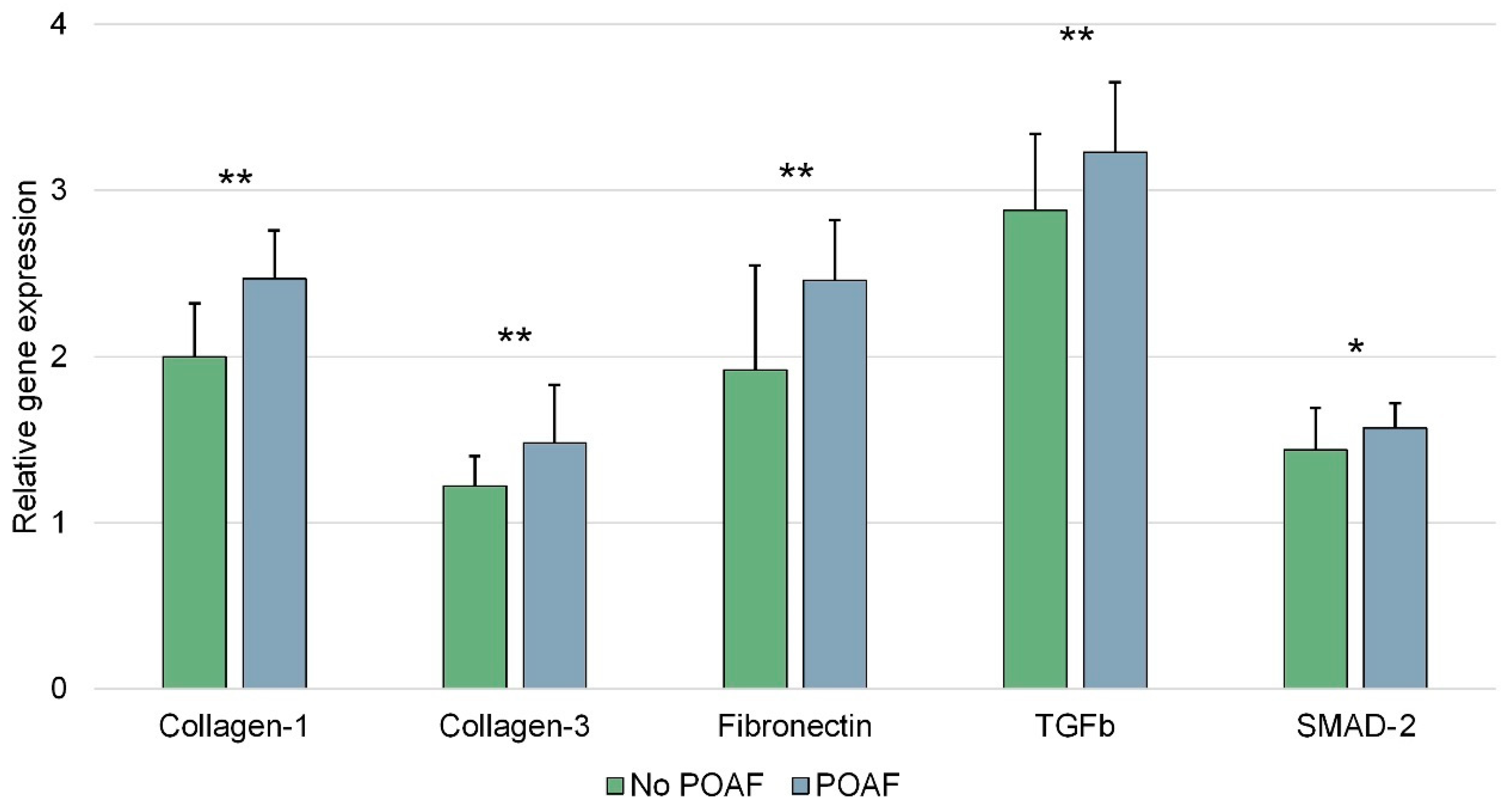
| Collagen-1 | 2.2 (1.9–2.5) 2.18 ± 0.39 | 2.4 (2.2–2.7) 2.47 ± 0.29 | 2.0 (1.8–2.3) 2.00 ± 0.32 | 0.001 |
| Collagen-3 | 1.3 (1.1–1.5) 1.32 ± 0.29 | 1.6 (1.1–1.8) 1.48 ± 0.35 | 1.2 (1.0–1.4) 1.22 ± 0.18 | 0.001 |
| Fibronectin | 2.2 (1.7–2.6) 2.13 ± 0.60 | 2.4 (2.1–2.8) 2.46 ± 0.36 | 2.0 (1.3–2.4) 1.92 ± 0.63 | 0.001 |
| TGFb | 3.0 (2.6–3.4) 2.98 ± 0.48 | 3.2 (2.9–3.6) 3.23 ± 0.42 | 2.9 (2.5–3.2) 2.88 ± 0.46 | 0.001 |
| SMAD-2 | 1.5 (1.3–1.7) 1.49 ± 0.22 | 1.6 (1.5–1.7) 1.57 ± 0.15 | 1.5 (1.3–1.6) 1.44 ± 0.25 | 0.030 |
| All Patients N = 100 | POAF N = 38 | No POAF N = 62 | p Value | |
|---|---|---|---|---|
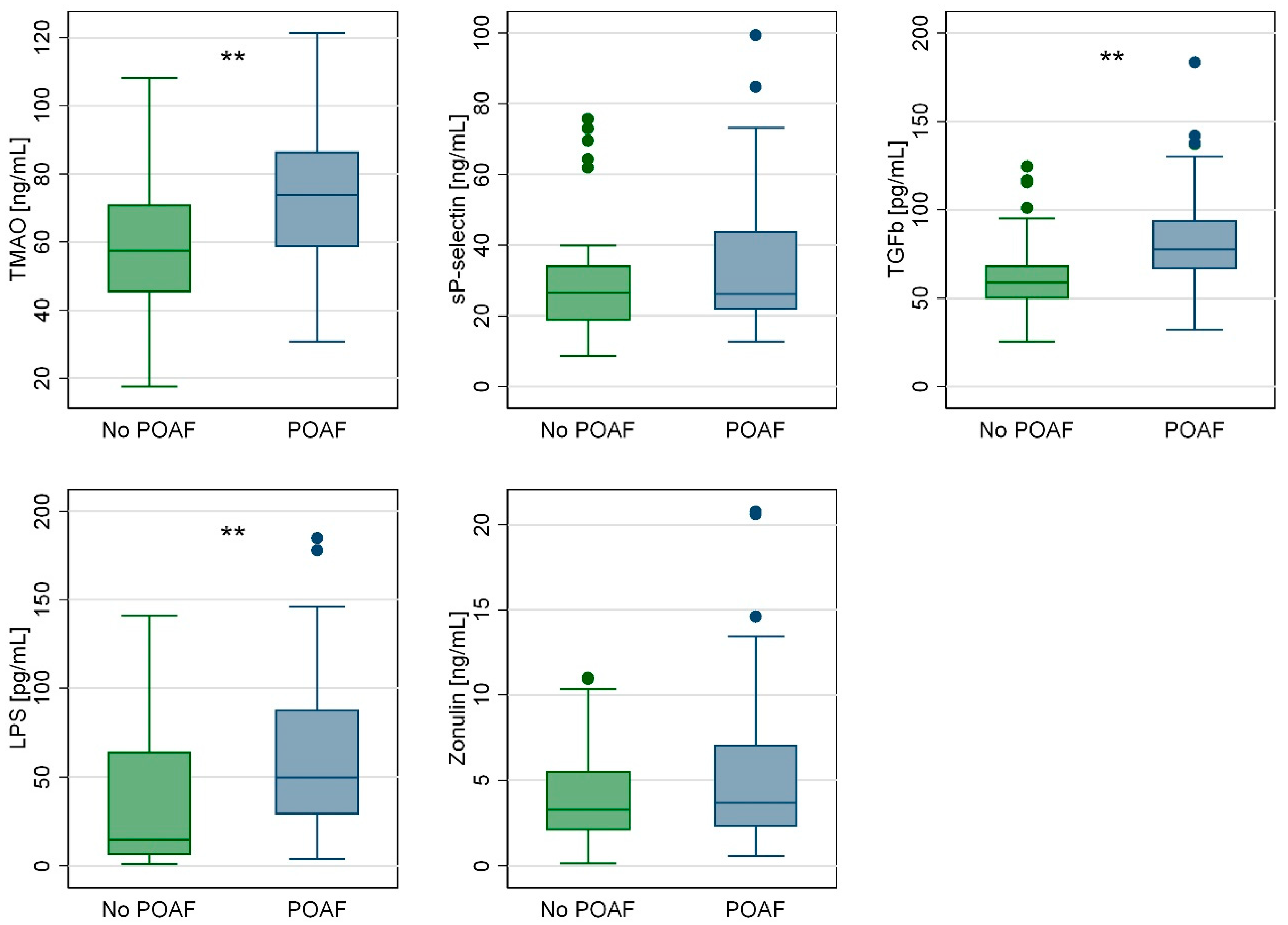
| TMAO [ng/mL] | 61.7 (51.6–75.2) | 73.8 (58.6–86.7) | 57.5 (45.2–71.1) | 0.001 |
| sP-selectin [ng/mL] | 26.6 (20.0–36.5) | 26.2 (21.7–44.0) | 26.6 (18.6–34.2) | 0.189 |
| TGFb [pg/mL] | 66.2 (51.5–75.8) | 77.3 (66.2–94.1) | 58.6 (49.6–68.5) | 0.001 |
| LPS [pg/mL] | 28.9 (10.9–68.0) | 49.7 (28.9–87.9) | 14.7 (6.3–64.6) | 0.001 |
| Zonulin [ng/mL] | 3.47 (2.13–5.94) | 3.70 (2.30–7.08) | 3.30 (2.08–5.56) | 0.218 |
| Univariable Analysis | Odds Ratio | 95% CI | p Value | Included in Multivariable? |
| Age | 0.99 | 0.93–1.04 | 0.729 | |
| Male sex | 1.41 | 0.62–3.22 | 0.414 | |
