Development of a Whole-Cell System Based on the Use of Genetically Modified Protoplasts to Detect Nickel Ions in Food Matrices
Abstract
1. Introduction
2. Results
2.1. Protoplasts Transformation and Immobilization
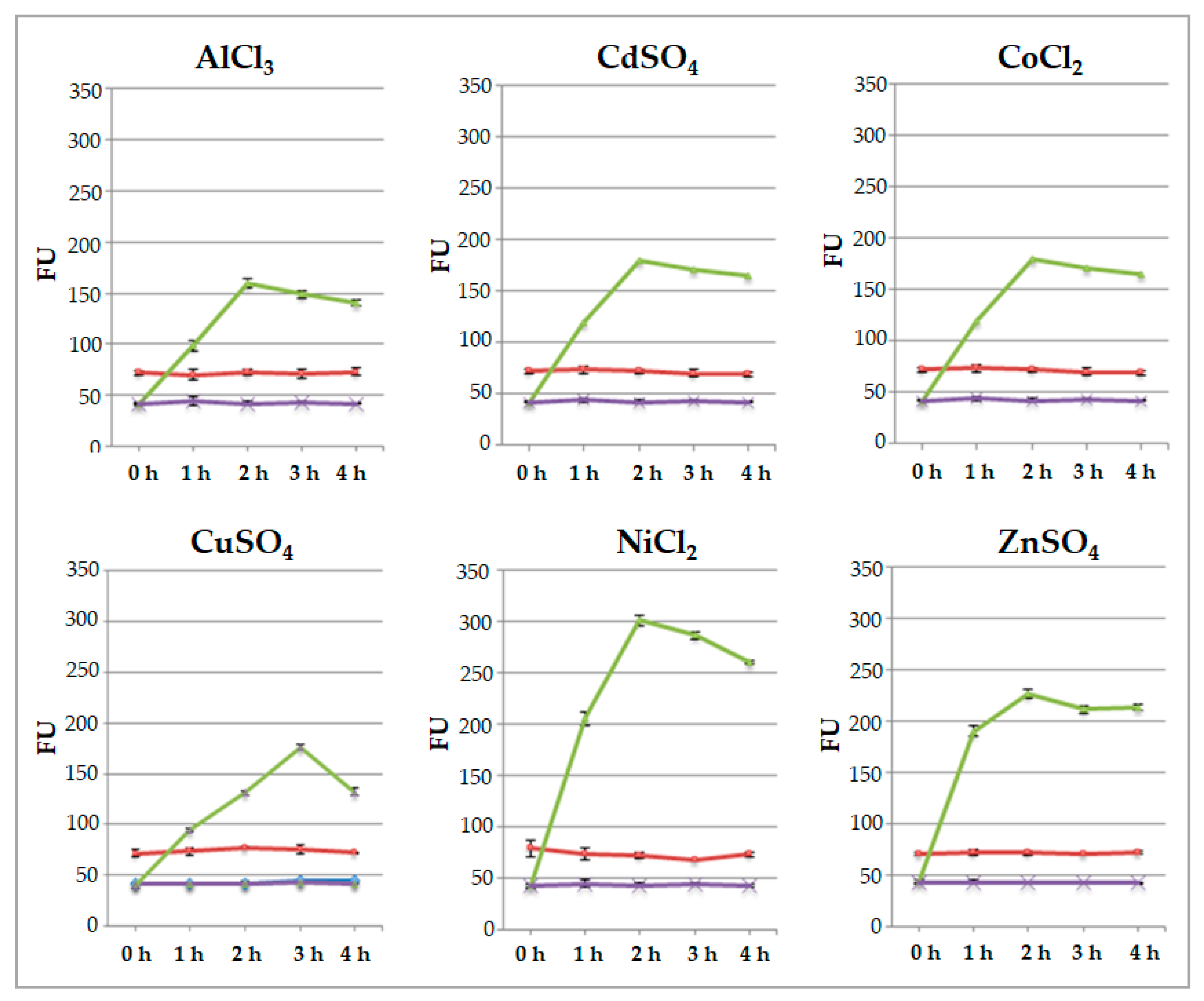
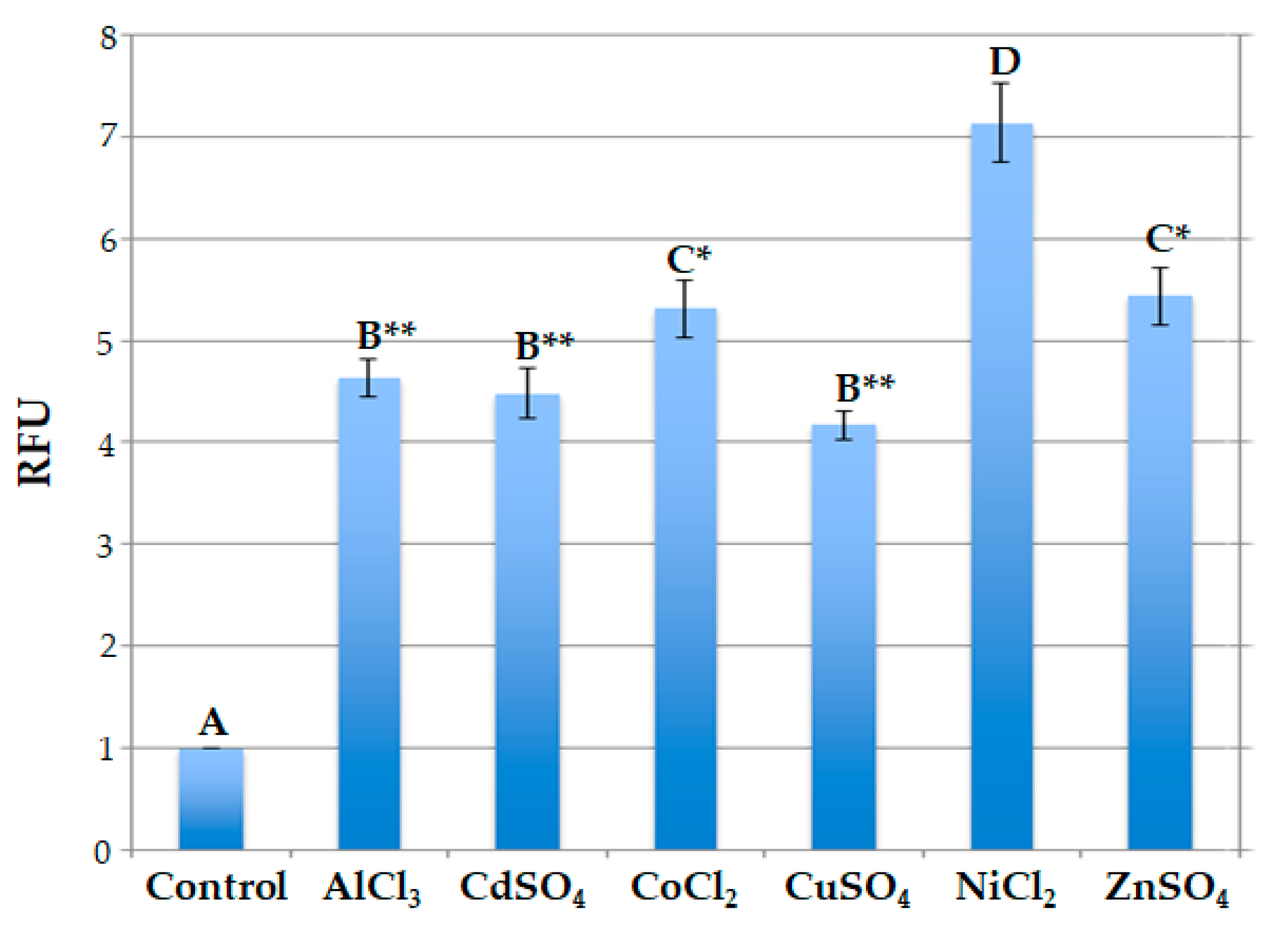
2.2. Responsiveness of Engineered Protoplast to Heavy Metal Ions
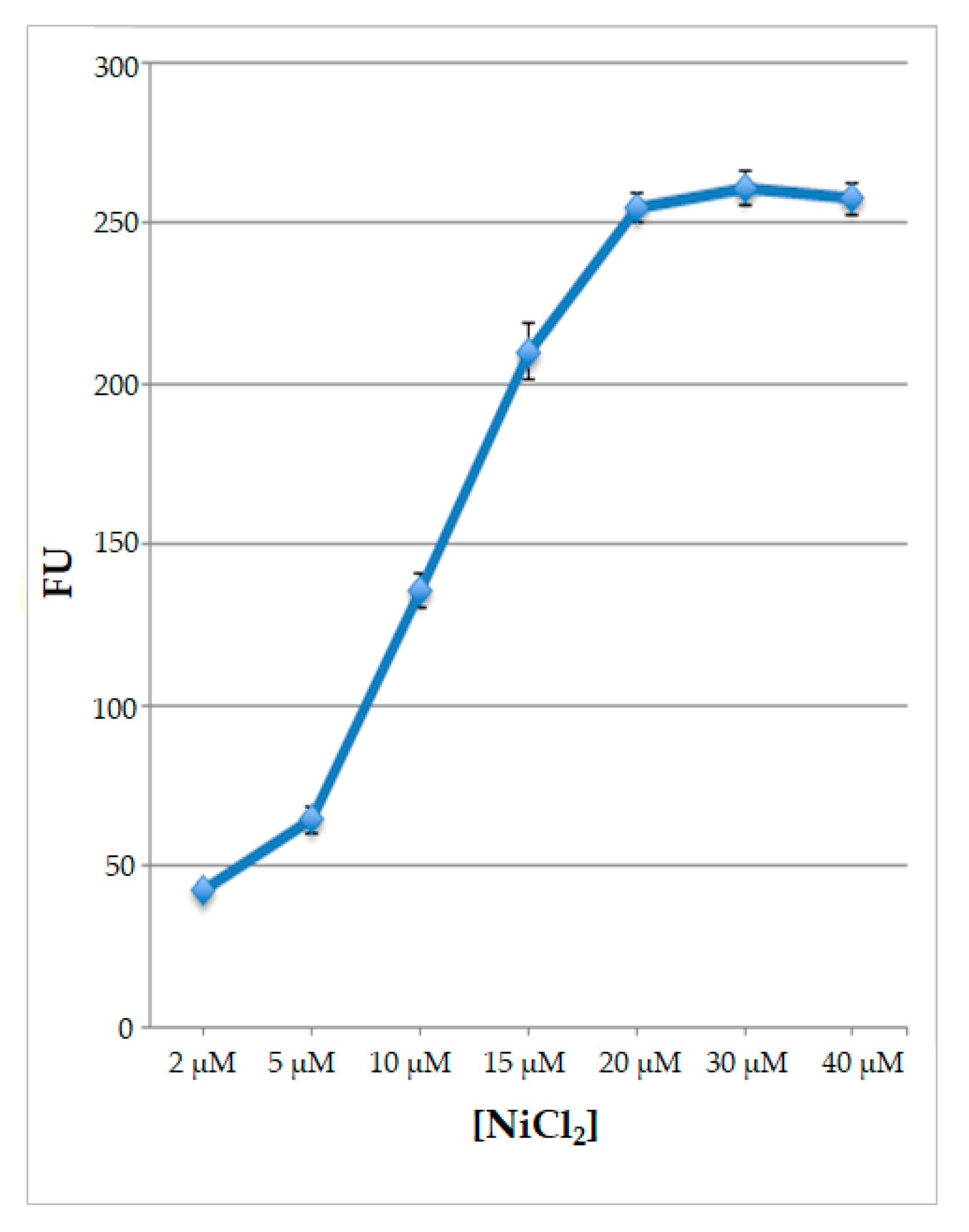
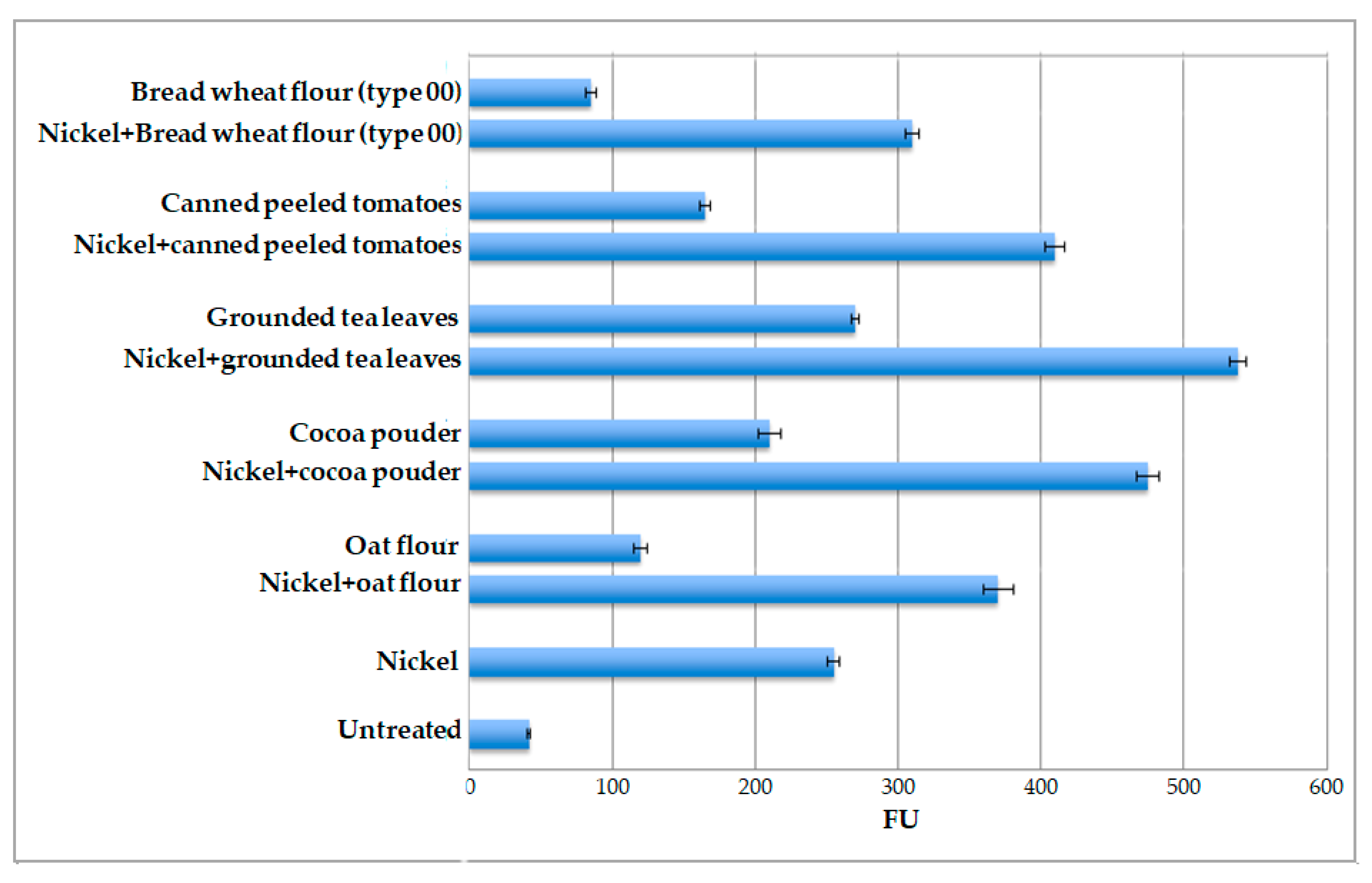
2.3. Nickel Ions Detection in Different Food Matrices
3. Discussion
4. Materials and Methods
4.1. Plasmids Construction
4.2. Protoplasts Preparation, Transformation and Immobilization
4.3. Protoplasts Stress Treatments with Different Heavy Metal Ions
4.4. FDA Assay for Protoplast Viability Estimation
4.5. Confocal Microscopy and Fluorescence Determination
4.6. Food Matrices Utilized for Nickel Ions Detection
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabata-Pendias, A.; Mukherjee, A.B. Soil. In Trace Elements from Soil to Human; Springer: Berlin/Heidelberg, Germany, 2007; pp. 9–38. [Google Scholar] [CrossRef]
