Diagnostic and Prognostic Ability of Pancreatic Stone Protein: A Scoping Review
Abstract
1. Introduction
2. Methods
Eligibility Criteria, Searches, and Data Collection
3. Results
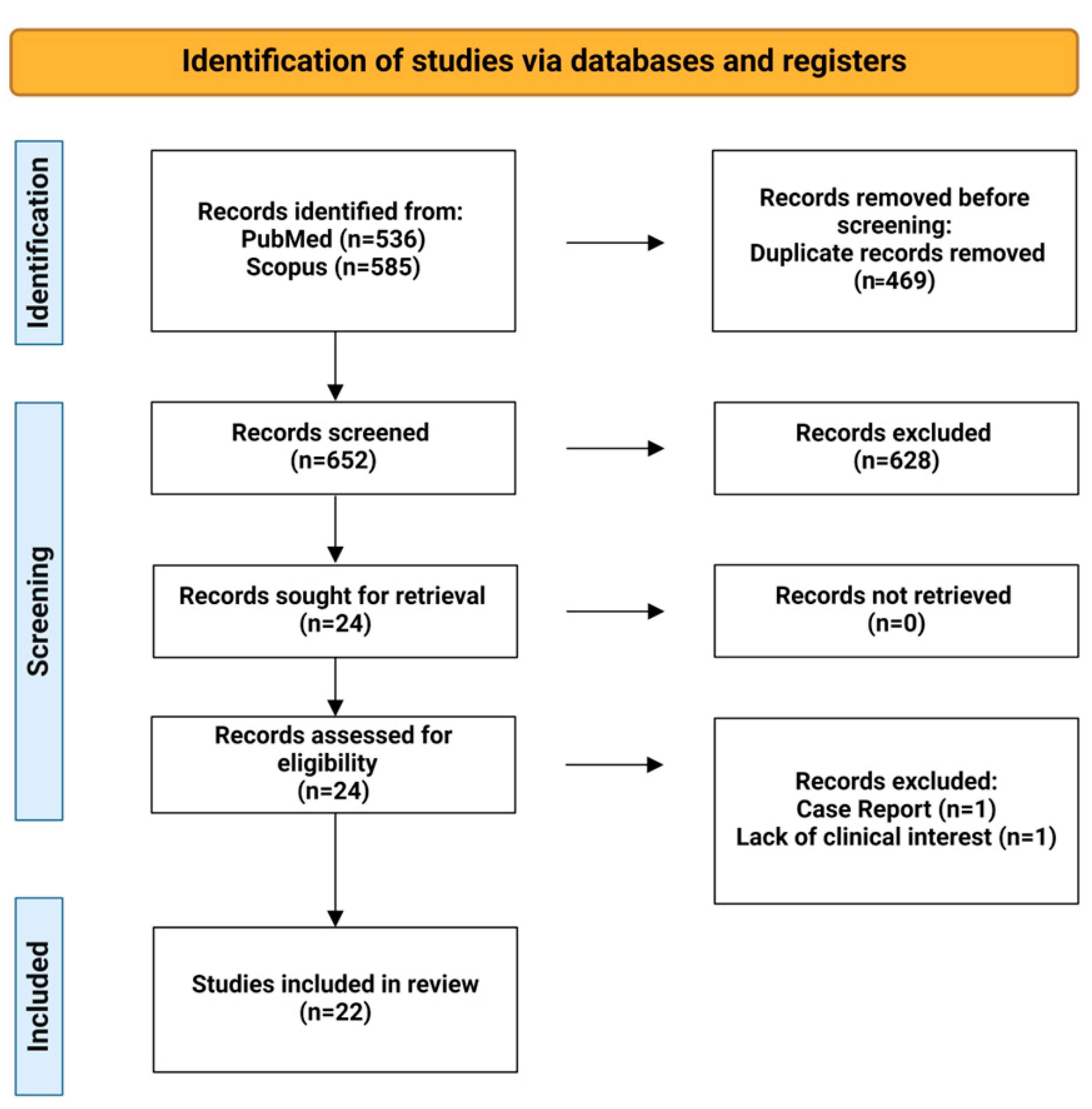
3.1. Flow Diagram
3.2. Type of Studies
3.3. Settings
3.4. Type of Infections
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.-H.; Huang, S.; Kerr, D. Biomarkers in clinical medicine. IARC Sci. Publ. 2011, 163, 303–322. [Google Scholar]
- Reinhart, K.; Bauer, M.; Riedemann, N.C.; Hartog, C.S. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin. Microbiol. Rev. 2012, 25, 609–634. [Google Scholar] [CrossRef] [PubMed]
- Keel, M.; Härter, L.; Reding, T.; Sun, L.K.; Hersberger, M.; Seifert, B.; Bimmler, D.; Graf, R. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit. Care Med. 2009, 37, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Ventura, F.; Eggimman, P.; Daix, T.; Francois, B.; Pugin, J. Pancreatic Stone Protein Measurement to Screen and Diagnose Sepsis in the Context of the Surviving Sepsis Campaign Recommendations. Med. Res. Arch. 2024, 11. [Google Scholar] [CrossRef]
- Hu, P.; Lu, Y.H.; Deng, W.; Li, Q.; Zhao, N.; Shao, Q.; Wu, L.; Wang, X.Z.; Qian, K.J.; Liu, F. The critical role of pancreatic stone protein/regenerating protein in sepsis-related multiorgan failure. Front. Med. 2023, 10, 1172529. [Google Scholar] [CrossRef] [PubMed]
- Michailides, C.; Lagadinou, M.; Paraskevas, T.; Papantoniou, K.; Kavvousanos, M.; Vasileiou, A.; Thomopoulos, K.; Velissaris, D.; Marangos, M. The Role of the Pancreatic Stone Protein in Predicting Intra-Abdominal Infection-Related Complications: A Prospective Observational Single-Center Cohort Study. Microorganisms 2023, 11, 2579. [Google Scholar] [CrossRef] [PubMed]
- Lagadinou, M.; Paraskevas, T.; Velissaris, D.; Michailides, C.; Eleftherakis, G.; Sampsonas, F.; Siakallis, G.; Assimakopoulos, S.F.; Marangos, M. The role of pancreatic stone protein as a prognostic factor for COVID-19 patients. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6391–6395. [Google Scholar] [CrossRef] [PubMed]
- Van Singer, M.; Brahier, T.; Brochu Vez, M.J.; Gerhard Donnet, H.; Hugli, O.; Boillat-Blanco, N. Pancreatic stone protein for early mortality prediction in COVID-19 patients. Crit. Care 2021, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- García de Guadiana-Romualdo, L.; Albaladejo-Otón, M.D.; Berger, M.; Jiménez-Santos, E.; Jiménez-Sánchez, R.; Esteban-Torrella, P.; Rebollo-Acebes, S.; Hernando-Holgado, A.; Ortín-Freire, A.; Trujillo-Santos, J. Prognostic performance of pancreatic stone protein in critically ill patients with sepsis. Biomark. Med. 2019, 13, 1469–1480. [Google Scholar] [CrossRef]
- Que, Y.A.; Guessous, I.; Dupuis-Lozeron, E.; de Oliveira, C.R.A.; Oliveira, C.F.; Graf, R.; Seematter, G.; Revelly, J.P.; Pagani, J.L.; Liaudet, L.; et al. Prognostication of Mortality in Critically Ill Patients With Severe Infections. Chest 2015, 148, 674–682. [Google Scholar] [CrossRef]