| Hypertension | 1.23 | 0.11–14.08 | 0.866 | |
| Dyslipidemia | 0.89 | 0.35–2.25 | 0.812 | |
| Diabetes | 1.82 | 0.80–4.17 | 0.152 | + |
| Smoking habit | 0.62 | 0.22–1.69 | 0.352 | |
| Alcohol use | 0.79 | 0.65–0.97 | 0.028 | + |
| Hemoglobin | 0.77 | 0.54–1.08 | 0.138 | + |
| Creatinine | 0.50 | 0.09–2.64 | 0.418 | |
| BMI | 0.95 | 0.84–1.09 | 0.511 | |
| LVEDD | 0.89 | 0.78–1.02 | 0.104 | + |
| LVEF | 0.89 | 0.81–0.98 | 0.024 | + |
| LAVI | 0.96 | 0.82–1.13 | 0.643 | |
| LAVI ≥ 34 | 1.04 | 0.46–2.36 | 0.907 | |
| TAPSE | 0.89 | 0.78–1.02 | 0.093 | + |
| Surgery type | ||||
| Myocard. revasc | Ref. | Ref. | Ref. | |
| Aortic valve replac. | 0.93 | 0.21–4.19 | 0.933 | |
| Ascending aorta | 0.67 | 0.16–2.78 | 0.581 | |
| Duration CPB | 1.01 | 0.98–1.03 | 0.629 | |
| Duration XCL | 1.01 | 0.98–1.03 | 0.618 | |
| EuroSCORE II | 1.28 | 0.78–2.10 | 0.322 | |
| TMAO | 1.05 | 1.02–1.08 | 0.001 | + |
| sP-selectin | 1.03 | 1.00–1.05 | 0.035 | + |
| TGFb | 1.04 | 1.02–1.06 | 0.001 | + |
| LPS | 1.01 | 1.00–1.02 | 0.003 | + |
| Zonulin | 1.10 | 0.99–1.23 | 0.059 | + |
| Multivariable model | Odds ratio | 95% CI | p value | |
| Hemoglobin | 0.75 | 0.59–0.97 | 0.030 | |
| TAPSE | 0.85 | 0.73–0.99 | 0.044 | |
| TMAO | 1.05 | 1.02–1.08 | 0.001 | |
| TGFb | 1.04 | 1.01–1.06 | 0.001 | |
