Antimicrobial Packaging for Plum Tomatoes Based on ZnO Modified Low-Density Polyethylene
Abstract
:1. Introduction
2. Results and Discussion
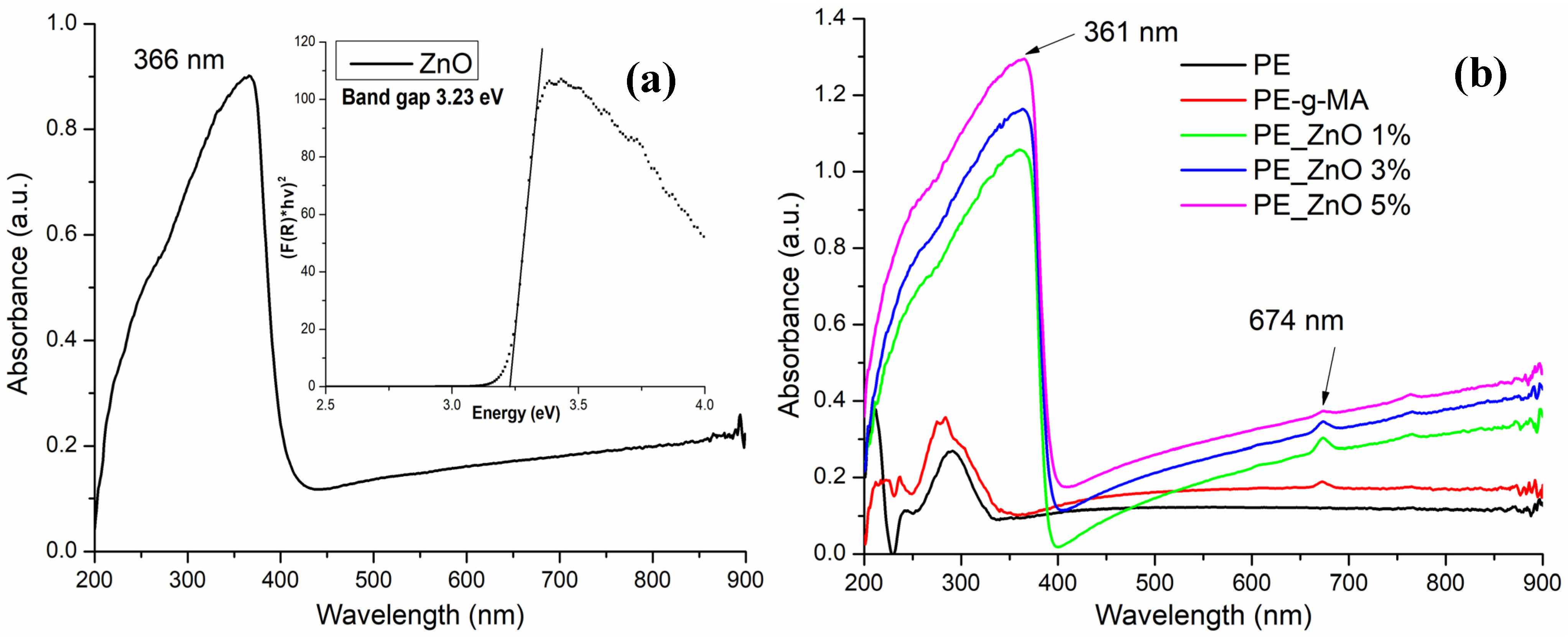
2.1. UV-Vis Spectroscopy
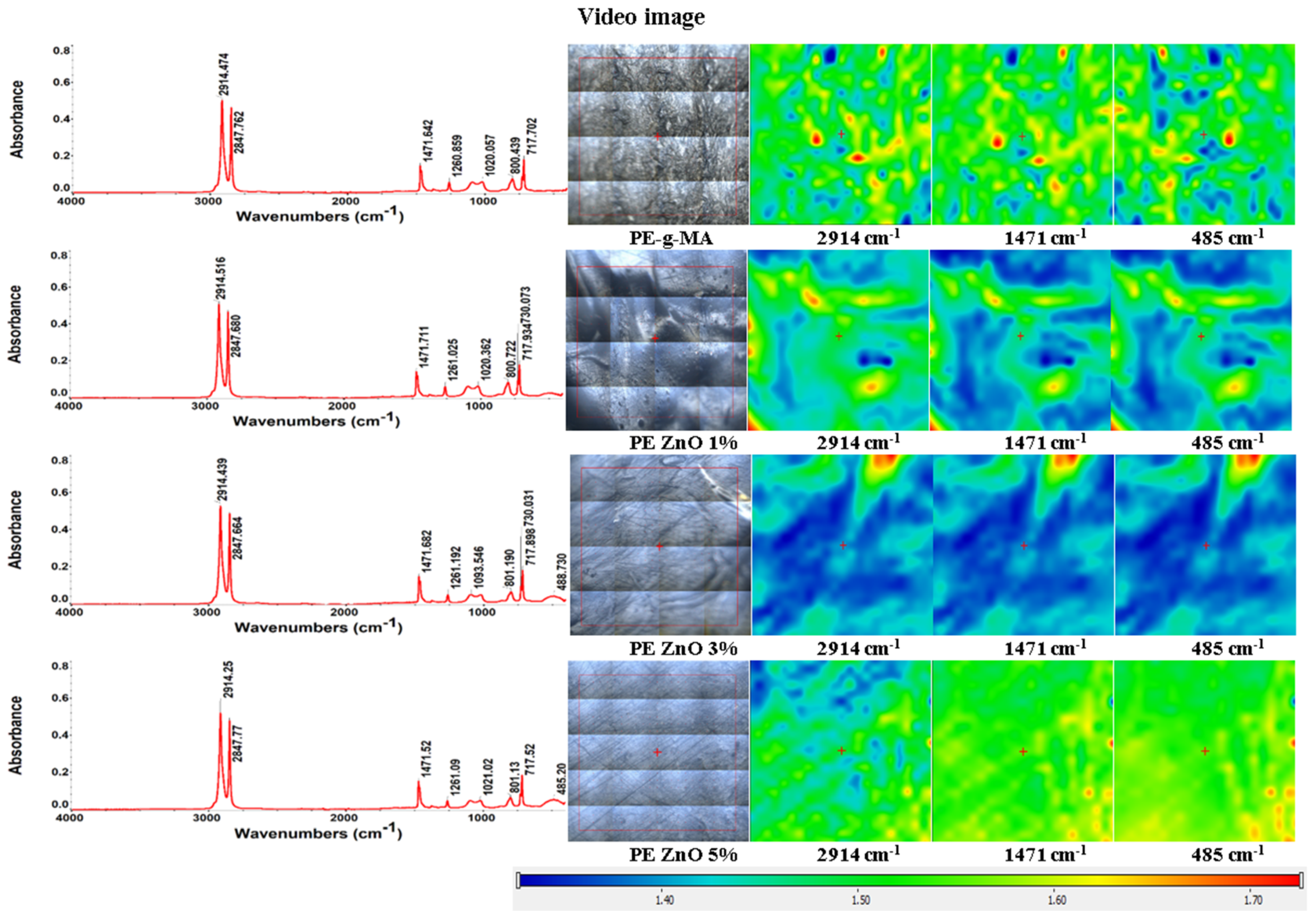
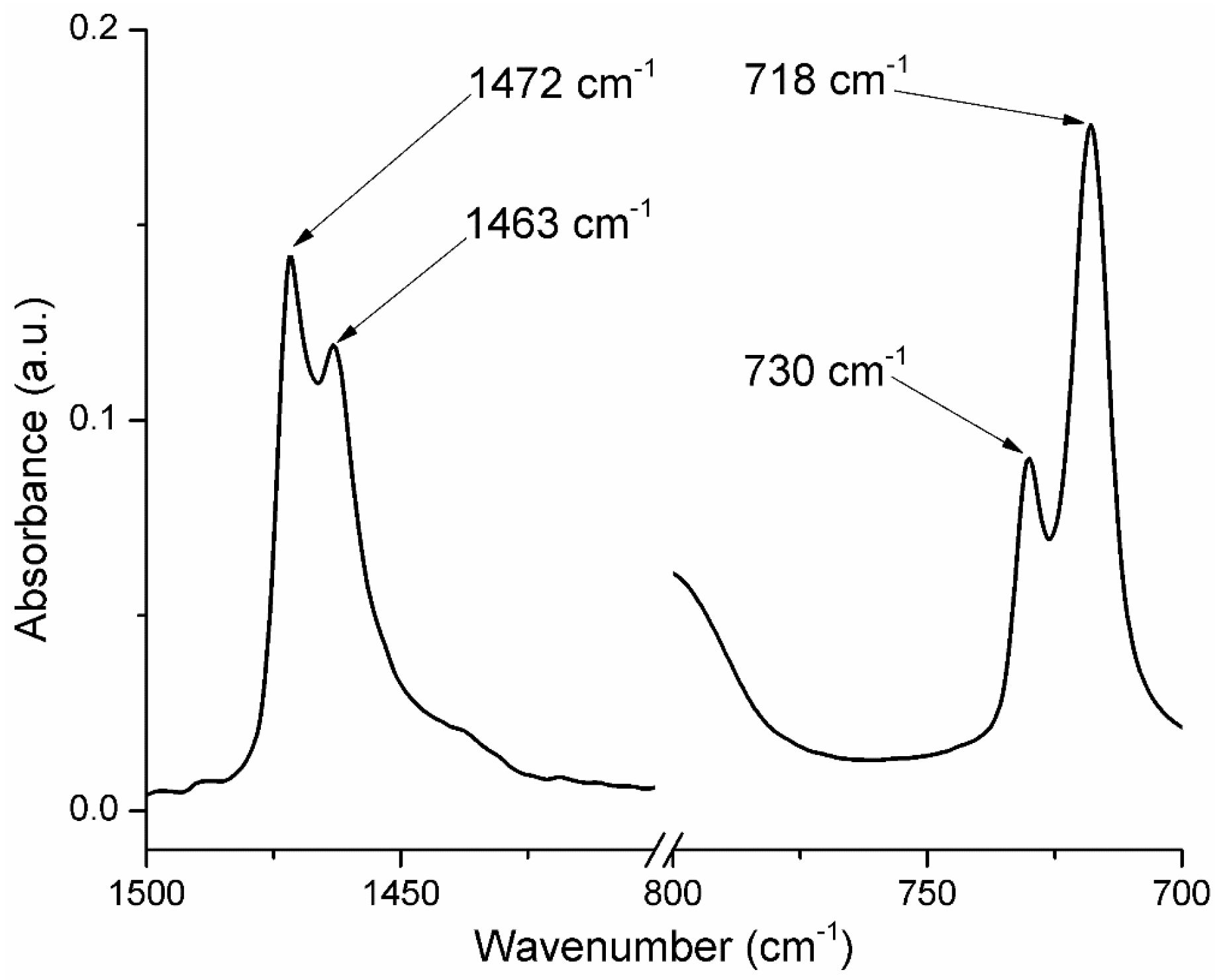
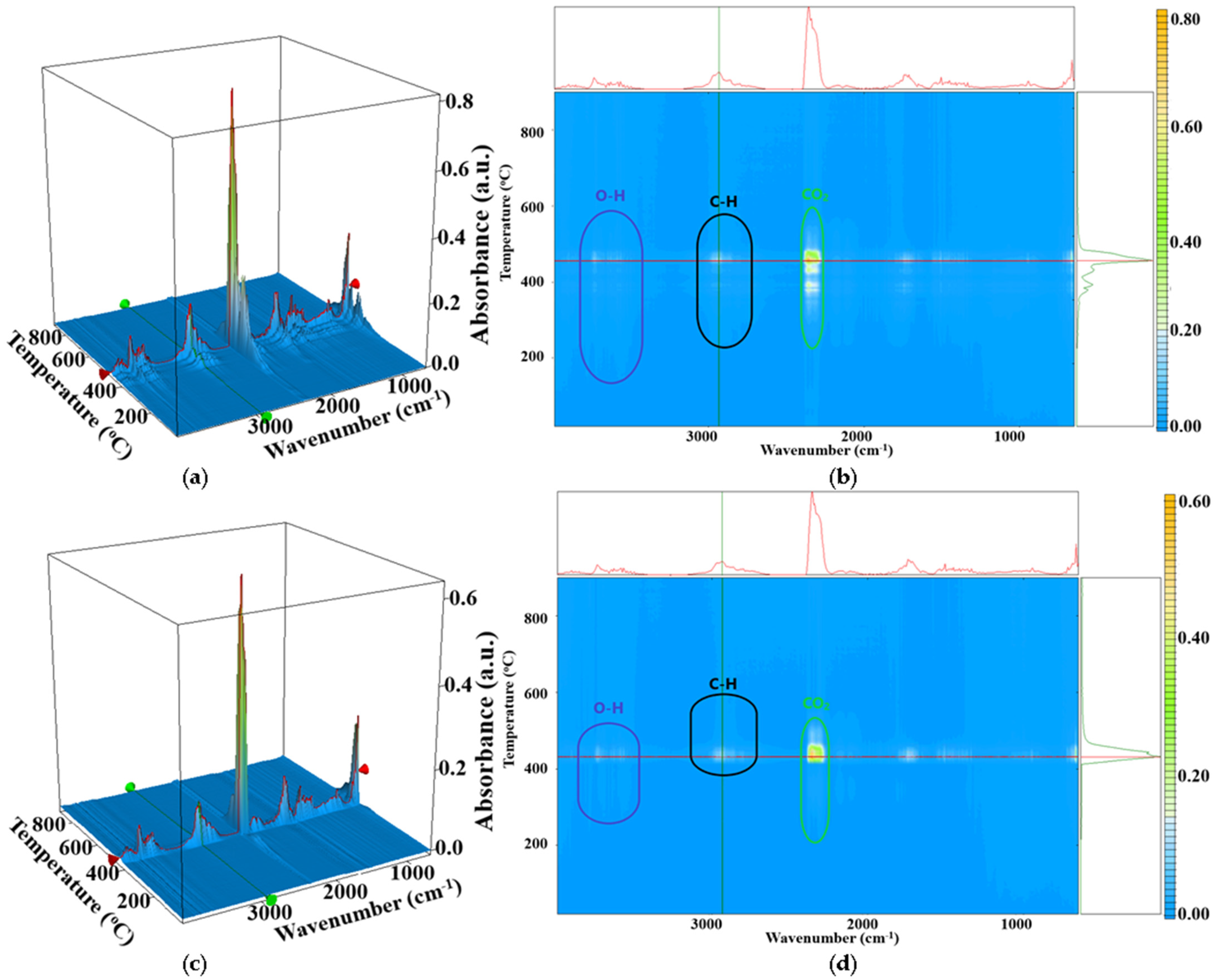
2.2. FT-IR Spectroscopy and Microscopy
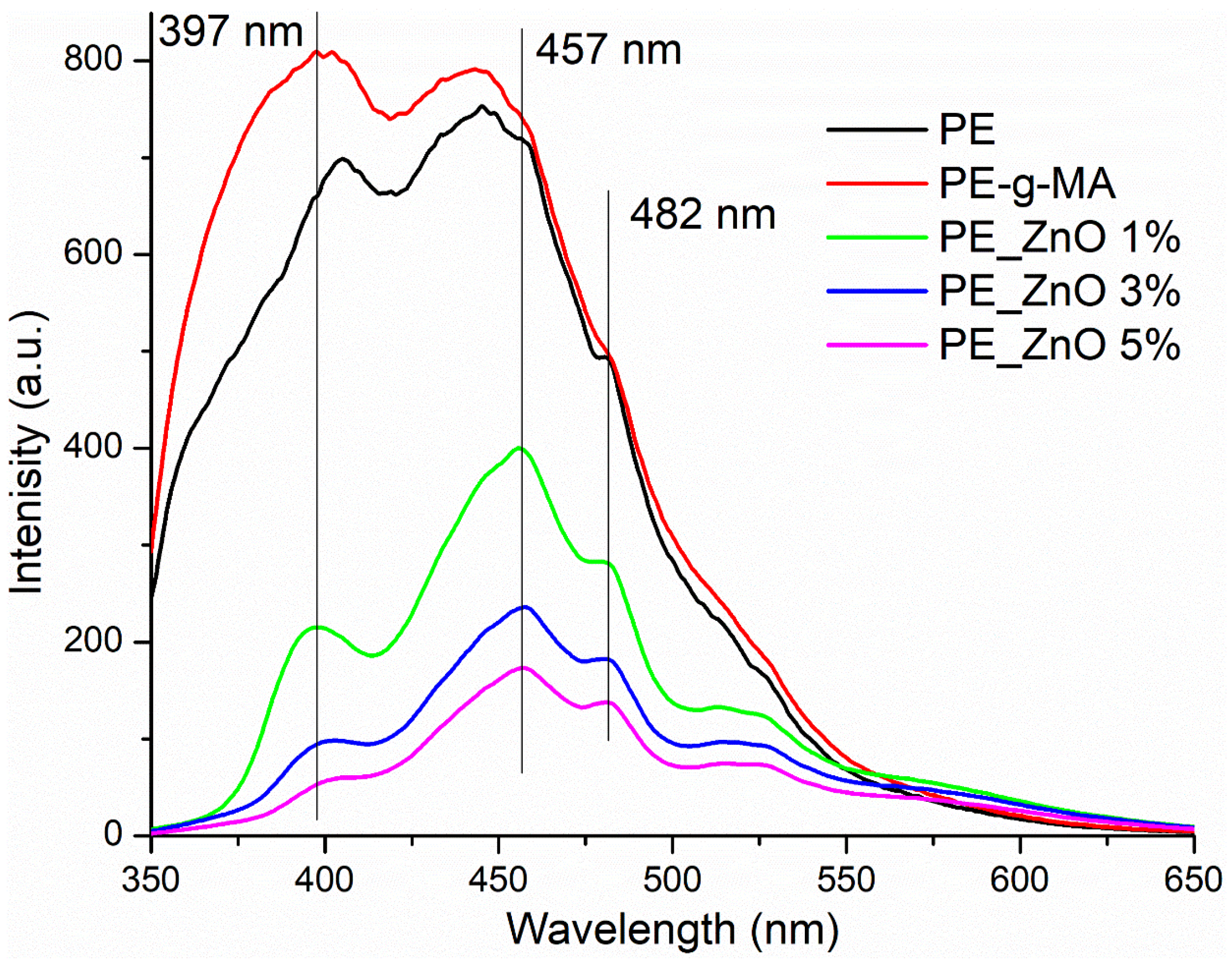
2.3. Photoluminescence Spectroscopy
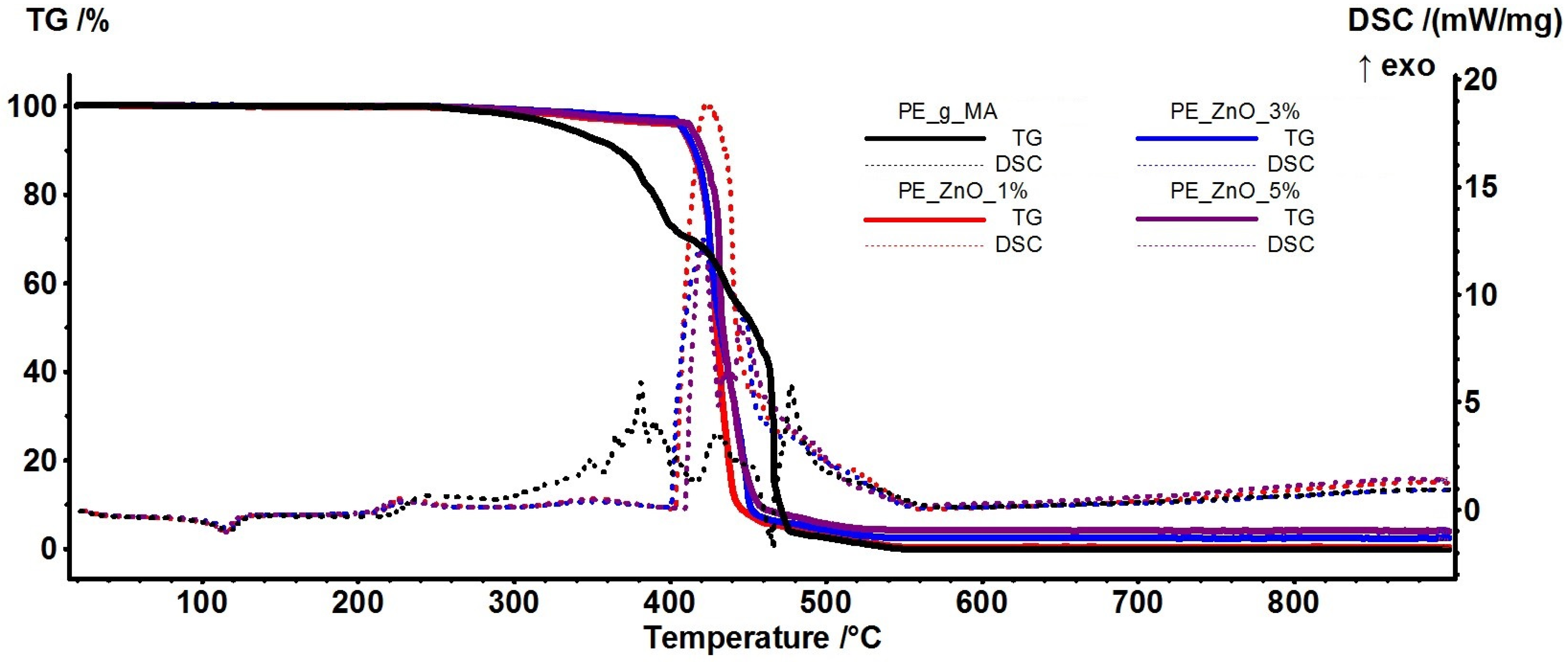
2.4. Thermal Analysis TG-DSC
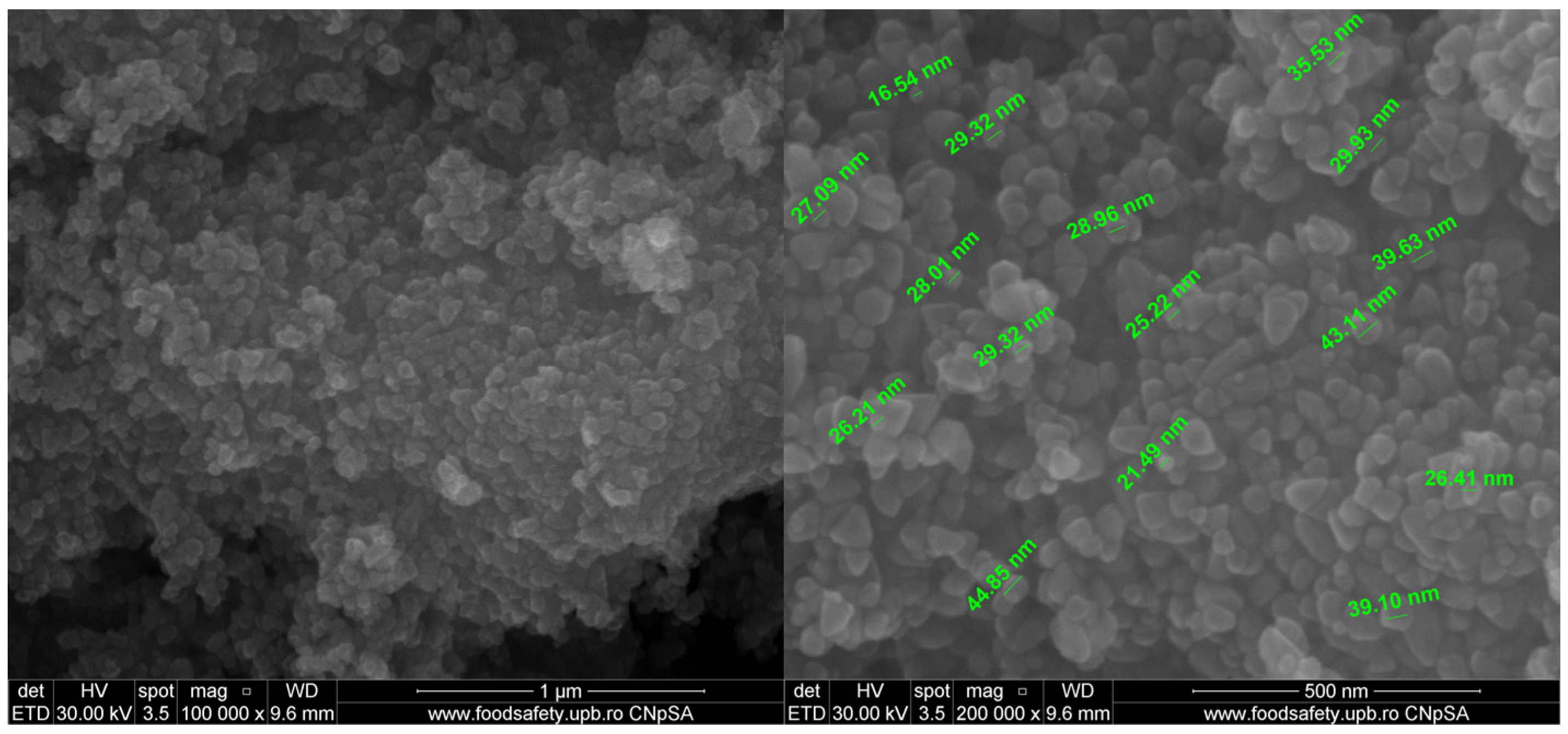
2.5. Scanning Electron Microscopy (SEM) Characterization
2.6. Mechanical and Barrier Properties
2.7. Antimicrobial Activity Assay
2.8. Preliminary Tests on Preserving Tomatoes
3. Experimental Section
3.1. Materials and Methods
3.1.1. Materials
3.1.2. Instrumental Analysis
3.1.3. Antimicrobial Assay
3.2. Synthesis of ZnO Nanoparticles
3.3. Synthesis of Polyethylene/ZnO Composite Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Andronescu, E. Smart Food Packaging Designed by Nanotechnological and Drug Delivery Approaches. Coatings 2020, 10, 806. [Google Scholar] [CrossRef]
- Radulescu, M.; Ficai, D.; Oprea, O.; Ficai, A.; Andronescu, E.; Holban, A.M. Antimicrobial Chitosan based Formulations with Impact on Different Biomedical Applications. Curr. Pharm. Biotechnol. 2015, 16, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Trusca, R.D.; Andronescu, E.; Holban, A. Biodegradable Alginate Films with ZnO Nanoparticles and Citronella Essential Oil—A Novel Antimicrobial Structure. Pharmaceutics 2021, 13, 1020. [Google Scholar] [CrossRef] [PubMed]
- Lemnaru (Popa), G.-M.; Trusca, R.D.; Ilie, C.-I.; Tiplea, R.D.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Ditu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef] [PubMed]
- Wilpiszewska, K.; Antosik, A.K.; Schmidt, B.; Janik, J.; Rokicka, J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers 2020, 12, 2447. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Yi, J.J.; Yu, X.M.; Wang, Z.Y.; Wang, L. Preparation and characterization of pullulan derivative/chitosan composite film for potential antimicrobial applications. Int. J. Biol. Macromol. 2020, 148, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Conte, A.; Longano, D.; Costa, C.; Ditaranto, N.; Ancona, A.; Cioffi, N.; Scrocco, C.; Sabbatini, L.; Conto, F.; Del Nobile, M.A. A novel preservation technique applied to fiordilatte cheese. Innov. Food Sci. Emerg. 2013, 19, 158–165. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.-S.; Ficai, A.; Surdu, V.-A.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Mustățea, G.; Ungureanu, E.L.; Dobre, A.A. Antibacterial activity of zinc oxide nanoparticles loaded with essential oils. Pharmaceutics 2023, 15, 2470. [Google Scholar] [CrossRef]
- Motelica, L.; Oprea, O.C.; Vasile, B.S.; Ficai, A.; Ficai, D.; Andronescu, E.; Holban, A.M. Antibacterial Activity of Solvothermal Obtained ZnO Nanoparticles with Different Morphology and Photocatalytic Activity against a Dye Mixture: Methylene Blue, Rhodamine B and Methyl Orange. Int. J. Mol. Sci. 2023, 24, 5677. [Google Scholar] [CrossRef]
- Nituica, M.; Oprea, O.; Stelescu, M.D.; Sonmez, M.; Georgescu, M.; Alexandrescu, L.; Motelica, L. Polymeric Biocomposite Based on Thermoplastic Polyurethane (TPU) and Protein and Elastomeric Waste Mixture. Materials 2023, 16, 5279. [Google Scholar] [CrossRef] [PubMed]
- Kuplennik, N.; Tchoudakov, R.; Zelas, Z.B.B.; Sadovski, A.; Fishman, A.; Narkis, M. Antimicrobial packaging based on linear low-density polyethylene compounded with potassium sorbate. LWT-Food Sci. Technol. 2015, 62, 278–286. [Google Scholar] [CrossRef]
