Meta-QTL and Candidate Gene Analyses of Agronomic Salt Tolerance and Related Traits in an RIL Population Derived from Solanum pimpinellifolium
Abstract
:1. Introduction
2. Results
3. Discussion
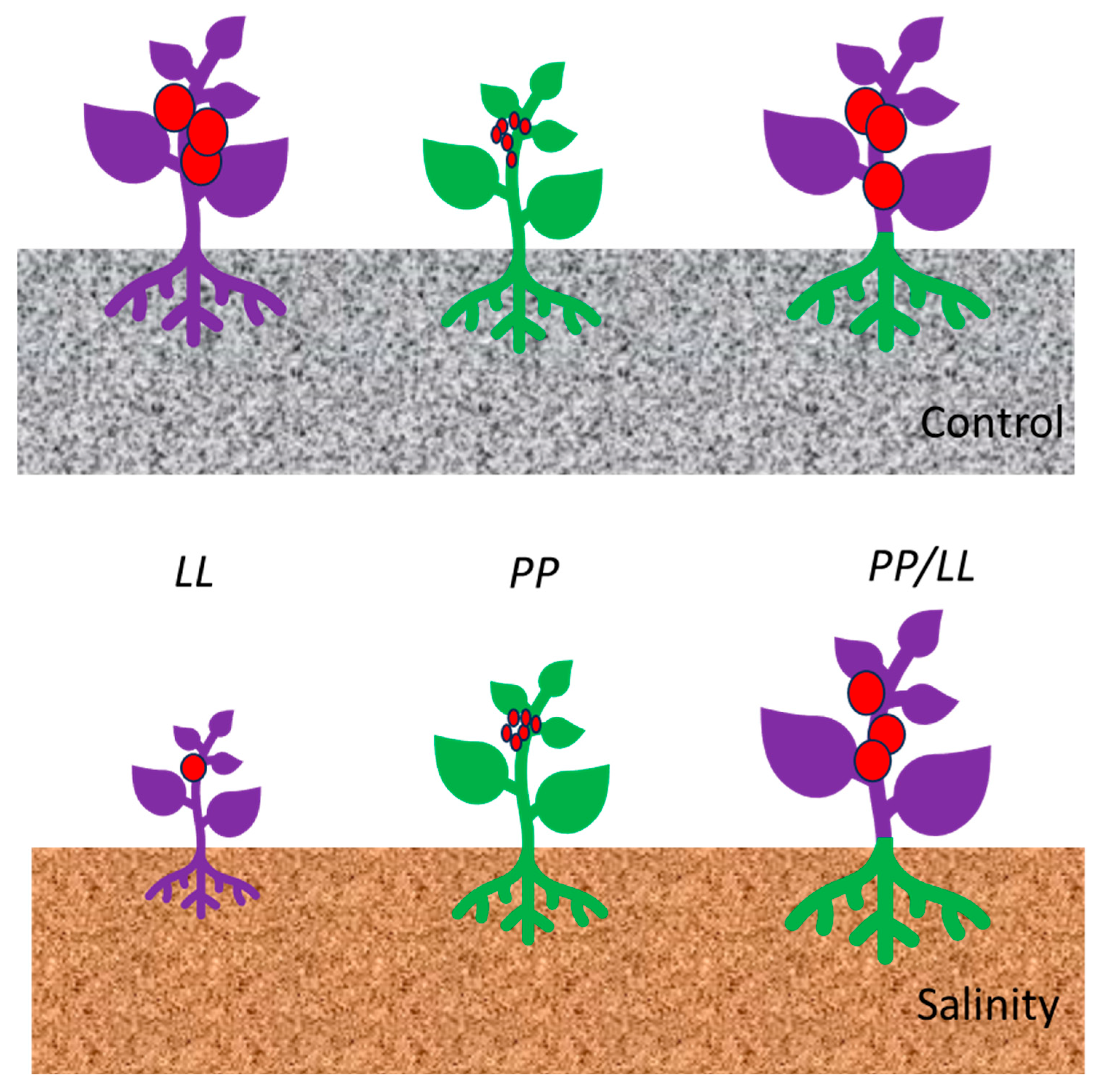
3.1. Fruit Yield Is Frequently Associated with Leaf Water Content in the RIL Population
3.2. Leaf Nutrient Content and Tomato Productivity Are Connected in Most MQTLs
3.3. From Genetic Knowledge to Improving Salt Tolerance in the Field
| MQTL | References | ||||
|---|---|---|---|---|---|
| 1.1 | Pons et al. [24] | Tsutsumi-Morita et al. [56] | |||
| 2.1 | Tsutsumi-Morita et al. [56] | ||||
| 2.2 | Frary et al. [52] | Ampomah-Dwamena et al. [53] | Diouf et al. [54] | Mata-Nicolas et al. [21] | Pons et al. [24] |
| 3 | Chakrabarti et al. [55] | Diouf et al. [54] | Ye et al. [23] | ||
| 4.1 | Ye et al. [23] | Kim et al. [22] | Tsutsumi-Morita et al. [56] | ||
| 4.2 | Liu et al. 2018 [61] | Ye et al. [23] | |||
| 5 | Ye et al. [23] | Tsutsumi-Morita et al. [56] | Pons et al. [24] | ||
| 6 | Tsutsumi-Morita et al. [56] | ||||
| 7.2 | Nitsch et al. [57] | Tsutsumi-Morita et al. [56] | Torgeman and Zamir [58] | ||
| 11 | Diouf et al. [54] | ||||
4. Materials and Methods
4.1. Experiments, Traits, and QTL Analysis
4.2. QTL Projection and Meta-QTL Analysis
4.3. Identification of Candidate Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marsh, J.I.; Hu, H.; Gill, M.; Batley, J.; Edwards, D. Crop breeding for a changing climate: Integrating phenomics and genomics with bioinformatics. Theor. Appl. Genet. 2021, 134, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Ray, D.K.; West, P.C.; Clark, M.; Gerber, J.S.; Prishchepov, A.V.; Chatterjee, S. Climate change has likely already affected global food production. PLoS ONE 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hong, Y.; Zhu, G.; Li, Y.; Niu, Q.; Yao, J.; Hua, K.; Bai, J.; Zhu, Y.; Shi, H.; et al. Loss of salt tolerance during tomato domestication conferred by variation in a Na+/K+ transporter. EMBO J. 2020, 39, e103256. [Google Scholar] [CrossRef] [PubMed]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Witcombe, J.R.; Hollington, P.A.; Howarth, C.J.; Reader, S.; Steele, K.A. Breeding for abiotic stresses for sustainable agriculture. Philos. Trans. R. Soc. B 2008, 363, 703–716. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 February 2024).
- Bonarota, M.-S.; Kosma, D.K.; Barrios-Masias, F.H. Salt tolerance mechanisms in the Lycopersicon clade and their trade-offs. AoB Plants 2022, 14, plab072. [Google Scholar] [CrossRef] [PubMed]