| Variable | AUC | 95% CI | Cut-Off | Se | Sp | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Hemoglobin | 0.41 | 0.32–0.52 | 13.1 | 39.5 | 48.4 | 31.9 | 56.6 |
| TAPSE | 0.40 | 0.30–0.50 | 20 | 50.0 | 37.1 | 32.8 | 54.8 |
| TMAO | 0.77 | 0.68–0.85 | 61.8 | 71.1 | 85.5 | 73.5 | 80.3 |
| TGFb | 0.74 | 0.65–0.82 | 72.1 | 65.8 | 62.9 | 54.0 | 78.0 |
| Odds Ratio | 95% CI | p Value | Included in Final Model? | |
|---|---|---|---|---|
| Hb ≥ 13.1 g/dL | 0.40 | 0.17–0.97 | 0.042 | + |
| TAPSE ≥ 20 mm | 0.23 | 0.10–0.56 | 0.001 | + |
| TMAO ≥ 61.8 ng/mL | 7.18 | 2.57–20.03 | 0.001 | + |
| TGFb ≥ 72.1 pg/mL | 1.77 | 0.75–4.19 | 0.195 |
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
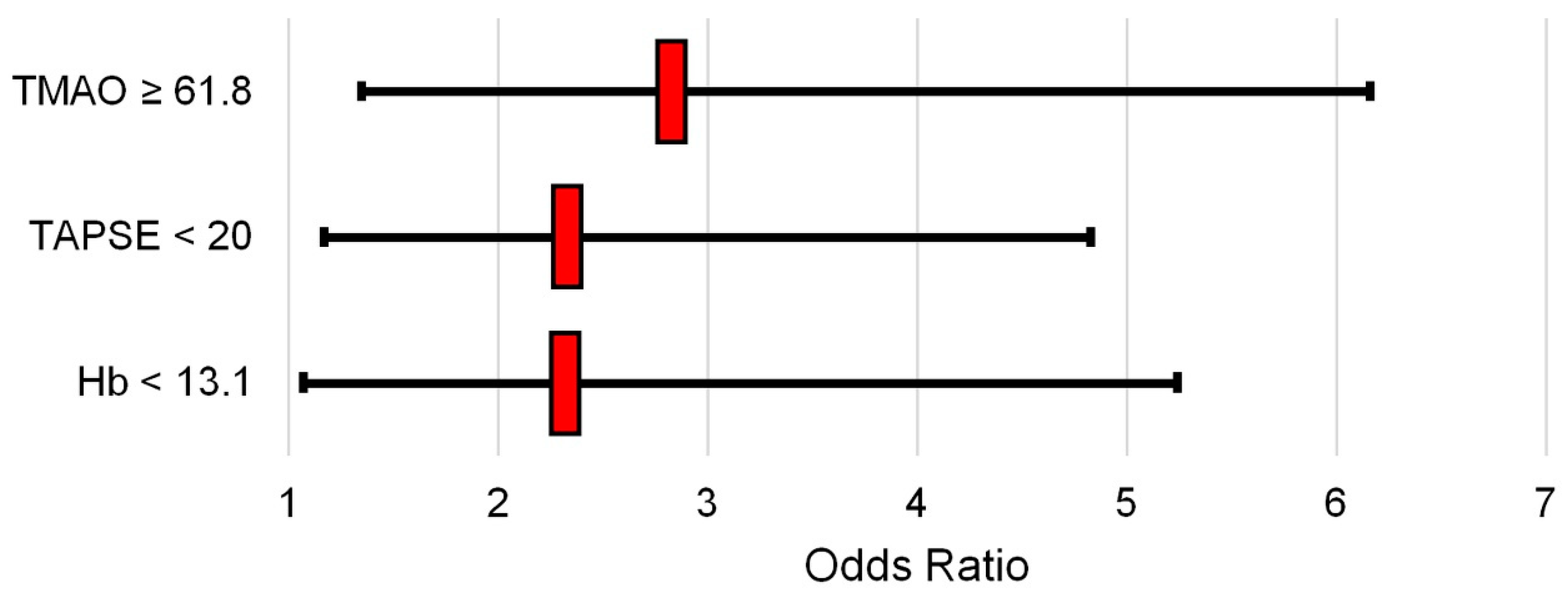
| Hb < 13.1 g/dL | 2.37 | 1.07–5.24 | 0.033 |
| TAPSE < 20 mm | 2.38 | 1.17–4.83 | 0.017 |
| TMAO ≥ 61.8 ng/mL | 2.88 | 1.35–6.16 | 0.006 |
| Logit Regression with Interaction between TMAO and Fibrosis | |||
| Odds Ratio | 95% CI | p Value | |
| Dependent variable: fibrosis (grade ≥ 2) | |||
| TMAO (≥61.8 ng/mL) | 2.67 | 1.19–5.98 | 0.017 |
| Dependent variable: POAF (univariable) | |||
| TMAO (≥61.8 ng/mL) | 4.16 | 1.74–9.93 | 0.001 |
| Fibrosis (grade ≥ 2) | 15.33 | 5.41–43.43 | 0.001 |
| Dependent variable: POAF (two variables) | |||
| TMAO (≥61.8 ng/mL) | 3.43 | 1.23–9.58 | 0.019 |
