Endophytic Fungi from the Four Staple Crops and Their Secondary Metabolites
Abstract
1. Introduction
2. Cultivation History and Pests and Diseases of the Four Staple Crops
2.1. Wheat
2.2. Rice
2.3. Maize
2.4. Potato
3. Endophytic Fungi
3.1. Wheat Endophytic Fungi
3.2. Rice Endophytic Fungi
3.3. Maize Endophytic Fungi
3.4. Potato Endophytic Fungi
| Fungal Endophytes | Organs | Fungal Endophytes | Organs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roots | Leaves | Stems | Fruits | Roots | Leaves | Stems | Fruits | ||
| Achroiostachys sp. [42] | √ | √ | Gibellulopsis sp. [42] | √ | |||||
| Acremonium sclerotigenum [42] | √ | √ | Gloeotinia sp. [42] | √ | |||||
| Acremonium sp. [42] | √ | Helicocephalum sp. [46] | √ | ||||||
| Akanthomyces sp. [42] | √ | Isaria farinose [42] | √ | ||||||
| Alternaria alternata [42,60] | √ | √ | √ | Leptobacillium leptobactrum [42] | √ | √ | |||
| Alternaria chalastospora [49] | √ | √ | Marasmius sp. [42] | √ | |||||
| Alternaria conjuncta [42] | √ | Meyerozyma sp. [42] | √ | √ | |||||
| Alternaria hordeicola [42] | √ | Microdochium bolleyi [42,61,62] | √ | √ | |||||
| Alternaria infectoria [42,46] | √ | √ | Microdochium nivale [61] | √ | √ | ||||
| Alternaria rosae [42] | √ | Microdochium sp. [42] | √ | √ | |||||
| Alternaria sp. [42] | √ | √ | √ | √ | Moesziomyces bullatus [42] | √ | |||
| Alternaria tenuissima [42,61] | √ | Moesziomyces sp. [42] | √ | ||||||
| Anthracocystis sp. [42] | √ | √ | Neonectria sp. [42] | √ | √ | ||||
| Arthrinium sp. [42] | √ | √ | Neosetophoma samarorum [62] | √ | |||||
| Aureobasidium pullulans [42] | √ | Nigrospora gorlenkoana [42] | √ | √ | √ | ||||
| Backusella sp. [42] | √ | √ | Nigrospora sp. [46] | √ | √ | ||||
| Bipolaris cynodontis [60] | √ | Penicilium amphipolaria [42] | √ | ||||||
| Bipolaris sorokiniana [42,60,62] | √ | Penicillium chrysogenum [42] | √ | ||||||
| Bipolaris sp. [60] | √ | √ | Penicillium crustosum [42] | √ | √ | √ | √ | ||
| Cadophora sp. [42] | √ | Penicillium digitatum [42] | √ | √ | |||||
| Candida albicans [46] | √ | Penicillium expansum [42] | √ | √ | √ | ||||
| Candida sake [63] | √ | Penicillium olsonii [42] | √ | ||||||
| Cephalosporium sp. [46] | √ | Penicillium sp. [42] | √ | √ | √ | √ | |||
| Chaetomium globosum [60] | √ | Periconia macrospinosa [42,62] | √ | √ | √ | √ | |||
| Chaetomium sp. [42,49] | √ | Periconia sp. [42] | √ | √ | |||||
| Chrysosporium pseudomerdarium [42] | √ | Phaeosphaeria nodorum [62] | √ | ||||||
| Cladorrhinum flexuosum [62] | √ | Phlebia sp. [42] | √ | √ | √ | ||||
| Cladosporium allicinum [42] | √ | Phoma eupyrena [42] | √ | ||||||
| Cladosporium cladosporioides [42] | √ | √ | √ | Phoma sp. [42] | √ | ||||
| Cladosporium delicatulum [63] | √ | Phomopsis sp. [60] | √ | ||||||
| Cladosporium herbarum [60] | √ | Plectosphaerella cucumerina [42] | √ | √ | |||||
| Cladosporium oxysporum [61] | √ | Pleospora herbarum [60] | √ | ||||||
| Cladosporium sp. [42] | √ | Pseudogymnoascus pannorum [46] | √ | ||||||
| Clonostachys candelabrum [42] | √ | √ | Pseudozyma flocculosa [42] | √ | |||||
| Cochliobolus spicifer [46] | √ | √ | √ | √ | Pyrenochaeta sp. [62] | √ | √ | √ | |
| Cryptococcus sp. [60] | √ | Rhizoctonia solani [42] | √ | ||||||
| Curvularia lunata [46] | √ | Rhodotorula rubra [60] | √ | ||||||
| Curvularia spicifera [46] | √ | Sarocladium sp. [42] | √ | √ | √ | √ | |||
| Didymella exitialis [61] | √ | Sarocladium strictum [42] | √ | √ | √ | √ | |||
| Didymella pomorum [42] | √ | √ | Septoria tritici [46] | √ | |||||
| Didymella sp. [42] | √ | Setophoma terrestris [42] | √ | √ | |||||
| Engyodontium album [42] | √ | Setosphaeria pedicellata [42] | √ | √ | |||||
| Epicoccum nigrum [60,61,62] | √ | Simplicillium lamellicola [62] | √ | √ | √ | ||||
| Epicoccum sp. [42] | √ | Stachybotrys sp. [46] | √ | ||||||
| Filobasidium chernovii [63] | √ | Stagonospora nodorum [61] | √ | ||||||
| Fusarium avenaceum [42] | √ | Stemphylium botryosum [46] | √ | ||||||
| Fusarium culmorum [61] | √ | √ | Stemphylium vesicarium [42,63] | √ | |||||
| Fusarium equiseti [62] | √ | √ | √ | Stemphylium sp. [60] | √ | ||||
| Fusarium graminearum [61] | √ | √ | Talaromyces aculeatus [42] | √ | |||||
| Fusarium incarnatum [62] | √ | √ | √ | Trichoderma hamatum [42] | √ | ||||
| Fusarium oxysporum [42,46,62] | √ | √ | √ | √ | Trichoderma koningii [42] | √ | |||
| Fusarium poae [42] | √ | Trichoderma viride [42] | √ | √ | |||||