- The Tomato Genome Consortium. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Chibon, P.-Y.; Schoof, H.; Visser, R.G.F.; Finkers, R. Marker2sequence, mine your QTL regions for candidate genes. Bioinformatics 2012, 28, 1921–1922. [Google Scholar] [CrossRef] [PubMed]
- Aflitos, S.; Schijlen, E.; de Jong, H.; de Ridder, D.; Smit, S.; Finkers, R.; Wang, J.; Zhang, G.; Li, N.; Mao, L.; et al. Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J. 2014, 80, 136–148. [Google Scholar]
- Fernandez-Pozo, N.; Menda, N.; Edwards, J.D.; Saha, S.; Tecle, I.Y.; Strickler, S.R.; Bombarely, A.; Fisher-York, T.; Pujar, A.; Foerster, H.; et al. The Sol Genomics Network (SGN)—From genotype to phenotype to breeding. Nucleic Acids Res. 2015, 43, D1036–D1041. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Nicolas, P.; Fernandez-Pozo, N.; Ma, Q.; Evanich, D.J.; Shi, Y.; Xu, Y.; Zheng, Y.; Snyder, S.I.; Martin, L.B.B.; et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat. Commun. 2018, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; He, Q.; Wang, J.; Wang, B.; Zhao, J.; Huang, S.; Yang, T.; Tang, Y.; Yang, S.; Aisimutuola, P.; et al. Super-pangenome analyses highlight genomic diversity and structural variation across wild and cultivated tomato species. Nat. Genet. 2023, 55, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xu, X.; Zhu, H.; Liu, A.; Liu, L.; Li, J.; Hua, X. Comparative Transcriptomic Profiling of a Salt-Tolerant Wild Tomato Species and a Salt-Sensitive Tomato Cultivar. Plant Cell Physiol. 2010, 51, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.; Scossa, F.; Bolger, M.; Lanz, C.; Maumus, F.; Tohge, T.; Quesneville, H.; Alseekh, S.; Sørensen, I.; Lichtenstein, G.; et al. The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat. Genet. 2014, 46, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.; Fiene, G.; Ahmed, H.I.; Rey, E.; Abrouk, M.; Angel, Y.; Johansen, K.; Saber, N.O.; Malbeteau, Y.; Al-Mashharawi, S.; et al. Deciphering Salt Stress Responses in Solanum pimpinellifolium through High-Throughput Phenotyping. bioRxiv 2023. preprint. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Asins, M.J.; Romero-Aranda, M.R.; Espinosa, J.; González-Fernández, P.; Jaime-Fernández, E.; Traverso, J.A.; Carbonell, E.A.; Belver, A. HKT1;1 and HKT1;2 Na+ Transporters from Solanum galapagense Play Different Roles in the Plant Na+ Distribution under Salinity. Int. J. Mol. Sci. 2022, 23, 5130. [Google Scholar] [CrossRef] [PubMed]
