Molecular Mechanisms of N-Acetylcysteine in RSV Infections and Air Pollution-Induced Alterations: A Scoping Review
Abstract
1. Introduction
2. Material and Methods
2.1. Study Protocol
2.1.1. Conceptual Framework
2.1.2. Eligibility Criteria
2.1.3. Search Strategy
2.1.4. Data Extraction
2.1.5. Synthesis of the Results
3. Results
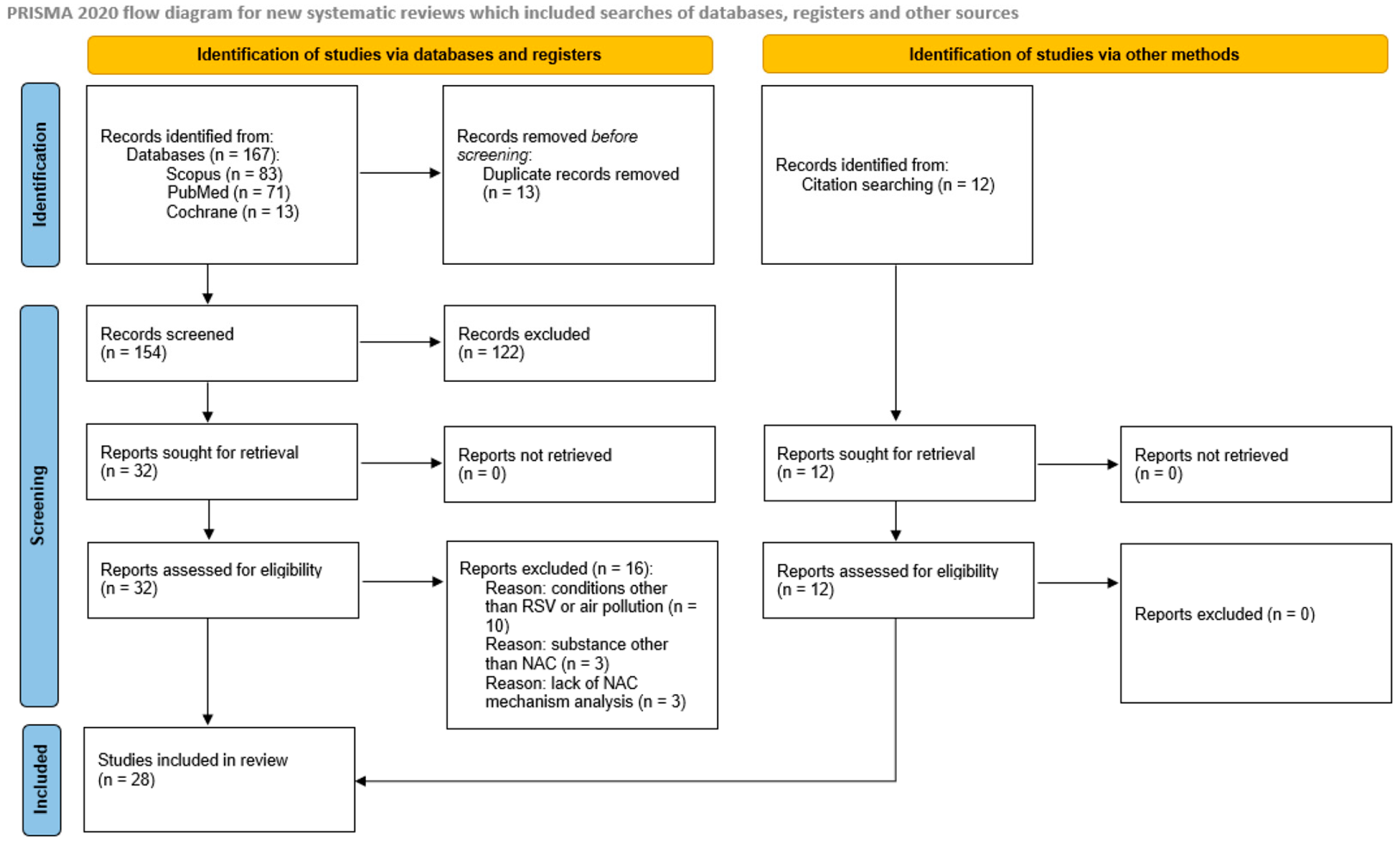
3.1. Search Results
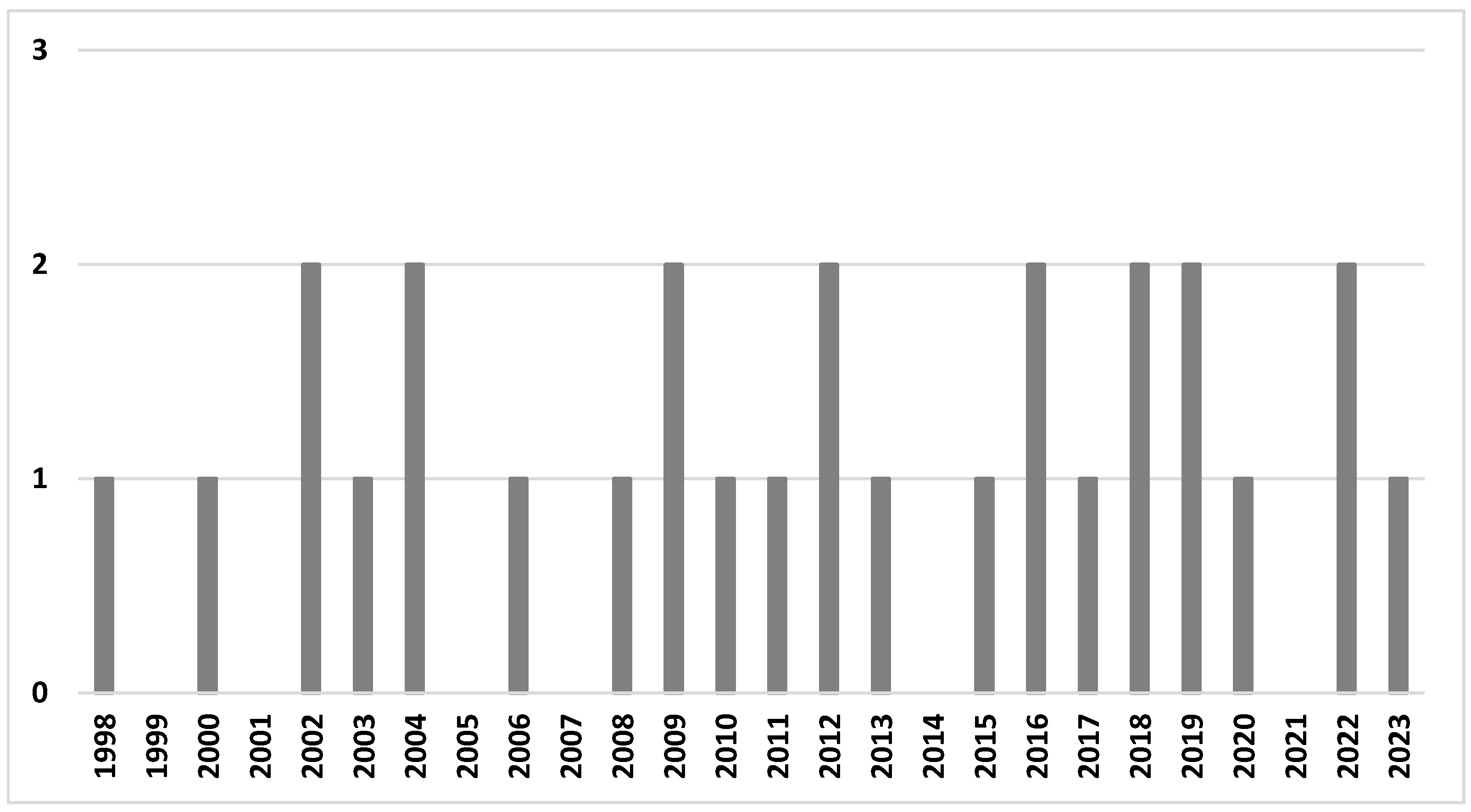
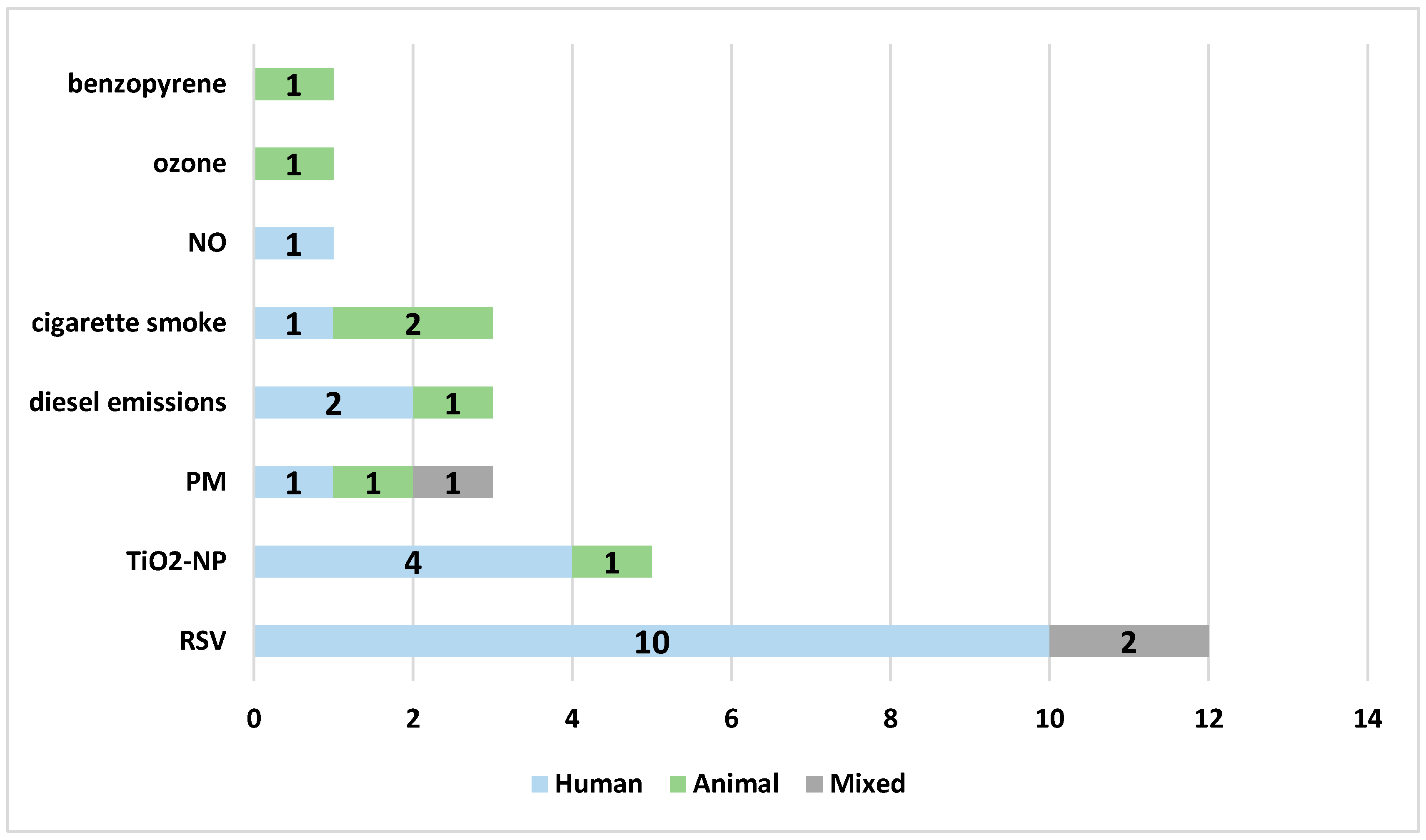
3.2. Study Characteristics
3.3. Molecular Mechanisms
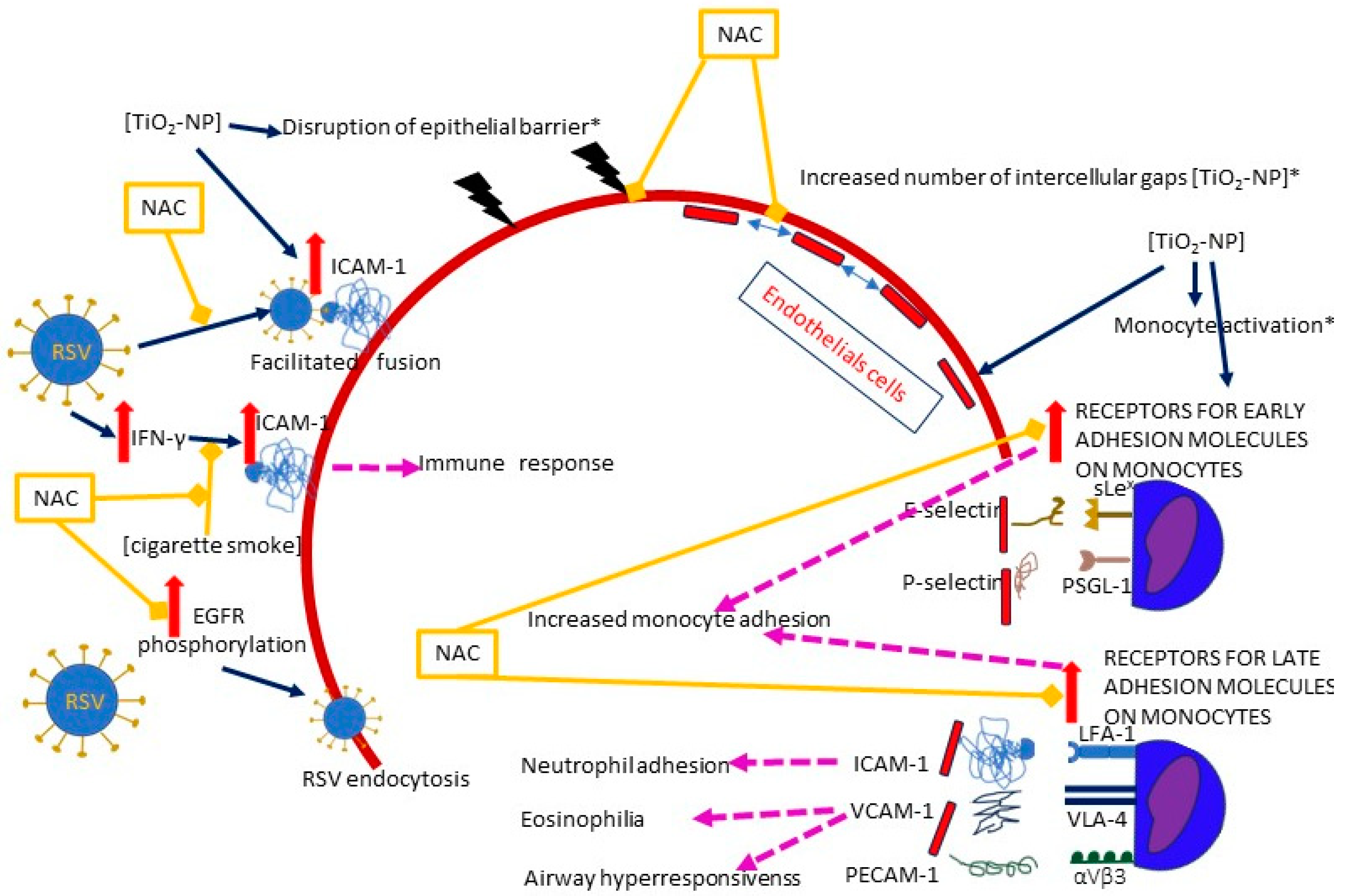
3.3.1. Facilitated Viral Entry
- Epithelial barrier
- ICAM-1
- EGFR
3.3.2. Increased Viral Load
Facilitated Virus Release
- A shift from apoptosis towards necrosis and ROS generation
3.3.3. Exaggerated Host Response
Exaggerated Proinflammatory Response
- Production of proinflammatory chemokines and cytokines
- The effects of NF-κB stimulation
- Oxidative stress
- PAMPs
Inappropriate Mucus Secretion
- MUC5AC
Structural Abnormalities
- Emphysema
- DNA damage and senescence
3.3.4. Airway Hyperresponsiveness (AHR)
4. Discussion
- -
- RSV: There is limited interest in the use of NAC for RSV infections, which could be an important direction for future studies, given the lack of registered, effective, widely used, and available drugs for RSV-caused diseases, and the lack of targeted anti-RSV treatment;
- -
- Air pollution: There is a lack of a detailed analysis of interventions for specific air pollutants; while the majority of studies focused on TiO2-NP or cigarette smoke, very little attention is paid to the six air pollutants acknowledged by the WHO as the most important ones (PM2.5, PM10, O₃, NO₂, SO₂, and CO);
- -
- Mechanisms:
- o
- The choice of the best route of administration needs to be verified;
- o
- A key role for the timing, duration, and dose of NAC might be expected. While some studies have tested the effects of different NAC doses, there seems to be another important and underestimated problem—the effects of timing (NAC pretreatment versus treatment)—where head-to-head comparisons would give the most accurate answers. Little is known about the optimal duration of NAC administration;
- o
- The lack of focus on long-term sequelae of RSV and/or air pollution; the studies tend to focus on the immediate effects, although from a clinical perspective, long-term complications are more important.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Web Annex, A. World Health Organization Model List of Essential Medicines—23rd List 2023. In The Selection and Use of Essential Medicines 2023: Executive Summary of the Report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines, 24–28 April 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.D.; Goulart, M.O.F. N-acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.D.; Grimwood, K.; Sly, P.D.; Lambert, S.B.; Chappell, K.J.; Watterson, D.; Ware, R.S. Epidemiology of respiratory syncytial virus in a community birth cohort of infants in the first 2 years of life. Eur. J. Pediatr. 2021, 180, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, J.; Ihling, C.M.; Manuel, B.; Rehbein, R.M.; Schnee, S.V.; Hoos, J.; Pfeil, J.; Grulich-Henn, J.; Schnitzler, P. Viral Etiology and Clinical Characteristics of Acute Respiratory Tract Infections in Hospitalized Children in Southern Germany (2014–2018). Open Forum. Infect. Dis. 2023, 10, ofad110. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Joy, E.A.; Hofmann, M.G.; Gesteland, P.H.; Cannon, J.B.; Lefler, J.S.; Blagev, D.P.; Korgenski, E.K.; Torosyan, N.; Hansen, G.I.; et al. Short-term elevation of fine particulate matter air pollution and acute lower respiratory infection. Am. J. Respir. Crit. Care Med. 2018, 198, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Karr, C.J.; Rudra, C.B.; Miller, K.A.; Gould, T.R.; Larson, T.; Sathyanarayana, S.; Koenig, J.Q. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ. Res. 2009, 109, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Vandini, S.; Corvaglia, L.; Alessandroni, R.; Aquilano, G.; Marsico, C.; Spinelli, M.; Lanari, M.; Faldella, G. Respiratory syncytial virus infection in infants and correlation with meteorological factors and air pollutants. Ital. J. Pediatr. 2013, 39, 1. [Google Scholar] [CrossRef] [PubMed]
- Nenna, R.; Evangelisti, M.; Frassanito, A.; Scagnolari, C.; Pierangeli, A.; Antonelli, G.; Nicolai, A.; Arima, S.; Moretti, C.; Papoff, P.; et al. Respiratory syncytial virus bronchiolitis, weather conditions and air pollution in an Italian urban area: An observational study. Environ. Res. 2017, 158, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Fu, J.-F.; Mao, J.-H.; Shang, S.-Q. Haze is a risk factor contributing to the rapid spread of respiratory syncytial virus in children. Environ. Sci. Pollut. Res. 2016, 23, 20178–20185. [Google Scholar] [CrossRef]
- Wrotek, A.; Badyda, A.; Czechowski, P.O.; Owczarek, T.; Dąbrowiecki, P.; Jackowska, T. Air Pollutants’ Concentrations Are Associated with Increased Number of RSV Hospitalizations in Polish Children. J. Clin. Med. 2021, 10, 3224. [Google Scholar] [CrossRef]
- Carugno, M.; Dentali, F.; Mathieu, G.; Fontanella, A.; Mariani, J.; Bordini, L.; Milani, G.P.; Consonni, D.; Bonzini, M.; Bollati, V.; et al. PM10 exposure is associated with increased hospitalizations for respiratory syncytial virus bronchiolitis among infants in Lombardy, Italy. Environ. Res. 2018, 166, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Maedel, C.; Kainz, K.; Frischer, T.; Reinweber, M.; Zacharasiewicz, A. Increased severity of respiratory syncytial virus airway infection due to passive smoke exposure. Pediatr. Pulmonol. 2018, 53, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Air Quality Guidelines. 2021. Available online: https://www.who.int/news-room/questions-and-answers/item/who-global-air-quality-guidelines (accessed on 10 May 2023).
- Wrotek, A.; Jackowska, T. Molecular Mechanisms of RSV and Air Pollution Interaction: A Scoping Review. Int. J. Mol. Sci. 2022, 23, 12704. [Google Scholar] [CrossRef] [PubMed]
- Schichlein, K.D.; Smith, G.J.; Jaspers, I. Protective effects of inhaled antioxidants against air pollution-induced pathological responses. Respir. Res. 2023, 24, 187. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhang, J.; Wang, Z.; Wu, Q.; Xianmei Zhou, X. Efficacy and safety of N-acetylcysteine therapy for idiopathic pulmonary fibrosis: An updated systematic review and meta-analysis. Exp. Ther. Med. 2019, 18, 802–816. [Google Scholar] [PubMed]
- Ciofu, O.; Lykkesfeldt, J. Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database Syst. Rev. 2019, 10, CD007020. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Morcillo, E.; Gimeno, C.; Cortijo, J. N-acetyl-L-cysteine (NAC) inhibit mucin synthesis and pro-inflammatory mediators in alveolar type II epithelial cells infected with influenza virus A and B and with respiratory syncytial virus (RSV). Biochem. Pharmacol. 2011, 82, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-Z.; Douglas, S.D. Glutathione and N-acetylcysteine suppression of human immunodeficiency virus replication in human monocyte/macrophages in vitro. AIDS Res. Hum. Retroviruses 1992, 8, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Alonso, J.L.; Pérez-Rial, S.; Rivera, C.G.; Peces-Barba, G. N-acetylcysteine for prevention and treatment of COVID-19: Current state of evidence and future directions. J. Infect. Public Health 2022, 15, 1477–1483. [Google Scholar] [CrossRef]
