Nerve Growth Factor Signaling Modulates the Expression of Glutaminase in Dorsal Root Ganglion Neurons during Peripheral Inflammation
Abstract
:1. Introduction
2. Results
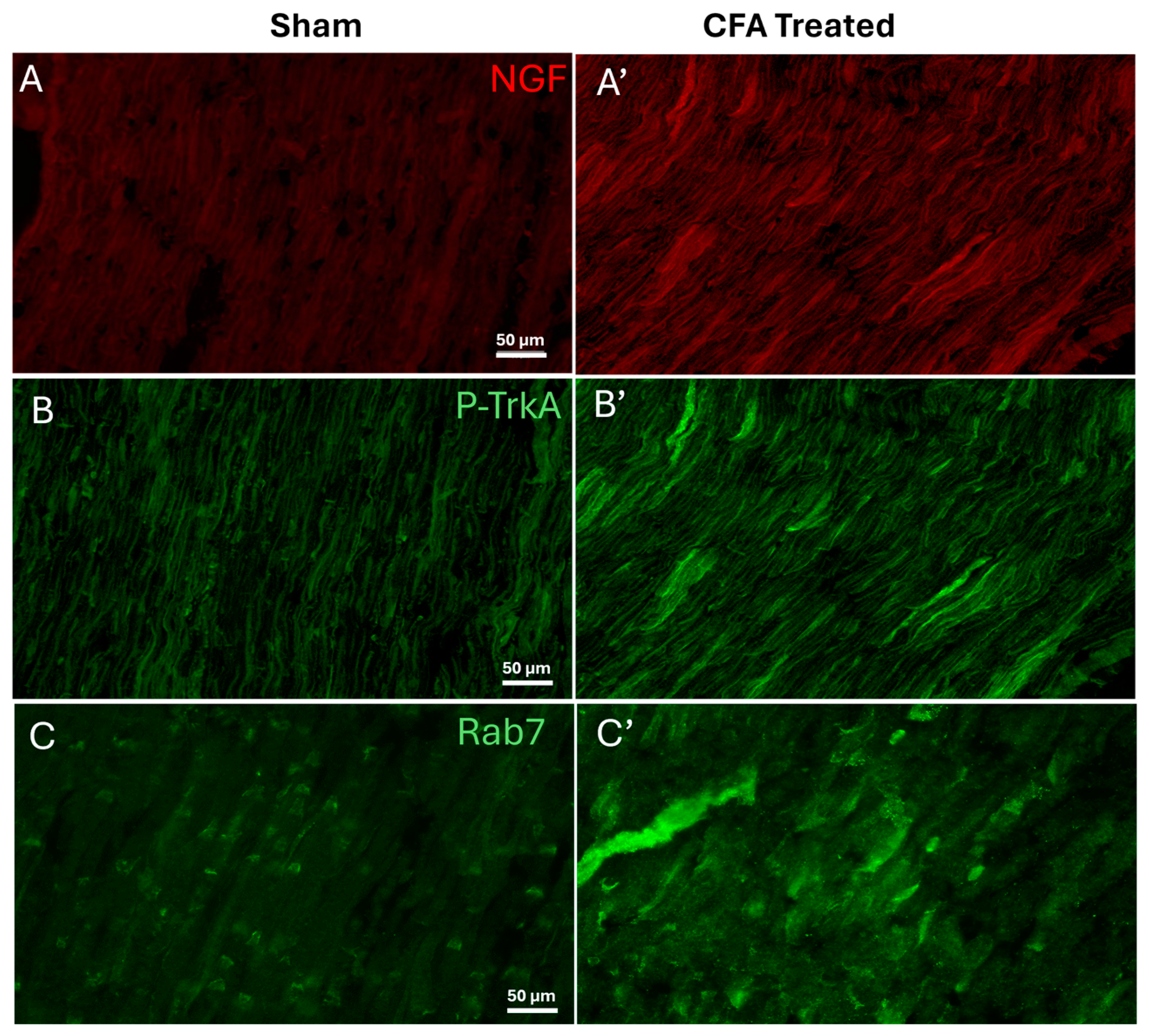
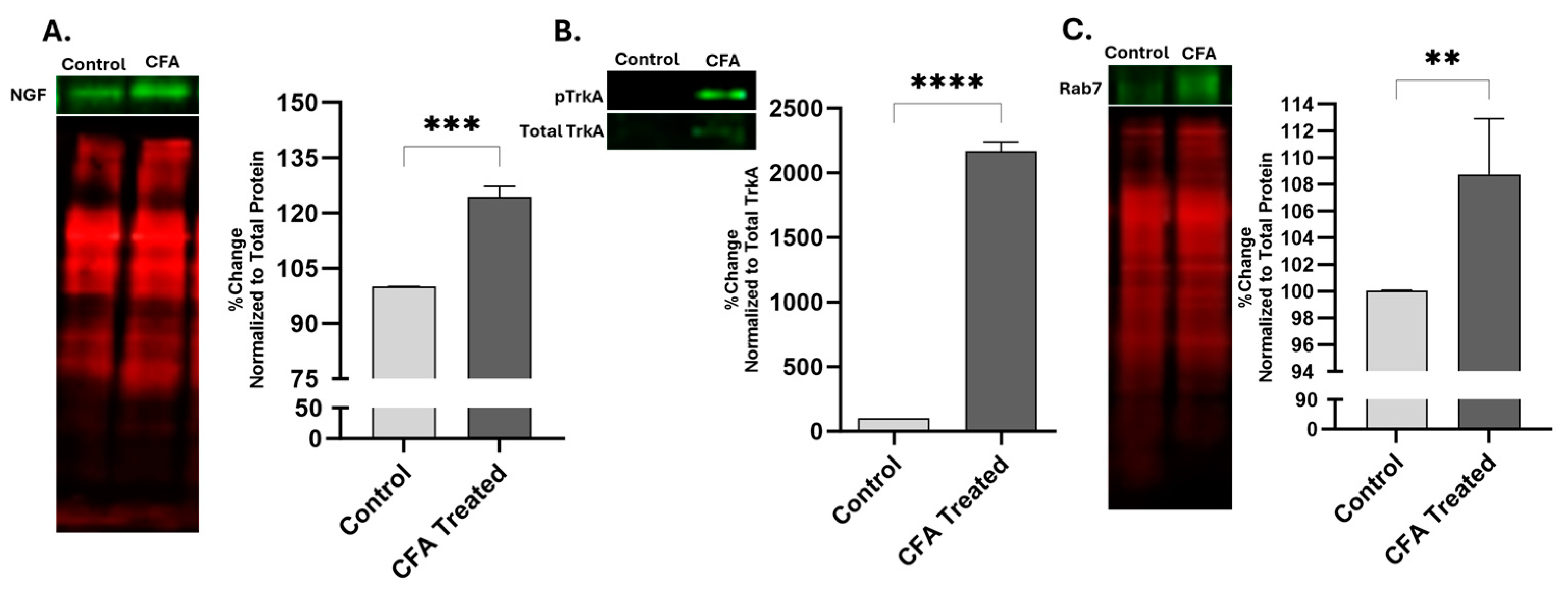
2.1. Effect of AIA on Levels of NGF, pTrkA, and Rab7 in Sciatic Nerve
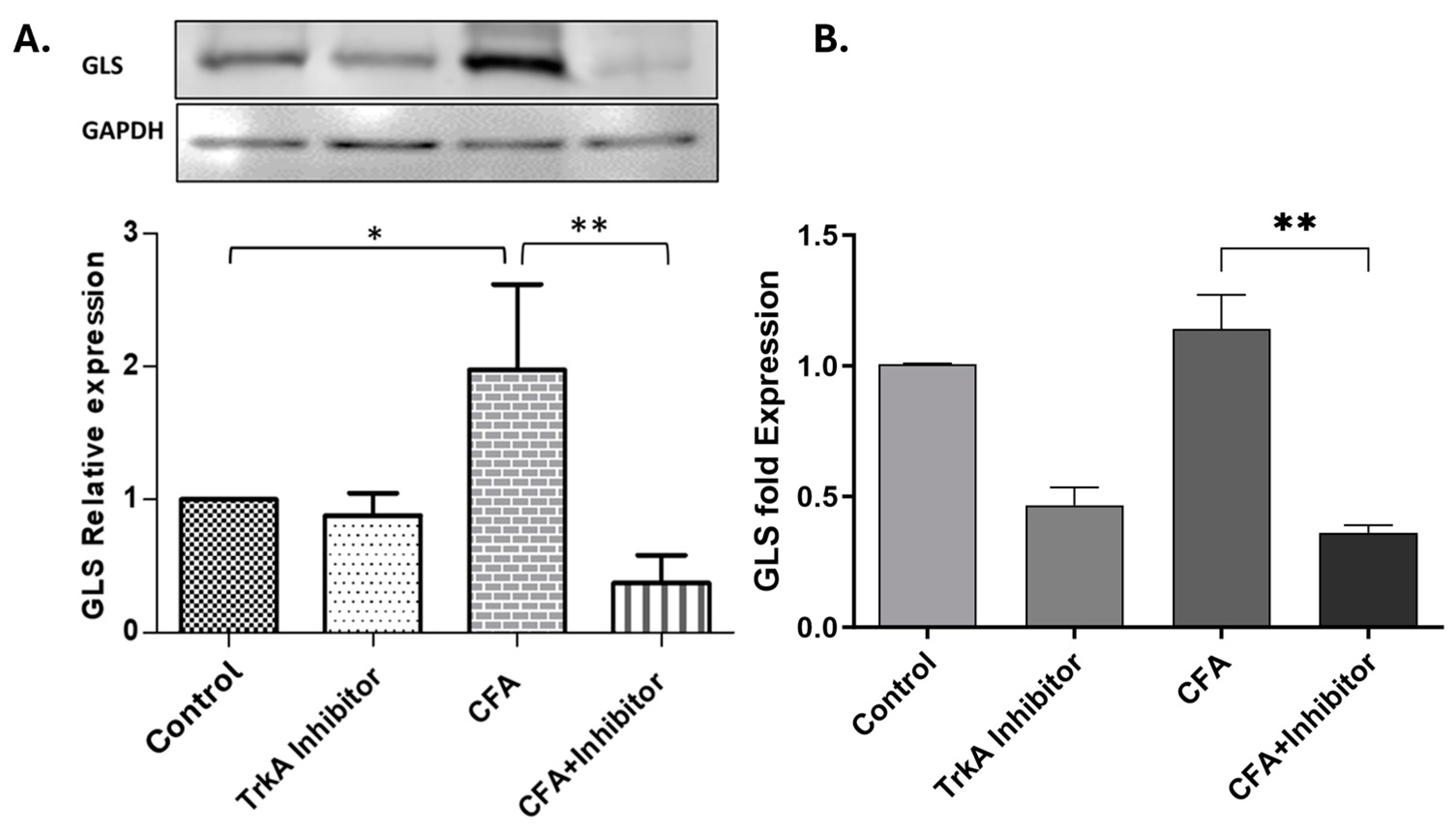
2.2. Effect of TrkA Inhibition on GLS Expression during AIA
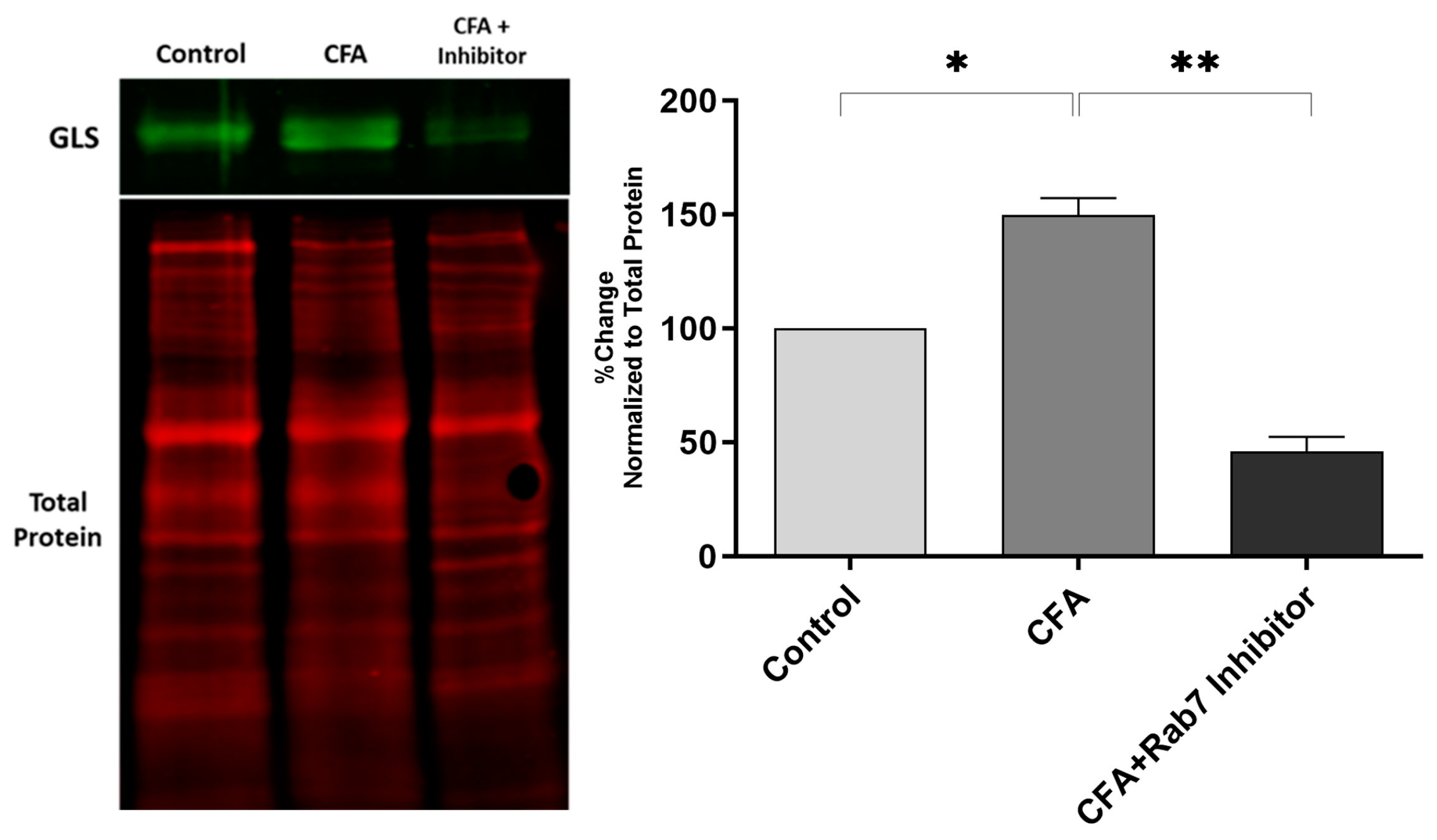
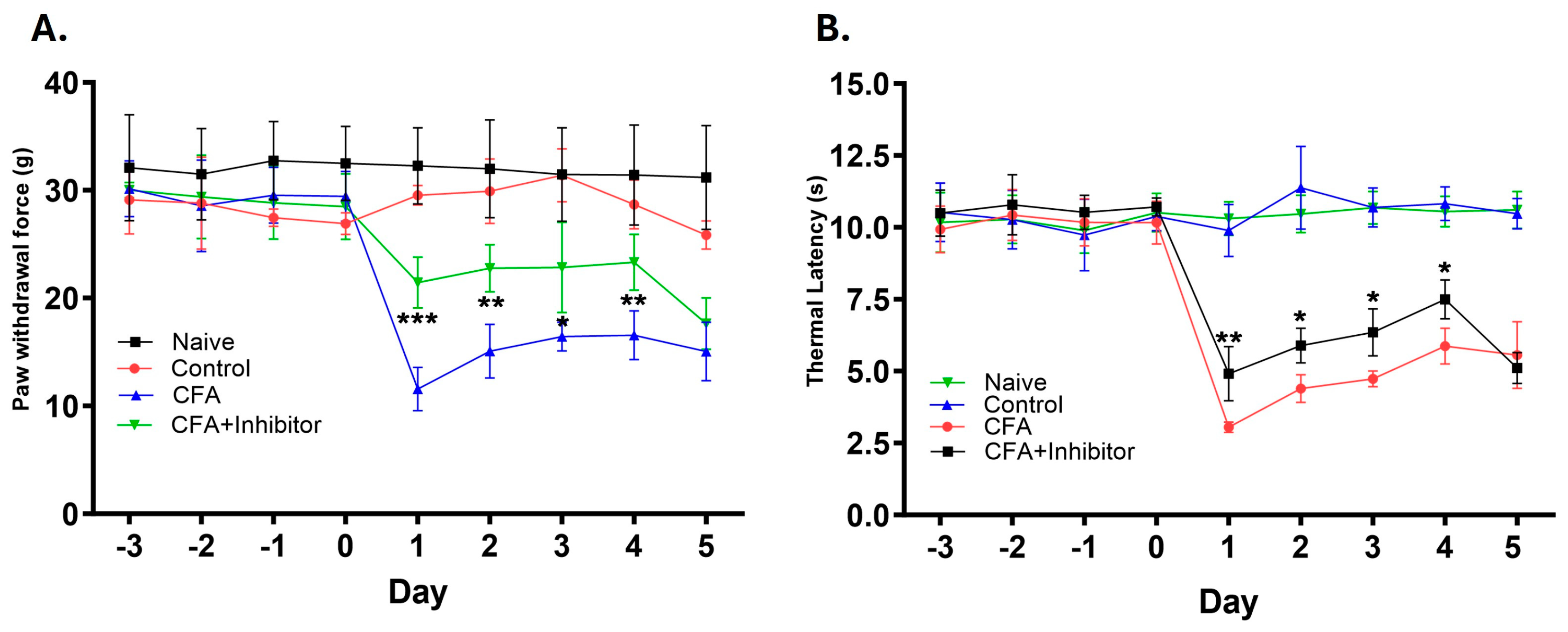
2.3. Effect of Rab7 Inhibition on GLS Expression and Pain Behavior during AIA
3. Discussion
3.1. Levels of NGF, pTrkA, and Rab7 Increases during AIA in Sciatic Nerve
3.2. Selective TrkA Inhibition Attenuates GLS Expression during Peripheral Inflammation
3.3. Peripheral Inhibition of Rab7GTPase Decreases the GLS Expression and Provides Analgesia
4. Materials and Methods
4.1. Animals
4.2. Induction of Adjuvant-Induced Arthritis (AIA) and Sciatic Nerve Ligation
4.3. Adjuvant-Induced Arthritis (AIA) and Pharmacological Interventions
4.4. Immunofluorescence
4.5. Western Blot Analysis
4.6. Quantitative Reverse Transcription PCR
4.7. Pain Behavior
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levi-Montalcini, R.; Hamburger, V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zoöl. 1951, 116, 321–361. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Phenotypic modification of primary sensory neurons: The role of nerve growth factor in the production of persistent pain. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.; Safieh-Garabedian, B.; Ma, Q.-P.; Crilly, P.; Winter, J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience 1994, 62, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Gujar, V.; Pande, R.D.; Das, S. Nerve Growth Factor Shows Biphasic Expression during Adjuvant-Induced Neurogenic Inflammation. Int. J. Mol. Sci. 2024, 25, 4029. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Kuroda, Y.; Murata, E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Pract. 