An Amino Acid Mixture to Counteract Skeletal Muscle Atrophy: Impact on Mitochondrial Bioenergetics
Abstract
:1. Introduction
2. Results
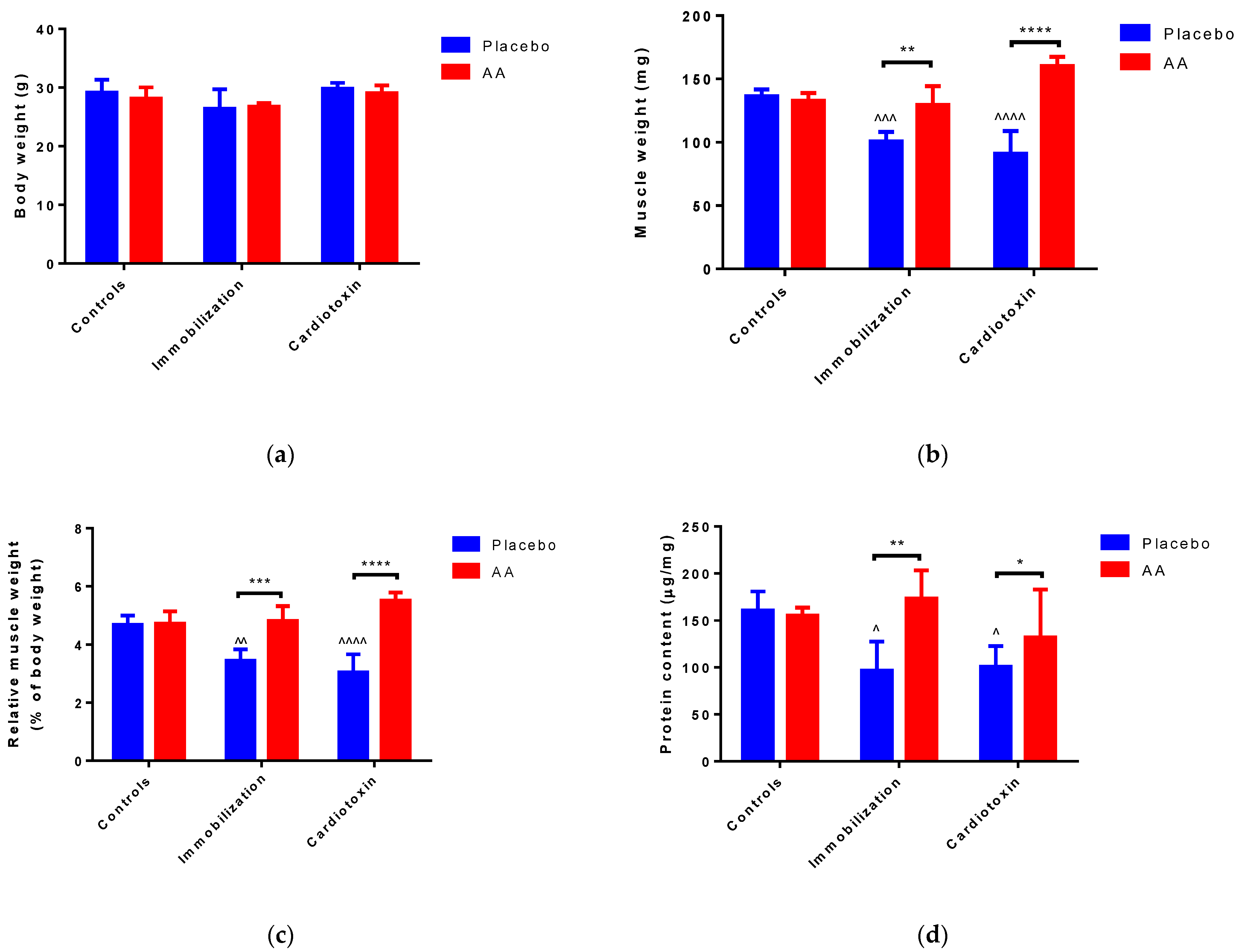
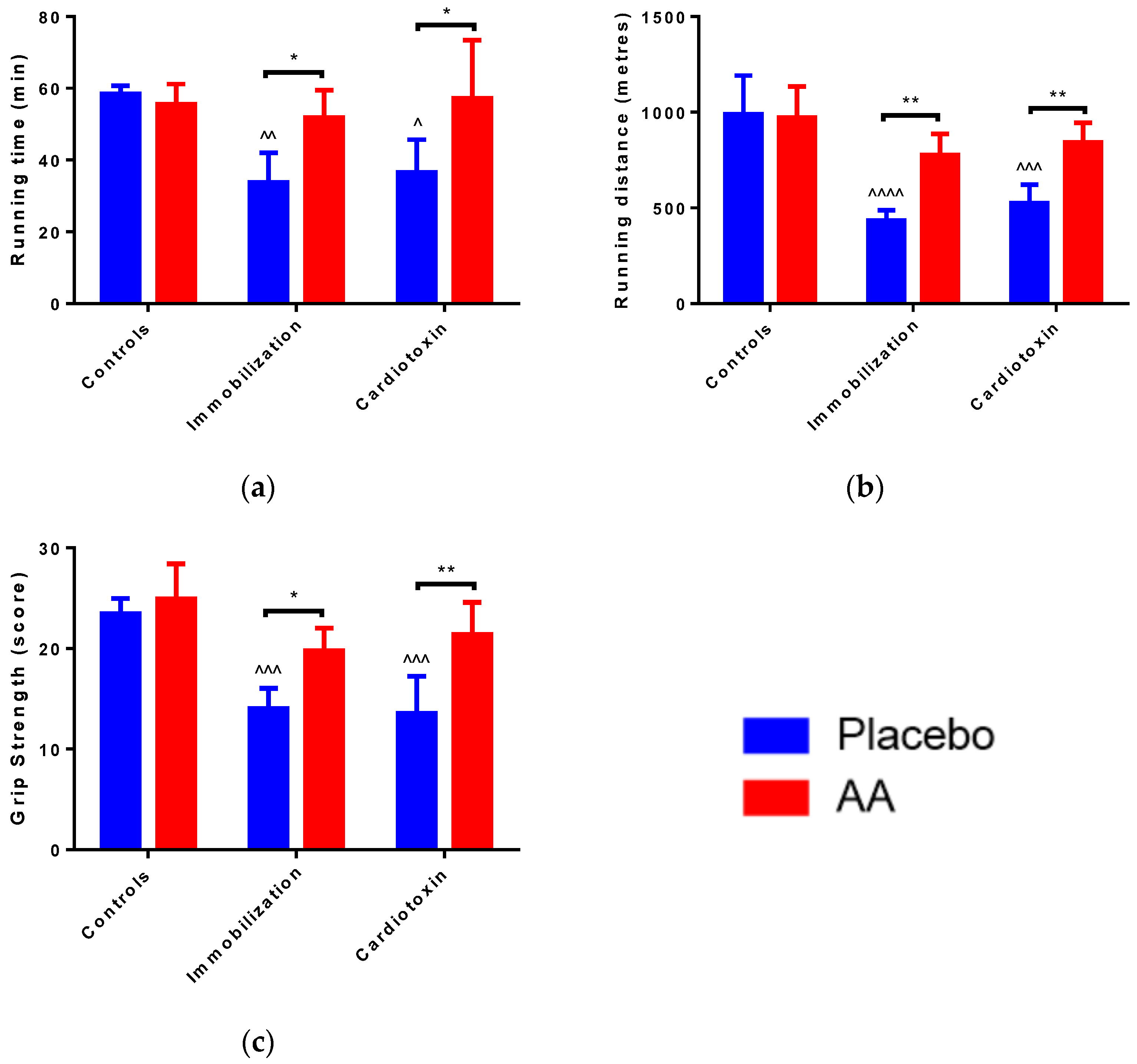
