Functional Characterization of the Effects of CsDGAT1 and CsDGAT2 on Fatty Acid Composition in Camelina sativa
Abstract
1. Introduction
2. Results
2.1. Phylogenetic Relationships of CsDGAT1s and CsDGAT2s
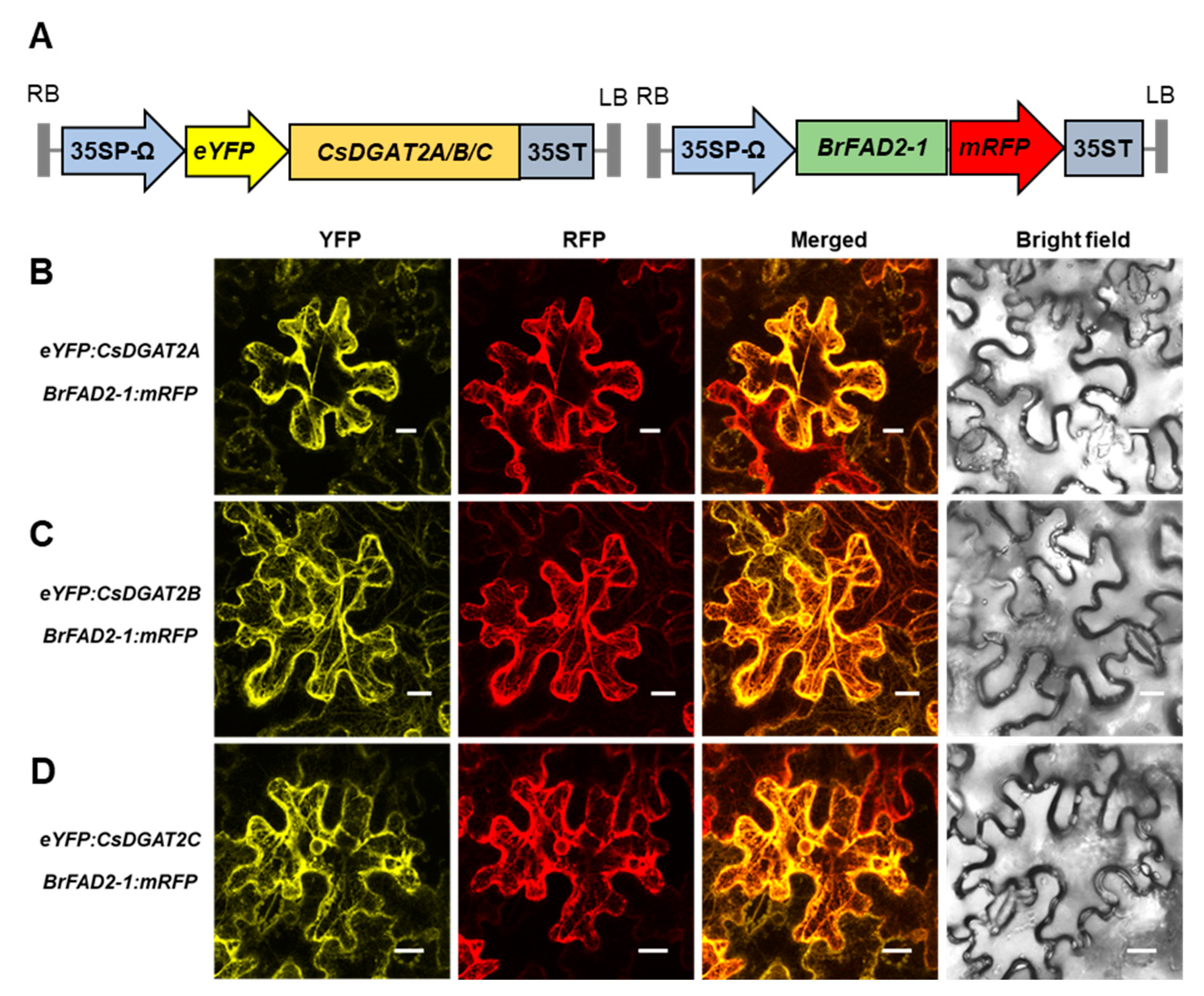
2.2. Both DGAT1 and DGAT2 Are Membrane-Bound Proteins Localized to the Endoplasmic Reticulum (ER)
2.3. CsDGAT1 and CsDGAT2 Have Seed-Specific Expression, but the Expression Level of CsDGAT1 Was Higher
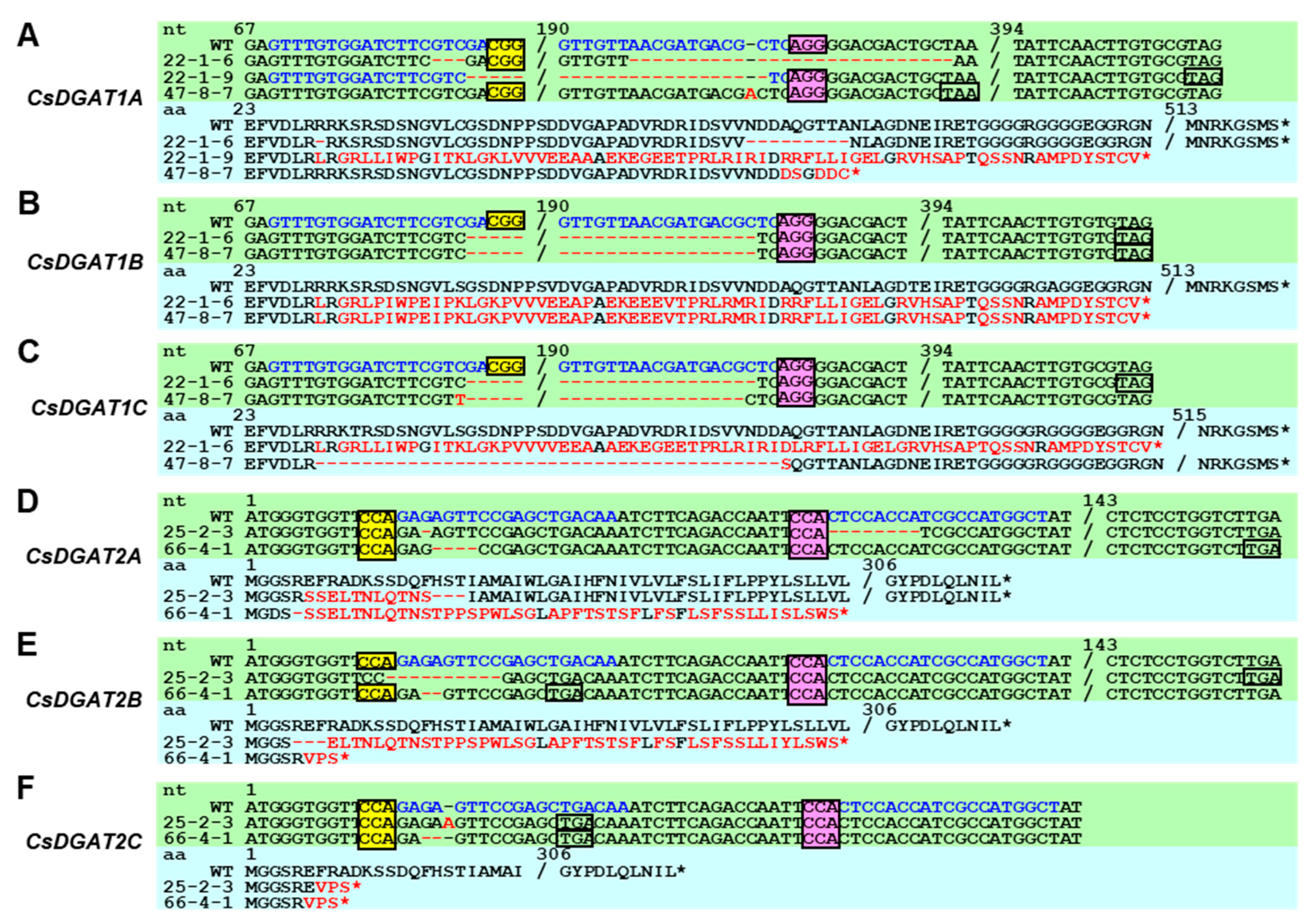
2.4. Csdgat1 KO Lines and Csdgat2 KO Lines Were Obtained Using CRISPR/Cas
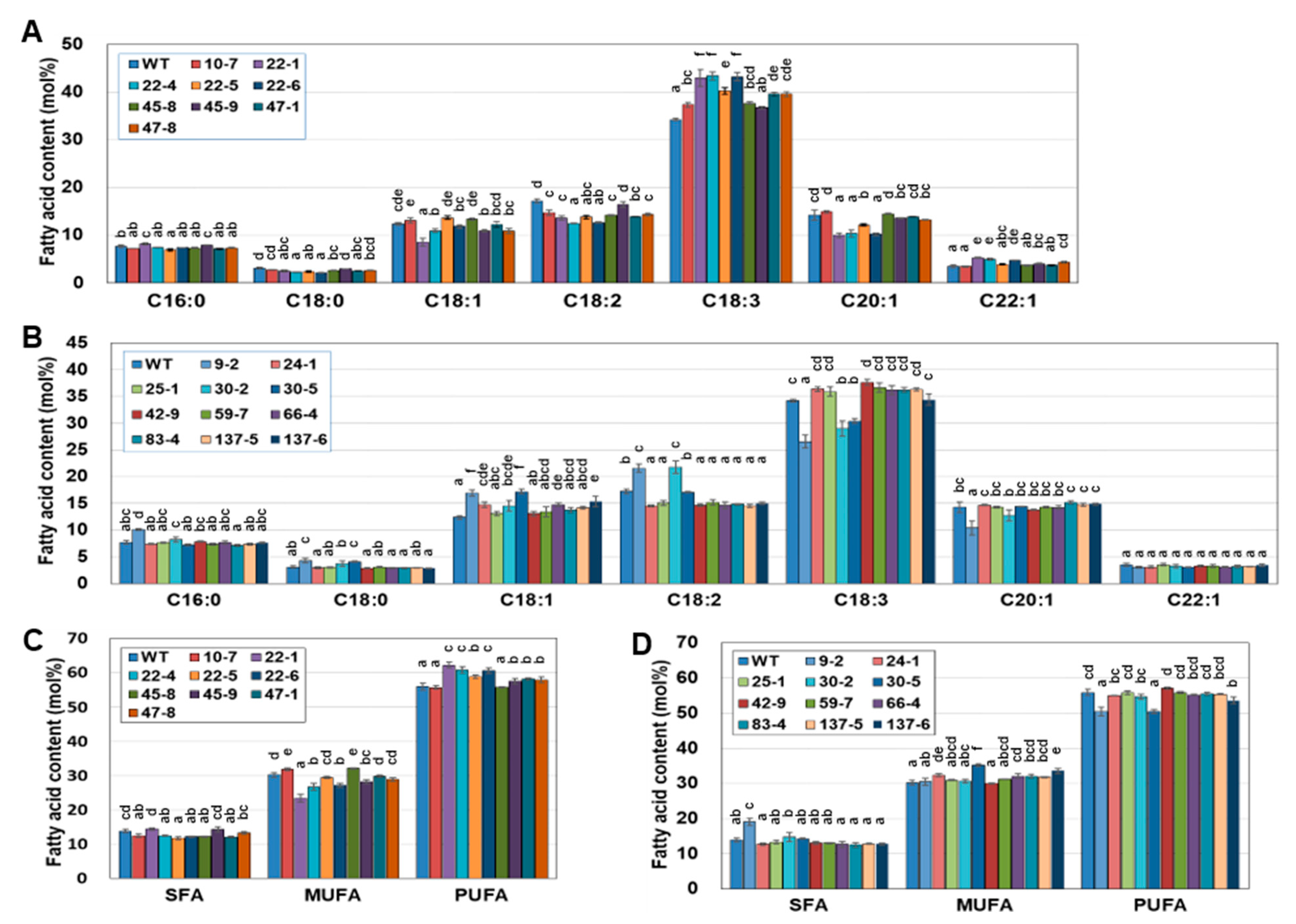
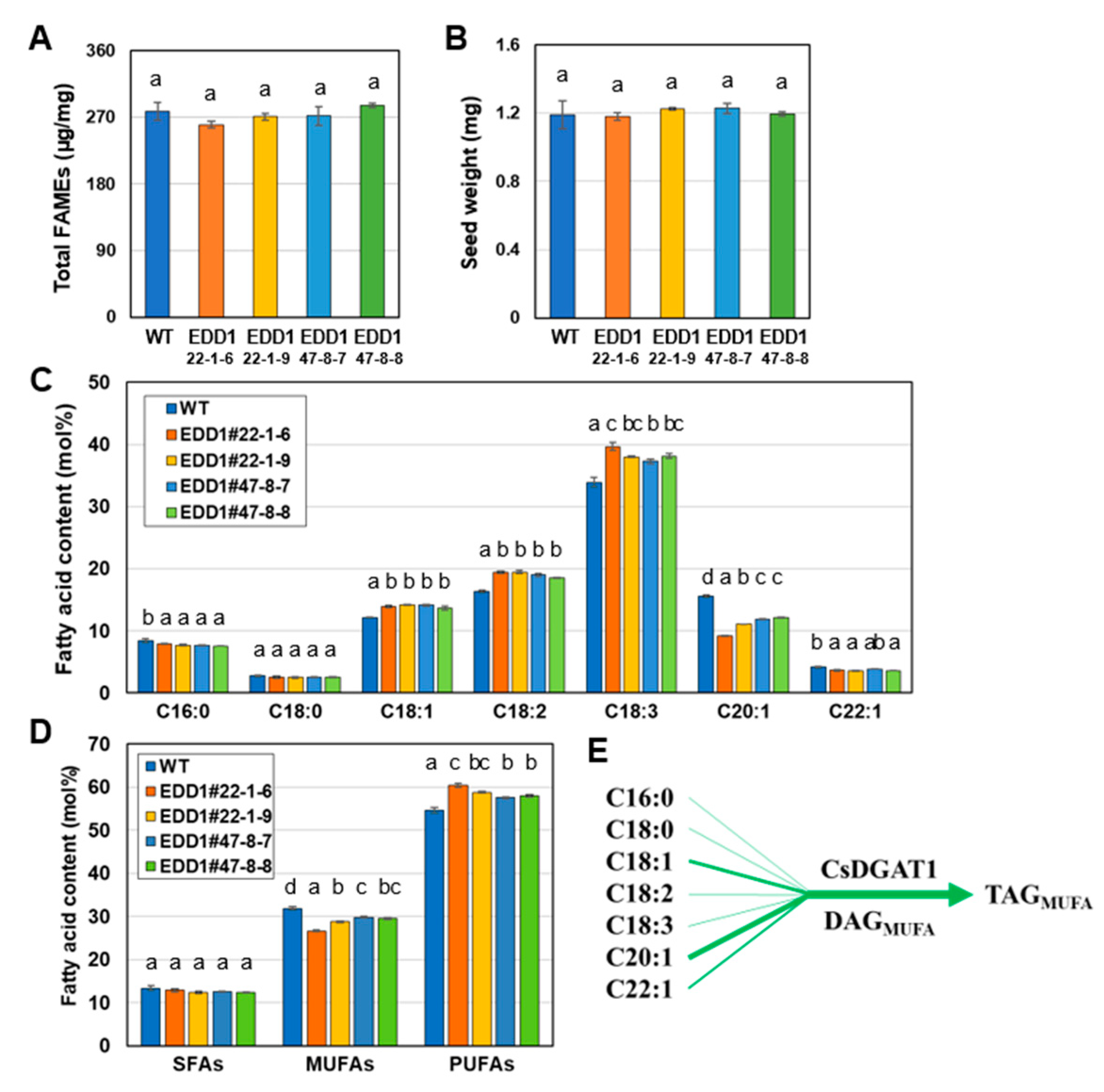
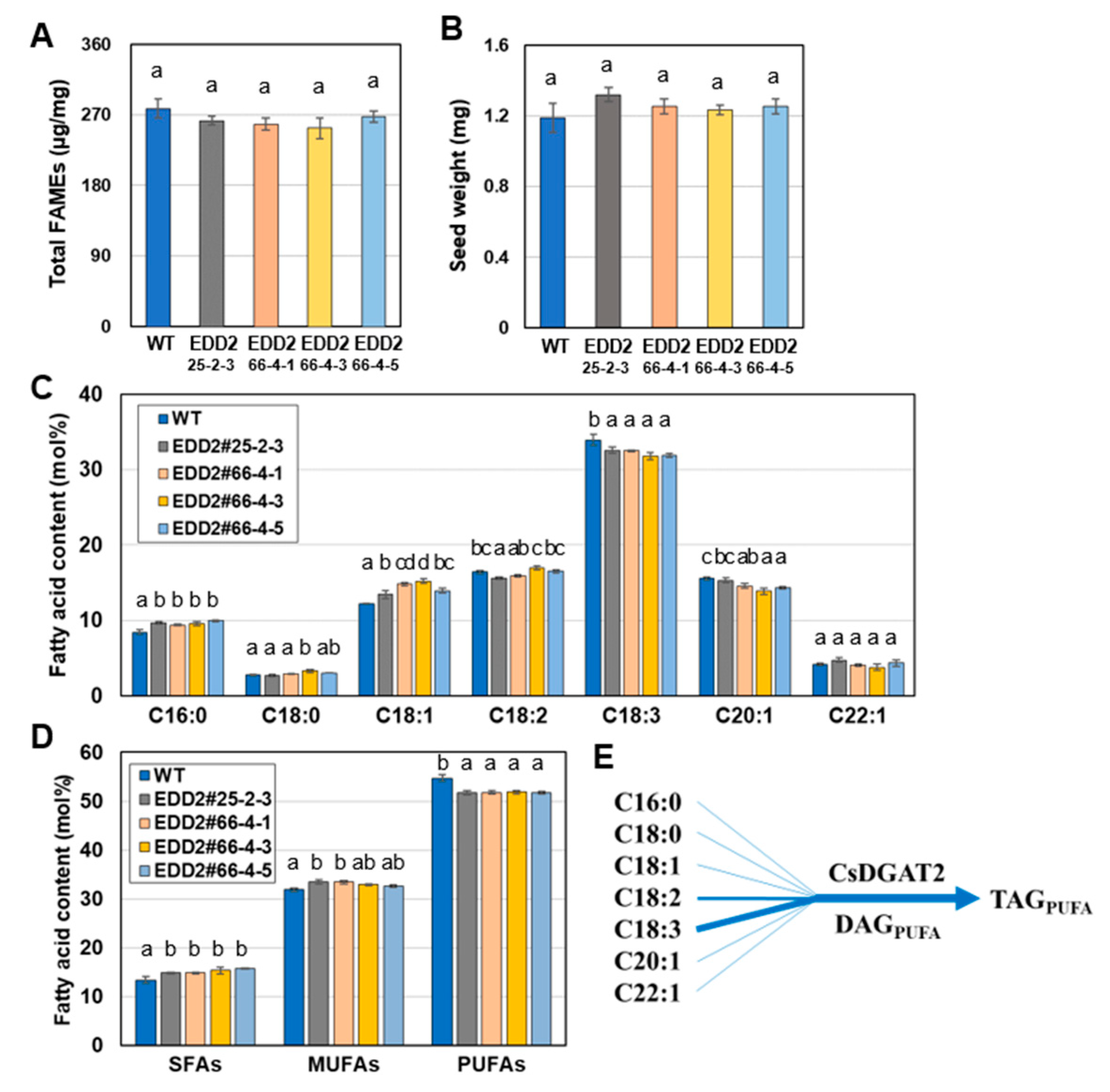
2.5. EDD1 and EDD2 Seeds Have Changed Fatty Acid Content Compared to WT Seeds
2.6. EDD1 and EDD2 Lines Were Phenotypically Similar to WT
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Subcellular Localization Analysis Using Nicotiana benthamiana Leaves
4.3. Reverse-Transcription Quantitative PCR Analysis
4.4. Guide RNA Design and Vector Construction
4.5. Camelina Transformation and Selection of Transformants
4.6. Homoeolog-Specific PCR and Sanger Sequencing–Based Genotyping
4.7. Fatty Acid Analysis
4.8. Phylogenetic Analysis and Multiple Alignment
4.9. Seed Phenotyping
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, E.P. Biosynthesis of complex lipids. Fed. Proc. 1961, 20, 934–940. [Google Scholar] [PubMed]
- Dahlqvist, A.; Ståhl, U.; Lenman, M.; Banaś, A.; Lee, M.; Sandager, L.; Ronne, H.; Stymne, S. Phospholipid:diacylglycerol acyltransferase: An enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl. Acad. Sci. USA 2000, 97, 6487–6492. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, U.; Carlsson, A.S.; Lenman, M.; Dahlqvist, A.; Huang, B.; Banaś, W.; Banaś, A.; Stymne, S. Cloning and functional characterization of a phospholipid:diacylglycerol acyltransferase from Arabidopsis. Plant Physiol. 2004, 135, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Ichihara, K.; Takahashi, T.; Fujii, S. Diacylglycerol acyltransferase in maturing safflower seeds: Its influences on the fatty acid composition of triacylglycerol and on the rate of triacylglycerol synthesis. Biochim. Biophys. Acta 1988, 958, 125–129. [Google Scholar] [CrossRef]
- Cases, S.; Smith, S.J.; Zheng, Y.W.; Myers, H.M.; Lear, S.R.; Sande, E.; Novak, S.; Collins, C.; Welch, C.B.; Lusis, A.J.; et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc. Natl. Acad. Sci. USA 1998, 95, 13018–13023. [Google Scholar] [CrossRef] [PubMed]