2015, 16, 175–182. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.B.; Bennett, D.L.; Priestley, J.V.; Shelton, D.L. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat. Med. 1995, 1, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Shelton, D.L.; McMahon, S.; Winslow, J.W.; Qao, W.-Q. Neurotrophins and neurotrophin antagonists as potential therapeutics. Restor. Neurol. Neurosci. 1995, 8, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Mantyh, W.G.; Bloom, A.P.; Kuskowski, M.A.; Mantyh, P.W. Administration of a tropomyosin receptor kinase inhibitor attenuates sarcoma-induced nerve sprouting, neuroma formation and bone cancer pain. Mol. Pain 2010, 6, 87. [Google Scholar] [CrossRef]
- Lane, N.E.; Schnitzer, T.J.; Birbara, C.A.; Mokhtarani, M.; Shelton, D.L.; Smith, M.D.; Brown, M.T. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 2010, 363, 1521–1531. [Google Scholar] [CrossRef]
- Ueda, K.; Hirose, M.; Murata, E.; Takatori, M.; Ueda, M.; Ikeda, H.; Shigemi, K. Local administration of a synthetic cell-penetrating peptide antagonizing TrKa function suppresses inflammatory pain in rats. J. Pharmacol. Sci. 2010, 112, 438–443. [Google Scholar] [CrossRef]
- Urschel, B.A.; Brown, P.N.; Hulsebosch, C.E. Differential effects on sensory nerve processes and behavioral alterations in the rat after treatment with antibodies to nerve growth factor. Exp. Neurol. 1991, 114, 44–52. [Google Scholar] [CrossRef]
- Saxena, S.; Bucci, C.; Weis, J.; Kruttgen, A. The small GTPase Rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor TrkA. J. Neurosci. 2005, 25, 10930–10940. [Google Scholar] [CrossRef]
- Delcroix, J.D.; Valletta, J.S.; Wu, C.; Hunt, S.J.; Kowal, A.S.; Mobley, W.C. NGF signaling in sensory neurons: Evidence that early endosomes carry NGF retrograde signals. Neuron 2003, 39, 69–84. [Google Scholar] [CrossRef]