- Shi, Z.; Puyo, C.A. N-Acetylcysteine to Combat COVID-19: An Evidence Review. Ther. Clin. Risk Manag. 2020, 16, 1047–1055. [Google Scholar] [CrossRef]
- Cai, S.; Chen, P.; Zhang, C.; Chen, J.; Wu, J. Oral N-acetylcysteine attenuates pulmonary emphysema and alveolar septal cell apoptosis in smoking-induced COPD in rats. Respirology 2009, 14, 354–359. [Google Scholar] [CrossRef]
- Carpenter, L.R.; Moy, J.N.; Roebuck, K.A. Respiratory syncytial virus and TNF alpha induction of chemokine gene expression involves differential activation of Rel A and NF-kappa B1. BMC Infect. Dis. 2002, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Shan, Y.; Cui, Z. N-Acetyl-L-Cysteine Protects Airway Epithelial Cells during Respiratory Syncytial Virus Infection against Mucin Synthesis, Oxidative Stress, and Inflammatory Response and Inhibits HSPA6 Expression. Anal. Cell Pathol. 2022, 2022, 4846336. [Google Scholar] [CrossRef] [PubMed]
- Dick, C.A.J.; Brown, D.M.; Donaldson, K.; Stone, V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal. Toxicol. 2003, 15, 39–52. [Google Scholar] [CrossRef]
- Groskreutz, D.J.; Monick, M.M.; Babor, E.C.; Nyunoya, T.; Varga, S.M.; Look, D.C.; Hunninghake, G.W. Cigarette smoke alters respiratory syncytial virus-induced apoptosis and replication. Am. J. Respir. Cell Mol. Biol. 2009, 41, 189–198. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gon, Y.; Takeshita, I.; Matsumoto, K.; Jibiki, I.; Takizawa, H.; Kudoh, S.; Horie, T. Diesel exhaust particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am. J. Respir. Crit. Care Med. 2000, 161, 280–285. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, B.M.; Lee, Y.J.; Chung, H.W. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ. Mol. Mutagen. 2008, 49, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wiegman, C.; Seiffert, J.M.; Zhu, J.; Clarke, C.; Chang, Y.; Bhavsar, P.; Adcock, I.; Zhang, J.; Zhou, X.; et al. Effects of N-acetylcysteine in ozone-induced chronic obstructive pulmonary disease model. PLoS ONE 2013, 8, e80782. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Zeng, R.; Yang, J.; Liu, J.; Zhang, Z.; Song, X.; Yao, Z.; Ma, C.; Li, W.; et al. Respiratory Syncytial Virus Replication Is Promoted by Autophagy-Mediated Inhibition of Apoptosis. J. Virol. 2018, 92, e02193-17. [Google Scholar] [CrossRef]
- March, T.H.; Wilder, J.A.; Esparza, D.C.; Cossey, P.Y.; Blair, L.F.; Herrera, L.K.; McDonald, J.D.; Campen, M.J.; Mauderly, J.L.; Seagrave, J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol. Sci. 2006, 92, 545–559. [Google Scholar] [CrossRef]
- Martínez, I.; García-Carpizo, V.; Guijarro, T.; García-Gomez, A.; Navarro, D.; Aranda, A.; Zambrano, A. Induction of DNA double-strand breaks and cellular senescence by human respiratory syncytial virus. Virulence 2016, 7, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Mastronarde, J.G.; Monick, M.M.; Mukaida, N.; Matsushima, K.; Hunninghake, G.W. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-kappaB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J. Infect. Dis. 1998, 177, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Sarrion, I.; Armengot, M.; Carda, C.; Martinez, I.; Melero, J.A.; Cortijo, J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: Effectiveness of N-acetylcysteine. PLoS ONE 2012, 7, e48037. [Google Scholar] [CrossRef] [PubMed]