| Fibrosis (grade ≥ 2) | 13.86 | 4.74–40.51 | 0.001 |
| Dependent variable: POAF (interaction) | |||
| Fibrosis # TMAO | |||
| no yes | 0.43 | 0.12–1.21 | 0.063 |
| yes no | 1.25 | 0.49–3.17 | 0.638 |
| yes yes | 2.75 | 1.22–6.17 | 0.014 |
| Dependent variable: POAF (factorial model) | |||
| Fibrosis (grade ≥ 2) | 1.25 | 0.49–3.17 | 0.638 |
| TMAO (≥61.8 ng/mL) | 0.43 | 0.12–1.21 | 0.063 |
| Fibrosis # TMAO | |||
| yes yes | 6.60 | 1.34–32.52 | 0.020 |
| Probit regression with endogenous covariates | |||
| Coefficient | 95% CI | p value | |
| Dependent variable: POAF | |||
| Fibrosis (grade ≥ 2) | 2.51 | 2.05–2.96 | 0.001 |
| TMAO | 2.08 | 1.75–2.1 | 0.001 |
| Dependent variable: fibrosis | |||
| TMAO | 1.21 | 1.03–1.40 | 0.034 |
| TGFb (ELISA) | 1.51 | 1.32–1.71 | 0.018 |
| /athrho2_1 | −1.82 | −2.85/−0.79 | 0.001 |
| /lnsigma2 | −0.74 | −0.89/−0.61 | 0.001 |
| Err. corr (e.fibrosis, e.POAF) | −0.95 | −0.99/−0.66 | - |
| SD (e.fibrosis) | 0.47 | 0.41–0.54 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nenna, A.; Laudisio, A.; Taffon, C.; Fogolari, M.; Spadaccio, C.; Ferrisi, C.; Loreni, F.; Giacinto, O.; Mastroianni, C.; Barbato, R.; et al. Intestinal Microbiota and Derived Metabolites in Myocardial Fibrosis and Postoperative Atrial Fibrillation. Int. J. Mol. Sci. 2024, 25, 6037. https://doi.org/10.3390/ijms25116037
