Blastocyst-like Structures in the Peripheral Retina of Young Adult Beagles
Abstract
1. Introduction
2. Results
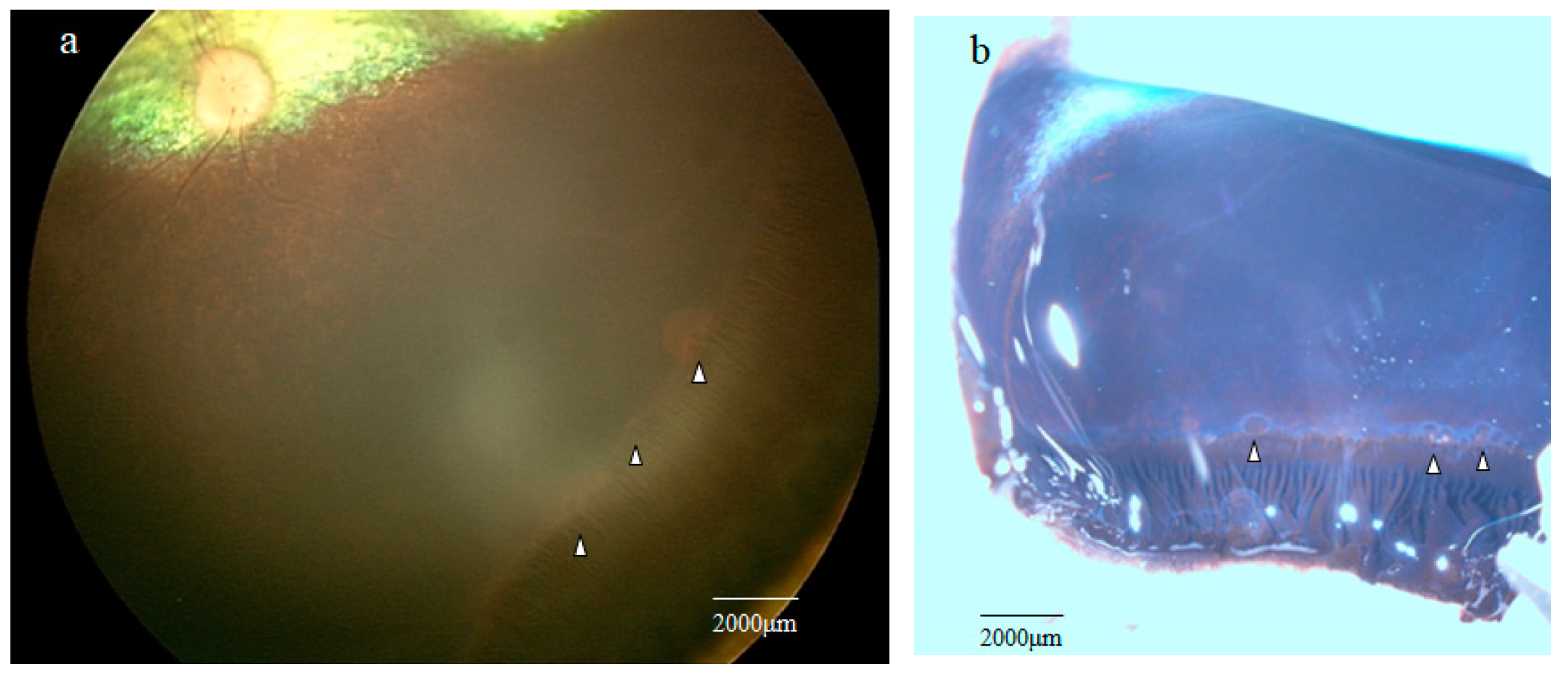
2.1. Fundoscopic Examination of the Peripheral Retina
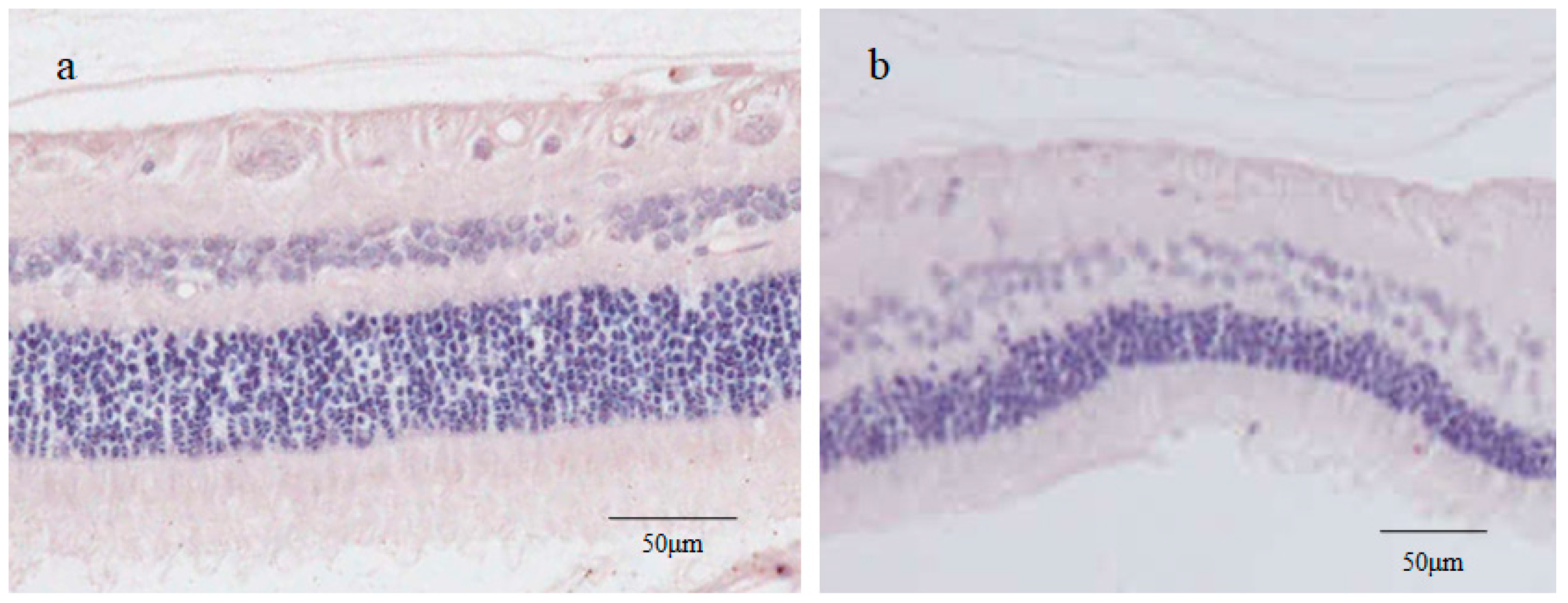
2.2. Stereomicroscopy Findings of the Peripheral Retina Specimens
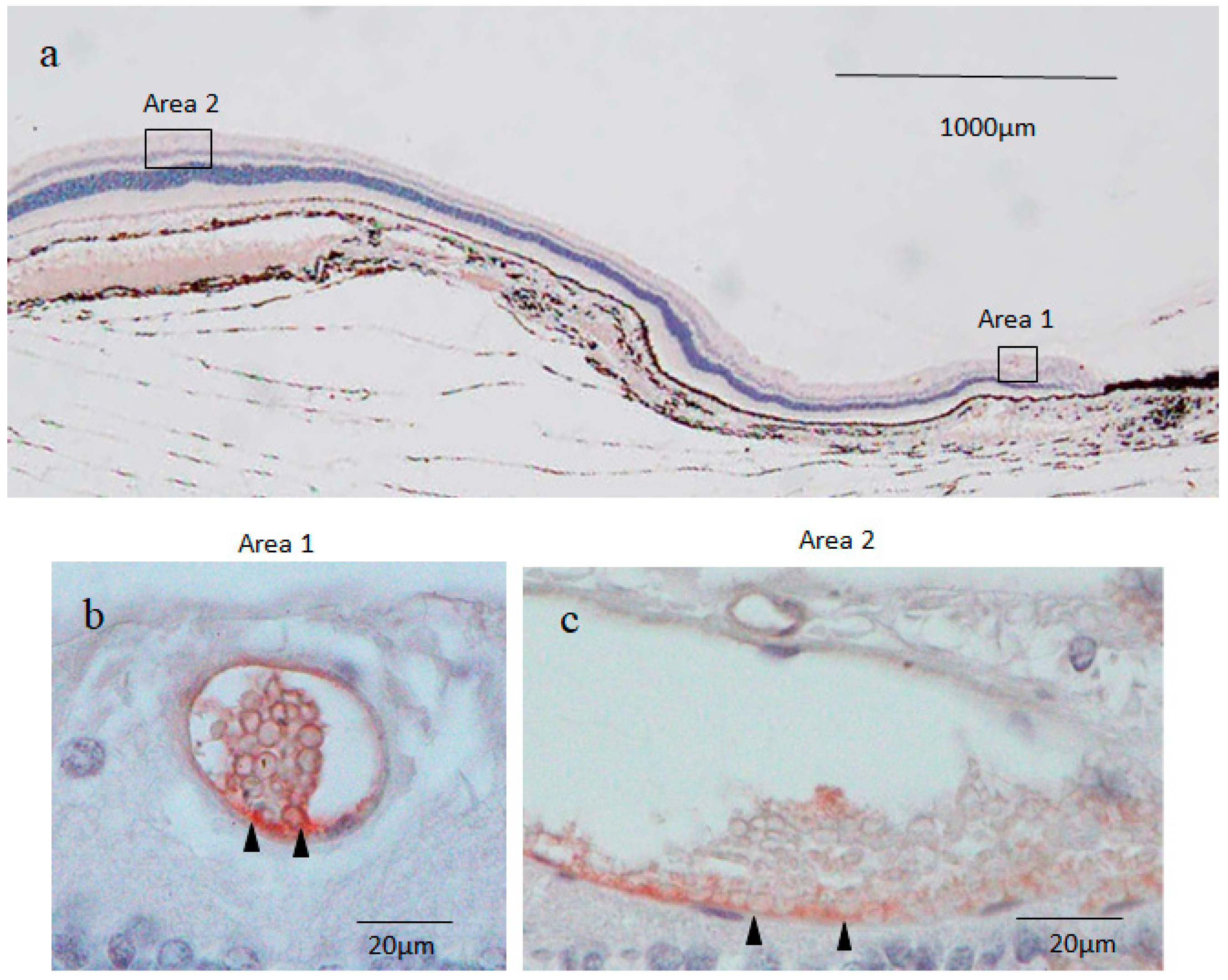
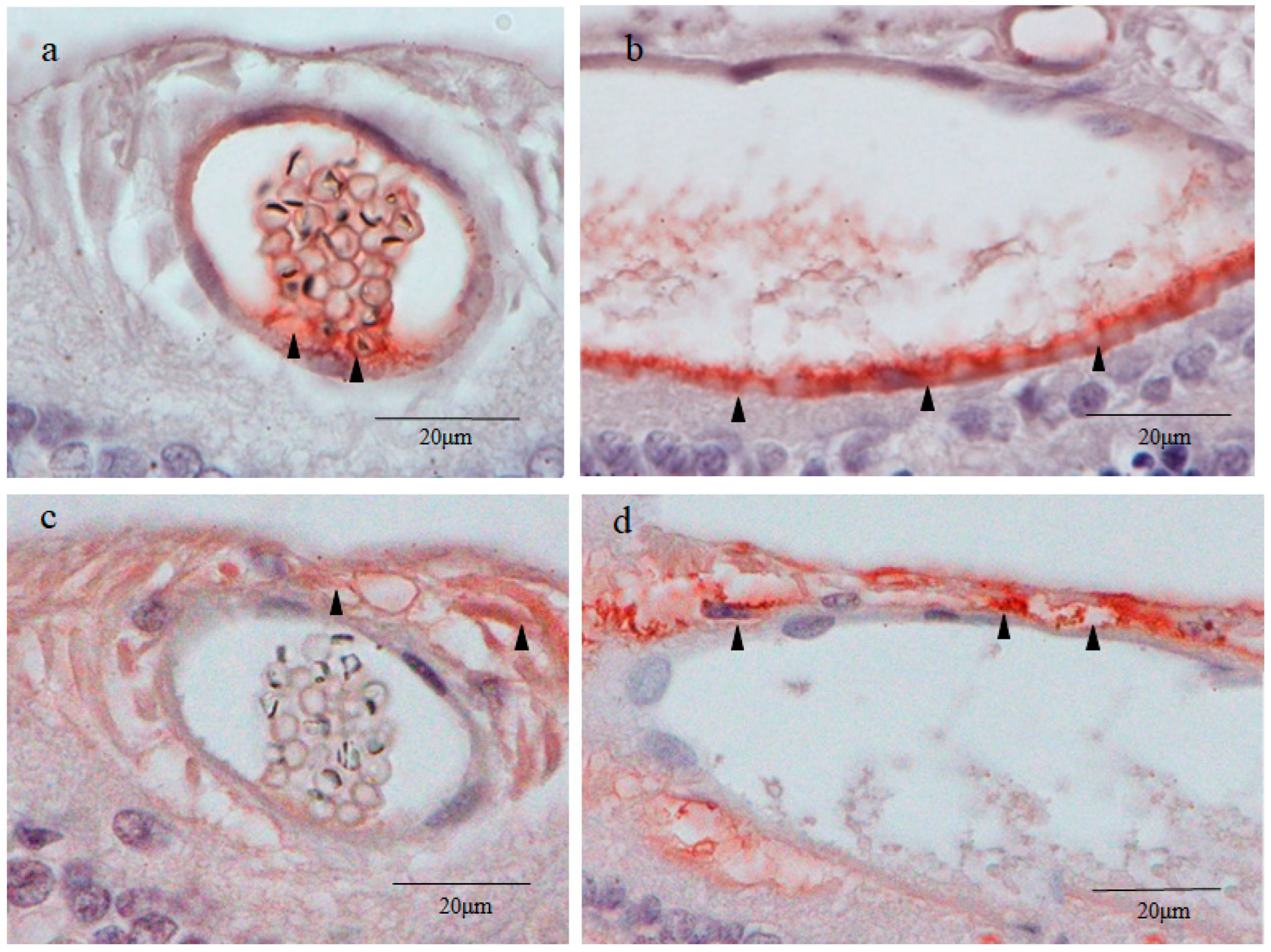
2.3. Nestin Immunostaining
2.4. 4′,6-Diamidino-2-phenylindole (DAPI) Fluorescence Staining
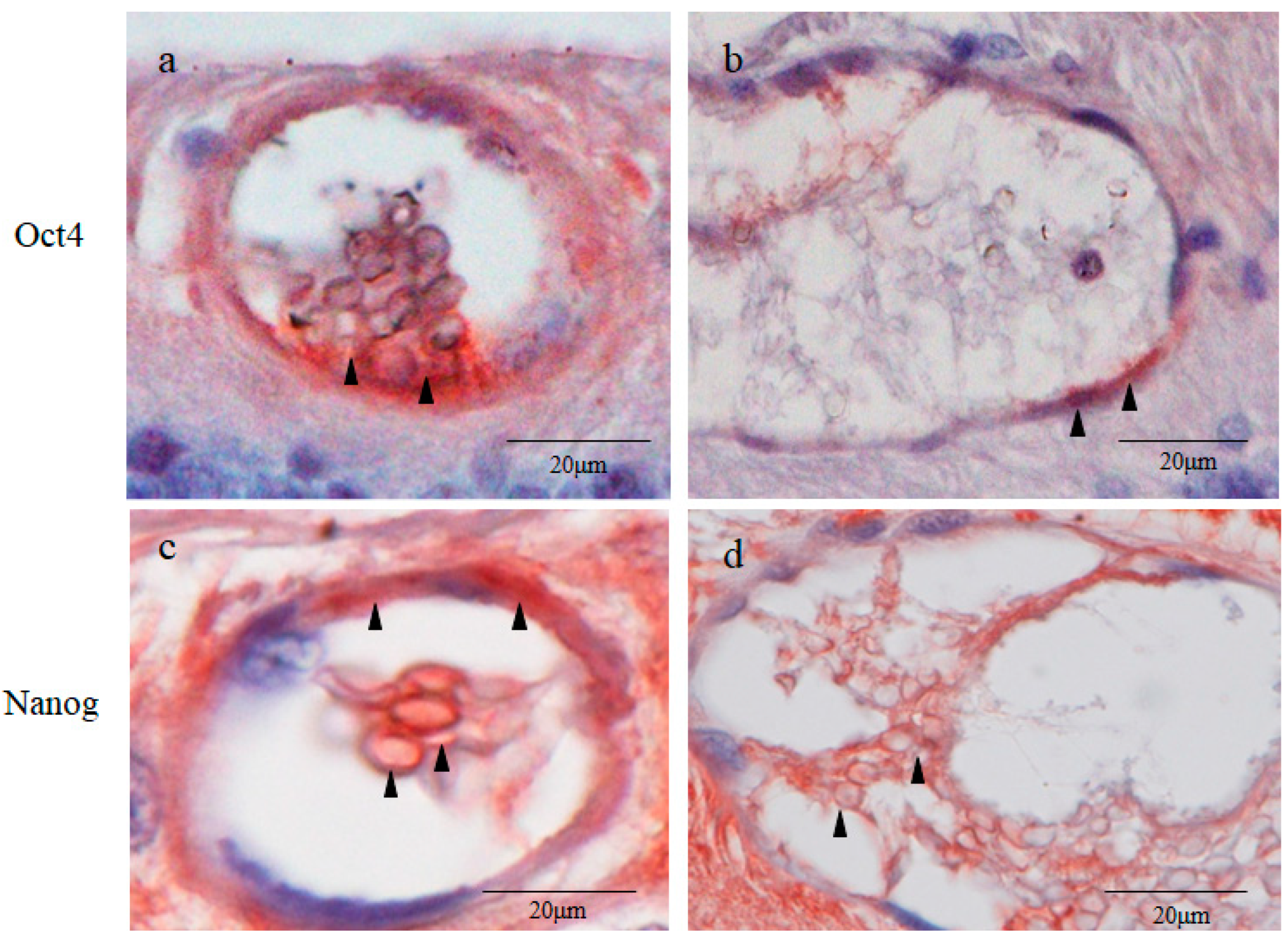
2.5. Octamer-Binding Transcription Factor 4 (Oct4) and Nanog Immunostaining
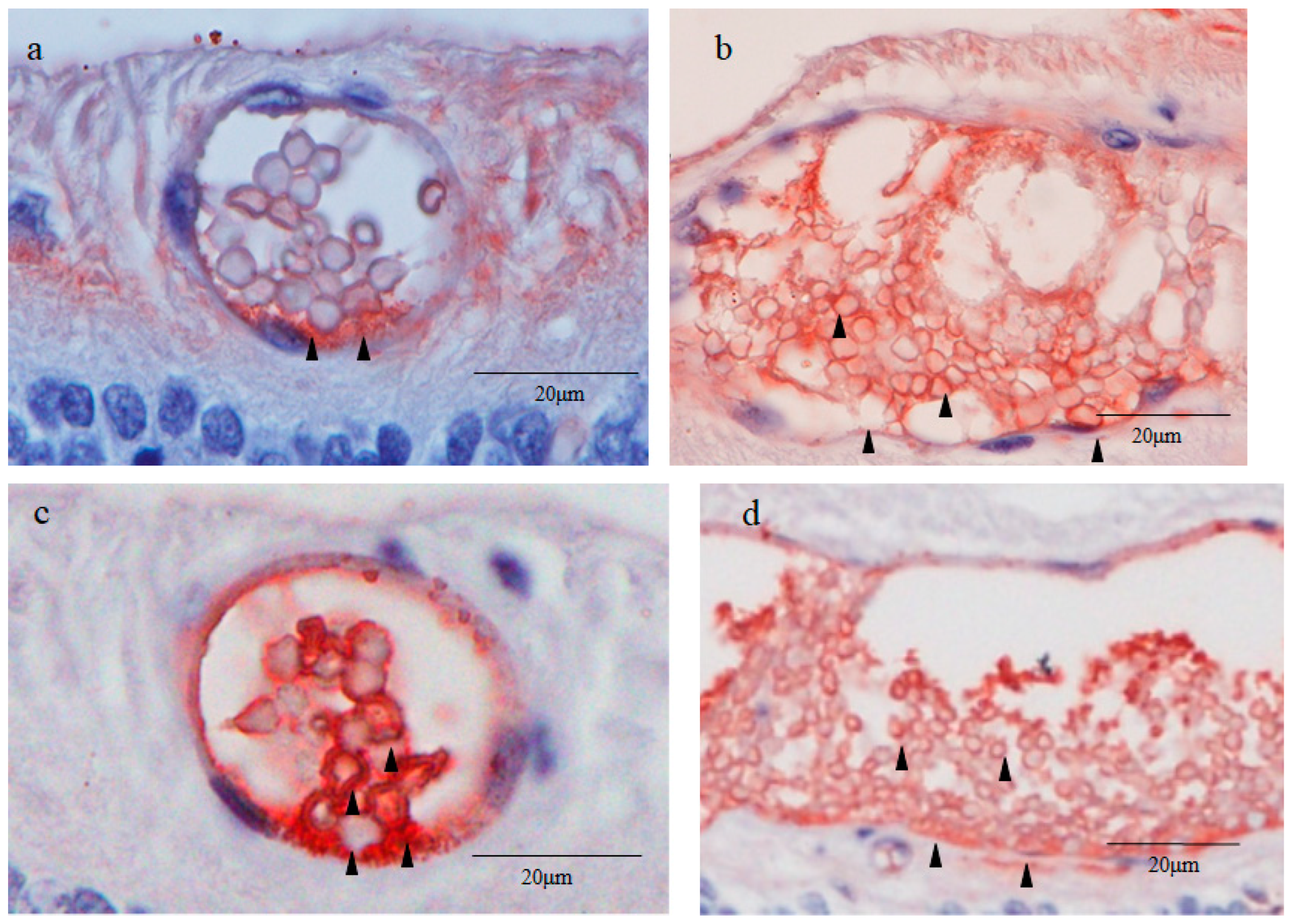
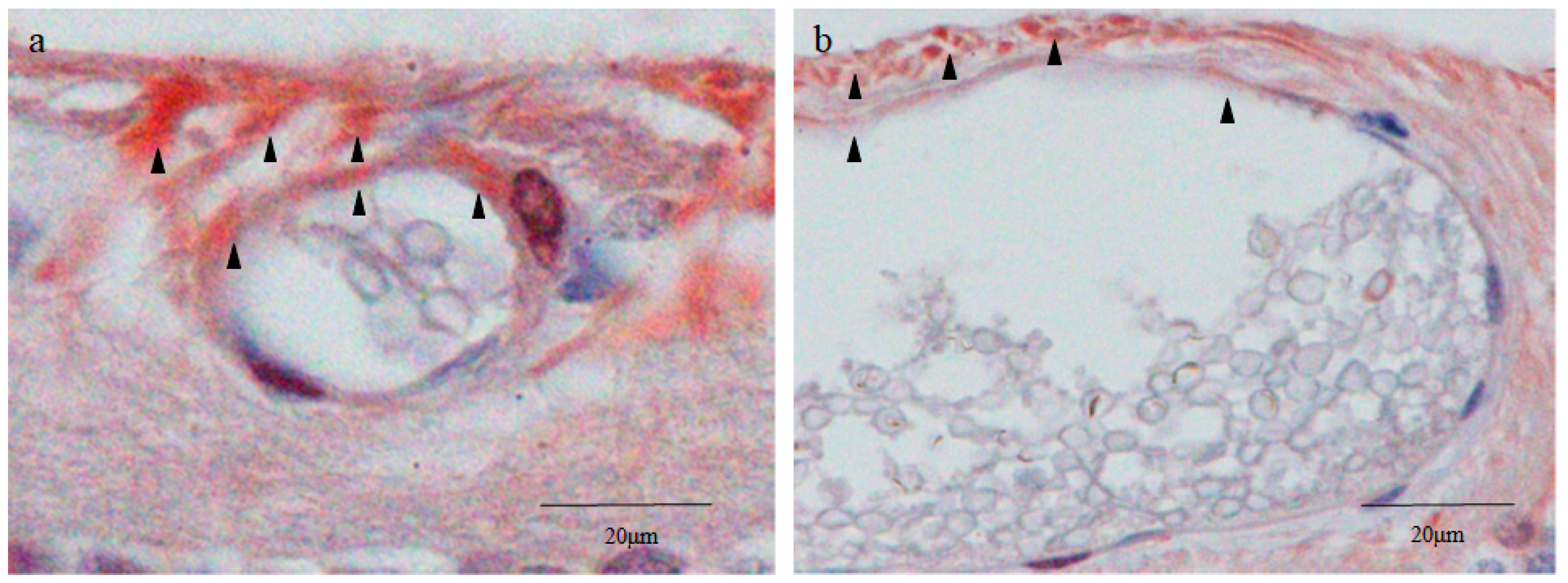
2.6. Sex-Determining Region Y-Box 2 (Sox2) and Caudal Type Homeobox 2 (CDX2) Immunostaining
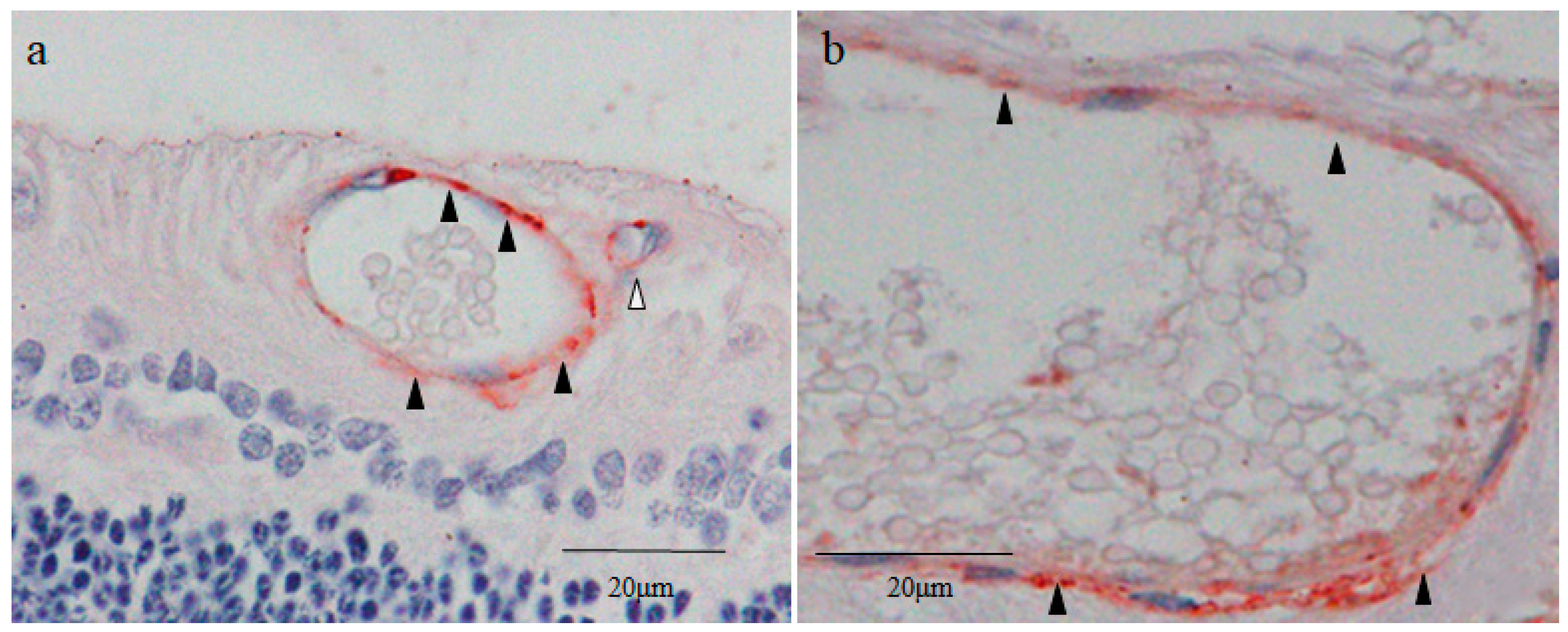
2.7. Cytokeratin 18 (CK18) and RPE65 Immunostaining
2.8. Yes-Associated Protein 1 (YAP1) Immunostaining
2.9. Von Willebrand Factor (vWF) Immunostaining
3. Discussion
4. Materials and Methods
4.1. Animals and Fundoscopic Examination of the Peripheral Retina of the Dogs
4.2. Preparation of Histological Sections of the Canine Peripheral Retina
4.3. Nestin Immunostaining
4.4. DAPI Nuclear Staining
4.5. Oct4, Nanog, Sox2, and CDX2 Immunostaining
4.6. CK18 and RPE65 Immunostaining
4.7. YAP1 Immunostaining
4.8. vWF Immunostaining
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grossniklaus, H.G.; Nickerson, J.M.; Edelhauser, H.F.; Bergman, L.A.M.K.; Bergman, L.A.M.K.; Berglin, L. Anatomic alterations in aging and age-related diseases of the eye. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF23–ORSF27. [Google Scholar] [CrossRef] [PubMed]
- Foos, R.Y.; Feman, S.S. Reticular cystoid degeneration of the peripheral retina. Am. J. Ophthalmol. 1970, 69, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Byer, N.E. Lattice degeneration of the retina. Surv. Ophthalmol. 1979, 23, 213–248. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, P.; Allen, R.A.; Straatsma, B.R.; O’Malley, C.C. Paving-stone degeneration of the retina. Arch. Ophthalmol. 1965, 73, 169–182. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, P.F.; Allen, R.A. Peripheral cystoid degeneration of the retina. Incidence and distribution in 1,000 autopsy eyes. Arch. Ophthalmol. 1967, 77, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Lewis, H. Peripheral retinal degenerations and the risk of retinal detachment. Am. J. Ophthalmol. 2003, 136, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.F.; Smelser, G.K. Some fine structural features of the ora serrata region in primate eyes. Investig. Ophthalmol. 1968, 7, 672–688. [Google Scholar]
- Johnsen, E.O.; Frøen, R.C.; Albert, R.; Omdal, B.K.; Sarang, Z.; Berta, A.; Nicolaissen, B.; Petrovski, G.; Moe, M.C. Activation of neural progenitor cells in human eyes with proliferative vitreoretinopathy. Exp. Eye Res. 2012, 98, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Francois, J.; Rabaey, M. Histopathological examination of a bilateral symmetrical cyst of the retina. Br. J. Ophthalmol. 1953, 37, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.; Moore, C.; Si, X.; Richer, S.; Jackson, J.; Wang, W. Aging dogs manifest myopia as measured by autorefractor. PLoS ONE 2016, 11, e0148436. [Google Scholar] [CrossRef]
- Okun, E.; Rubin, L.F.; Collins, E.M. Retinal breaks in the senile dog eye. Arch. Ophthalmol. 1961, 66, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Bellhorn, R.W.; Haring, B.C. Peripheral retinal cysts. Vet. Med. Small Anim. Clin. 1974, 69, 1528–1530. [Google Scholar] [PubMed]