- Modestou, M.A.; Manzel, L.J.; El-Mahdy, S.; Look, D.C. Inhibition of IFN-gamma-dependent antiviral airway epithelial defense by cigarette smoke. Respir. Res. 2010, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Rhoden, C.R.; Lawrence, J.; Godleski, J.J.; González-Flecha, B. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol. Sci. 2004, 79, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Romero, C.; Hernández-Pérez, G.; Ramos-Godínez, P.; Vázquez-López, I.; Quintana-Belmares, R.O.; Huerta-García, E.; Stepien, E.; López-Marure, R.; Montiel-Dávalos, A.; Alfaro-Moreno, E. Titanium dioxide nanoparticles induce the expression of early and late receptors for adhesion molecules on monocytes. Part. Fibre Toxicol. 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Smallcombe, C.C.; Harford, T.J.; Linfield, D.T.; Lechuga, S.; Bokun, V.; Piedimonte, G.; Rezaee, F. Titanium dioxide nanoparticles exaggerate respiratory syncytial virus-induced airway epithelial barrier dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L481–l496. [Google Scholar] [CrossRef] [PubMed]
- Sparkman, L.; Boggaram, V. Nitric oxide increases IL-8 gene transcription and mRNA stability to enhance IL-8 gene expression in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, L764–L773. [Google Scholar] [CrossRef]
- Vaughan, A.; Stevanovic, S.; Jafari, M.; Rahman, M.; Bowman, R.V.; Fong, K.M.; Ristovski, Z.; Yang, I.A. The effect of diesel emission exposure on primary human bronchial epithelial cells from a COPD cohort: N-acetylcysteine as a potential protective intervention. Environ. Res. 2019, 170, 194–202. [Google Scholar] [CrossRef]
- Wan, R.; Mo, Y.; Feng, L.; Chien, S.; Tollerud, D.J.; Zhang, Q. DNA damage caused by metal nanoparticles: Involvement of oxidative stress and activation of ATM. Chem. Res. Toxicol. 2012, 25, 1402–1411. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-κB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lu, M.; Zhang, C.; Wu, X.; Chen, J.; Lv, W.; Sun, T.; Qiu, H.; Huang, S. Oxidative stress modulates the expression of toll-like receptor 3 during respiratory syncytial virus infection in human lung epithelial A549 cells. Mol. Med. Rep. 2018, 18, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Yang, A.; Piao, L.; Hao, S.; Du, L.; Meng, J.; Liu, J.; Xu, H. Comparative study of in vitro effects of different nanoparticles at non-cytotoxic concentration on the adherens junction of human vascular endothelial cells. Int. J. Nanomed. 2019, 14, 4475–4489. [Google Scholar] [CrossRef] [PubMed]
- Whitekus, M.J.; Li, N.; Zhang, M.; Wang, M.; Horwitz, M.A.; Nelson, S.K.; Horwitz, L.D.; Brechun, N.; Diaz-Sanchez, D.; Nel, A.E. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J. Immunol. 2002, 168, 2560–2567. [Google Scholar] [CrossRef]
- Wong, K.K.; Kua, K.P.; Ooi, K.S.; Cheah, F.C. The effects of N-acetylcysteine on lung alveolar epithelial cells infected with respiratory syncytial virus. Malays. J. Pathol. 2023, 45, 43–50. [Google Scholar] [PubMed]
- Yan, Z.; Wang, J.; Li, J.; Jiang, N.; Zhang, R.; Yang, W.; Yao, W.; Wu, W. Oxidative stress and endocytosis are involved in upregulation of interleukin-8 expression in airway cells exposed to PM2.5. Environ. Toxicol. 2016, 31, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Fu, L.; Xiang, H.-X.; Xiang, Y.; Li, M.-D.; Lv, B.-B.; Tan, Z.-X.; Gao, L.; Zhang, C.; Xu, D.-X. N-acetylcysteine alleviates pulmonary inflammatory response during benzo[a]pyrene-evoked acute lung injury. Environ. Sci. Pollut. Res. Int. 2022, 29, 3474–3486. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Cruz, C.; Camacho, J.L.V.; De Ávila-Arias, M.; Hurtado-Gomez, L.; Rodriguez, A.; San-Juan-Vergara, H. Respiratory syncytial virus entry mechanism in host cells: A general overview. Mol. Microbiol. 2023, 120, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.K.; Matsuse, H.; Kumar, M.; Kong, X.; Lockey, R.F.; Mohapatra, S.S. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem. Biophys. Res. Commun. 2001, 280, 188–195. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Hubbard, A.K.; Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 2000, 28, 1379–1386. [Google Scholar] [CrossRef]