2.1. Effect of AA Supplementation on Skeletal Muscle Mass and Performance
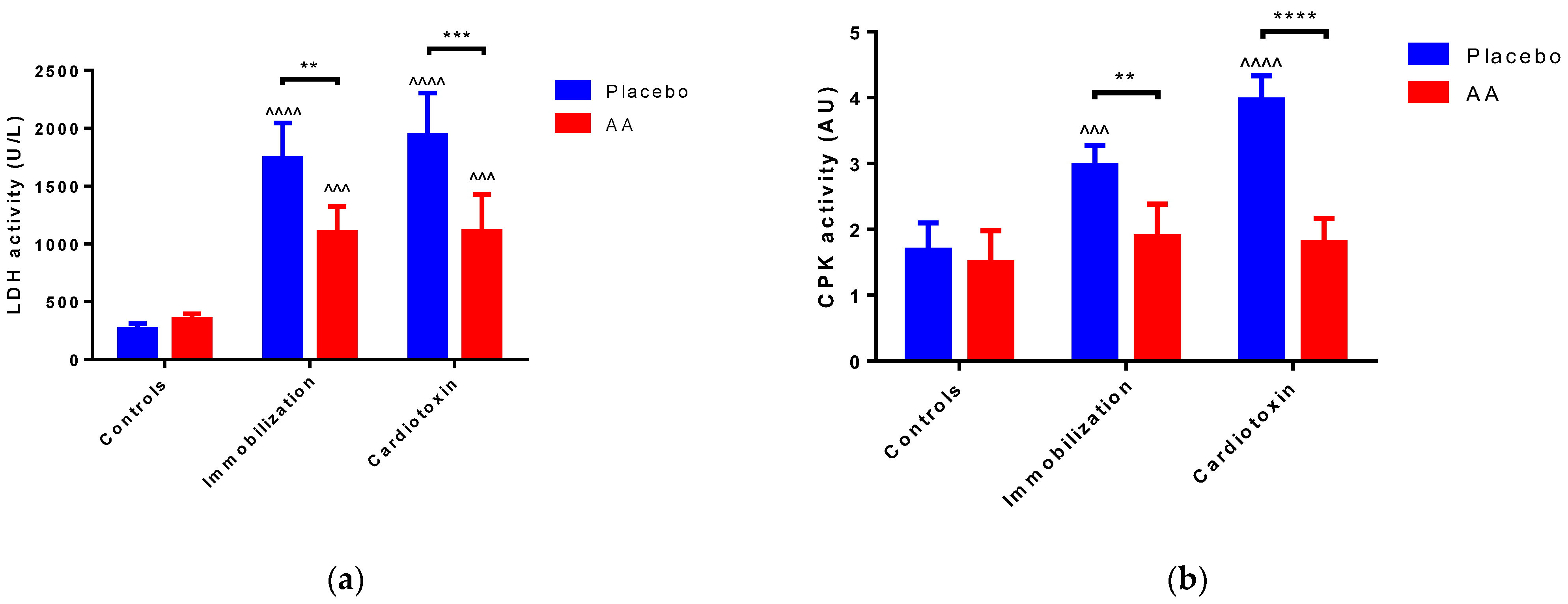
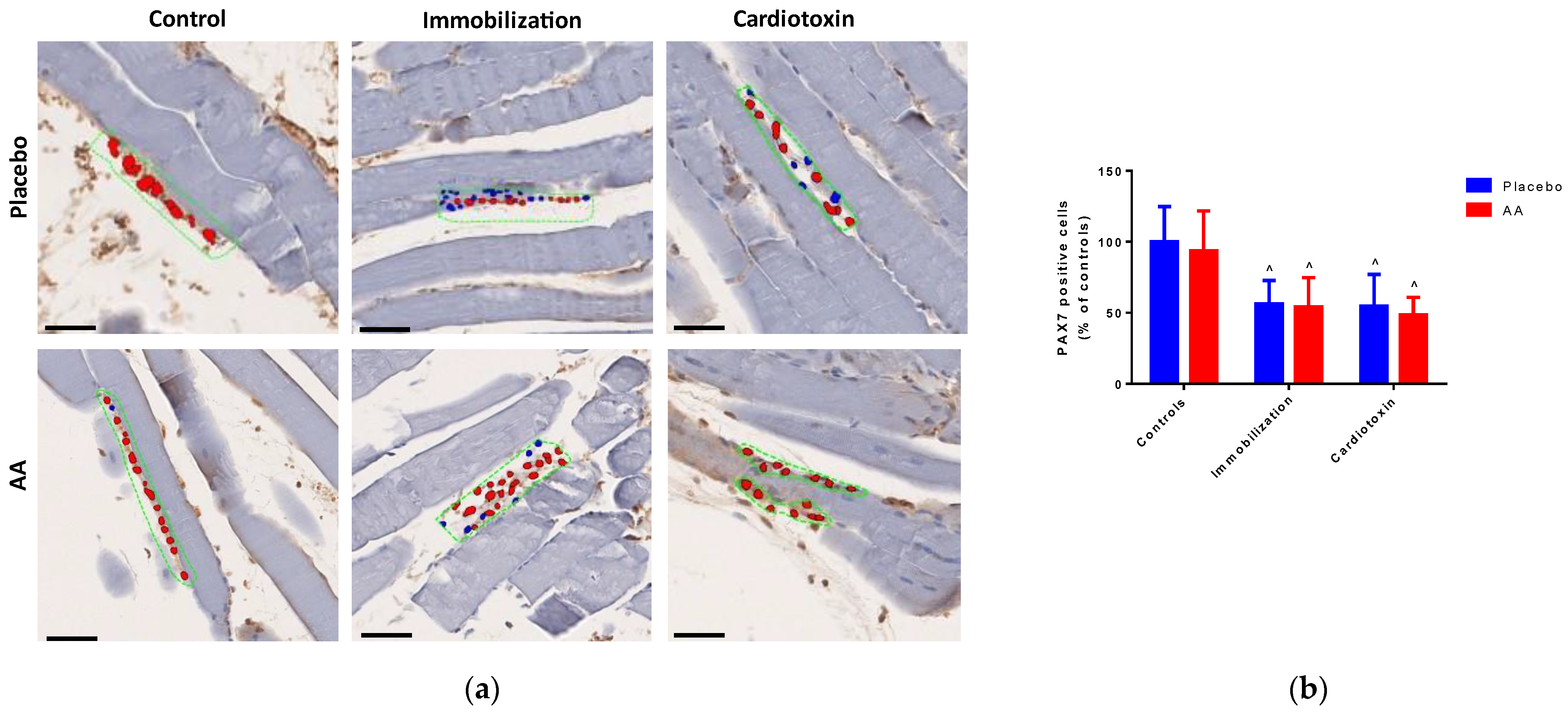
2.2. Effect of AA Supplementation on Skeletal Muscle Injury, Architecture and Regeneration
2.3. Effect of AA Supplementation on Skeletal Muscle Mitochondria Content and Bioenergetics
3. Discussion
4. Materials and Methods
4.1. Study Protocol
- Control + placebo.