- Tarditi, A.; Klipfel, M.; Rodriguez, A.; Suvire, F.; Chasse, G.; Farkas, O.; Perczel, A.; Enriz, R. An ab initio exploratory study of side chain conformations for selected backbone conformations of N-acetyl-L-glutamine-N-methylamide. J. Mol. Struct. THEOCHEM 2001, 545, 29–47. [Google Scholar] [CrossRef]
- Hoffman, E.M.; Miller, K.E. Peripheral inhibition of glutaminase reduces carrageenan-induced Fos expression in the superficial dorsal horn of the rat. Neurosci. Lett. 2010, 472, 157–160. [Google Scholar] [CrossRef]
- McNearney, T.; Speegle, D.; Lawand, N.; Lisse, J.; Westlund, K.N. Excitatory amino acid profiles of synovial fluid from patients with arthritis. J. Rheumatol. 2000, 27, 739–745. [Google Scholar]
- Nordlind, K.; Johansson, O.; Lidén, S.; Hökfelt, T. Glutamate- and aspartate-like immunoreactivities in human normal and inflamed skin. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1993, 64, 75–82. [Google Scholar] [CrossRef]
- Wallace, D.R.; Dawson, R. Regional differences in glutaminase activation by phosphate and calcium in rat brain: Impairment in aged rats and implications for regional glutaminase isozymes. Neurochem. Res. 1993, 18, 1271–1279. [Google Scholar] [CrossRef]
- Hoffman, E.M. The Role of Dorsal Root Ganglion Glutaminase in Nociception—A Novel Therapeutic Target for Inflammatory Pain. Ph.D. Thesis, Oklahoma State University, Ann Arbor, MI, USA, 2009. Volume 3356494. [Google Scholar]
- Miller, K.E. Method of Alleviating Chronic Pain via Peripheral Glutaminase Regulation. U.S. Patent US7288246B2, 30 October 2007. [Google Scholar]
- Miller, K.E.; Balbás, J.C.; Benton, R.L.; Lam, T.S.; Edwards, K.M.; Kriebel, R.M.; Schechter, R. Glutaminase immunoreactivity and enzyme activity is increased in the rat dorsal root ganglion following peripheral inflammation. Pain Res. Treat. 2011, 2012, 414697. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.; Zhang, Z.; Anderson, M.; Schechter, R.; Miller, K. Potential mechanisms for hypoalgesia induced by anti-nerve growth factor immunoglobulin are identified using autoimmune nerve growth factor deprivation. Neuroscience 2011, 193, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Gujar, V.; Anderson, M.B.; Miller, K.E.; Pande, R.D.; Nawani, P.; Das, S. Separation of Rat Epidermis and Dermis with Thermolysin to Detect Site-Specific Inflammatory mRNA and Protein. J. Vis. Exp. 2021, 175, e59708. [Google Scholar]
- Miller, K.E.; Douglas, V.D.; Kaneko, T. Glutaminase immunoreactive neurons in the rat dorsal root ganglion contain calcitonin gene-related peptide (CGRP). Neurosci. Lett. 1993, 160, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Richards, B.A.; Kriebel, R.M. Glutamine-, glutamine synthetase-, glutamate dehydrogenase- and pyruvate carbox-ylase-immunoreactivities in the rat dorsal root ganglion and peripheral nerve. Brain Res. 2002, 945, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. The Role of Dorsal Root Ganglion Glutaminase in Acute and Chronic Inflammatory Pain. Ph.D. Thesis, Oklahoma State University, Ann Arbor, MI, USA, 2013. Available online: http://argo.library.okstate.edu/login?url=https://search.proquest.com/docview/1562918358?accountid=4117 (accessed on 7 July 2019).
- Mesentier-Louro, L.A.; De Nicolò, S.; Rosso, P.; De Vitis, L.A.; Castoldi, V.; Leocani, L.; Mendez-Otero, R.; Santiago, M.F.; Tirassa, P.; Rama, P.; et al. Time-Dependent Nerve Growth Factor Signaling Changes in the Rat Retina During Optic Nerve Crush-Induced Degeneration of Retinal Ganglion Cells. Int. J. Mol. Sci. 2017, 18, 98. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Allchorne, A.; Safieh-Garabedian, B.; Poole, S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997, 121, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Tuveri, M.A.; Carcassi, U.; Levi-Montalcini, R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992, 35, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Falcini, F.; Cerinic, M.M.; Lombardi, A.; Generini, S.; Pignone, A.; Tirassa, P.; Ermini, M.; Lepore, L.; Partsch, G.; Aloe, L. Increased