- Arnold, R.; König, W. Respiratory syncytial virus infection of human lung endothelial cells enhances selectively intercellular adhesion molecule-1 expression. J. Immunol. 2005, 174, 7359–7367. [Google Scholar] [CrossRef] [PubMed]
- Chini, B.A.; Fiedler, M.A.; Milligan, L.; Hopkins, T.; Stark, J.M. Essential roles of NF-kappaB and C/EBP in the regulation of intercellular adhesion molecule-1 after respiratory syncytial virus infection of human respiratory epithelial cell cultures. J. Virol. 1998, 72, 1623–1626. [Google Scholar] [CrossRef]
- Markoutsa, E.; McGill, A.R.; Singer, A.; Jadhav, H.; Mohapatra, S.; Mohapatra, S.S. A multifunctional nanoparticle as a prophylactic and therapeutic approach targeting respiratory syncytial virus. Nanomedicine 2021, 32, 102325. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.S.; Lockey, R.F. Respiratory syncytial virus infection: From biology to therapy: A perspective. World Allergy Organ. J. 2008, 1, 21–28. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Hallsworth, P.; Dowling, K.; Alpers, J.; Bowden, J.; Forsyth, K. Adhesion molecule expression on epithelial cells infected with respiratory syncytial virus. Eur. Respir. J. 2000, 15, 358–366. [Google Scholar] [CrossRef]
- Montiel-Dávalos, A.; Ventura-Gallegos, J.L.; Alfaro-Moreno, E.; Soria-Castro, E.; García-Latorre, E.; Cabañas-Moreno, J.G.; Ramos-Godinez, M.d.P.; López-Marure, R. TiO2 nanoparticles induce dysfunction and activation of human endothelial cells. Chem. Res. Toxicol. 2012, 25, 920–930. [Google Scholar] [CrossRef]
- Schwarze, J.; Cieslewicz, G.; Hamelmann, E.; Joetham, A.; Shultz, L.D.; Lamers, M.C.; Gelfand, E.W. IL-5 and eosinophils are essential for the development of airway hyperresponsiveness following acute respiratory syncytial virus infection. J. Immunol. 1999, 162, 2997–3004. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.A.; Deng, Y.; Hardelid, P.; Robinson, E.; Ren, L.; Moulding, D.; Smyth, R.L.; Claire Mary Smith, C.M. β2-integrin LFA1 mediates airway damage following neutrophil transepithelial migration during respiratory syncytial virus infection. Eur. Respir. J. 2020, 56, 1902216. [Google Scholar] [CrossRef]
- Krzyzaniak, M.A.; Zumstein, M.T.; Gerez, J.A.; Picotti, P.; Helenius, A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013, 9, e1003309. [Google Scholar] [CrossRef]
- Greenlund, A.C.; Morales, M.O.; Viviano, B.L.; Yan, H.; Krolewski, J.; Schreiber, R.D. Stat recruitment by tyrosine-phosphorylated cytokine receptors: An ordered reversible affinity-driven process. Immunity 1995, 2, 677–687. [Google Scholar] [CrossRef]
- Cha, S.-R.; Jang, J.; Park, S.-M.; Ryu, S.M.; Cho, S.-J.; Yang, S.-R. Cigarette Smoke-Induced Respiratory Response: Insights into Cellular Processes and Biomarkers. Antioxidants 2023, 12, 1210. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Wedzicha, J.A.; Celli, B.R.; Anzueto, A.; Matera, M.G. Use of thiols and implications for the use of inhaled corticosteroids in the presence of oxidative stress in COPD. Respir. Res. 2023, 24, 194. [Google Scholar] [CrossRef]