- Charoensri, K.; Shin, Y.J.; Park, H.J. Innovative HDPE Composites Enriched with UV Stabilizer and Diatomaceous Earth/Zinc Oxide for Enhanced Seafood Packaging and Antimicrobial Properties. Polymers 2023, 15, 4577. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tenorio, Y.; Ramírez-Corona, N.; Jiménez-Munguia, M.T.; López-Malo, A.; Mani-López, E. Development of Antifungal Packaging From Low-Density Polyethylene With Essential Oil of Oregano and Potassium Sorbate. Packag. Technol. Sci. 2024; early view. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Trusca, R.D.; Ilie, C.I.; Oprea, O.C.; Andronescu, E. Innovative Antimicrobial Chitosan/ZnO/Ag NPs/Citronella Essential Oil Nanocomposite—Potential Coating for Grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Motelica, L.; Ficai, D.; Ilie, C.I.; Trusca, R.D.; Surdu, V.A.; Oprea, O.C.; Mirt, A.L.; Vasilievici, G.; Semenescu, A.; et al. Increasing Bioavailability of Trans-Ferulic Acid by Encapsulation in Functionalized Mesoporous Silica. Pharmaceutics 2023, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Fasihnia, S.H.; Khosrowshahi, N.K.; Gullón, B.; Lorenzo, J.M. Optimization of the Amount of ZnO, CuO, and Ag Nanoparticles on Antibacterial Properties of Low-Density Polyethylene (LDPE) Films Using the Response Surface Method. Food Anal. Methods 2021, 14, 98–107. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Duan, G.G.; Zhang, G.Y.; Yang, H.Q.; He, S.J.; Jiang, S.H. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Xing, Y.G.; Li, X.L.; Guo, X.L.; Li, W.X.; Chen, J.W.; Liu, Q.; Xu, Q.L.; Wang, Q.; Yang, H.; Shui, Y.R.; et al. Effects of Different TiO2 Nanoparticles Concentrations on the Physical and Antibacterial Activities of Chitosan-Based Coating Film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Viktorova, J.; Stupak, M.; Rehorova, K.; Dobiasova, S.; Hoang, L.; Hajslova, J.; Thanh, T.V.; Tri, L.V.; Tuan, N.V.; Ruml, T. Lemon Grass Essential Oil does not Modulate Cancer Cells Multidrug Resistance by Citral-Its Dominant and Strongly Antimicrobial Compound. Foods 2020, 9, 585. [Google Scholar] [CrossRef]
- Fleancu, M.C.; Olteanu, N.L.; Rogozea, A.E.; Crisciu, A.V.; Pincovschi, I.; Mihaly, M. Physical-chemical parameters promoting phase changes in non-ionic environmental-friendly microemulsions. Fluid. Phase Equilibr 2013, 337, 18–25. [Google Scholar] [CrossRef]
- Sonseca, A.; Madani, S.; Rodriguez, G.; Hevilla, V.; Echeverria, C.; Fernandez-Garcia, M.; Munoz-Bonilla, A.; Charef, N.; Lopez, D. Multifunctional PLA Blends Containing Chitosan Mediated Silver Nanoparticles: Thermal, Mechanical, Antibacterial, and Degradation Properties. Nanomaterials 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Patel, S.K.S.; Rasool, K.; Lone, N.; Bhatia, S.K.; Seth, C.S.; Ghodake, G.S. Bioinspired silver nanoparticle-based nanocomposites for effective control of plant pathogens: A review. Sci. Total Environ. 2024, 908, 168318. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Ene, V.L.; Vasile, B.S.; Andronescu, E.; Holban, A.M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil-Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Senturk Parreidt, T.; Muller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc oxide nanoparticles in meat packaging: A systematic review of recent literature. Food Packag. Shelf 2023, 36, 101045. [Google Scholar] [CrossRef]
- Bolognesi, C.; Castle, L.; Cravedi, J.P.; Engel, K.H.; Fowler, P.; Grob, K.; Gürtler, R.; Husoy, T.; Mennes, W.; Milana, M.R.; et al. Scientific Opinion on the safety evaluation of the substance zinc oxide, nanoparticles, uncoated and coated with [3-(methacryloxy) propyl] trimethoxysilane, for use in food contact materials. EFSA J. 2015, 13, 4063. [Google Scholar] [CrossRef]
- Bolognesi, C.; Castle, L.; Cravedi, J.P.; Engel, K.H.; Franz, R.; Fowler, P.; Grob, K.; Gürtler, R.; Husoy, T.; Kärenlampi, S.; et al. Safety assessment of the substance zinc oxide, nanoparticles, for use in food contact materials. EFSA J. 2016, 14, 4408. [Google Scholar] [CrossRef]