| Fusarium proliferatum [42] | √ | √ | Trichoderma sp. [42] | √ | √ | ||||
| Fusarium redolens [42] | √ | Ulocladium sp. [46] | √ | ||||||
| Fusarium solani [42] | √ | √ | Umbelopsis sp. [42] | √ | |||||
| Fusarium sp. [42] | √ | √ | √ | √ | Valsa friesii [62] | √ | |||
| Fusarium temperatum [42] | √ | Waitea circinata [42] | √ | ||||||
| Fungal Endophytes | Organs | Fungal Endophytes | Organs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roots | Leaves | Stems | Fruits | Roots | Leaves | Stems | Fruits | ||
| Absidia sp. [64] | √ | √ | Penicillium chrysogenum [50] | √ | √ | ||||
| Acremonium cellulolyticus [65] | √ | Penicillium citrinum [65] | √ | √ | |||||
| Acremonium sp. [64] | √ | √ | √ | √ | Penicillium decumbens [50] | √ | √ | ||
| Arthrobotrys sp. [64] | √ | √ | √ | √ | Penicillium griseofulvum [65] | √ | √ | ||
| Aspergillus aureolus [65] | √ | √ | Penicillium limosum [65] | √ | √ | ||||
| Aspergillus flavus [66] | √ | √ | √ | Penicillium pinophilum [65] | √ | √ | |||
| Aspergillus ochraceous [50] | √ | √ | Penicillium rubens [67] | √ | √ | √ | |||
| Aspergillus udagawae [65] | √ | √ | Penicillium simplicissimum [68] | √ | √ | ||||
| Aspergillus ustus [69] | √ | √ | Penicillium sp. [65] | √ | √ | ||||
| Aspergillus welwitschiae Ocstreb1 [70] | √ | √ | Pestalotiopsis disseminata [65] | √ | |||||
| Aspergillus sp. [64] | √ | √ | √ | Phialophora verrucosa [50] | √ | √ | |||
| Candida tropicalis [51] | √ | Phoma sp. [68] | √ | ||||||
| Ceriporia lacerata [65] | √ | Piriformospora indica [71] | √ | ||||||
| Chaetomium brasiliense [69] | √ | Pseudophialophora oryzae sp. Nov [72] | √ | √ | |||||
| Chaetomium globosum [50] | √ | √ | Pyricularia sacc [64] | √ | √ | ||||
| Chaetomium pilosum [65] | √ | Rhizoctonia solani [50] | √ | ||||||
| Cladosporium cladosporioides [50] | √ | √ | Sarocladium oryzae [65] | √ | |||||
| Cladosporium sphaerospermum [51] | √ | Sarocladium oryzae DX-THL3 [73] | √ | √ | |||||
| Coniothyrium fuckelli [50] | √ | √ | Speiropsis pedatospora [50] | √ | √ | ||||
| Cylindrocladium sp. [64] | √ | √ | Stemphylium botryosum [50] | √ | √ | ||||
| Emmia lacerata [65] | √ | Talaromyces adpressus [70] | √ | √ | |||||
| Eupenicillium javanicum [67] | √ | √ | √ | Talaromyces argentinensis [70] | √ | ||||
| Fusarium oxysporum [50] | √ | √ | Talaromyces cellulolyticus [65] | √ | |||||
| Fusarium solani [65] | √ | √ | Talaromyces funiculosus [65] | √ | √ | √ | |||
| Fusarium sp. [64] | √ | Talaromyces pinophilus [66] | √ | ||||||
| Galactomyces geotrichum [68] | √ | √ | Talaromyces purpureogenus [65] | √ | |||||
| Humicola fuscoatra [50] | √ | Talaromyces sp. [65] | √ | ||||||
| Marasmius nigrobrunneus [65] | √ | √ | √ | Thielavia sp. [65] | √ | ||||
| Microsphaeropsis arundinis [67] | √ | Thielavia terricola [65] | √ | ||||||
| Mucor irregularis [65] | √ | Trichocomaceae sp. [65] | √ | ||||||
| Neocosmospora rubicola [65] | √ | Trichoderma hamatum [65] | √ | ||||||
| Neosartorya fischeri [65] | √ | Trichoderma paraviridescens [65] | √ | ||||||
| Neosartorya sp. [65] | √ | Trichoderma sp. [65] | √ | √ | |||||
| Nigrospora oryzae [60] | √ | √ | Trichoderma viridae [50] | √ | √ | √ | |||
| Paecilomyces varioti [50] | √ | √ | Trichoderma zelobreve [66] | √ | |||||
| Fungal Endophytes | Organs | Fungal Endophytes | Organs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roots | Leaves | Stems | Fruits | Roots | Leaves | Stems | Fruits | ||
| Acremonium sp. [68] | √ | Fusarium ventricosum [74] | √ | ||||||
| Acremonium strictum [75] | √ | Fusarium verticillioides [75,76] | √ | ||||||
| Alternaria alternata [74,77] | √ | Gibberella circinata [68] | √ | ||||||
| Aspergillus carneus [68] | √ | √ | √ | Gibberella fujikuroi [68] | √ | ||||
| Aspergillus flavus [75] | √ | Gibberella intermedia [68] | √ | ||||||
| Aspergillus fumigatus [75] | √ | Gibberella moniliformis [68] | √ | ||||||
| Aspergillus insuetu80s [54] | √ | Microsphaeropsis arundinis [54] | √ | √ | |||||
| Aspergillus niger [75] | √ | √ | Monocillium mucidum [54] | √ | |||||
| Aspergillus terreus [77] | √ | Mucor circinelloides [77] | √ | ||||||
| Aspergillus tubingensis [68] | √ | √ | √ | Penicillium aurantiogriseum [75] | √ | √ | |||
| Bipolaris tetramera [77] | √ | Penicillium citrinum [74,76] | √ | ||||||
| Bipolaris zeicola [75] | √ | √ | Penicillium glaucoroseum [54] | √ | |||||