- Control + AA.
- Immobilization + placebo.
- Immobilization + AA.
- Cardiotoxin + placebo.
- Cardiotoxin + AA.

4.2. Immobilization Procedure
4.3. Cardiotoxin Injection
4.4. Running Test
4.5. Muscle Strength
4.6. Serum Creatin Phosphokinase (CPK) and Lactate Dehydrogenase (LDH) Measurement
4.7. Histology and Immunohistochemistry
4.8. Mitochondria Isolation and Bioenergetic Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes, J.; Russell, D.M.; Whitwell, J.; Jeejeebhoy, K.N. Skeletal muscle function in malnutrition. Am. J. Clin. Nutr. 1982, 36, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L.M.; Hughes, D.C.; Waddell, D.S.; Bodine, S.C. SnapShot: Skeletal muscle atrophy. Cell 2022, 185, 1618. [Google Scholar] [CrossRef] [PubMed]
- Wall, B.T.; Dirks, M.L.; van Loon, L.J. Skeletal muscle atrophy during short-term disuse: Implications for age-related sarcopenia. Ageing Res. Rev. 2013, 12, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Janssen, L.; Allard, N.A.E.; Saris, C.G.J.; Keijer, J.; Hopman, M.T.E.; Timmers, S. Muscle Toxicity of Drugs: When Drugs Turn Physiology into Pathophysiology. Physiol. Rev. 2020, 100, 633–672. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, A.A.; Tipton, K.D.; Bamman, M.M.; Wolfe, R.R. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J. Appl. Physiol. (1985) 1997, 82, 807–810. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Ye, H. Exercise and Muscle Atrophy. Adv. Exp. Med. Biol. 2020, 1228, 255–267. [Google Scholar] [PubMed]
- Ferrando, A.A.; Paddon-Jones, D.; Hays, N.P.; Kortebein, P.; Ronsen, O.; Williams, R.H.; McComb, A.; Symons, T.B.; Wolfe, R.R.; Evans, W. EAA supplementation to increase nitrogen intake improves muscle function during bed rest in the elderly. Clin. Nutr. 2010, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Dirks, M.L.; Wall, B.T.; Nilwik, R.; Weerts, D.H.; Verdijk, L.B.; van Loon, L.J. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J. Nutr. 2014, 144, 1196–1203. [Google Scholar] [CrossRef]
- English, K.L.; Mettler, J.A.; Ellison, J.B.; Mamerow, M.M.; Arentson-Lantz, E.; Pattarini, J.M.; Ploutz-Snyder, R.; Sheffield-Moore, M.; Paddon-Jones, D. Leucine partially protects muscle mass and function during bed rest in middle-aged adults. Am. J. Clin. Nutr. 2016, 103, 465–473. [Google Scholar] [CrossRef]
- Tipton, K.D.; Gurkin, B.E.; Matin, S.; Wolfe, R.R. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J. Nutr. Biochem. 1999, 10, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Droge, W. Oxidative stress and ageing: Is ageing a cysteine deficiency syndrome? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 2355–2372. [Google Scholar] [CrossRef] [PubMed]
- Nishizaki, K.; Ikegami, H.; Tanaka, Y.; Imai, R.; Matsumura, H. Effects of supplementation with a combination of beta-hydroxy-beta-methyl butyrate, L-arginine, and L-glutamine on postoperative recovery of quadriceps muscle strength after total knee arthroplasty. Asia Pac. J. Clin. Nutr. 2015, 24, 412–420. [Google Scholar] [PubMed]