- Pavoski, G.; Kalikoski, R.; Souza, G.; Baldisserotto, D.; Brum, L.F.W.; dos Santos, C.; Bergmann, C.; Brandelli, A.; Jacobi, M.; Galland, G. Antimicrobial High-Density Polyethylene (HDPE)/ZnO Nanocomposites Obtained by Polymerization. J. Braz. Chem. Soc. 2020, 31, 1566–1574. [Google Scholar] [CrossRef]
- Dumbrava, A.; Berger, D.; Matei, C.; Prodan, G.; Aonofriesei, F.; Radu, M.D.; Moscalu, F. New Composite Nanomaterials with Antimicrobial and Photocatalytic Properties Based on Silver and Zinc Oxide. J. Inorg. Organomet. Polym. Mater. 2019, 29, 2072–2082. [Google Scholar] [CrossRef]
- Lungu, I.I.; Holban, A.M.; Ficai, A.; Grumezescu, A.M. Zinc Oxide Nanostrucures: New Trends in Antimicrobial Therapy. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 503–514. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef]
- Gao, W.; Li, Y.F.; Zhao, J.T.; Zhang, Z.; Tang, W.W. Influence of surface modification of zinc oxide on physical properties of high density polyethylene. Colloid Surf. A 2022, 653, 130000. [Google Scholar] [CrossRef]
- Emamifar, A.; Mohammadizadeh, M. Preparation and Application of LDPE/ZnO Nanocomposites for Extending Shelf Life of Fresh Strawberries. Food Technol. Biotechnol. 2015, 53, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Alim, A.A.A.; Baharum, A.; Shirajuddin, S.S.M.; Anuar, F.H. Blending of Low-Density Polyethylene and Poly(Butylene Succinate) (LDPE/PBS) with Polyethylene-Graft-Maleic Anhydride (PE-g-MA) as a Compatibilizer on the Phase Morphology, Mechanical and Thermal Properties. Polymers 2023, 15, 261. [Google Scholar] [CrossRef] [PubMed]
- Stelescu, M.D.; Oprea, O.C.; Motelica, L.; Ficai, A.; Trusca, R.D.; Sonmez, M.; Nituica, M.; Georgescu, M. Obtaining and Characterizing New Types of Materials Based on Low-Density Polyethylene and Thermoplastic Starch. J. Compos. Sci. 2024, 8, 134. [Google Scholar] [CrossRef]
- Yu, D.R.; Liu, S.Q.; Xin, Y. Air Plasma-Nano ZnO Coating Improves the Impact Resistance of Ultra-High Molecular Weight Polyethylene Fiber. Compos. Interface 2023, 30, 1247–1267. [Google Scholar] [CrossRef]
- Agbozouhoue, K.K.; Koffi, D.; Erchiqui, F.; Barnabé, S. ZnO Treatment on Mechanical Behavior of Polyethylene/Yellow Birch Fiber Composites When Exposed to Fungal Wood Rot. Polymers 2023, 15, 3664. [Google Scholar] [CrossRef] [PubMed]
- Barabaszová, K.C.; Holesová, S.; Hundáková, M.; Kalendová, A. Tribo-Mechanical Properties of the Antimicrobial Low-Density Polyethylene (LDPE) Nanocomposite with Hybrid ZnO-Vermiculite-Chlorhexidine Nanofillers. Polymers 2020, 12, 2811. [Google Scholar] [CrossRef]
- Alsayed, Z.; Awad, R.; Badawi, M.S. Thermo-mechanical properties of high density polyethylene with zinc oxide as a filler. Iran. Polym. J. 2020, 29, 309–320. [Google Scholar] [CrossRef]
- Vahidi, G.; Bajwa, D.S.; Shojaeiarani, J.; Stark, N.M. Experimental investigation into the direct feeding of coupling agent, cellulose nanocrystals, and nano zinc oxide in high-density polyethylene. Compos. Part. C-Open 2022, 8, 100287. [Google Scholar] [CrossRef]
- Yao, Y.L.; De Guzman, M.R.; Duan, H.; Gao, C.; Lin, X.; Wen, Y.H.; Du, J.; Lin, L.; Chen, J.C.; Wu, C.S.; et al. Infusing High-density Polyethylene with Graphene-Zinc Oxide to Produce Antibacterial Nanocomposites with Improved Properties. Chin. J. Polym. Sci. 2020, 38, 898–907. [Google Scholar] [CrossRef]
- Mwafy, E.A.; Abd-Elmgeed, A.A.; Kandil, A.A.; Elsabbagh, I.A.; Elfass, M.M.; Gaafar, M.S. High UV-shielding Performance of Zinc Oxide/High-Density Polyethylene Nanocomposites. Spectrosc. Lett. 2015, 48, 646–652. [Google Scholar] [CrossRef]