- Gukasjan, R.; Raptis, D.A.; Schulz, H.U.; Halangk, W.; Graf, R. Pancreatic stone protein predicts outcome in patients with peritonitis in the ICU. Crit. Care Med. 2013, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Scherr, A.; Graf, R.; Bain, M.; Christ-Crain, M.; Müller, B.; Tamm, M.; Stolz, D. Pancreatic stone protein predicts positive sputum bacteriology in exacerbations of COPD. Chest 2013, 143, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.A.; Delodder, F.; Guessous, I.; Graf, R.; Bain, M.; Calandra, T.; Liaudet, L.; Eggimann, P. Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Crit. Care 2012, 16, R114. [Google Scholar] [CrossRef] [PubMed]
- Boeck, L.; Graf, R.; Eggimann, P.; Pargger, H.; Raptis, D.A.; Smyrnios, N.; Thakkar, N.; Siegemund, M.; Rakic, J.; Tamm, M.; et al. Pancreatic stone protein: A marker of organ failure and outcome in ventilator-associated pneumonia. Chest 2011, 140, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Loots, F.J.; Smits, M.; Jenniskens, K.; van Zanten, A.R.H.; Kusters, R.; Verheij, T.J.M.; Hopstaken, R.M. Added Diagnostic Value of Biomarkers in Patients with Suspected Sepsis: A Prospective Cohort Study in Out-Of-Hours Primary Care. J. Appl. Lab. Med. 2022, 7, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Fisher, O.M.; Oberkofler, C.E.; Raptis, D.A.; Soll, C.; Béchir, M.; Schiesser, M.; Graf, R. Pancreatic stone protein (PSP) and pancreatitis-associated protein (PAP): A protocol of a cohort study on the diagnostic efficacy and prognostic value of PSP and PAP as postoperative markers of septic complications in patients undergoing abdominal surgery (PSP study). BMJ Open 2014, 4, e004914. [Google Scholar] [CrossRef] [PubMed]
- de Hond, T.A.P.; Oosterheert, J.J.; van Hemert-Glaubitz, S.J.M.; Musson, R.E.A.; Kaasjager, K.A.H. Pancreatic Stone Protein as a Biomarker for Sepsis at the Emergency Department of a Large Tertiary Hospital. Pathogens 2022, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- García de Guadiana-Romualdo, L.; Berger, M.; Jiménez-Santos, E.; Rebollo-Acebes, S.; Jiménez-Sánchez, R.; Esteban-Torrella, P.; Hernando-Holgado, A.; Ortín-Freire, A.; Albaladejo-Otón, M.D. Pancreatic stone protein and soluble CD25 for infection and sepsis in an emergency department. Eur. J. Clin. Investig. 2017, 47, 297–304. [Google Scholar] [CrossRef] [PubMed]
- García de Guadiana-Romualdo, L.; Jiménez-Santos, E.; Cerezuela-Fuentes, P.; Español-Morales, I.; Berger, M.; Esteban-Torrella, P.; Hernando-Holgado, A.; Albaladejo-Otón, M.D. Analyzing the capability of PSP, PCT and sCD25 to support the diagnosis of infection in cancer patients with febrile neutropenia. Clin. Chem. Lab. Med. 2019, 57, 540–548. [Google Scholar] [CrossRef]
- Niggemann, P.; Rittirsch, D.; Buehler, P.K.; Schweizer, R.; Giovanoli, P.; Reding, T.; Graf, R.; Plock, J.A.; Klein, H.J. Incidence and Time Point of Sepsis Detection as Related to Different Sepsis Definitions in Severely Burned Patients and Their Accompanying Time Course of Pro-Inflammatory Biomarkers. J. Pers. Med. 2021, 11, 701. [Google Scholar] [CrossRef]