- Luckose, F.; Pandey, M.C.; Radhakrishna, K. Effects of amino acid derivatives on physical, mental, and physiological activities. Crit. Rev. Food Sci. Nutr. 2015, 55, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Custureri, R.; Puntoni, M.; Bianchi, L.; Daragjati, J.; Garaboldi, S.; Simonato, M.; Greco, A.; Rizzo, E.; Santo, P.D.; et al. Effects of oral amino acid supplementation on Multidimensional Prognostic Index in hospitalized older patients: A multicenter randomized, double-blind, placebo-controlled pilot study. Clin. Interv. Aging 2018, 13, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Di Stasio, E.; Di Bello, G.; Tamborra, R.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Vendemiale, G. An open-label, single-center pilot study to test the effects of an amino acid mixture in older patients admitted to internal medicine wards. Nutrition 2020, 69, 110588. [Google Scholar] [CrossRef] [PubMed]
- Romanello, V.; Sandri, M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front. Physiol. 2015, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Vendemiale, G. Muscle Delivery of Mitochondria-Targeted Drugs for the Treatment of Sarcopenia: Rationale and Perspectives. Pharmaceutics 2022, 14, 2588. [Google Scholar] [CrossRef] [PubMed]
- Memme, J.M.; Slavin, M.; Moradi, N.; Hood, D.A. Mitochondrial Bioenergetics and Turnover during Chronic Muscle Disuse. Int. J. Mol. Sci. 2021, 22, 5179. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Musarò, A. Muscle Homeostasis and Regeneration: From Molecular Mechanisms to Therapeutic Opportunities. Cells 2020, 9, 2033. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, M.; Deng, C.; Qiu, J.; Wang, K.; Chang, M.; Zhou, S.; Gu, Y.; Shen, Y.; Wang, W.; et al. Potential Therapeutic Strategies for Skeletal Muscle Atrophy. Antioxidants 2022, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shen, D.; Zhang, L.; Ji, Y.; Xu, L.; Chen, Z.; Shen, Y.; Gong, L.; Zhang, Q.; Shen, M.; et al. SKP-SC-EVs Mitigate Denervated Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation and Improving Microcirculation. Antioxidants 2021, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D‘Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatabolic syndrome: Molecular basis and effects of nutritional supplements with amino acids. Am. J. Cardiol. 2008, 101, 11E–15E. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Volpi, E. Protein Requirements in Critically Ill Older Adults. Nutrients 2018, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- Renzini, A.; Riera, C.S.; Minic, I.; D‘Ercole, C.; Lozanoska-Ochser, B.; Cedola, A.; Gigli, G.; Moresi, V.; Madaro, L. Metabolic Remodeling in Skeletal Muscle Atrophy as a Therapeutic Target. Metabolites 2021, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Magne, H.; Savary-Auzeloux, I.; Remond, D.; Dardevet, D. Nutritional strategies to counteract muscle atrophy caused by disuse and to improve recovery. Nutr. Res. Rev. 2013, 26, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Wandrag, L.; Brett, S.J.; Frost, G.; Hickson, M. Impact of supplementation with amino acids or their metabolites on muscle wasting in patients with critical illness or other muscle wasting illness: A systematic review. J. Hum. Nutr. Diet. 2015, 28, 313–330. [Google Scholar] [CrossRef]