| Chaetomium cochliodes [74] | √ | Penicillium griseofulvum [54] | √ | ||||||
| Chaetomium murorum [75] | √ | Penicillium janthinellum [54] | √ | ||||||
| Chaetomium sp. [75] | √ | Penicillium ludwigii [54] | √ | ||||||
| Chaetomium subaffine [74] | √ | Penicillium ochrochloron [68] | √ | ||||||
| Cladosporium cladosporioides [74] | √ | Penicillium oxalicum [75] | √ | ||||||
| Cladosporium sphaerospermum [75] | √ | Penicillium polonicum [75] | √ | ||||||
| Clonostachys rosea [54] | √ | Penicillium pulvillorum [54] | √ | ||||||
| Diaporthe longicolla [57] | √ | Penicillium subrubescens [54] | √ | ||||||
| Didymella americana [54] | √ | Periconia macrospinosa [57] | √ | ||||||
| Didymella heteroderae [54] | √ | Pleosporales sp. [68] | √ | ||||||
| Didymella pomorum [54] | √ | Pyrenochaetopsis microspora [54] | √ | ||||||
| Drechslera sp. [57] | √ | Rhizomucor pusillus [75] | √ | ||||||
| Epicoccum purpurascens [74] | √ | Rhizomucor sp. [68] | √ | ||||||
| Epicoccum sorghi [68] | √ | Rhizopus oryzae [74] | √ | ||||||
| Eupenicillium javanicum [68] | √ | Sarocladium zae [54] | √ | ||||||
| Eutypella scoparia [68] | √ | Sarocladium zeae [75,78] | √ | √ | |||||
| Fusarium andiyazi [68] | √ | Setophoma terrestris [57] | √ | ||||||
| Fusarium concentricum [68] | √ | Sordariomycetes sp. [68] | √ | ||||||
| Fusarium denticulatum [68] | √ | Talaromyces calidicanicus [54] | √ | ||||||
| Fusarium equiseti [68] | √ | Talaromyces pinophilus [68] | √ | ||||||
| Fusarium graminearum [77] | √ | Talaromyces verroculosus [54] | √ | ||||||
| Fusarium incarnatum [68] | √ | √ | Thermomyces dupontii [75] | √ | |||||
| Fusarium lateritium [54] | √ | Trichoderma asperellum [74] | √ | ||||||
| Fusarium moniliformis [54] | √ | Trichoderma gamsii [75] | √ | ||||||
| Fusarium oxysporum [54,57,74] | √ | Trichoderma harzianum [75] | √ | ||||||
| Fusarium proliferatum [74,75,77] | √ | √ | √ | √ | Trichoderma koningiopsis [68] | √ | |||
| Fusarium sacchari [76] | √ | Ustilago sp. [75] | √ | ||||||
| Fusarium sp. [68] | √ | Verticillium lecanii [74] | √ | ||||||
| Fusarium succisae [68] | √ | √ | |||||||
| Fungal Endophytes | Organs | Fungal Endophytes | Organs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Roots | Leaves | Stems | Fruits | Roots | Leaves | Stems | Fruits | ||
| Acremonium sp. [59] | √ | Fusarium sp. [59] | √ | ||||||
| Aspergillus carneus [79] | √ | √ | √ | Microdochium sp. [59] | √ | ||||
| Bipolaris eleusines [80] | √ | √ | √ | Mycelium sterile [59] | √ | ||||
| Boeremia exigua [58] | √ | √ | √ | Plectosporium tabacinum [59] | √ | ||||
| Cephalotrichum asperulum [81] | √ | √ | Trichosporon sp. [59] | √ | |||||
| Cephalotrichum gorgonifer [81] | √ | √ | √ | Trichothecium crotocinigenum [82] | √ | √ | √ | ||
| Cephalotrichum tenuissimum [81] | √ | √ | Ulocladium sp. [59] | √ | |||||
| Chaetomium globosum [83] | √ | √ | Verticillium dahliae [59] | √ | |||||
| Chaetomium subaffine [81] | √ | √ | √ | Xylaria curta E10 [84,85] | √ | √ | √ | ||
| Colletotrichum coccodes [59] | √ | Xylaria cf. curta [86,87] | √ | √ | √ | ||||
| Cylindrocarpon destructans [59] | √ | ||||||||
4. Secondary Metabolites
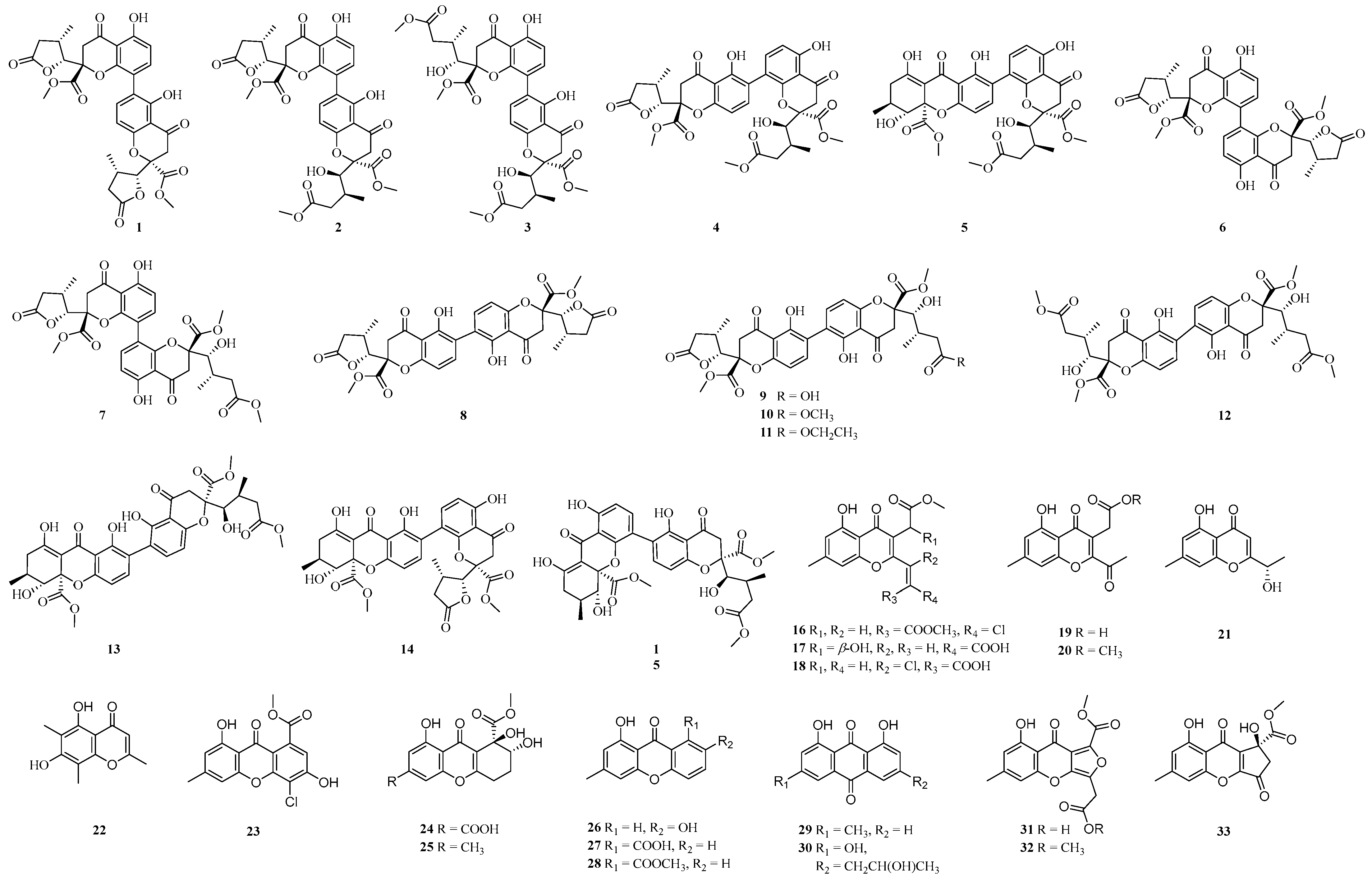
4.1. Ketone Compounds
4.1.1. Chromones
4.1.2. Other Ketones
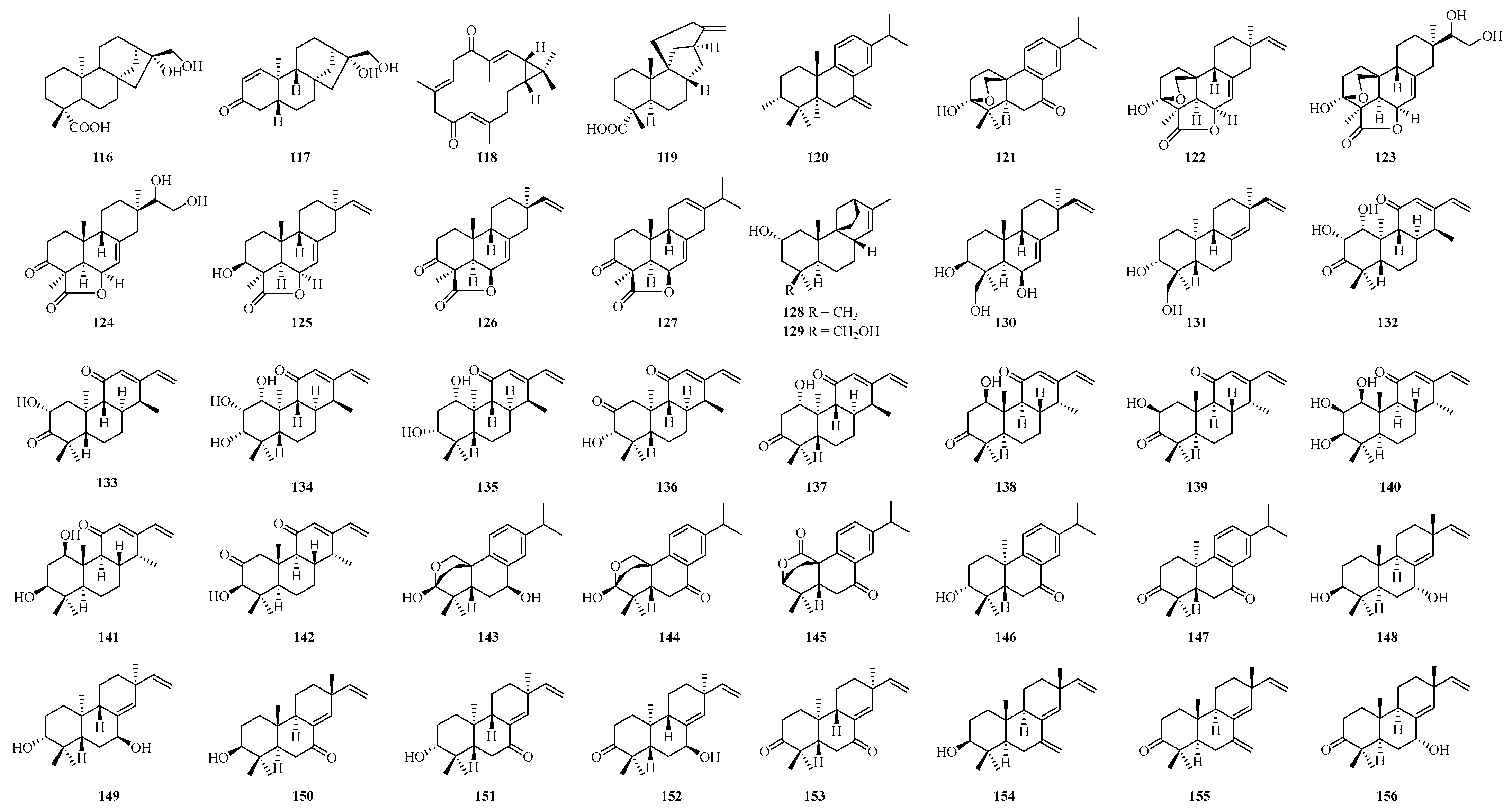
4.2. Terpenoid Compounds
4.2.1. Sesquiterpenoids
4.2.2. Diterpenoids
4.2.3. Sesterterpenoids
4.2.4. Other Terpenoids
4.3. Alkaloid Compounds
4.3.1. Cytochalasins
4.3.2. Other Alkaloids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olmos, E.; Roman-Garcia, I.; Reguera, M.; Mestanza, C.; Fernandez-Garcia, N. An update on the nutritional profiles of quinoa (Chenopodium quinoa Willd.), amaranth (Amaranthus spp.), and chia (Salvia hispanica L.), three key species with the potential to contribute to food security worldwide. JSFA Rep. 2022, 2, 591–602. [Google Scholar] [CrossRef]
- Gruber, K. Agrobiodiversity: The living library. Nature 2017, 544, S8–S10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, F.; Wu, Y.; Hu, H.H.; Dai, X.F. Progress of potato staple food research and industry development in China. J. Integr. Agric. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Our World in Data. 2024. Available online: https://ourworldindata.org/explorers/global-food?tab=table&time=latest&facet=none&Food=Potatoes&Metric=Production&Per+Capita=false&country=ZAF%3BhideControls~OWID_WRL (accessed on 17 April 2024).