- Klein, H.J.; Niggemann, P.; Buehler, P.K.; Lehner, F.; Schweizer, R.; Rittirsch, D.; Fuchs, N.; Waldner, M.; Steiger, P.; Giovanoli, P.; et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study. Ann. Surg. 2021, 274, e1179–e1186. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Rittirsch, D.; Buehler, P.K.; Schweizer, R.; Giovanoli, P.; Cinelli, P.; Plock, J.A.; Reding, T.; Graf, R. Response of routine inflammatory biomarkers and novel Pancreatic Stone Protein to inhalation injury and its interference with sepsis detection in severely burned patients. Burns 2021, 47, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Tschuor, C.; Raptis, D.A.; Limani, P.; Bächler, T.; Oberkofler, C.E.; Breitenstein, S.; Graf, R. The value of pancreatic stone protein in predicting acute appendicitis in patients presenting at the emergency department with abdominal pain. BMC Gastroenterol. 2012, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J.; Daix, T.; Pagani, J.L.; Morri, D.; Giacomucci, A.; Dequin, P.F.; Guitton, C.; Que, Y.A.; Zani, G.; Brealey, D.; et al. Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: A prospective multicentric study. Crit. Care 2021, 25, 151. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Csordas, A.; Falk, V.; Slankamenac, K.; Rudiger, A.; Schönrath, F.; Rodriguez Cetina Biefer, H.; Starck, C.T.; Graf, R. Pancreatic stone protein predicts postoperative infection in cardiac surgery patients irrespective of cardiopulmonary bypass or surgical technique. PLoS ONE 2015, 10, e0120276. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.J.; Berger, M.; Gregory, M.; Ramaiah, R.; Taylor, A.L.; Curdt, I.; Lajaunias, F.; Graf, R.; Blincko, S.J.; Drage, S.; et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit. Care 2013, 17, R60. [Google Scholar] [CrossRef] [PubMed]
- Prazak, J.; Irincheeva, I.; Llewelyn, M.J.; Stolz, D.; García de Guadiana Romualdo, L.; Graf, R.; Reding, T.; Klein, H.J.; Eggimann, P.; Que, Y.A. Accuracy of pancreatic stone protein for the diagnosis of infection in hospitalized adults: A systematic review and individual patient level meta-analysis. Crit. Care 2021, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Zhou, L.; Wang, Q.; Ding, B.; Zhan, Y.; Qin, S.; Zhu, N.; Li, Z.; Lei, Z. Diagnostic accuracy of pancreatic stone protein in patients with sepsis: A systematic review and meta-analysis. BMC Infect. Dis. 2024, 24, 472. [Google Scholar] [CrossRef]
- Zuercher, P.; Moser, A.; Garcia de Guadiana-Romualdo, L.; Llewelyn, M.J.; Graf, R.; Reding, T.; Eggimann, P.; Que, Y.A.; Prazak, J. Discriminative performance of pancreatic stone protein in predicting ICU mortality and infection severity in adult patients with infection: A systematic review and individual patient level meta-analysis. Infection 2023, 51, 1797–1807. [Google Scholar] [CrossRef]
| Title | Author | Year | Study Design | Settings | Type | Population | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age | Sex (no. (%)) | Subgroup | |||||||
| The critical role of pancreatic stone protein/regenerating protein in sepsis-related multiorgan failure [5] | Hu P | 2023 | Prospective study | ICU | Prognostic | 141 | Median 61 (IQR: 50–72) | Male 91 (64) | 1. MODS 48–72 h after admission 2. 28-day mortality | |