- Routaboul, J.M.; Benning, C.; Bechtold, N.; Caboche, M.; Lepiniec, L. The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol. Biochem. 1999, 37, 831–840. [Google Scholar] [CrossRef]
- Zou, J.; Wei, Y.; Jako, C.; Kumar, A.; Selvaraj, G.; Taylor, D.C. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J. 1999, 19, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Nykiforuk, C.L.; Furukawa-Stoffer, T.L.; Huff, P.W.; Sarna, M.; Laroche, A.; Moloney, M.M.; Weselake, R.J. Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose-mediated induction of enzyme biosynthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2002, 1580, 95–109. [Google Scholar] [CrossRef]
- Xu, J.; Francis, T.; Mietkiewska, E.; Giblin, E.M.; Barton, D.L.; Zhang, Y.; Zhang, M.; Taylor, D.C. Cloning and characterization of an acyl-CoA-dependent diacylglycerol acyltransferase 1 (DGAT1) gene from Tropaeolum majus, and a study of the functional motifs of the DGAT protein using site-directed mutagenesis to modify enzyme activity and oil content. Plant Biotechnol. J. 2008, 6, 799–818. [Google Scholar] [CrossRef]
- Cao, H. Structure-Function Analysis of Diacylglycerol Acyltransferase Sequences from 70 Organisms. BMC Res. Notes 2011, 4, 249. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.H.; Kim, D.J.; Kim, A.Y.; Suh, M.C. Functional analysis of diacylglycerol acyltransferase 1 genes from Camelina sativa and effects of CsDGAT1B overexpression on seed mass and storage oil content in C. sativa. Plant Biotechnol. Rep. 2016, 10, 141–153. [Google Scholar] [CrossRef]
- Torabi, S.; Sukumaran, A.; Dhaubhadel, S.; Johnson, S.E.; LaFayette, P.; Parrot, W.A.; Rajcan, I.; Eskandari, M. Effects of type I Diacylglycerol O-acyltransferase (DGAT1) genes on soybean (Glycine max L.) seed composition. Sci. Rep. 2021, 11, 2556. [Google Scholar] [CrossRef] [PubMed]
- Katavic, V.; Reed, D.W.; Taylor, D.C.; Giblin, E.M.; Barton, D.L.; Zou, J.; Mackenzie, S.L.; Covello, P.S.; Kunst, L. Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol. 1995, 108, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Lardizabal, K.D.; Mai, J.T.; Wagner, N.W.; Wyrick, A.; Voelker, T.; Hawkins, D.J. DGAT2 Is a New Diacylglycerol Acyltransferase Gene Family: Purification, cloning, and expression in insect cells of two polypeptides from mortierella ramanniana with diacylglycerol acyltransferase activity. J. Biol. Chem. 2001, 276, 38862–38869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fan, J.; Taylor, D.C.; Ohlrogge, J.B. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 2009, 21, 3885–3901. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, K.; Wu, Y.; Tateno, M.; Hatanaka, T.; Hildebrand, D.F. Vernonia DGATs can complement the disrupted oil and protein metabolism in epoxygenase expressing soybean seeds. Metab. Eng. 2012, 14, 29–38. [Google Scholar] [CrossRef]
- Shockey, J.; Gidda, S.; Chapital, D.; Kuan, J.; Dhanoa, P.; Bland, J.; Rothstein, S.; Mullen, R.; Dyer, J. Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 2006, 18, 2294–2313. [Google Scholar] [CrossRef] [PubMed]
- Kroon, J.T.; Wei, W.; Simon, W.J.; Slabas, A.R. Identification and functional expression of a type 2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 2006, 67, 2541–2549. [Google Scholar] [CrossRef]
- Burgal, J.; Shockey, J.; Lu, C.; Dyer, J.; Larson, T.; Graham, I.; Browse, J. Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol. J. 2008, 6, 819–831. [Google Scholar] [CrossRef]
- Saha, S.; Enugutti, B.; Rajakumari, S.; Rajasekharan, R. Cytosolic triacylglycerol biosynthetic pathway in oilseeds. molecular cloning and expression of peanut cytosolic diacylglycerol acyltransferase. Plant Physiol. 2006, 141, 1533–1543. [Google Scholar] [CrossRef]
- Hernández, M.L.; Whitehead, L.; He, Z.; Gazda, V.; Gilday, A.; Kozhevnikova, E.; Vaistij, F.E.; Larson, T.R.; Graham, I.A. A cytosolic acyltransferase contributes to triacylglycerol synthesis in sucrose-rescued Arabidopsis seed oil catabolism mutants. Plant Physiol. 2012, 160, 215–225. [Google Scholar] [CrossRef]
- Cao, H.; Shockey, J.M.; Klasson, K.T.; Chapital, D.C.; Mason, C.B.; Scheffler, B.E. Developmental Regulation of Diacylglycerol Acyltransferase Family Gene Expression in Tung Tree Tissues. PLoS ONE 2013, 8, e76946. [Google Scholar] [CrossRef] [PubMed]
- Zubr, J. Oil-seed crop: Camelina sativa. Ind. Crops Prod. 1997, 6, 113–119. [Google Scholar] [CrossRef]
- Iskandarov, U.; Kim, H.J.; Cahoon, E.B. Camelina: An Emerging oilseed platform for advanced biofuels and bio-based materials: In Plants and BioEnergy; Springer: New York, NY, USA, 2014; pp. 131–140. [Google Scholar]
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina uses, genetics, genomics, production, and management. Ind. Crop. Prod. 2016, 94, 690–710. [Google Scholar] [CrossRef]