circulating nerve growth factor is directly correlated with disease activity in juvenile chronic arthritis. Ann. Rheum. Dis. 1996, 55, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Aloe, L. Role of IL-1 beta and TNF-alpha in the regulation of NGF in experimentally induced arthritis in mice. Rheumatol. Int. 1998, 18, 97–102. [Google Scholar] [CrossRef]
- Steiner, P.; Pfeilschifter, J.; Boeckh, C.; Radeke, H.; Otten, U. Interleukin-1 beta and tumor necrosis factor-alpha synergistically stimulate nerve growth factor synthesis in rat mesangial cells. Am. J. Physiol. Physiol. 1991, 261, F792–F798. [Google Scholar] [CrossRef]
- Edwards, R.H.; Selby, M.J.; Garcia, P.D.; Rutter, W.J. Processing of the native nerve growth factor precursor to form biologically active nerve growth factor. J. Biol. Chem. 1988, 263, 6810–6815. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Martin-Zanca, D.; Parada, L.F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature 1991, 350, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Minnone, G.; De Benedetti, F.; Bracci-Laudiero, L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int. J. Mol. Sci. 2017, 18, 1028. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Vizzard, M.A. Cystitis-induced upregulation of tyrosine kinase (TrkA, TrkB) receptor expression and phosphorylation in rat micturition pathways. J. Comp. Neurol. 2002, 454, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Press, B.; Wandinger-Ness, A. Rab 7: An important regulator of late endocytic membrane traffic. J. Cell Biol. 1995, 131, 1435–1452. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Salinas, S.; Verastegui, C.; Watson, R.; Worth, D.; Hanrahan, S.; Bucci, C.; Schiavo, G. Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron 2006, 52, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ben Kenan, R.F.; Osakada, Y.; Xu, W.; Sinit, R.S.; Chen, L.; Zhao, X.; Chen, J.-Y.; Cui, B.; Wu, C. Defective axonal transport of Rab7 gtpase results in dysregulated trophic signaling. J. Neurosci. 2013, 33, 7451–7462. [Google Scholar] [CrossRef] [PubMed]