- Estrada, Y.; Plasencia, F.; Ortíz-Atienza, A.; Faura, C.; Flores, F.B.; Lozano, R.; Egea, I. A novel function of the tomato CALCINEURIN-B LIKE 10 gene as a root-located negative regulator of salt stress. Plant Cell Environ. 2023, 46, 3433–3444. [Google Scholar] [CrossRef] [PubMed]
- Villalta, I.; Bernet, G.P.; Carbonell, E.A.; Asins, M.J. Comparative QTL analysis of salinity tolerance in terms of fruit yield using two Solanum populations of F7 lines. Theor. Appl. Genet. 2007, 114, 1001–1017. [Google Scholar] [CrossRef]
- Mata-Nicolás, E.; Montero-Pau, J.; Gimeno-Paez, E.; Garcia-Carpintero, V.; Ziarsolo, P.; Menda, N.; Mueller, L.A.; Blanca, J.; Cañizares, J.; van der Knaap, E.; et al. Exploiting the diversity of tomato: The development of a phenotypically and genetically detailed germplasm collection. Hortic. Res. 2020, 7, 66. [Google Scholar] [CrossRef]
- Kim, M.; Nguyen, T.T.P.; Ahn, J.H.; Kim, G.-J.; Sim, S.-C. Genome-wide association study identifies QTL for eight fruit traits in cultivated tomato (Solanum lycopersicum L.). Hortic. Res. 2021, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wang, X.; Wang, W.; Yu, H.; Ai, G.; Li, C.; Sun, P.; Wang, X.; Li, H.; Ouyang, B.; et al. Genome-wide association study reveals the genetic architecture of 27 agronomic traits in tomato. Plant Physiol. 2021, 186, 2078–2092. [Google Scholar] [CrossRef]
- Pons, C.; Joan Casals, J.; Brower, M.; Sacco, A.; Riccini, A.; Hendrickx, P.; Figás, M.d.R.; Fisher, J.; Grandillo, S.; Mazzucato, A.; et al. Diversity and genetic architecture of agro-morphological traits in a core collection of European traditional tomato. J. Exp. Bot. 2023, 74, 5896–5916. [Google Scholar] [CrossRef] [PubMed]
- Estañ, M.T.; Villalta, I.; Bolarín, M.C.; Carbonell, E.A.; Asins, M.J. Identification of fruit yield loci controlling the salt tolerance conferred by Solanum rootstocks. Theor. Appl. Genet. 2009, 118, 305–312. [Google Scholar] [CrossRef]
- Asins, M.J.; Raga, V.; Roca, D.; Carbonell, E.A. Genetic dissection of tomato rootstock effects on scion traits under moderate salinity. Theor. Appl. Genet. 2015, 128, 667–679. [Google Scholar] [CrossRef]