- Fiedot-Tobola, M.; Ciesielska, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Wozniak, P.; Teterycz, H.; Bryjak, M. Deposition of Zinc Oxide on Different Polymer Textiles and Their Antibacterial Properties. Materials 2018, 11, 707. [Google Scholar] [CrossRef]
- Vatanpour, V.; Nekouhi, G.N.; Esmaeili, M. Preparation, characterization and performance evaluation of ZnO deposited polyethylene ultrafiltration membranes for dye and protein separation. J. Taiwan Inst. Chem. Eng. 2020, 114, 153–167. [Google Scholar] [CrossRef]
- Faraj, M.G.; Ibrahim, K.; Eisa, M.H.; Pakhuruddin, M.K.M.; Pakhuruddin, M.Z. Comparison of Zinc Oxide thin films deposited on the glass and polyethylene terephthalate substrates by thermal evaporation technique for applications in solar cells. Optoelectron. Adv. Mater. 2010, 4, 1587–1590. [Google Scholar]
- Alluhaibi, A.A.; Sendi, R.K. Effect of Plasticisers and Zinc Oxide Nanoparticle Concentration on the Properties of Polyethylene Thin Films. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1269, 012002. [Google Scholar] [CrossRef]
- Zhu, K.X.; Wang, H.D.; Chen, C.W.; Xie, J. Development of polyethylene antifogging and antibacterial packaging films for lettuce preservation. LWT-Food Sci. Technol. 2023, 181, 114772. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Liu, L.Z.; Wang, Y.X.; Song, L.X.; Shi, Y. Effect of nanometer zinc oxide and processing technology on the properties of antibacterial composites. Polym. Bull. 2023, 81, 7193–7210. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Stefan, A.; Sonmez, M.; Nituica, M.; Georgescu, M. Elasto-Plastic Materials Based on EPDM Rubber, LDPE, Plasticized Starch and OMMT. Mater. Plast. 2024, 61, 131–140. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M.; Popov, A.I. A few common misconceptions in the interpretation of experimental spectroscopic data. Opt. Mater. 2022, 127, 112276. [Google Scholar] [CrossRef]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.M. Optimized Synthesis Approaches of Metal Nanoparticles with Antimicrobial Applications. J. Nanomater. 2020, 2020, 6651207. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using leaf extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1751–1761. [Google Scholar] [CrossRef]
- Chaurasia, V.; Chand, N.; Bajpai, S.K. Water Sorption Properties and Antimicrobial Action of Zinc Oxide Nanoparticles-Loaded Cellulose Acetate Films. J. Macromol. Sci. A 2010, 47, 309–317. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.V.B. Novel microbial route to synthesize ZnO nanoparticles using and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Huang, X.; Zhang, B.N.; He, M.; Yin, G.Q.; Cui, Y.D. Preparation and characterization of a dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers II: Polyethylene. Spectroscopy 2021, 36, 24–29. [Google Scholar] [CrossRef]
- Depan, D.; Chirdon, W.; Khattab, A. Morphological and Chemical Analysis of Low-Density Polyethylene Crystallized on Carbon and Clay Nanofillers. Polymers 2021, 13, 1558. [Google Scholar] [CrossRef]
- Agosti, E.; Zerbi, G.; Ward, I.M. Structure of the Skin and Core of Ultradrawn Polyethylene Films by Vibrational Spectroscopy. Polymer 1992, 33, 4219–4229. [Google Scholar] [CrossRef]
- Painter, P.C.; Havens, J.; Hart, W.W.; Koenig, J.L. Fourier-Transform Ir Spectroscopic Investigation of Polyethylene Single-Crystals. II. Fine-Structure of CH2 Rocking Mode. J. Polym. Sci. Pol. Phys. 1977, 15, 1237–1249. [Google Scholar] [CrossRef]
- Huang, J.B.; Hong, J.W.; Urban, M.W. Attenuated Total Reflectance Fourier-Transform Infrared Studies of Crystalline Amorphous Content on Polyethylene Surfaces. Polymer 1992, 33, 5173–5178. [Google Scholar] [CrossRef]