- Putnam, D.H.; Budin, J.T.; Field, L.A.; Breene, W.M. Camelina: A Promising Low Input Oilseed. New Crops; Wiley: New York, NY, USA, 1993; pp. 314–322. [Google Scholar]
- Kagale, S.; Koh, C.; Nixon, J.; Bollina, V.; Clarke, W.E.; Tuteja, R.; Spillane, C.; Robinson, S.J.; Links, M.G.; Clarke, C.; et al. The emerging biofuel crop Camelina sativa retains a highly undifferentiated hexaploid genome structure. Nat. Comm. 2014, 5, 3706. [Google Scholar] [CrossRef] [PubMed]
- Hutcheon, C.; Ditt, R.F.; Beilstein, M.; Comai, L.; Schroeder, J.; Goldstein, E.; Shewmaker, C.K.; Nguyen, T.; Rocher, J.D.; Kiser, J. Polyploid genome of Camelina sativa revealed by isolation of fatty acid synthesis genes. BMC Plant Biol. 2010, 10, 233–247. [Google Scholar] [CrossRef]
- Lu, C.; Kang, J. Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep. 2008, 27, 273–278. [Google Scholar] [CrossRef]
- Liu, X.; Brost, J.; Hutcheon, C.; Guilfoil, R.; Wilson, A.K.; Leung, S.; Shewmaker, C.K.; Rooke, S.; Nguyen, T.; Kiser, J.; et al. Transformation of the oilseed crop Camelina sativa by Agrobacterium-mediated floral dip and simple large-scale screening of transformants. In Vitro Cell. Dev. Biol.–Plant 2012, 48, 462–468. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Bansal, S.; Durrett, T.P. Camelina sativa: An ideal platform for the metabolic engineering and field production of industrial lipids. Biochimie 2016, 120, 9–16. [Google Scholar] [CrossRef]
- Marmon, S.; Sturtevant, D.; Herrfurth, C.; Chapman, K.; Stymne, S.; Feussner, I. Two Acyltransferases Contribute Differently to Linolenic Acid Levels in Seed Oil. Plant Physiol. 2017, 173, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Lager, I.; Jeppson, S.; Gippert, A.-L.; Feussner, I.; Stymne, S.; Marmon, S. Acyltransferases Regulate Oil Quality in Camelina sativa Through Both Acyl Donor and Acyl Acceptor Specificities. Front. Plant Sci. 2020, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Gao, Y.; Zhang, F.; Liu, B.; Ji, C.; Xue, J.; Yuan, L.; Li, R. Functional characterization of an novel acyl-CoA:diacylglycerol acyltransferase 3-3 (CsDGAT3-3) gene from Camelina sativa. Plant Sci. 2021, 303, 110752. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Kim, Y.M.; Yeo, Y.; Kim, S.; Suh, M.C. Camelina cytosol-localized diacylglycerol acyltransferase 3 contributes to the accumulation of seed storage oils. Ind. Crop. Prod. 2022, 189, 115808. [Google Scholar] [CrossRef]