- Villalta, I.; Reina-Sánchez, A.; Bolarín, M.C.; Cuartero, J.; Belver, A.; Venema, K.; Carbonell, E.A.; Asins, M.J. Genetic analysis of Na+ and K+ concentrations in leaf and stem as physiological components of salt tolerance in tomato. Theor. Appl. Genet. 2008, 116, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Asins, M.J.; Bolarín, M.C.; Pérez-Alfocea, F.; Estañ, M.T.; Martinez-Andújar, C.; Albacete, A.; Villalta, I.; Bernet, G.P.; Dodd, I.; Carbonell, E.A. Genetic analysis of physiological components of salt tolerance conferred by Solanum rootstocks. What is the rootstock doing for the scion? Theor. Appl. Genet. 2010, 121, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Goffinet, B.; Gerber, S. Quantitative Trait Loci: A Meta-analysis. Genetics 2000, 155, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Driedonks, N.; Rutten, M.J.M.; Vriezen, W.H.; de Boer, G.-J.; Rieu, I. Mapping quantitative trait loci for heat tolerance of reproductive traits in tomato (Solanum lycopersicum). Mol. Breed. 2017, 37, 58. [Google Scholar] [CrossRef]
- Gonzalo, M.J.; da Maia, L.C.; Nájera, I.; Baixauli, C.; Giuliano, G.; Ferrante, P.; Granell, A.; Asins, M.J.; Monforte, A.J. Genetic Control of Reproductive Traits under Different Temperature Regimes in Inbred Line Populations Derived from Crosses between S. pimpinellifolium and S. lycopersicum Accessions. Plants 2022, 11, 1069. [Google Scholar] [CrossRef]
- Asins, M.J.; Villalta, I.; Aly, M.M.; Olías, R.; Álvarez De Morales, P.; Huertas, R.; Li, J.; Jaime-Pérez, N.; Haro, R.; Raga, V.; et al. Two closely linked tomato HKT coding genes are positional candidates for the major tomato QTL involved in Na+/K+ homeostasis. Plant Cell Environ. 2013, 36, 1171–1191. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Pérez, N.; Pineda, B.; García-Sogo, B.; Atares, A.; Athman, A.; Byrt, C.S.; Olias, R.; Asins, M.J.; Gilliham, M.; Moreno, V.; et al. The Na+ transporter encoded by the HKT1;2 gene modulates Na+/K+ homeostasis in tomato shoots under salinity. Plant Cell Environ. 2017, 40, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aranda, M.R.; González Fernández, P.; López-Tienda, J.R.; López-Diaz, M.R.; Espinosa, J.; Granum, E.; Traverso, J.A.; Pineda, B.; Garcia-Sogo, B.; Moreno, V.; et al. Na+ transporter HKT1;2 reduces flower Na+ content and considerably mitigates the decline in tomato fruit yields under saline conditions. Plant Physiol. Biochem. 2020, 154, 341–352. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 40–433. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops—What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Gilliham, M.; Flowers, T.J.; Colmer, T.D. Tissue tolerance: An essential but elusive trait for salt-tolerant crops. Funct. Plant Biol. 2016, 43, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Marowa, P.; Ding, A.; Kong, Y. Expansins: Roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016, 35, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Bolarin, M.C.; Estañ, M.T.; Caro, M.; Romero-Aranda, R.; Cuartero, J. Relationship between tomato fruit growth and fruit osmotic potential under salinity. Plant Sci. 2001, 160, 1153–1159. [Google Scholar] [CrossRef]
- Gharbi, E.; Martínez, J.P.; Benahmed, H.; Hichri, I.; Dobrev, P.I.; Motyka, V.; Quinet, M.; Lutts, S. Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Sci. 2017, 258, 77–89. [Google Scholar] [CrossRef]
- Han, W.; Jia, J.; Hu, Y.; Liu, J.; Guo, J.; Shi, Y.; Huo, H.; Gong, H. Maintenance of Root Water Uptake Contributes to Salt-Tolerance of a Wild Tomato Species under Salt Stress. Arch. Agron. Soil Sci. 2021, 67, 205–217. [Google Scholar] [CrossRef]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the Move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef]
- Geilfus, C.M. Chloride: From Nutrient to Toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Rosales, M.A.; Franco-Navarro, J.D.; Peinado-Torrubia, P.; Díaz-Rueda, P.; Álvarez, R.; Colmenero-Flores, J.M. Chloride improves nitrate utilization and NUE in plants. Front. Plant Sci. 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Shabala, L.; Zhang, J.; Pottosin, I.; Bose, J.; Zhu, M.; Fuglsang, A.T.; Velarde-Buendia, A.; Massart, A.; Hill, C.B.; Roessner, U.; et al. Cell-type-specific H+-ATPase activity in root tissues enables K+ retention and mediates acclimation of barley (Hordeum vulgare) to salinity stress. Plant Physiol. 2016, 172, 2445–2458. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Rubio, F.; Nieves-Cordones, M.; Horie, T.; Shabala, S. Doing ‘business as usual’ comes with a cost: Evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol. 2020, 225, 1097–1104. [Google Scholar] [CrossRef]
- Asins, M.J.; Albacete, A.; Martinez-Andujar, C.; Pérez-Alfocea, F.; Dodd, I.C.; Carbonell, E.A.; Dieleman, J.A. Genetic analysis of rootstock-mediated nitrogen (N) uptake and root-to-shoot signalling at contrasting N availabilities in tomato. Plant Sci. 2017, 263, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aduragbemi, A.; Wei, D.; Zheng, J.; Qiao, P.; Cui, C.; Lu, S.; Chen, L.; Hu, Y.-G. Large-scale integration of meta-QTL and genome-wide association study discovers the genomic regions and candidate genes for yield and yield-related traits in bread wheat. Theor. Appl. Genet. 2021, 134, 3083–3109. [Google Scholar] [CrossRef]