- Li, J.; Zhao, J.L.; Xu, L.J.; Zhou, L.G.; Li, X.L.; Wang, J.G. Endophytic fungi from rhizomes of Paris polyphylla var. yunnanensis. World J. Microbiol. Biotechnol. 2008, 24, 733–737. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.W.; Liu, H.Y.; Wang, C.L.; Chen, X.; Huang, Y.X.; Zhang, M.M.; Huang, Q.L.; Zhang, G.F. Isolation of endophytic fungi from Cotoneaster multiflorus and screening of drought-tolerant fungi and evaluation of their growth-promoting effects. Front. Microbiol. 2023, 14, 1267404. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.A.; da Mata, T.B.; Canuto, G.A.B.; Silva, E.O. Chemical Diversity of Secondary Metabolites Produced by Brazilian Endophytic Fungi. Curr. Microbiol. 2021, 78, 33–54. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Hyde, K.D.; McKenzie, E.H.C.; Saxena, R.K.; Li, Q.R. Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 2023, 125, 73–98. [Google Scholar] [CrossRef]
- Ratnaweera, P.B.; de Silva, E.D. Endophytic Fungi: A Remarkable Source of Biologically Active Secondary Metabolites. In Endophytes: Crop Productivity and Protection. Sustainable Development and Biodiversity; Maheshwari, D., Annapurna, K., Eds.; Springer: Cham, Switzerland, 2017; Volume 16. [Google Scholar]
- de Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- Hatziminaoglou, Y.; Boyazoglu, J. The goat in ancient civilisations: From the Fertile Crescent to the Aegean Sea. Small Rumin. Res. 2004, 51, 123–129. [Google Scholar] [CrossRef]
- Saffirio, L. Food and dietary habits in ancient Egypt. J. Hum. Evol. 1972, 1, 297–305. [Google Scholar] [CrossRef]
- Capparelli, A.; Lema, V.; Giovannetti, M.; Raffino, R. The introduction of Old World crops (wheat, barley and peach) in Andean Argentina during the 16th century a.d.: Archaeobotanical and ethnohistorical evidence. Veg. Hist. Archaeobot. 2005, 14, 472–484. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Gutaker, R.M.; Groen, S.C.; Bellis, E.S.; Choi, J.Y.; Pires, I.S.; Bocinsky, R.K.; Slayton, E.R.; Wilkins, O.; Castillo, C.C.; Negrão, S.; et al. Genomic history and ecology of the geographic spread of rice. Nat. Plants 2020, 6, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Ding, J.L.; Shu, J.W.; Chen, W. Exploration of early rice farming in China. Quat. Int. 2010, 227, 22–28. [Google Scholar] [CrossRef]
- Taylor, E. A Log-Book of Magellan’s Voyage, 1519–1522. J. Navig. 1964, 17, 83–87. [Google Scholar] [CrossRef]
- Bellwood, P. The Checkered Prehistory of Rice Movement Southwards as a Domesticated Cereal—From the Yangzi to the Equator. Rice 2011, 4, 93–103. [Google Scholar] [CrossRef]
- Carney, J. The African origins of Carolina rice culture. Ecumene 2000, 7, 125–149. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Y.; Zhang, W. Changes in rice yields in China since 1980 associated with cultivar improvement, climate and crop management. Field. Crop. Res. 2012, 136, 65–75. [Google Scholar] [CrossRef]
- Peng, S.; Khush, G.S.; Virk, P.; Tang, Q.; Zou, Y. Progress in ideotype breeding to increase rice yield potential. Field Crop. Res. 2008, 108, 32–38. [Google Scholar] [CrossRef]
- Peng, S.; Tang, Q.; Zou, Y. Current Status and Challenges of Rice Production in China. Plant. Prod. Sci. 2009, 12, 3–8. [Google Scholar] [CrossRef]
- Salakinkop, S.R.; Talekar, S.C.; Patil, C.R.; Patil, S.B.; Jat, S.L.; Iliger, K.S.; Manjulatha, G.; Harlapur, S.I.; Kachapur, R.M. Sustainable intensification of climate-resilient maize–chickpea system in semi-arid tropics through assessing factor productivity. Sci. Rep. 2024, 14, 3958. [Google Scholar] [CrossRef] [PubMed]
- García-Lara, S.; Serna-Saldivar, S.O. Chapter 1-Corn History and Culture. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019. [Google Scholar]
- Cherniwchan, J.; Moreno-Cruz, J. Maize and precolonial Africa. J. Dev. Econ. 2019, 136, 137–150. [Google Scholar] [CrossRef]
- Chen, S.; Kung, J.K. Of maize and men: The effect of a New World crop on population and economic growth in China. J. Econ. Growth. 2016, 21, 71–99. [Google Scholar] [CrossRef]
- Bannert, M.; Stamp, P. Cross-pollination of maize at long distance. Eur. J. Agron. 2007, 27, 44–51. [Google Scholar] [CrossRef]
- Singh, N.; Vasudev, S.; Yadava, D.K.; Chaudhary, D.P.; Prabhu, K.V. Oil Improvement in Maize: Potential and Prospects. In Maize: Nutrition Dynamics and Novel Uses; Chaudhary, D.P., Kumar, S., Langyan, S., Eds.; Springer: New Delhi, India, 2014. [Google Scholar]
- White, J.S.; Nicklas, T.A. High-Fructose Corn Syrup Use in Beverages: Composition, Manufacturing, Properties, Consumption, and Health Effects. In Beverage Impacts on Health and Nutrition, 2nd ed.; Wilson, T., Temple, N.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Han, X.; Chen, Y.; Wang, X. Impacts of China’s bioethanol policy on the global maize market: A partial equilibrium analysis to 2030. Food. Secur. 2022, 14, 147–163. [Google Scholar] [CrossRef] [PubMed]
- De Jong, H. Impact of the Potato on Society. Am. J. Potato Res. 2016, 93, 415–429. [Google Scholar] [CrossRef]
- Ugent, D.; Dillehay, T.; Ramirez, C. Potato remains from a late pleistocene settlement in southcentral Chile. Econ. Bot. 1987, 41, 17–27. [Google Scholar] [CrossRef]
- Ugent, D.; Peterson, L.W. Archeologial remains of potato and sweet potato in Peru. CIP Circular. 1988, 16, 1–10. [Google Scholar]