- Ascano, M.; Bodmer, D.; Kuruvilla, R. Endocytic trafficking of neurotrophins in neural development. Trends Cell Biol. 2012, 22, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.W.; Ginty, D.D. Long-distance retrograde neurotrophic factor signalling in neurons. Nat. Rev. Neurosci. 2013, 14, 177–187. [Google Scholar] [CrossRef]
- Wehrman, T.; He, X.; Raab, B.; Dukipatti, A.; Blau, H.; Garcia, K.C. Structural and mechanistic insights into nerve growth factor inter-actions with the TrkA and p75 receptors. Neuron 2007, 53, 25–38. [Google Scholar] [CrossRef]
- Bullock, C.M.; Wookey, P.; Bennett, A.; Mobasheri, A.; Dickerson, I.; Kelly, S. Peripheral calcitonin gene-related peptide receptor ac-tivation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol. 2014, 66, 2188–2200. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.M.; O’Connell, J.; O’Brien, D.I.; Goode, T.; Bredin, C.P.; Shanahan, F. The role of substance P in inflammatory disease. J. Cell. Physiol. 2004, 201, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Bouhana, K.S.; Pheneger, J.; Andrews, S.W.; Walsh, D.A. Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis. Arthritis Res. Ther. 2016, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, L.N.; Mapp, P.I.; Chapman, V.; Walsh, D.A. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain be-haviour in two rat models of osteoarthritis. Ann. Rheum. Dis. 2016, 75, 1246–1254. [Google Scholar] [CrossRef]
- Ginty, D.D.; A Segal, R. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr. Opin. Neurobiol. 2002, 12, 268–274. [Google Scholar] [CrossRef]
- Girard, E.; Chmiest, D.; Fournier, N.; Johannes, L.; Paul, J.; Vedie, B.; Lamaze, C. Rab7 is functionally required for selective cargo sorting at the early endosome. Traffic 2014, 15, 309–326. [Google Scholar] [CrossRef]