- Asins, M.J.; Raga, M.V.; Torrent, D.; Roca, D.; Carbonell, E.A. QTL and candidate gene analyses of rootstock-mediated tomato fruit yield and quality traits under low iron stress. Euphytica 2020, 216, 63. [Google Scholar] [CrossRef]
- Frary, A.; Nesbitt, T.C.; Frary, A.; Grandillo, S.; Knaap, E.v.d.; Cong, B.; Liu, J.; Meller, J.; Elber, R.; Alpert, K.B.; et al. fw2. 2: A quantitative trait locus key to the evolution of tomato fruit size. Science 2000, 289, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Ampomah-Dwamena, C.; Morris, B.A.; Sutherland, P.; Veit, B.; Yao, J.L. Down-regulation of TM29, a tomato SEPALLATA homolog, causes parthenocarpic fruit development and floral reversion. Plant Physiol. 2002, 130, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Diouf, I.A.; Derivot, L.; Bitton, F.; Pascual, L.; Causse, M. Water Deficit and Salinity Stress Reveal Many Specific QTL for Plant Growth and Fruit Quality Traits in Tomato. Front. Plant Sci. 2018, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, M.; Zhang, N.; Sauvage, C.; Muños, S.; Blanca, J.; Cañizares, J.; Diez, M.J.; Schneider, R.; Mazourek, M.; McClead, J.; et al. A cytochrome P450 CYP78A regulates a domestication trait in tomato (Solanum lycopersicum). Proc. Natl. Acad. Sci. USA 2013, 110, 17125–17130. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi-Morita, Y.; Heuvelink, E.; Khaleghi, S.; Bustos-Korts, D.; Marcelis, L.F.M.; Vermeer, K.M.C.A.; van Dijk, H.; Millenaar, F.F.; Van Voorn, G.A.K.; Van Eeuwijk, F.A. Yield dissection models to improve yield: A case study in tomato, in silico. Plants 2021, 3, diab012. [Google Scholar] [CrossRef]
- Nitsch, L.; Kohlen, W.; Oplaat, C.; Charnikhova, T.; Cristescu, S.; Michieli, P.; Wolters-Arts, M.; Bouwmeester, H.; Mariani, C.; Vriezen, W.H.; et al. ABA-deficiency results in reduced plant and fruit size in tomato. J. Plant Physiol. 2012, 169, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Torgeman, S.; Zamir, D. Epistatic QTLs for yield heterosis in tomato. Proc. Natl. Acad. Sci. USA 2023, 120, e2205787119. [Google Scholar] [CrossRef]
- Mu, Q.I.; Huang, Z.; Chakrabarti, M.; Illa-Berenguer, E.; Liu, X.; Wang, Y.; Ramos, A.; van der Knaap, E. Fruit weight is controlled by Cell Size Regulator encoding a novel protein that is expressed in maturing tomato fruits. PLoS Genet. 2017, 13, e1006930. [Google Scholar] [CrossRef]