- Watson, A.J. The cell biology of blastocyst development. Mol. Reprod. Dev. 1992, 33, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J. Stem cells from the Mammalian blastocyst. Stem Cells 2001, 19, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, D.; Hou, Y.; Jiao, L.; Zheng, X.; Wang, W.H. Isolation and culture of embryonic stem cells from porcine blastocysts. Mol. Reprod. Dev. 2003, 65, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.C.; VandeVoort, C.A.; Kumar, P.; Chang, T.C.; Golos, T.G. Trophoblast stem cells: Models for investigating trophectoderm differentiation and placental development. Endocr. Rev. 2009, 30, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Suwińska, A.; Czołowska, R.; Ozdzeński, W.; Tarkowski, A.K. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: Expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev. Biol. 2008, 322, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Fukumoto, M.; Sato, T.; Horie, T.; Kida, T.; Oku, H.; Nakamura, K.; Jin, D.; Takai, S.; Ikeda, T. Involvement of the retinal pigment epithelium in the development of retinal lattice degeneration. Int. J. Mol. Sci. 2020, 21, 7347. [Google Scholar] [CrossRef]
- Miura, M.; Makita, S.; Sugiyama, S.; Hong, Y.J.; Yasuno, Y.; Elsner, A.E.; Tamiya, S.; Tsukahara, R.; Iwasaki, T.; Goto, H. Evaluation of intraretinal migration of retinal pigment epithelial cells in age-related macular degeneration using polarimetric imaging. Sci. Rep. 2017, 7, 3150. [Google Scholar] [CrossRef]
- Chen, K.C.; Jung, J.J.; Curcio, C.A.; Balaratnasingam, C.; Gallego-Pinazo, R.; Dolz-Marco, R.; Freund, R.K.; Yannuzzi, L.A. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: Clinical and histologic study. Am. J. Ophthalmol. 2016, 164, 89–98. [Google Scholar] [CrossRef]
- Bogolyubova, I.; Bogolyubov, D. Heterochromatin morphodynamics in late oogenesis and early embryogenesis of mammals. Cells 2020, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Ralston, A.; Rossant, J. Cdx2 acts downstream of cell polarization to cell-autonomously promote trophectoderm fate in the early mouse embryo. Dev. Biol. 2008, 313, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.H.; Buhusi, M.C.; Ma, J.X.; Crouch, R.K. RPE65 is present in human green/red cones and promotes photopigment regeneration in an in vitro cone cell model. J. Neurosci. 2011, 31, 18618–18626. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, T.; Røsland, V.G.; Sakariassen, P.O.; Kavalar, R.; Lah, T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: Correlation of nestin with prognosis of patient survival. Surg. Neurol. 2007, 68, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, L.; Marcus, S.G.; Vasile, J.; Dobryansky, M.; Cohen, H.; Eng, K.; Shamamian, P.; Mignatti, P. Expression of Von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: A potential method for objective assessment of tumor angiogenesis. Int. J. Cancer 2000, 85, 281–288. [Google Scholar] [CrossRef]
- Ball, B.; Bulmer, J.N.; Ayis, S.; Lyall, F.; Robson, S.C. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J. Pathol. 2006, 208, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bosco, C.; González, J.; Gutiérrez, R.; Parra-Cordero, M.; Barja, P.; Rodrigo, R. Oxidative damage to pre-eclamptic placenta: Immunohistochemical expression of VEGF, nitrotyrosine residues and von Willebrand factor. J. Matern. Fetal Neonatal Med. 2012, 25, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Fetsch, J.F. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: Implications in tumor diagnosis. Hum. Pathol. 2000, 31, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, T.; Kageyama, N. Cdx2 homeodomain protein regulates the expression of MOK, a member of the mitogen-activated protein kinase superfamily, in the intestinal epithelial cells. FEBS Lett. 2004, 573, 147–154. [Google Scholar] [CrossRef]