- Fontana, L.; Santoro, M.; Bini, R.; Vinh, D.Q.; Scandolo, S. High-pressure vibrational properties of polyethylene. J. Chem. Phys. 2010, 133, 204502. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Popescu, A.; Razvan, A.G.; Oprea, O.; Trusca, R.D.; Vasile, B.S.; Dumitru, F.; Holban, A.M. Facile Use of ZnO Nanopowders to Protect Old Manual Paper Documents. Materials 2020, 13, 5452. [Google Scholar] [CrossRef] [PubMed]
- Ghifari, N.; Cinquin, B.; Chahboun, A.; El Abed, A.I. Rhodamine B Doped ZnO Monodisperse Microcapsules: Droplet-Based Synthesis, Dynamics and Self-Organization of ZnO Nanoparticles and Dye Molecules. Nanomaterials 2020, 10, 2351. [Google Scholar] [CrossRef] [PubMed]
- Pomastowski, P.; Krol-Gorniak, A.; Railean-Plugaru, V.; Buszewski, B. Zinc Oxide Nanocomposites-Extracellular Synthesis, Physicochemical Characterization and Antibacterial Potential. Materials 2020, 13, 4347. [Google Scholar] [CrossRef] [PubMed]
- Asamoah, R.B.; Yaya, A.; Nbelayim, P.; Annan, E.; Onwona-Agyeman, B. Development and Characterization of Clay-Nanocomposites for Water Purification. Materials 2020, 13, 3793. [Google Scholar] [CrossRef] [PubMed]
- Tachikawa, S.; Noguchi, A.; Tsuge, T.; Hara, M.; Odawara, O.; Wada, H. Optical Properties of ZnO Nanoparticles Capped with Polymers. Materials 2011, 4, 1132–1143. [Google Scholar] [CrossRef]
- Stanculescu, A.; Socol, M.; Rasoga, O.; Breazu, C.; Preda, N.; Stanculescu, F.; Socol, G.; Vacareanu, L.; Girtan, M.; Doroshkevich, A.S. Arylenevinylene Oligomer-Based Heterostructures on Flexible AZO Electrodes. Materials 2021, 14, 7688. [Google Scholar] [CrossRef]
- Charoensri, K.; Shin, Y.J.; Kim, K.C.; Park, H.J. Active Packaging Material Based on Immobilized Diatomaceous Earth/Zinc Oxide/High-Density Polyethylene Composite for Sea Food and Products. Polymers 2022, 14, 5228. [Google Scholar] [CrossRef]
- Poh, L.; Wu, Q.; Chen, Y.D.; Narimissa, E. Characterization of industrial low-density polyethylene: A thermal, dynamic mechanical, and rheological investigation. Rheol. Acta 2022, 61, 701–720. [Google Scholar] [CrossRef]
- Wunderlich, B. (Ed.) Appendix—ATHAS Table of Thermal Properties of Linear Macromolecules; Thermal Analysis; Academic Press: London, UK, 1990; pp. 417–431. [Google Scholar]
- Naidu, D.S.; John, M.J. Effect of Clay Nanofillers on the Mechanical and Water Vapor Permeability Properties of Xylan-Alginate Films. Polymers 2020, 12, 2279. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial Mechanisms and Biomedical Application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Kang, S.; Park, D.H.; Hwang, J. Hierarchical ZnO nano-spines grown on a carbon fiber seed layer for efficient VOC removal and airborne virus and bacteria inactivation. J. Hazard. Mater. 2022, 424, 127262. [Google Scholar] [CrossRef]
- Yongabi, D.; Jooken, S.; Givanoudi, S.; Khorshid, M.; Deschaume, O.; Bartic, C.; Losada-Pérez, P.; Wübbenhorst, M.; Wagner, P. Ionic strength controls long-term cell-surface interactions—A QCM-D study of S. cerevisiae adhesion, retention and detachment. J. Colloid Interface Sci. 2021, 585, 583–595. [Google Scholar] [CrossRef]
- ISO 527; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2019. Available online: https://standards.iteh.ai/catalog/standards/sist/926d5ea4-e0ad-4a58-b318-520f53bb3c85/iso-527-1-2019 (accessed on 5 April 2024).
- ISO 15106-1; Plastics—Film and Sheeting—Determination of Water Vapour Transmission Rate. Part 1: Humidity Detection Sensor Method. ISO: Geneva, Switzerland, 2003. Available online: https://standards.iteh.ai/catalog/standards/sist/c15de716-9989-4c60-ba8a-3efcf6bda179/iso-15106-1-2003 (accessed on 5 April 2024).
- ISO 2556:2000; Plastics-Determination of the Gas Transmission Rate of Films and Thin Sheets under Atmospheric Pressure-Manometric Method. ISO: Geneva, Switzerland, 2000. Available online: https://standards.iteh.ai/catalog/standards/cen/7d33cd43-472d-45aa-87af-206abdd15656/en-iso-2556-2000 (accessed on 5 April 2024).