- Taghavi, M.; Darvishiyan, M.; Momeni, M.; Eslami, H.; Fallahzadeh, R.A.; Zarei, A. Ecological risk assessment of trace elements (TEs) pollution and human health risk exposure in agricultural soils used for saffron cultivation. Sci. Rep. 2023, 13, 4556. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Jahangeer, M.; Bouyahya, A.; Omari, N.E.; Ghchime, R.; Balahbib, A.; Aboulaghras, S.; Mahmood, Z.; Akram, M.; Shah, S.M.A.; et al. Heavy Metal Contamination of Natural Foods Is a Serious Health Issue: A Review. Sustainability 2022, 14, 161. [Google Scholar] [CrossRef]
- Gutiérrez, J.C.; Amaro, F.; Martin-Gonzáles, A. Heavy metal whole-cell biosensor using eukaryotic microorganism: An update critical review. Front. Microbiol. 2015, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Lusi, E.A.; Di Ciommo, V.M.; Patrissi, T.; Guarascio, P. High prevalence of nickel allergy in an overweight female population: A pilot observational analysis. PLoS ONE 2015, 10, e0123265. [Google Scholar] [CrossRef] [PubMed]
- Venter, C. Food Hypersensitivity: Diagnosing and Managing Food Allergies and Intolerances. J. Allergy 2012, 2012, 576017. [Google Scholar] [CrossRef] [PubMed]
- Anchidin-Norocel, L.; Savage, W.K.; Gutt, G.; Amariei, S. Development, Optimization, Characterization, and Application of Electrochemical Biosensors for Detecting Nickel Ions in Food. Biosensors 2021, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Belkin, S. Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol. 2003, 6, 206–212. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Zhang, B.; Liu, S.; Liu, C.-g.; Li, F.; Song, H. Engineering whole-cell microbial biosensors: Design principles and applications in monitoring and treatment of heavy metals and organic pollutants. Biotechnol. Adv. 2022, 60, 108019. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Su, H.; Guo, M.; Liu, H. Advances in Synthetic-Biology-Based Whole-Cell Biosensors: Principles, Genetic Modules, and Applications in Food Safety. Int. J. Mol. Sci. 2023, 24, 7989. [Google Scholar] [CrossRef]
- Schoor, S.; Lung, S.C.; Sigurdson, D.; Chuong, S.D. Fluorescent Staining of Living Plant Cells. In Plant Microtechniques and Protocols; Yeung, E.C.T., Stasolla, C., Sumner, M.J., Huang, B.Q., Eds.; Springer: Cham, Switzerland, 2015; pp. 153–165. [Google Scholar] [CrossRef]
- Widholm, J.M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Borghini, R.; De Amicis, N.; Bella, A.; Greco, N.; Donato, G.; Picarelli, A. Beneficial Effects of a Low-Nickel Diet on Relapsing IBS-Like and Extraintestinal Symptoms of Celiac Patients during a Proper Gluten-Free Diet: Nickel Allergic Contact Mucositis in Suspected Non-Responsive Celiac Disease. Nutrients 2020, 12, 2277. [Google Scholar] [CrossRef]