- Finkelstein, E.I.; Nardini, M.; van der Vliet, A. Inhibition of neutrophil apoptosis by acrolein: A mechanism of tobacco-related lung disease? Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L732–L739. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.I.; Ruben, J.; Koot, C.W.; Hristova, M.; van der Vliet, A. Regulation of constitutive neutrophil apoptosis by the alpha,beta-unsaturated aldehydes acrolein and 4-hydroxynonenal. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L1019–L1028. [Google Scholar] [CrossRef] [PubMed]
- Van der Toorn, M.; Smit-de Vries, M.P.; Slebos, D.J.; de Bruin, H.G.; Abello, N.; van Oosterhout, A.J.; Bischoff, R.; Kauffman, H.F. Cigarette smoke irreversibly modifies glutathione in airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L1156–L1162. [Google Scholar] [CrossRef]
- Hristova, M.; Heuvelmans, S.; van der Vliet, A. GSH-dependent regulation of Fas-mediated caspase-8 activation by acrolein. FEBS Lett. 2007, 581, 361–367. [Google Scholar] [CrossRef] [PubMed]
- van der Toorn, M.; Slebos, D.J.; de Bruin, H.G.; Leuvenink, H.G.; Bakker, S.J.; Gans, R.O.; Koëter, G.H.; van Oosterhout, A.J.; Kauffman, H.F. Cigarette smoke-induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L1211–L1218. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Castranova, V.; Perez, M.K.; Piedimonte, G. Nanoparticles increase human bronchial epithelial cell susceptibility to respiratory syncytial virus infection via nerve growth factor-induced autophagy. Physiol. Rep. 2017, 5, e13344. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Sun, Y.; Xie, M.; Lin, J. Clinical value of N-acetylcysteine combined with terbutaline sulfate in elderly patients with chronic obstructive pulmonary disease and its effect on apoptosis/anti-apoptosis mechanism. Ann. Palliat. Med. 2020, 9, 3393–3401. [Google Scholar] [CrossRef]
- Castro, S.M.; Kolli, D.; Guerrero-Plata, A.; Garofalo, R.P.; Casola, A. Cigarette smoke condensate enhances respiratory syncytial virus-induced chemokine release by modulating NF-kappa B and interferon regulatory factor activation. Toxicol. Sci. 2008, 106, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, B.; Kluza, J.; Antherieu, S.; Sotty, J.; Alleman, L.Y.; Perdrix, E.; Loyens, A.; Coddeville, P.; Guidice, J.-M.L.; Marchetti, P.; et al. Air pollution-derived PM2.5 impairs mitochondrial function in healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells. Environ. Pollut. 2018, 243 Pt B, 1434–1449. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Nan, G. The Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway as a Discovery Target in Stroke. J. Mol. Neurosci. 2016, 59, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Jang, S.H.; Jang, Y.J.; Na, S.W.; Yoon, J.J.; Moon, H.G.; Kim, S.Y.; Seo, C.S.; Lee, H.S.; Lee, Y.M.; et al. Diesel vehicles-derived PM2.5 induces lung and cardiovascular injury attenuates by Securiniga suffruticosa: Involvement of NF-κB-mediated NLRP3 inflammasome activation pathway. Biomed. Pharmacother. 2023, 162, 114637. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wright, M.M.; Jackson, R.M. Reactive species mediated injury of human lung epithelial cells after hypoxia-reoxygenation. Exp. Lung Res. 2002, 28, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Garcia, G.; Irudayam, J.I.; Jeyachandran, A.V.; Dubey, S.; Chang, C.; Cario, S.C.; Price, N.; Arumugam, S.; Marquez, A.L.; Shah, A.; et al. Innate immune pathway modulator screen identifies STING pathway activation as a strategy to inhibit multiple families of arbo and respiratory viruses. Cell Rep. Med. 2023, 4, 101024. [Google Scholar] [CrossRef]