- Xu, Y.; Watanabe, T.; Tanigawa, T.; Machida, H.; Okazaki, H.; Yamagami, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; et al. Bile acids induce cdx2 expression through the farnesoid x receptor in gastric epithelial cells. J. Clin. Biochem. Nutr. 2010, 46, 81–86. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; So, C.K.; Wang, S.; Wang, P.; Yan, L.; Myers, R.; Chen, Z.; Patterson, A.P.; Yang, C.S.; et al. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis 2007, 28, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Fransz, P.; Soppe, W.; Schubert, I. Heterochromatin in interphase nuclei of Arabidopsis thaliana. Chromosome Res. 2003, 11, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Sato, G.; Amai, T.; Ueda, M.; Kuroda, K. Development of artificial system to induce chromatin loosening in saccharomyces cerevisiae. Biomolecules 2022, 12, 1138. [Google Scholar] [CrossRef] [PubMed]
- Martins-Taylor, K.; Xu, R.H. Determinants of pluripotency: From avian, rodents, to primates. J. Cell Biochem. 2010, 109, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Ohbo, K.; Tomizawa, S. Epigenetic regulation in stem cell development, cell fate conversion, and reprogramming. Biomol. Concepts 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, E.; Hwang, S.U.; Yoon, J.D.; Jeon, Y.; Park, K.M.; Kim, K.J.; Jin, M.; Lee, C.K.; Lee, E.; et al. Ultrastructural comparison of porcine putative embryonic stem cells derived by in vitro fertilization and somatic cell nuclear transfer. J. Reprod. Dev. 2016, 62, 177–185. [Google Scholar] [CrossRef]
- Gauster, M.; Moser, G.; Wernitznig, S.; Kupper, N.; Huppertz, B. Early human trophoblast development: From morphology to function. Cell Mol. Life Sci. 2022, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Madeja, Z.E.; Hryniewicz, K.; Orsztynowicz, M.; Pawlak, P.; Perkowska, A. WNT/β-catenin signaling affects cell lineage and pluripotency-specific gene expression in bovine blastocysts: Prospects for bovine embryonic stem cell derivation. Stem Cells Dev. 2015, 24, 2437–2454. [Google Scholar] [CrossRef]
- Bou, G.; Liu, S.; Guo, J.; Zhao, Y.; Sun, M.; Xue, B.; Wang, J.; Wei, Y.; Kong, Q.; Liu, Z. Cdx2 represses Oct4 function via inducing its proteasome-dependent degradation in early porcine embryos. Dev. Biol. 2016, 410, 36–44. [Google Scholar] [CrossRef]
- Cui, W.; Mager, J. Transcriptional regulation and genes involved in first lineage specification during preimplantation development. Adv. Anat. Embryol. Cell Biol. 2018, 229, 31–46. [Google Scholar] [CrossRef]
- Strumpf, D.; Mao, C.A.; Yamanaka, Y.; Ralston, A.; Chawengsaksophak, K.; Beck, F.; Rossant, J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 2005, 132, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Karoubi, G.; Cortes-Dericks, L.; Gugger, M.; Galetta, D.; Spaggiari, L.; Schmid, R.A. Atypical expression and distribution of embryonic stem cell marker, OCT4, in human lung adenocarcinoma. J. Surg. Oncol. 2010, 102, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Haghpanah, V.; Malehmir, M.; Larijani, B.; Ahmadian, S.; Alimoghaddam, K.; Heshmat, R.; Ghavamzadeh, A.; Adabi, K.; Ghaffari, S.H. The beneficial effects of valproic acid in thyroid cancer are mediated through promoting redifferentiation and reducing stemness level: An in vitro study. J. Thyroid Res. 2014, 2014, 218763. [Google Scholar] [CrossRef] [PubMed]