- Cappelli, A.; Bini, A.; Cini, E. The Effects of Storage Time and Environmental Storage Conditions on Flour Quality, Dough Rheology, and Biscuit Characteristics: The Case Study of a Traditional Italian Biscuit (Biscotto di Prato). Foods 2022, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Pisano, A.; Mezzatesta, L.; Pettinelli, M.; Meacci, A.; Pignataro, M.G.; Giordano, C.; Picarelli, A. New Insights and Evidence on “Food Intolerances”: Non-Celiac Gluten Sensitivity and Nickel Allergic Contact Mucositis. Nutrients 2023, 15, 2353. [Google Scholar] [CrossRef] [PubMed]
- Scutarsu, E.C.; Trinca, L.C. Heavy Metals in Foods and Beverages: Global Situation, Health Risks and Reduction Methods. Foods 2023, 12, 3340. [Google Scholar] [CrossRef]
- Lamers, J.; van der Meer, T.; Testerink, C. How Plants Sense and Respond to Stressful Environments. Plant Physiol. 2022, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, T.; Hu, J.; Zhao, L.; Yu, C.; Ma, F. Research advances in function and regulation mechanisms of plant small heat shock proteins (sHSPs) under environmental stresses. Sci. Total Environ. 2022, 825, 154054. [Google Scholar] [CrossRef]