- Caron, A.Z.; Drouin, G.; Desrosiers, J.; Trensz, F.; Grenier, G. A novel hindlimb immobilization procedure for studying skeletal muscle atrophy and recovery in mouse. J. Appl. Physiol. (1985) 2009, 106, 2049–2059. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Liu, Y. Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models. Int. J. Mol. Sci. 2022, 23, 13380. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.; Besnard, A.; Latil, M.; Jouvion, G.; Briand, D.; Thepenier, C.; Pascal, Q.; Guguin, A.; Gayraud-Morel, B.; Cavaillon, J.M.; et al. Comparative Study of Injury Models for Studying Muscle Regeneration in Mice. PLoS ONE 2016, 11, e0147198. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Yun, H.Y.; Park, J.; Lim, K. Protective effect of branched chain amino acids on hindlimb suspension-induced muscle atrophy in growing rats. J. Exerc. Nutr. Biochem. 2015, 19, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, P.; Boccanegra, B.; Bianchini, G.; Conte, E.; De Bellis, M.; Sanarica, F.; Camerino, G.M.; Pierno, S.; Cappellari, O.; Allegretti, M.; et al. BCAAs and Di-Alanine supplementation in the prevention of skeletal muscle atrophy: Preclinical evaluation in a murine model of hind limb unloading. Pharmacol. Res. 2021, 171, 105798. [Google Scholar] [CrossRef] [PubMed]
- Maki, T.; Yamamoto, D.; Nakanishi, S.; Iida, K.; Iguchi, G.; Takahashi, Y.; Kaji, H.; Chihara, K.; Okimura, Y. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutr. Res. 2012, 32, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. Br. J. Pharmacol. 2017, 174, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo, R.M.; Noel, K.K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Petry, E.R.; Dresch, D.F.; Carvalho, C.; Medeiros, P.C.; Rosa, T.G.; de Oliveira, C.M.; Martins, L.A.M.; Guma, F.C.R.; Marroni, N.P.; Wannmacher, C.M.D. Oral glutamine supplementation relieves muscle loss in immobilized rats, altering p38MAPK and FOXO3a signaling pathways. Nutrition 2024, 118, 112273. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Sheffield-Moore, M.; Urban, R.J.; Sanford, A.P.; Aarsland, A.; Wolfe, R.R.; Ferrando, A.A. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J. Clin. Endocrinol. Metab. 2004, 89, 4351–4358. [Google Scholar] [CrossRef]
- Coqueiro, A.Y.; Rogero, M.M.; Tirapegui, J. Glutamine as an Anti-Fatigue Amino Acid in Sports Nutrition. Nutrients 2019, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.J.; Pereira, R.; Machado, M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Interact. 2014, 14, 68–77. [Google Scholar] [PubMed]