- Osawa, Y.; Miyamoto, T.; Ohno, S.; Ohno, E. Morphological analysis of live undifferentiated cells derived from induced pluripotent stem cells. Stem Cells Dev. 2018, 27, 1–9. [Google Scholar] [CrossRef]
- Liu, J. The “life code”: A theory that unifies the human life cycle and the origin of human tumors. Semin. Cancer Biol. 2020, 60, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bou, G.; Sun, R.; Guo, S.; Xue, B.; Wei, R.; Cooney, A.J.; Liu, Z. Sox2 is the faithful marker for pluripotency in pig: Evidence from embryonic studies. Dev. Dyn. 2015, 244, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.P.; Kwok, J.C.F.; Vecino, E.; Martin, K.R.; Fawcett, J.W. A method for the isolation and culture of adult rat retinal pigment epithelial (RPE) cells to study retinal diseases. Front. Cell Neurosci. 2015, 9, 449. [Google Scholar] [CrossRef]
- Krugluger, W.; Seidel, S.; Steindl, K.; Binder, S. Epidermal growth factor inhibits glycogen synthase kinase-3 (GSK-3) and beta-catenin transcription in cultured ARPE-19 cells. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, M.A.; Maumenee, I.H.; Green, W.R.; Kenyon, K.R. Histopathology of Sanfilippo’s syndrome. Arch. Ophthalmol. 1983, 101, 1255–1262. [Google Scholar] [CrossRef]
- O’Driscoll, E.; Hughes, E.; Irnaten, M.; Kuehn, M.; Wallace, D.; O’Brien, C. Role of epithelial-to-mesenchymal transition of retinal pigment epithelial cells in glaucoma cupping. J. Clin. Med. 2023, 12, 2737. [Google Scholar] [CrossRef]
- Casaroli-Marano, R.P.; Pagan, R.; Vilaró, S. Epithelial-mesenchymal transition in proliferative vitreoretinopathy: Intermediate filament protein expression in retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2062–2072. [Google Scholar] [PubMed]
- Lu, B.; Hu, W.; Zhang, X.; Yang, N.; Bressler, N.; Kong, J. Epithelial-mesenchymal transition of retinal pigment epithelium from different sources in vitro. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3009. [Google Scholar]
- Kadyrov, M.; Kaufmann, P.; Huppertz, B. Expression of a cytokeratin 18 neo-epitope is a specific marker for trophoblast apoptosis in human placenta. Placenta 2001, 22, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Lian, C.; Shi, F.; Chen, L.; Qian, S.; Wang, H.; Zhao, X.; Ji, X.; Zhang, J.; Xu, G. The Petri Dish-N2B27 Culture Condition Maintains RPE Phenotype by Inhibiting Cell Proliferation and mTOR Activation. J. Ophthalmol. 2020, 2020, 4892978. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Leong, B.; Messinger, J.D.; Kar, D.; Ach, T.; Yannuzzi, L.A.; Freund, K.B.; Curcio, C.A. Hyperreflective foci, optical coherence tomography progression indicators in age-related macular degeneration, include transdifferentiated retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2021, 62, 34. [Google Scholar] [CrossRef] [PubMed]
- Soler, M.V.C.; Gallo, J.E.; Dodds, R.A.; Suburo, A.M. A mouse model of proliferative vitreoretinopathy induced by disperse. Exp. Eye Res. 2002, 75, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Straatsma, B.R.; Zeegen, P.D.; Foos, R.Y.; Feman, S.S.; Shabo, A.L. Lattice degeneration of the retina. XXX Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 1974, 77, 619–649. [Google Scholar] [CrossRef] [PubMed]