- Liu, Q.; Siloto, R.M.P.; Lehner, R.; Stone, S.J.; Weselake, R.J. Acyl-CoA:diacylglycerol acyltransferase: Molecular biology, biochemistry and biotechnology. Prog. Lipid Res. 2012, 51, 350–377. [Google Scholar] [CrossRef] [PubMed]
- Jako, C.; Kumar, A.; Wei, Y.; Zou, J.; Barton, D.L.; Giblin, E.M.; Covello, P.S.; Taylor, D.C. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol. 2001, 126, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.L.; Nobre, T.M.; Cilli, E.M.; Beltramini, L.M.; Araujo, A.P.; Wallace, B.A. Deconstructing the DGAT1 enzyme: Binding sites and substrate interactions. Biochim. Biophys. Acta 2014, 1838, 3145–3152. [Google Scholar] [CrossRef]
- Lopes, J.L.; Beltramini, L.M.; Wallace, B.A.; Araujo, A.P. Deconstructing the DGAT1 enzyme: Membrane interactions at substrate binding sites. PLoS ONE 2015, 10, e0118407. [Google Scholar] [CrossRef]
- Zhou, X.-R.; Shrestha, P.; Yin, F.; Petrie, J.R.; Singh, S.P. AtDGAT2 is a functional acyl-CoA:diacylglycerol acyltransferase and displays different acyl-CoA substrate preferences than AtDGAT1. FEBS Lett. 2013, 587, 2371–2376. [Google Scholar] [CrossRef]
- Hayashi, K.; Hashimoto, N.; Daigen, M.; Ashikawa, I. Development of PCR-based SNP markers for rice blast resistance genes at the Piz locus. Theor. Appl. Genet. 2004, 108, 1212–1220. [Google Scholar] [CrossRef]
- Park, J.; Bae, S.; Kim, J.S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef]
- Liu, W.; Xie, X.; Ma, X.; Li, J. DSDecode: A Web-Based Tool for Decoding of Sequencing Chromatograms for Genotyping of Targeted Mutations. Mol. Plant 2015, 8, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- McCartney, A.W.; Dyer, J.M.; Dhanoa, P.K.; Kim, P.K.; Andrews, D.W.; McNew, J.A.; Mullen, R.T. Membrane-bound fatty acid desaturases are inserted co-translationally into the ER and contain different ER retrieval motifs at their carboxy termini. Plant J. 2004, 37, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Hills, M.J. Arabidopsis mutants deficient in diacylglycerol acyltransferase display increased sensitivity to abscisic acid, sugars, and osmotic stress during germination and seedling development. Plant Physiol. 2002, 129, 1352–1358. [Google Scholar] [CrossRef]