- Zhang, Y.; Huang, J.J.; Wang, Z.Q.; Wang, N.; Wu, Z.Y. Value of muscle enzyme measurement in evaluating different neuromuscular diseases. Clin. Chim. Acta 2012, 413, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.F.; Rogero, M.M.; Tirapegui, J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem. Funct. 2010, 28, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Trabelsi, K.; Jahrami, H.; AlRasheed, M.M.; Boukhris, O.; Puce, L.; Bragazzi, N.L.; Ammar, A.; Glenn, J.M.; Chtourou, H. Branched-Chain Amino Acids Supplementation and Post-Exercise Recovery: An Overview of Systematic Reviews. J. Am. Nutr. Assoc. 2024, 43, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Michele, D.E. Mechanisms of skeletal muscle repair and regeneration in health and disease. FEBS J. 2022, 289, 6460–6462. [Google Scholar] [CrossRef] [PubMed]
- Kubat, G.B.; Bouhamida, E.; Ulger, O.; Turkel, I.; Pedriali, G.; Ramaccini, D.; Ekinci, O.; Ozerklig, B.; Atalay, O.; Patergnani, S.; et al. Mitochondrial dysfunction and skeletal muscle atrophy: Causes, mechanisms, and treatment strategies. Mitochondrion 2023, 72, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Yeo, D.; Ji, L.L. Muscle immobilization activates mitophagy and disrupts mitochondrial dynamics in mice. Acta Physiol. 2016, 218, 188–197. [Google Scholar] [CrossRef]
- Kunzke, T.; Buck, A.; Prade, V.M.; Feuchtinger, A.; Prokopchuk, O.; Martignoni, M.E.; Heisz, S.; Hauner, H.; Janssen, K.P.; Walch, A.; et al. Derangements of amino acids in cachectic skeletal muscle are caused by mitochondrial dysfunction. J. Cachexia Sarcopenia Muscle 2020, 11, 226–240. [Google Scholar] [CrossRef]
- Sirago, G.; Pellegrino, M.A.; Bottinelli, R.; Franchi, M.V.; Narici, M.V. Loss of neuromuscular junction integrity and muscle atrophy in skeletal muscle disuse. Ageing Res. Rev. 2023, 83, 101810. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.; D‘Antona, G.; Nisoli, E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: An evolutionary perspective. Aging 2011, 3, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Romani, M.; Berger, M.M.; D‘Amelio, P. From the Bench to the Bedside: Branched Amino Acid and Micronutrient Strategies to Improve Mitochondrial Dysfunction Leading to Sarcopenia. Nutrients 2022, 14, 483. [Google Scholar] [CrossRef] [PubMed]
- Hirofuji, C.; Ishihara, A.; Roy, R.R.; Itoh, K.; Itoh, M.; Edgerton, V.R.; Katsuta, S. SDH activity and cell size of tibialis anterior motoneurons and muscle fibers in SAMP6. Neuroreport 2000, 11, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Benard, G.; Bellance, N.; Jose, C.; Melser, S.; Nouette-Gaulain, K.; Rossignol, R. Multi-site control and regulation of mitochondrial energy production. Biochim. Biophys. Acta 2010, 1797, 698–709. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Bogin, E.; Chechick, A.; Rzetelny, V. The effect of single hind-limb immobilization on the contralateral limb in the rat: A morphometric and biochemical study. Am. J. Orthop. 1999, 28, 706–708. [Google Scholar] [PubMed]