- Grisanti, S.; Esser, P.; Schraermeyer, U. Retinal pigment epithelial cells: Autocrine and paracrine stimulation of extracellular matrix contraction. Graefes Arch. Clin. Exp. Ophthalmol. 1997, 235, 587–598. [Google Scholar] [CrossRef]
- Luz-Madrigal, A.; Grajales-Esquivel, E.; McCorkle, A.; DiLoenzo, A.M.; Barbosa-Sabanero, K.; Tsonis, P.A.; Rio-Tsonis, K.D. Reprogramming of the chick retinal pigmented epithelium after retinal injury. BMC Biol. 2014, 12, 28. [Google Scholar] [CrossRef]
- Milyushina, L.A.; Verdiev, B.I.; Kuznetsova, A.V.; Aleksandrova, M.A. Expression of multipotent and retinal markers in pigment epithelium of adult human in vitro. Bull. Exp. Biol. Med. 2012, 153, 157–162. [Google Scholar] [CrossRef]
- Zhou, M.; Geathers, J.S.; Grillo, S.L.; Weber, S.R.; Wang, W.; Zhao, Y.; Sundstrom, J.M. Role of epithelial-mesenchymal transition in retinal pigment epithelium dysfunction. Front. Cell Dev. Biol. 2020, 8, 501. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J. Yes-associated protein is essential for proliferative vitreoretinopathy development via the epithelial-mesenchymal transition in retinal pigment epithelial fibrosis. J. Cell. Mol. Med. 2021, 25, 10213–10223. [Google Scholar] [CrossRef]
- Yamamura, S.; Goda, N.; Akizawa, H.; Kohri, N.; Balboula, A.Z.; Kobayashi, K.; Bai, H.; Takahashi, M.; Kawahara, M. Yes-associated protein 1 translocation through actin cytoskeleton organization in trophectoderm cells. Dev. Biol. 2020, 468, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fu, W.; Long, M.; Yuan, X.; Zhao, K.; Hu, X.; Liu, J.; Liu, W.; Peng, L.; Xiao, Y. Rapid establishment of Oct4: EGFP transgenic zebrafish homozygote through gynogenesis for monitoring the pluripotency during induction of pluripotent stem cells. Reprod. Breed. 2022, 3, 106–111. [Google Scholar] [CrossRef]
- Buehr, M.; Nichols, J.; Stenhouse, F.; Mountford, P.; Greenhalgh, C.J.; Kantachuvesiri, S.; Brooker, G.; Mullins, J.; Smith, A.G. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol. Reprod. 2003, 68, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Jinno, M.; Ozaki, T.; Iwashita, M.; Nakamura, Y.; Kudo, A.; Hirano, H. Measurement of endometrial tissue blood flow: A novel way to assess uterine receptivity for implantation. Fertil. Steril. 2001, 76, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Orsi, N.M.; Tribe, R.M. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J. Neuroendocrinol. 2008, 20, 462–469. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, Z.; Myers, D.P.; Terada, L.S. Mechanotransduction and anoikis: Death and the homeless cell. Cell Cycle 2008, 7, 2462–2465. [Google Scholar] [CrossRef]
- Wan, Y.; Almeida, A.D.; Rulands, S.; Chalour, N.; Muresan, L.; Wu, Y.; Simons, B.D.; He, J.; Harris, W.A. The ciliary marginal zone of the zebrafish retina: Clonal and time-lapse analysis of a continuously growing tissue. Development 2016, 143, 1099–1107. [Google Scholar] [CrossRef]
- Reh, T.A.; Levine, E.M. Multipotential stem cells and progenitors in the vertebrate retina. J. Neurobiol. 1998, 36, 206–220. [Google Scholar] [CrossRef]
- Kubota, R.; Hokoc, J.N.; Moshiri, A.; McGuire, C.; Reh, T.A. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res. Dev. Brain Res. 2002, 134, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Ooto, S.; Akagi, T.; Mandai, M.; Akaike, A.; Takahashi, M. Wnt signaling promotes regeneration in the retina of adult mammals. J. Neurosci. 2007, 27, 4210–4219. [Google Scholar] [CrossRef] [PubMed]