- Gullì, M.; Rampino, P.; Lupotto, E.; Marmiroli, N.; Perrotta, C. The effect of heat stress and cadmium ions on the expression of a small hsp gene in barley and maize. J. Cer. Sci. 2005, 42, 25–31. [Google Scholar] [CrossRef]
- Rampino, P.; Mita, G.; Assab, E.; De Pascali, M.; Giangrande, E.; Treglia, A.S.; Perrotta, C. Two sunflower 17.6HSP genes, arranged in tandem and highly homologous, are induced differently by various elicitors. Plant Biol. 2010, 12, 13–22. [Google Scholar] [CrossRef]
- Picarelli, A.; Di Tola, M.; Vallecoccia, A.; Libanori, V.; Magrelli, M.; Carlesimo, M.; Rossi, A. Oral mucosa patch test: A new tool to recognize and study the adverse effects of dietary nickel exposure. Biol. Trace Elem. Res. 2011, 139, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Pizzutelli, S. Systemic nickel hypersensitivity and diet: Myth or reality? Eur. Ann. Allergy Clin. Immunol. 2011, 43, 5–18. [Google Scholar] [PubMed]
- Rizzi, A.; Nucera, E.; Laterza, L.; Gaetani, E.; Valenza, V.; Corbo, G.M.; Inchingolo, R.; Buonomo, A.; Schiavino, D.; Gasbarrini, A. Irritable Bowel Syndrome and Nickel Allergy: What Is the Role of the Low Nickel Diet? J. Neurogastroenterol. Motil. 