- Engler, C.; Kandzia, R.; Marillonnet, S. A One Pot, One Step, Precision Cloning Method with High Throughput Capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, H.; Go, Y.S.; Lee, S.B.; Hur, C.-G.; Kim, H.U.; Suh, M.C. Identification of functional BrFAD2-1 gene encoding microsomal delta-12 fatty acid desaturase from Brassica rapa and development of Brassica napus containing high oleic acid contents. Plant Cell Rep. 2011, 30, 1881–1892. [Google Scholar] [CrossRef]
- Qiu, W.; Park, J.W.; Scholthof, H.B. Tombusvirus P19-mediated suppression of virus-induced gene silencing is controlled by genetic and dosage features that influence pathogenicity. Mol. Plant Microbe Interact. 2002, 15, 269–280. [Google Scholar] [CrossRef]
- Wang, Z.P.; Xing, H.L.; Dong, L.; Zhang, H.Y.; Han, C.Y.; Wang, X.C.; Chen, Q.J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015, 16, 144. [Google Scholar] [CrossRef]
- Höfgen, R.; and Willmitzer, L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988, 16, 9877. [Google Scholar] [CrossRef]
- Lee, K.R.; Jeon, I.; Yu, H.; Kim, S.G.; Kim, H.S.; Ahn, S.J.; Lee, J.; Lee, S.K.; Kim, H.U. Increasing Monounsaturated Fatty Acid Contents in Hexaploid Camelina sativa Seed Oil by FAD2 Gene Knockout Using CRISPR-Cas9. Front. Plant Sci. 2021, 12, 702930. [Google Scholar] [CrossRef] [PubMed]
| Line No. | CsDGAT1A | CsDGAT1B | CsDGAT1C | Predicted Genotype | |||
|---|---|---|---|---|---|---|---|
| Indel | Zygosity | Indel | Zygosity | Indel | Zygosity | ||
| EDD1#10-1, -3, -4, -10 | D6 | Het | - | WT | - | WT | Aa′BBCC |
| EDD1#10-5 | D39 + D6 | Het | - | WT | - | WT | Aa′BBCC |
| EDD1#10-6, -7 | D39 + D6 | Hom | - | WT | - | WT | a′a′BBCC |
| EDD1#22-1, -4 | D3 + D27, D121 | Biallele | D121 | Hom | D121 | Hom | aa′bbcc |
| EDD1#22-2 | D3 + D27, D121 | Biallele | D121 | Het | D121 | Het | aa′BbCc |
| EDD1#22-3 | D3 + D27 | Hom | D121 | Hom | D121 | Het | a′a′bbCc |
| EDD1#22-5 | D3 + D27 | Hom | D121 | Het | D121 | Hom | a′a′Bbcc |
| EDD1#22-6, -7 | D3 + D27, D121 | Biallele | D121 | Hom | D121 | Het | aa′bbCc |
| EDD1#22-8 | D3 + D27, D121 | Biallele | D121 | Het | D121 | Hom | aa′Bbcc |
| EDD1#45-1, -3, -6 | - | WT | - | WT | D14 | Het | AABBCc |
| EDD1#45-8, -9 | - | WT | - | WT | D14 | Hom | AABBcc |
| EDD1#47-1, -2, -3, -8 | I1 | Hom | D121 | Hom | D120 | Hom | aabbc′c′ |
| EDD1#47-4, -9, -10 | Chi | - | D121 | Hom | D120 | Het | ??bbCc′ |
| EDD1#47-5, -6 | Chi | - | D121 | Hom | D120 | Hom | ??bbc′c′ |
| Line No. | CsDGAT2A | CsDGAT2B | CsDGAT2C | Predicted Genotype | |||
|---|---|---|---|---|---|---|---|