- Beauchet, A.; Gévaudant, F.; Gonzalez, N.; Chevalier, C. In search of the still unknown function of FW2.2/CELL NUMBER REGULATOR, a major regulator of fruit size in tomato. J. Exp. Bot. 2021, 72, 5300–5311. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Feng, Q.; Qin, L.; Pan, C.; Lamin-Samu, A.T.; Lu, G. Tomato AUXIN RESPONSE FACTOR 5 regulates fruit set and development via the mediation of auxin and gibberellin signaling. Sci. Rep. 2018, 8, 2971. [Google Scholar] [CrossRef]
- Thomas, H.; Van den Broeck, L.; Spurney, R.; Sozzani, R.; Frank, M. Gene regulatory networks for compatible versus incompatible grafts identify a role for SlWOX4 during junction formation. Plant Cell 2022, 34, 535–556. [Google Scholar] [CrossRef] [PubMed]
- Cazzonelli, C.I.; Vanstraelen, M.; Simon, S.; Yin, K.; Carron-Arthur, A.; Nisar, N.; Tarle, G.; Cuttriss, A.J.; Searle, I.R.; Benkova, E.; et al. Role of the Arabidopsis PIN6 Auxin Transporter in Auxin Homeostasis and Auxin-Mediated Development. PLoS ONE 2013, 8, e70069. [Google Scholar] [CrossRef] [PubMed]
- Bouzroud, S.; Gouiaa, S.; Hu, N.; Bernadac, A.; Mila, I.; Bendaou, N.; Smouni, A.; Bouzayen, M.; Zouine, M. Auxin response factors (ARFs) are potential mediators of auxin action in tomato response to biotic and abiotic stress (Solanum lycopersicum). PLoS ONE 2018, 13, e0193517. [Google Scholar] [CrossRef] [PubMed]
- Bouzroud, S.; Gasparini, K.; Hu, G.; Barbosa, M.A.M.; Rosa, B.L.; Fahr, M.; Bendaou, N.; Bouzayen, M.; Zsögön, A.; Smouni, A.; et al. Down Regulation and Loss of Auxin Response Factor 4 Function Using CRISPR/Cas9 Alters Plant Growth, Stomatal Function and Improves Tomato Tolerance to Salinity and Osmotic Stress. Genes 2020, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; He, S.; Zhai, H.; Li, R.; Zhao, N.; Liu, Q. A Sweetpotato Auxin Response Factor Gene (IbARF5) Is Involved in Carotenoid Biosynthesis and Salt and Drought Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
- Van Ooijen, J.W. MapQTL® 6, Software for the Mapping of Quantitative Trait Loci in Experimental Populations of Diploid Species; Kyazma BV: Wageningen, The Netherlands, 2009. [Google Scholar]
- Sosnowski, O.; Charcosset, A.; Joets, J. BioMercator V3: An upgrade of genetic map compilation and quantitative trait loci meta-analysis algorithms. Bioinformatics 2012, 28, 2082–2083. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Sleper, D.A.; Lu, P.; Shannon, J.G.; Nguyen, H.T.; Arelli, P.R. QTLs Associated with Resistance to Soybean Cyst Nematode in Soybean: Meta-Analysis of QTL Locations. Crop Sci. 2006, 46, 595–602. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.; Yao, R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef]