- Garzón, F. Sociedades precolombinas asociadas a la domesticación y cultivo de la papa (Solanum tuberosum) en Sudamérica. Rev. Latinoam. Papa 2016, 14, 1–9. [Google Scholar]
- Wang, Z.J.; Liu, H.; Zeng, F.K.; Yang, Y.C.; Xu, D.; Zhao, Y.C.; Liu, X.F.; Kaur, L.; Liu, G.; Singh, J. Potato Processing Industry in China: Current Scenario, Future Trends and Global Impact. Potato Res. 2023, 66, 543–562. [Google Scholar] [CrossRef] [PubMed]
- Alam, B.; Lǐ, J.; Gě, Q.; Khan, M.A.; Gōng, J.; Mehmood, S.; Yuán, Y.; Gǒng, W. Endophytic Fungi: From Symbiosis to Secondary Metabolite Communications or Vice Versa? Front. Plant. Sci. 2021, 12, 791033. [Google Scholar] [CrossRef] [PubMed]
- Buddhika, U.V.A.; Abeysinghe, S. Chapter 5-Plant endophytic microorganisms enhancing crop productivity and yield. In New and Future Developments in Microbial Biotechnology and Bioengineering; Verma, J.P., Macdonald, C., Gupta, V.K., Podile, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Burragoni, S.G.; Jeon, J. Applications of endophytic microbes in agriculture, biotechnology, medicine, and beyond. Microbiol. Res. 2021, 245, 126691. [Google Scholar] [CrossRef]
- Salamon, S.; Mikołajczak, K.; Błaszczyk, L. Constellation of the endophytic mycobiome in spring and winter wheat cultivars grown under various conditions. Sci. Rep. 2023, 13, 6089. [Google Scholar] [CrossRef] [PubMed]
- Latz, M.A.C.; Kerrn, M.H.; Sørensen, H.; Collinge, D.B.; Jensen, B.; Brown, J.K.M.; Madsen, A.M.; Jørgensen, H.J.L. Succession of the fungal endophytic microbiome of wheat is dependent on tissue-specific interactions between host genotype and environment. Sci. Total Environ. 2021, 759, 143804. [Google Scholar] [CrossRef] [PubMed]
- Lenc, L.; Kwaśna, H.; Sadowski, C.; Grabowski, A. Microbiota in wheat roots, rhizosphere and soil in crops grown in organic and other production systems. J. Phytopathol. 2015, 163, 245–263. [Google Scholar] [CrossRef]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.L.; Latz, M.A.C.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of fungal endophytes with biocontrol potential against Fusarium head blight in wheat. Biol. Control 2020, 144, 104222. [Google Scholar] [CrossRef]
- Larran, S.; Perelló, A.; Simón, M.R.; Moreno, V. The endophytic fungi from wheat (Triticum aestivum L.). World J. Microbiol. Biotechnol. 2007, 23, 565–572. [Google Scholar] [CrossRef]
- Vujanovic, V.; Mavragani, D.; Hamel, C. Fungal communities associated with durum wheat production system: A characterization by growth stage, plant organ and preceding crop. Crop Prot. 2012, 37, 26–34. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Justesen, A.F.; Knorr, K.; Wang, J.; Pinnschmidt, H.O. Fungal communities in wheat grain show significant co-existence patterns among species. Fungal Ecol. 2014, 11, 145–153. [Google Scholar] [CrossRef]
- Ofek-Lalzar, M.; Gur, Y.; Ben-Moshe, S.; Sharon, O.; Kosman, E.; Mochli, E.; Sharon, A. Diversity of fungal endophytes in recent and ancient wheat ancestors Triticum dicoccoides and Aegilops sharonensis. FEMS Microbiol. Ecol. 2016, 92, fiw152. [Google Scholar] [CrossRef]
- Wang, W.F.; Zhai, Y.Y.; Cao, L.X.; Tan, H.M.; Zhang, R.D. Endophytic bacterial and fungal microbiota in sprouts, roots and stems of rice (Oryza sativa L.). Mcrobiol. Res. 2016, 188–189, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Bhat, J.A.; Devoto, A. The influence of endophytes on rice fitness under environmental stresses. Plant. Mol. Biol. 2022, 109, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Ahmad, N.; Tang, D.S.; Kang, S.M.; Na, C.I.; Sohn, E.Y.; Hwang, Y.H.; Shin, D.H.; Lee, B.H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.L.; Li, X.X.; Zhang, Z.B.; Yan, R.M.; Yang, H.L.; Zhu, D. Phylogenetic diversity of culturable endophytic fungi in Dongxiang wild rice (Oryza rufipogon Griff), detection of polyketide synthase gene and their antagonistic activity analysis. Fungal Biol. 2015, 119, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Zelaya-Molina, L.X.; Sanchez-Lima, A.D.; Arteaga-Garibay, R.I.; Bustamante-Brito, R.; Vásquez-Murrieta, M.S.; Martínez-Romero, E.; Ramos-Garza, J. Functional characterization of culturable fungi from microbiomes of the “conical cobs” Mexican maize (Zea mays L.) landrace. Arch. Microbiol. 2021, 204, 57. [Google Scholar] [CrossRef]
- Yadav, A.N.; Verma, P.; Kumar, V.; Sangwan, P.; Mishra, S.; Panjiar, N.; Saxena, A.K. Biodiversity of the genus Penicillium in different habitats. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–18. [Google Scholar]
- Park, Y.H.; Mishra, R.C.; Yoon, S.; Kim, H.; Park, C.; Seo, S.T.; Bae, H. Endophytic Trichoderma citrinoviride isolated from mountain-cultivated ginseng (Panax ginseng) has great potential as a biocontrol agent against ginseng pathogens. J. Ginseng. Res. 2019, 43, 408–420. [Google Scholar] [CrossRef]
- Strom, N.; Hu, W.; Haarith, D.; Chen, S.; Bushley, K. Corn and Soybean Host Root Endophytic Fungi with Toxicity Toward the Soybean Cyst Nematode. Phytopathology® 2019, 110, 603–614. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.T.; Yang, H.X.; Li, Z.H.; Liu, J.K.; Ai, H.L.; Wang, G.K.; Feng, T. Depsidones and diaryl ethers from potato endophytic fungus Boeremia exigua. Fitoterapia 2020, 141, 104483. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Nirenberg, H.; Krause, S.; Wolters, H.; Draeger, S.; Buchner, A.; Lottmann, J.; Berg, G.; Smalla, K. Fungal endophytes in potato roots studied by traditional isolation and cultivation-independent DNA-based methods. FEMS Microbiol. Ecol. 2006, 58, 404–413. [Google Scholar] [CrossRef]