2019, 23, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Gullì, M.; De Pascali, M.; Perrotta, C.; Rampino, P. A stress-related transcription factor belonging to the YL-1 family is differently regulated in durum wheat cultivars differing in drought sensitivity. Plant Physiol. Biochem. 2022, 170, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Freydl, E.; Meins, F., Jr.; Boller, T.; Neuhaus, J.M. Kinetics of prolyl hydroxylation, intracellular transport and C-terminal processing of the tobacco vacuolar chitinase. Planta 1995, 197, 250–256. [Google Scholar] [CrossRef]
- De Caroli, M.; Lenucci, M.S.; Manualdi, F.; Dalessandro, G.; De Lorenzo, G.; Piro, G. Molecular dissection of Phaseolus vulgaris polygalacturonase-inhibiting protein 2 reveals the presence of hold/release domains affecting protein trafficking toward the cell wall. Front. Plant Sci. 2015, 6, 660. [Google Scholar] [CrossRef]


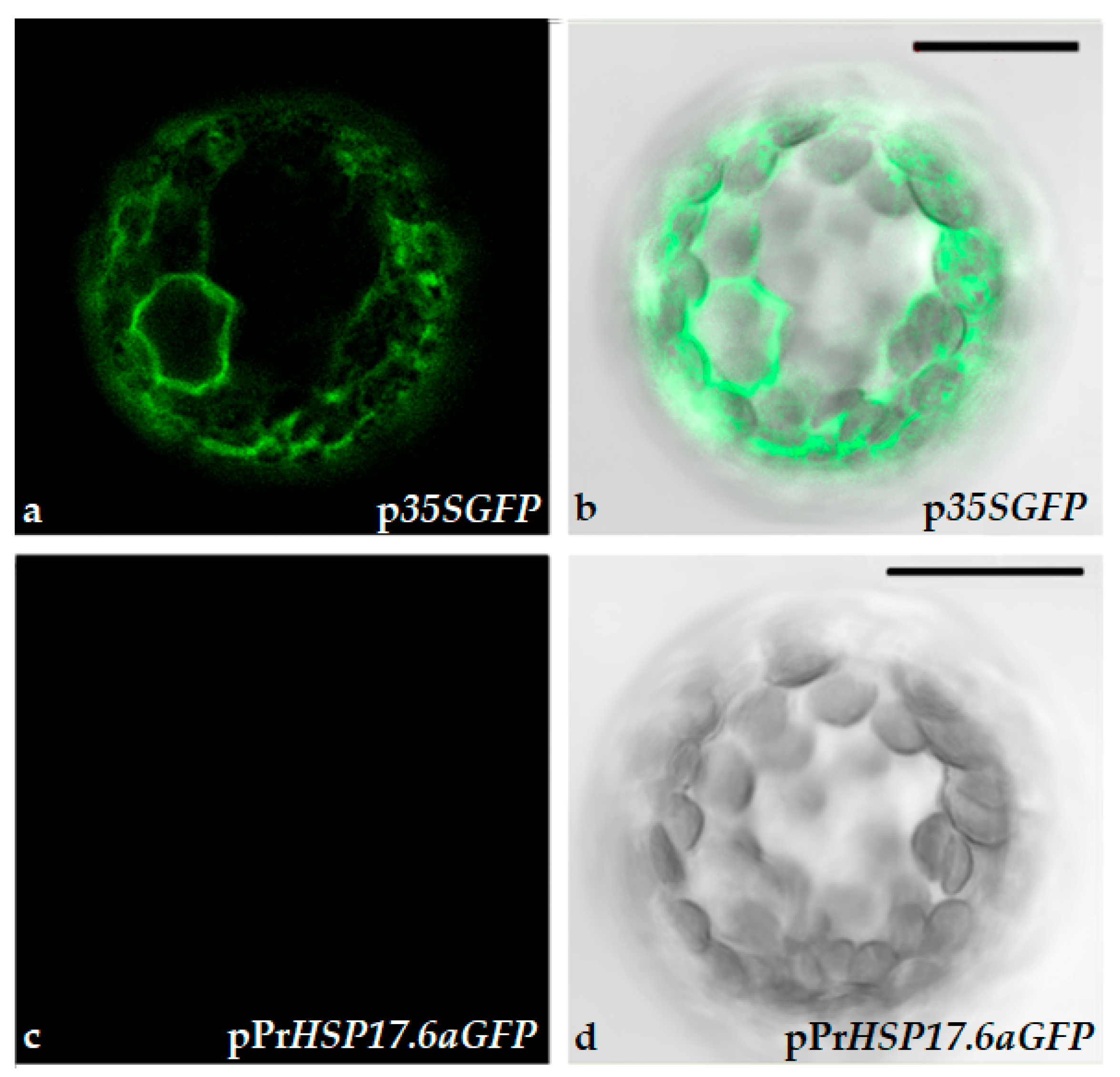

| Floating Protoplasts (%) | Immobilized Protoplasts (%) | |
|---|---|---|
| WT | 98.77 ± 1.23 | 97.70 ± 2.30 |
| p35SGFP | 99.05 ± 0.95 | 98.44 ± 1.56 |
| pPrHSP17.6aGFP | 97.87 ± 2.13 | 98.65 ± 1.35 |
| Mean ± SD of GFP Pixel Intensity per Protoplast | |
|---|---|
| AlCl3 (2 h) | 4.76 ± 0.43 ** |
| CdSO4 (2 h) | 4.21 ± 0.46 ** |
| CoCl2 (2 h) | 5.27 ± 0.66 ** |