| The role of the pancreatic stone protein in predicting intra-abdominal infection-related complications: A prospective observational single-center cohort study [6] | Michailides C | 2023 | Prospective study | Department of Internal Medicine | Prognostic | 40 | Mean 64.2 ± 22.8 | Intra-abdominal infection (IAI) | 1. Sepsis 2. Hospital readmission 3. Need of treatment escalation 4. Need of surgery treatment 5. Mortality 6. Length of hospital stay (LOS) | |
| The role of pancreatic stone protein as a prognostic factor for COVID-19 patients [7] | Lagadinou M | 2022 | Prospective study | Department of Internal Medicine | Prognostic | 55 | Mean 68.8 ± 14 | Male (51.9) | COVID-19 patients | 1. In-hospital mortality of patients with COVID-19 2. Non-invasive mechanical ventilation |
| Pancreatic stone protein for early mortality prediction in COVID-19 patients [8] | Van Singer M | 2021 | Prospective study | ED and ICU | Prognostic | 173 | Survival group: median 64.0 (IQR: 52.0–75.0), Dead group: median 81.50 (IQR: 70.3–83.3) | Female survival group, 102; death group, 5 | COVID-19 patients | 1. 7-day mortality 2. 7-day ICU admission |
| Prognostic performance of pancreatic stone protein in critically ill patients with sepsis [9] | García de Guadiana-Romualdo L | 2019 | Single-center, prospective, and observational study | ICU | Prognostic | 122 | Median 62 (IQR: 52–72) | Male, 68 (55.7) | 28-day mortality of PSP in critically ill patients with sepsis | |
| Prognostication of mortality in critically ill patients with severe infections [10] | Que YA | 2015 | Prospective study | ICU | Prognostic | 158 Verification Group, 91 Validation Group | Verification group: mean 61.2 ± 18.2, Validation group: mean 59.9 ± 16.1 | Derivation group, female, 93 (65); Validation group, female 54 (37) | Hospital mortality | |
| Pancreatic stone protein predicts outcome in patients with peritonitis in the ICU [11] | Gukasjan R | 2013 | Prospective study | ICU | Prognostic | 91 | Median 66 (IQR: 50–72) | Male, 53 (58); Female, 38 (42) | 1. Organ failure 2. Multiorgan failure 3. Death in the ICU | |
| Pancreatic stone protein predicts positive sputum bacteriology in exacerbations of COPD [12] | Scherr A | 2013 | Prospective, monocentric study | ED | Prognostic | 200 | Median 70 (IQR: 42–91) | Male, 114 (57) | Exacerbations of COPD | Lung bacterial infection in AECOPD |
| Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management [13] | Que YA | 2012 | Prospective cohort study | ICU | Prognostic | 107 | Mean 59 ± 17.5 | Male, 93; Female, 65 | Hospital mortality | |
| Pancreatic stone protein: a marker of organ failure and outcome in ventilator-associated pneumonia [14] | Boeck L | Retrospective study | ICU | Prognostic | 101 | Median 57 (IQR: 43–70) | Male 74 | Ventilator-associated pneumonia (VAP) | 1. Organ failure 2. Mortality in VAP | |
| Added diagnostic value of biomarkers in patients with suspected sepsis: A prospective cohort study in out-of-hours primary care [15] | Loots FJ | 2022 | Prospective study | Primary care | Diagnostic and Prognostic | 336 | Median 80 (IQR: 74–85) | Male, 123 (60); Female; 83 (40) | Septic patients | 1. Sepsis (no. = 141) within 72 h of inclusion 2. ICU admission within 72 h or 30-day mortality |