- Larran, S.; Perelló, A.; Simón, M.R.; Moreno, V. Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J. Microbiol. Biotechnol. 2002, 18, 683–686. [Google Scholar] [CrossRef]
- Sieber, T.; Riesen, T.K.; Müller, E.; Fried, P.M. Endophytic fungi in four winter wheat cultivars (Triticum aestivum L.) differing in resistance against Stagonospora nodorum (Berk.) Cast. & Germ. = Septoria nodorum (Berk.) Berk. J. Phytopathol. 1988, 122, 289–306. [Google Scholar]
- Abaya, A.; Xue, A.; Hsiang, T. Selection and screening of fungal endophytes against wheat pathogens. Biol. Control 2021, 154, 104511. [Google Scholar] [CrossRef]
- Sun, X.; Kosman, E.; Sharon, A. Stem endophytic mycobiota in wild and domesticated wheat: Structural differences and hidden resources for wheat improvement. J. Fungi 2020, 6, 180. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Islam, M.M.; Mandal, S. Endophytic Microbiota of Rice and Their Collective Impact on Host Fitness. Curr. Microbiol. 2022, 79, 37. [Google Scholar] [CrossRef]
- Pang, Z.Q.; Zhao, Y.; Xu, P.; Yu, D.Q. Microbial Diversity of Upland Rice Roots and Their Influence on Rice Growth and Drought Tolerance. Microorganisms 2020, 8, 1329. [Google Scholar] [CrossRef] [PubMed]
- Gateta, T.; Nacoon, S.; Seemakram, W.; Ekprasert, J.; Theerakulpisut, P.; Sanitchon, J.; Suwannarach, N.; Boonlue, S. The Potential of Endophytic Fungi for Enhancing the Growth and Accumulation of Phenolic Compounds and Anthocyanin in Maled Phai Rice (Oryza sativa L.). J. Fungi 2023, 9, 937. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Z.; Liang, W.; Gao, B.; Wang, Y.; Chang, J.; Zhu, D. Endophytic fungi from Dongxiang wild rice (Oryza rufipogon Griff.) show diverse catalytic potential for converting glycyrrhizin. 3 Biotech 2022, 12, 79. [Google Scholar] [CrossRef]
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional Characterization of Endophytic Fungal Community Associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Promgool, T.; Kanokmedhakul, K.; Leewijit, T.; Song, J.; Soytong, K.; Yahuafai, J.; Kudera, T.; Kokoska, L.; Kanokmedhakul, S. Cytotoxic and antibacterial depsidones from the endophytic fungus Chaetomium brasiliense isolated from Thai rice. Nat. Prod. Res. 2022, 36, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Airin, A.A.; Arafat, M.I.; Begum, R.A.; Islam, M.R.; Seraj, Z.I. Plant growth-promoting endophytic fungi of the wild halophytic rice Oryza coarctata. Ann. Microbiol. 2023, 73, 36. [Google Scholar] [CrossRef]
- Tsai, H.J.; Shao, K.H.; Chan, M.T.; Cheng, C.P.; Yeh, K.W.; Oelmüller, R.; Wang, S.J. Piriformospora indica symbiosis improves water stress tolerance of rice through regulating stomata behavior and ROS scavenging systems. Plant Signal. Behav. 2020, 15, 1722447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.N.; Yu, Y.J.; Dai, M.D.; Zeng, Y.L.; Lu, X.J.; Wang, L.; Liu, X.H.; Su, Z.Z.; Lin, F.C. A New Species in Pseudophialophora from Wild Rice and Beneficial Potential. Front. Microbiol. 2022, 13, 845104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Du, S.Y.; Ji, B.; Ji, C.J.; Xiao, Y.W.; Yan, R.M.; Zhu, D. New Helvolic Acid Derivatives with Antibacterial Activities from Sarocladium oryzae DX-THL3, an Endophytic Fungus from Dongxiang Wild Rice (Oryza rufipogon Griff.). Molecules 2021, 26, 1828. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.G. Maize seed endophytes. Mol. Plant. Pathol. 2023, 24, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Goodwin, S.B. Exploring the Corn Microbiome: A Detailed Review on Current Knowledge, Techniques, and Future Directions. PhytoFrontiers™ 2022, 2, 158–175. [Google Scholar] [CrossRef]
- Terna, T.P.; Mohamed Nor, N.M.I.; Zakaria, L. Histopathology of Corn Plants Infected by Endophytic Fungi. Biology 2022, 11, 641. [Google Scholar] [CrossRef]
- Russo, M.L.; Pelizza, S.A.; Cabello, M.N.; Stenglein, S.A.; Vianna, M.F.; Scorsetti, A.C. Endophytic fungi from selected varieties of soybean (Glycine max L. Merr.) and corn (Zea mays L.) grown in an agricultural area of Argentina. Rev. Argent. Microbiol. 2016, 48, 154–160. [Google Scholar] [CrossRef]
- de Almeida, M.N.; Guimarães, V.M.; Falkoski, D.L.; Visser, E.M.; Siqueira, G.A.; Milagres, A.M.; de Rezende, S.T. Direct ethanol production from glucose, xylose and sugarcane bagasse by the corn endophytic fungi Fusarium verticillioides and Acremonium zeae. J. Biotechnol. 2013, 168, 71–77. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, F.L.; Wu, X.; Ye, K.; Lv, X.; Ai, H.L.; Liu, J.K. Bioactive Polyketides From the Potato Endophytic Fungus Aspergillus carneus. Nat. Prod. Commun. 2020, 15, 1934578X2098522. [Google Scholar] [CrossRef]
- Ai, H.L.; Shi, B.B.; Li, W.; He, J.; Li, Z.H.; Feng, T.; Liu, J.K. Bipolarithizole A, an antifungal phenylthiazole-sativene meroses-quiterpenoid from the potato endophytic fungus Bipolaris eleusines. Org. Chem. Front. 2022, 9, 1814–1819. [Google Scholar] [CrossRef]