- Walcott, J.C.; Provis, J.M. Müller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin. Exp. Ophthalmol. 2003, 31, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Tackenberg, M.A.; Tucker, B.A.; Swift, J.S.; Jiang, C.; Redenti, S.; Greenberg, K.P.; Flannery, J.G.; Reichenbach, A.; Young, M.J. Müller cell activation, proliferation and migration following laser injury. Mol. Vis. 2009, 15, 1886–1896. [Google Scholar]
- Valamanesh, F.; Monnin, J.; Morand-Villeneuve, N.; Michel, G.; Zaher, M.; Miloudi, S.; Chemouni, D.; Jeanny, J.C.; Versaux-Botteri, C. Nestin expression in the retina of rats with inherited retinal degeneration. Exp. Eye Res. 2013, 110, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Singhal, S.; Lawrence, J.M.; Khaw, P.T.; Limb, G.A. Distribution of Müller stem cells within the neural retina: Evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp. Eye Res. 2009, 89, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarrete, G.C.; Angulo, A.; Martín-Nieto, J.; Cuenca, N. Gradual morphogenesis of retinal neurons in the peripheral retinal margin of adult monkeys and humans. J. Comp. Neurol. 2008, 511, 557–580. [Google Scholar] [CrossRef] [PubMed]
- Gilyarov, A.V. Nestin in central nervous system cells. Neurosci. Behav. Physiol. 2008, 38, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Nakamura, K.; Oku, H.; Horie, T.; Kida, T.; Takai, S. Immunohistological study of monkey foveal retina. Sci. Rep. 2019, 9, 5258. [Google Scholar] [CrossRef]
- Hendrickson, M.L.; Rao, A.J.; Demerdash, O.N.; Kalil, R.E. Expression of nestin by neural cells in the adult rat and human brain. PLoS ONE 2011, 6, e18535. [Google Scholar] [CrossRef]
- Viets, K.; Eldred, K.; Johnston, R.J., Jr. Mechanisms of photoreceptor patterning in vertebrates and invertebrates. Trends Genet. 2016, 32, 638–665. [Google Scholar] [CrossRef] [PubMed]
- Ahnelt, P.K. The photoreceptor mosaic. Eye 1998, 12, 531–540. [Google Scholar] [CrossRef]
- Williams, R.W. The human retina has a cone-enriched rim. Vis. Neurosci. 1991, 6, 403–406. [Google Scholar] [CrossRef]
- Quinn, N.; Csincsik, L.; Flynn, E.; Curcio, C.A.; Kiss, A.; Sadda, S.V.R.; Hogg, R.; Peto, T.; Lengyel, I. The clinical relevance of visualising the peripheral retina. Prog. Retin Eye Res. 2019, 68, 83–109. [Google Scholar] [CrossRef]
- Tschulakow, A.V.; Oltrup, T.; Bende, T.; Schmelzle, S. The anatomy of the foveola reinvestigated. PeerJ 2018, 6, e4482. [Google Scholar] [CrossRef] [PubMed]
- Ooto, S.; Hangai, M.; Takayama, K.; Ueda-Arakawa, N.; Makiyama, H.; Yoshimura, Y. High-resolution imaging of photoreceptors in macular microholes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5932–5943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Chang, Y.C.; Wu, W.C. Spectral-domain optical coherence tomography finding of acute retinal pigment epitheliitis. Taiwan J. Ophthalmol. 2019, 9, 276–279. [Google Scholar] [CrossRef]
- Mtanes, K.; Mimouni, M.; Zayit-Soudry, S. Laser pointer-induced maculopathy: More than meets the eye. J. Pediatr. Ophthalmol. Strabismus 2018, 55, 312–318. [Google Scholar] [CrossRef]
- Litts, K.M.; Messinger, J.D.; Freund, K.B.; Zhang, Y.; Curisio, C.A. Inner segment remodeling and mitochondrial translocation in cone photoreceptors in age-related macular degeneration with outer retinal tubulation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2243–2253. [Google Scholar] [CrossRef]
- Hendrickson, A.; Drucker, D. The development of parafoveal and mid-peripheral human retina. Behav. Brain Res. 1992, 49, 21–31. [Google Scholar]