- Kato, K.; Chang, E.H.; Chen, Y.; Lu, W.; Kim, M.M.; Niihori, M.; Hecker, L.; Kim, K.C. MUC1 contributes to goblet cell metaplasia and MUC5AC expression in response to cigarette smoke in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L82–L90. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, Y.; Yang, M.; Yuan, L.; Wang, L.; Wu, M.; Zhou, K.; Li, W.; Xiang, Y.; Qu, X.; et al. ITGB4 deficiency induces mucus hypersecretion by upregulating MUC5AC in RSV-infected airway epithelial cells. Int. J. Biol. Sci. 2022, 18, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Vallath, S.; Hynds, R.E.; Succony, L.; Janes, S.M.; Giangreco, A. Targeting EGFR signalling in chronic lung disease: Therapeutic challenges and opportunities. Eur. Respir. J. 2014, 44, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Mebratu, Y.A.; Smith, K.R.; Agga, G.E.; Tesfaigzi, Y. Inflammation and emphysema in cigarette smoke-exposed mice when instilled with poly (I:C) or infected with influenza A or respiratory syncytial viruses. Respir. Res. 2016, 17, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pociask, D.A.; McAleer, J.P.; Chan, Y.R.; Alcorn, J.F.; Kreindler, J.L.; Keyser, M.R.; Shapiro, S.D.; Houghton, A.M.; Kolls, J.K.; et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS ONE 2011, 6, e20333. [Google Scholar] [CrossRef] [PubMed]
- Triantaphyllopoulos, K.; Hussain, F.; Pinart, M.; Zhang, M.; Li, F.; Adcock, I.; Kirkham, P.; Zhu, J.; Chung, K.F. A model of chronic inflammation and pulmonary emphysema after multiple ozone exposures in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L691–L700. [Google Scholar] [CrossRef] [PubMed]
- Assavanopakun, P.; Sapbamrer, R.; Kumfu, S.; Chattipakorn, N.; Chattipakorn, S.C. Effects of air pollution on telomere length: Evidence from in vitro to clinical studies. Environ. Pollut. 2022, 312, 120096. [Google Scholar] [CrossRef]
- Mumby, S.; Adcock, I.M. Recent evidence from omic analysis for redox signalling and mitochondrial oxidative stress in COPD. J. Inflamm. 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef]
- Montero, P.; Roger, I.; Estornut, C.; Milara, J.; Cortijo, J. Influence of dose and exposition time in the effectiveness of N-Acetyl-L-cysteine treatment in A549 human epithelial cells. Heliyon 2023, 9, e15613. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Facciolo, F.; Rogliani, P.; Matera, M.G. Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir. Res. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- El Hafiz, A.M.A.; El Wakeel, L.M.; El Hady, H.M.; Mourad, A.E.R. High dose N-acetyl cysteine improves inflammatory response and outcome in patients with COPD exacerbations. Egypt. J. Chest Dis. Tuberc. 2013, 62, 51–57. [Google Scholar] [CrossRef]
- Wong, K.K.; Lee, S.W.H.; Kua, K.P. N-Acetylcysteine as Adjuvant Therapy for COVID-19—A Perspective on the Current State of the Evidence. J. Inflamm. Res. 2021, 14, 2993–3013. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, C.; MacNutt, M.J.; Zhang, Z.; Sava, F.; Pui, M.M. Anti-oxidant N-acetylcysteine diminishes diesel exhaust-induced increased airway responsiveness in person with airway hyper-reactivity. Toxicol. Sci. 2014, 139, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Raza, A.B.; Ijaz, I.; Kazi, M.Y. Effectiveness of nebulized N-acetylcysteine solution in children with acute bronchiolitis. J. Coll. Physicians Surg. Pak. 2014, 24, 408–411. [Google Scholar]
- Ralston, S.L.; Lieberthal, A.S.; Meissner, H.C.; Alverson, B.K.; Baley, J.E.; Gadomski, A.M.; Johnson, D.W.; Light, M.J.; Maraqa, N.F.; Mendonca, E.A.; et al. Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014, 134, e1474–e1502. [Google Scholar] [CrossRef]
- Trials, E.C. EudraCT. 2023. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=acetylcysteine (accessed on 6 September 2023).
- clinicaltrials.gov. 2023. Available online: https://www.clinicaltrials.gov/search?cond=n-acetylcysteine (accessed on 6 September 2023).