| Indel | Zygosity | Indel | Zygosity | Indel | Zygosity | ||
| EDD2#9-2, -4, -5 -8 | D37 | Hom | D2 | Hom | D9 | Hom | aabbc′c′ |
| EDD2#9-3, -7, -9, -10 | D37 | Hom | D37, D2 | Biallele | D9 | Hom | aabbc′c′ |
| EDD2#24-1 | D9 | Hom | D2 | Hom | D10, D2 | Biallele | a′a′bbcc |
| EDD2#24-2 | D9 | Hom | D45 | Hom | D10 | Hom | a′a′bbcc |
| EDD2#24-3, -4, -6, -8 | D9 | Hom | D45, D2 | Biallele | D2 | Hom | a′a′bbcc |
| EDD2#24-7 | D9 | Hom | D45 | Hom | D10, D2 | Biallele | a′a′bbcc |
| EDD2#24-9, -10 | D9 | Hom | D45, D2 | Biallele | D10, D2 | Biallele | a′a′bbcc |
| EDD2#25-1 | D1 + D8 | Hom | D5 | Hom | D2 | Hom | a′a′bbcc |
| EDD2#25-2, -5 | D1 + D8 | Hom | D5, D10 | Biallele | I1 | Hom | a′a′bbcc |
| EDD2#25-3, -9 -10 | D1 + D8 | Hom | D5, D10 | Biallele | D2, I1 | Biallele | a′a′bbcc |
| EDD2#25-6 | D1 + D8 | Hom | D10 | Hom | D2, I1 | Biallele | a′a′bbcc |
| EDD2#25-7, -8 | D1 + D8 | Hom | D5, D10 | Biallele | D2 | Hom | a′a′bbcc |
| EDD2#30-1, -2 -4, -6, -9 | D36 | Hom | D9, D10 | Biallele | D38, D9 | Biallele | a′a′b′bcc′ |
| EDD2#30-3 | D36 | Hom | D9, D10 | Biallele | D9 | Hom | a′a′b′bc′c′ |
| EDD2#30-5 | D36 | Hom | D10 | Hom | D38 | Hom | a′a′bbcc |
| EDD2#30-7 | D36 | Hom | D9 | Hom | D38, D9 | Biallele | a′a′b′b′cc′ |
| EDD2#30-8 | D36 | Hom | D9, D10 | Biallele | D38 | Hom | a′a′b′bcc |
| EDD2#30-10 | D36 | Hom | D10 | Hom | D38, D9 | Biallele | a′a′bbcc′ |
| EDD2#42-1, -2 | D4 | Hom | I3, D5 | Biallele | D38, D10 | Biallele | aab′bcc |
| EDD2#42-3, -5 | D2, D4 | Biallele | I3, D5 | Biallele | D38, D10 | Biallele | aab′bcc |
| EDD2#42-4 | D2 | Hom | I3, D5 | Biallele | D38 | Hom | aab′bcc |
| EDD2#42-6 | D2, D4 | Biallele | I3 | Hom | D38, D10 | Biallele | aab′b′cc |
| EDD2#42-7 | D4 | Hom | I3 | Hom | D38, D10 | Biallele | aab′b′cc |
| EDD2#42-8 | D2, D4 | Biallele | D5 | Hom | D38, D10 | Biallele | aabbcc |
| EDD2#42-9 | D2 | Hom | D5+I1 | Biallele | D38 | Hom | aabbcc |
| EDD2#42-10 | D4 | Hom | D5 | Hom | D38, D10 | Biallele | aabbcc |
| EDD2#44-1, -2, -3 | D2, D9 | Biallele | D1, I1 | Biallele | D3 | Hom | aa′bbc′c′ |
| EDD2#44-4, -8 | D2, D9 | Biallele | D1 | Hom | D3 | Hom | aa′bbc′c′ |
| EDD2#44-5, -7 | D2 | Hom | D1, I1 | Biallele | D10, D3 | Biallele | aabbcc′ |
| EDD2#44-6 | D9 | Hom | D1, I1 | Biallele | D10, D3 | Biallele | a′a′bbcc′ |
| EDD2#44-9 | D2 | Hom | D1, I1 | Biallele | D3 | Hom | aabbc′c′ |
| EDD2#44-10 | D2, D9 | Biallele | D1, I1 | Biallele | D10, D3 | Biallele | aa′bbcc′ |