- Lau, J.; Goh, C.C.; Devi, S.; Keeble, J.; See, P.; Ginhoux, F.; Ng, L.G. Intravital multiphoton imaging of mouse tibialis anterior muscle. Intravital 2016, 5, e1156272. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.V.; Mohanty, A. Immunohistochemical Identification of Muscle Fiber Types in Mice Tibialis Anterior Sections. Bio Protoc. 2019, 9, e3400. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, O.; Andolfi, G.; Tirone, M.; Iavarone, F.; Brunelli, S.; Minchiotti, G. Induction of Acute Skeletal Muscle Regeneration by Cardiotoxin Injection. J. Vis. Exp. 2017, 119, 54515. [Google Scholar] [CrossRef]
- Bouganim, S.; Bergdahl, A. Constructing an inexpensive and versatile homemade rodent treadmill. Lab. Anim. 2017, 46, 67–69. [Google Scholar] [CrossRef]
- Deacon, R.M. Measuring the strength of mice. J. Vis. Exp. 2013, 76, 2610. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Romano, A.D.; Giudetti, A.M.; Rollo, T.; Blonda, M.; Tamborra, R.; Vendemiale, G.; Serviddio, G. Many faces of mitochondrial uncoupling during age: Damage or defense? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cazarin, M.L.; Snider, N.N.; Andrade, F.H. Mitochondrial isolation from skeletal muscle. J. Vis. Exp. 2011, 49, 2452. [Google Scholar] [CrossRef] [PubMed]

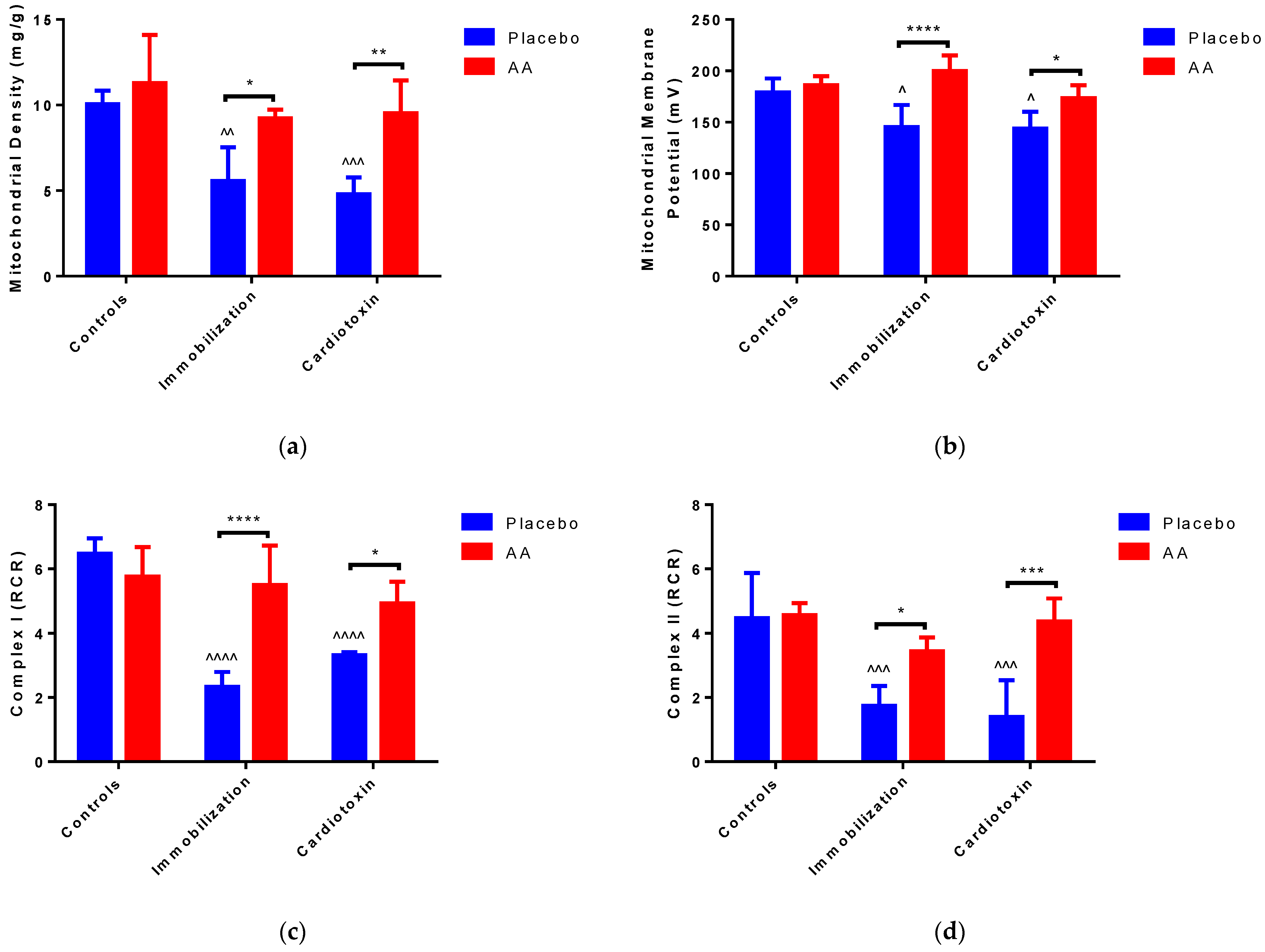
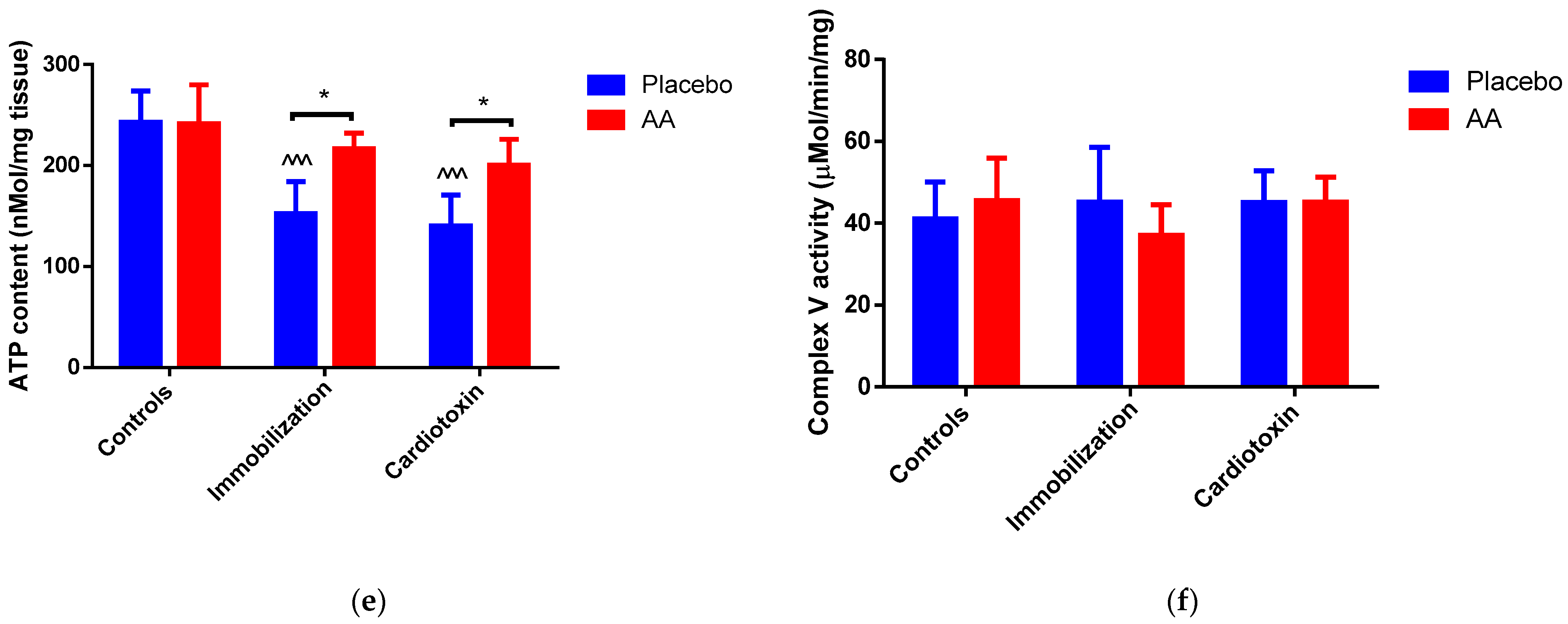
| Glutamate + Malate | State 3 Respiration (nmol O2/min/mg) | State 4 Respiration (nmol O2/min/mg) |
|---|---|---|
| Controls + Placebo | 10.09 ± 2.04 | 1.56 ± 0.21 |
| Controls + AA | 7.94 ± 2.18 | 1.38 ± 0.17 |
| Immobilization + Placebo | 3.49 ± 0.83 ^^^^ | 1.49 ± 0.19 |
| Immobilization + AA | 7.09 ± 1.44 * | 1.29 ± 0.12 |
| Cardiotoxin + Placebo | 4.35 ± 1.03 ^^^ | 1.31 ± 0.24 |
| Cardiotoxin + AA | 7.54 ± 1.44 * | 1.53 ± 0.21 |
| Succinate | State 3 Respiration (nmol O2/min/mg) | State 4 Respiration (nmol O2/min/mg) |
|---|---|---|
| Controls + Placebo | 19.27 ± 4.88 | 4.31 ± 1.15 |
| Controls + AA | 18.15 ± 4.25 | 3.98 ± 1.32 |
| Immobilization + Placebo | 7.20 ± 2.79 ^^^ | 4.14 ± 0.99 |
| Immobilization + AA | 15.51 ± 4.07 * | 4.51 ± 1.91 |
| Cardiotoxin + Placebo | 5.89 ± 2.15 ^^^ | 4.24 ± 0.94 |
| Cardiotoxin + AA | 17.87 ± 4.14 *** | 4.09 ± 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellanti, F.; Lo Buglio, A.; Pannone, G.; Pedicillo, M.C.; De Stefano, I.S.; Pignataro, A.; Capurso, C.; Vendemiale, G. An Amino Acid Mixture to Counteract Skeletal Muscle Atrophy: Impact on Mitochondrial Bioenergetics. Int. J. Mol. Sci. 2024, 25, 6056. https://doi.org/10.3390/ijms25116056
Bellanti F, Lo Buglio A, Pannone G, Pedicillo MC, De Stefano IS, Pignataro A, Capurso C, Vendemiale G. An Amino Acid Mixture to Counteract Skeletal Muscle Atrophy: Impact on Mitochondrial Bioenergetics. International Journal of Molecular Sciences. 2024; 25(11):6056. https://doi.org/10.3390/ijms25116056
Chicago/Turabian StyleBellanti, Francesco, Aurelio Lo Buglio, Giuseppe Pannone, Maria Carmela Pedicillo, Ilenia Sara De Stefano, Angela Pignataro, Cristiano Capurso, and Gianluigi Vendemiale. 2024. "An Amino Acid Mixture to Counteract Skeletal Muscle Atrophy: Impact on Mitochondrial Bioenergetics" International Journal of Molecular Sciences 25, no. 11: 6056. https://doi.org/10.3390/ijms25116056