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Original research | Review study |
| Publication date: 1998–2023 | Publication before 1998 |
| Accepted after a peer-review process | Lack of peer-review (e.g., gray literature, preprints, etc.) |
| Animal, human cellular, or mixed model | Lack of a model (e.g., epidemiological studies, clinical studies) |
| Analysis of NAC molecular mechanism(s) of action | Lack of analysis of NAC molecular mechanisms |
| RSV infection or air pollution model | Lack of analysis of RSV or the influence of air pollution |
| Only maternal/in utero exposure analysis | |
| NAC mechanism in condition other than RSV or air pollution | |
| Study on combined effects only (e.g., various etiological agents, or allergy models) | |
| Analysis of effects other than in the respiratory tract |
| First Author, Publication Year | Model | Air Pollutant | RSV | Material/Subjects | Country | |
|---|---|---|---|---|---|---|
| 1 | Cai, 2009 [23] | A | cigarette smoke | male Sprague–Dawley rats | China | |
| 2 | Carpenter, 2002 [24] | H | + | A549 human type II lung carcinoma cell line | USA | |
| 3 | Chi, 2022 [25] | H | + | human bronchial epithelial cells BEAS-2B | China | |
| 4 | Dick, 2003 [26] | A | four different ultrafine particles (carbon black, cobalt, nickel, and titanium dioxide) | male Wistar rats | UK | |
| 5 | Groskreutz, 2009 [27] | H | cigarette smoke extract | + | primary human tracheobronchial epithelial | USA |
| 6 | Hashimoto, 2000 [28] | H | diesel exhaust particles | transformed human bronchial epithelial cell line BET-1A | Japan | |
| 7 | Kang, 2008 [29] | H | TiO2-NP | peripheral blood lymphocytes from a healthy female adult donor | South Korea | |
| 8 | Li, 2013 [30] | A | ozone | C57/BL6 mice | UK, China, USA | |
| 9 | Li, 2018 [31] | H/A | + | human laryngeal epithelial cell line HEp-2 and HEK 293T cells and BALB/c female mice | China/USA | |
| 10 | March, 2006 [32] | A | cigarette smoke | male and female A/J mice | USA | |
| 11 | Martinez, 2016 [33] | H | + | HEp-2 and A549 cells | Spain | |
| 12 | Mastronarde, 1998 [34] | H | + | alveolar epithelial cells A549 | USA/Japan | |
| 13 | Mata, 2011 [19] | H | + | human pulmonary epithelial cell line A549 | Spain | |
| 14 | Mata, 2012 [35] | H | + | primary normal human bronchial epithelial cell (NHBEC) | Spain | |
| 15 | Modestou, 2010 [36] | H | cigarette smoke extract | + | human trachea and bronchial samples, primary human tracheobronchial epithelial cells | USA |
| 16 | Rhoden, 2004 [37] | A | concentrated ambient particles (CAPs) | adult male Sprague-Dawley rats | USA | |
| 17 | Rueda-Romero, 2016 [38] | H | TiO2-NP | human cell line U937 as a monocyte cell model and HUVECs as a model for endothelial cells | Mexico, Poland, Sweden | |
| 18 | Smallcombe, 2020 [39] | H/A | TiO2-NP | + | immortalized human bronchial epithelial cells; C57BL/6 mice | USA |
| 19 | Sparkman, 2004 [40] | H | nitric oxide | NCI-H441 cells, a human lung adenocarcinoma cell line of bronchiolar (Clara) cell lineage, and BEAS-2B cells, an SV40 transformed human bronchial epithelial cell line | USA | |
| 20 | Vaughan, 2019 [41] | H | diesel emission | primary human bronchial epithelial cells (pHBEC) from patients with/without COPD | Australia | |
| 21 | Wan, 2012 [42] | H | nano-sized cobalt (nano-Co) and titanium dioxide (nano-TiO2) | human lung epithelial cell lines A549 | USA | |
| 22 | Wang, 2017 [43] | H/A | urban particulate matter 1649b | human bronchial epithelial cells (HBECs) Male C57 mice mice | China | |
| 23 | Wang, 2018 [44] | H | + | human lung adenocarcinoma alveolar basal epithelial cell line A549 and laryngeal epithelial carcinoma HEp-2 cell line | China/USA | |
| 24 | Wen, 2019 [45] | H | nanoparticles including gold (Au), platinum (Pt), silica (SiO2), titanium dioxide (TiO2), ferric oxide (Fe2O3), oxidized multi-walled carbon nanotubes (MWCNTs) | primary human umbilical vein endothelial cells (HUVECs) | China | |
| 25 | Whitekus, 2002 [46] | A | diesel exhaust particles | murine macrophage cell line, RAW 264.7 cells | USA | |
| 26 | Wong, 2023 [47] | H | + | human type II pulmonary epithelial cell line A549 | Malaysia | |
| 27 | Yan, 2015 [48] | H | PM2.5 | human bronchial epithelial cell line (BEAS-2B cells) and human macrophage-like cell line (THP-1 cells) | China | |
| 28 | Zhao, 2022 [49] | A | benzo[a]pyrene | C57BL/6J male mice | China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrotek, A.; Badyda, A.; Jackowska, T. Molecular Mechanisms of N-Acetylcysteine in RSV Infections and Air Pollution-Induced Alterations: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 6051. https://doi.org/10.3390/ijms25116051
Wrotek A, Badyda A, Jackowska T. Molecular Mechanisms of N-Acetylcysteine in RSV Infections and Air Pollution-Induced Alterations: A Scoping Review. International Journal of Molecular Sciences. 2024; 25(11):6051. https://doi.org/10.3390/ijms25116051
Chicago/Turabian StyleWrotek, August, Artur Badyda, and Teresa Jackowska. 2024. "Molecular Mechanisms of N-Acetylcysteine in RSV Infections and Air Pollution-Induced Alterations: A Scoping Review" International Journal of Molecular Sciences 25, no. 11: 6051. https://doi.org/10.3390/ijms25116051
APA StyleWrotek, A., Badyda, A., & Jackowska, T. (2024). Molecular Mechanisms of N-Acetylcysteine in RSV Infections and Air Pollution-Induced Alterations: A Scoping Review. International Journal of Molecular Sciences, 25(11), 6051. https://doi.org/10.3390/ijms25116051