- Oprea, O.; Andronescu, E.; Vasile, B.S.; Voicu, G.; Covaliu, C. Synthesis and Characterization of Zno Nanopowder by Non-Basic Route. Dig. J. Nanomater. Biostruct. 2011, 6, 1393–1401. [Google Scholar]
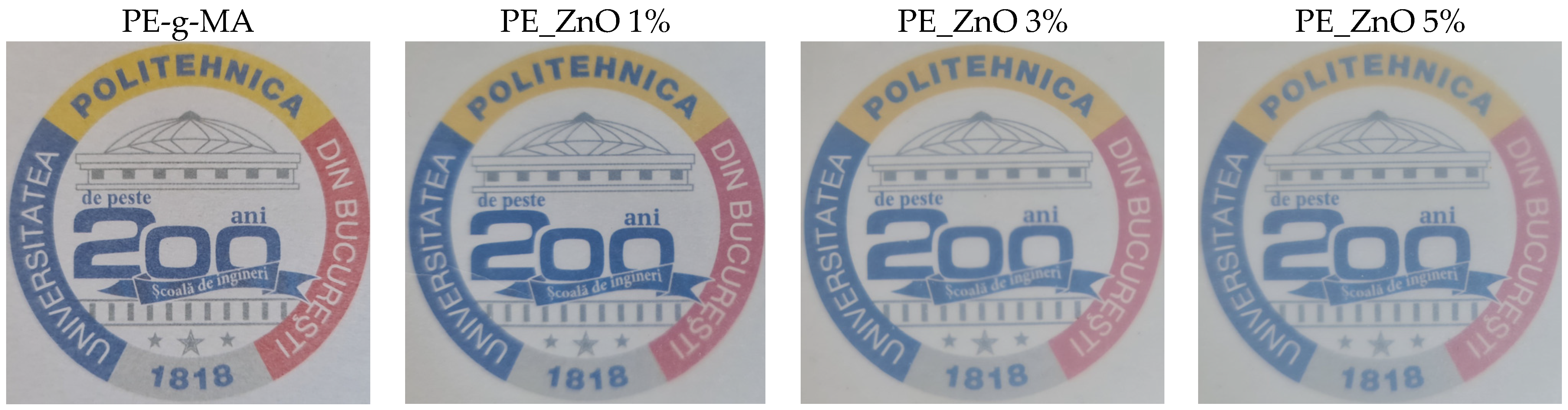
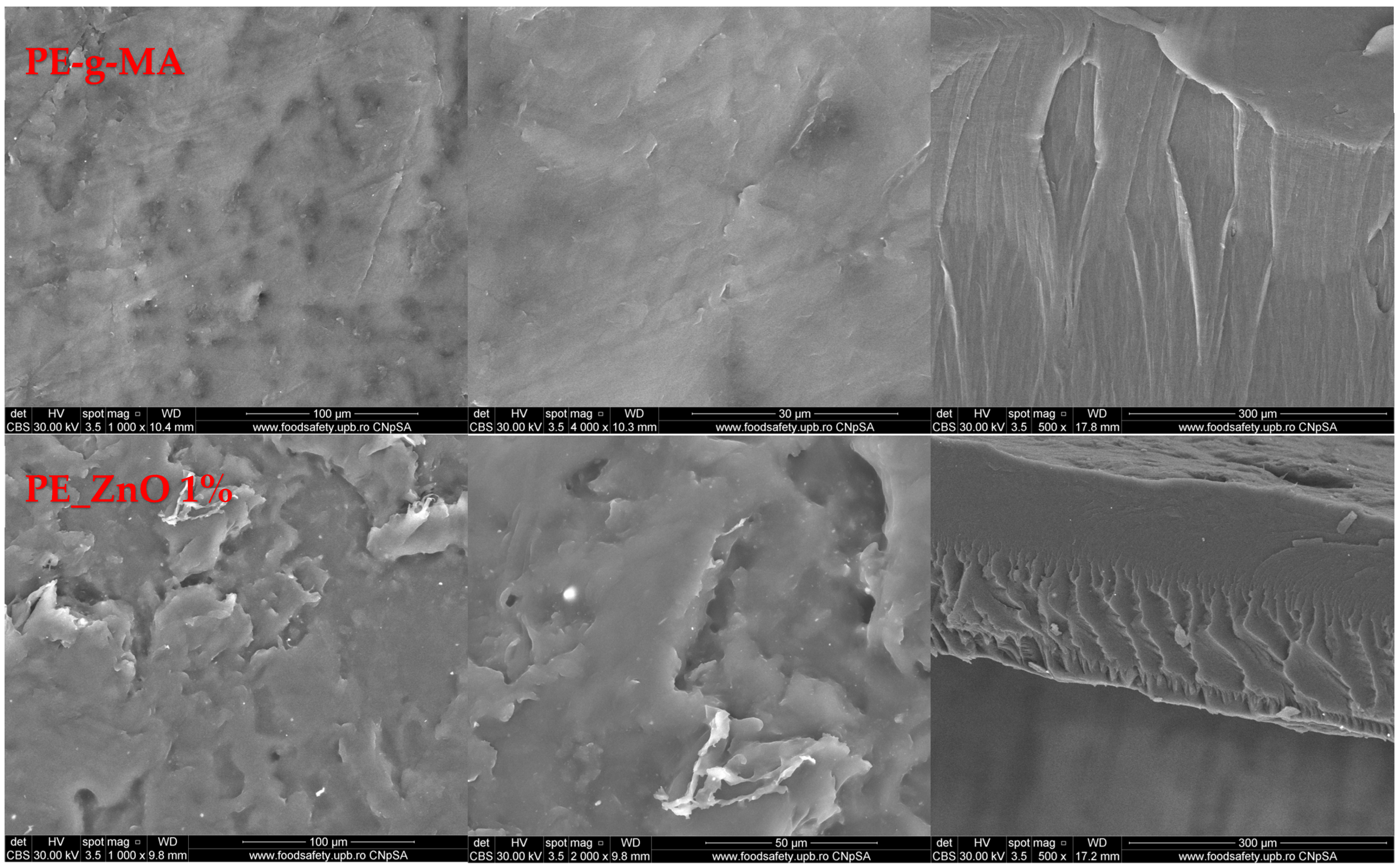
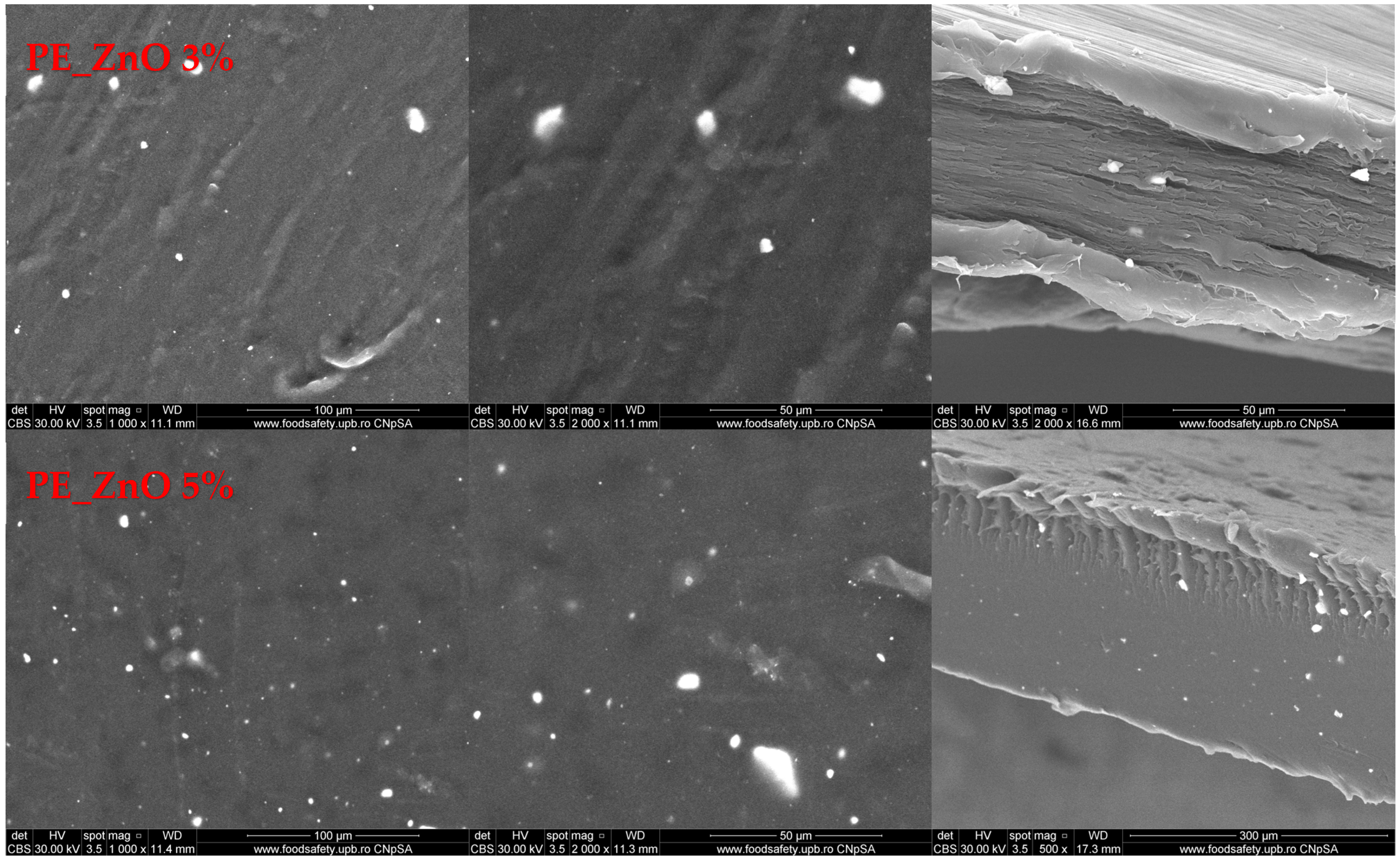

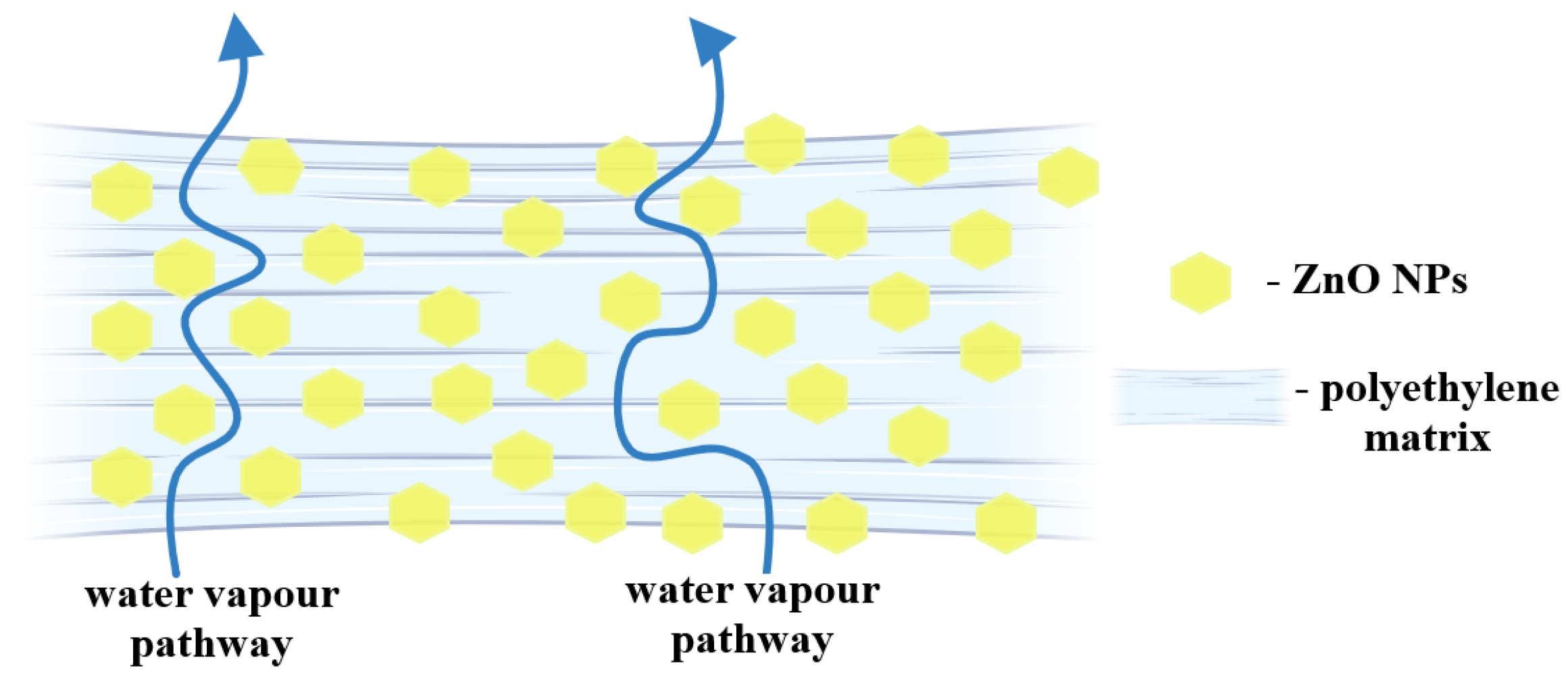
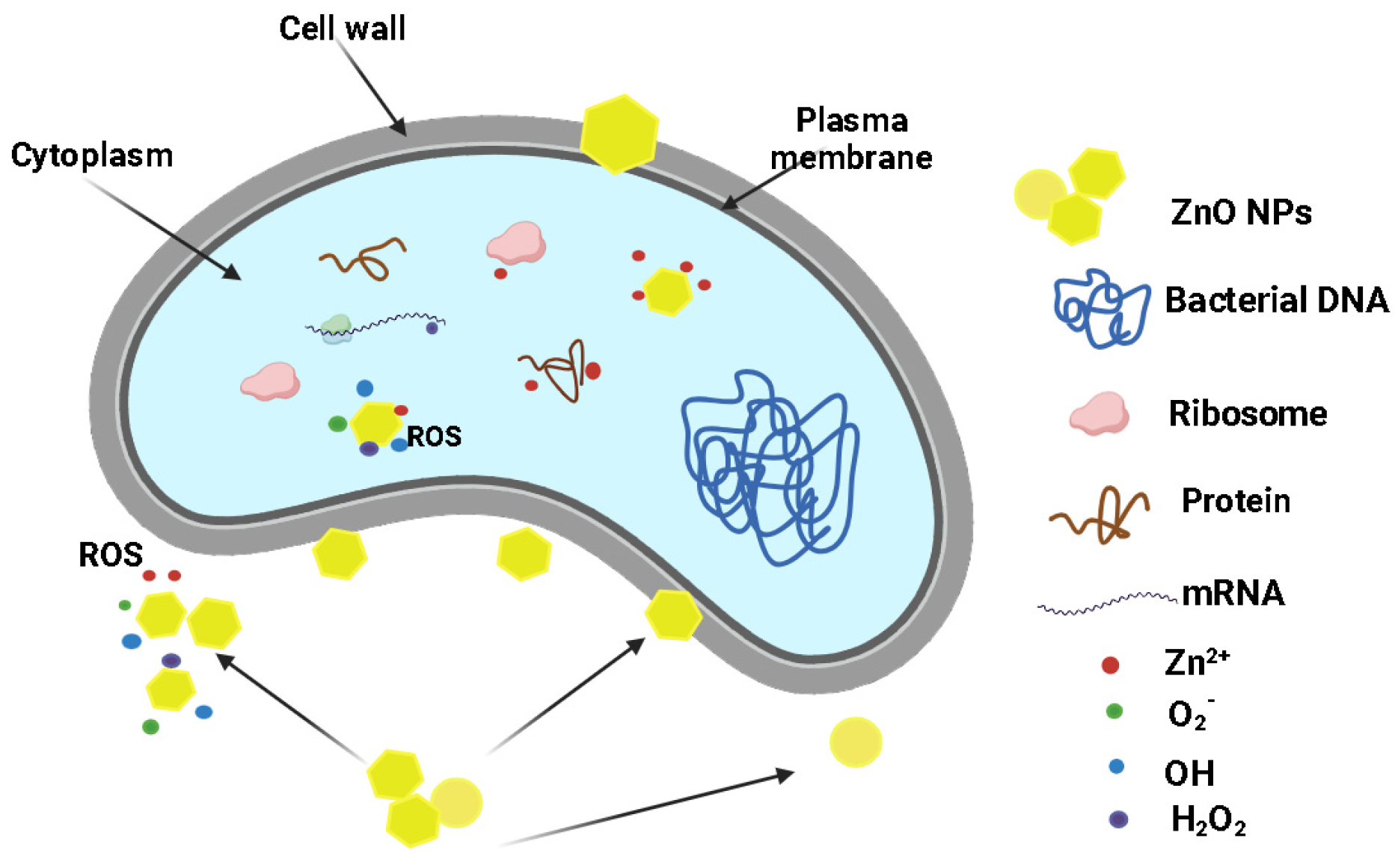

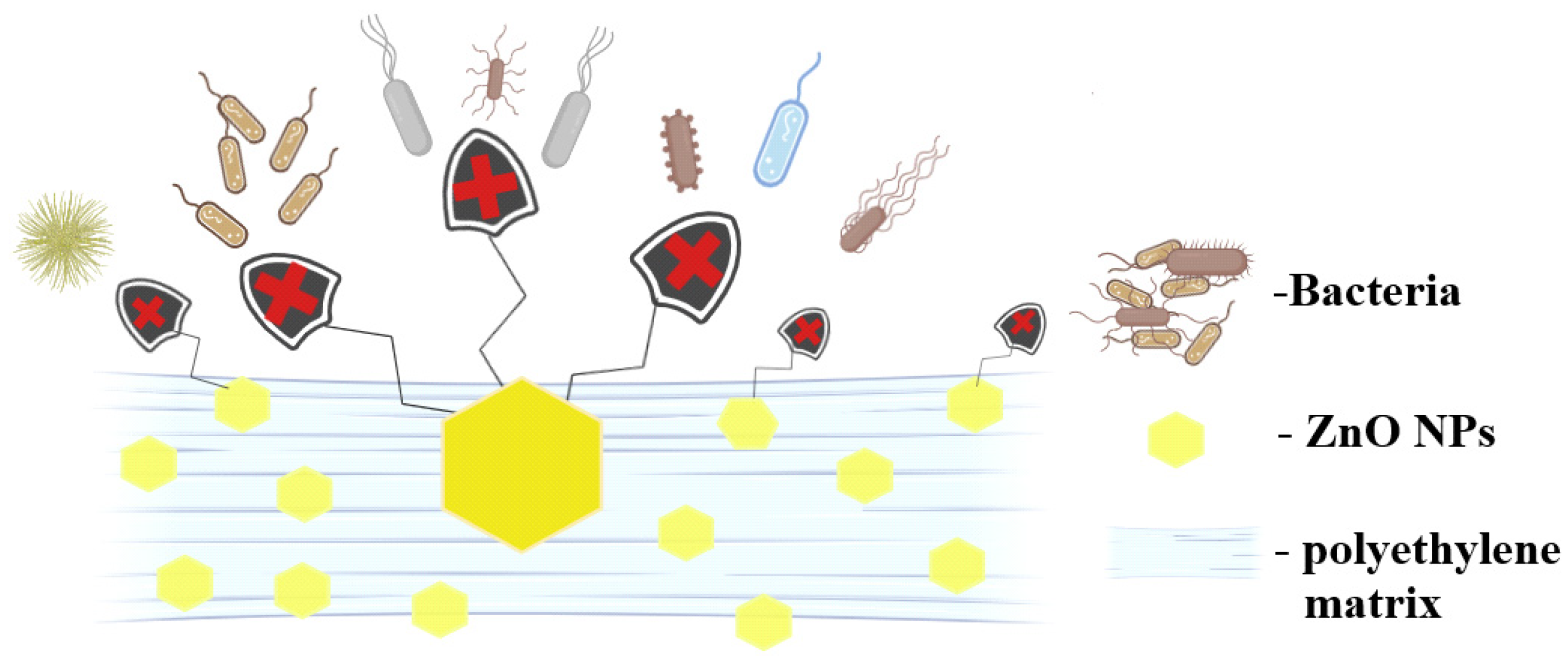

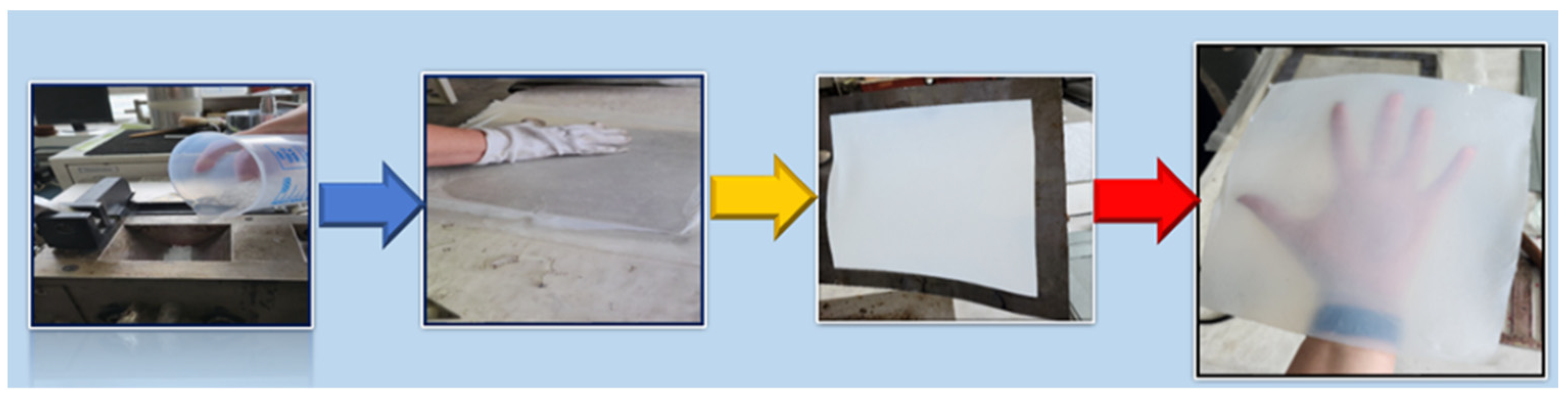
| Sample | PE | PE-g-MA | PE_ZnO 1% | PE_ZnO 3% | PE_ZnO 5% |
|---|---|---|---|---|---|
| Absorbance (A600 nm) | 0.121 | 0.171 | 0.224 | 0.278 | 0.323 |
| Thickness (mm) | 0.31 ± 0.01 | 0.36 ± 0.06 | 0.29 ± 0.01 | 0.30 ± 0.02 | 0.27 ± 0.02 |
| Opacity | 0.39 | 0.47 | 0.77 | 0.92 | 1.19 |
| Group Frequency (cm−1) | Functional Group/Assignment |
|---|---|
| 2914 | methylene asymmetric stretch (from LDPE) |
| 2848 | methylene symmetric stretch (from LDPE) |
| 1472 | crystalline bending methylene (CH2) |
| 1463 | amorphous bending methylene (CH2) |
| 729–730 | crystalline rock methylene (CH2) |
| 717–718 | amorphous rock methylene (CH2) |
| 484–488 | Zn-O bond |
| Sample | T5% (°C) | T10% (°C) | T15% (°C) | Mass Loss (%) RT-300 °C | Tonset (°C) (melting) | Melting Peak (°C) | Mass loss (%) 300–400 °C | ΔHmelting (J/g) | Crystallinity Degree (%) |
|---|---|---|---|---|---|---|---|---|---|
| PE-g-MA | 334.0 | 366.5 | 380.3 | 2.06% | 108.6 | 117.0 | 24.87 | 88.08 | 30.06 |