- Alijanimamaghani, N.; Saremi, H.; Javan-Nikkhah, M.; Sophie, D.R.; Pianta, E.; Tonolla, M. Endophytic Cephalotrichum spp. from Solanum tuberosum (potato) in Iran—A polyphasic analysis. Sydowia 2022, 74, 287–301. [Google Scholar]
- Devaux, A.; Goffart, J.P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The Potato of the Future: Opportunities and Challenges in Sustainable Agri-food Systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef]
- Zhang, J.; Islam, M.S.; Wang, J.; Zhao, Y.; Dong, W. Isolation of Potato Endophytes and Screening of Chaetomium globosum Antimicrobial Genes. Int. J. Mol. Sci. 2022, 23, 4611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, Y.Z.; Ye, K.; Pan, X.Y.; Ma, X.J.; Ai, H.L.; Shi, B.B.; Liu, J.K. Six Unprecedented Cytochalasin Derivatives from the Potato Endophytic Fungus Xylaria curta E10 and Their Cytotoxicity. Pharmaceuticals 2023, 16, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Li, Z.H.; Feng, T.; Li, J.; Sun, H.; Huang, R.; Yuan, Q.X.; Ai, H.L.; Liu, J.K. Curtachalasins A and B, Two Cyto-chalasans with a Tetracyclic Skeleton from the Endophytic Fungus Xylaria curta E10. Org. Lett. 2018, 20, 7758–7761. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Lei, X.; Yang, Y.L.; Li, Z.H.; Ai, H.L.; Li, J.; Feng, T.; Liu, J.K. Xylarichalasin A, a Halogenated Hexacyclic Cyto-chalasan from the Fungus Xylaria cf. curta. Org. Lett. 2019, 21, 6957–6960. [Google Scholar] [CrossRef]
- Wang, W.X.; Li, Z.H.; Ai, H.L.; Li, J.; He, J.; Zheng, Y.S.; Feng, T.; Liu, J.K. Cytotoxic 19,20-epoxycytochalasans from endophyt-ic fungus Xylaria cf. curta. Fitoterapia 2019, 137, 104253. [Google Scholar] [CrossRef]
- Kliebenstein, D.J. Use of Secondary Metabolite Variation in Crop Improvement. In Plant-Derived Natural Products; Osbourn, A., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Wink, M. Plant Breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Chakraborty, R. Role of Secondary Metabolites and Prospects of Engineering Secondary Metabolite Production for Crop Im-provement. In Plant Stress: Challenges and Management in the New Decade. Advances in Science, Technology & Innovation; Roy, S., Mathur, P., Chakraborty, A.P., Saha, S.P., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Gaspar, A.; Matos, J.M.; Garrido, J.; Uriarte, E.; Borges, F. Chromone: A Valid Scaffold in Medicinal Chemistry. Chem. Rev. 2014, 114, 4960–4992. [Google Scholar] [CrossRef]
- Wei, P.P.; Ai, H.L.; Shi, B.B.; Ye, K.; Lv, X.; Pan, X.Y.; Ma, X.J.; Xiao, D.; Li, Z.H.; Lei, X.X. Paecilins F–P, new dimeric chroma-nones isolated from the endophytic fungus Xylaria curta E10, and structural revision of paecilin A. Front. Microbiol. 2022, 13, 922444. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Z.; Tian, C.; Tong, S.Y.; Liu, Q.; Xu, F.; Shi, B.B.; Ai, H.L.; Liu, J.K. Chromones from the endophytic fungus Bipolaris eleu-sines. Phytochemistry 2024, 221, 114046. [Google Scholar] [CrossRef]
- He, J.; Li, Z.H.; Ai, H.L.; Feng, T.; Liu, J.K. Anti-bacterial chromones from cultures of the endophytic fungus Bipolaris eleusines. Nat. Prod. Res. 2019, 33, 3515–3520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wang, Z.Z.; Song, Z.J.; Karthik, L.; Hou, C.J.; Zhu, G.L.; Jiang, L.; Han, J.Y.; Ma, R.; Li, L.; et al. Brocaeloid D, a novel compound isolated from a wheat pathogenic fungus, Microdochium majus 99049. Synth. Syst. Biotechnol. 2019, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, M.S.; Wang, W.X.; Li, Z.H.; Elkhateeb, W.A.M.; Wen, T.C.; Ai, H.L.; Feng, T. Anti-phytopathogenic sesquiterpe-noid-xanthone adducts from potato endophytic fungus Bipolaris eleusines. RSC Adv. 2019, 9, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, X.Q.; Wan, J.L.; Han, H.L.; Zhao, Y.D.; Cai, L.; Yang, Y.B.; Ding, Z.T. The antifungal metabolites isolated from maize endophytic fungus Fusarium sp. induced by OSMAC strategy. Fitoterapia 2023, 171, 105710. [Google Scholar] [CrossRef]
- Zhang, X.T.; Zheng, M.J.; Fu, A.M.; Li, Q.; Chen, C.M.; Zhu, H.C.; Zhang, Y.H. Natural Sesquiterpenoids, Diterpenoids, Ses-terterpenoids, and Triterpenoids with Intriguing Structures from 2017 to 2022. Chin. J. Chem. 2023, 41, 3115–3132. [Google Scholar] [CrossRef]
- Li, Z.H.; Ai, H.L.; Yang, M.S.; He, J.; Feng, T. Bioactive sativene sesquiterpenoids from cultures of the endophytic fungus Bipolaris eleusines. Phytochem. Lett. 2018, 27, 87–89. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Tian, C.; Tong, S.Y.; Liu, Q.; Xu, F.; Shi, B.B.; Ai, H.L.; Liu, J.K. The antifungal properties of terpenoids from the en-dophytic fungus Bipolaris eleusines. Nat. Prod. Bioprospect. 2023, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Cai, X.Y.; He, Y.Y.; Lu, M.Y.; Liu, S.; Wang, W.X.; Li, Z.H.; Ai, H.L.; Feng, T. Seco-sativene and Seco-longifolene Sesquiterpenoids from Cultures of Endophytic Fungus Bipolaris eleusines. Nat. Prod. Bioprospect. 2017, 7, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Ai, H.L.; Feng, T.; Wang, W.X.; Wu, B.; Zheng, Y.S.; Sun, H.; He, J.; Li, Z.H.; Liu, J.K. Trichothecrotocins A–C, An-tiphytopathogenic Agents from Potato Endophytic Fungus Trichothecium crotocinigenum. Org. Lett. 2018, 20, 8069–8072. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; Wu, X.; Chi, M.J.; Li, Z.H.; Feng, T.; Ai, H.L.; Liu, J.K. Structure and cytotoxicity of trichothecenes produced by the potato-associated fungus Trichothecium crotocinigenum. Bioorg. Chem. 2021, 111, 104874. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.X.; He, J.; Zhang, F.L.; Zhang, X.D.; Li, Z.H.; Feng, T.; Ai, H.L.; Liu, J.K. Trichothecrotocins D–L, Antifungal Agents from a Potato-Associated Trichothecium crotocinigenum. J. Nat. Prod. 2020, 83, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Toshima, H. Identification of a novel casbene-type diterpene phytoalexin, ent-10-oxodepressin, from rice leaves. Biosci. Biotechnol. Biochem. 2013, 77, 760–765. [Google Scholar] [CrossRef]