| CuSO4 (3 h) | 4.02 ± 0.51 ** |
| NiCl2 (2 h) | 7.30 ± 0.46 |
| ZnSO4 (2 h) | 5.18 ± 0.52 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caroli, M.; Perrotta, C.; Rampino, P. Development of a Whole-Cell System Based on the Use of Genetically Modified Protoplasts to Detect Nickel Ions in Food Matrices. Int. J. Mol. Sci. 2024, 25, 6090. https://doi.org/10.3390/ijms25116090
De Caroli M, Perrotta C, Rampino P. Development of a Whole-Cell System Based on the Use of Genetically Modified Protoplasts to Detect Nickel Ions in Food Matrices. International Journal of Molecular Sciences. 2024; 25(11):6090. https://doi.org/10.3390/ijms25116090
Chicago/Turabian StyleDe Caroli, Monica, Carla Perrotta, and Patrizia Rampino. 2024. "Development of a Whole-Cell System Based on the Use of Genetically Modified Protoplasts to Detect Nickel Ions in Food Matrices" International Journal of Molecular Sciences 25, no. 11: 6090. https://doi.org/10.3390/ijms25116090
APA StyleDe Caroli, M., Perrotta, C., & Rampino, P. (2024). Development of a Whole-Cell System Based on the Use of Genetically Modified Protoplasts to Detect Nickel Ions in Food Matrices. International Journal of Molecular Sciences, 25(11), 6090. https://doi.org/10.3390/ijms25116090