| PE_ZnO 1% | 407.5 | 414.6 | 419.0 | 1.19% | 106.1 | 115.5 | 2.69 | 87.93 | 30.01 |
| PE_ZnO 3% | 407.9 | 415.7 | 419.9 | 1.00% | 106.8 | 117.4 | 2.01 | 80.61 | 27.51 |
| PE_ZnO 5% | 413.6 | 420.7 | 425.3 | 1.24% | 106.8 | 114.3 | 2.68 | 81.73 | 27.89 |
| Sample | PE-g-MA | PE_ZnO 1% | PE_ZnO 3% | PE_ZnO 5% |
|---|---|---|---|---|
| Thickness (mm) | 0.36 ± 0.06 | 0.29 ± 0.01 | 0.30 ± 0.02 | 0.27 ± 0.02 |
| Elongation at break (%) | 32.5 ± 2.9 | 38.0 ± 1.6 | 46.1 ± 3.2 | 14.6 ± 1.5 |
| Tensile strength (MPa) | 15.2 ± 0.33 | 17.1 ± 0.33 | 18.9 ± 0.16 | 15.5 ± 0.16 |
| O2 permeability (cm3/m2·bar·day) | 65.00 ± 0.02 | 63.02 ± 0.01 | 49.56 ± 0.02 | 36.78 ± 0.02 |
| H2O vapor permeability (g/m2·day) | 0.280 ± 0.007 | 0.125 ± 0.005 | 0.091 ± 0.002 | 0.088 ± 0.002 |
| Sample/ Strain | PE_ZnO 1% | PE_ZnO 3% | PE_ZnO 5% |
|---|---|---|---|
| S. aureus | 9 ± 1.0 | 10 ± 1.0 | 11 ± 1.0 |
| E. coli | 10 ± 1.0 | 10 ± 1.0 | 12 ± 1.0 |
| Sample | Weight Loss (%) | |||
|---|---|---|---|---|
| 1 Day | 3 Days | 10 Days | 14 Days | |
| PE-g-MA | 0.31 ± 0.05 | 0.96 ± 0.12 | 3.71 ± 0.16 | 6.10 ± 0.25 |
| PE_ZnO 1% | 0.11 ± 0.03 | 0.23 ± 0.07 | 0.91 ± 0.11 | 1.74 ± 0.16 |
| PE_ZnO 3% | 0.07 ± 0.02 | 0.16 ± 0.03 | 0.70 ± 0.11 | 1.46 ± 0.11 |
| PE_ZnO 5% | 0.03 ± 0.01 | 0.11 ± 0.03 | 0.31 ± 0.07 | 0.82 ± 0.06 |
| Sample Code | LDPE (g) | LDPE-g-MA | ZnO NPs (g) | Glycerol (g) |
|---|---|---|---|---|
| PE | 248 | 0 | 0 | 2 |
| PE-g-MA | 238 | 10 | 0 | 2 |
| PE_ZnO 1% | 235.5 | 10 | 2.5 | 2 |