- Wang, W.L.; Zhang, K.Y.; Yuan, M.Q.; Yang, M.; Wang, A.D.; Huang, L.; Li, J.L. α-Glucosidase inhibitors from the husks of rice Oryza sativa L. Fitoterapia 2023, 171, 105688. [Google Scholar] [CrossRef] [PubMed]
- Kariya, K.; Ube, N.; Ueno, M.; Teraishi, M.; Okumoto, Y.; Mori, N.; Ueno, K.; Ishihara, A. Natural variation of diterpenoid phytoalexins in cultivated and wild rice species. Phytochemistry 2020, 180, 112518. [Google Scholar] [CrossRef]
- Horie, K.; Inoue, Y.; Sakaki, M.; Yao, Q.; Tanimoto, Y.; Koga, J.; Toshima, H.; Hasegawa, M. Identification of UV-Induced diterpenes including a new diterpene phytoalexin, phytocassane F, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food. Chem. 2015, 63, 4050–4059. [Google Scholar] [CrossRef]
- Koga, J.; Shimura, M.; Oshima, K.; Ogawa, N.; Yamauchi, T.; Ogasawara, N. Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron 1995, 51, 7907–7918. [Google Scholar] [CrossRef]
- Koga, J.; Ogawa, N.; Yamauchi, T.; Kikuchi, M.; Ogasawara, N.; Shimura, N. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry 1997, 44, 249–253. [Google Scholar] [CrossRef]
- Kariya, K.; Fujita, A.; Ueno, M.; Yoshikawa, T.; Teraishi, M.; Taniguchi, Y.; Ueno, K.; Ishihara, A. Natural variation of diterpe-noid phytoalexins in rice: Aromatic diterpenoid phytoalexins in specific cultivars. Phytochemistry 2023, 211, 113708. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, T.; Kodama, O.; Kato, H.; Kono, Y.; Takeuchi, S. 3-Hydroxy-7-oxo- sandaraco-pimaradiene (oryzalexin A), a new phytoalexin isolated from rice blast leaves. Agric. Biol. Chem. 1983, 47, 445–447. [Google Scholar] [CrossRef]
- Kono, Y.; Takeuchi, S.; Kodama, O.; Akatsuka, T. Absolute configuration of oryzalexin A and structures of its related phytoa-lexins isolated from rice blast leaves infected with Pyricularia oryzae. Agric. Biol. Chem. 1984, 48, 253–255. [Google Scholar]
- Sekido, H.; Endo, T.; Suga, R.; Kodama, O.; Akatsuka, T.; Kono, Y.; Takeuchi, S. Oryzalexin D (3,7-dihydroxy-(+)-sandaracopimaradiene), a new phytoalexin isolated from blast-infected rice leaves. J. Pestic. Sci. 1986, 11, 369–372. [Google Scholar] [CrossRef]
- Lichman, B.R. The scaffold-forming steps of plant alkaloid biosynthesis. Nat. Prod. Rep. 2021, 38, 103–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Cheng, G.G.; Li, Z.H.; Ai, H.L.; He, J.; Li, J.; Feng, T.; Liu, J.K. Curtachalasins, immunosuppressive agents from the endophytic fungus Xylaria cf. curta. Org. Biomol. Chem. 2019, 17, 7985–7994. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Feng, T.; Li, Z.H.; Li, J.; Ai, H.L.; Liu, J.K. Cytochalasins D1 and C1, unique cytochalasans from endophytic fungus Xylaria cf. curta. Tetrahedron. Lett. 2019, 60, 150952. [Google Scholar] [CrossRef]
- Wang, W.X.; Lei, X.; Ai, H.L.; Bai, X.; Li, J.; He, J.; Li, Z.H.; Zheng, Y.S.; Feng, T.; Liu, J.K. Cytochalasans from the Endophytic Fungus Xylaria cf. curta with Resistance Reversal Activity against Fluconazole-Resistant Candida albicans. Org. Lett. 2019, 21, 1108–1111. [Google Scholar] [CrossRef]
- Teng, S.Q.; Zhang, X.F.; Li, H.F.; Luo, X.W.; Zhou, Y.S.; Liu, H.; Liu, J.K.; Feng, T. Boerechalasins A–G, cytochalasans from the fungus Boeremia exigua with anti-inflammatory and cytotoxic activities. Phytochemistry 2023, 215, 113861. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pastor, I.; González-Menéndez, V.; Annang, F.; Toro, C.; Mackenzie, T.A.; Bosch-Navarrete, C.; Genilloud, O.; Reyes, F. Pipecolisporin, a Novel Cyclic Peptide with Antimalarial and Antitrypanosome Activities from a Wheat Endophytic Nigrospora oryzae. Pharmaceuticals 2021, 14, 268. [Google Scholar] [CrossRef] [PubMed]

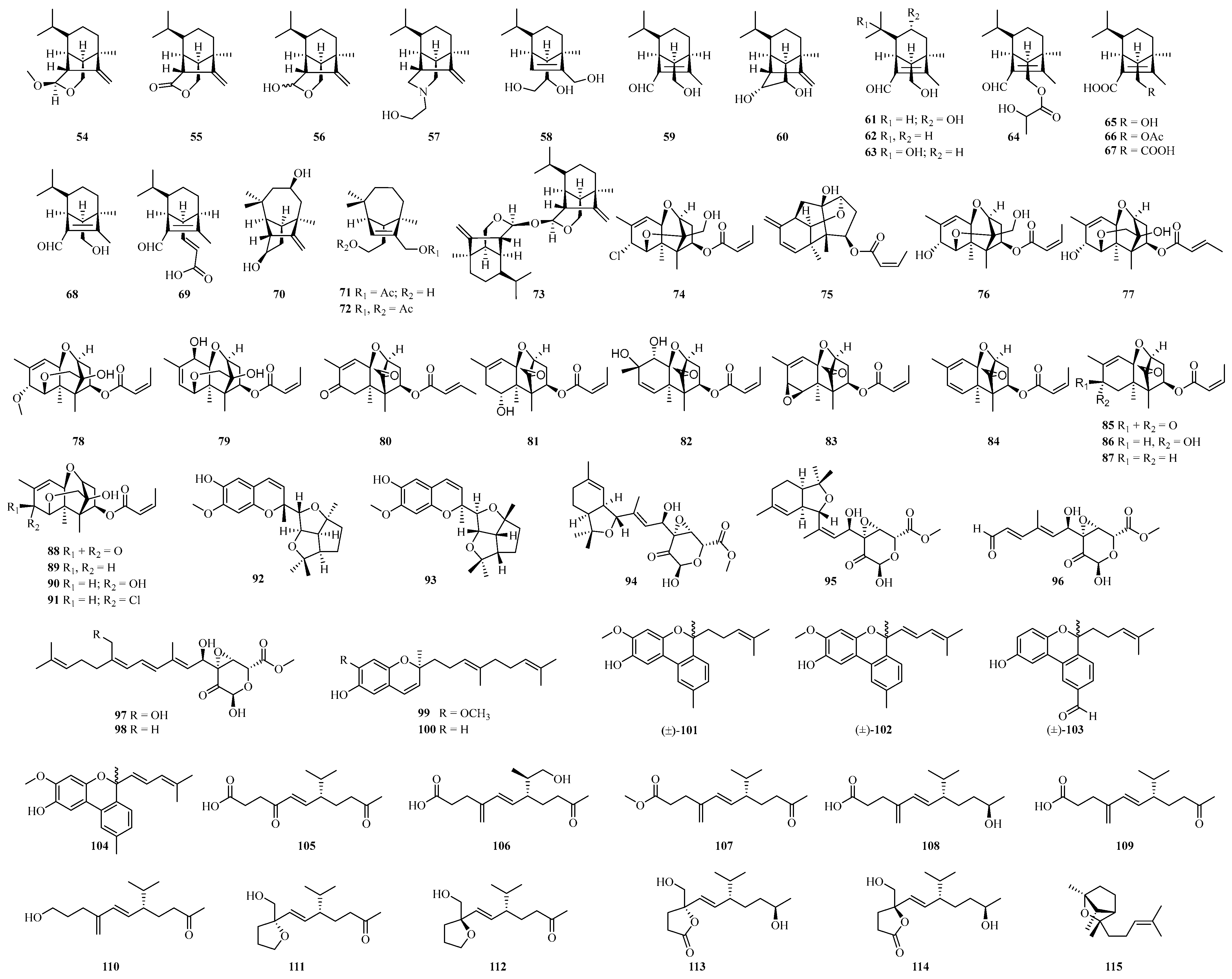


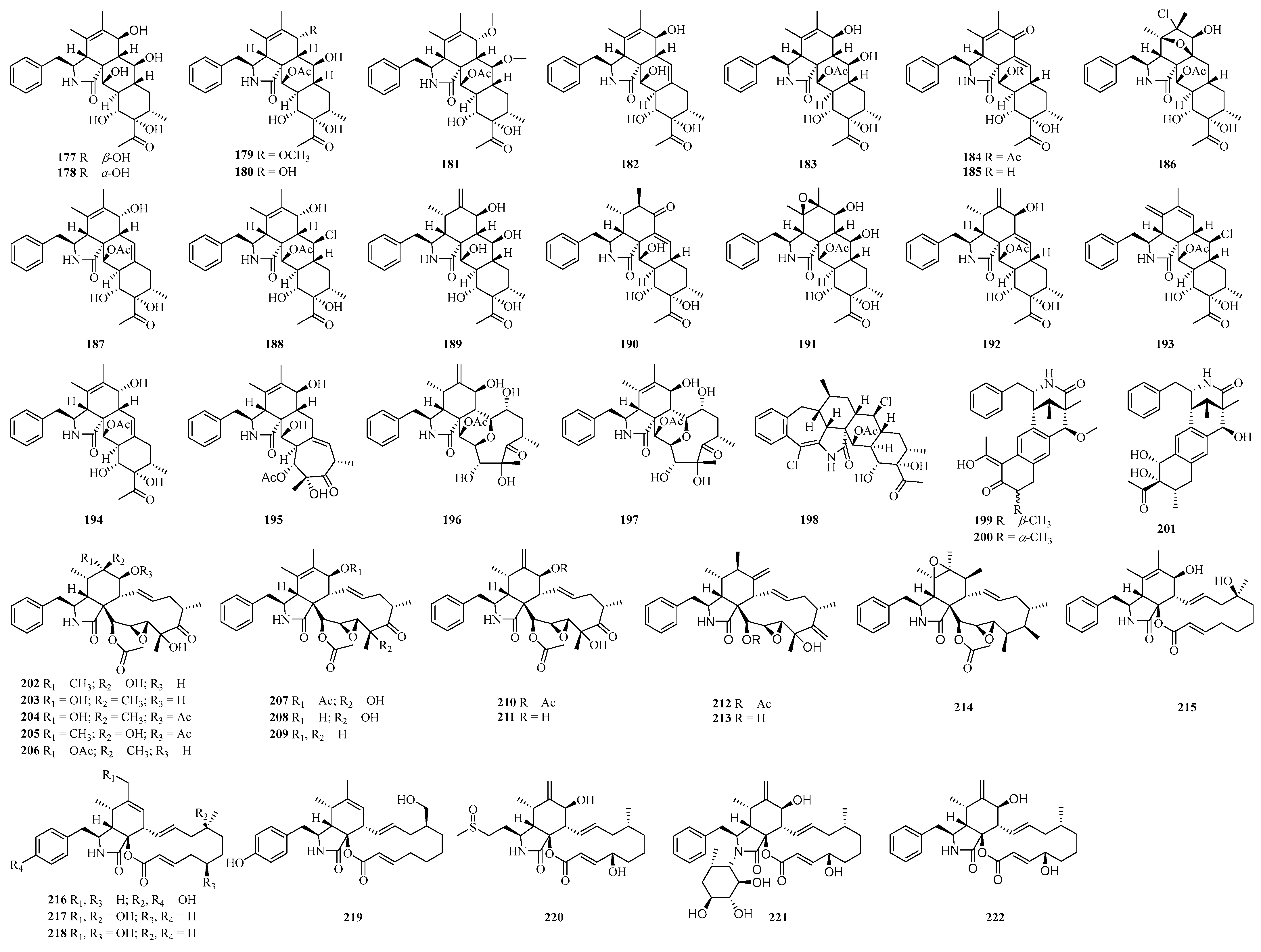

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Shi, B. Endophytic Fungi from the Four Staple Crops and Their Secondary Metabolites. Int. J. Mol. Sci. 2024, 25, 6057. https://doi.org/10.3390/ijms25116057
Fan Y, Shi B. Endophytic Fungi from the Four Staple Crops and Their Secondary Metabolites. International Journal of Molecular Sciences. 2024; 25(11):6057. https://doi.org/10.3390/ijms25116057
Chicago/Turabian StyleFan, Yinzhong, and Baobao Shi. 2024. "Endophytic Fungi from the Four Staple Crops and Their Secondary Metabolites" International Journal of Molecular Sciences 25, no. 11: 6057. https://doi.org/10.3390/ijms25116057
APA StyleFan, Y., & Shi, B. (2024). Endophytic Fungi from the Four Staple Crops and Their Secondary Metabolites. International Journal of Molecular Sciences, 25(11), 6057. https://doi.org/10.3390/ijms25116057