- Kevei, Z.; King, R.C.; Mohareb, F.; Sergeant, M.J.; Awan, S.Z.; Thompson, A.J. Resequencing at ≥40-Fold Depth of the Parental Genomes of a Solanum lycopersicum x S. pimpinellifolium Recombinant Inbred Line Population and Characterization of Frame-Shift InDels That Are Highly Likely to Perturb Protein Function. G3 Genes/Genomes/Genet. 2015, 5, 971–981. [Google Scholar] [CrossRef]
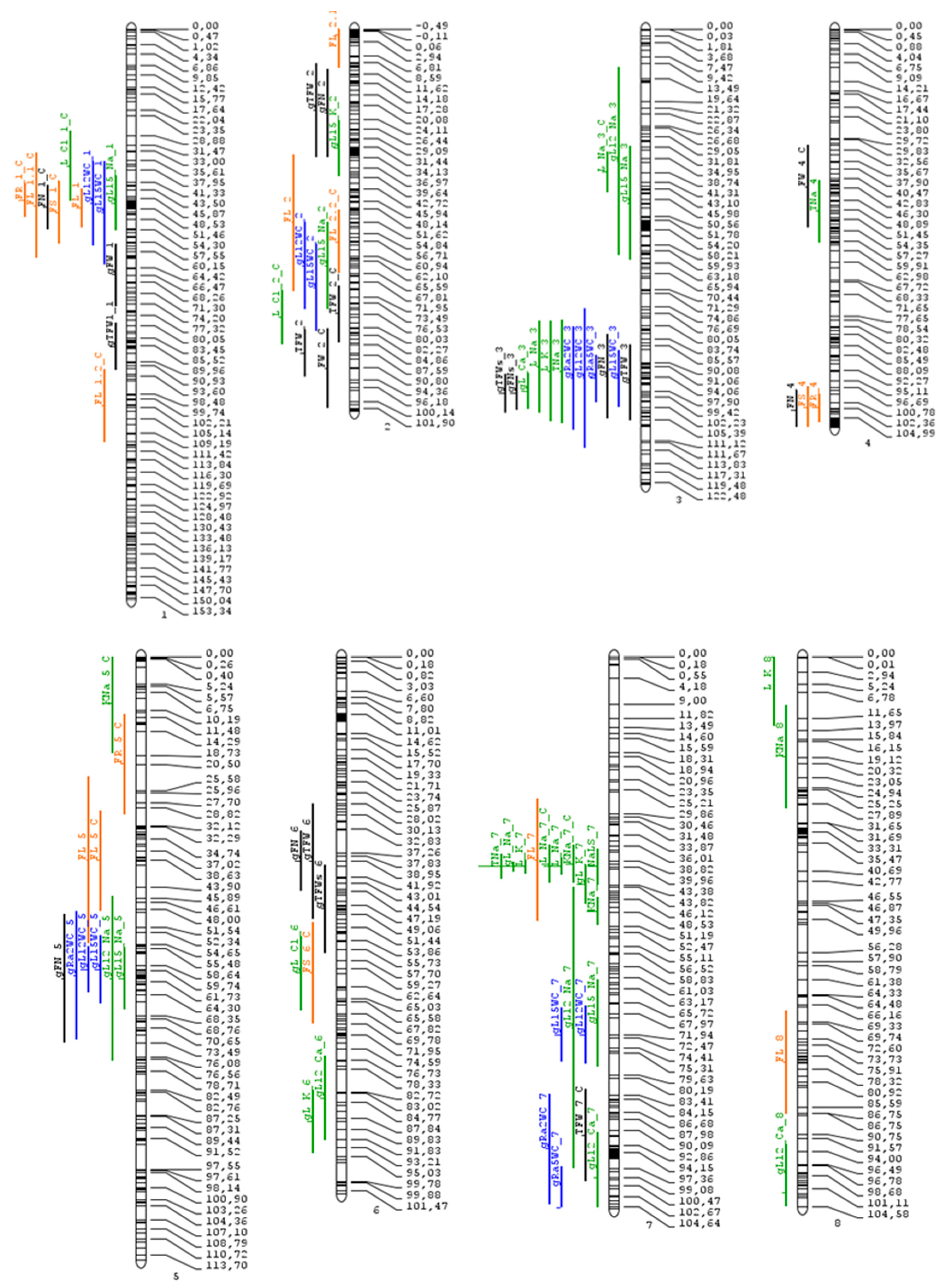
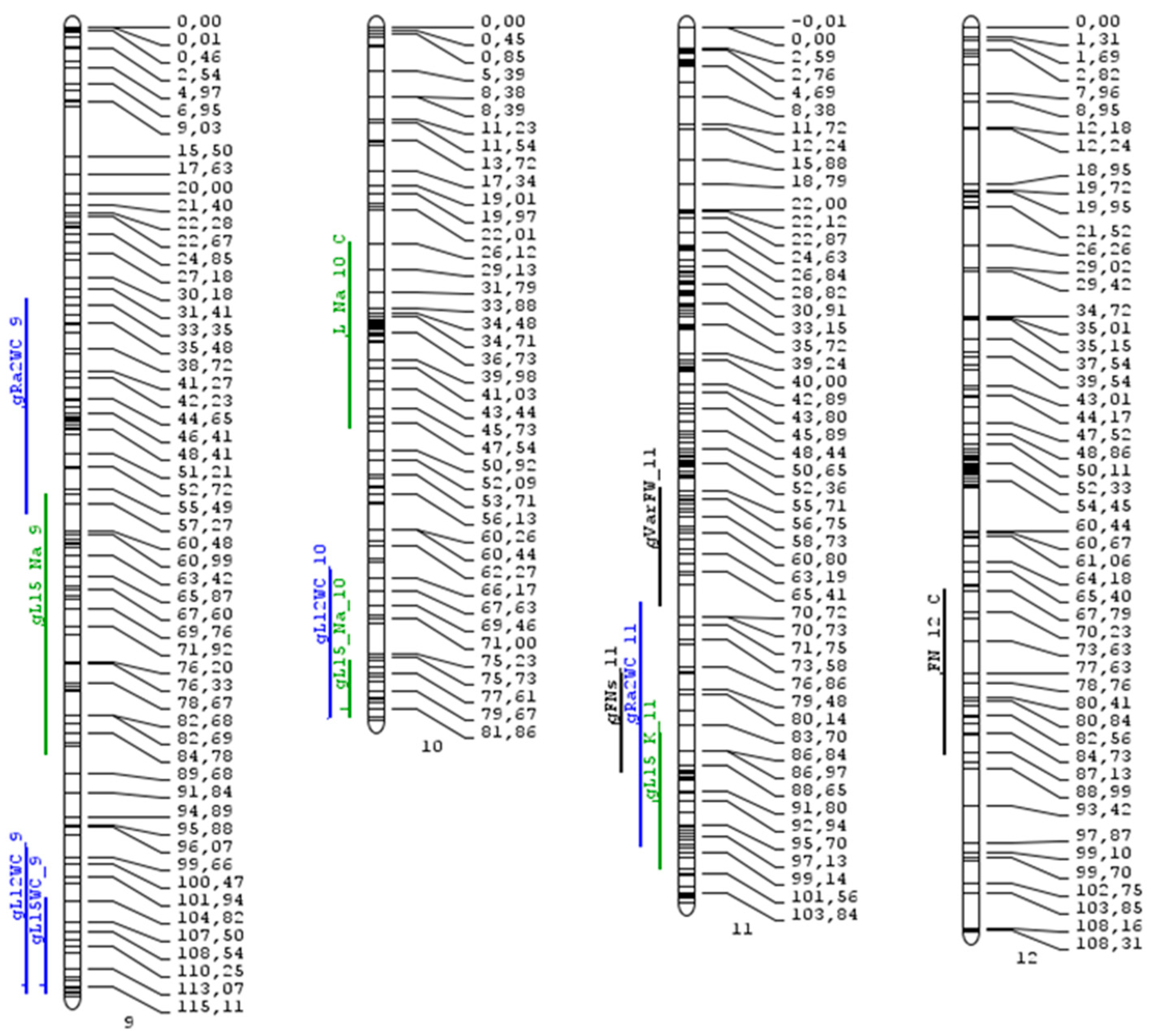
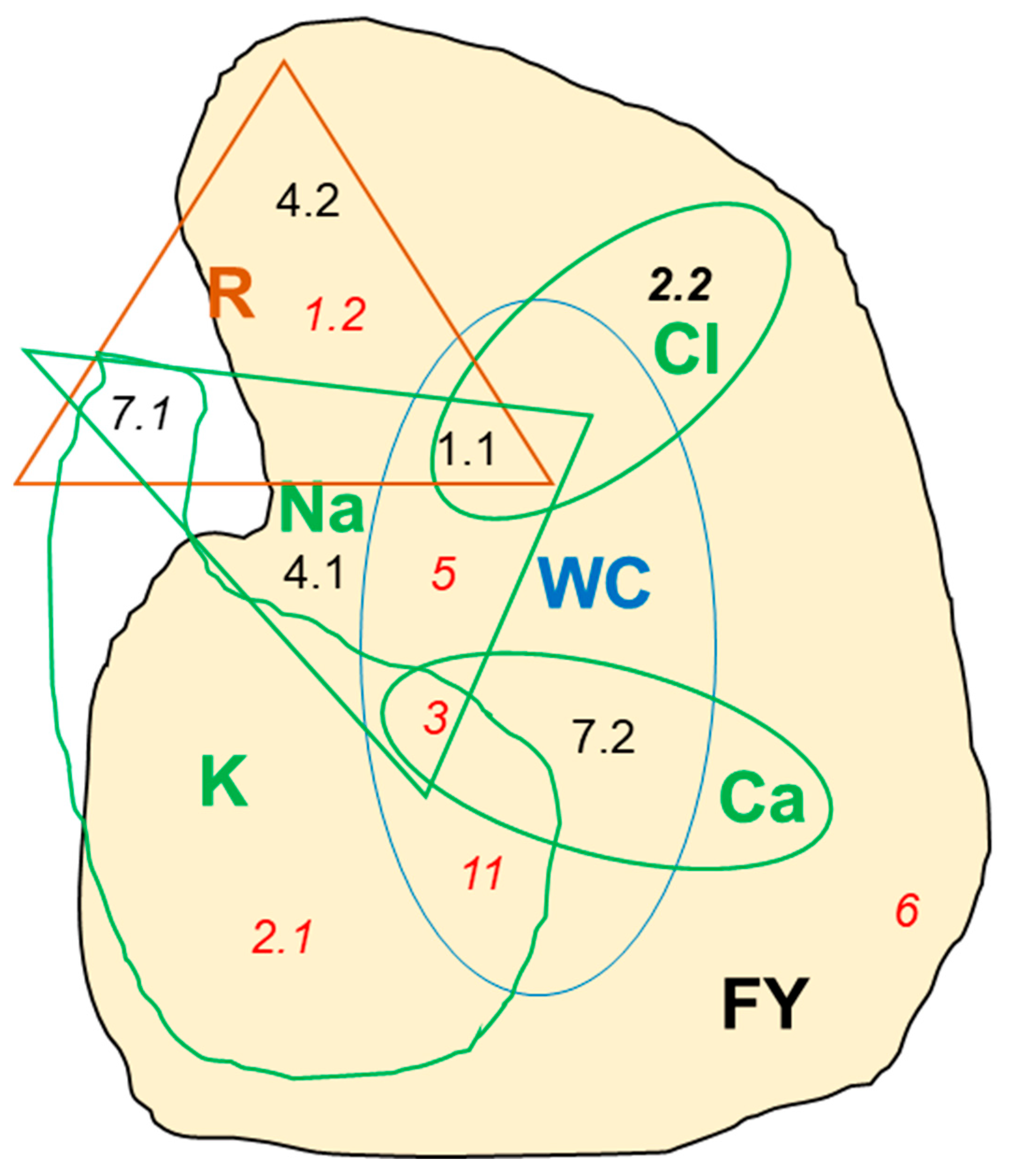
| MQTL | Pos. | CI_lo (cM) | CI_lo SNP | CI_hi (cM) | CI_hi SNP | CI (Mbp) | CI Genes |
|---|---|---|---|---|---|---|---|
| 1.1 | 47.03 | 44.6 | 33677 | 49.46 | 50504 | 71.06 | 1210 |
| 1.2 | 90.68 | 85.24 | 34545 | 96.12 | 28168 | 2.34 | 291 |
| 2.1 | 27.64 | 21.89 | 33633 | 33.38 | 29543 | 2.7 | 358 |
| 2.2 | 82.34 | 78.48 | 50066 | 86.2 | 67052 | 2.07 | 276 |
| 3 | 96.53 | 94.32 | 62180 | 98.74 | 61967 | 0.88 | 123 |
| 4.1 | 46.91 | 40.1 | 41577 | 53.73 | 24557 | 50.33 | 1096 |
| 4.2 | 102.54 | 99.77 | 47540 | 105.32 | 3896 | 0.87 | 113 |
| 5 | 60.13 | 57.24 | 50872 | 60.03 | 51127 | 4.05 | 209 |
| 6 | 41.38 | 37.01 | 55858 | 45.75 | 1360 | 1.95 | 198 |
| 7.1 | 40.04 | 39.4 | 57002 | 40.67 | 38698 | 2.59 | 94 |
| 7.2 | 97.53 | 92.4 | 55453 | 102.67 | 100289 | 3.08 | 407 |
| 11 | 86.61 | 81.96 | 100998 | 91.27 | 100965 | 0.85 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asins, M.J.; Carbonell, E.A. Meta-QTL and Candidate Gene Analyses of Agronomic Salt Tolerance and Related Traits in an RIL Population Derived from Solanum pimpinellifolium. Int. J. Mol. Sci. 2024, 25, 6055. https://doi.org/10.3390/ijms25116055
Asins MJ, Carbonell EA. Meta-QTL and Candidate Gene Analyses of Agronomic Salt Tolerance and Related Traits in an RIL Population Derived from Solanum pimpinellifolium. International Journal of Molecular Sciences. 2024; 25(11):6055. https://doi.org/10.3390/ijms25116055
Chicago/Turabian StyleAsins, Maria J., and Emilio A. Carbonell. 2024. "Meta-QTL and Candidate Gene Analyses of Agronomic Salt Tolerance and Related Traits in an RIL Population Derived from Solanum pimpinellifolium" International Journal of Molecular Sciences 25, no. 11: 6055. https://doi.org/10.3390/ijms25116055