| PE_ZnO 3% | 230.5 | 10 | 7.5 | 2 |
| PE_ZnO 5% | 225.5 | 10 | 12.5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motelica, L.; Ficai, D.; Oprea, O.-C.; Trusca, R.-D.; Ficai, A.; Stelescu, M.D.; Sonmez, M.; Nituica, M.; Mustatea, G.; Holban, A.M. Antimicrobial Packaging for Plum Tomatoes Based on ZnO Modified Low-Density Polyethylene. Int. J. Mol. Sci. 2024, 25, 6073. https://doi.org/10.3390/ijms25116073
Motelica L, Ficai D, Oprea O-C, Trusca R-D, Ficai A, Stelescu MD, Sonmez M, Nituica M, Mustatea G, Holban AM. Antimicrobial Packaging for Plum Tomatoes Based on ZnO Modified Low-Density Polyethylene. International Journal of Molecular Sciences. 2024; 25(11):6073. https://doi.org/10.3390/ijms25116073
Chicago/Turabian StyleMotelica, Ludmila, Denisa Ficai, Ovidiu-Cristian Oprea, Roxana-Doina Trusca, Anton Ficai, Maria Daniela Stelescu, Maria Sonmez, Mihaela Nituica, Gabriel Mustatea, and Alina Maria Holban. 2024. "Antimicrobial Packaging for Plum Tomatoes Based on ZnO Modified Low-Density Polyethylene" International Journal of Molecular Sciences 25, no. 11: 6073. https://doi.org/10.3390/ijms25116073
APA StyleMotelica, L., Ficai, D., Oprea, O. -C., Trusca, R. -D., Ficai, A., Stelescu, M. D., Sonmez, M., Nituica, M., Mustatea, G., & Holban, A. M. (2024). Antimicrobial Packaging for Plum Tomatoes Based on ZnO Modified Low-Density Polyethylene. International Journal of Molecular Sciences, 25(11), 6073. https://doi.org/10.3390/ijms25116073