- Saga, T.; Scheurer, D.; Adler, R. Development and maintenance of outer segments by isolated chick embryo photoreceptor cells in culture. Investig. Ophthalmol. Vis. Sci. 1996, 37, 561–573. [Google Scholar] [PubMed]
- Gunzinger, J.M.; Fasler, K.; Barthelmes, D.; Maloca, P.; Hasler, P.W.; Böni, C.; Zweifel, S.A. En face optical coherence tomography imaging ellipsoid zone regeneration in laser-induced and solar maculopathies. Case Rep. Ophthalmol. Med. 2019, 2019, 3849871. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, F.; Fossataro, F.; Fossataro, C.; Rizzo, C.; Rizzo, S. Spontaneous closure of full-thickness macular hole after pars plana vitrectomy for retinal detachment: Evidence of ellipsoid zone restoration. Retin. Cases Brief Rep. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Shifa, J.Z.; Gezum, A.M. Presenting signs of retinoblastoma at a tertiary level teaching hospital in Ethiopia. Pan. Afr. Med. J. 2017, 28, 66. [Google Scholar] [CrossRef] [PubMed]
- Nork, T.M.; Schwartz, T.L.; Doshi, H.M.; Millecchia, L.L. Retinoblastoma. Cell of origin. Arch. Ophthalmol. 1995, 113, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Bogenmann, E.; Lochrie, M.A.; Simon, M.L. Cone cell-specific genes expressed in retinoblastoma. Science 1988, 240, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Fang, Y.; Lee, T.C.; Almeida, D.; Jhanwar, S.; Abramson, D.H.; Cobrinik, D. A cone precursor phenotype distinguishes human retinoblastoma from mouse retinoblastoma models. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2012. [Google Scholar]
- Xu, X.L.; Fang, Y.; Lee, T.C.; Forrest, D.; Gregory-Evans, C.; Almeida, D.; Liu, A.; Jhanwar, S.C.; Abramson, D.H.; Cobrinik, D. Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell 2009, 137, 1018–10131. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, D.L.; Fagoonee, S.; Qiao, X.T.; Liebert, M.; Merchant, J.L.; Altruda, F.; Rizzetto, M. Tissue stem cells and cancer stem cells: Potential implications for gastric cancer. Panminerva Med. 2008, 50, 65–71. [Google Scholar]
- Wicha, M.C.; Liu, S.; Dontu, G. Cancer stem cells: An old idea—A paradigm shift. Cancer Res. 2006, 66, 1883–1890; discussion 1895–1896. [Google Scholar] [CrossRef]
- Luu, P.L.; Gerovska, D.; Schöler, H.R.; Araúzo-Bravo, M.J. Rules governing the mechanism of epigenetic reprogramming memory. Epigenomics 2018, 10, 149–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Li, Y.; Hua, B. A chromosomal investigation of four species of Chinese Panorpidae (Insecta, Mecoptera). Comp. Cytogenet. 2013, 7, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Masui, Y.; Wang, P. Cell cycle transition in early embryonic development of Xenopus laevis. Biol. Cell 1998, 90, 537–548. [Google Scholar] [CrossRef]
- Wickramasinghe, D.; Ebert, K.M.; Albertini, D.F. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev. Biol. 1991, 143, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, D.; Li, W.; Gao, Q.; Tao, J.; Liu, H. Comprehensive analysis of nonsurrounded nucleolus and surrounded nucleolus oocytes on chromatin accessibility using ATAC-seq. Mol. Reprod. Dev. 2023, 90, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Nakajima, R.; Nagata, M.; Aoki, F. Contribution of the oocyte nucleus and cytoplasm to the determination of meiotic and developmental competence in mice. Hum. Reprod. 2008, 23, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Harumoto, T.; Miyake, A. Chemical induction of conjugation by DAPI (4′, 6-diamidino-2-phenylindole) and other DNA-binding compounds in Paramecium caudatum. Eur. J. Protistol. 1996, 32, 37–45. [Google Scholar] [CrossRef]