- Kallenborn-Gerhardt, W.; Möser, C.V.; Lorenz, J.E.; Steger, M.; Heidler, J.; Scheving, R.; Petersen, J.; Kennel, L.; Flauaus, C.; Lu, R.; et al. Rab7—A novel redox target that modulates inflammatory pain processing. Pain 2017, 158, 1354–1365. [Google Scholar] [CrossRef]
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Puehler, W.; Zollner, C.; Brack, A.; Shaqura, M.A.; Krause, H.; Schafer, M.; Stein, C. Rapid upregulation of mu opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience 2004, 129, 473–479. [Google Scholar] [CrossRef]
- Crosby, H.A.; Ihnat, M.; Miller, K.E. Evaluating the Toxicity of the Analgesic Glutaminase Inhibitor 6-Diazo-5-Oxo-L-Norleucine in vitro and on Rat Dermal Skin Fibroblasts. MOJ Toxicol. 2015, 1, 00005. [Google Scholar] [CrossRef]
- Hoffman, E.M.; Schechter, R.; Miller, K.E. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J. Histochem. Cytochem. 2009, 58, 329–344. [Google Scholar] [CrossRef] [PubMed]

| Tissue | Primary Antibodies | Dilutions | Secondary Antibodies | Dilutions |
|---|---|---|---|---|
| Sciatic Nerve | NGF Anti-mouse (E-12, Santa Cruz, TX, USA) | 1:1000 | Donkey anti-mouse Alexa Flour 555 (Invitrogen, Carlsbad, CA, USA) | 1:1000 |
| pTrkA Anti-rabbit (4168S, Cell Signaling, Danvers, MA, USA) | 1:2000 | Donkey anti-rabbit FITC 488 (Invitrogen, Carlsbad, CA, USA) | 1:1000 | |
| Rab7 Anti-rabbit (55469-1-AP, Proteintech, Rosemont, IL, USA) | 1:2000 | Donkey anti-rabbit FITC 488 (Invitrogen, Carlsbad, CA, USA) | 1:1000 | |
| L4 and L5 DRG | GLS Anti-rabbit (Norman Curthoys, Colorado State University, Fort Collins, CO, USA) | 1:1000 | Donkey anti-rabbit FITC 488 (Invitrogen, Carlsbad, CA, USA) | 1:1000 |
| Study/Tissue | Primary Antibodies | Dilutions | Secondary Antibodies | Dilutions |
|---|---|---|---|---|
| Sciatic Nerve Ligation/Sciatic Nerve | NGF Anti-Mouse (E-12, Santa Cruz, TX, USA) | 1:1000 | IRDye 800CW Donkey anti-mouse (Li-Cor, Lincoln, NE, USA) | 1:20,000 |