Nenna A, Laudisio A, Taffon C, Fogolari M, Spadaccio C, Ferrisi C, Loreni F, Giacinto O, Mastroianni C, Barbato R, et al. Intestinal Microbiota and Derived Metabolites in Myocardial Fibrosis and Postoperative Atrial Fibrillation. International Journal of Molecular Sciences. 2024; 25(11):6037. https://doi.org/10.3390/ijms25116037
Chicago/Turabian StyleNenna, Antonio, Alice Laudisio, Chiara Taffon, Marta Fogolari, Cristiano Spadaccio, Chiara Ferrisi, Francesco Loreni, Omar Giacinto, Ciro Mastroianni, Raffaele Barbato, and et al. 2024. "Intestinal Microbiota and Derived Metabolites in Myocardial Fibrosis and Postoperative Atrial Fibrillation" International Journal of Molecular Sciences 25, no. 11: 6037. https://doi.org/10.3390/ijms25116037
APA StyleNenna, A., Laudisio, A., Taffon, C., Fogolari, M., Spadaccio, C., Ferrisi, C., Loreni, F., Giacinto, O., Mastroianni, C., Barbato, R., Rose, D., Salsano, A., Santini, F., Angeletti, S., Crescenzi, A., Antonelli Incalzi, R., Chello, M., & Lusini, M. (2024). Intestinal Microbiota and Derived Metabolites in Myocardial Fibrosis and Postoperative Atrial Fibrillation. International Journal of Molecular Sciences, 25(11), 6037. https://doi.org/10.3390/ijms25116037