- Ishiwata, K.; Oikawa, A. Actions of human DNA glycosylases on uracil-containing DNA, methylated DNA and their reconstituted chromatins. Biochim. Biophys. Acta 1979, 563, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Halley-Stott, R.P.; Pasque, V.; Astrand, C.; Miyamoto, K.; Simeoni, I.; Jullien, J.; Gurdon, J.B. Mammalian nuclear transplantation to Germinal Vesicle stage Xenopus oocytes—A method for quantitative transcriptional reprogramming. Methods 2010, 51, 56–65. [Google Scholar] [CrossRef]
- Andrés-Manzano, M.J.; Andrés, V.; Dorado, B. Oil Red O and hematoxylin and eosin staining for quantification of atherosclerosis burden in mouse aorta and aortic root. Methods Mol. Biol. 2015, 1339, 85–99. [Google Scholar] [CrossRef]
- Kime, C.; Kiyonari, H.; Ohtsuka, S.; Kohbayashi, E.; Asahi, M.; Yamanaka, S.; Takahashi, M.; Tomoda, K. Induced 2C expression and implantation-competent blastocyst-like cysts from primed pluripotent stem cells. Stem Cell Rep. 2019, 13, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhong, C.; Yu, Y.; Liu, H.; Sakurai, M.; Yu, L.; Min, Z.; Shi, L.; Wei, Y.; Takahashi, Y.; et al. Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell 2019, 179, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Pour, M.; Nachman, I. Building blastocysts from stem cells. Stem Cell Rep. 2019, 13, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Virant-Klun, I.; Rozman, B.; Vrtacnik-Bokal, E.; Novakovic, S.; Rülicke, T.; Dovc, P.; Meden-Vrtovec, H. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev. 2009, 18, 137–149. [Google Scholar] [CrossRef]

| Small Cysts | Large Cysts | |||||||
|---|---|---|---|---|---|---|---|---|
| Marker | Inner Cell Nuclei | Inner Cell Cytoplasms | Cyst Wall Cells | Surrounding Cells | Inner Cell Nuclei | Inner Cell Cytoplasms | Cyst Wall Cells | Surrounding Cells |
| Nestin | ― | +++ | +++ | ― | ― | + | ++ | ― |
| Oct4 | ++ | +++ | +++ | ++ | ― | ― | ++ | + |
| Nanog | + | +++ | +++ | +++ | ― | ++ | + | ++ |
| Sox2 | ++ | ++ | ++ | ++ | + | ++ | + | + |
| CDX2 | ++ | +++ | +++ | ― | + | ++ | ++ | ― |
| CK18 | + | +++ | +++ | ― | ― | + | +++ | ― |
| RPE65 | ― | ― | ― | +++ | ― | ― | ― | +++ |
| YAP1 | ― | ― | +++ | +++ | ― | ― | ++ | +++ |
| vWF | ― | ― | +++ | ― | ― | ― | +++ | ― |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, T.; Jin, D.; Takai, S.; Nakamura, K.; Nemoto, E.; Kojima, S.; Oku, H. Blastocyst-like Structures in the Peripheral Retina of Young Adult Beagles. Int. J. Mol. Sci. 2024, 25, 6045. https://doi.org/10.3390/ijms25116045
Ikeda T, Jin D, Takai S, Nakamura K, Nemoto E, Kojima S, Oku H. Blastocyst-like Structures in the Peripheral Retina of Young Adult Beagles. International Journal of Molecular Sciences. 2024; 25(11):6045. https://doi.org/10.3390/ijms25116045
Chicago/Turabian StyleIkeda, Tsunehiko, Denan Jin, Shinji Takai, Kimitoshi Nakamura, Emika Nemoto, Shota Kojima, and Hidehiro Oku. 2024. "Blastocyst-like Structures in the Peripheral Retina of Young Adult Beagles" International Journal of Molecular Sciences 25, no. 11: 6045. https://doi.org/10.3390/ijms25116045
APA StyleIkeda, T., Jin, D., Takai, S., Nakamura, K., Nemoto, E., Kojima, S., & Oku, H. (2024). Blastocyst-like Structures in the Peripheral Retina of Young Adult Beagles. International Journal of Molecular Sciences, 25(11), 6045. https://doi.org/10.3390/ijms25116045