| pTrkA Anti-rabbit (4168S, Cell Signaling, Danvers, MA, USA) | 1:2000 | IRDye 800CW Donkey anti-rabbit (Li-Cor, Lincoln, NE, USA) | 1:20,000 | |
| Rab7 Anti-rabbit (55469-1-AP, Proteintech, Rosemont, IL, USA) | 1:2000 | IRDye 800CW Donkey anti-rabbit (Li-Cor, Lincoln, NE, USA) | 1:20,000 | |
| Total TrkA Anti-rabbit (2505S, Cell Signaling, Danvers, MA, USA) | 1:2000 | IRDye 800CW Donkey anti-rabbit (Li-Cor, Lincoln, NE, USA) | 1:20,000 | |
| TrkA Inhibition/L4, L5 DRG | GLS Anti-rabbit (Norman Curthoys, Colorado State University, Ft. Collins, CO, USA) | 1:1000 | AP labeled Anti-rabbit IgG (Promega, Madison, WI, USA) | 1:1000 |
| Rab7 Inhibition/L4, L5 DRG | GLS Anti-rabbit (Norman Curthoys, Colorado State University, Ft. Collins, CO, USA) | 1:1000 | IRDye 800CW Donkey anti-rabbit (Li-Cor, Lincoln, NE, USA) | 1:20,000 |
| Gene | Primer Sequence |
|---|---|
| GLS | GLS-F: 5′-GGGTCTGTTACCTAGCTTGGAAGATTTGC-3′ GLS-R: 5′-GAGTTAATCTTAACATATCCATACACT-3′ |
| GAPDH | GAPDH-F: 5′-GAACCACGAGAAATATGACAACTCCCTCAAG-3′ GAPDH-R: 5′-GCAGTGATGGCATGGACTGTGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gujar, V.; Pande, R.D.; Hardas, B.M.; Das, S. Nerve Growth Factor Signaling Modulates the Expression of Glutaminase in Dorsal Root Ganglion Neurons during Peripheral Inflammation. Int. J. Mol. Sci. 2024, 25, 6053. https://doi.org/10.3390/ijms25116053
Gujar V, Pande RD, Hardas BM, Das S. Nerve Growth Factor Signaling Modulates the Expression of Glutaminase in Dorsal Root Ganglion Neurons during Peripheral Inflammation. International Journal of Molecular Sciences. 2024; 25(11):6053. https://doi.org/10.3390/ijms25116053
Chicago/Turabian StyleGujar, Vikramsingh, Radhika D. Pande, Bhalchandra M. Hardas, and Subhas Das. 2024. "Nerve Growth Factor Signaling Modulates the Expression of Glutaminase in Dorsal Root Ganglion Neurons during Peripheral Inflammation" International Journal of Molecular Sciences 25, no. 11: 6053. https://doi.org/10.3390/ijms25116053