| Pancreatic stone protein (PSP) and pancreatitis-associated protein (PAP): a protocol of a cohort study on the diagnostic efficacy and prognostic value of PSP and PAP as postoperative markers of septic complications in patients undergoing abdominal surgery (PSP study) [16] | Fisher OM | 2014 | Prospective monocentric cohort study | Surgical ICU | Diagnostic and Prognostic | 160 | Unclear | Unclear | Unclear | 1. Sepsis 2. Mortality |
| Title | Author | Year | Study Design | Settings | Type | Population | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Age | Sex (no. (%)) | Subgroup | |||||||
| Pancreatic stone protein as a biomarker for sepsis at the emergency department of a large tertiary hospital [17] | de Hond TAP | 2022 | Semi-prospective, observational cohort study, mono-center | ED | Diagnostic | 156 | Median 60.0 (IQR: 44.5–73.0) | Male, 82 (52.6) | Sepsis diagnosis (no. = 26) | |
| Incidence and time point of sepsis detection as related to different sepsis definitions in severely burned patients and their accompanying time course of pro-inflammatory biomarkers [20] | Niggemann P | 2021 | Retrospective study | Burn Center | Diagnostic | 90 | Mean 48.5 ± 18.8 | Female, 18 (20); Male, 72 (80) | Severely Burned Patients | 1. Sepsis-3 (no. = 46) 2. Sepsis ABA 2007 (no. = 33) 3. Sepsis Zurich Burn Center (no. = 24) |
| Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: a prospective multicentric study [24] | Pugin J | 2021 | Prospective observational clinical study | ICU | Diagnostic | 243 | Median 65.0 (IQR: 54.0–73.0) | Female, 90 (37); Male, 153 (63) | Sepsis | |
| Response of routine inflammatory biomarkers and novel Pancreatic Stone Protein to inhalation injury and its interference with sepsis detection in severely burned patients [22] | Klein HJ | 2020 | Longitudinal, observational study | Burn Center | Diagnostic | 90 | Median 52 (IQR: 9) | Female, 18 (20); Male, 72 (80) | Inhalation Injury no. = 27, ARDS (32%) | Sepsis |
| Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity: A Monocentric Observational Study [21] | Klein HJ | 2021 | Observational study | Burn Center | Diagnostic | 90 | Mean 48.5 ± 18.8 | Female, 18 (20); Male, n 72 (80) | Severely burned patients | 1. Sepsis 2. Infection |
| Analyzing the capability of PSP, PCT and sCD25 to support the diagnosis of infection in cancer patients with febrile neutropenia [19] | García de Guadiana-Romualdo L | 2018 | Single-center prospective observational cohort study | ED | Diagnostic | 105 | Median 63 (IQR: 50–70) | Male, 43 (37.7) | Cancer patients with chemotherapy-associated febrile neutropenia (FN) | Infection |
| Pancreatic stone protein and soluble CD25 for infection and sepsis in an emergency department [18] | García de Guadiana-Romualdo L | 2017 | Prospective observational study | ED | Diagnostic | 152 | Median 66 (IQR: 33) | Male, 88 (57.9) | 1. Sepsis 2. Infection | |
| Pancreatic stone protein predicts postoperative infection in cardiac surgery patients irrespective of cardiopulmonary bypass or surgical technique [25] | Klein HJ | 2015 | Prospective, single-center cohort study | Cardiosurgical ICU | Diagnostic | 120 | Median 66.5 (IQR: 54.2–75.0) | Female, (27); Male (73) | Infection | |
| Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care [26] | Llewelyn MJ | 2013 | Observational study | ICU, high-dependency care | Diagnostic | 219 | Median 65.9 (IQR: 52.0–76) | Female, 93 (42) | 1. Sepsis diagnosis 2. Discrimination severe sepsis from non-infective SIRS | |
| The value of pancreatic stone protein in predicting acute appendicitis in patients presenting at the emergency department with abdominal pain [23] | Tschuor C | 2012 | Prospective, multi-center, cohort study, clinical Trial | ED, Department of Surgery, Division of Visceral and Transplantation Surgery | Diagnostic | 245 (Interim analysis will be performed once 123 patients are recruited.) | Unclear | Unclear | Acute appendicitis diagnosis | |
| Added Diagnostic Value of Biomarkers in Patients with Suspected Sepsis: A Prospective Cohort Study in Out-Of-Hours Primary Care [15] | Loots FJ | 2022 | Prospective study | Primary care | Diagnostic and Prognostic | 336 | Median 80 (IQR: 74–85) | Male, 123 (60); Female, 83 (40) | Septic patients | 1. Sepsis (no. = 141) within 72 h of inclusion 2. ICU admission within 72 h or 30-day mortality |
| Pancreatic stone protein (PSP) and pancreatitis-associated protein (PAP): a protocol of a cohort study on the diagnostic efficacy and prognostic value of PSP and PAP as postoperative markers of septic complications in patients undergoing abdominal surgery (PSP study) [16] | Fisher OM | 2014 | Prospective monocentric cohort study | Surgical ICU | Diagnostic and Prognostic | 160 | Unclear | Unclear | Unclear | 1. Sepsis 2. Mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailides, C.; Paraskevas, T.; Demiri, S.; Chourpiliadi, C.; Papantoniou, K.; Aggeletopoulou, I.; Velissari, E.K.; Lagadinou, M.; Triantos, C.; Velissaris, D. Diagnostic and Prognostic Ability of Pancreatic Stone Protein: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 6046. https://doi.org/10.3390/ijms25116046
Michailides C, Paraskevas T, Demiri S, Chourpiliadi C, Papantoniou K, Aggeletopoulou I, Velissari EK, Lagadinou M, Triantos C, Velissaris D. Diagnostic and Prognostic Ability of Pancreatic Stone Protein: A Scoping Review. International Journal of Molecular Sciences. 2024; 25(11):6046. https://doi.org/10.3390/ijms25116046
Chicago/Turabian StyleMichailides, Christos, Themistoklis Paraskevas, Silvia Demiri, Charikleia Chourpiliadi, Konstantinos Papantoniou, Ioanna Aggeletopoulou, Eleni Konstantina Velissari, Maria Lagadinou, Christos Triantos, and Dimitrios Velissaris. 2024. "Diagnostic and Prognostic Ability of Pancreatic Stone Protein: A Scoping Review" International Journal of Molecular Sciences 25, no. 11: 6046. https://doi.org/10.3390/ijms25116046
APA StyleMichailides, C., Paraskevas, T., Demiri, S., Chourpiliadi, C., Papantoniou, K., Aggeletopoulou, I., Velissari, E. K., Lagadinou, M., Triantos, C., & Velissaris, D. (2024). Diagnostic and Prognostic Ability of Pancreatic Stone Protein: A Scoping Review. International Journal of Molecular Sciences, 25(11), 6046. https://doi.org/10.3390/ijms25116046