| EDD2#59-2, -3, -4, -6 | D4 | Hom | D37, D10 | Biallele | D10, D2 | Biallele | aabbcc |
| EDD2#59-5 | D4 | Hom | D10 | Hom | D10, D2 | Biallele | aabbcc |
| EDD2#59-7, -10 | D4 | Hom | D10 | Hom | D2 | Hom | aabbcc |
| EDD2#59-8, -9 | D4 | Hom | D37, D10 | Biallele | D2 | Hom | aabbcc |
| EDD2#66-1, -7, -9 | D2, D4 | Biallele | D2 | Hom | D2 | Hom | aabbcc |
| EDD2#66-4, -6, -8 | D4 | Hom | D2 | Hom | D2 | Hom | aabbcc |
| EDD2#66-5, -10 | D2 | Hom | D2 | Hom | D2 | Hom | aabbcc |
| EDD2#137-2, -3, -4, -8, -9 | D36 | Hom | D2 | Hom | D4 + I1, D41 | Biallele | a′a′bbc′c |
| EDD2#137-5 | D36 | Hom | D2 | Hom | D4 + I1 | Hom | a′a′bbc′c′ |
| EDD2#137-6, -7 | D36 | Hom | D2 | Hom | D41 | Hom | a′a′bbcc |
| EDD2#147-2 | D9 | Hom | D37, D10 | Biallele | D39, D2 | Biallele | a′a′bbc′c |
| EDD2#147-3, -7, -9 | D9 | Hom | D37, D10 | Biallele | D39 | Hom | a′a′bbc′c′ |
| EDD2#147-4, -8 | D9 | Hom | D37 | Hom | D39 | Hom | a′a′bbc′c′ |
| EDD2#147-5 | D9 | Hom | D37 | Hom | D2 | Hom | a′a′bbcc |
| EDD2#147-10 | D9 | Hom | D10 | Hom | D39, D2 | Biallele | a′a′bbc′c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-R.; Yeo, Y.; Lee, J.; Kim, S.; Im, C.; Kim, I.; Lee, J.; Lee, S.-K.; Suh, M.C.; Kim, H.U. Functional Characterization of the Effects of CsDGAT1 and CsDGAT2 on Fatty Acid Composition in Camelina sativa. Int. J. Mol. Sci. 2024, 25, 6944. https://doi.org/10.3390/ijms25136944
Lee K-R, Yeo Y, Lee J, Kim S, Im C, Kim I, Lee J, Lee S-K, Suh MC, Kim HU. Functional Characterization of the Effects of CsDGAT1 and CsDGAT2 on Fatty Acid Composition in Camelina sativa. International Journal of Molecular Sciences. 2024; 25(13):6944. https://doi.org/10.3390/ijms25136944
Chicago/Turabian StyleLee, Kyeong-Ryeol, Yumi Yeo, Jihyea Lee, Semi Kim, Chorong Im, Inyoung Kim, Juho Lee, Seon-Kyeong Lee, Mi Chung Suh, and Hyun Uk Kim. 2024. "Functional Characterization of the Effects of CsDGAT1 and CsDGAT2 on Fatty Acid Composition in Camelina sativa" International Journal of Molecular Sciences 25, no. 13: 6944. https://doi.org/10.3390/ijms25136944
APA StyleLee, K.-R., Yeo, Y., Lee, J., Kim, S., Im, C., Kim, I., Lee, J., Lee, S.-K., Suh, M. C., & Kim, H. U. (2024). Functional Characterization of the Effects of CsDGAT1 and CsDGAT2 on Fatty Acid Composition in Camelina sativa. International Journal of Molecular Sciences, 25(13), 6944. https://doi.org/10.3390/ijms25136944







